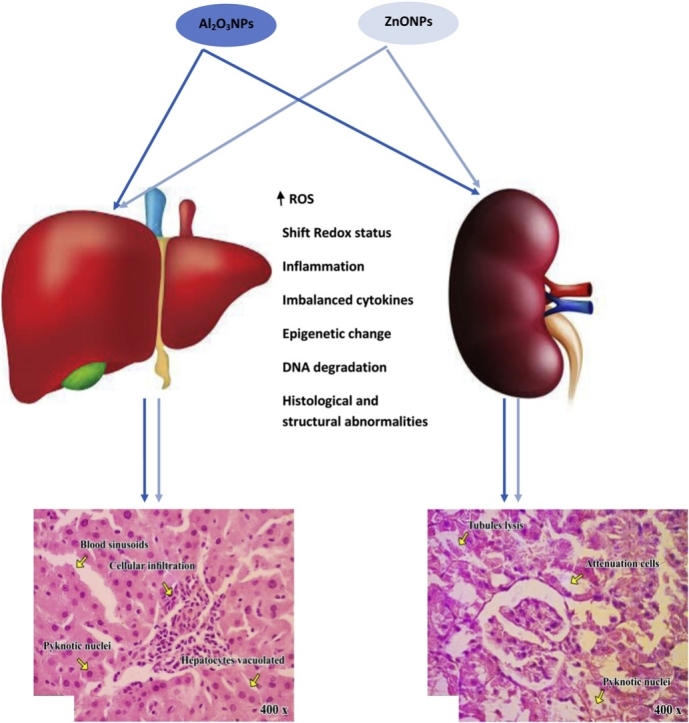
Graphical abstract
Abbreviations: TBARS, thiobarbituric acid-reactive substances; GSH, reduced glutathione; GST, glutathione S-transferase; SOD, superoxide dismutase; CAT, catalase; ROS, reactive oxygen species; GPX, glutathione peroxidase; AST, aspartate transaminase; ALT, alanine transaminase; ACP, acid phosphatase; AlP, alkaline phosphatase; GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase; mtTFA, mitochondrial transcription factor A; PGC-1α, peroxisome proliferator activator receptor gamma-coactivator 1α
Keywords: Aluminum oxide nanoparticles, Zinc oxide nanoparticles, Gene expression, DNA fragmentation, Cytokines and p53, Oxidative stress
Highlights
-
•
Oral sub-chronic exposure to Aluminum oxide or zinc oxide nanoparticles has hepato-renal toxicity.
-
•
The toxicities of Aluminum oxide and/or zinc oxide NPs mediated through different correlated pathways.
-
•
The pathways including; epigenetic changes, impaired antioxidant systems, induced oxidative stress and disturbed cytokine production.
-
•
Exaggerated hepatic and renal toxicities of combined exposure to both NPs.
Abstract
Aluminum oxide nanoparticles (Al2O3NPs) and zinc oxide nanoparticles (ZnONPs) have been involved in many industries and they are extensively abundant in many aspects of human life. Consequently, concerns have been raised about their potentially harmful effects. However the toxicities of Al2O3NPs and ZnONPs are well documented, the effect of co-exposure to both nanoparticles remains strictly obscure. Therefore, the present study was undertaken to address this issue. Four groups of male Wistar rats (10 rats each) were used; control, Al2O3NPs treated, ZnONPs treated and Co-treated groups. Rats were orally administered their respective treatment daily for 75 days. The effects of each nanoparticle alone or in combination were assessed at different levels including; hepatic and renal function, structure, and redox status, nuclear DNA fragmentation, hepatic expression of mitochondrial transcription factor A (mtTFA) gene and peroxisome proliferator-activated receptor gamma-coactivator 1α (PGC-1α), systemic inflammation, and hematologic parameters. The results confirmed the hepatorenal toxicities of each nanoparticle used at the level of all parameters with suppression of the hepatic expression of mtTFA and PGC-1α. The co-exposure to both nanoparticles results in synergistic effects. From these results, we can conclude that co-exposure to aluminum oxide nanoparticles and zinc oxide nanoparticles results in more pronounced hepatorenal toxicities and systemic inflammation.
1. Introduction
Nanotechnology is a new branch of science that based on the creation and manipulation of nanoparticles (NPs) (particle size range from 1 to 100 nm) to create products that exhibit novel properties [1]. With the immense spread of nanotechnology in industrial and consumer markets, the environmental accumulation of nanomaterials and human exposure to nanoparticles (NPs) became inevitable [2]. Nanoparticles affect various physiological systems [3], such as the respiratory system [4] and male reproductive system [5]. Among NPs, zinc oxide nanoparticles (ZnONPs), and due to their excellent antibacterial activity and absorption of ultraviolet radiation, they have been commercially available and are widely used as active component of food additives, toothpaste, food packaging, sunscreens, and other pharmaceuticals [6,7]. Subsequently, with the increased use of ZnONPs, the exposure to these nanoparticles has been rising steadily, causing more concerns about the extent of their potential toxicity, including cytotoxic, genotoxic, and proinflammatory effects [8]. This has raised concerns in public and scientific communities regarding their unanticipated and adverse health effects [9]. The toxicity of ZnONPs has been evaluated in different biological systems, such as; bacteria [10], mammalian cells [11] and in vivo models [12]. In mammalian cells, the toxic effects of ZnONPs such as membrane injury, inflammatory response, DNA damage, and apoptosis have been demonstrated (Gojova et al., 2007). However, studies have shown that the toxicity of ZnONPs is due to their possible role in increased reactive oxygen species (ROS) [13]. ROS generation is linked to DNA damage and cellular apoptosis [14].
The toxicity of ZnONPs has been studied extensively (see [15]), revealing different inauspicious effects induced by ZnONPs in different cell lines oxidative stress such as; lipid peroxidation, cell membrane leakage, oxidative DNA damage, increasing intracellular calcium, and even antiproliferative activity [16].
Aluminum oxide nanoparticles (Al2O3NPs) have been used in industrial and biomedical applications. However, studies on Al2O3NPs ecotoxicology are mainly limited to reports on acute exposure [17]. Aluminum oxide nanoparticles may possibly enter the food chain and be responsible for toxicity in animals [18]. The oral exposure of rats to Al2O3NPs has been implicated to cause genotoxic damage [19]. Prabhakar et al. [20] have illustrated the possible involvement of oxidative stress and altered antioxidant status in eliciting toxicity of Al2O3NPs after acute oral treatment. Several studies have shown that the in vitro and in vivo toxicity of Al2O3 nanoparticles negatively affect cellular morphology and cellular components, which lead to apoptosis and damage to DNA and proteins [21]. Also, the exposure to Al2O3NPs may lead to adverse effects, such as genetic damage [19], inflammatory response [22], carcinogenicity [23], cytotoxicity [24], ROS generation and mitochondrial dysfunction [24].
The toxicity of NPs may affect the whole cell and tissue through changing the architecture of the cell by the induction of toxic effects on different cellular components. At the molecular level, the toxicity of nanoparticles is ranging from direct effects on protein structure and function (by activation or inhibition) to effects on the expression of genes encoding these proteins. Understanding the effect of NPs on the expression of genes encoding master regulators of cellular metabolism is of great importance to achieve a real understanding of NPs toxicity.
Mitochondria are the powerhouse of the cell which responsible for the production of adenosine triphosphate (ATP) which is the main player in cellular metabolism. So, the disruption of mitochondrial homeostasis is a key event in a wide variety of diseases and toxicological effects [25]. The liver and kidney are a highly metabolic tissue that needs an intense demand for mitochondria. Mitochondrial biogenesis plays an essential role in maintaining mitochondrial homeostasis to meet the physiological needs of eukaryotic cells. The factors regulating mitochondrial biogenesis include mitochondrial transcription factor A (mtTFA), which drives transcription and replication of mtDNA. The expression of mtTFA is regulated by peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1 α), the master regulator of mitochondrial biogenesis [26].
However the toxicities of Al2O3NPs and ZnONPs are well documented, the effect of co-exposure to both nanoparticles remains strictly obscure. Only one recent study by Benavides et al. [27] on zebra fish indicated that single and combined exposure to aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish are capable of causing sub-lethal effects, but when combined, NPs seem to be more toxic. Therefore, the present study was undertaken to address this issue. Also we hypothesized that, the documented oxidative stress associated with Al2O3 and ZnO NPs exposure may results from impaired mitochondrial biogenesis so we undertaken to explore the effects their exposure on the rat hepatic expression of genes controlling the mitochondrial biogenesis beside the conventional parameters hepatotoxicity and nephrotoxicity including; hepatic and renal function, structure, and redox status, nuclear DNA fragmentation, systemic inflammation, and hematologic parameters.
