Abstract
Ticks act as vectors of pathogens affecting human and animal health worldwide, and recent research has focused on the characterization of tick-pathogen interactions using omics technologies to identify new targets for developing novel control interventions. The regulome (transcription factors-target genes interactions) plays a critical role in cell response to pathogen infection. Therefore, the application of regulomics to tick-pathogen interactions would advance our understanding of these molecular interactions and contribute to the identification of novel control targets for the prevention and control of tick infestations and tick-borne diseases. However, limited information is available on the role of tick regulome in response to pathogen infection. In this study, we applied complementary in silico approaches to modeling how Anaplasma phagocytophilum infection modulates tick vector regulome. This proof-of-concept research provided support for the use of network analysis in the study of regulome response to infection, resulting in new information on tick-pathogen interactions and potential targets for developing interventions for the control of tick infestations and pathogen transmission. Deciphering the precise nature of circuits that shape the tick regulome in response to pathogen infection is an area of research that in the future will advance our knowledge of tick-pathogen interactions, and the identification of new antigens for the control of tick infestations and pathogen infection/transmission.
Keywords: regulome, transcription, tick, Ixodes scapularis, Anaplasma phagocytophilum, ISE6 cells, vaccine
Introduction
Ticks (Acari: Ixodida) are major vectors of pathogens affecting human and animal health worldwide, and consequently the focus of research for developing novel control interventions (de la Fuente, 2018). Among tick-transmitted pathogens, Anaplasma phagocytophilum (Alphaproteobacteria: Rickettsiales) is mainly transmitted by Ixodes spp. and the causative agent of human and animal anaplasmosis and tick-borne fever in small ruminants (Severo et al., 2015).
Recent developments in tick genomics have advanced research using latest omics technologies for the characterization of tick-host-pathogen interactions and the identification of candidate protective antigens (de la Fuente et al., 2016c,a, 2017; Gulia-Nuss et al., 2016; Shaw et al., 2017; de la Fuente, 2018). Vaccinomics, a holistic perspective based on the use of omics technologies and bioinformatics in a systems biology approach for the characterization of tick-host-pathogen molecular interactions is our platform for the identification of candidate vaccine antigens (de la Fuente and Merino, 2013; de la Fuente et al., 2016a, 2018; Contreras et al., 2017). In this context, tick cell lines constitute a valuable resource because it is a proven model for the study of tick-pathogen and particularly tick-A. phagocytophilum interactions, easy manipulation without animal experimentation, and the fact that A. phagocytophilum infects mainly one cell type in vertebrates (neutrophils) but multiple cell types in ticks better resembled by these cell lines (Munderloh et al., 1994; Severo et al., 2015; Villar et al., 2015; Bell-Sakyi et al., 2018).
The regulome (transcription factors-target genes interactions) and interactome (protein-protein physical and functional interactions) play a critical role in cell response to different stimuli including pathogen infection. Both regulome and interactome are implicated in transcriptional regulation, which is one of the most fundamental mechanisms for controlling the amount of protein produced by cells under different environmental and physiological conditions and developmental stages (Gronostajski et al., 2011; Vaquerizas et al., 2012; Shih et al., 2016; Rioualen et al., 2017). Therefore, the application of regulomics and interactomics to host/tick-pathogen interactions would advance our understanding of these molecular interactions and contribute to the identification of new control targets for the prevention and control of tick infestations and tick-borne diseases (de la Fuente et al., 2018; Artigas-Jerónimo et al., 2018a,b; Estrada-Peña et al., 2018).
Few studies have addressed the role of the regulome or regulon (part of the regulome including a set of genes that share a common regulatory element binding site) in the interaction between tick-borne pathogens and vertebrate hosts (i.e., Bugrysheva et al., 2015; Boyle et al., 2019). However, limited information is available on the role of tick regulome in response to pathogen infection (Artigas-Jerónimo et al., 2018b).
In this study, we applied complementary in silico approaches to modeling how A. phagoctophilum infection modulates tick vector regulome, and the possibilities for the identification of new control target antigens. This proof-of-concept research provided new information on tick-pathogen interactions and potential targets for developing interventions for the control of tick infestations and pathogen infection.
Materials and Methods
Datasets
The RNA sequencing (RNAseq) datasets of differential expression of I. scapularis transcription factors (TF) and target genes (TG) in response to A. phagocytophilum infection was obtained from previously published transcriptomics analyses in ISE6 cells, and fed adult female midguts and salivary glands (Ayllón et al., 2015; Villar et al., 2015). Gene ontology (GO) level-3 annotations for biological processes (BP) were conducted using Blast2GO software (version 3.0) 1 (Villar et al., 2014; Supplementary Dataset 1). The RNAseq data is available at https://doi.org/10.5061/dryad.50kt0 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE68881.
Network Analysis of the Tick Regulome in Response to Infection
A network of interactions followed by a co-correspondence analysis (CoCA) was used for the integration of TF and TG interactions (regulome) of I. scapularis tick response to A. phagocytophilum infection. The methodology to build the network of interactions between proteins and functional metabolic processes has been previously described and validated (Estrada-Peña et al., 2018). This network consists of a set of nodes that are connected by edges where nodes are the interacting items, and links between nodes represent the strength with which they interact. In this development, a TF or TG is the source node and the cell metabolic process(es) in which it is involved are the target(s). The edge linking both nodes has a weight, which is the expression of either TF or TG. Networks were built separately for infected and uninfected I. scapularis ISE6 cells, salivary glands and midguts. Only TF and TG with GO functional annotations were included in the networks (Supplementary Dataset 1). Centrality is a fundamental property of a network because it refers to nodes that connect high score nodes (Opsahl et al., 2010; Estrada-Peña et al., 2018). In this context, “high score” applies to other nodes with high importance in the network. We calculated the importance of a node in the “traffic” between different nodes of the network using Betweenness Centrality (BNC), giving a higher score to a node that sits on many shortest paths of other node pairs (Barthelemy, 2004; Estrada-Peña et al., 2018). In our context, it is an indicator of the relative importance of a TF/TG in the links between two or more processes. Other calculated indexes included the PageRank (PR), a measure of the importance of the nodes linking with a given node, and the Weighted Degree (WD), which was calculated from the expression profile of each TF/TG linking to a cell process (Estrada-Peña et al., 2018).
