Abstract
Very low-density lipoprotein (VLDL) is the main plasma carrier of triacylglycerol that is elevated in pathological conditions such as diabetes, metabolic syndrome, obesity and dyslipidemia. How variations in triacylglycerol levels influence structural stability and remodeling of VLDL and its metabolic product, low-density lipoproteins (LDL), is unknown. We applied a biochemical and biophysical approach using lipoprotein remodeling by lipoprotein lipase and cholesterol ester transfer protein, along with thermal denaturation that mimics key aspects of lipoprotein remodeling in vivo. The results revealed that increasing the triacylglycerol content in VLDL promotes changes in the lipoprotein size and release of the exchangeable apolipoproteins. Similarly, increased triacylglycerol content in LDL promotes lipoprotein remodeling and fusion. These effects were observed in single-donor lipoproteins from healthy subjects enriched in exogenous triolein, in single-donor lipoproteins from healthy subjects with naturally occurring differences in endogenous triacylglycerol, and in LDL and VLDL from pooled plasma of diabetic and normolipidemic patients. Consequently, triacylglycerol-induced destabilization is a general property of plasma lipoproteins. This destabilization reflects a direct effect of triacylglycerol on lipoproteins. Moreover, we show that TG can act indirectly by increasing lipoprotein susceptibility to oxidation and lipolysis and thereby promoting the generation of free fatty acids that augment fusion. These in vitro findings are relevant to lipoprotein remodeling and fusion in vivo. In fact, fusion of LDL and VLDL enhances their retention in the arterial wall and, according to the response-to-retention hypothesis, triggers atherosclerosis. Therefore, enhanced fusion of triacylglycerol-rich lipoproteins suggests a new causative link between elevated plasma triacylglycerol and atherosclerosis.
Keywords: Lipoprotein remodeling and fusion, apolipoprotein dissociation, free fatty acids, lipoprotein oxidation and lipolysis, diabetes and atherosclerosis
Graphical Abstract
1. Introduction
Very-low, low- and high-density lipoproteins (VLDL, LDL and HDL) are heterogeneous non-covalent assemblies of lipids and apolipoproteins (apos) that solubilize lipids and direct their plasma transport and metabolism [1]. Lipoprotein surface is comprised of amphipathic apolipoproteins and polar lipids, mainly phospholipids and cholesterol, while apolar lipids, mainly cholesterol esters and triacylglycerols (TG), are sequestered in the lipoprotein core. During metabolism, lipoproteins are continuously remodeled by plasma factors such as hydrolases and lipid transfer proteins, which alter the biochemical composition, structure and function of lipoproteins. One example is TG hydrolysis into di- and monoacylglycerides and free fatty acids (FFA) by lipoprotein lipase (LpL) ([2] and references therein), which occurs at an obligatory step during maturation of VLDL (diameter 40-100 nm) into LDL (20-24 nm). Upon lipolysis of core TG, the excess surface material including exchangeable proteins (mainly apoE and apoCs) is released from VLDL in the form of free proteins or small particles that join the pool of plasma HDL (~10 nm) [3]. Another example is lipoprotein remodeling by cholesterol ester transfer protein (CETP) that shuttles TG and cholesterol esters among lipoproteins and is responsible for loading TG into the HDL core (for a recent review see [4]). Remodeling by these and other plasma factors, such as the phospholipid transfer protein and various lipases, can shift the balance between the apolar core of a lipoprotein and its polar surface. This balance can be restored upon release of excess surface materials, such as exchangeable apolipoproteins and phospholipids, and/or upon lipoprotein aggregation, fusion and coalescence into droplets, which alleviates the energetically unfavorable effects of insufficient protein surface coverage ([5-10] and references therein). Lipoprotein remodeling in vivo and in vitro can be influenced by multiple factors, such as the levels of FFA that are potent lipid fusogens: increased FFA levels generally promote lipoprotein remodeling and fusion, while FFA removal by albumin counteracts it [11]. While HDL fusion is a part of normal metabolism [10], fusion of LDL and VLDL, which occurs upon their hydrolytic and oxidative modifications [6-9,12], enhances their retention in the arterial wall matrix and is thought to be an early trigger of atherosclerosis ([13,14] and references therein). To understand and modulate lipoprotein function in health and disease, it is important to determine what factors influence lipoprotein susceptibility to remodeling such as hydrolysis, oxidation, apolipoprotein release and lipoprotein fusion.
Here we determine how variations in TG levels influence lipoprotein susceptibility to biochemical and structural remodeling in vitro. The focus is on LDL and VLDL, the major plasma carrier of TG. Non-fasting levels of plasma TG, which can vary in healthy subjects depending on the genetic and environmental factors, are elevated in diabetes, metabolic syndrome, obesity and dyslipidemia, and represent an independent causative risk factor for atherosclerosis [15-19]. Despite their importance, the effects of the naturally occurring variations in TG on the structural stability and remodeling of LDL and VLDL are unknown due to experimental challenges in stability studies of these large apoB-containing particles. We have developed a biophysical approach that uses thermal or chemical denaturation as an experimental model to mimic key aspects of lipoprotein remodeling, such as the protein release and lipoprotein fusion [8,20,21]. Previously we used this approach to show that elevated TG destabilize HDL and augment protein release and lipoprotein fusion [22]; other HDL studies using different approaches reached a similar conclusion [23,24]. We also showed that elevated FFA promote LDL fusion and coalescence into lipid droplets [11]. This effect that may contribute to the pro-atherogenic action of elevated FFA, which accompany elevated TG in conditions such as obesity and type-2 diabetes [25,26] and are a hallmark of inflammation and an independent risk factor for cardiovascular disease [27-29]. Here, we combine our biophysical approach with biochemical remodeling by plasma factors, such as CETP and LpL, to determine how naturally occurring variations in TG levels influence the structural stability of VLDL and LDL and their susceptibility to oxidation and lipolysis. The results shed new light on the causal link between elevated plasma TG and FFA and the risk of atherosclerosis.
2. Materials and Methods
2.1. Lipids and proteins
Palmitoyl-oleoyl phosphatidylcholine (POPC) was from Avanti Polar Lipids. Triolein (TO) and lipoprotein lipase (LpL) from porcine pancreas (# L3126) were from Sigma. Cholesterol ester transfer protein (CETP), which was purified from human plasma, was a generous gift from Prof. Kerry-Ann Rye, University of New South Wales, Australia. CETP was obtained and purified as previously described [30]; the protein purity was confirmed by mass spectrometry as described in the on-line supplement. All chemicals used in this study were of highest purity analytical grade.
2.2. Lipoprotein preparation
Single-donor human VLDL, LDL and HDL were isolated from plasma of anonymous healthy volunteers. The plasma was purchased from a local blood bank (Research Blood Components LLC, Boston, USA) in full compliance with the Institutional Review Board. Lipoproteins were isolated following established protocols [31] from fresh EDTA-containing plasma by KBr density gradient ultracentrifugation in the density range 0.94–1.006 g/mL for VLDL, 1.019–1.063 g/mL for LDL, and 1.063–1.21 g/mL for HDL. Each isolated lipoprotein fraction migrated as a single band on the agarose gel. Lipoproteins were dialyzed against the standard buffer (10 mM Na phosphate, pH 7.5) containing 0.25 mM EDTA, degassed, and stored in the dark at 4 °C. Prior to each experiment, aliquots of the stock solutions were dialyzed against the EDTA-free standard buffer. The stock solutions were used within 2-3 weeks, during which no protein degradation was detected by SDS PAGE and no changes in the lipoprotein electrophoretic mobility were seen on the agarose gel.