2. Materials and methods
2.1. Tested compounds and doses
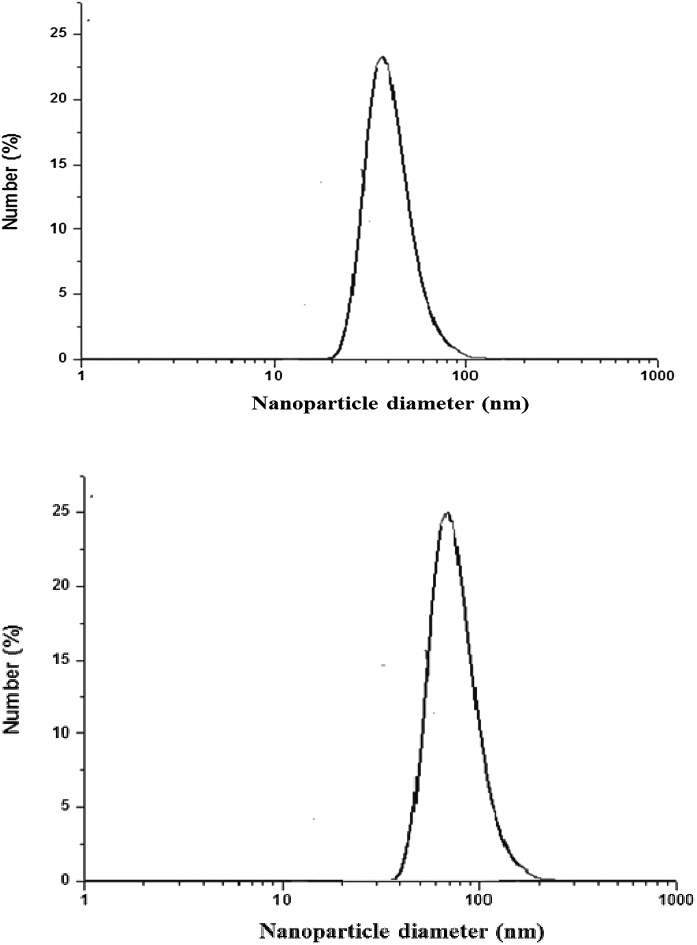
Al2O3NPs nanopowder (about 50 nm particle size) and ZnONPs nanopowder (about 100 nm particle size), were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). The dose of aluminum oxide nanoparticles was 70 mg/kg BW (aqueous suspension) and was chosen according to Park et al. [28]. The dose of ZnONPs was 100 mg/kg BW (aqueous suspension) and was chosen according to Saman et al. [29]. The hydrodynamic size distribution of each nanoparticles in the aqueous diluted solutions (5 mg/ml) were determined by Dynamic Light Scattering (DLS) using a Zetasizer Nano ZS from Malvern (Fig. 1).
Fig. 1.
DLS size distribution of hydrodynamic size of Aluminum oxide nanoparticles (A) and Zinc oxide nanoparticles (B).
2.2. Animals and experimental groups
Forty Wistar male albino rats 4–5 months age and weighing 160–170 g were used in the present study. Animals were obtained from Faculty of Medicine, Alexandria University, Alexandria, Egypt. The local committee approved the design of the experiments, and the protocol conforms to the guidelines of the National Institutes of Health (NIH). Animals were housed in a stainless steel wire cages and kept on a standard diet (9% fat, 20% protein, 53% starch, 5% fiber) and given food and water ad libitum. Animals were maintained in a controlled atmosphere, a temperature of 25 ± 5 °C and 50–70% humidity. After two weeks of acclimation, animals were divided into four equal groups as follows: a control group and 3 treated groups; group 2, 3 and 4 which were orally treated with Al2O3NPs (70 mg/kg BW), ZnONPs (100 mg/kg BW) and Al2O3NPs plus ZnONPs, respectively. Rats were orally administered their respective doses daily for 75 consecutive days.
2.3. Blood samples collection and tissue preparations
At the end of the 75th day of the experimental period, all animals of each group were anesthetized with diethyl ether and sacrificed. Blood samples were collected by cardiac puncture from anesthetized rats in test tubes containing heparin as an anticoagulant and placed immediately on ice. The collected blood was centrifuged at 860×gfor 20 min for the separation of plasma. The plasma was kept at −80 °C until the analysis of the tested parameters. Livers and kidneys were immediately removed, washed using chilled saline solution and the adhering fat and connective tissues were cautiously removed. Livers and kidneys were divided each into 4 different parts; the first part was used for DNA isolation for the assessment of DNA fragmentation, the second part was used for RNA isolation for gene expression analysis, the third part was immersed immediately in formalin for histological analysis, and the last part was minced and homogenized (10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a Potter–Elvehjem type homogenizer, then the homogenates were centrifuged at 10,000 × g for 20 min at 4 °C, to pellet the cell debris and the supernatant was collected and stored at −80 °C for the determination of the rest of parameters.
2.4. Body and organs weights
Initial and final body weights of male rats were recorded and subsequently, body weight gain (g/75 days) was calculated. Body weight gain (g/75 days) = Final weight – Initial weight. The organs (liver and kidney) were immediately removed, washed using chilled saline solution, the adhering fat and connective tissues were removed, then dried on tissue papers and weighed.
2.5. Quantitative analysis of hepatic gene expression of mitochondrial transcription factor A (mtTFA) and peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α) using qRT-PCR
Total RNA was isolated from liver tissues using GF-1 Total RNA Extraction Kit (Vivantis, Malaysia).ViPrimePLUS One-Step Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Green Master Mix (Vivantis, Malaysia) was used for the relative quantitative determination of the gene expression of mtTFA [30] and PGC-1α [31] at mRNA level using GAPDH as an internal reference gene for validation of the extraction procedure and calculation of relative expression.
2.6. Assay of DNA fragmentation
Total DNA in the tissues was isolated using DNeasy kit (Qiagen, Germany) and the concentration and purity DNA were assessed using Nanodrop2000@ (Thermo Fisher Scientific, USA). Then DNA fragmentation, as a marker of cell death, was assayed using agarose gel electrophoresis according to the method of Miller et al. [32].
2.7. ELISA measurements
Tumor suppressor gene p53, tumor necrosis factor- alpha (TNF-α) and interleukin-6 (IL-6) were assayed using Enzyme-linked Immunosorbent Assay (ELISA) kits (Abcam, Cambridge, UK) in liver and kidney tissue homogenates according to the manufacturer instructions using the serial standard of each parameter.
2.8. Markers of oxidative stress
The process of lipid peroxidation resulted in the end product of malondialdehyde (MDA). The MDA was determined as thiobarbituric acid-reactive substances (TBARS) assay in which MDA was heated with thiobarbituric acid (TBA) at a low pH to produce a pink chromogen with a maximum absorbance at 532 nm. The level of TBARS was calculated from a standard curve constructed using serial concentration of tetramethoxypropane (TMP) [66].
The Griess reaction was used to determine the concentration of nitrite and nitrate as nitric oxide end products (NOx) in the deproteinized samples. The Griess reaction was supplemented with the reduction of nitrate to nitrite by NADPH-dependent nitrate reductase. The assay procedure consisted of two steps: the first required the diazotization of sulphanilic acid with nitrite ions followed by the second step of coupling this product with a diamine, resulting in a measurable pink metabolite 540 nm. The level of NOx was determined from the slope of the standard curve constructed using serial concentration of sodium nitrite.
The total antioxidant capacity (TAC) and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S-transferase (GST) and catalase (CAT) in the tissue homogenates were measured using colorimetric kits (Biodiagnostic, Egypt) according to the manufacturer instructions and using specific standard for each parameter. Reduced glutathione content was assayed after protein precipitation using a metaphosphoric acid reagent. The assay was based on the oxidation of GSH by 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to yield GSSG and 5-thio-2-nitrobenzoic acid (TNB). The rate of TNB formation was assayed at 412 nm and was proportional to the GSH present in the sample. The rate of formation of TNB was monitored by recording the change in the absorbance. This was found to be 412 nm per minute (ΔA/min). The total glutathione content in the samples was determined from a GSH standard curve and the results were subsequently expressed as nmol/g tissue by dividing the concentration of glutathione in the sample by the weight (in grams) of tissue used to prepare the sample [67].
2.9. Biochemical parameters
Plasma total protein, albumin, urea, creatinine, uric acid and total bilirubin were measured using kits from Biosystems S.A (Biosystems S.A. Costa Brava 30, Barcelona, Spain). The activities of plasma and liver aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), acid phosphatase (AcP), gamma-glutamyl transferase (GGT) and lactate dehydrogenase (LDH)were measured also using kits from Biosystems S.A (Biosystems S.A. Costa Brava 30, Barcelona, Spain) the validity of these kits was assayed using Quality Control Reagent.