The interactions between TF and TG were demonstrated using CoCA. Only TF/TG with values of BNC or PR higher than zero were included. We did the CoCA using the indexes of centrality obtained from the network explained above. The function “coca” of the package “cocorresp” was used for the R programming environment (Simpson, 2016). Data on BNC, PR and WD of each infected and uninfected datasets from ISE6 cells, salivary glands and midguts were entered into separate CoCAs. The analysis aimed to relate two different datasets from uninfected and infected samples to find patterns that are common to both and associating the TF and TG that are close in the reduced multivariate space and establishing correspondences. The plotting of the scores in the two first axes of the reduced space gives the interaction between TF and TG, i.e., the closer they are in the space, the higher is the expected interaction. The method produces a cloud of interacting TF and TG. To improve the resolution of the charts, we plotted only TF/TG that were at a maximum of two score units of distance. We assumed that other TG separated by more than 2 score units from the values of TF were not interacting with these TF.
In silico Prediction of TF-TG Interactions
Putative DNA binding sites in TG for TF present only in infected tick ISE6 cells were predicted based on published information for TF-interacting sequences in other species, and the I. scapularis genomic scaffold whole genome shotgun sequence using cisTargetX2 and direct search for TF binding sequences in the predicted 5′ gene regulatory regions of the I. scapularis genome (Supplementary Dataset 2; Potier et al., 2012; Rougemont and Naef, 2012; Vaquerizas et al., 2012). The BP with higher representation in the upregulated than in downregulated regulome (peptidase inhibitor and stress response) in response to infection were selected for further characterization of TF-TG interactions.
RNA Interference (RNAi) for Gene Knockdown in Tick ISE6 Cells
The I. scapularis ISE6 cells (provided by U.G. Munderloh, University of Minnesota, United States) was maintained in L-15B300 medium as described previously (Munderloh et al., 1994). Four different TF (HSF, Ap-2, Arx and Hox; Supplementary Dataset 2) were silenced using two siRNAs for each TF (HSF: 5′ GCA CUC AGG GCC AGG AUU A 3′ and 5′ CCU CGG AAG CAG ACA GGA A 3′; Ap-2: 5′ AGA AAG AGG ACA CGA AGA A 3′ and 5′ CCA AGA AAG AGG ACA CGA A 3′; Arx: 5′ CCA AGA AAG AGG ACA CGA A 3′ and 5′ GAC CGA AGC CAG AGU GCA A 3′; Hox: 5′ CCU CCA GCU UCA ACA CAU A 3′ and 5′ ACG CCA CGG CCG AGC UUA A 3′) provided by Dharmacon (GE Healthcare Dharmacon Inc., Lafayette, CO, United States). As control, two Rs86 siRNAs (5′ CGG UAA AUG UCG AAG CAA A 3′ and 5′ GCG AAU AUG AAG UCG GUA A 3′) were used. The siRNA experiments were conducted by incubating ISE6 tick cells with 100 nM of each siRNA diluted in 100 μl of serum-free medium in 24-well plates using four wells per treatment. To facilitate siRNA transfection, DharmaFECT (GE Healthcare Dharmacon Inc.) was used following manufacturer’s recommendations. After 24 h, 0.5 ml/well of fresh medium was added. After 48 h of siRNA exposure, medium containing siRNA was removed and replaced with 1 ml fresh medium alone or containing cell free A. phagocytophilum NY18 obtained as previously reported (de la Fuente et al., 2005). Cells were incubated for a total of 72 h, and then collected for DNA and RNA extraction.
Determination of Gene Knockdown and TG mRNA Levels by RT-qPCR
Total RNA was extracted from ISE6 cells using All Prep DNA/RNA/PROTEIN Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s recommendations. Gene knockdown levels after TF RNAi were assessed for TF and TG by RT-qPCR on RNA samples using gene-specific oligonucleotide primers (Supplementary Table 1), the Kapa SYBR Fast One-Step RT-qPCR Kit (Kapa Biosystems, Roche Holding AG, Basel, Switzerland), and the QIAGEN Rotor-Gene Real-Time PCR Detection System (Qiagen). A dissociation curve was run at the end of the reaction to ensure that only one amplicon was formed and that the amplicons denatured consistently in the same temperature range for every sample. The mRNA levels were normalized against tick rps4 using the genNorm method [Delta-Delta-Ct (ddCt) method] as described previously (Ayllón et al., 2013). Normalized Ct values were compared between test siRNAs-treated tick cells and controls treated with Rs86 siRNA by Chi2-test (p = 0.05; n = 4 biological replicates).
Determination of A. phagocytophilum DNA Levels by qPCR
Total DNA was extracted from infected cells using an All Prep DNA/RNA/Protein Mini Kit (Qiagen, Hilden, Germany). DNA samples were analyzed by qPCR using gene-specific primers for A. phagocytophilum msp4 as previously described (Ayllón et al., 2013). Normalized against tick rps4 Ct values were compared between test siRNAs-treated tick cells and controls treated with Rs86 siRNA by Chi2-test (p = 0.001; n = 2–4 biological replicates).
Results and Discussion
Rationale and Experimental Design
The tick regulome in response to A. phagocytophilum infection was characterized in the I. scapularis tick vector to provide insights into tissue-specific regulome profiles, and the identification of potential targets for the control of tick infestations and pathogen infection/transmission. The experimental design included two independent methods for the in silico characterization of the tick regulome in response to A. phagocytophilum using transcriptomics data previously obtained from infected I. scapularis ISE6 cells, and fed female midguts and salivary glands (Supplementary Figure 1A). The first approach was based on a network analysis in which the nodes were either TF or TG together with their corresponding GO BP annotations, and the link between two nodes represented the expression of the gene (Supplementary Figure 1B). The indexes of centrality were calculated separately for each network of uninfected and infected samples, and only nodes of TF and TG with indexes of centrality higher than zero were used for co-correspondence CoCA analysis (Supplementary Figure 1B). The second approach was used in parallel with network analysis, and consisted in the in silico prediction of TF-TG interactions based on described TF recognition sequences by searching in the I. scapularis genomic scaffold whole genome shotgun sequence (Supplementary Figure 1C). This analysis was focused on TF present only in infected ISE6 cells, and TG in BP overrepresented in the upregulated than in the downregulated regulome in response to infection as a proof-of-concept to facilitate the identification of candidate target antigens for development of vaccines and other control measures. The results of the network analysis were then plotted with TF and TG together in the reduced space to demonstrate that the position of the TF correlates with the TG that are near to these TF after the CoCA (Supplementary Figure 1D). Finally, the results of both approaches were compared, and the TF-TG interactions predicted by both methods were functionally characterized by RNAi in A. phagocytophilum-infected and uninfected tick ISE6 cells (Supplementary Figure 1E).