To enrich lipoproteins in exogenous TG, phospholipid/TO emulsions termed Intralipid were used as donors following published protocols [22,30,32]. While TG found in the lipoprotein core are asymmetric triglycerides containing three acyl chains of various length, TO is a symmetrical model TG that contains three units of oleic acid. To prepare Intralipid, TO (255 mg) and POPC (45 mg) were dissolved in chloroform and dried under a nitrogen stream for 2 h. Five ml of 10 mM PBS solution was added, and the mixture was sonicated continuously for 60 min using a Branson sonicator. The partially clarified mixture was centrifuged at a low speed, layered in tubes of a SW 50.1 rotor (Beckman) beneath a KBr solution of density 1.006 g/ml, and centrifuged for 1 h at 10 °C and 48,000 rpm. This technique was employed to remove excess phospholipids, since phospholipid vesicles with 1.03 g/ml density do not float under these conditions, whereas the lighter emulsions float. Next, the top layer containing the emulsion particles was carefully removed. The excess vesicles, visible as a band at the interface of the density solutions, were discarded. The purified Intralipid, which contained 27% phospholipids and 73% TO measured by enzymatic assays, was used within a day for lipid transfer studies.
To obtain plasma LDL and VLDL enriched with TO, single-donor lipoproteins (final TG concentration of 1.0 mM) were mixed with Intralipid (final TO concentration of 4.0 mM), either alone or with CETP (52.6 activity units/ml), and incubated at 37 °C for 3 h. As a control, lipoproteins and CETP were incubated at 37 °C for 3 h without Intralipid. LDL and VLDL were re-isolated in the density range 1.006-1.063 g/ml, with two spins at each density, followed by dialysis against the standard buffer. Table 1 lists TG composition in these lipoproteins.
Table 1.
Triglyceride levels in single-donor plasma VLDL and LDL that were either intact or enriched with exogenous TO (+TO). Lipoproteins were prepared and analyzed as described in Methods parts 2.2 and 2.4. Data represents the mean of three independent measurements with variability of ±2%.
| Lipoprotein | TG, mM/L |
|---|---|
| VLDL | |
| Intact | 0.95 |
| +TO | 1.23 |
| LDL | |
| Intact | 0.59 |
| +TO | 1.89 |
In another series of studies, we used plasma lipoproteins with naturally occurring low and high levels of endogenous TG, which were isolated from single-donor plasma of two healthy volunteer donors. The TG levels were 0.89 and 1.32 mM/L in low- and high-TG VLDL, 0.63 and 0.98 mM/L in low- and high-TG LDL, and 0.47 and 0.78 mM/L in low- and high-TG HDL. There values were measured in technical triplicates with 2% error.
To compare human lipoproteins from healthy and diabetic subjects, two different batches of pooled plasma were used in the current study. Both batches contained plasma from 20 healthy normolipemic and normoglycemic volunteers (NL) and from 20 patients who have been diagnosed with type-2 diabetes mellitus and presented a poorly controlled glycemic index (PC, hemoglobin A1c: HbA1c ≥7%). These patients were subjected to hypoglycemic treatment (diet, exercise, metformin, insulin depending on the clinical situation) to improve their glycemic control (good control, GC, HbA1c 1.5% below baseline value and always lower than 7%). Plasma was obtained at the Lipid Laboratory of Hospital de Sant Pau (Barcelona, Spain) with written informed consent by the patients and upon approval by the institutional ethics committee. The plasma was pooled for NL, GC and PC patients and the lipoproteins were isolated by density gradient ultracentrifugation. Protein and lipid composition in lipoproteins from each batch was determined as previously described [22]. The results are shown in Table S1 of the online supplement.
2.3. Transmission electron microscopy, gel electrophoresis and size exclusion chromatography
Lipoproteins were visualized using negative-stain transmission electron microscopy with a CM12 transmission electron microscope (Philips Electron Optics, the Netherlands) as previously described [8,20].
For non-denaturing PAGE, Novex™ 4-20% or 4% Tris-glycine gels (from Invitrogen) were loaded with 6 μg protein per lane and run to termination at 1,500 V-h under non-denaturing conditions in Tris-glycine buffer. For SDS PAGE, the gels were loaded with 5 μg protein per lane and run at 200 V for 1 h under denaturing conditions in SDS-Tris-glycine buffer. The gels were stained with Denville blue protein stain (Denville Scientific).
Agarose gel electrophoresis was done using TITAN lipoprotein gel electrophoresis system. Lipoprotein samples containing 4 μg protein were loaded on the pre-cast gels that were run in barbital-sodium barbital buffer at 60 V for 40 min and at 125 V for 7 min. The gels were dried at 70 °C for 20 min, stained with 0.1 % w/v Fat red 7B stain in 95% methanol, destained in 75% methanol, and dried at 70 °C.
Size exclusion chromatography (SEC) was performed with a Superdex 200 prep grade XK 16/100 column or a Superose 6 10/300 GL column controlled by an ÄKTA UPC 10 FPLC system (GE Healthcare). Elution by phosphate buffer saline (10 mM Na phosphate, 150 mM NaCl, pH 7.5) was carried out at a flow rate of 0.5 ml/min.
2.4. Lipoprotein biochemical analyses and remodeling
Cholesterol, TG, and phospholipids were quantified using enzymatic assays following published protocols [33]. Protein concentration was determined using a modified Lowry assay with bovine serum albumin as a standard. FFA were measured using an enzymatic colorimetric assay (Enzymchrom™ free fatty acid kit). All concentrations were measured in technical triplicates.
Lipid composition of selected lipoproteins before and after thermal or chemical denaturation was assessed by thin-layer chromatography. The lipids were extracted by Folch method with 2:1 chloroform :methanol, and were dried under nitrogen [34]. Known amounts of dry lipids were analyzed using hexane:ether:acetic acid (70:30:1) to separate apolar lipids, or chloroform:methanol:water:acetic acid (65:25:4:1) to separate polar lipids.
To selectively hydrolyze TG in the lipoprotein core, LDL and VLDL were remodeled by secretory LpL that hydrolyzes TG in these lipoproteins in vivo. Lipoproteins (0.5 mg/ml total protein) were incubated with LpL (24 U/ml) at 37 °C for 1 h in 10 mM Tris buffer saline containing 2.5 mM CaCl2 at pH 7.7. The reaction was quenched with EDTA (final concentration 20 mM) in Tris buffer saline. The extent of the reaction was quantified by measuring the FFA generated upon TG hydrolysis. At this relatively short incubation time with LpL, no significant changes in the lipoprotein size and morphology were detected.
To analyze HDL remodeling by CETP, HDL (final protein concentration 1 mg/ml) was incubated with VLDL as TG donors (final TG concentration 5 mM) and CETP as a TG transporter (final concentration 5 units/ml) in standard buffer at 37 °C for 24 h. The samples were cooled on ice to terminate the reaction. Next, the samples were analyzed by SEC using a 7 Superdex 200 preparatory grade XK 16/100 column; the lipoproteins were eluted in 10 mM PBS, pH 7.5 at a flow rate of 0.5 ml/min.
Oxidation of lipoproteins by Cu2+ was carried out as previously described [35]. The reaction time course was monitored continuously by UV absorbance at 234 nm that reports mainly on the conjugated diene formation. Lipoprotein solutions (0.1 mg/ml protein) were equilibrated at 37 °C and the oxidation was initiated by adding CuSO4 to a final concentration of 5 μM. Lipid oxidation at 37 °C was monitored using Varian Cary Biomelt-300 UV-Vis absorption spectrometer with thermoelectric temperature controller.
2.5. Lipoprotein secondary structure and stability studied by circular dichroism and calorimetry
Secondary structure and stability of lipoproteins were characterized by circular dichroism (CD) spectroscopy, turbidity and differential scanning calorimetry using an experimental approach and sample conditions developed in our previous studies [8,21,36]. Lipoprotein solutions in standard buffer containing 150 mM NaCl were used. CD data were recorded by using an AVIV-400 spectropolarimeter equipped with thermoelectric temperature controller. To characterize protein secondary structure, far-UV CD spectra (190-250 nm) were recorded as previously described [8,36]. To compare thermal stability, lipoprotein solutions containing 0.1 mg/ml protein were heated and cooled from 5 to 98 °C at a constant rate of 11 °C/h. Heat-induced changes in far-UV CD were monitored at 220 nm or 222 nm for secondary structural unfolding. This method was well-suited for HDL and VLDL whose exchangeable apolipoproteins undergo cooperative thermal unfolding [8,36]. Far-UV CD data were normalized to the protein concentration and reported in units of molar residue ellipticity, [Θ]. ORIGIN software was used for data processing and display.
