Abstract
Poly(amidoamine) and Poly(propylenimine) dendrimers with different generations and peripheral groups were studied as solubility enhancers and nanocarriers for 7-bromo-2-hydroxy-phenazine N5,N10-dioxide. This compound possesses potential antitumoral and anti-trypanosomal activity, but its low solubility in physiological media precludes its possible application as therapeutic drug. The amino terminated dendrimers association with the active compounds as observed trough NMR studies showed that electrostatic interactions are essential in the solubilization enhancement process. The obtaining of a stable and no cytotoxic formulation makes the drug-carried association a suitable strategy for the generation of a drug delivery system for phenazine derivatives.
Keywords: Organic chemistry, Physical chemistry, Pharmaceutical chemistry
1. Introduction
Cancer is the second worldwide leading cause of death [1]. Cancer cases are increasing due to growth and the aging of the population, as well as a growing prevalence of risk factors such as smoking, overweight and sedentary lifestyle [2]. In the last decades, scientific and economic efforts around this issue have been successful in the reduction of the overall rate of deaths originated by cancer, as well as in the increase in the survival years [3]. However, the World Health Organization (WHO) estimates that cancer death will continue to rise worldwide to reach 13.1 million by 2030 [4]. These trends show that although progresses are being made against the disease, much work remains to be done. One of the challenges is focused on the optimization of chemotherapies carried out with conventional drugs, which involves the creation of even more effective treatments with fewer side effects [5]. In this sense, the use of nanocarriers for diagnosis or treatment has allowed to improve the aqueous solubility of active compounds, and consequently its bioavailability [6]. Also, this strategy can increase the local concentration of the drug in the tumoral area, due to the enhanced of permeability and retention (EPR) effect [7, 8]. Furthermore, the use of nanostructured host-guest systems for antitumor drug delivery facilitates the drug localization in the site to be treated, often through pathways based in specific interaction between a ligand located in the carrier and some cellular receptor [9, 10]. The drug concentration increment in the affected area (and the consequent diminution in systemic concentration), increases the treatment efficiency and reduces adverse collateral effects [11, 12].
The research and development of new materials for drug delivery includes the application, of nanoparticles, liposomes, polymers, hydrogels and dendrimers, among others [5, 13, 14, 15]. In particular the dendrimers have been extensively studied for this purpose [16, 17, 18]. These macromolecules belonging to the polymer family have a well-defined and highly branched structure, with very low polydispersity [19]. This last characteristic differentiate dendrimers from other polymers, making these materials of great utility in medicinal uses, because they monodispersity results in a high pharmacokinetic and pharmacodynamic reproducibility [20, 21]. Poly(amidoamine) and Poly(propylenimine) (PAMAM and PPI respectively) are the most studied dendrimers in drug delivery research and development [22]. This is in part due to their high aqueous solubility and biocompatibility, low immunogenicity, and the presence of modifiable peripheral functional groups [23, 24]. The high density of terminal groups and internal cavities in the PAMAM and PPI dendrimers promotes drug association, resulting in the improvement of aqueous solubility of lipophilic therapeutic compounds [25] such as candersatan cilexetil [26], hydrochlorothiazide [27], crocetin [28] and doxorubicin [29]. Also, several ionic drugs such as mycophenolic acid [30], daidzein [31] phenobarbital and zulfamethoxazole [32] have been associate to dendrimeric carriers.
On the other hand, a class of compounds that have been tested as anticancer agents are phenazines [33, 34, 35], in particular N,N′-phenazine dioxide derivatives [36]. The studies carried out to date have shown that these last compounds are selective cytotoxic agents under hypoxia condition, a particular characteristic that is present in solid tumors [37]. These compounds combine two bioactive chemical clusters: a bioreducible system, the N-oxide function, and a flat structure, phenazine, which can interact with DNA [38]. One of this antitumoral derivatives is 7-bromo-2-hydroxy-phenazine N5,N10-dioxide (Phenazine, Fig. 1). This compound exhibited in vitro antitumoral activity and high selectivity for hypoxia conditions [39]. However, the low water solubility decreases its bioavailability and effectiveness for successful in vivo usage [27]. Thus, the incorporation of this active compound to a water soluble dendrimeric carrier would allow the development of clinical formulations. Up to the present we have achieved, using various dendrimeric systems, the association and release of different hydrophobic compounds with biological activity [40, 41, 42, 43, 44]. In this work, we studied the association of the anionic Phenazine drug with four low generation dendrimers (PAMAM and PPI types, Fig. 2) with the aim of enhancing its aqueous solubility. It has been reported [45, 56] that generations smaller than 5 of PAMAM dendrimers do not present significant in vivo toxicity, suggesting that formulations involving this carriers appear to have little or no deleterious effects in biological applications. Nevertheless, the cytotoxicity of the formulation in normal Madin Darby Canine Kidney cells (MDCK) was evaluated.