The I. scapularis Regulome Shows Tissue-Specific Signatures in Response to A. phagocytophilum Infection
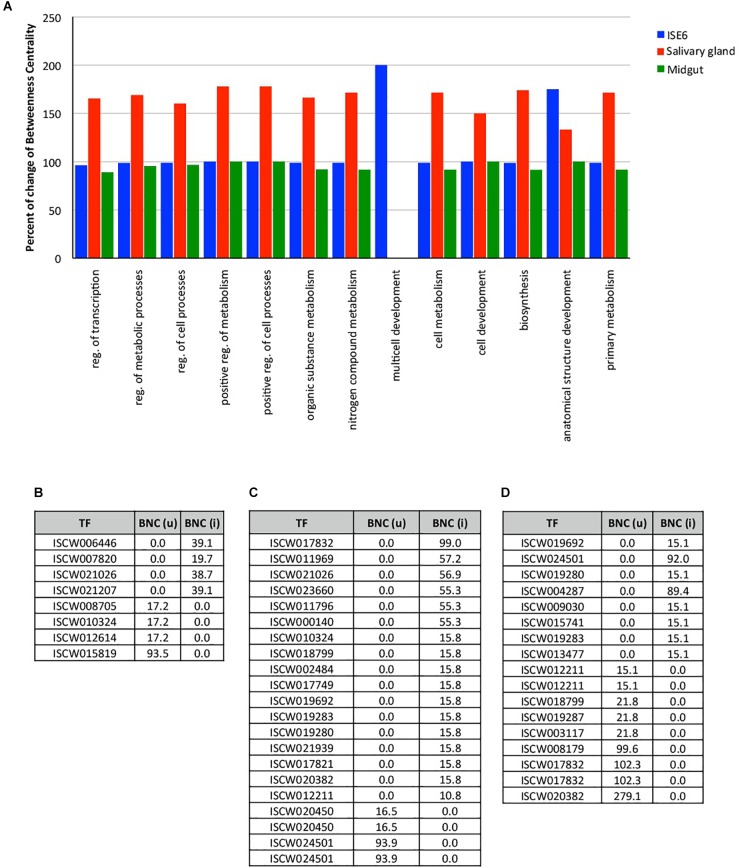
For the construction of networks, a total of 144, 86, and 93 TF (Supplementary Dataset 3), and 5225, 3919, and 4341 TG (Supplementary Dataset 4) were used derived from tick ISE6 cells, salivary glands and midguts, respectively. Tick midgut did not show detectable differences in the network indexes of TF BP between uninfected and infected samples (close to 100% BNC; Figure 1A). However, the TF multicell development and anatomical structure development processes increased to near 200% in infected versus uninfected ISE6 cells (Figure 1A). The network centrality of all the TF processes showed a clear increase in infected salivary glands when compared to unifected controls (Figure 1A). The multicell development process was represented in TF from ISE6 cells only (Figure 1A).
FIGURE 1.
Changes in the expression of TF in tick ISE6 cells, salivary gland and midgut in response to A. phagocytophilum infection. (A) The percentage change of the index Betweenness Centrality (BNC) infection among uninfected and infected target organs in the 13 BP GO annotations. (B) Values of BNC of the 8 TF that showed the highest changes between uninfected BNC (u) and infected BNC (i) ISE6 cells. (C) Values of BNC of the 21 TF that showed the highest changes between uninfected BNC (u) and infected BNC (i) salivary glands. (D) Values of BNC of the 17 TF that showed the highest changes between uninfected BNC (u) and infected BNC (i) midgut. Abbreviations: reg., regulation; (u), uninfected; (i), infected.
Other than minor variations in the network indexes of the TF, each test showed different TF that were present or absent in either uninfected or infected samples. Four TF were detected only in uninfected ISE6 cells, and other 4 were recorded only in infected ISE6 cells (Figure 1B). The most prominent TF in ISE6 cells (ISCW01819) was completely inhibited in infected ISE6 cells (Figure 1B). The pattern was more complex in the salivary glands showing up to 17 TF recorded only in infected, and 4 in uninfected samples (Figure 1C). Nine TF were recorded only in uninfected and 8 only in infected midgut (Figure 1D). As in ISE6 cells, the three most highly represented TF in uninfected midgut were not recorded in infected samples (Figure 1D). The number of TG detected only in uninfected or infected samples varied from 197 (uninfected) to 206 (infected) ISE6 cells, 159 (uninfected) to 585 (infected) salivary glands, and 360 (uninfected) to 129 (infected) midgut.
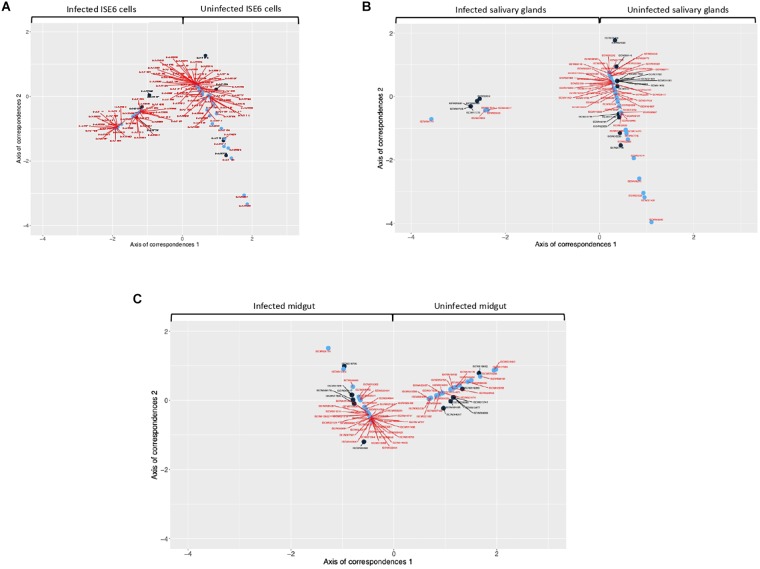
Every detected TF that was unique for uninfected or infected samples was included in CoCA. Based on the values of network indexes of TG, 70 TG in uninfected and 88 TG in infected ISE6 cells, 5 TG in uninfected and 58 TG in infected salivary glands, and 43 TG in uninfected and 25 TG in infected midgut were included in the analysis. The results from multivariate analyses showed a clear correspondence between TF and TG recorded only in uninfected or infected samples while the origin of the first correspondence axis (value = 0) separated completely TF and TG occurring only in either uninfected or infected samples (Figure 2A–C and Supplementary Figures 2A–C). These analyses suggested that the TF closer to individual TG are likely to regulate the expression of these genes (Figure 2A–C and Supplementary Figures 2A–C).