As a complementary method to assess lipoprotein stability, changes in turbidity were monitored during heating and cooling at 320 nm by using dynode voltage (in Volts) measured in CD experiments, as previously described [8]. This method enabled us to monitor changes in the particle size resulting from the lipoprotein remodeling upon heating. Previously we showed that such a remodeling involves release and unfolding of exchangeable proteins along with lipoprotein fusion, followed by lipoprotein rupture and release of apolar core lipids that coalesce into droplets and their aggregates [8,21,36]. These morphological transitions cause an initial increase in turbidity due to increased particle size upon fusion, aggregation and droplet formation; this increase is followed by a drop in turbidity upon phase separation in aqueous suspensions of fully denatured lipoproteins [8].
Differential scanning calorimetry was used to assess lipoprotein thermal stability as previously described [36]. Lipoprotein fusion and rupture manifest themselves as distinct peaks in the heat capacity function, CP(T). Since this method enables data collection up to 115 °C, it is particularly useful for HDL that are highly thermostable and undergo rupture above 110 °C, which is outside the range accessible by conventional CD spectrometers. Briefly, HDL solutions (3 mg/mL protein in standard buffer containing 150 mM NaCl) were heated from 5 °C to 120 °C at a rate of 90 °C/h, and the heat capacity CP(T) was recorded using VP-DSC microcalorimeter (MicroCal, MA, USA). The buffer baselines were subtracted from the data. ORIGIN software was used for the data collection, analysis and display.
2.6. Reproducibility
Unless otherwise stated, all experiments in this study were repeated at least three times to ensure reproducibility. Studies of TO-enriched single-donor lipoproteins were performed in technical triplicates of biological duplicates. Studies of single-donor lipoproteins with naturally occurring high-TG and low-TG levels were performed in technical triplicates using two different lipoprotein batches. Comparative studies of pooled lipoproteins from plasma of normolipidemic and diabetic patients were performed in technical triplicates of biological duplicates using two lipoprotein batches (batch 1 and 2, Table S1). Both batches showed similar trends; the data for batch 1 are shown. Statistical analysis was performed using ANOVA t-test.
3. Results
3.1. Human LDL and VLDL enriched with exogenous TO show decreased thermal stability
To determine how increased TG levels influence the structure and stability of VLDL and LDL, single-donor lipoproteins from normolipidemic plasma were enriched with exogenous TO using Intralipid and CETP. Table 1 lists the TG content of these VLDL and LDL before (intact) and after the enrichment (+TO); for both lipoproteins, the TG levels upon enrichment were in the physiological range. No other changes in the lipid or protein composition of VLDL and LDL occurred upon enrichment. SDS PAGE and transmission electron microscopy detected no changes in the protein composition or in the size and morphology of lipoproteins upon their enrichment in TO (Figure S1).
Far-UV CD spectra of VLDL showed a marginally significant decrease in amplitude upon TO enrichment, indicating little if any decrease in the ordered protein secondary structure (Figure 1A). However, thermal denaturation revealed large effects of TO enrichment on the structural integrity of VLDL. Melting data of intact VLDL recorded at a rate of 11 °C/h showed a large irreversible increase in turbidity upon heating above 75 °C (Figure 1B, black line), which reflected lipoprotein fusion and rupture (described in Methods part 2.5; for details see [8]). In TO-enriched VLDL, the onset of this transition was observed at similar temperatures but the changes in turbidity occurred in a much narrower temperature range, suggesting increased cooperativity (Figure 1B, gray line). As a result, the apparent transition midpoint, Tm,app, shifted to lower temperatures: in intact VLDL increase in turbidity was observed between 75 and 98 °C, with 50% change at Tm,app= 84 °C, while in TO-enriched VLDL the turbidity reached maximum circa 82 °C and then rapidly dropped upon phase separation in fully denatured lipoprotein (Figure 1B). Hence, compared to intact VLDL,TO-enriched VLDL underwent a faster more extensive denaturation.
Figure 1.
Secondary structure and thermal stability of single-donor lipoproteins enriched with exogenous TO. Total TG content in these lipoproteins is shown in Table 1. Far-UV CD spectra were recorded of intact (black) and TO-enriched (gray) VLDL (A) and LDL (C). To record the melting data of VLDL (B) and LDL (D), the samples were heated and cooled at a rate of 11 °C/h, and changes in the particle size were monitored by turbidity at 320 nm. Arrows in panels B and D show the directions of temperature changes. An increase in turbidity observed upon heating above 70-75 °C reflects irreversible lipoprotein fusion, rupture and coalescence into aggregated lipid droplets, while the sharp drop in turbidity upon further heating results from the phase separation in aqueous suspensions of denatured lipoproteins (for details see Methods part 2.5 and [8,20]). The heating data in panels B, D show that the transition midpoint is observed at lower temperatures in high-TG lipoproteins (gray lines), suggesting their increased susceptibility to thermal remodeling as compared to their low-TG counterparts (black lines).
Similar to VLDL, far-UV CD spectra of LDL suggested little if any decrease in the ordered secondary structure upon TO enrichment (Fig. 1C, gray and black lines). Irreversible thermal denaturation of LDL was observed upon heating above 75 °C. Again, the cooperativity of this transition greatly increased upon TO enrichment, as evident from the increased slope of the turbidity heating curves (Figure 1D). As a result, the apparent transition midpoint decreased from Tm,app=90 °C in intact LDL to ~83°C in TO-enriched LDL (Figure 1D, black and gray lines).
In summary, enrichment in TO did not significantly alter the size and morphology of lipoproteins (Figure S1) and had little if any disordering effect on the secondary structure (Figure 1A, C). However, TO enrichment destabilized VLDL and LDL and greatly increased the cooperativity and the extent of their thermal denaturation (Figure 1B, D).
3.2. Single-donor human LDL and VLDL differing in endogenous TG levels differ in stability
Next, we tested whether naturally occurring variations in plasma TG levels significantly influence lipoprotein stability. To this end, we compared the biochemical composition, structure and stability of single-donor lipoproteins from two different healthy donors, one normolipidemic (termed low-TG) and another with elevated plasma TG (high-TG), but with otherwise similar biochemical composition. SDS PAGE and transmission electron microscopic data were very similar for the high-TG and low-TG lipoproteins (supplemental Figure S2). Far-UV CD spectra suggested little if any decrease in the ordered secondary structure of the high-TG compared to low-TG lipoproteins (Figure 2A and C, gray and black lines). Notably, a much steeper thermal denaturation was observed in high-TG VLDL and LDL as compared to their low-TG counterparts, suggesting that higher TG levels increased the cooperativity of the lipoprotein fusion and rupture (Figure 2B and D, gray and black lines).
Figure 2.
Secondary structure and thermal stability of single-donor VLDL and LDL with naturally occurring high and low levels of endogenous TG. TG content in these lipoproteins is listed in . Far-UV CD spectra and the melting data were recorded of VLDL (A, B) and LDL (C, D) as described in Figure 1 legend. Arrows in panels B and D show directions of temperature changes. The heating data in panels B, D show that the transition midpoint is observed at lower temperatures in high-TG lipoproteins (gray lines), suggesting their increased susceptibility to thermal remodeling as compared to their low-TG counterparts (black lines).
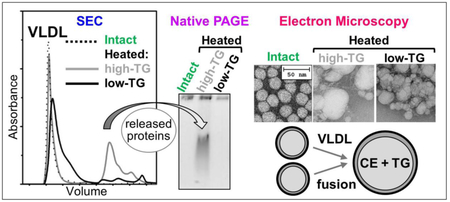
To probe in greater detail the effects of TG on the remodeling of VLDL and LDL, we incubated low-TG and high-TG lipoproteins for 10 min at 85 °C, cooled them to 25 °C, and analyzed them by SEC, non-denaturing PAGE and transmission electron microscopy (Figure 3). Intact VLDL migrated in a void volume in SEC (Figure 3A, dotted line), while heated VLDL showed an additional peak corresponding to smaller species (Figure 3A, solid lines). Previously we showed that such smaller species released from VLDL upon heating comprise HDL-size particles and free proteins [8]. Importantly, the release of such species was strongly TG- dependent: only high-TG VLDL showed a large SEC peak at 16-20 nm (gray line, Figure 3A) that corresponded to an additional band on the non-denaturing PAGE (Figure 3B). Mass spectrometry analysis of this SEC fraction identified apoE and apoCs, the major exchangeable apolipoproteins in VLDL (supplemental Figure S3). Furthermore, electron microscopy showed more extensive morphological changes and formation of larger particles in high-TG vis a vis low-TG VLDL (Figure 3C). Together, these results revealed that, under otherwise identical conditions, high-TG VLDL undergo much more extensive heat-induced structural remodeling and protein release compared to their low-TG counterparts.