2.10. Hematological parameters
Blood samples were collected in tubes containing heparin (anticoagulant).HA-VET CLINDIAG (Alfa swelab, Sweden) was used to measure the following parameters: white blood cells (WBCs), red blood cells (RBCs), hemoglobin (HGB), hematocrit value (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelets (PLT) counts according to the manufacturing instructions and using Quality Control Reagent to assess the validity of the assays.
2.11. Histological section preparation of liver and kidney
Livers and kidneys were obtained from rats, and immediately fixed in 10% formalin, and then treated with a conventional grade of alcohol and xylol, embedded in paraffin and sectioned at 4-6 μm thickness. The sections were stained with Haematoxylin and Eosin (H&E) stains and photographed on the PC screen using a light microscope (Olympus BH-2; Japan) with a digital
color camera attachment (Sanyo VVC-6975 P; Japan) and dial indicator for studying the histopathological changes [33]. The severity of routine histological and histopathological findings was scored semi-quantitatively as 0 (minimal), +, (mild), ++ (moderate), +++ (marked), or ++++ (marked) as compared with the control group.
2.12. Statistical analysis
Results are reported as means ± SE. Statistical analysis for all studied parameters was performed using the general linear model (GLM) produced by Statistical Analysis Systems Institute [34]. Duncan's New Multiple Range Test was used to test the significance of the differences between means [35]. Values of p<0.05 were considered statistically significant.
3. Results
3.1. Initial and final body weights, body weight gain, and organs weights
The initial body weights of all groups were comparable to each other; however, the final body weights of rat treated with the nanoparticles were significantly lower than control rats with no significant difference between the rats treated with each NP alone or in combination (Table 1). The rats treated with ZnONPs alone or combination of Al2O3NPs have lower body weight gain (BWG) compared to control rats and rats treated with Al2O3NPs alone. The liver and kidney weight showed a similar pattern of change between groups (Table 1).
Table 1.
The body and organs weights of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination.
| Parameter | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| IBW (gm) | 169 ± 3.71a | 167 ± 2.38a | 170 ± 2.24a | 168 ± 2.91a |
| FBW (gm) | 222 ± 6.16a | 207 ± 3.76 b | 200 ± 5.50b | 195 ± 4.53b |
| BWG (gm/75 days) | 53.50 ± 6.54a | 40 ± 5.37ab | 30.50 ± 5.40b | 27 ± 5.12b |
| Liver weight (gm) | 5.83 ± 0.28a | 5.09 ± 0.22b | 5.13 ± 0.25b | 4.67 ± 0.16b |
| Kidney weight (gm) | 1.61 ± 0.06a | 1.43 ± 0.07b | 1.45 ± 0.05ab | 1.38 ± 0.04b |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05. (IBW = Initial body weight, FBW = Final body weight, BWG= Body weight gain).
3.2. Hepatic expression of PGC-1α and mtTFA
The results of the present study showed significant suppression of the hepatic gene expression mtTFA in rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles, and their combination by about 29%, 62% and 68% of control value, respectively (Table 2). Also, the hepatic gene expression of PGC-1α showed significant suppression in the rats treated with Al2O3NPs, ZnONPs and their combination by about 23%, 51% and 63% of control value, respectively. It was clear that the suppression of mtTFA and PGC-1αexpression was more pronounced in the rats treated with the combination of Al2O3NPs and ZnONPs compared to the rats treated with Al2O3NPs or ZnONPs solely.
Table 2.
The effect of aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination on the hepatic gene expression of mitochondrial transcription factor-A (mtTFA) and peroxisome proliferator activator receptor gamma-coactivator 1α (PGC-1α).
| Parameter | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
|
mtTFA (Fold control) |
1.0 ± 0.06a | 0.71 ± 0.03b (-29 %) |
0.38 ± 0.02c (-62 %) |
0.32 ± 0.02d (-68 %) |
|
PGC-1α (Fold control) |
1.0 ± 0.06a | 0.77 ± 0.05b (-23 %) |
0.49 ± 0.04c (-51 %) |
0.37 ± 0.03d (-63 %) |
Data presented as Mean±SE. M For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.. (Numbers between parentheses represent the percentage change from control value).
3.3. DNA fragmentation
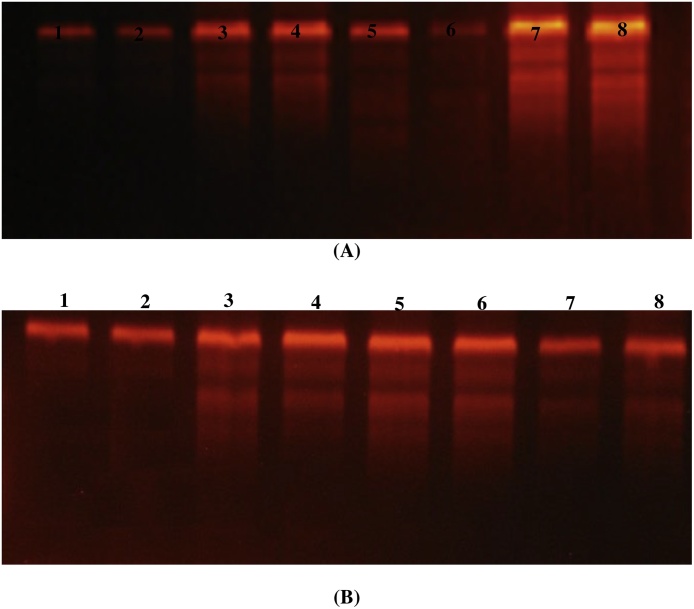
The genomic DNA fragmentation in liver and kidney tissues of rats treated with Al2O3NPs and ZnONPs and their combination were presented in (Fig. 2A and B). The agarose gel electrophoresis of liver and kidney genomic DNA showed very low or undetectable DNA laddering (DNA fragmentation) in the control rats. The DNA intact band appears to be condensed near the application point with no DNA smearing suggesting no DNA fragmentation. On the other hand, Al2O3NPs and ZnONPs treatment alone or in combination resulted in DNA fragmentations in the liver which appear as DNA smearing (Fig. 2A). Kidney tissues showed a similar pattern of DNA fragmentation as that observed in the liver (Fig. 2B). DNA fragmentation was more pronounced in the combination group than the groups treated with Al2O3NPs or ZnONPs solely.
Fig. 2.
Stained agarose gel of genomic DNA of liver (A) and kidney(B) demonstrated apoptotic and necrotic cell deaths induced by nanoparticles. Lanes 1 and 2: Control, lanes 3 and 4: Aluminum oxide nanoparticles treated rats, lanes 5 and 6: Zinc oxide nanoparticles treated rats, lanes 7and 8: Combination of aluminum oxide and zinc oxide nanoparticles treated rats.
3.4. Tissues levels of p53, TNF-α, and IL-6
The rats treated with NPs alone or in combination showed significantly higher liver and kidney levels of p53, TNF-α and IL-6 compared to the control group. While the rats treated with Al2O3NPs have significantly higher liver and kidney levels of p53 and TNF- α, they have significantly lower levels of IL-6, compared to rats treated with ZnONPs. The rats treated with both NPs significantly have higher tissues levels of p53, TNF-α and IL-6 compared to rats treated with single NP (Table 3).
Table 3.
Hepatic and kidney levels of tumor suppressor p53 (p53), tumor necrosis factor-α (TNF-α)and interliukin-6 (IL-6) of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination.
| Parameter | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| Liver | ||||
| p53(ng /mg protein) | 8.34 ± 0.42 c | 12.15 ± 0.58 b | 12.44 ± 0.49 b | 18.31 ± 0.51 a |
| TNF-α(ng/gm tissue) | 173 ± 5.66d | 325 ± 4.22c | 424 ± 4.37b | 462 ± 7.47 a |
| IL-6(ng/gm tissue) | 153 ± 3.7d | 226 ± 2.3 b | 187 ± 2.5 c | 258 ± 5.2 a |
| Kidney | ||||
| p53 (ng /mg protein) | 9.76 ± 0.36 c | 12.5 ± 0.32 b | 13.5 ± 0.39 b | 17.7 ± 0.22 a |
| TNF-α (ng/gm tissue) | 165 ± 6.0 d | 284 ± 7.4 c | 387 ± 8.3 b | 431 ± 7.5 a |
| IL-6 (ng/gm tissue) | 174 ± 4.4d | 271 ± 3.8b | 223 ± 4.3c | 393 ± 3.2a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.