FIGURE 2.
Co-correspondence analysis (CoCA) of TF and TG in uninfected and A. phagocytophilum-infected samples. CoCA was conducted in I. scapularis (A) ISE6 cells, (B) salivary glands and (C) midgut. The charts show the position of TF (black symbol and label) and TG (blue symbol and red label) after the CoCA of the indexes of centrality. The TF and associated TG with highest values of centrality in the network of infected cells appear together at negative values of the Axis 1 (n = 4, 4, and 9 in ISE6 cells, salivary glands and midgut, respectively). The TF and the associated TG with highest values of centrality in the network of uninfected cells appear together at positive values of the Axis 1 (n = 4, 17, and 8 in ISE6 cells, salivary glands and midgut, respectively). High-resolution images are shown in Supplementary Figures 2A–C.
These results evidenced tissue-specific differences between infected and uninfected cells, thus supporting previous findings at the mRNA, protein and metabolic levels in I. scapularis ISE6 cells, a model for hemocytes, midgut and salivary glands, which are involved in A. phagocytophilum life cycle in the tick vector (Ayllón et al., 2015; Villar et al., 2015; reviewed by de la Fuente et al., 2017). These results evidenced that the regulome regulates various BP involved in tick-A. phagocytophilum interactions (Figure 1A, 3A and Supplementary Dataset 2), a finding previously reported in other organisms (Shih et al., 2016; Casella et al., 2017).
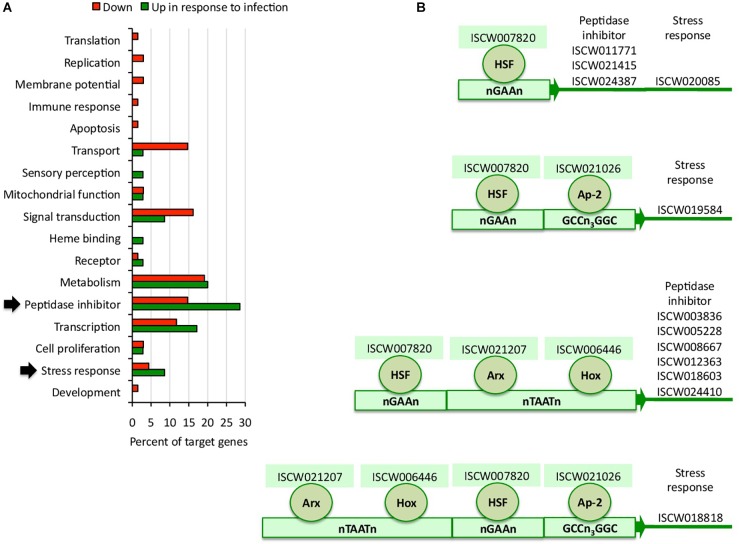
FIGURE 3.
Biological processes affected by the tick ISE6 cells regulome in response to A. phagocytophilum infection. (A) Upregulated and downregulated target genes in the in silico predicted tick ISE6 cells regulome in response to A. phagocytophilum infection were grouped according to their BP. The BP with higher representation in the upregulated than in downregulated regulome in response to infection (arrows) were selected for characterization of TF-TG interactions. (B) Predicted regulatory DNA motifs according to regulatory factors identified by RNAseq in infected cells only and involved in the control of upregulated target genes annotated in the peptidase inhibitor and stress response BP with higher representation in the upregulated than in downregulated regulome.
A. phagocytophilum Modulates the Tick Regulome to Upregulate Biological Processes That Facilitate Pathogen Infection
To complement the network analysis approach to tick regulome study, the putative DNA binding sites were characterized in silico for TF and TG in the upregulated regulome in response to infection in tick vector ISE6 cells (Figure 3A,B and Supplementary Dataset 2). In particular, the peptidase inhibitor and stress response BP with higher representation in the upregulated than in downregulated regulome (Figure 3A) were selected for further characterization of TF-TG interactions (Figure 3B).
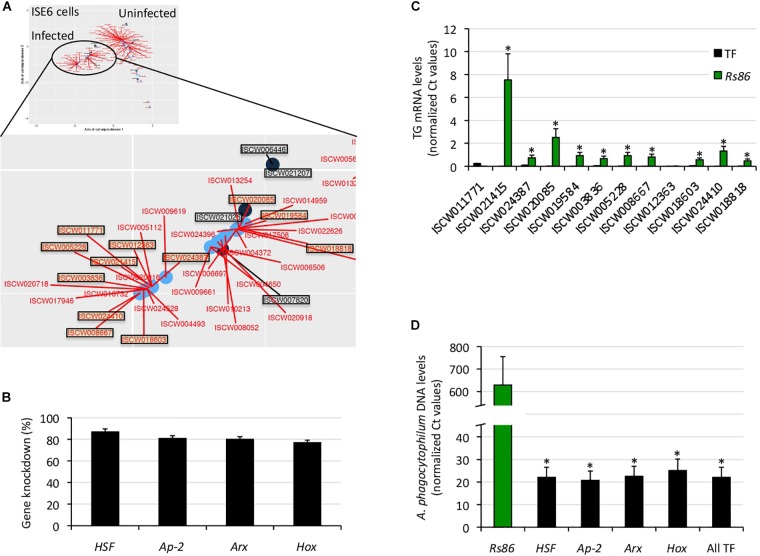
The results showed a correlation between complementary in silico approaches (Figure 4A), therefore providing support for the network analysis of the regulome to predict at the transcriptomics level the most significant TF-TG interactions in response to stimuli such as pathogen infection.
FIGURE 4.