Figure 3.
Effects of endogenous TG levels on the lipoprotein remodeling and fusion. Single-donor VLDL and LDL with naturally occurring high and low TG were same as those in Figure 2. To compare the extent of thermal remodeling, the lipoproteins were incubated for 10 min at 85 °C followed by cooling to 25 °C and analysis by SEC using Superose 6 10/300 GL column (A, C), non-denaturing PAGE, 4% (B, D), and transmission electron microscopy (E) as described in part 2.3 Methods. Intact (unheated) lipoproteins were used as controls; SEC profiles for high-TG and low-TG controls fully superimposed. In panels A and B, exchangeable proteins released from VLDL are indicated; these proteins have been identified by mass spectrometry (Figure S3). In panels D and E, numbers mark intact-size LDL (1), fused LDL (2), and ruptured/aggregated LDL (3). White lines between the lanes indicate split gels (panel E and other gels throughout this paper).
Similar to VLDL, high-TG LDL showed more extensive thermal remodeling compared to their low-TG counterparts (Figure 3D-F). Intact LDL migrated as a single peak circa 12 ml on SEC (Figure 3D, dotted line). Heated LDL showed additional peaks (Figure 3D) corresponding to larger particles formed upon LDL fusion, rupture and coalescence into droplets and their aggregates [21]. SEC and non-denaturing PAGE showed that in low-TG LDL, such fused and ruptured/aggregated particles were a minority, while in high-TG LDL the large aggregated particles became the majority (peak 3 in Figure 3D, E). Electron microscopy supported this observation: low-TG LDL showed little change in the particle size and morphology in these experiments, while high-TG LDL were extensively remodeled and coalesced into droplets and aggregates (Figure 3F).
In summary, the results in 1-3 showed that, regardless of their source (exogenous or endogenous), increased TG levels destabilize VLDL and LDL and promote their remodeling, including fusion, rupture and aggregation, along with the release of the exchangeable apolipoproteins from VLDL. Similar effects of exogenous and endogenous TG on the structural stability, fusion and protein release were detected previously in human plasma HDL using thermal and chemical denaturation, which was monitored by biochemical, spectroscopic and calorimetric methods [22]. Figure S4 shows representative SEC data illustrating such a remodeling of high-TG and low-TG HDL during thermal and chemical denaturation. Taken together, these results revealed that the destabilizing effects of TG are not limited to a particular lipoprotein class (VLDL, LDL or HDL), the source of TG (endogenous or exogenous), and the method used to perturb lipoproteins (thermal or chemical denaturation) or to monitor their structural integrity (CD spectroscopy, turbidity, calorimetry, gel electrophoresis, SEC, electron microscopy). Rather, these effects represent a common property of plasma lipoproteins: the higher are the TG levels, the lower the lipoprotein stability and the more extensive lipoprotein remodeling.
3.3. Thermal and chemical denaturation of lipoproteins is accompanied by lipolysis
Lipoprotein denaturation at high temperatures may involve spontaneous lipolysis; the hydrolytic products such as FFA are expected to influence lipoprotein remodeling and promote fusion [11]. To test whether thermal denaturation involved FFA generation in our studies, agarose gel electrophoresis was used to compare VLDL, LDL and HDL before and after incubation at 90 °C for 30 min (Figure 4). Heated lipoproteins consistently showed higher net negative charge, suggesting an increased content of FFA. These FFA would be generated upon hydrolysis of lipoprotein lipids such as phospholipids and TG, which are particularly susceptible to hydrolysis.
Figure 4.
Thermal denaturation of lipoproteins leads to lipolysis and generation of FFA. (A) Agarose gel electrophoresis (Fat red lipid stain) of single-donor normolipidemic VLDL, HDL and LDL, which were either intact or have been incubated for 30 min at 90 °C followed by cooling to 25 °C. (B) HDL remodeling during thermal denaturation is accompanied by FFA generation. HDL were heated from 25 °C at a rate of 90 °C/h; sample aliquots were taken at various temperatures from 60 to 95 °C (indicated on the lanes) and analyzed. Non-denaturing PAGE (4-20%) depicts structural remodeling of HDL upon heating above 60 °C. Agarose gel electrophoresis shows a progressive increase in the net negative charge on HDL upon FFA generation during this remodeling.
To test for a possible coupling between the lipoprotein disintegration upon thermal denaturation and the generation of FFA, HDL were heated at a constant rate, and sample aliquots taken at various temperatures were analyzed (Figure 4B). Non-denaturing PAGE showed that heating above 60 °C lead to progressive HDL disintegration including fusion, rupture and protein release. Agarose gel electrophoresis showed that this disintegration was concomitant with a progressive increase in the net negative charge of HDL, which was observed upon heating beyond 60 °C (Figure 4B). We conclude that the lipoprotein disintegration upon thermal denaturation was concomitant with the generation of FFA.
This observation helps explain increased cooperativity of thermal denaturation observed in TG-rich VLDL, LDL (Figures 1B, C and 2B, C) and HDL [22]. In fact, FFA are potent lipid fusogens that promote fusion and rupture of lipoproteins [11,37]. Therefore, FFA generated during thermal denaturation of lipoproteins further promote their fusion and rupture, leading to increased cooperativity of the denaturation reaction.
To test whether spontaneous lipolysis that accompanied lipoprotein denaturation at high temperatures also occurs at ambient temperatures, chemical denaturation of lipoproteins was explored as previously described [20]. VLDL and LDL were incubated with 6 M guanidinium hydrochloride for 3 to 6 hours at 37 °C. Non-denaturing PAGE of VLDL showed that after the incubation intact-size lipoproteins disappeared due to fusion and rupture that was accompanied by a release of exchangeable proteins (supplemental Figure S5 A). Non-denaturing PAGE of LDL after 3 h of incubation with the denaturant showed intact-size, fused and ruptured lipoproteins, while after 6 h of incubation only ruptured particles were observed, indicating complete denaturation of LDL (Figure S5 A). Thin-layer chromatography showed a gradual increase in FFA levels of VLDL during incubation with the denaturant for 3 to 6 h (Figure S5 B). In LDL where the initial FFA content was lower, a significant increase in FFA levels was detected by thin-layer chromatography after 6 h of incubation (Figure S5 B). Notably, control lipoproteins that were incubated at 37 °C in the absence of the denaturant showed no changes in the particle size on the non-denaturing PAGE, and no increase in the FFA levels were seen by thin-layer chromatography. Together, the results in Figure S5 suggest strongly that generation of FFA is coupled to lipoprotein disintegration during chemical denaturation.
Taken together, our results show that thermal and chemical denaturation of VLDL, LDL and HDL is accompanied by lipolysis and generation of FFA. Consequently, spontaneous hydrolysis during lipoprotein disintegration is an inherent property of lipoproteins which affects their denaturation data, particularly the transition cooperativity. Therefore, lipolysis should be taken into account in the interpretation of thermal and chemical denaturation data. Despite this caveat, lipoprotein denaturation provides a useful tool for comparative analysis of the overall particle stability.
3.4. Increased endogenous TG promote lipoprotein remodeling by plasma factors
To determine direct effects of TG on the lipoprotein remodeling in the absence of spontaneous lipolysis, we utilized two plasma factors that remodel lipoproteins in vivo. One factor is LpL that hydrolyses TG in plasma lipoproteins [1,2]. VLDL remodeling by LpL into smaller particles is accompanied by a release of exchangeable apolipoproteins, either in a free form or as HDL-size particles [3].