3.5. Lipid peroxidation and antioxidants
The liver and kidney tissues of rats treated to NPs have significantly lower levels of all antioxidant parameters assayed including; GSH, SOD, CAT, GPx, GST, and TAC, while having significantly higher levels of TBARS and NO, compared to control group. For most parameters, there is no significant difference between the rats treated with Al2O3NPs or ZnONPs, while the rats treated with the combination have significant lower antioxidant parameters and higher TBARS and NO levels in the liver and kidney tissues compared to the rats treated with single NPs (Table 4)
Table 4.
Liver and kidney glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), total antioxidant activity (TAC), nitric oxide (NO) and thiobarbituric acid-reactive substances (TBARS) of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination.
| Parameter | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| Liver | ||||
| GSH(μmole/gm tissue) | 5.4 ± 0.27a | 3.9 ± 0.07b | 3.8 ± 0.09b | 3.1 ± 0.07c |
| SOD (U/mg protein) | 53.1 ± 2.5a | 40.6 ± 2.4b | 42.6 ± 3.4b | 35.5 ± 1.4c |
| CAT (U/mg protein) | 66 ± 0.68a | 50 ± 0.77c | 53 ± 0.62b | 40 ± 0.42d |
| GPx (U/mg protein) | 25.9 ± 0.45a | 19.8 ± 0.39 b | 18.9 ± 0.44b | 15.8 ± 0.35 c |
| GST(μmole /hr/mg protein) | 1.77 ± 0.05a | 1.38 ± 0.05 b | 1.39 ± 0.05b | 1.06 ± 0.05c |
| TAC (μmole/gm tissue) | 2.5 ± 0.09a | 1.97 ± 0.05b | 1.9 ± 0.11b | 1.58 ± 0.05c |
| NO (μmole/gm tissue) | 0.47 ± 0.03c | 0.60 ± 0.03b | 0.63 ± 0.05b | 0.77 ± 0.04a |
| TBARS (nmole/gm tissue) | 45 ± 2.0c | 71 ± 2.2b | 75 ± 4.4b | 87 ± 3.2 a |
| Kidney | ||||
| GSH (μ mole/gm tissue) | 5.7 ± 0.14a | 4.5 ± 0.09b | 4.5 ± 0.12b | 3.9 ± 0.09c |
| SOD (U/mg protein) | 60.8 ± 1.1 a | 36.5 ± 1.1 c | 39.0 ± 1.0 b | 33.1 ± 0.8d |
| CAT (U/mg protein) | 53.9 ± 2.36a | 34.7 ± 0.79 b | 34.3 ± 1.02 b | 25.7 ± 1.10c |
| GPx (U/mg protein) | 84.1 ± 0.93a | 65.6 ± 1.13 b | 63.6 ± 1.16 b | 51.6 ± 1.86c |
| GST(μmole /hr/mg protein) | 1.16 ± 0.03a | 0.92 ± 0.02 b | 0.93 ± 0.02 b | 0.77 ± 0.02c |
| TAC (μmole/gm tissue) | 2.5 ± 0.06a | 1.8 ± 0.06b | 1.9 ± 0.08b | 1.5 ± 0.06c |
| NO (μmole/gm tissue) | 0.5 ± 0.02c | 0.6 ± 0.04b | 0.7 ± 0.03b | 0.8 ± 0.03a |
| TBARS (nmole/gm tissue) | 22.4 ± 1.53c | 68.2 ± 1.29 b | 70.4 ± 1.19 b | 80.8 ± 1.27a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.
3.6. Biochemical parameters
The rat's groups treated with Al2O3NPs and ZnONPs showed higher plasma activities of AST, ALT, GGT, AcP, AlP, and LDH compared to the control rats. There are no significant differences in these parameters between the rats treated with one NP alone, while the rats treated with the combination showed significantly higher plasma activities of these enzymes compared to rat treated with each NPs alone. The hepatic activities of these enzymes showed an opposite pattern of changes to that observed in the plasma (Table 5).
Table 5.
Plasma and liver alanine transaminase (ALT), aspartate transaminase (AST),acid phosphatase (AcP), alkaline phosphatase (AlP), gama-glutamyl transferase (GGT) and lactate dehydrogenase(LDH) of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination.
| Parameter | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| Plasma | ||||
| ALT (U/L) | 64 ± 1.8 c | 104 ± 1.8 b | 108 ± 2.1 b | 169 ± 2.4 a |
| AST (U/L) | 58 ± 1.1 c | 97 ± 1.5 b | 97 ± 1.8 b | 125 ± 1.8 a |
| AcP (U/L) | 14.3 ± 0.3 d | 18.5 ± 0.3 c | 19.3 ± 0.3 b | 23.2 ± 0.6 a |
| AlP (U/L) | 134 ± 3.41c | 183 ± 1.98 b | 180 ± 2.84 b | 210 ± 2.86 a |
| GGT (U/L) | 17.6 ± 1.24 d | 45.8 ± 1.38 c | 54.9 ± 1.44 b | 75.2 ± 1.24 a |
| LDH (U/L) | 1098 ± 24.7 c | 1307 ± 22.2 b | 1306 ± 20.9 b | 1488 ± 29.8 a |
| Liver | ||||
| ALT (U/mg) | 391 ± 5.4 a | 292 ± 5.8 b | 287 ± 5.5 b | 223 ± 4.6 c |
| AST (U/mg) | 216 ± 3.04 a | 164 ± 3.04 b | 160 ± 3.25 b | 118 ± 2.31 c |
| AcP (U/mg) | 19.8 ± 0.36 a | 11.8 ± 0.61 b | 11.1 ± 0.94 c | 9.6 ± 0.45 d |
| AlP (U/mg) | 202 ± 2.51 a | 139 ± 2.36 b | 133 ± 2.55 b | 82 ± 2.35 c |
| GGT (U/mg) | 28.3 ± 0.84 a | 15.6 ± 0.59b | 14.0 ± 0.36 c | 9.0 ± 0.43 d |
| LDH (U/mg) | 964 ± 9.3 a | 742 ± 9.8 b | 726 ± 12.1 b | 571 ± 10.6 c |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.
The NPs treated rats showed higher plasma levels of bilirubin, urea, blood urea nitrogen, creatinine, and uric acid compared to control rats with the highest levels observed in the rats treated with the combination. On the other hand, the NPs treated rats have lower plasma level of total protein, albumin and globulin compared to control rats with the lowest levels observed in the rats treated with the combination (Table 6).
Table 6.
Plasma total protein, albumin, globulin, total bilirubin, urea, blood urea nitrogen, uric acid and creatinine of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination.
| Parameters | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| Total protein (gm/dl) | 7.9 ± 0.07 a | 6.1 ± 0.17 b | 6.2 ± 0.17 b | 5.0 ± 0.11 c |
| Albumin (gm/dl) | 4.5 ± 0.14 a | 3.3 ± 0.17 b | 3.2 ± 0.08 b | 2.5 ± 0.06 c |
| Globulin (gm/dl) | 3.5 ± 0.04a | 3.0 ± 0.07b | 2.0 ± 0.08b | 2.7 ± 0.07c |
| Total bilirubin (mg/dl) | 0.85 ± 0.05 c | 2.20 ± 0.07 b | 2.29 ± 0.07 b | 3.22 ± 0.05 a |
| Urea (mg/dl) | 29 ± 1.62 c | 69 ± 1.87 b | 61 ± 1.97 b | 77 ± 3.03 a |
| Blood urea nitrogen (mg/dl) | 12 ± 0.35 c | 30 ± 0.72 b | 29 ± 1.07 b | 36 ± 0.86 a |
| Uric acid (mg/dl) | 4 ± 0.11 d | 9 ± 0.21 c | 10 ± 0.40 b | 14 ± 0.28a |
| Creatinine(mg/dl) | 0.98 ± 0.04 d | 2.33 ± 0.05 c | 2.65 ± 0.08b | 3.28 ± 0.07 a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.
3.7. Hematological parameters
Compared to control rats, the three groups of rats treated with NPs showed lower values of all red blood cell indices including; red blood cells count (RBC), hemoglobin (HB), hematocrit value (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) and also platelets count (PLT). All of these parameters not significantly differ between rats treated with Al2O3NPs and ZnONPs alone while the group treated with the combination showed significantly lower values compared to the rats treated with any of the NPs alone. One the other hand, the white blood cells count (WBC) showed the opposite pattern of changes to that observed with the red cell indices and platelets (Table 7).
Table 7.