Functional characterization of selected TF-TG components of the tick ISE6 cells regulome. (A) The predictive results of network analysis and in silico prediction of TF-TG interactions were compared in infected ISE6 cells. The TF-TG interactions predicted by both methods (squared in black letter for TF and red letter for TG) were then functionally characterized by RNAi in tick ISE6 cells. (B) Percentage of TF gene knockdown with respect to Rs86 siRNA control in ISE6 cells. Normalized against tick rps4 Ct values were compared between test siRNAs-treated tick cells and controls treated with Rs86 siRNA by Chi2-test (p < 0.05; n = 4 biological replicates). (C) The TG mRNA levels were determined by qRT-PCR in ISE6 cells after TF gene knockdown or treatment with control Rs86 siRNA. Normalized against tick rps4 Ct values (average + S.E.) were compared between test siRNAs-treated tick cells and controls treated with Rs86 siRNA by Chi2-test (∗p < 0.001; n = 4 biological replicates). (D) The A. phagocytophilum DNA levels were determined by qPCR in ISE6 cells after TF gene knockdown or treatment with control Rs86 siRNA. Normalized against tick rps4 Ct values (average + S.D.) were compared between test siRNAs-treated tick cells and controls treated with Rs86 siRNA by Chi2-test (∗p < 0.001; n = 2–4 biological replicates).
To characterize the functional implications of selected TF-TG interactions predicted by both methodological approaches (Figure 3B, 4A), RNAi was used in ISE6 cells to knockdown the expression of TF and characterize the effect on TG mRNA and A. phagocytophilum DNA levels (Figure 4B–D). The results showed that after 77–87% (average ± S.D., 81 ± 4%) TF silencing (Figure 4B), the levels of all predicted TG except for ISCW011771 and ISCW012363 decreased when compared to Rs86 siRNA-treated controls (Figure 4C). The two TG that were not downregulated after TF knockdown had the lowest mRNA levels (Figure 4C), which could affect the possibility of detecting differences between test and control cells. Alternatively, other TF or interacting proteins could be involved in the regulation of these genes. Nevertheless, except for ISCW011771 and ISCW012363 the results supported the prediction that these TF are implicated in the regulation of TG. Nevertheless, these TF-TG interactions should be corroborated in future experiments using different in vitro protein-DNA binding assays (Yang, 1998; Forde and McCutchen-Maloney, 2002; Deplancke and Gheldof, 2012; Ogawa and Biggin, 2012).
Regarding A. phagocytophilum infection, the results showed a 96–97% decrease in pathogen DNA levels after TF knockdown (Figure 4D). These results suggested that the TF and corresponding TG are upregulated by A. phagocytophilum to facilitate pathogen infection.
Characterization of TF and Upregulated TG in Response to Infection as Putative Control Targets
The TF implicated in the regulation of selected TG included heat shock transcription factor (HSF), Ap-2, Aristaless-related homeobox gene (Arx) and Hox (Figure 3B). These TF has been described before to function in different transcriptionally regulated processes in other species. The mammalian Ap-2 TF has been shown to be involved in transcriptional activation and DNA binding/dimerization (Williams and Tjian, 1991). The HSF family has been implicated in the regulation of different physiological processes including cell response to stress and infection (Gomez-Pastor et al., 2018). Hox and Arx are members of a family of essential developmental regulators that bind to homeodomains target DNA sequences to regulate embryogenesis and neuronal processes in different organisms (Pellerin et al., 1994; Cho et al., 2012).
Few studies in other host-pathogen models have shown that some of these TF facilitate pathogen infection and therefore has been proposed as potential targets for control interventions. In primary peripheral blood monocytes, HSF1 is upregulated by human cytomegalovirus (HCMV) for pathogen survival, and has been suggested as a potential control target (Peppenelli et al., 2018). The tick-borne pathogen, Ehrlichia chaffeensis, upregulates the expression of certain Hox genes to facilitate infection through epigenetic mechanisms in human monocytic leukemia cells (THP-1) (Mitra et al., 2018). However, the role of these TF during pathogen infection in ticks has not been investigated before.
The BP modulated by the regulome of selected TF-TG interactions with a putative role in facilitating A. phagocytophilum infection included peptidase inhibitor and stress response (Figure 3A and Supplementary Dataset 2). The stress response upregulated TG included genes coding for peroxinectin and uncharacterized protein with heme binding and peroxidase activity (n = 2), and glutathione peroxidase with glutathione peroxidase activity. The peptidase inhibitor TG encoded for a carboxypeptidase inhibitor precursor with carboxipeptidase and metalloendopeptidase inhibitor activity, uncharacterized protein, Kunitz-type proteinase inhibitor 5 II, serpin-2 precursors and secreted salivary gland peptides with serine-type endopeptidase inhibitor activity (n = 6), and cystatin and salivary cystatin-L with cysteine-type endopeptidase inhibitor activity (n = 2).