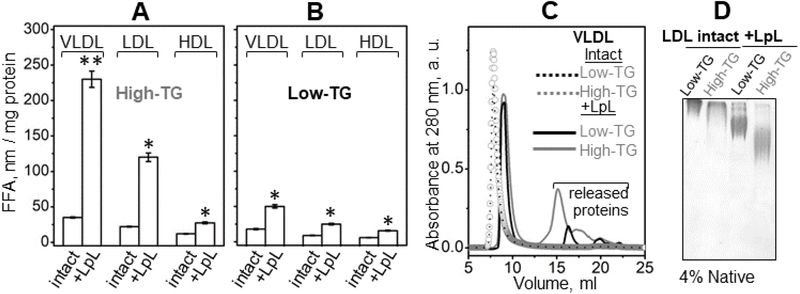
We used LpL to hydrolyze high-TG and low-TG VLDL and LDL as described in part 2.4 Methods. Under these conditions, LpL hydrolyzes TG but not phospholipids. The extent of TG hydrolysis was monitored by measuring FFA using a colorimetric assay. Figure 5A, B shows that the amount of FFA generated by LpL in high-TG VLDL was ~4.5 fold greater than that in low-TG VLDL. A ~2.5 fold increase in FFA generated by LpL was observed in high-TG versus low-TG LDL; a smaller but significant increase was also detected in HDL (Figure 5A, B). Next, we characterized structural remodeling of VLDL and LDL upon hydrolysis by LpL. As expected, SEC of VLDL showed that TG hydrolysis by LpL led to a small but significant increase in the elution volume of the main peak, from about 8 ml to 9 ml, reflecting a decrease in the VLDL particle size. This decrease in size was accompanied by formation of additional peaks at 14-22 ml corresponding to small HDL-size particles and free proteins released from VLDL surface upon hydrolysis of the core TG (Figure 5C). Notably, compared to low-TG VLDL, more surface material was released from high-TG VLDL (gray line in Figure 5C). This finding is consistent with more extensive TG hydrolysis and FFA generation in high-TG VLDL (Figure 5A). We conclude that high-TG VLDL undergo more extensive biochemical and structural remodeling by LpL compared to their low-TG counterparts.
Figure 5.
Effects of endogenous TG levels on the lipoprotein remodeling by LpL. Single-donor VLDL, LDL and HDL with naturally occurring high and low TG levels were the same as those shown in Figures S2, 2 and 3. The TG hydrolysis by LpL and the measurements of FFA generated in this reaction were performed as described in part 2.4 Methods. FFA quantification in (A) high-TG and (B) low-TG lipoproteins upon hydrolysis by LpL is shown. For each lipoprotein, mean values from three independent measurements ±STD are shown. Comparisons are shown for lipoproteins that were either intact or hydrolyzed (marked “intact” or “+LpL”). For each comparison, significant differences in the FFA content are indicated by asterisks, with p<0.05 (*) and p<0.01 (**) according to ANOVA t-test. (C) SEC data of VLDL that were either intact or hydrolyzed with LpL. (D) Non-denaturing PAGE (4%) of LDL that were intact or hydrolyzed with LpL.
Since the non-exchangeable apoB comprises nearly all LDL protein, with only minor amounts of exchangeable proteins present, structural remodeling of LDL by LpL did not involve any significant protein release. Non-denaturing PAGE showed a significant change in the electrophoretic mobility of LDL upon TG hydrolysis (Figure 5D), which was greater in high-TG than in low-TG LDL. This result was consistent with higher negative charge of the LpL-remodeled high-TG LDL and perhaps their smaller size. Together, the results in Figure 5 clearly show that high-TG VLDL and LDL undergo more extensive biochemical and structural remodeling by LpL compared to their low-TG counterparts.
In the next series of experiments, we probed the effects of endogenous TG levels on HDL remodeling by plasma factors. To this end we used CETP to remodel high-TG and low-TG HDL. CETP mediates the exchange of TG and cholesterol esters among plasma lipoproteins and is necessary to load TG in the HDL core. CETP-induced HDL remodeling in vitro has been previously shown to induce HDL fusion and release of apoA-I [5]; a similar remodeling of HDL by this and other plasma factors occurs in vivo and is an important step in HDL metabolism (reviewed in [10]). Notably, remodeling by CETP does not include lipolysis of HDL lipids. We used HDL as TG acceptors, VLDL as TG donors, and CETP as a TG transporter as described in part 2.4 Methods. Importantly, non-denaturing PAGE and SEC clearly showed a more extensive CETP-induced remodeling of high-TG as compared to low-TG HDL, which is evident from the extent of the protein release and lipoprotein fusion (Figure 6). Since FFA levels were invariant in these experiments, this result reflects a direct effect of TG: high-TG lipoproteins are more structurally labile and are remodeled by CETP more readily than their low-TGcounterparts.
Figure 6.
Effects of endogenous TG levels on HDL remodeling by CETP. TG content in single-donor low-TG and high-TG HDL was 0.47 and 0.78 nM/L, respectively. (A) Non-denaturing PAGE (4-20%) of HDL before and after incubation with CETP. (B) SEC profiles (Superdex 200 prep grade XK 16/100 column) of HDL before (intact) and after remodeling by CETP. Sample line coding is shown in the box. Intact-size HDL, fused HDL and released proteins are indicated.
In summary, the results in Figures 5 and 6 reveal that TG-rich lipoproteins undergo more extensive biochemical and structural remodeling by plasma factors such as LpL and CETP. These findings are consistent with the observation that TG-rich VLDL, LDL (Figures 1-3) and HDL [22] undergo more extensive thermal remodeling compared to their TG-poor counterparts. Together, the results in Figures 1-6 show that elevated TG content in the lipoprotein core promotes lipoprotein remodeling both directly (by destabilizing the lipoprotein particle) and indirectly (by increasing its susceptibility to lipolysis and promoting the generation of FFA).
3.5. Increased endogenous TG promote lipoprotein oxidation
In addition to the dual effects of TG described in part 3.4, increased oxidation of the TG-rich lipoproteins is also expected to contribute to the observed reduction in their structural stability. In fact, previous studies by us and others showed that oxidation significantly influences lipoprotein remodeling and fusion in vitro and in vivo [38,39]. Moreover, TG-rich lipoproteins are more susceptible to peroxidation, which was proposed to contribute to the increased atherogenic potential of the hypertriglyceridemic state [18]. In the current study, we tested whether single-donor lipoproteins with high and low levels of endogenous TG differed in their susceptibility to oxidation. To this end, the time course of the copper-mediated oxidation was monitored for high-TG and low-TG lipoproteins. A shorter lag phase was observed in high-TG VLDL, LDL and, to a lesser extent, HDL as compared to their low-TG counterparts (Figure 7). Consequently, high-TG lipoproteins were more susceptible to oxidation.
Figure 7.
Effects of endogenous TG levels on the lipoprotein susceptibility to oxidation. TG content in single-donor lipoproteins is listed in part 2.2 Methods. Time course of copper-induced oxidation of high-TG and low-TG VLDL (A), LDL (B) and HDL (C) at 37 °C was monitored by absorbance at 234 nm for conjugated diene formation. Lag (I), propagation (II) and saturation phases (III) of oxidation are indicated.
In summary, the results in Figures 1-7 revealed that several factors can contribute to the decreased structural stability of TG-rich VLDL, LDL and HDL. These factors include direct destabilizing effects of TG on the lipoprotein particle, as well as indirect effects resulting from increased susceptibility of TG-rich lipoproteins to spontaneous and enzymatic lipolysis and to oxidation.