Fig. 1.

Molecular structure of 7-bromo-2-hydroxy-phenazine N5, N10-dioxide (Phenazine).
Fig. 2.
Chemical structure of PAMAM-AT (G = 1), PAMAM-AT (G = 3), PAMAM-OHT (G = 3) and PPI (G = 4).
2. Materials and methods
2.1. Materials
Generation 1 and 3 ethylenediamine cored and amine terminated PAMAM dendrimers (PAMAM-AT (G = 1), PAMAM-AT (G = 3)), and generation 3 ethylenediamine cored and hydroxy terminated PAMAM dendrimer (PAMAM-OHT (G = 3)), were obtained from Sigma-Aldrich (solutions 20 wt.% in methanol). Generation 4 diamine butane cored and amine terminated PPI dendrimer (PPI (G = 4)) was obtained from Sigma-Aldrich. 7-bromo-2-hydroxy-phenazine N5,N10-dioxide (Phenazine) was synthesized as previously described [33] and stored at room temperature under vacuum. The pKa of Phenazine was determined by spectrophotometric titration [46, 47]. Methanol was purchased from Sintorgan in HPLC quality and deionized water was obtained from Elga Classic equipment (resistivity of 18 MΩ cm). Phosphate-buffered saline solution (PBS pH = 7.4) was prepared (150 mM NaCl; 1.9 mM NaH2PO4; 8.1 mM Na2HPO4). All the chemicals were used as received without further purification.
2.2. Formation of phenazine/dendrimer conjugates
Appropriate aliquots of stock solution of PPI (0.01 M in methanol) or PAMAM dendrimers methanolic solutions were transferred to 5 mL flasks and the methanol was evaporated under nitrogen stream. Then, solid Phenazine was added to ensure the obtaining of a saturated solution. The samples were diluted to the appropriate volume with water or PBS. These solutions were then sonicated for 30 minutes and vigorously stirred for 2 hours. The samples were stored at room temperature in darkness overnight. The next day the samples were shaken again for 2 hours and stored at room temperature in absence of light for 48 hs. Finally, the solutions were sonicated for 10 minutes and shaken for 2 hours. The unsolubilized Phenazine was removed via filtration through a 0.45 μm Millipore membrane. The same procedure was also carried out with Phenazine solutions in dendrimer absence. The samples of guest-host Phenazine/dendrimer conjugates were studied by UV-visible light absorption (Shimadzu UV 2401 PC spectrophotometer) and 1H Nuclear Magnetic Resonance spectroscopies (Bruker 400 MHz Advance II NMR spectrometer). In the case of the analysis of the electronic transitions the same were observed for the composite solutions in both, water and PBS medium, in the range of 420 and 700 nm, where the Phenazine compound exhibit light absorption in the two states, neutral and as phenolate anion. Aqueous solutions of Phenazine-PAMAM and Phenazine-PPI dendrimers composites were lyophilized and the solids were resolubilized in D2O for NMR analysis. Phenazine-dendrimer formulation stability in water was evaluated. Samples of the solutions were maintained at both, room temperature and 4 °C and analyzed periodically by UV-vis spectroscopy for two months. In addition, the possible occurrence of precipitate, turbidity, crystallization and color change were controlled by periodic eye observation and by UV-visible light absorption spectroscopy. As control, the stability of a saturated Phenazine solution in water in the absence of dendrimer was also tested using the same methodology.
2.3. Cells and cells culture
In order to analyze Phenazine-dendrimer systems biocompatibility, MDCK (derived from canine kidney) cell lines were used. Cells were cultured in Dulbecco's Modified Eagle Culture Medium (DMEM) (Gibco®) supplemented with fetal bovine serum 10% (Natocor®) and antibiotic-antimycotic (Gibco®) at 37 °C, in a humidity saturated atmosphere of 95% air – CO2 5%. For experiments, cells were plated in 96 wells (Biofil®) at a density of 5000 cells/well in complete media for 24 h and treated with phenazin 2 × 10−5 M in PBS, dendrimers (0.5 × 10−5 M, 1.25 × 10−5 M and 1.5 × 10−5 M PAMAM-AT (G = 3), 1.25 × 10−5 M PAMAM-AT (G = 1) and 1 × 10−5 M PPI (G = 4)) and Phenazine-dendrimer complexes (0.5 × 10−5 M PAMAM-AT (G = 3) + 1.8 × 10−4 M Phenazine, 1.25 × 10−5 M PAMAM-AT (G = 3) + 3.8 × 10−4 M Phenazine, 1.5 × 10−5 M PAMAM-AT (G = 3) + 5 × 10−4 M Phenazine, 1.25 × 10−5 M PAMAM-AT (G = 1) + 1.27 × 10−4 M Phenazine, 1 × 10−5 M PPI (G = 4) + 1.6 × 10−4 M Phenazine). The dendrimers and Phenazine/dendrimers concentrations were choose according to the studies reported by Malik et al [48] that showed that at very low dendrimers concentration there is not noticeable effects on cells viability, and at higher concentrations the cytotoxic effect is nearly concentration independent. Each experimental condition, including positive viability controls (cells cultured in complete DMEM), were run in six times.
