Abstract
Objective:
Azathioprine (AZA) and mycophenolate mofetil (MMF) are frequently used immunosuppressives for moderate-to-severe SLE. We studied longitudinal patterns and predictors of adherence to AZA and MMF in a nationwide U.S. SLE cohort.
Methods:
In the Medicaid Analytic eXtract (2000–2010), we identified SLE patients who initiated AZA or MMF (no use in prior 6 months) with ≥12 months of continuous follow-up. We dichotomized adherence at 80% with ≥24/30 days/month considered adherent. We used group-based trajectory models to estimate monthly adherence patterns and multivariable multinomial logistic regression to determine the association between demographic, SLE and utilization-related predictors and the odds ratios (OR) of belonging to a nonadherent vs. the adherent trajectory, separately for AZA and MMF.
Results:
We identified 2,309 AZA initiators and 2,070 MMF initiators with SLE. Four-group trajectory models classified 17% of AZA and 21% of MMF initiators as adherent. AZA and MMF nonadherers followed similar trajectory patterns. Black race (OR 1.67, 95% CI 1.20–2.31) and Hispanic ethnicity (OR 1.58, 95% CI 1.06–2.35) increased odds of AZA nonadherence; there were no significant associations between race/ethnicity and MMF nonadherence. Male sex and polypharmacy were associated with lower odds of nonadherence to both medications; lupus nephritis was associated with lower odds of nonadherence to MMF (OR 0.74, 95% CI 0.55–0.99).
Conclusions:
Adherence to AZA or MMF over the first year of use was rare. Race, sex and lupus nephritis were modestly associated with adherence, but the magnitude, direction and significance of predictors differed by medication suggesting the complexity of predicting adherence behavior.
Keywords: Adherence, azathioprine, mycophenolate mofetil, systemic lupus erythematosus, pharmacoepidemiology, observational study, health services research
Background
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with a range of organ system manifestations. Patients with moderate-to-severe disease often receive immunosuppressives, either azathioprine (AZA) or mycophenolate mofetil (MMF) often interchangeably, to control lupus nephritis, serositis, hematologic abnormalities, arthritis and cutaneous disease.(1, 2) Adherence to medications for SLE varies from 20–80% depending on the population studied, the medication, and the method used to measure adherence (e.g. self-reported surveys, blood levels, prescription refill data).(3–6) Higher rates of nonadherence have been observed among younger age groups, black and Hispanic patients, and individuals with less education.(4, 7, 8) Studies have varied as to whether polypharmacy and disease severity affect the risk for nonadherence.
In Medicaid, the largest public insurance in the U.S. primarily for low income individuals, our prior work demonstrated that under 20% of patients were adherent to hydroxychloroquine (HCQ), defined as ≥80% of days covered with prescription refills during the first year of use.(8) There were higher odds of nonadherence among SLE patients who were young, black, taking fewer medications, and with less severe disease. We also observed that HCQ adherence was dynamic and for most, declined over the first year of use. In this study, we aimed to assess patterns of adherence over the first year of AZA or MMF use. We hypothesized that like HCQ, adherence would decline over time and that while predictors of nonadherence would be similar, patterns would suggest better adherence to MMF because patients may be slightly sicker and therefore more invested in continuing their medication.
Methods
Patient Cohort
We used the Medicaid Analytic eXtract (MAX) with demographic data, billing claims, healthcare utilization, and drug dispensing data from 2000–2010 for the 29 most populated U.S. states (86% of Medicaid beneficiaries nationwide). We excluded all claims from Ohio because detailed medication dispensing data were not available, and additionally excluded all individuals without drug dispensing data, including those who were hospitalized for the entire follow-up period. We identified two cohorts of SLE patients (≥2 ICD-9 SLE codes (710.0) for discharge diagnoses or physician claims ≥30 days apart) with either AZA or MMF dispensing within 365 days of a SLE code.(8) We required ≥6 months of continuous enrollment without use of AZA or MMF prior to the date of initiation (index date). We allowed AZA initiators to previously receive MMF and vice versa. We required ≥365 days of continuous enrollment following the index date to assess adherence.
Measures of Adherence
We used prescription refill data to measure adherence, which has previously been validated in claims data.(9) We calculated the proportion of days covered (PDC) beginning at the index date for 365 days (number of days covered divided by 365 times 100, subtracting hospitalized days from numerator and denominator) and classified individuals with PDC≥80% as adherent.(10) We also measured adherence monthly to MMF and to AZA over the 12-month period and each month was classified either as adherent (1) or nonadherent (0) depending on whether ≥24 of 30 days (80%) were covered. The majority (>85%) of our cohort received a one-month supply of their AZA or MMF in accordance with Medicaid policies in most states.