Hematological parameters of red blood cells, hemoglobin, hematocrit value, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets and white blood cells of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination.
| Parameters | Experimental groups |
|||
|---|---|---|---|---|
| Control | Al2O3NPs | ZnONPs | Combination | |
| RBC (106/ml) | 4.72 ± 0.05a | 3.74 ± 0.05c | 3.96 ± 0.04b | 2.92 ± 0.08d |
| HB (g/dl) | 12.96 ± 0.36a | 8.96 ± 0.38b | 8.80 ± 0.37cb | 7.82 ± 0.45c |
| HCT (%) | 44.6 ± 0.81a | 28.4 ± 0.93b | 29.8 ± 0.86b | 23.4 ± 0.51c |
| MCV(fl) | 111 ± 2.46a | 75.3 ± 1.60b | 76.4 ± 2.20b | 63.4 ± 2.73c |
| MCH (pg) | 30.6 ± 0.68a | 25.8 ± 0.59b | 23.1 ± 0.70c | 20.4 ± 0.51d |
| MCHC (pg) | 36.2 ± 0.66a | 29.5 ± 0.61b | 29.1 ± 0.60b | 23.0 ± 0.71c |
| PLT (103/ ml) | 303 ± 4.44a | 188 ± 3.97b | 187 ± 3.79b | 126 ± 2.80c |
| WBC (103/ ml) | 8219 ± 125.5c | 9654 ± 179.5b | 9555 ± 256.1b | 11903 ± 144.3a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p < 0.05.
3.8. Histopathology
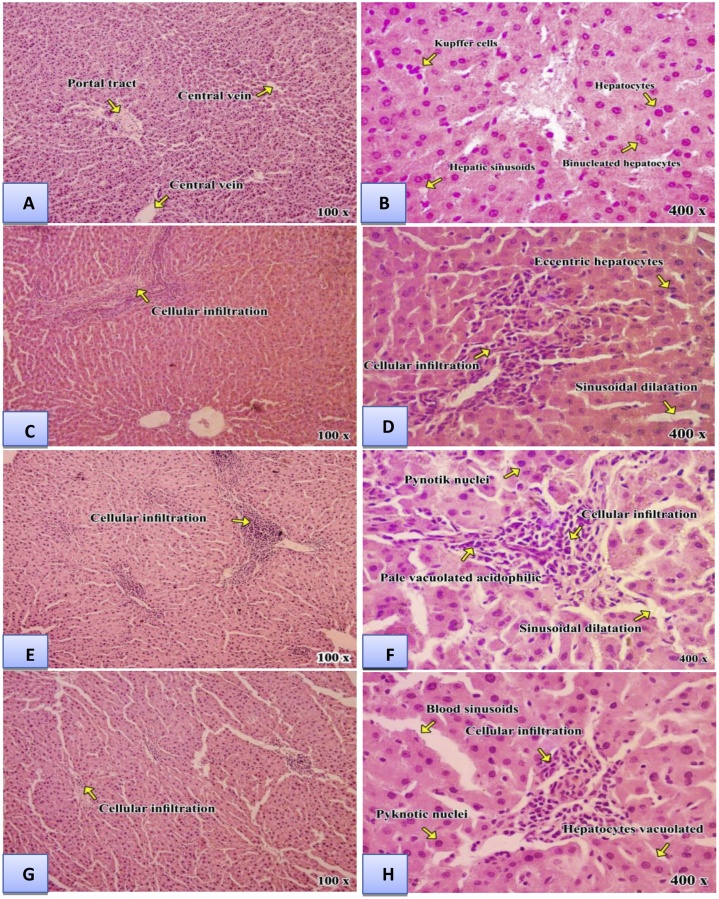
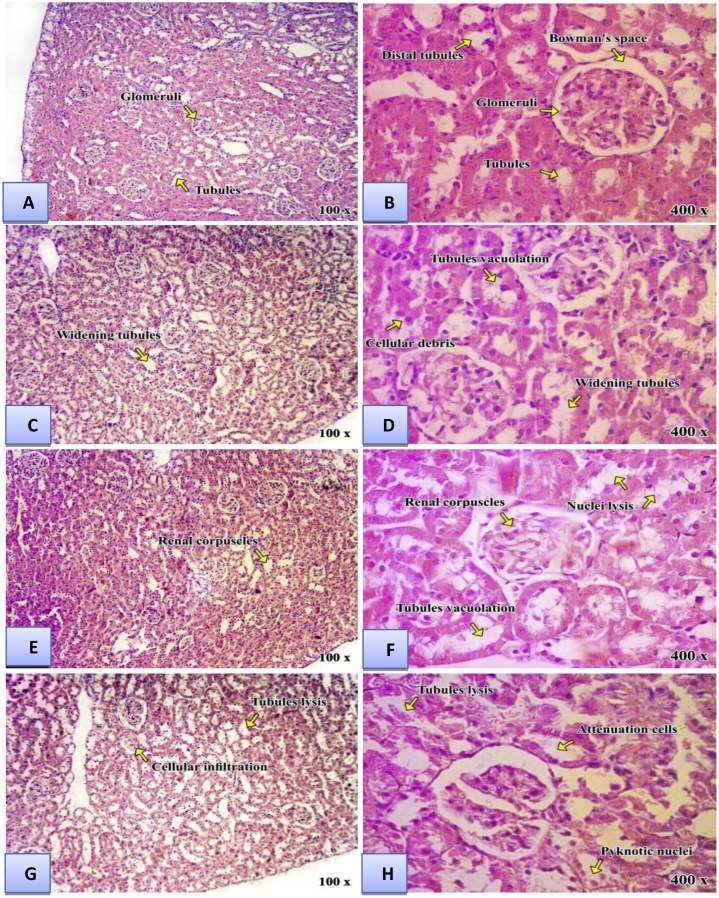
The histopathological changes of liver were shown in (Fig. 3) and Table 8, and kidney was shown in (Fig. 4) and Table 9. In liver, cellular necrosis, congestion, and hepatocytes appear with vacuolated acidophilic were observed in rats treated with Al2O3NPs or ZnONPs. These changes were more prominent in the rats treated with the combination of Al2O3NPs and ZnONPs. Renal damages appeared as hypertrophy, glomeruli segmentation, hydropic degeneration in epithelial cells, necrosis of epithelial cells in tubules, and swelling in epithelial cells of proximal tubules were found in kidney tissues treated with Al2O3NPs or ZnONPs. These changes were more pronounced in the kidney of rats treated with the combination of Al2O3NPs and ZnONPs.
Fig. 3.
Light micrographs of liver sections: Control group (A&B) showingnormal hepatic architecture and hepatic sinusoids are seen lined by endothelial cells and a Kupffer cell is seen. Aluminum oxide nanoparticles group(C &D) showing degenerative hydropicchanges and cellular infiltration in numerous hepatocytes, piecemeal necrosis, lyticnecrosis, congestion of sinusoidal blood vessels. Zinc oxide nanoparticles group (E&F) showing distended and hemorrhage in the portal veinlymphocytes aggregation, degenerated hepatocytes with pyknotic nuclei, hepatocyte vacuolization and cellular infiltration. Combination group (G&H) showing disturbed hepatic architecture in most of the lobule. Many of hepatocytes are vacuolated. While other are apoptotic, shrunken with pyknotic nuclei. Inflammatory cellular infiltration is evident in periportal areas and around the central veins. Widely dilated hepatic blood sinusoids are observed.(H & E stain. Mic.Mag. × 100 and ×400).
Table 8.
Quantitative analysis of liver histology of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their mixture.
| No | Quantitative analysis | Control | Al2O3NPs | ZnONPs | Combination |
|---|---|---|---|---|---|
| 1 | Normal hepatic architecture | – | + | ++ | ++ + |
| 2 | Congestion of sinusoidal blood vessels | – | + | ++ | + + + |
| 3 | Degenerated hepatocytes with pyknotic nuclei | – | + | ++ | + + + |
| 4 | Disturbed hepatic architecture in most of the lobule | – | + | ++ | + + + |
(-) No change, (+) mild change, (++) moderate change, (+++) moderate to marked change, (++++) marked change.
Fig. 4.
Light micrographs of kidneysections: Control group (A&B) showing normal renal corpuscles with normal glomeruli and normal Bowman's space. The distal tubules appeared having wider lumina lined by cuboidal cells with less acidophilic cytoplasm and rounded nuclei. Aluminum oxide nanoparticles group(C&D) revealed renal damages appeared as hypertrophy and degeneration of epithelia renal tubules with distinct of mononuclear cells infiltration. Zinc oxide nanoparticles group(E &F)degeneration of the tubules in the form of cytoplasmic vacuolation and distorted the renal corpuscles.Also some vascular glomeruli were apparently enlarged, tightly filling the Bowman’s capsule with absence of the capsular spaces was observed.Combination group(G&H) revealed several histopathological changes such as shrinkage of capillaries in the glomerulus with the capsular space and slightly degeneration in the epithelial cells of both proximal and distal tubules with pyknotic nuclei.(H & E stain. Mic.Mag. × 100 and ×400).