2.4. Cytotoxicity assays
Adherent cell viability was evaluated as described previously by Rivero et al. [49] MTT technique measures 3- (4,5-dimethylthiazol-2 -yl) -2,5-diphenyltetrazolium bromide (MTT) conversion to an insoluble formazan salt. The formazan is solubilized in dimethyl sulfoxide (DMSO) and its absorbance, which is proportional to adherent cell number was measured at 540 nm wavelength. Briefly, after 24 h of treatment the media were removed and cells were incubated with MTT reagent (5 mg/mL in media) during 3 h. The reaction mixture was removed and formazan precipitated were dissolved with 100 μl DMSO then, OD (540 nm) was registered. Data were evaluated by one-way analysis of variance and Bonferroni post-hoc test using Infostat software 2017 version (Universidad Nacional de Córdoba (FCA-UNC), Córdoba, Argentina). Differences were considered significant at p value less than 0.05.
3. Results and discussion
3.1. Phenazine solubilization mediated by PAMAM and PPI dendrimers in water
As was already mentioned, Phenazine has been shown to be a selective cytotoxic agent under hypoxic conditions and, in addition, has anti Trypanosoma cruzi activity [36]. However, its water solubility is low for successful use in vivo, reducing its bioavailability and effectiveness. The association of this type of compounds with PAMAM and PPI dendrimers could increase their aqueous solubility. Therefore, the ability of PAMAM-AT (G = 1), PAMAM-AT (G = 3), PAMAM-OHT (G = 3) and PPI (G = 4) dendrimers to be associated with Phenazine was evaluated in order to select the most suitable dendrimer for to act as molecular vehicle.
In Fig. 3, the UV-vis absorption spectra of Phenazine in aqueous solutions of the studied PAMAM dendrimers are shown. In the figure it is also included the spectrum obtained for a saturated solution of the drug in pure water, corresponding to its maximum aqueous solubility (water solubility (Sw) = 2 × 10−4 M). As can be seen in Fig. 3, in dendrimers solutions the light absorption due to Phenazine markedly increases with respect to the observed in pure water saturated solution, indicating a significant enhancement in drug solubility. However, in the presence of PAMAM-AT (G = 1) and (G = 3) amino terminal dendrimers, the Phenazine spectrum shows a bell-shaped band with wavelength maxima at λmax = 560 nm. This band is in agreement with the presence of Phenazine predominately as Phenolate anion (see Fig. A1 in Additional Information). On the other hand, in PAMAM-OHT (G = 3) dendrimer aqueous solution, the Phenazine spectrum is different, exhibiting an enhancement at short wavelength, which is in agreement with the presence of Phenazine molecules in his neutral state in a higher proportion (Fig. A1). These facts are due to the different pH of the dendrimer aqueous solutions. In the case of amine terminated dendrimers the pH at the used concentration are 9.0 for PAMAM G1, 9.1 for PMAMA-G3 and 8.0 for PPI G4 dendrimer. As the Phenazine pKa is 5.6 (see Fig. A2 in Additional Information), in PAMAM-AT solutions Phenazine predominates as Phenolate anion. On the other hand, the pH of PAMAM-OHT (G = 3) aqueous solution is 7.5, increasing the presence of neutral Phenazine in the protonation-deprotonation equilibrium.
Fig. 3.
UV-vis absorption spectra of Phenazine in aqueous solution of PAMAM-AT (G = 3) (a), PPI (G = 4) (b), PAMAM-OHT (G = 3) (c) and PAMAM-AT (G = 1) (d). Dendrimer concentration: 1 × 10−4 M. The spectrum of a saturated Phenazine solution in pure water is also shown (e).