Potential Correlates of Adherence
We measured potential predictors during the 6 months prior to and including the index date in the AZA and MMF cohorts. Demographic factors included age and state of residence at the index date, sex, race/ethnicity and region from MAX, and zip code-level median household income from American Community Survey data.(11) We included diabetes, smoking, lupus nephritis, antidepressant medication use, SLE-related laboratory tests and medications (HCQ, immunosuppressives and corticosteroids), number of medications at the index date, days’ supply of first AZA/MMF fill, vaccinations, healthcare utilization, and the SLE risk-adjustment index (Supplemental Table 1).(8, 12, 13) We also examined models that included comorbidities (thromboembolism, pulmonary, hepatic, cardiovascular and cerebrovascular disease, substance abuse, obesity, and malignancy) but did not include them in our final models as they did not contribute significantly and have not been shown in prior studies to be strongly associated with adherence.
Statistical Analyses
We compared baseline characteristics and PDC for AZA and MMF using descriptive statistics. We used our binary indicators of monthly adherence to construct group-based trajectory models (GBTM) to classify patients by adherence, separately for AZA and MMF. GBTM are used to identify latent patterns in longitudinal data with repeated measures and has been previously applied to prescription refill data to uncover adherence patterns over the first year of use.(8, 14) We evaluated AZA and MMF GBTMs ranging from three to six trajectory groups and based our model choice on a combination of Bayesian information criteria with lower values considered preferable, reasonable distribution across groups, posterior probabilities ≥80% for each group, and explanatory potential.(15) We then used multinomial logistic regression models for both AZA and MMF to determine the odds of belonging to a nonadherent trajectory compared to the persistently adherent trajectory for demographic, utilization and SLE-related predictors.
We conducted sensitivity analyses censoring at potential conditions that may have resulted in physician-recommended discontinuation rather than nonadherence. We censored at the beginning of the nearest preceding refill for first discharge diagnosis code for serious infection, and for any code for neutropenia or transaminitis for both cohorts, and additionally for pregnancy or colitis for the MMF cohort (Supplemental Table 1). We conducted all analyses using SAS 9.4 (Cary, NC) and used the “Proc Traj” add-on package for GBTM. Data were obtained from the Centers for Medicare and Medicaid Services through a Data Use Agreement and data are presented in accordance with their policies. The Partners Healthcare IRB approved this study.
Results
We identified 2,309 AZA initiators and 2,070 MMF initiators. AZA initiators were slightly older with a mean ± SD age of 36.1 ± 11.8 compared to 33.4 ± 11.6 for MMF (Table 1). The percentage of females was slightly higher among AZA initiators, as was the percentage of black individuals. On average, at baseline AZA initiators had less severe SLE with a lower mean SLE risk adjustment index, lower prevalence of lupus nephritis, fewer overall medications, and less immunosuppressant use. Corticosteroid use was comparable between initiators of the two drugs. The mean ± SD PDC for AZA initiators beginning at the index date of new use was 40% ± 29, with 15% classified as adherent (PDC ≥80%) compared to 44% ± 30% for MMF, with 18% classified as adherent (p<0.001). We did not observe significant changes in either AZA or MMF adherence by index date year (Supplemental Figure 1). When we varied the adherence threshold, 7.3% of AZA initiators and 8.6% of MMF initiators had PDCs ≥90%, and 21.9% of AZA initiators and 25.7% of MMF initiators had PDCs ≥70%.
Table 1.