3.6. Effects of TG on the oxidation, structure and stability of pooled VLDL, LDL, and HDL from diabetic and normolipidemic patients
Naturally occurring high TG are a hallmark of diabetes mellitus. To test whether structural stability of lipoproteins is decreased in diabetes mellitus, we explored VLDL, LDL, and HDL isolated from pooled plasma of diabetic and healthy subjects. The patients were divided into three cohorts on the basis of their medical history and hemoglobin glycemic index, A1c: healthy normolipidemic (NL) subjects had A1c<7%; diabetic patients with A1c<7% due to treatment had good glycemic control (GC); and diabetic patients with A1c≥7% had poor glycemic control (PC) (see Methods part 2.2 for details). Plasma from each cohort was pooled and the lipoproteins were isolated and analyzed for biochemical composition, structure and stability. Supplemental Table S1 lists the composition of VLDL, LDL and HDL from these three cohorts. Lipoproteins isolated from two independent batches of pooled plasma were explored in the current study (Table S1). The results for batch 1 are shown below (Figures 8, 9 and S7); the trends observed in lipoproteins from batches 1 and 2 were similar.
Figure 8.
Secondary structure and thermal stability of VLDL and LDL from healthy and diabetic subjects. Lipoproteins (batch 1, Table S1) were isolated from pooled plasma of normolipidemic subjects (NL, black) and of diabetic patients with either good glycemic control, A1c<7% (GC, light gray), or poor glycemic control, A1c≥7% (PC, dark gray). The lipoproteins were obtained as described in part 2.2 and the data were recorded as described in part 2.5 Methods. For VLDL (top panels), far-UV CD spectra (A) and the CD melting data at 220 nm (B) are shown. The melting data monitor changes in the protein secondary structure upon heating and cooling. For LDL (bottom panels), far-UV CD spectra (C) and the melting data recorded by turbidity at 320 nm (D) are shown. The turbidity data monitor changes in the particle size during thermal remodeling. Arrows in panels B and D show directions of the temperature changes.
Figure 9.
Secondary structure and thermal stability of HDL from normolipidemic and diabetic subjects. Lipoproteins were obtained from pooled plasma of the same cohorts of patients as those in Figure 8. (A) Far-UV CD spectra and (B) the CD melting data at 222 nm to monitor α-helical unfolding upon heating and cooling at a rate of 11 °C/h were recorded as described in part 2.5 Methods. (C) Heat capacity, Cp(T), was recorded by differential scanning calorimetry as described in part 2.5 during HDL heating at a rate of 90 °C/h. The calorimetric data are shifted along the Y-axis to avoid overlap. Two peaks in the Cp(T) function, indicated by vertical bars, represent HDL fusion (a broad peak below 100 °C) followed by rupture and release of core lipids (a sharp peak above 100 °C). Arrows show directions of the temperature changes.
SDS PAGE and transmission electron microscopy did not detect any significant differences among lipoproteins obtained from these cohorts of patients; representative results for VLDL, LDL and HDL from batch 1 are shown in the supplemental Figure S6 A-D. However, intact VLDL, LDL and HDL showed significantly higher levels of the endogenous FFA in diabetic versus normolipidemic patients (Figure S6 E); the FFA levels correlated directly with the TG content in these lipoproteins (Table S1, batch 1).
To test whether the lipoproteins from diabetic patients were more susceptible to oxidation, the time course of copper-mediated peroxidation was monitored for all lipoprotein classes from the three cohorts (supplemental Figure S7). VLDL and LDL from diabetic patients showed faster oxidation compared to their normolipidemic counterparts (Figure S7 A, B). The rank order of oxidizability, NL<GC≤PC, was related directly to the content of TG and FFA in these lipoproteins. In HDL, which have lower TG levels compared to LDL and VLDL, only minor differences in the final signal but no large differences in the oxidation rate were detected by absorbance at 234 nm (Figure S7 C). Hence, all our studies of pooled as well as single-donor lipoproteins consistently showed that TG-rich lipoproteins were more susceptible to oxidation compared to their TG-poor counterparts (Figures 7, S7). We conclude that increased TG levels in lipoprotein from various classes lead to their increased oxidizability.
Secondary structural and stability studies of pooled VLDL, LDL and HDL revealed clear cohort-specific differences (Figures 8, 9). Far-UV CD spectra of VLDL showed less well-ordered secondary structure in VLDL of diabetic patients (Figure 8A), with the most ordered structure observed in NL VLDL (black) and least order in PC VLDL (grey). Importantly, studies of VLDL structural stability monitored by turbidity for changes in the particle size and by CD at 220 nm for secondary structural unfolding upon heating showed a rank order of stability NL>GC>PC; Figure 8B shows representative CD melting data of VLDL.
A similar rank order was observed in the structure and stability of LDL, which was monitored by far-UV CD and turbidity (Figure 8C, D). Thus, NL lipoproteins consistently showed more well-ordered protein structure and highest thermal stability, while PC lipoproteins were least ordered and least stable (Figure 8).
Similar to VLDL and LDL, the rank order for the well-ordered secondary structure in HDL indicated by far-UV CD spectra was NL>GC>PC (Figure 9A). However, CD melting data of HDL recorded at 222 nm for α-helical unfolding did not detect any cohort-specific differences in the temperature range of denaturation (Figure 9B). This observation could reflect the fact that the highest temperature attained in CD experiments was 98 °C, which was insufficient to fully denature HDL upon relatively fast heating in these experiments (see part 2.5 Methods for detail). To completely denature HDL, we used differential scanning calorimetry that enables data collection up to 115°C and can clearly distinguish between lipoprotein fusion and rupture [11,36]. The calorimetric peaks corresponding to HDL fusion and rupture showed no significant cohort-dependent differences (Figure 9C). Relatively low absolute values of TG in these HDL and relatively small differences in the TG content of HDL from different cohorts (Table S1) help explain why these HDL showed no cohort-dependent differences in stability. In fact, previous studies of pooled HDL that had higher absolute TG levels and a larger difference in the TG content of diabetic versus normolipidemic lipoproteins showed that HDL with the highest TG content had lowest structural stability and was most susceptible to oxidation and lipolysis [22]. Hence, despite these batch-to-batch variations, all our studies showed a similar trend: HDL with higher TG levels are more labile structurally as well as biochemically.
In summary, our results consistently showed that the structural stability of VLDL and LDL declines in order NL>GC>PC (Figures 9B, D). This rank order was inverse of the cohort-dependent differences in the levels of endogenous TG (Table S1) and FFA (Figure S6 E) of VLDL and LDL, NL<GC≤PC, and of the cohort-dependent lipoprotein susceptibility to lipolysis and oxidation (Figures S6F, S7). Hence, our results consistently showed that structural stability of pooled plasma VLDL and LDL was related inversely to the TG and FFA content, while the lipoprotein susceptibility to lipolysis and peroxidation was related directly to the TG content. We conclude that TG-rich lipoproteins are more labile to structural remodeling and biochemical modifications compared their TG-poor counterparts.
4. Discussion
Although VLDL followed by LDL are the major carriers of plasma TG, the effects of the naturally occurring variations in the TG cargo on the structural stability and remodeling of VLDL and LDL have not been previously explored because of the challenges in the stability studies of these large apoB-containing lipoproteins. To determine these effects, we combined a biophysical approach based on thermal remodeling, which was developed in our previous lipoprotein studies [8,21,22,36], with the biochemical remodeling of these lipoproteins by LpL and CETP and with copper oxidation. Our goal was to elucidate how the naturally occurring variations in the TG levels of plasma lipoproteins influence their biochemical and structural remodeling by these and perhaps other plasma factors.
Thermal remodeling studies revealed that, similar to HDL [22], increased TG levels in human plasma VLDL and LDL reduced their structural stability and augmented their fusion and rupture (Figures 1- 3 and 8). Moreover, like in HDL, in VLDL increased TG levels also promoted the heat-induced release of the exchangeable apolipoproteins (Figure 3A, B). These effects were observed in single-donor plasma VLDL and LDL from healthy subjects enriched with exogenous TO (Figure 1), in single-donor VLDL and LDL from healthy subjects with natural variations in the endogenous TG levels (Figures 2, 3), and in pooled VLDL and LDL from normolipidemic and diabetic subjects (Figure 8). Collectively, these findings revealed that naturally occurring variations in TG levels significantly influence structural stability and thermal remodeling of VLDL and LDL: the higher the TG levels the less stable the lipoproteins are and the more extensive is their remodeling.