Although the dendrimer concentration is the same in all cases, the solubility increment of the drug is noticeably different. In solution of PAMAM-AT (G = 3) 1 × 10−4 M, an enhancement of 15-folds in the solubility of Phenazine with respect to its solubility in water was achieved, whereas when using the PAMAM-OHT (G = 3) dendrimer in the same concentration the solubility was increased 5 times. These two dendrimers have the same size and number of terminal groups; however, they differ in the terminal group type, PAMAM-AT possesses 32 amine terminal groups, whereas in PAMAM-OHT there is the same number of hydroxyl groups. On the other hand, the PAMAM-AT (G = 1) that holds only 8 amine terminal groups increase the Phenazine solubility 4 times at the same dendrimer concentration. Therefore, the enhancement in solubility of Phenazine depends on both, the type of terminal groups in the PAMAM dendrimer, and its generation (Table 1).
Table 1.
Characteristics of the PAMAM and PPI dendrimers and increment in water solubility of Phenazine (Dendrimer concentration: 1 × 10−4 M).
| Dendrimer | Terminal group | Surface charge | Molecular weight (g/mol) | Phenazine Solubility enhancement |
|---|---|---|---|---|
| PAMAM AT (G = 1) | 8 NH2 | Cationic | 1430 | 4 Swa |
| PAMAM AT (G = 3) | 32 NH2 | Cationic | 6909 | 15 Swa |
| PAMAM OHT (G = 3) | 32 OH | Neutral | 6941 | 5 Swa |
| PPI (G = 4) | 32 NH2 | Cationic | 3513 | 9 Swa |
Sw (Phenazine water solubility) = 2 × 10−4 M.
The active compound solubility enhancement in aqueous solutions of PAMAM dendrimers can be attributed to different mechanisms of interaction between the dendrimer and the guest [50]. The existence of relatively nonpolar cavities within the dendrimeric structure gives them the ability to encapsulate molecules through hydrophobic interactions [24, 51, 52]. On the other hand, the tertiary amine and amide groups in the dendrimers nanocavity can interact with guests molecules by hydrogen-bond formation [24, 53]. In addition, if the dendrimer has charged terminal groups, as is the case of PAMAM-AT, it can be associated with charged drug molecules through electrostatic interactions [24, 54, 55, 56]. In the aqueous solutions of the PAMAM-AT (G = 1 and G = 3) and PAMAM-OHT (G = 3) dendrimers, Phenazine is mainly found in anionic form. Thus, in the solutions of the amine terminal dendrimers the drug can be associated through electrostatic interactions with the cationic peripheral groups of the macromolecule. On the contrary, in the case of the PAMAM-OHT dendrimer this type of interaction is not possible, but the drug could be associated through hydrophobic interaction and hydrogen-bonds in the nanocavities, as well as through hydrogen-bond with the hydroxyl terminal groups [44].
Several studies demonstrate that the electrostatic interaction between ionized drugs and dendrimeric charged end-groups is often more important than other types of interactions, such as hydrophobic encapsulation or hydrogen-bond interactions [24, 25] Accordingly, in the present case, while PAMAM-AT (G = 3) and PAMAM-OHT (G = 3) have the same size and interior, the lack of positively charged surface groups in PAMAM-OHT (G = 3) limits the Phenazine solubility enhancement. In turn, although both PAMAM-AT (G = 3) and PAMAM-AT (G = 1) have positively charged surface groups capable of being associated with Phenazine through electrostatic interactions, the former holds a higher number of cationic terminal groups, which is reflected in a greater increase of Phenazine water solubility.
Fig. 4(a) shows the Phenazine solubility obtained using different concentrations of the PAMAM dendrimers. It can be seen that increasing the concentration of the dendrimers, the amount of solubilized Phenazine growths. In the case of PAMAM-AT (G = 3) dendrimer the enhancement of drug solubility is more noticeable. As previously mentioned, for the same dendrimer concentration, PAMAM-AT (G = 3) is able to increase the Phenazine solubility more than PAMAM-AT (G = 1) since the (G = 3) dendrimer has more surface groups than that of (G = 1) (32 and 8 groups respectively) and therefore more opportunities for electrostatic interaction with the negatively charged drug molecules. Nevertheless, if the comparison is made for the same concentration of terminal amino groups, it is found that both dendrimers hold a similar capacity for increasing the drug solubility in water (Fig. 4(b)), indicating that the amine terminal groups are directly involved in the solubility enhancement effect.
Fig. 4.
Aqueous solubility of Phenazine in the presence of increasing concentrations of PAMAM-AT (G = 1), PAMAM-AT (G = 3) and PAMAM OHT (G = 3) dendrimers (a) and as a function of the concentration of terminal amino groups of the dendrimers PAMAM-AT (G = 1) and PAMAM-AT (G = 3) (b).