Baseline characteristics of azathioprine and mycophenolate initiators with SLE in Medicaid, 2000–2010
| Characteristics | Azathioprine (N=2309) | Mycophenolate mofetil (N=2070) |
|---|---|---|
| Age – mean ± SD | 36.1 ± 11.8 | 33.4 ± 11.6 |
| Female sex – N (%) | 2138 (92.6) | 1857 (89.7) |
| Race/Ethnicity | ||
| Black | 1089 (47.2) | 925 (44.7) |
| White | 579 (25.1) | 506 (24.4) |
| Hispanic | 476 (20.6) | 453 (21.9) |
| Asian | 93 (4.0) | 114 (5.5) |
| American Indian/Alaska Native | 22 (1.0) | 31 (1.5) |
| Region – N (%) | ||
| Northeast | 523 (22.7) | 577 (27.9) |
| South | 837 (36.3) | 680 (32.9) |
| Midwest | 378 (16.4) | 368 (17.8) |
| West | 571 (24.7) | 445 (21.5) |
| Zip code median household income- median $ (25th, 75th) | 41,643 (33,659–51,948) | 42,557 (33,995–55,565) |
| Diabetes- N (%) | 297 (12.9) | 242 (11.7) |
| SLE risk adjustment index – mean ± SD | 1.4 ± 2.2 | 2.1 ± 2.5 |
| Lupus nephritis – N (%) | 503 (21.8) | 1162 (56.1) |
| Number of drugs – mean ± SD | 5.0 ± 3.6 | 5.5 ± 3.8 |
| Antidepressant use – N (%) | 439 (19.0) | 209 (10.1) |
| Corticosteroid use – N (%) | 1898 (82.2) | 1704 (82.3) |
| Hydroxychloroquine use – N (%) | 1315 (57.0) | 938 (45.3) |
| Immunosuppressant use* - N (%) | 374 (16.2) | 526 (25.4) |
| Number of SLE-related laboratory tests+ – mean (SD) | 3.3 (4.2) | 3.71 (5.0) |
Immunosuppressants include methotrexate, leflunomide, tacrolimus, sulfasalazine, cyclosporine, cyclophosphamide, azathioprine (for mycophenolate mofetil initiators) and mycophenolate mofetil (for azathioprine initiators)
Laboratory tests include BUN, creatinine, complement (C3 and C4), ESR, CRP, anti-dsDNA and urinalysis
We examined three to six-group GBTMs for both AZA and MMF initiators. We aimed to balance model fit with explanatory power and to compare similar numbers of trajectories for both medications. A four-group model provided an adequate fit for both drugs. The mean posterior probabilities for each trajectory were greater than 80%, and each trajectory had a reasonably balanced distribution of individuals. The Bayesian information criteria for the three-group models were slightly smaller than for the four-group models but we chose the four-group models because we felt that the explanatory potential was greater and the other model fit criteria were met.(15)
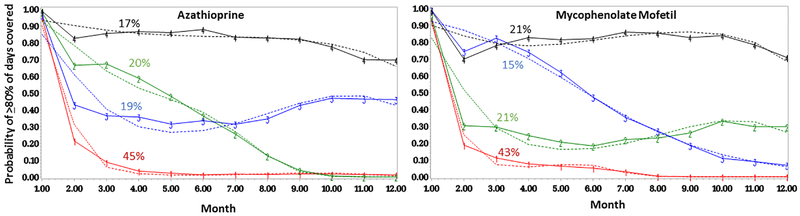
Overall, we observed similar patterns for the four-group trajectory model (Figure 1). In the persistently adherent trajectory (group 4, in black), there were 384 (17%) AZA initiators and 441 (21%) MMF initiators. In the persistently nonadherent trajectory (group 1, in red), there were 1030 (45%) AZA initiators and 883 (43%) MMF initiators. Among AZA initiators, two groups with more dynamic nonadherent patterns (group 2, in green and group 3, in blue), steadily declined until between 6–7 months at which point adherence for group 3 plateaued at about 45% of days/month covered, while group 2 continued to decline. Among MMF initiators, among those with more dynamic nonadherent patterns, group 2 (in green) precipitously declined initially and then plateaued at about 30% of days/month covered. Group 3 (in blue) declined more slowly over the course of use, remaining just below the adherent range of ≥80% of days covered until between months 4–5.
Figure 1.
Group-based trajectory models demonstrating monthly adherence patterns for SLE patients enrolled in Medicaid over the first year of azathioprine and mycophenolate mofetil use, with group 4 (in black) as the persistently adherent trajectory and group 1 (in red) as the persistently nonadherent trajectory
We examined multinomial logistic regression models comparing the odds of belonging to the nonadherent trajectories (groups 1–3) vs. the persistently adherent trajectory (group 4) for both AZA and MMF initiators (Table 2). Among AZA initiators, we observed increased odds of belonging to the persistently nonadherent trajectory (group 1) vs. the persistently adherent (group 4) among SLE patients who were black (OR 1.67, 95% CI 1.20–2.31) or Hispanic (OR 1.58, 95% CI 1.06–2.35) compared to White, and in the 18–35-year age group (OR 1.60, 95% CI 1.10–2.34) compared to the oldest (age 51–65 years). We found greater than two times higher odds of belonging to the declining and then plateauing nonadherent trajectory (group 3), vs. the persistently adherent trajectory (group 4) for black race and Hispanic ethnicity, and reduced odds of belonging to group 3 vs. 4 among individuals living in areas with less than or equal to the median of the zip code median household income, compared to areas above the median (OR 0.63, 95% CI 0.47–0.86). Male sex and increased number of medications were associated with reduced odds of belonging to nearly all nonadherent trajectories compared to the most adherent (group 4).