Table 9.
Quantitative analysis of kidney histology of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their mixture.
| No | Quantitative analysis | Control | Al2O3NPs | ZnONPs | Combination |
|---|---|---|---|---|---|
| 1 | normal renal corpuscles with normal glomeruli | – | ++ | ++ | ++ + |
| 2 | degeneration of epithelia renal tubules with distinct of mononuclearcells infiltration | – | ++ | ++ | + + + |
| 3 | vacuolation and distorted the renal corpuscles | – | ++ | ++ | + + + |
| 4 | Shrinkage of capillaries in the glomerulus with the capsular space and cellular infiltration in both proximal and distal tubules with pyknotic nuclei. | – | ++ | ++ | + + + |
(-) No change, (+) mild change, (++) moderate change, (+++) moderate to marked change, (++++) marked change.
4. Discussion
The present study confirmed the hepatorenal toxicities of Al2O3NPs and ZnONPs [9,[36], [37], [38], [39], [40], [41], [42]] and indicated that these toxicities may be mediated through induction of lipid peroxidation, DNA degradation, induction of systemic inflammation, anemia, and disturbances in the hepatic expression of the genes controlling mitochondrial biogenesis. Also, the study clearly indicated the synergistic (potentiation) toxic effects between Al2O3NPs and ZnONPs.
Nanoparticles, when ingested into the body, can travel to different regions and different organs of the body due to their small size. They can cross the small intestine and then find their way into the blood, brain, lung, heart, kidney, spleen, liver, intestine and stomach [43]. Biodistribution experiments have revealed that the liver, kidneys, and spleen are the main target organs for engineered nanoparticles after uptake by the gastrointestinal tract [6,36].
Both nanoparticles significantly affect the liver and kidney function tests which are in parallel with many previous studies. Sharma et al. [37]Xia et al. [44]Mansouri et al. [41] and Almansour et al. [45] reported that ZnONPs caused dose-dependent liver injury in rodents. Furthermore, Yan et al. [38] demonstrated that ZnONPs exhibited toxicological symptom like diarrhea, increases in serum blood urea nitrogen and creatinine, implying the potential renal damage.
The liver and kidney damages caused by ZnONPs and Al2O3NPs are further confirmed by histopathological examination, which showed focal hepatocellular necrosis, congestive dilation of central veins, edema and degeneration in the hepatocytes, degeneration of epithelial renal tubules, vacuolation and distorted the renal corpuscles, shrinkage of capillaries in the glomerulus with the capsular space and cellular infiltration in both proximal and distal tubules with pyknotic nuclei. All of these abnormalities appear to be more pronounced in the rats treated with the combination of both nanoparticles which may indicate synergy between them. These data are in line with the previous studies of Xia et al. [44,46], Mansouri et al. [41] and Almansour et al. [45].
The mechanism(s) of liver and kidney cytotoxicity induced by nanoparticles is of scientific interest. There are many mechanisms by which nanoparticles can induce cytotoxic effects including enhanced production of reactive oxygen species (ROS) and accompanied oxidative stress and lipid peroxidation, genotoxicity, and induction of inflammatory pathways. The present study indicated enhanced oxidative stress in the liver and kidney tissues of rats treated with ZnONPs and Al2O3NPs as indicated by higher levels of lipid peroxidation end products (TBARS) and nitric oxide end product levels. The observed status of oxidative stress was associated with low antioxidant status as indicated by lower tissues levels of glutathione and total antioxidant capacity and inhibited activities of the antioxidant enzymes including; superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase. All of these abnormalities are significantly higher in the rats co-treated with both NPs which confirm the synergism between them. In line with these, it was reported that ZnONPs interact with proteins and enzymes within mammalian cells and they can interfere with the antioxidant defense mechanism leading to ROS generation, the initiation of an inflammatory response and perturbation and destruction of the mitochondria causing apoptosis or necrosis [9,47].
Li et al. [48] reported that nanoparticles exert their toxic effects through the generation of various deleterious ROS like hydrogen peroxide, hydroxyl radical species, nitric oxide or superoxide anion. Previous studies had proved that ZnONPs could induce toxicity response by increasing the generation of ROS, inflammation, oxidative stress, and cell organelle disruption [[49], [50], [51]]. ZnONPs also caused apoptosis in human hepatocytes (HepG2), human lung epithelial cells and some human cancer cells through ROS production [16]. Nano-alumina has the capability to induce the production of free radicals, thereby resulting in oxidative stress in cells (Zhang et al., 2010). Klotz and Sies [52] reported that the uptake of metal oxide nanoparticles can lose the metallic ions which directly interact with NADPH oxidases from the plasma membrane or mitochondria thus disturbing the electron transport chain and generating a superoxide anion. ZnONPs may directly interact with external membrane surface, resulting in membrane damage via generation of ROS [53]. Al2O3NPs may exert its toxicity by the direct interaction with cell organelles, the formation of chemical compounds with DNA, RNA, proteins and so on, and by its accumulation in cells, tissues, and organs, leading to oxidative damage of organs [42].
The inflammatory status was confirmed in the present study by higher circulatory levels of TNF-α and IL-6 in the rats treated with ZnONPs, Al2O3NPs or both. In line with these data, Monteiller et al. [54] reported that exposure to nanoparticles has been found to result in oxidative stress-induced activation of pro-inflammatory factors such as IL-1, IL-6, IL-8, and macrophage inflammatory proteins (MIP) at both mRNA and protein levels in vitro. Also, Faddahet al. (2012) found that ZnONPs induced nephrotoxicity was associated with an elevation in serum inflammatory markers including TNF-α, IL-6 and C-reactive protein (CRP). Furthermore, Hou et al. [55] described that when nanoparticles enter the systemic circulation, they encounter a complex web of immune cells and plasma proteins. The recognition of nanoparticles as foreign particles by the immune cells may lead to the generation of ROS, reactive nitrogen species (RNS) and altered cytokine levels.
The present study showed that Al2O3NPs and ZnONPs alone or in combination caused an elevation in the tumor suppressor p53 which may participate in the induction of the apoptosis in liver and kidney tissues. Vurusaner et al. [56] described that oxidative stress cause activation of p53 which in turn performs antioxidant functions.
Patil et al. [57] illustrated that the production of ROS as a result to nanotoxicity is merely responsible for cell death. ZnONPs caused oxidative DNA damage and ROS-triggered mitochondrial-mediated apoptosis in hepatocytes and kidney tissues [38]. Also, accumulation of ZnONPs in the liver can generate excessive oxidative stress, causing DNA damage and cell apoptosis [37,58]. Al2O3NPs caused mitochondria-mediated oxidative stress and cytotoxicity in human mesenchymal stem cells (Alshatwiet al., 2013). The pulmonary artery endothelial cells and human umbilical vein endothelial cells exposed to Al2O3NPs showed increased mRNA and protein expression of adhesion molecules, possibly due to the generation of ROS and the activation of redox-sensitive signaling pathways, that could be implicated in cardiovascular health risks [22]. In line with the previous studies, the present data indicated the NPs-induced oxidative stress status in liver and kidney tissues associated with severe DNA fragmentation. Damage to DNA is a fundamental example of cellular toxicity, and it is critical to assess such damage for various nanoparticles that humans encounter, given that damage to DNA is highly correlated with an increased risk of cancer.
The main feature of apoptosis is the degradation of genomic DNA at the internucleosomal level. Endonucleases catalyze the internucleosomal fragmentation of DNA to produce a ladder pattern. In necrosis, however, DNA degradation is a later phenomenon after cell rupture, the chromatin is digested by proteases and endonucleases into a smear pattern instead of a ladder pattern since the proteases destroy the histones and expose the entire length of DNA to the nucleases, consequently necrosis is characterized by random DNA fragmentation which forms a “smear” on agarose gels. In the present results, the DNA fragmentation showed a mixed pattern of smearing and “laddering” of DNA fragments due to the nonspecific DNA fragmentation process caused by necrosis and apoptosis. Measurements of the indicators of apoptosis and/or necrosis directly reveal the ability of nanoparticles to induce cell destruction or cell death [59]. Jennifer and Maciej [60] reported that Al2O3NPs and ZnONPs induced genotoxicity and cytotoxicity in liver cells.