However, it has been showed that the increment in dendrimer generation produces congestion of terminal amino groups on the surface [25, 57], increasing the steric hindrance for the electrostatic association with drug molecules and reducing the relative ability for drug solubility improvement. This is not the case in the present study, at least until generation G = 3. In this sense, like PAMAM-AT (G = 3) dendrimer, PPI (G = 4) has 32 primary amine groups on its surface that could potentially interact with Phenazine through electrostatic interactions. However, the interior of the PPI dendrimer is more hydrophobic than that of PAMAM [58] having in consequence greater ability for association with hydrophobic compounds in the inner dendrimer nanocavities. Nevertheless, contrary to expectations, PPI (G = 4) increases the Phenazine solubility with less efficiency than PAMAM-AT with the same number of amine peripheral groups (Table 1). Therefore, the different Phenazine solubilization capabilities of the PAMAM and PPI dendrimers cannot be attributed to a difference in the number of surface charges or to the medium pH (pKa of amine terminal groups are 9.75 for PPI [59] and 10.5 for PAMAM [60]). However, the branching units of PPI dendrimers are shorter than those of PAMAM dendrimers (4 versus 7 bonds), so PPI (G = 4) is smaller than PAMAM-AT (G = 3) (PPI diameter = 2.3 nm [61] versus PAMAM diameter = 3.0 nm [62]). Therefore, in the PPI dendrimer, the density of surface groups is higher; and could drive to a greater steric hindrance in the binding of Phenazine molecules, affecting the efficiency of the drug-dendrimer association for solubility enhancement. Also, this result is consequent with the hypothesis that the surface ionic interactions contribute much more to the drug solubility improvement than the encapsulation in the nanocavities through hydrophobic and hydrogen-bond interactions [24].
3.2. Analysis of the association of phenazine with PAMAM and PPI dendrimers by 1H-NMR spectroscopy
The association of Phenazine with dendrimers in water was studied by 1H-NMR spectroscopy. NMR techniques are very useful tools for characterizing dendrimer-guest complexes, as they provide information on the intermolecular interactions present in the system [63].
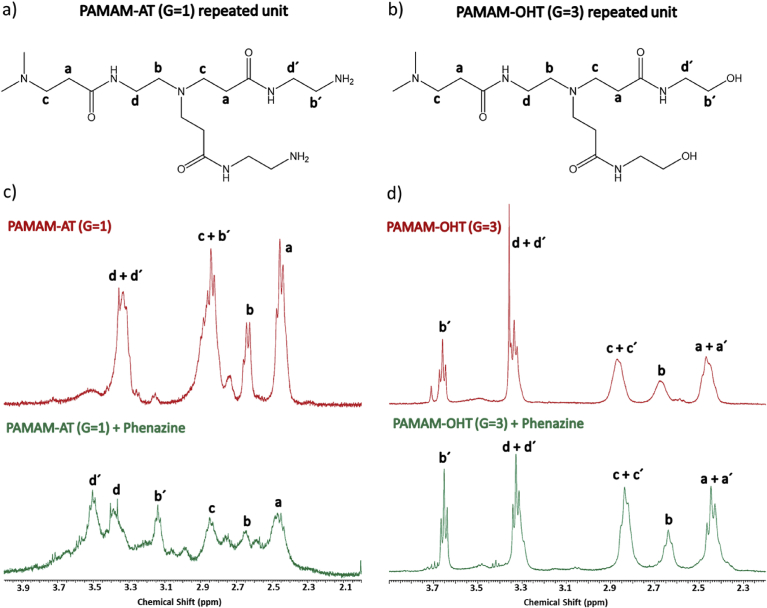
The PAMAM-AT and PAMAM-OHT dendrimers have six types of protons signals (Fig. 5a and b): four corresponding to the inner methylenes (groups a, b, c, d) and two corresponding to the methylene groups of the outermost layer of the dendrimer, close to the terminal groups (b'y d' in Fig. 5) [64, 65]. In the 1H-NMR spectra of these dendrimers, the peak for protons in d' generally overlaps with protons in d, whereas protons in b' methylene groups overlaps with that in c [42]. In the 1H-NMR spectra of PAMAM-AT in the absence and presence of Phenazine, dendrimer proton signals appear in the zone of aliphatic hydrogens, between 2 and 4 ppm (Fig. 5c). In presence of Phenazine, a significant downfield shift of the signals of the methylene protons located in the outermost layer of the dendrimer (Hb′ y Hd′) is observed. This indicates that the association occurs on the surface of the dendrimer, by ionic interactions between the PAMAM amino terminal groups and the Phenazine phenolic group [23, 25, 42, 66]. Furthermore, previous studies showed that the guest encapsulation inside of the dendrimers shift the signals of internal protons to high field [25]. In the case of the Phenazine-PAMAM-AT system, no signal upfield shift of the internal proton is observed in the presence of the drug. Therefore, as has been demonstrated in previous studies [24], terminal amino groups are the predominant factor in the solubilization enhancement of the anionic drug by the cationic dendrimers.