Table 2.
Multivariable multinomial regression comparing the odds of belonging to a nonadherent trajectory (groups 1–3) to the most adherent trajectory (group 4, reference) for azathioprine and mycophenolate mofetil initiators with SLE
| Predictors | Azathioprine (N=2309) OR (95% CI) |
Mycophenolate Mofetil (N=2070) OR (95% CI) |
||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |
| N (%) | 1030 (44.6) | 459 (19.9) | 436 (18.9) | 883 (42.7) | 441 (21.3) | 305 (14.7) |
| Age (ref=51–65) | ||||||
| 18–34 years | 1.60 (1.10–2.34) | 1.75 (1.12–2.73) | 1.48 (0.96–2.29) | 1.14 (0.76–1.71) | 1.15 (0.71–1.86) | 1.95 (1.12–3.42) |
| 35–50 years | 1.36 (0.95–1.96) | 1.67 (1.09–2.57) | 1.17 (0.76–1.79) | 0.83 (0.55–1.26) | 0.96 (0.59–1.56) | 1.44 (0.82–2.54) |
| Male sex (ref=female) | 0.59 (0.39–0.91) | 0.68 (0.42–1.11) | 0.54 (0.32–0.92) | 0.67 (0.45–0.99) | 0.85 (0.56–1.31) | 0.83 (0.51–1.34) |
| Race/ethnicity (ref=White) | ||||||
| Black | 1.67 (1.20–2.31) | 1.62 (1.11–2.35) | 2.05 (1.39–3.02) | 1.33 (0.95–1.86) | 1.20 (0.82–1.76) | 0.72 (0.47–1.09) |
| Hispanic | 1.58 (1.06–2.35) | 1.37 (0.87–2.15) | 2.00 (1.26–3.19) | 0.94 (0.64–1.39) | 0.85 (0.55–1.32) | 0.88 (0.56–1.40) |
| Asian | 1.02 (0.52–1.99) | 0.85 (0.40–1.84) | 1.52 (0.72–3.18) | 0.64 (0.36–1.13) | 0.62 (0.33–1.16) | 0.59 (0.30–1.17) |
| American Indian/Alaska Native | 0.78 (0.24–2.51) | 0.61 (0.15–2.52) | NR | 1.88 (0.57–6.25) | 1.49 (0.39–5.65) | 0.77 (0.16–3.83) |
| SLE risk adjustment index | 0.98 (0.91–1.05) | 0.97 (0.90–1.05) | 0.98 (0.90–1.06) | 0.99 (0.92–1.05) | 0.97 (0.90–1.04) | 1.00 (0.93–1.08) |
| Lupus nephritis | 1.06 (0.75–1.49) | 1.22 (0.83–1.79) | 1.31 (0.89–1.93) | 0.74 (0.55–0.99) | 0.98 (0.70–1.36) | 0.95 (0.66–1.37) |
| Diabetes mellitus | 1.11 (0.74–1.67) | 1.74 (1.11–2.71) | 1.30 (0.81–2.08) | 1.05 (0.70–1.59) | 0.95 (0.59–1.53) | 1.02 (0.61–1.68) |
| Median household income ≤ median (ref= >median) | 0.80 (0.61–1.04) | 0.99 (0.73–1.34) | 0.63 (0.47–0.86) | 1.33 (1.02–1.72) | 1.28 (0.95–1.72) | 1.26 (0.92–1.75) |
| Number of medications | 0.90 (0.86–0.93) | 0.94 (0.90–0.98) | 0.92 (0.88–0.96) | 0.90 (0.87–0.94) | 0.90 (0.86–0.94) | 0.99 (0.95–1.03) |
| Outpatient visits | 1.01 (0.98–1.03) | 0.99 (0.97–1.02) | 0.99 (0.9–1.01) | 0.99 (0.96–1.01) | 0.99 (0.96–1.01) | 0.98 (0.95–1.00) |
| Hospitalizations | 1.08 (0.96–1.21) | 1.05 (0.93–1.20) | 1.09 (0.96–1.24) | 0.98 (0.89–1.08) | 0.96 (0.86–1.08) | 0.98 (0.87–1.10) |
| Emergency Department Visits | 1.05 (0.99–1.12) | 1.05 (0.98–1.13) | 1.01 (0.94–1.09) | 1.16 (1.08–1.25) | 1.14 (1.05–1.23) | 1.06 (0.97–1.15) |
Models additionally adjusted for calendar year of index date, state of residence at index date, days’ supply at first dispensing, SLE-related medication use (hydroxychloroquine, immunosuppressants, corticosteroids), number of SLE-related laboratory tests, antidepressant use, smoking, obesity, and influenza and pneumococcal vaccinations
Among MMF initiators, we did not observe statistically significant associations by race/ethnicity comparing any of the nonadherent trajectories (groups 1–3) to the persistently adherent trajectory (group 4). Like AZA, we observed reduced odds of belonging to the persistently nonadherent trajectory (group 1) vs. the persistently adherent (group 4) among males compared to females (OR 0.67, 95% CI 0.45–0.99) and for increased medication use (OR 0.90, 95% CI 0.87–0.94). Specific to MMF initiators, we observed reduced odds of belonging to the persistently nonadherent vs. the persistently adherent group among SLE patients with lupus nephritis (OR 0.74, 95% CI 0.55–0.99) and increased odds among those living in areas below or equal to the median household income compared to above (OR 1.33, 95% CI 1.02–1.72). Increased number of emergency department visits were also associated with increased odds of belonging to least adherent groups (1 and 2) vs. the most adherent group (4).