A similar trend was observed during biochemical and structural remodeling of VLDL and LDL by LpL (Figure 5) and of HDL by CETP (Figure 6): TG-rich lipoproteins were more susceptible to remodeling. Moreover, studies of copper-induced oxidation showed a similar trend: TG-rich VLDL, LDL and HDL were more susceptible to phospholipid peroxidation as compared to their low-TG counterparts (Figures 7, S7). Taken together, these results suggest strongly that lipoproteins with elevated TG levels are more labile structurally as well as biochemically, and are more extensively remodeled upon various perturbations (thermal, enzymatic, hydrolytic, oxidative) in vitro.
These in vitro findings are relevant to the lipoprotein remodeling and fusion in vivo. According to the response–to-retention hypothesis of atherosclerosis [13,14], fusion of LDL and VLDL promotes lipoprotein retention in the arterial wall matrix and is an early triggering event in atherogenesis [7,9,12]. Lipoprotein oxidation is thought to importantly contribute to this event ([40] and references therein). The results reported here suggest that increased levels of plasma TG, which are a hallmark of diabetes, metabolic syndrome, obesity and dyslipidemia and an independent risk factor for atherosclerosis ([12,15-19] and references therein), promote pro-atherogenic lipoprotein oxidation and fusion. Therefore, our findings suggest an additional causative link between elevated levels of plasma TG in various pathologic conditions and an increased risk of atherosclerosis.
Although the effects of TG on the structural stability of VLDL and LDL have not been reported previously, TG-rich HDL have long been known to have reduced structural stability, altered apolipoprotein conformation, and increased propensity to undergo structural remodeling, fusion, rupture and protein release [22,23,32]. The molecular underpinnings for these effects were unknown. The current study helped establish such underpinnings for the three major classes of plasma lipoproteins.
First, we show that elevated TG destabilize not only on HDL (which contain exchangeable proteins, mainly apoA-I and apoA-II) but also LDL (which contain one copy of the non-exchangeable apoB and minor amounts of exchangeable proteins) and VLDL (which contain one copy of apoB and multiple copies of exchangeable proteins, apoE and apoCs). Consequently, the destabilizing effects of TG are not limited to a particular apolipoprotein or lipoprotein class and may (in VLDL and HDL) or may not (in LDL) involve a substantial protein release from lipoproteins. Rather, these TG effects represent a common property of various lipoprotein classes.
Second, the current study reveals that increased TG content decreases the lipoprotein stability via several mechanisms. Our results show that TG have a direct destabilizing effect on the lipoprotein particle evident from the more extensive CETP-induced remodeling of TG-rich HDL (Figure 6). Additional destabilization comes from indirect effects of TG, such as increased susceptibility of TG-rich lipoproteins to spontaneous and enzymatic hydrolysis (Figures 5, S5, S6 D) and oxidation (Figures 7, S7). The products of these reactions, particularly FFA, further augment lipoprotein destabilization and fusion.
Third, we show that increased TG content leads to increased cooperativity of the lipoprotein disintegration during thermal remodeling (gray and black lines in Figure 1B, D, Figure 2B, D, and Figure 8B, D). Since this transition involves particle fusion, rupture and coalescence into droplets, its increased cooperativity suggests that in TG-rich lipoproteins a greater number of lipoproteins coalesce together, forming larger particles. This idea is supported strongly by our SEC, non-denaturing PAGE and electron microscopic data of VLDL and LDL: high-TG lipoproteins not only undergo more extensive thermal remodeling but also coalesce into larger particles compared to their low-TG counterparts (Figure 3C, F). These results resemble closely the effects of FFA on the remodeling of plasma HDL, LDL and VLDL reported previously: an increase in the FFA content greatly enhanced the cooperativity of the lipoprotein fusion and rupture, leading to formation of larger-size particles, whereas FFA removal by albumin had an opposite effect [11]. Importantly, the current study shows that lipoprotein disintegration during thermal or chemical denaturation is accompanied by spontaneous lipolysis and generation of FFA (Figures 4, S5). Therefore, increased cooperativity of thermal denaturation observed in TG-rich VLDL and LDL (gray lines in Figures 1B, D and 2B, D) results, in part, from higher amounts of FFA generated upon disintegration of these lipoproteins.
FFA are potent lipid fusogens that perturb acyl chain packing in phospholipid monolayers and bilayers and promote their fusion in various systems including lipoproteins ([11,37] and references therein). Elevated levels of plasma FFA are a biomarker of inflammation and are associated with insulin resistance in conditions such as diabetes and obesity ([25,26] and references therein). The results of the current study suggest that increased levels of plasma FFA contribute to the enhanced remodeling, fusion and rupture of TG-rich lipoproteins. Moreover, our results show that TG-rich lipoproteins are more susceptible to lipolysis (Figure S5), which further augments their remodeling and fusion. In addition, increased oxidizability of TG-rich lipoproteins (Figures 7, S7) is expected to impact lipoprotein fusion directly or indirectly by promoting lipid hydrolysis and FFA generation.
These findings are relevant to the established link between atherosclerosis and other pathologic conditions such as diabetes, metabolic syndrome, obesity and dyslipidemia [15-19,25-29], which are associated with elevated levels of TG, FFA and oxidation. Our results suggest that all these factors can adversely affect lipoprotein remodeling and fusion, and thereby contribute to the development of atherosclerosis.
5. Conclusions
This study reveals that naturally occurring variations in plasma TG levels have profound effects on the stability of human VLDL and LDL and their susceptibility to hydrolysis and oxidation: elevated TG make lipoproteins more susceptible to biochemical and structural remodeling culminating in lipoprotein fusion, disintegration and release of the exchangeable apolipoproteins from VLDL. Similar effects of TG on human plasma HDL were previously reported ([22] and references therein). The current study suggests a mechanistic explanation for these effects. We propose that the TG-induced lipoprotein destabilization stems for the direct effects of the TG cargo on the lipoprotein particle as well as the indirect effects, such as higher susceptibility of TG-rich lipoproteins to hydrolysis and oxidation. Moreover, high-TG conditions in vivo are associated with higher levels of endogenous FFA that contribute to lipoprotein destabilization and fusion, since FFA are potent lipid fusogens [11,37]. According to the response-to-retention hypothesis of atherosclerosis [13,14], LDL and VLDL fusion in the arterial wall is an early trigger of atherogenesis [7,9,12]. Therefore, the current study of lipoprotein fusion in vitro is relevant to in vivo conditions wherein TG are elevated, such as the diabetes, metabolic syndrome, obesity and dyslipidemia. We propose that fusion of LDL and VLDL as well as their hydrolytic and oxidative remodeling, which is augmented upon increasing TG content in these lipoproteins, provides an additional causative link between elevated TG levels and an increased risk of atherosclerosis.
Supplementary Material
Highlights:
Elevated triacylglycerol promotes structural and biochemical remodeling of lipoproteins
VLDL and LDL from diabetic patients are less stable than those from healthy subjects
Triacylglycerol destabilizes lipoprotein particles directly as well as indirectly
The indirect effect stems, in part, from elevated free fatty acids that promote lipoprotein fusion
The results suggest a new causative link between diabetes and atherosclerosis
Acknowledgements
We thank Professor Kerry-Ann Rye from University of New South Wales, Australia, for her generous gift of CETP. We are grateful to Donald L. Gantz for expert help with transmission electron microscopy, and to Michael Gigliotti and Cheryl England for help with thin-layer chromatography and agarose gel electrophoresis. This work was supported by the National Institutes of Health grants RO1 GM026267, T32 HL007224, and T32 HL007969, and by ISCIII/FIS PI16-00471 from the Spanish Ministry of Health with FEDER funds.