These proteins are involved in key physiological processes during tick life cycle such as heme/iron metabolism and detoxification and tick-host interactions (Chmelar et al., 2016; de la Fuente et al., 2016a). Glutathione peroxidase belongs to the glutathione antioxidant defense system and protects eukaryotic cells from oxidative damage (Espinosa-Diez et al., 2015). Glutathione peroxidase levels and activity are affected in different ways by Anaplasma marginale infection in both vertebrate and tick cells (Reddy et al., 1988; More et al., 1989; Kalil et al., 2017; Esmaeilnejad et al., 2018). While the activity and expression of glutathione peroxidase and other components of the antioxidant system was lower in A. marginale-infected cattle and water buffaloes (Reddy et al., 1988; More et al., 1989; Esmaeilnejad et al., 2018), glutathione peroxidase coding gene was upregulated in embryonic Rhipicephalus microplus BME26 cells in response to A. marginale infection (Kalil et al., 2017). These results showed that A. marginale infection induces a differential response of the glutathione antioxidant defense system in the vertebrate and tick hosts. RNAi-mediated gene silencing of glutathione peroxidase and other antioxidant defense system genes increased A. marginale infection in BME26 cells, suggesting that the antioxidant response mediated by this molecule might play a role in the control of infection in ticks (Kalil et al., 2017). Another of the identified tick TG, peroxinectin, is a cell adhesion protein involved in melanization of pathogens in invertebrates (Sritunyalucksana et al., 2001; Cerenius and Söderhäll, 2004), was upregulated in crayfish resistant to white spot syndrome virus, and susceptible crayfish failed to upregulate this gene in response to viral infection (Yi et al., 2017). Strong cellular adhesion in response to the invading agent during crustacean encapsulation defense reaction was proposed as a protective mechanism mediated by peroxinectin in infected crayfish. The heme-binding lipoprotein (HELP), a transporter of heme in ticks, was previously found to be upregulated and downregulated in midguts and salivary glands, respectively, of A. phagocytophilum-infected ticks (Villar et al., 2016). HELP, together with Vitellogenin 1 and 2, was proposed to transport heme to other tick tissues such as salivary glands (Hajdusek et al., 2009). The uncharacterized protein with heme binding activity identified in this study may have a function similar to HELP, suggesting that A. phagocytophilum affects hemoglobin primary cleavage and heme transport in tick midguts and salivary glands, possibly to regulate the levels of heme in a tissue-specific manner with potential effects for pathogen and vector survival. The expression of Kunitz-type proteinase inhibitors have been found to be modified in several tick species in response to infection by tick-borne pathogens such as Bartonella henselae (Liu et al., 2014), flavivirus (McNally et al., 2012), Babesia bigemina (Antunes et al., 2012), and A. marginale (Zivkovic et al., 2010). Kunitz peptides are moonlighting proteins that perform multiple functions within the feeding lesion (Schwarz et al., 2014). Upregulation of Kunitz proteins in salivary glands of ticks infected with B. henselae (Liu et al., 2014) and flavivirus (McNally et al., 2012) may be associated with host immunity modulation at the feeding site. In contrast to upregulation, it is less clear why Kunitz peptides, including a Kunitz-type proteinase inhibitor 5, would be dowregulated in salivary glands following A. marginale infection (Zivkovic et al., 2010). Additional studies show that the expression of Kunitz peptides is complex and may be related to the tick and pathogen species (Rachinsky et al., 2007; Antunes et al., 2012).
These preliminary evidences based on selected TF-TG interactions support that the analysis of tick regulome in response to different stimuli such as pathogen infection could provide potential targets for the control of tick infestations and pathogen infection/transmission. Furthermore, some of these protein families have been proposed as protective antigens using a rational approach for the identification of tick vaccine protective antigens (de la Fuente et al., 2016a). As recently proposed (de la Fuente et al., 2018), the combination of regulomics with intelligent Big Data analytic techniques may contribute to the high throughput identification of candidate vaccine antigens.
Conclusion
Our modeling of the modulation of the tick regulome in response to A. phagocytophilum infection provided new insights into the mechanisms that target specific functions in different tick tissues. These results supported the use of network analysis for the study of regulome response to infection. Although general mechanisms affected by A. phagocytophilum infection may be conserved even between tick and human cells (de la Fuente et al., 2016b), the effect of vector-pathogen co-evolution on pathogen isolates adaptation to grow in tick cells (Alberdi et al., 2015) may result in differences between isolates in the modulation of the tick cell regulome. Future research should be directed at validating the results of the network analysis for regulomics studies and the characterization of TF-TG interactions. Deciphering the precise nature of circuits that shape the tick regulome in response to pathogen infection is an area of research that in the future will advance our knowledge of tick-pathogen interactions, and the identification of new targets for the control of tick infestations and pathogen infection/transmission.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Author Contributions
MV, AE-P, AC-C, and JdlF conceived the study and designed the experiments. SA-J, PA, and MV performed the experiments. AE-P, MV, AC-C, and JdlF performed the data analysis. JdlF, SA-J, AE-P, and AC-C wrote the manuscript. All authors approved and contributed to the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This research was financially supported by the Ministerio de Economía, Industria y Competitividad, Spain grant BFU2016-79892-P. MV was funded by the Universidad de Castilla La Mancha, Spain.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00462/full#supplementary-material
References
- Alberdi P., Ayllón N., Cabezas-Cruz A., Bell-Sakyi L., Zweygarth E., Stuen S., et al. (2015). Infection of Ixodes spp. tick cells with different Anaplasma phagocytophilum isolates induces the inhibition of apoptotic cell death. Ticks Tick Borne Dis. 6 758–767. 10.1016/j.ttbdis.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Antunes S., Galindo R. C., Almazán C., Rudenko N., Golovchenko M., Grubhoffer L., et al. (2012). Functional genomics studies of Rhipicephalus (Boophilus) annulatus ticks in response to infection with the cattle protozoan parasite, Babesia bigemina. Int. J. Parasitol. 42 187–195. 10.1016/j.ijpara.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Artigas-Jerónimo S., de la Fuente J., Villar M. (2018a). Interactomics and tick vaccine development: new directions for the control of tick-borne diseases. Expert Rev. Proteomics 15 627–635. 10.1080/14789450.2018.1506701 [DOI] [PubMed] [Google Scholar]