Fig. 5.
Hydrogen atom labeling in the chemical structure of PAMAM-AT (G = 1) (a) and PAMAM-OHT (G = 3) (b) dendrimer. Expanded region of the 1H NMR spectra of PAMAM-AT (G = 1) (c) and PAMAM-OHT (G = 3) (d) in absence and in presence of Phenazine, in D2O.
Moreover, in the case of PPI dendrimers, the association with Phenazine produces a downfield shift of the NMR signals attributed to the aliphatic protons located in immediate vicinity of the terminal amino groups (see the NMR spectra, Fig. A3, in Additional Information) [67]. This fact is in fully agreement with our hypothesis that the guest-host association occurs by ionic interactions between the amines located in the outermost layer of the PAMAM and PPI dendrimers and the Phenazine phenolic group [31, 56].
On the other hand, when analyzing the 1H-NMR spectrum of the PAMAM-OHT dendrimer in the absence and in the presence of Phenazine, in D2O, it can be seen that the positions of the dendrimer signals practically do not change in the presence of drug (Fig. 5d). This allows us to infer that Phenazine does not associate with the PAMAM-OHT dendrimer since there is no evidence of ionic, hydrophobic or hydrogen-bond interaction between the drug and the macromolecule. However, in the UV-vis spectroscopy studies, an increase in the solubility of the drug in aqueous PAMAM-OHT solution was observed with respect to the saturated Phenazine solution in pure water (Fig. 3). This increase in solubility can be attributed to the fact that aqueous solutions of PAMAM-OHT dendrimer have a more basic pH than pure water. Therefore, in the presence of the PAMAM-OHT dendrimer, Phenazine is more ionized than in pure water; as the ionized drug is more soluble in water than when it is in the neutral state, its solubility increases in the presence of the dendrimer. In the case of PAMAM-AT dendrimers whose aqueous solutions are also more basic than pure water, the increase in Phenazine solubility would also partly be due to an increase in the pH of the solution. However, unlike for OHT dendrimer, in amino-terminal dendrimers the solubility of the drug would also be due to the association with the macromolecule, according to the evidence found by 1H-NMR spectroscopy. To corroborate this hypothesis, the solubility of Phenazine in PBS and in solutions of the PAMAM-AT and OHT dendrimers in PBS was analyzed.
3.3. Phenazine solubilization mediated by PAMAM dendrimers in PBS
Due to the fact that the presence of PAMAM dendrimers affect the water pH, and that in aqueous solution, neutral Phenazine (phenolic form) and the corresponding phenolate anion coexist in equilibrium, the drug-dendrimer association was investigated in buffered media (PBS pH = 7.4). At physiological pH Phenazine is mainly found as phenolate anion (pKa = 5.6). Fig. 6 shows the UV-vis absorption spectra of Phenazine in the presence of PAMAM-AT (G = 3) and PAMAM-OHT (G = 3), in PBS. In addition, the spectrum of a saturated solution of the drug in PBS, corresponding to its maximum solubility in PBS, is shown for comparison (PBS solubility (SPBS) = 5 × 10−4 M). As can be clearly see in Fig. 6, the Phenazine absorbance in PBS is almost the same with and without PAMAM-OHT (G = 3). Thus there is not solubility enhancement due to the presence of OHT terminal dendrimer, and the effect observed in pure water was due to the changes in the media pH. This show that there is not specific interaction between PAMAM-OHT (G = 3) dendrimer and Phenazine, in agreement with the H1NMR studies.
Fig. 6.
UV-vis absorption spectra of Phenazine in presence of PAMAM-AT (G = 3) (a), PPI (G = 4) (b) and PAMAM-OHT (G = 3) (c) dendrimer in PBS. Dendrimer concentration: 1 × 10−4 M. The spectrum of a saturated Phenazine solution in PBS is also shown (d).