In sensitivity analyses, censoring at indications that may have resulted in physician recommended discontinuation of AZA or MMF, we observed only modestly increased adherence estimates. Among AZA initiators, censoring at ≥1 ICD-9 code for serious infection, transaminitis or neutropenia, the mean ± SD PDC was 45% ± 30 with 18.8% categorized as adherent (PDC ≥80%). Among MMF initiators, censoring at serious infection, transaminitis, neutropenia, colitis or pregnancy, the mean (SD) PDC was 49% ± 29 with 22.7% categorized as adherent.
Discussion
Overall, we observed profoundly poor adherence among Medicaid beneficiaries with SLE who initiated AZA or MMF over the first year of use; less than a quarter of patients refilled their medications 80% of the time or more. These rates of nonadherence were similar among HCQ initiators also enrolled in Medicaid.(8) Our findings were also in line with a prior study that utilized trajectory models to describe adherence patterns among statin initiators; only 23.4% were persistently adherent over the first 15 months of use.(14)
In our cohorts, adherence was slightly better among MMF initiators compared to AZA initiators, and groups of MMF nonadherers appeared to remain at least partially adherent for longer. The populations of AZA and MMF initiators were somewhat different – AZA initiators were slightly older, included more females, likely because this medication is compatible with pregnancy whereas MMF is not, and had less severe SLE, with a lower prevalence of lupus nephritis and prior immunosuppressive use. Predictors of nonadherence differed as well. We observed primarily demographic associations with AZA nonadherence; black race, Hispanic ethnicity, female sex, and younger age were associated with increased odds of nonadherence. However, among MMF initiators, while we did see an association between female sex, younger age and nonadherence, the findings were less consistent across nonadherent trajectories, and we did not observe significant associations by race/ethnicity. Among MMF initiators, we observed reduced odds of persistent nonadherence associated with lupus nephritis and increased medication use and increased odds associated with visiting the emergency room more. The median zip-code level household income was slightly higher for MMF initiators compared to AZA, and interestingly, while we observed increased odds of nonadherence among MMF initiators living in lower vs. higher median income zip codes, the trend was in the opposite direction among AZA initiators for unclear reasons.
In accordance with prior studies, we did not find one dominant factor to be consistently associated with all nonadherence patterns across different SLE-related medications. While many factors likely contribute to adherence behavior, their relationship is not necessarily constant across medications, populations, or over time of use. Differences between SLE-related medications including affordability, tolerability, regimen complexity, interactions with other medications or lifestyle factors, likely contribute to varying patterns of adherence. In addition, aspects that cannot be measured in a study like ours that relies on claims data including the doctor-patient relationship, or beliefs about SLE and about medication safety, play a role as well. This complexity suggests that a simple variable-based algorithm to predict a person’s adherence pattern over time to multiple medications is likely unrealistic.