- Artigas-Jerónimo S., Villar M., Cabezas-Cruz A., Valdés J. J., Estrada-Peña A., Alberdi P., et al. (2018b). Functional evolution of Subolesin/Akirin. Front. Physiol. 9:1612. 10.3389/fphys.2018.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayllón N., Villar M., Busby A. T., Kocan K. M., Blouin E. F., Bonzón-Kulichenko E., et al. (2013). Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 81 2415–2425. 10.1128/IAI.00194-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayllón N., Villar M., Galindo R. C., Kocan K. M., Šíma R. L, ópez J. A., et al. (2015). Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS Genet. 11:e1005120. 10.1371/journal.pgen.1005120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy M. (2004). Betweenness centrality in large complex networks. Eur. Phys. J. B 38 163–168. 10.1140/epjb/e2004-00111-4 [DOI] [Google Scholar]
- Bell-Sakyi L., Darby A., Baylis M., Makepeace B. L. (2018). The Tick Cell Biobank: a global resource for in vitro research on ticks, other arthropods and the pathogens they transmit. Ticks Tick Borne Dis. 9 1364–1371. 10.1016/j.ttbdis.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. K., Groshong A. M., Drecktrah D., Boylan J. A., Gherardini F. C., Blevins J. S., et al. (2019). DksA controls the response of the Lyme disease spirochete Borrelia burgdorferi to starvation. J. Bacteriol. 201:e00582-18. 10.1128/JB.00582-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J. V., Pappas C. J., Terekhova D. A., Iyer R., Godfrey H. P., Schwartz I., et al. (2015). Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One 10:e0118063. 10.1371/journal.pone.0118063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella L. G., Weiss A., Pérez-Rueda E., Ibarra J. A., Shaw L. N. (2017). Towards the complete proteinaceous regulome of Acinetobacter baumannii. Microb. Genom. 3:mgen000107. 10.1099/mgen.0.000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L., Söderhäll K. (2004). The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198 116–126. 10.1111/j.0105-2896.2004.00116.x [DOI] [PubMed] [Google Scholar]
- Chmelar J., Kotal J., Karim S., Kopacek P., Francischetti I. M. B., Pedra J. H. F., et al. (2016). Sialomes and mialomes: a systems-biology view of tick tissues and tick-host interactions. Trends Parasitol. 32 242–254. 10.1016/j.pt.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. J., Valles Y., Kim K. M., Ji S. C., Han S. J., Park S. C. (2012). Additional duplicated Hox genes in the earthworm: Perionyx excavatus Hox genes consist of eleven paralog groups. Gene 493 260–266. 10.1016/j.gene.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Contreras M., Alberdi P., Fernández de Mera I. G., Krull C., Nijhof A., Villar M., et al. (2017). Vaccinomics approach to the identification of candidate protective antigens for the control of tick vector infestations and Anaplasma phagocytophilum infection. Front. Cell. Infect. Microbiol. 7:360. 10.3389/fcimb.2017.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J. (2018). Controlling ticks and tick-borne diseases...looking forward. Ticks Tick Borne Dis. 9 1354–1357. 10.1016/j.ttbdis.2018.04.001 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Antunes S., Bonnet S., Cabezas-Cruz A., Domingos A., Estrada-Peña A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7:114. 10.3389/fcimb.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Ayoubi P., Blouin E. F., Almazán C., Naranjo V., Kocan K. M. (2005). Gene expression profiling of human promyelocytic cells in response to infection with Anaplasma phagocytophilum. Cell. Microbiol. 7 549–559. 10.1111/j.1462-5822.2004.00485.x [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Merino O. (2013). Vaccinomics, the new road to tick vaccines. Vaccine 31 5923–5929. 10.1016/j.ttbdis.2018.04.001 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Villar M., Estrada-Peña A., Olivas J. A. (2018). High throughput discovery and characterization of tick and pathogen vaccine protective antigens using vaccinomics with intelligent Big Data analytic techniques. Expert Rev. Vaccines 17 569–576. 10.1080/14760584.2018 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Kopáček P., Lew-Tabor A., Maritz-Olivier C. (2016a). Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 38 754–769. 10.1111/pim.12339 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Villar M., Cabezas-Cruz A., Estrada-Peña A., Ayllón N., Alberdi P. (2016b). Tick-host-pathogen interactions: conflict and cooperation. PLoS Pathog. 12:e1005488. 10.1371/journal.ppat.1005488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Waterhouse R. M., Sonenshine D. E., Roe M. R., Ribeiro J. M., Sattelle D. B., et al. (2016c). Tick genome assembled: new opportunities for research on tick-host-pathogen interactions. Front. Cell. Infect. Microbiol. 6:103. 10.3389/fcimb.2016.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B., Gheldof N. (2012). Gene Regulatory Networks Methods and Protocols. New York, NY: Human Press. [Google Scholar]
- Esmaeilnejad B., Tavassoli M., Samiei A., Hajipour N., Imani-Baran A., Farhang-Pajuh F. (2018). Evaluation of oxidative stress and antioxidant status, serum trace mineral levels and cholinesterases activity in cattle infected with Anaplasma marginale. Microb. Pathog. 123 402–409. 10.1016/j.micpath.2018.07.039 [DOI] [PubMed] [Google Scholar]
- Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., et al. (2015). Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 6 183–197. 10.1016/j.redox.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A., Villar M., Artigas-Jerónimo S., López V., Alberdi P., Cabezas-Cruz A., et al. (2018). Use of graph theory to characterize human and arthropod vector cell protein response to infection. Front. Cell. Infect. Microbiol. 8:265. 10.3389/fcimb.2018.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde C. E., McCutchen-Maloney S. L. (2002). Characterization of transcription factors by mass spectrometry and the role of SELDI-MS. Mass Spectrom. Rev. 21 419–439. 10.1002/mas.10040 [DOI] [PubMed] [Google Scholar]
- Gomez-Pastor R., Burchfiel E. T., Thiele D. J. (2018). Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 19 4–19. 10.1038/nrm.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Guaneri J., Lee D. H., Gallo S. M. (2011). The NFI-Regulome Database: a tool for annotation and analysis of control regions of genes regulated by Nuclear Factor I transcription factors. J. Clin. Bioinforma. 1:4. 10.1186/2043-9113-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M., Nuss A. B., Meyer J. M., Sonenshine D. E., Roe R. M., Waterhouse R. M., et al. (2016). Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 7:10507. 10.1038/ncomms10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdusek O., Sojka D., Kopacek P., Buresova V., Franta Z., Sauman I., et al. (2009). Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proc. Natl. Acad. Sci. U.S.A. 106 1033–1038. 10.1073/pnas.0807961106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil S. P., Rosa R. D. D., Capelli-Peixoto J., Pohl P. C., Oliveira P. L., Fogaça A. C., et al. (2017). Immune-related redox metabolism of embryonic cells of the tick Rhipicephalus microplus (BME26) in response to infection with Anaplasma marginale. Parasit. Vectors. 