On the other hand, in PAMAM-AT (G = 3) dendrimer solution the absorbance of Phenazine increases relative to the saturated drug solution in PBS, indicating that the drug solubility is remarkably enhanced (Fig. 6). At pH = 7.4 both, the terminal amino groups of the dendrimer [68] and the phenol group of the drug are ionized. Therefore, the increase in Phenazine solubility can be attributed to the drug-dendrimer association through the ionic interactions between the terminal groups of the dendrimer and the phenolate group of the therapeutic compound (Fig. 7), in agreement with NMR shift observed in the protons that belongs to the peripheral groups in PAMAM-AT dendrimer when Phenazine is present in the aqueous media.
Fig. 7.
Schematic illustration of Phenazine in presence of PAMAM-AT and PAMAM-OHT dendrimer in PBS. (a) Proposed ionic interaction between cationic PAMAM dendrimer and deprotonated Phenazine molecules. (b) Phenazine does not associate with PAMAM-OHT dendrimer.
Regarding PPI dendrimer, the Phenazine solubility increase as compared with pure PBS media, but this enhancement is low in comparison with the same effect obtained with PAMAM-AT (G = 3) dendrimer, in agreement with the results obtained in pure water, that was attributed to the steric hindrance produced in PPI- Phenazine association.
3.4. Stability of the phenazine-dendrimer complexes
In order to evaluate the stability of Phenazine-dendrimer associations, solutions of the complexes maintained at 4 °C in refrigerator and at room temperature were analyzed periodically. Possible precipitation, turbidity, crystallization, spectral changes and drug release were monitored. The stability of Phenazine in the absence and presence of the dendrimers in water at room temperature over time is shown in Fig. 8. It can be seen that there were no significant changes in the remaining percentage of Phenazine in solution as a function of time, whether the drug is in pure water or in aqueous solution of the PAMAM-AT (G = 1), PAMAM-AT (G = 3) or PPI (G = 4) dendrimers, indicating that drug-dendrimer associations are stable over time. Similar results were obtained for water solutions at 4 °C and for drug-dendrimer association in PBS media at both temperatures. This is very important considering that the study of such systems aims at the preparation of formulations for future clinical application.
Fig. 8.
Stability of Phenazine in absence and presence of the PAMAM and PPI dendrimers in water for two months. Dendrimer concentration: 1.0 × 10−4 M.
3.5. Biocompatibility analysis of phenazine-dendrimer complexes
Phenazine-dendrimer systems are investigated for their potential application in the development of new treatment for clinical use. In this way, the biocompatibility analysis of the Phenazine-dendrimer complexes was initiated through cytotoxicity assays. The cytotoxic effects of Phenazine, PAMAM-AT (G = 1), PAMAM-AT (G = 3) and PPI (G = 4) dendrimers and their corresponding complexes with the active compound were determined in normal MDCK cells by MTT assays as detailed in section 2.4. The percentages of cell viability obtained in each case are shown, as optical density (540 nm) in Fig. 9. The MDCK viability data showed that a single treatment of Phenazine and dendrimers did not produce cytotoxic effect, with the exception of treatment with PPI (G = 4). For cationic dendrimers, such as PAMAM-AT and PPI, the surface charge is known to be the predominant factor in polymer cytotoxicity. Both PAMAM-AT (G = 3) and PPI (G = 4) has 32 surface primary amine groups, so it would be expected that both dendrimers have similar cytotoxicity effect [25]. However, the PPI dendrimer (G = 4) has a more lipophilic areas than PAMAM-AT, being suitable to traversing the lipophilic region of cell membrane [50]. Thus, PPI dendrimer (G = 4) have a greater ability to perturb the cell membrane stability than the PAMAM-AT dendrimer, showing cytotoxicity. However, the Phenazine anionic drug association with PPI reduce the cytotoxic effects of the polycationic carrier (Fig. 9) by masking the surface charge [52].
Fig. 9.
MDCK cells viability treated with Phenazine (Phe), PAMAM-AT (G = 1), PAMAMAT (G = 3), PPI (G = 4) and their combination during 24 hs. Phenazine saturated solution. Dendrimer Concentration 1.0 × 10−4 M (highest studied concentration). Data represented media of absorbance ± Standard Error (n = 6) (p ≤ 0.05).
4. Conclusions
The use of amine terminal dendrimers such PAMAM-AT and PPI allows the generation of formulations that increase the water solubility of 7-bromo-2-hydroxy-phenazine N5,N10-dioxide, a compound that exhibited in vitro antitumoral activity whit high selectivity for hypoxia conditions and anti-trypanosomal activity against Trypanosoma cruzi, but whose low solubility in physiological media decreases its bioavailability and effectiveness for successful in vivo usage. NMR studies showed that the amino groups in dendrimeric periphery are essential in the solubilization enhancement. This process is originated in the association of Phenazine phenolic group trough ionic interactions with the dendrimers amino terminal residues. In fact, the PAMAM-AT (G = 3) dendrimer with 32 amine terminal groups generates the higher solubility increment in water and PBS media. The formulation is stable in time and exhibit no cytotoxic effects for normal MDCK cells, making the drug-carried association a suitable strategy for the generation of a drug delivery system for phenazine derivatives.