This study has limitations. We utilized prescription refill data to determine adherence, which may not always represent use, however prior studies have shown this to be a valid method.(9, 16) We did not have data regarding initial AZA or MMF prescribing and therefore could not capture primary nonadherence. We used a cutoff of ≥80% to indicate adherence, and while this is accepted in the chronic disease literature, it is unclear whether it correlates with the physiologic levels needed to have a clinically meaningful effect. In addition, we could not distinguish between medically-indicated discontinuation and nonadherence. However, we excluded all hospitalized time during which adherence could not be readily measured and conducted sensitivity analyses censoring at potential indications for physician recommended discontinuation which resulted in adherence estimates similar to our primary analyses. We lacked qualitative measures of potential predictors of nonadherence, as well as actual lab results and medical records to understand fluctuations in disease activity. We also lacked information on socioeconomic status, such as individual income or education, and we cannot exclude the possibility that racial/ethnic differences may be markers for differences in socioeconomic circumstances. We lacked more recent data past 2010, however between 2000–2010, we did not appreciate significant fluctuations in either AZA or MMF adherence.
In our study, we leveraged two large cohorts of AZA and MMF initiators to examine adherence patterns over time. While we found relatively similar patterns between the two medications, MMF initiators seemed to stay at least partially adherent for longer, possibly due to an understanding of the need to treat more severe manifestations such as lupus nephritis. Adherence overall however, was very poor and in this vulnerable, low-income SLE population, more needs to be done to facilitate persistent adherence to efficacious, standard-of-care medications to ultimately reduce disparities in outcomes.
Supplementary Material
Significance and Innovation.
Adherence to the two most frequently used immunosuppressive medications for SLE, azathioprine and mycophenolate, is overall extremely poor over the first year of use among a national cohort of Medicaid beneficiaries with SLE.
Adherence overall was slightly better to mycophenolate mofetil compared to azathioprine however adherence to both medications declined significantly for nearly 80 percent of patients over the first year of use.
While demographic factors including black race, Hispanic ethnicity and younger age were associated with higher odds of nonadherence among azathioprine initiators, they were significantly less strongly associated with nonadherence among mycophenolate mofetil initiators. This suggests that a single set of patient characteristics does not consistently predict nonadherence patterns across medications.
Acknowledgments
Funding: This study was funded by the Rheumatology Research Foundation Investigator Award (CH Feldman), NIH K23 AR071500 (CH Feldman), R01 057327 (KH Costenbader), K24 AR066109 (KH Costenbader), K24 AR055989 (DH Solomon) and P30 AR072577 (DH Solomon and KH Costenbader).
Footnotes
None of the authors have relevant financial disclosures.
References
- 1.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A. Diagnosis, Monitoring, and Treatment of Systemic Lupus Erythematosus: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res (Hoboken). 2015;67(10):1440–52. [DOI] [PubMed] [Google Scholar]
- 3.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum 2009;61(2):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine Blood Levels in Systemic Lupus Erythematosus: Clarifying Dosing Controversies and Improving Adherence. J Rheumatol 2015;42(11):2092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated With Increased Subsequent Acute Care Utilization Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2015;67(12):1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costedoat-Chalumeau N, Houssiau F, Izmirly P, Guern VL, Navarra S, Jolly M, et al. A Prospective International Study on Adherence to Treatment in 305 Patients With Flaring SLE: Assessment by Drug Levels and Self-Administered Questionnaires. Clin Pharmacol Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M, Cox V, Kallen MA, Janssen N, et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol 2008;27(7):883–9. [DOI] [PubMed] [Google Scholar]
- 8.Feldman CH, Collins J, Zhang Z, Subramanian SV, Solomon DH, Kawachi I, et al. Dynamic patterns and predictors of hydroxychloroquine nonadherence among Medicaid beneficiaries with systemic lupus erythematosus. Semin Arthritis Rheum 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. Journal of clinical epidemiology. 1997;50(1):105–16. [DOI] [PubMed] [Google Scholar]
- 10.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15(9):728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manson S, Schroeder J, Van Riper D, Ruggles S. IPUMS National Historical Geographic Information System, Version 12.0 [Database]. Minneapolis: University of Minnesota; 2017. [Google Scholar]
- 12.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol 2000;27(6):1408–13. [PubMed] [Google Scholar]
- 13.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010;19(6):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care 2013;51(9):789–96. [DOI] [PubMed] [Google Scholar]
- 15.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 16.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Medical care 1988;26(8):814–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.