10:613. 10.1186/s13071-017-2575-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., de la Fuente J., Cote M., Galindo R. C., Moutailler S., Vayssier-Taussat M., et al. (2014). IrSPI, a tick serine protease inhibitor involved in tick feeding and Bartonella henselae infection. PLoS Negl. Trop. Dis. 8:e2993. 10.1371/journal.pntd.0002993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K. L., Mitzel D. N., Anderson J. M., Ribeiro J. M., Valenzuela J. G., Myers T. G., et al. (2012). Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick Borne Dis. 3 18–26. 10.1016/j.ttbdis.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Dunphy P. S., Das S., Zhu B., Luo T., McBride J. W. (2018). Ehrlichia chaffeensis TRP120 effector targets and recruits host polycomb group proteins for degradation to promote intracellular infection. Infect. Immun. 86:e00845-17. 10.1128/IAI.00845-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More T., Reddy G. R., Sharma S. P., Singh L. N. (1989). Enzymes of oxidant defence system of leucocytes and erythrocytes in bovine anaplasmosis. Vet. Parasitol. 31 333–337. 10.1016/0304-4017(89)90082-4 [DOI] [PubMed] [Google Scholar]
- Munderloh U., Liu Y., Wang M., Chen C., Kurtti T. (1994). Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80 533–543. [PubMed] [Google Scholar]
- Ogawa N., Biggin M. D. (2012). High-throughput SELEX determination of DNA sequences bound by transcription factors in vitro. Methods Mol. Biol. 786 51–63. 10.1007/978-1-61779-292-2_3 [DOI] [PubMed] [Google Scholar]
- Opsahl T., Agneessens F., Skvoretz J. (2010). Node centrality in weighted networks: generalizing degree and shortest paths. Soc. Networks 32 245–251. 10.1016/j.socnet.2010.03.006 [DOI] [Google Scholar]
- Pellerin I., Schnabel C., Catron K. M., Abate C. (1994). Hox proteins have different affinities for a consensus DNA site that correlate with the positions of their genes on the Hox cluster. Mol. Cell Biol. 14 4532–4545. 10.1128/mcb.14.7.4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppenelli M. A., Miller M. J., Altman A. M., Cojohari O., Chan G. C. (2018). Aberrant regulation of the Akt signaling network by human cytomegalovirus allows for targeting of infected monocytes. Antiviral Res. 158 13–24. 10.1016/j.antiviral.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier D., Atak Z. K., Sanchez M. N., Herrmann C., Aerts S. (2012). Using cisTargetX to predict transcriptional targets and networks in Drosophila. Methods Mol. Biol. 786 291–314. 10.1007/978-1-61779-292-2_18 [DOI] [PubMed] [Google Scholar]
- Rachinsky A., Guerrero F. D., Scoles G. A. (2007). Differential protein expression in ovaries of uninfected and Babesia-infected southern cattle ticks, Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol. 37 1291–1308. 10.1016/j.ibmb.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Reddy G. R., More T., Sharma S. P., Singh L. N. (1988). The oxidant defence system in water-buffaloes (Bubalus bubalis) experimentally infected with Anaplasma marginale. Vet. Parasitol. 27 245–249. 10.1016/0304-4017(88)90039-8 [DOI] [PubMed] [Google Scholar]
- Rioualen C., Da Costa Q., Chetrit B., Charafe-Jauffret E., Ginestier C., Bidaut G. (2017). HTS-Net: an integrated regulome-interactome approach for establishing network regulation models in high-throughput screenings. PLoS One 12:e0185400. 10.1371/journal.pone.0185400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont J., Naef F. (2012). Computational analysis of protein-DNA interactions from ChIP-seq data. Methods Mol. Biol. 786 263–273. 10.1007/978-1-61779-292-2_16 [DOI] [PubMed] [Google Scholar]
- Schwarz A., Cabezas-Cruz A., Kopeckẏ J., Valdés J. J. (2014). Understanding the evolutionary structural variability and target specificity of tick salivary Kunitz peptides using next generation transcriptome data. BMC Evol. Biol. 14:4. 10.1186/1471-2148-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severo M. S., Pedra J. H. F., Ayllón N., Kocan K. M., de la Fuente J. (2015). “Anaplasma,” in Molecular Medical Microbiology, 2nd Edn Vol. 3 eds Tang Y. W., Sussman M., Liu D., Poxton I., Schwartzman J. (London: Academic Press; ), 2033–2042. [Google Scholar]
- Shaw D. K., Wang X., Brown L. J., Oliva Chávez A. S., Reif K. E., Smith A. A., et al. (2017). Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 8:14401. 10.1038/ncomms14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H. Y., Sciumè G., Mikami Y., Guo L., Sun H. W., Brooks S. R., et al. (2016). Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 165 1120–1133. 10.1016/j.cell.2016.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson G. L. (2016). cocorresp: Co-Correspondence Analysis Ordination Methods. R package version 0.3-0. Available at: http://cran.r-project.org/package=cocorresp (accessed June, 2018). [Google Scholar]
- Sritunyalucksana K., Wongsuebsantati K., Johansson M. W., Söderhäll K. (2001). Peroxinectin, a cell adhesive protein associated with the proPO system from the black tiger shrimp, Penaeus monodon. Dev. Comp. Immunol. 25 353–363. 10.1016/s0145-305x(01)00009-x [DOI] [PubMed] [Google Scholar]
- Vaquerizas J. M., Teichmann S. A., Luscombe N. M. (2012). How do you find transcription factors? computational approaches to compile and annotate repertoires of regulators for any genome. Methods Mol. Biol. 786 3–19. 10.1007/978-1-61779-292-2_1 [DOI] [PubMed] [Google Scholar]
- Villar M., Ayllón N., Alberdi P., Moreno A., Moreno M., Tobes R., et al. (2015). Integrated metabolomics, transcriptomics and proteomics identifies metabolic pathways affected by Anaplasma phagocytophilum infection in tick cells. Mol. Cell. Proteomics 14 3154–3172. 10.1074/mcp.M115.051938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M., López V., Ayllón N., Cabezas-Cruz A., López J. A., Vázquez J., et al. (2016). The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit. Vectors 9:467. 10.1186/s13071-016-1747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M., Popara M., Ayllón N., Fernández de Mera I. G., Mateos-Hernández L., Galindo R. C., et al. (2014). A systems biology approach to the characterization of stress response in Dermacentor reticulatus tick unfed larvae. PLoS One 9:e89564. 10.1371/journal.pone.0089564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Tjian R. (1991). Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 5 670–682. 10.1101/gad.5.4.670 [DOI] [PubMed] [Google Scholar]
- Yang V. W. (1998). Eukaryotic transcription factors: identification, characterization and functions. J. Nutr. 128 2045–2051. 10.1093/jn/128.11.2045 [DOI] [PubMed] [Google Scholar]
- Yi S., Li Y., Shi L., Zhang L. (2017). Novel Insights into Antiviral gene regulation of red swamp crayfish, Procambarus clarkii, infected with white spot syndrome virus. Genes 8:E320. 10.3390/genes8110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic Z., Esteves E., Almazán C., Daffre S., Nijhof A. M., Kocan K. M., et al. (2010). Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus) microplus in response to infection with Anaplasma marginale. BMC Genomics 11:186. 10.1186/1471-2164-11-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.