Abbreviations:
- apo
apolipoprotein
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- VLDL
very low-density lipoproteins
- TG
triacylglycerol
- TO
triolein
- FFA
free (unesterified) fatty acid
- POPC
palmitoyl-oleoyl phosphatidylcholine
- CETP
cholesterol ester transfer protein
- LpL
lipoprotein lipase
- CD
circular dichroism
- SEC
size exclusion chromatography
- NL
normolipidemic
- GC
good glycemic control
- PC
poor glycemic control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwiterovich PO, The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review. Am. J. Cardiol. 86(12), (2000) 5–10. [DOI] [PubMed] [Google Scholar]
- 2.Olivecrona G, Role of lipoprotein lipase in lipid metabolism, Curr. Opin. Lipidol. 27(3) (2016) 233–241. [DOI] [PubMed] [Google Scholar]
- 3.Musliner TA, Long MD, Forte TM, Nichols AV, Gong EL, Blanche PJ, Krauss RM, Dissociation of high density lipoprotein precursors from apolipoprotein B-containing lipoproteins in the presence of unesterified fatty acids and a source of apolipoprotein A-I, J. Lipid Res. 32(6) (1991) 917–933. [PubMed] [Google Scholar]
- 4.Shrestha S, Wu BJ, Guiney L, Barter PJ, Rye KA, Cholesteryl ester transfer protein and its inhibitors, J. Lipid Res. 59(5) (2018) 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rye KA, Hime NJ, Barter PJ, Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion, J. Biol. Chem. 272(7) (1997) 3953–3960. [DOI] [PubMed] [Google Scholar]
- 6.Oorni K, Pentikainen MO, Ala-Korpela M, Kovanen PT, Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions, J. Lipid Res. 41 (2000) 1703–1714. [PubMed] [Google Scholar]
- 7.Pentikainen MO, Oorni K, Ala-Korpela M, Kovanen PT, Modified LDL - trigger of atherosclerosis and inflammation in the arterial intima, J. Intern. Med. 247 (2000) 359–370. [DOI] [PubMed] [Google Scholar]
- 8.Guha M, England C, Herscovitz H, Gursky O, Thermal transitions in human very-low-density lipoprotein: fusion, rupture, and dissociation of HDL-like particles, Biochemistry 46 (2007) 6043–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu M, Gursky O, Aggregation and fusion of low-density lipoproteins in vivo and in vitro. Biomol Concepts, 4(5) (2013) 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gursky O, Structural stability and functional remodeling of high-density lipoproteins, FEBS Lett. 589(19A) (2015) 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraman S, Gantz DL, Gursky O, Effects of phospholipase A(2) and its products on structural stability of human LDL: relevance to formation of LDL-derived lipid droplets, J. Lipid Res. 52(3) (2011) 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Öörni K, Lehti S, Sjövall P, Kovanen PT, Triglyceride-rich lipoproteins as a source of proinflammatory lipids in the arterial wall. Curr. Med. Chem. 25 (2018) 1–10. [DOI] [PubMed] [Google Scholar]
- 13.Williams KJ, Tabas I, The response-to-retention hypothesis of early atherogenesis, Arterioscler. Thromb. Vasc. Biol. 15(5) (1995) 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.J Borén KJ Williams The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 27(5) (2016) 473–483. [DOI] [PubMed] [Google Scholar]
- 15.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V, Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 115 (2007) 450–458. [DOI] [PubMed] [Google Scholar]
- 16.Talayero BG, Sacks FM, The role of triglycerides in atherosclerosis Curr. Cardiol. Rep. 13(6) (2011) 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordestgaard BG, Varbo A, Triglycerides and cardiovascular disease. Lancet 384 (2014) 626–635. [DOI] [PubMed] [Google Scholar]
- 18.Toth PP, Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag. 12 (2016) 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallinga-Thie GM, Kroon J, Borén J, Chapman MJ, Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr. Cardiol. Rep. 18(7) (2016) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta R, Gantz DL, Gursky O, Human plasma high-density lipoproteins are stabilized by kinetic factors. J. Mol. Biol. 328 (2003) 183–192. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Gantz DL, Herscovitz H, Gursky O, Kinetic analysis of thermal stability of human low density lipoproteins: a model for LDL fusion in atherogenesis, J. Lipid Res. 53 (2012) 2175–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayaraman S, Sánchez-Quesada JL, Gursky O, Triglyceride increase in the core of high-density lipoproteins augments apolipoprotein dissociation from the surface: Potential implications for treatment of apolipoprotein deposition diseases, Biochim. Biophys. Acta. 1863 (2017) 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparks DL, Davidson WS, Lund-Katz S, Phillips MC, Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability, J. Biol. Chem. 270 (1995) 26910–26917. [DOI] [PubMed] [Google Scholar]
- 24.Braschi S, Coffill CR, Neville TA, Hutt DM, Sparks DL, Effect of acylglyceride content on the structure and function of reconstituted high density lipoprotein particles, J. Lipid. Res. 42(1) (2001) 79–87. [PubMed] [Google Scholar]
- 25.Boden G, Obesity and free fatty acids. Endocrinol Metab Clin North Am. 37(3) (2008) 635–646, viii-ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiller S, Blüher M, Hoffmann R, Plasma levels of free fatty acids correlate with type 2 diabetes mellitus, Diabetes Obes. Metab. 20(11) (2018) 2661–2669. [DOI] [PubMed] [Google Scholar]
- 27.Pirro M, Mauriège P, Tchernof A, Cantin B, Dagenais GR, Després JP, Lamarche B, Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Québec Cardiovascular Study, Atherosclerosis, 160 (2) (2002), 377–384. [DOI] [PubMed] [Google Scholar]
- 28.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, Schaefer JR, März W, Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J. Clin. Endocrinol. Metab. 91(7) (2006) 2542–2547. [DOI] [PubMed] [Google Scholar]
- 29.ledema MD, Maziarz M, Biggs ML, Zieman SJ, Kizer JR, Ix JH, Mozaffarian D, Tracy RP, Psaty BM, Siscovick DS, Mukamal KJ, Djousse L, Plasma free fatty acids, fatty acid-binding protein 4, and mortality in older adults (from the Cardiovascular Health Study), Am. J. Cardiol. 114 (6) (2014) 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rye KA, Hime NJ, Barter PJ, The influence of cholesteryl ester transfer protein on the composition, size, and structure of spherical, reconstituted high density lipoproteins. J. Biol. Chem. 270(1) (1995) 189–196. [DOI] [PubMed] [Google Scholar]
- 31.Schumaker VN, Puppione DL, Sequential flotation ultracentrifugation, Methods Enzymol. 128 (1986) 151–170. [DOI] [PubMed] [Google Scholar]
- 32.Rye KA, Jauhiainen M, Barter PJ, Ehnholm C, Triglyceride-enrichment of high density lipoproteins enhances their remodelling by phospholipid transfer protein, J. Lipid Res. 39 (3) (1998) 613–622. [PubMed] [Google Scholar]
- 33.Bergmeyer HU, Metabolites 3: lipids, amino acids and related compounds, Bergmeyer J, Grassl M (Eds.), Methods of Enzymatic Analysis, VIII, VCH Weinheim, Germany: (1985). [Google Scholar]
- 34.Folch J, Lees M, Sloane GH Stanley A simple method for the isolation and purification of total lipides from animal tissues J. Biol. Chem, 226 (1) (1957) 497–509 [PubMed] [Google Scholar]
- 35.Jayaraman S, Gantz DL, Gursky O, Effects of oxidation on the structure and stability of human low-density lipoprotein. Biochemistry 46(19) (2007) 5790–5797. [DOI] [PubMed] [Google Scholar]
- 36.Jayaraman S, Gantz DL, Gursky O Effects of salt on the thermal stability of human plasma high-density lipoprotein. Biochemistry 45(14) (2006) 4620–4628. [DOI] [PubMed] [Google Scholar]
- 37.Rull A, Jayaraman S, Gantz DL, Rivas-Urbina A, Pérez-Cuellara M, Ordóñez-Llanos J, Sánchez-Quesada JL, Gursky O, Thermal stability of human plasma electronegative low-density lipoprotein: a paradoxical behavior of low-density lipoprotein aggregation. BBA Mol. Cell. Biol. Lipids. 1861(9) (2016) 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Jayaraman S, Gursky O, Mild oxidation promotes and advanced oxidation impairs remodeling of human high-density lipoprotein in vitro, J. Mol. Biol. 376(4) (2008) 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guha M, Gursky O, Effects of oxidation on structural stability and remodeling of human very low density lipoprotein, Biochemistry, 49(44) (2010) 9584–9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg D, Witztum JL, Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30(12) (2010) 2311–2316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.