Understanding the effects of nanoparticles on the cellular genome is critical toward interpreting the extent of toxicity of any nanoparticle. Assessing the expression of genes implicated in various cellular processes may provide a molecular explanation of the toxicity of NPs on different rat organs. Given the importance of mitochondria in any cell metabolism, ATP level, ROS production, and apoptosis, make the disruption of mitochondrial homeostasis is a key trigger to a wide variety of diseases and toxicological effects [25,61]
The present work demonstrated significant suppression of hepatic expression of PGC-1α and mTFA by treatment with Al2O3NPs, ZnONPs or both. The combined treatment caused a marked decline in the expression of these genes which may indicate a decreased mitochondrial biogenesis and impaired mtDNA replication and transcription that may lead to mitochondrial dysfunction. In line with this assumption, it was documented that NPs exposure caused impairment of rat liver mitochondrial function, mainly due to alterations of mitochondrial membrane permeability because the disruption of mitochondrial membrane potential is a critical step in the apoptotic pathway. Aluminum oxide NP-induced cell death was associated with a disruption of the mitochondrial membrane potential, depletion of mitochondrial thiols, caspases activation and ROS generation [62]. Similar results showed that the liberation of Zn+ from ZnONPs can increase the inner mitochondrial membrane permeability and impair the respiratory chain, thus leading to energy dissipation, oxidative stress, and apoptosis. [63]. The nanoparticles compromised the electron transfer along the electron transport chain by affecting complex II and IV of the respiratory chain. This impairment of mitochondrial electron transport chain activity caused an accumulation of electrons in the electron transport chain complexes that can escape and directly react with oxygen to form the superoxide anion radical [64]. These data provide insight into the important crosstalk between the gene expression of nuclear transcription factors involved in mitochondrial biogenesis and function (namely; mtTFA and PGC-1α) and hepatotoxicity of Al2O3NPs and ZnONPs and their combination. In line with these results, Sharma et al. [37] demonstrated that ZnONPs exposure caused significant suppression of the hepatic gene expression of mTFA and PGC-1α.
The hepatorenal toxicities of Al2O3NPs and ZnONPs were associated with hematological abnormalities especially in the RBCs indices that manifested as severe microcytic hypochromic anemia that may be a direct consequence of liver and renal damages which play important role in heme synthesis and erythropoiesis, respectively. In accordance with these results, Ben-Slama et al. found that ZnONPs decreased the blood cell count and caused changes in the biochemical parameters in rats. Yan et al. [38] also demonstrated decreased hemoglobin level and red blood cell indices (HCT and MCHC) in ZnONPs treated animals, suggesting that the animals suffered from anemia which reinforces our findings.
5. Conclusion
The present study showed that Al2O3NPs and ZnONPs alone or in combination caused hepato- and nephrotoxicities through epigenetic changes in the gene expression of mtTFA, and PGC-1α that may subsequently cause mitochondrial dysfunctional which instigating the generation of ROS and oxidative stress. These effects are associated with impaired antioxidant defense systems and disturbed cytokines production and accelerated cell death through apoptosis and necrosis as indicated by the pattern of DNA fragmentation. The results of our study indicated more pronounced hepatic and renal toxicities of combined exposure to Al2O3NPs and ZnONPs which may suggest a synergistic relationship between these two nanoparticles which in line with the idea that, the synergy of the toxic stimuli in combination means lower levels of each stimulus is required to cause damage compared to exposure levels when tested in isolation [65]. The main drawback of the study is the application of only one dose of each NP that makes the final conclusion of the synergistic toxicity between the NPs used require further investigation using serial doses of both NPs.
Declaration of interest
The authors report no conflict of interest.
The authors declare that there are no conflicts of interest and agreed to submit this article to the journal “Toxicology Reports”
References
- 1.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolution from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dağlıoğlu Y., Öztürk B.Y. Effect of concentration and exposure time of ZnO-TiO2 nanocomposite on photosynthetic pigment contents, ROS production ability, and bioaccumulation of freshwater algae (Desmodesmus multivariabilis) Caryologia. 2017;71(1):13–23. [Google Scholar]
- 3.Zhang Y., Bai Y., Jia J., Gao N., Li Y., Zhang R., Jiang G., Yan B. Perturbation of physiological systems by nanoparticles. Chem. Soc. Rev. 2014;43(10):3762–3809. doi: 10.1039/c3cs60338e. [DOI] [PubMed] [Google Scholar]
- 4.Schinwald A., Murphy F.A., Jones A., MacNee W., Donaldson K. Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano. 2012;6(1):736–746. doi: 10.1021/nn204229f. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y., Zhang Y., Zhang J., Mu Q., Zhang W., Butch E.R., Snyder S.E., Yan B. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat. Nanotechnol. 2010;5(9):683–689. doi: 10.1038/nnano.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M., Lin D., Zhu L. Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ. Pollut. 2013;173:97–102. doi: 10.1016/j.envpol.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Espitia P.J.P., Soares N.D.F.F., dos Reis Coimbra J.S., de Andrade N.J., Cruz R.S., Medeiros E.A.A. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012;5(5):1447–1464. [Google Scholar]
- 8.Hackenberg S., Zimmermann F.Z., Scherzed A., Friehs G., Froelich K., Ginzkey C., Koehler C., Burghartz M., Hagen R., Kleinsasser N. Repetitive exposure to zinc oxide nanoparticles induces DNA damage in human nasal mucosa mini organ cultures. Environ. Mol. Mutagen. 2011;52(7):582–589. doi: 10.1002/em.20661. [DOI] [PubMed] [Google Scholar]
- 9.Schrand A.M., Rahman M.F., Hussain S.M., Schlager J.J., Smith D.A., Syed A.F. Metal‐based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2(5):544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 10.Miller R.J., Lenihan H.S., Muller E.B., Tseng N., Hanna S.K., Keller A.A. Impacts of metal oxide nanoparticles on marine phytoplankton. Environ. Sci. Technol. 2010;44(19):7329–7334. doi: 10.1021/es100247x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H.J., Growcock A.C., Tang T.H., O’Hara J., Huang Y.W., Aronstam R.S. Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathway. Toxicol. In Vitro. 2010;24(7):1953–1961. doi: 10.1016/j.tiv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang B., Feng W., Wang M., Wang T., Gu Y., Zhu M., Ouyang H., Shi J., Zhang F., Zhao Y., Chai Z. Acute toxicological impact of nano-and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanoparticle Res. 2008;10(2):263–276. [Google Scholar]
- 13.Moos P.J., Chung K., Woessner D., Honeggar M., Cutler N.S., Veranth J.M. ZnO particulate matter requires cell contact for toxicity in human colon cancer cells. Chem. Res. Toxicol. 2010;23(4):733–739. doi: 10.1021/tx900203v. [DOI] [PubMed] [Google Scholar]
- 14.Cadet J., Douki T., Ravanat J.L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010;49(1):9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Piperigkou Z., Karamanou K., Engin A.B., Gialeli C., Docea A.O., Vynios D.H., Pavão M.S., Golokhvast K.S., Shtilman M.I., Argiris A., Shishatskaya E., Tsatsakis A.M. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem. Toxicol. 2016;91:42–57. doi: 10.1016/j.fct.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang C.C., Aronstam R.S., Chen D.R., Huang Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. In Vitro. 2010;24(1):45–55. doi: 10.1016/j.tiv.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Wagner A.J., Bleckmann C.A., Murdock R.C., Schrand A.M., Schlager J.J., Hussain S.M. Cellular interaction of different forms of aluminum nanoparticles in rat alveolar macrophages. J. Phys. Chem. B. 2007;111(25):7353–7359. doi: 10.1021/jp068938n. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi A., Zivcak M., Sytar O., Kalaji H.M., He X., Mbarki S., Brestic M. Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 2017;5:78. doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasubramanyam A., Sailaja N., Mahboob M., Rahman M.F., Hussain S.M., Grover P. In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test. Mutagenesis. 2009;24(3):245–251. doi: 10.1093/mutage/gep003. [DOI] [PubMed] [Google Scholar]
- 20.Prabhakar P.V., Reddy U.A., Singh S.P., Balasubramanyam A., Rahman M.F., InduKumari S., Agawane S.B., Murty U.S.N., Grover P., Mahboob M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J. Appl. Toxicol. 2012;32(6):436–445. doi: 10.1002/jat.1775. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q.L., Li M.Q., Ji J.W., Gao F.P., Bai R., Chen C.Y., Wang Z.W., Zhang C., Niu Q. In vivo toxicity of nano-alumina on mice neurobehavioral profiles and the potential mechanisms. Int. J. Immunopathol. Pharmacol. 2011;24:23S–29S. [PubMed] [Google Scholar]
- 22.Oesterling E., Chopra N., Gavalas V., Arzuaga X., Lim E.J., Sultana R., Butterfield D.A., Bachas L., Hennig B. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol. Lett. 2008;178(3):160–166. doi: 10.1016/j.toxlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Dey S., Bakthavatchalu V., Tseng M.T., Wu P., Florence R.L., Grulke E.A., Yokel R.A., Dhar S.K., Yang H.S., Chen Y., St Clair D.K. Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells. Carcinogenesis. 2008;29(10):1920–1929. doi: 10.1093/carcin/bgn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L., Yokel R.A., Hennig B., Toborek M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J. Neuroimmune Pharmacol. 2008;3(4):286–295. doi: 10.1007/s11481-008-9131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer J.N., Leung M.C., Rooney J.P., Sendoel A., Hengartner M.O., Kisby G.E., Bess A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013;134(1):1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z., Puigserver P., Andersson U. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 27.Benavides M., Fernández-Lodeiro J., Coelho P., Lodeiro C., Diniz M.S. Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassiusauratus. Environ. Sci. Pollut. Res. 2016;23(24):24578–24591. doi: 10.1007/s11356-016-7915-3. [DOI] [PubMed] [Google Scholar]
- 28.Park E.J., Kim H., Kim Y., Choi K. Repeated-dose toxicity attributed to aluminum nanoparticles following 28-day oral administration, particularly on gene expression in mouse brain. Toxicol. Environ. Chem. Rev. 2011;93(1):120–133. [Google Scholar]
- 29.Saman S., Moradhaseli S., Shokouhian A., Ghorbani M. Histopathological effects of ZnO nanoparticles on liver and heart tissues in wistar rats. AdvBiores. 2013;4(2):83–88. [Google Scholar]
- 30.Piantadosi C.A., Suliman H.B. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 2006;281(1):324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 31.Li L., Pan R., Li R., Niemann B., Aurich A.C., Chen Y., Rohrbach S. Mitochondrial biogenesis and peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity intact adipocytokine signaling is required. Diabetes. 2011;60(1):157–167. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller S.A., Dykes D.D., Polesky H.F.R.N. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drury R.A., Wallington E.A. 6th edn. Oxford University Press; New York, Toronto, USA: 1980. Carleton’s Histological Techniques. [Google Scholar]
- 34.SAS . SAS Procedure Guide. release 6.03 edition. SAS Institute Inc.; Cary, Nc, U.S.A: 1998. Statistical analysis system. [Google Scholar]
- 35.Duncan D.B. Multiple ranges and multiple F tests. Biometrics. 1955;11(1):1–42. [Google Scholar]
- 36.Cui Y., Liu H., Zhou M., Duan Y., Li N., Gong X., Hu R., Hong M., Hong F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. Part A. 2011;96(1):221–229. doi: 10.1002/jbm.a.32976. [DOI] [PubMed] [Google Scholar]
- 37.Sharma V., Singh P., Pandey A.K., Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;745(1):84–91. doi: 10.1016/j.mrgentox.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Yan G., Huang Y., Bu Q., Lv L., Deng P., Zhou J., Wang Y., Yang Y., Mansouri Q., Cen X., Zhao Y. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J. Environ. Sci. Health Part A. 2012;47(4):577–588. doi: 10.1080/10934529.2012.650576. [DOI] [PubMed] [Google Scholar]
- 39.Faddah L.M., Baky N.A.A., Al-Rasheed N.M., Al-Rasheed N.M., Fatani A.J., Atteya M. Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC Complement. Altern. Med. 2012;12(1):1062. doi: 10.1186/1472-6882-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Slama I., Mrad I., Rihane N., Mir L.E., Sakly M., Amara S. Sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J. Nanomed. Nanotechnol. 2015;6(3):1. [Google Scholar]
- 41.Mansouri E., Khorsandi L., Orazizadeh M., Jozi Z. Dose-dependent hepatotoxicity effects of zinc oxide nanoparticles. Nanomed. J. 2015;2(4):273–282. [Google Scholar]
- 42.Morsy G.M., El-Ala K.S.A., Ali A.A. Studies on fate and toxicity of nanoalumina in male albino rats oxidative stress in the brain, liver and kidney. Toxicol. Ind. Health. 2016;32(2):200–214. doi: 10.1177/0748233713498462. [DOI] [PubMed] [Google Scholar]
- 43.Hillyer J.F., Albrecht R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001;90(12):1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 44.Xia T., Kovochich M., Liong M., Mädler L., Gilbert B., Shi H., Yeh J.I., Zink J.I., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almansour M.I., Alferah M.A., Shraideh Z.A., Jarrar B.M. Zinc oxide nanoparticles hepatotoxicity: histological and histochemical study. Environ. Toxicol. Pharmacol. 2017;51:124–130. doi: 10.1016/j.etap.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Asharani P.V., Low KahMun G., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2008;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 47.Babele P.K. Zinc oxide nanoparticles impose metabolic toxicity by de-regulating proteome and metabolome in Saccharomyces cerevisiae. Toxicol. Rep. 2019;6:64–73. doi: 10.1016/j.toxrep.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S.Q., Zhu R.R., Zhu H., Xue M., Sun X.Y., Yao S.D., Wang S.L. Nanotoxicity of TiO2 nanoparticles to erythrocyte in vitro. Food Chem. Toxicol. 2008;46(12):3626–3631. doi: 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Chen R., Huo L., Shi X., Bai R., Zhang Z., Zhao Y., Chang Y., Chen C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–2574. doi: 10.1021/nn406184r. [DOI] [PubMed] [Google Scholar]
- 50.Yu K.N., Chang S.H., Park S.J., Lim J., Lee J., Yoon T.J., Kim J.S., Cho M.H. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagiccell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS One. 2015;10(6):e0131208. doi: 10.1371/journal.pone.0131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehmas L.C., Anders C., Chess J., Punnoose A., Pereira C.B., Greenwood J., Tanguay R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015;2:702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klotz L.O., Sies H. Cellular generation of oxidants: relation to oxidative stress. Redox Signal. Regul. Biol. Med. 2009:45–61. [Google Scholar]
- 53.Jiang W., Mashayekhi H., Xing B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009;157(5):1619–1625. doi: 10.1016/j.envpol.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 54.Monteiller C., Tran L., MacNee W., Faux S., Jones A., Miller B., Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup. Environ. Med. 2007;64(9):609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou L., Xie K., Qin M., Peng D., Ma S., Shang L., Li N., Li S., Ji G., Lu Y., Xiong L. Effects of reactive oxygen species scavenger on the protective action of 100% oxygen treatment against sterile inflammation in mice. Shock. 2010;33(6):646–654. doi: 10.1097/SHK.0b013e3181c1b5d4. [DOI] [PubMed] [Google Scholar]
- 56.Vurusaner B., Poli G., Basaga H. Tumor suppressor genes and ROS: complex networks of interactions. Free Radic. Biol. Med. 2012;52(1):7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Patil U.S., Adireddy S., Jaiswal A., Mandava S., Lee B.R., Chrisey D.B. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015;16(10):24417–24450. doi: 10.3390/ijms161024417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pati R., Das I., Mehta R.K., Sahu R., Sonawane A. Zinc-oxide nanoparticles exhibit genotoxic, clastogenic, cytotoxic and actin depolymerization effects by inducing oxidative stress responses in macrophages and adult mice. Toxicol. Sci. 2016;150(2):454–472. doi: 10.1093/toxsci/kfw010. [DOI] [PubMed] [Google Scholar]
- 59.Love S.A., Maurer-Jones M.A., Thompson J.W., Lin Y.S., Haynes C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 60.Jennifer M., Maciej W. Nanoparticle technology as a double-edged sword: cytotoxic, genotoxic and epigenetic effects on living cells. J. Biomater. Nanobiotechnol. 2013;4(1):53–63. [Google Scholar]
- 61.Teodoro J.S., Rolo A.P., Palmeira C.M. The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods. 2013;23(5):297–302. doi: 10.3109/15376516.2012.759305. [DOI] [PubMed] [Google Scholar]
- 62.Alshatwi A.A., Subbarayan P.V., Ramesh E., Al-Hazzani A.A., Alsaif M.A., Alwarthan A.A. Aluminum oxide nanoparticles induce mitochondrial-mediated oxidative stress and alter the expression of antioxidant enzymes in human mesenchymal stem cells. Food Addit. Contam. Part A. 2013;30(1):1–10. doi: 10.1080/19440049.2012.729160. [DOI] [PubMed] [Google Scholar]
- 63.Li J.H., Liu X., Zhang Y., Tian F., Zhao G., Yu O., Jiang F., Liu Y. Toxicity of nano zinc oxide to mitochondria. Toxicol. Res. 2012;1:137–144. [Google Scholar]
- 64.Ribeiro M.P., Santos A.E., Custódio J.B. Mitochondria: the gateway for tamoxifen-induced liver injury. Toxicology. 2014;323:10–18. doi: 10.1016/j.tox.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Kostoff R.N. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol. Rep. 2018;5:1169–1172. doi: 10.1016/j.toxrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Draper H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 67.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]