Declarations
Author contribution statement
Nahir Dib, Fabrisio Alustiza, Ana Cecilia Liaudat, Pablo Bosch, M. Laura Lavaggi, Hugo Cerecetto, Mercedes González: Performed the experiments; Analyzed and interpreted the data.
Luciana Fernández, Marisa Santo, Luis Otero: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-Argentina PIP 112-20110101029). Agencia Nacional de Promoción Científica y Tecnológica (FONCYT-Argentina PICT 0415-2014; PICT-0654-2011). Secretaría de Ciencia y Técnica. Universidad Nacional de Río Cuarto (SECYT-UNRC18/C297).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Luciana Fernández, Email: lfernandez@exa.unrc.edu.ar.
Luis Otero, Email: lotero@exa.unrc.edu.ar.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol. Biomark. Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Gmeiner W.H., Ghosh S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2013;3(2):111–122. doi: 10.1515/ntrev-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodabandehloo H., Zahednasab H., Hafez A.A. Nanocarriers usage for drug delivery in cancer therapy. Iran. J. Cancer Prev. 2016;9(2):e3966. doi: 10.17795/ijcp-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanty C., Das M., Sahoo S.K. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin. Drug Deliv. 2012;9(11):1347–1364. doi: 10.1517/17425247.2012.724676. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu D., Auguste D.T. Cancer targeted therapeutics: from molecules to drug delivery vehicles. J. Control. Release. 2015;219:632–643. doi: 10.1016/j.jconrel.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y., Fu F., Zhang M., Shen M., Zhu M., Shi X. Multifunctional dendrimers modified with alpha-tocopheryl succinate for targeted cancer therapy. Med. Chem. Commun. 2014;5(7):879–885. [Google Scholar]
- 10.Pan D., Guo C., Luo K., Yi Q., Gu Z. PEGylated dendritic diaminocyclohexyl-platinum (II) conjugates as pH-responsive drug delivery vehicles with enhanced tumor accumulation and antitumor efficacy. Biomaterials. 2014;35(38):10080–10092. doi: 10.1016/j.biomaterials.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Guillaudeu S.J., Fox M.E., Haidar Y.M., Dy E.E., Szoka F.C., Fréchet J.M.J. PEGylated dendrimers with core functionality for biological applications. Bioconjug. Chem. 2008;19(2):461–469. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 12.Kaminskas L.M., McLeod V.M., Kelly B.D., Cullinane C., Sberna G., Williamson M., Boyd B.J., Owen D.J., Porter C.J.H. Doxorubicin-conjugated PEGylated dendrimers show similar tumoricidal activity but lower systemic toxicity when compared to PEGylated liposome and solution formulations in mouse and rat tumor models. Mol. Pharm. 2012;9(3):422–432. doi: 10.1021/mp200522d. [DOI] [PubMed] [Google Scholar]
- 13.Beija M., Salvayre R., Lauth-de Viguerie N., Mart J.D. Colloidal systems for drug delivery: from design to therapy. Trends Biotechnol. 2012;30(9):485–496. doi: 10.1016/j.tibtech.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Shen S., Liu M., Li T., Lin S., Mo R. Recent progress in nanomedicine-based combination cancer therapy using a site-specific co-delivery strategy. Biomater. Sci. 2017;5(8):1367–1381. doi: 10.1039/c7bm00297a. [DOI] [PubMed] [Google Scholar]
- 15.Pradeep P., Kumar P., Choonara Y.E., Pillay V. Targeted nanotechnologies for cancer intervention: a patent review (2010–2016) Expert Opin. Ther. Pat. 2017;27(9):1005–1019. doi: 10.1080/13543776.2017.1344216. [DOI] [PubMed] [Google Scholar]
- 16.Sheikhpour M., Barani L., Kasaeian A. Biomimetics in drug delivery systems: a critical review. J. Control. Release. 2017;253:97–109. doi: 10.1016/j.jconrel.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Gorzkiewicz M., Klajnert-Maculewicz B. Dendrimers as nanocarriers for nucleoside analogues. Eur. J. Pharm. Biopharm. 2017;114:43–56. doi: 10.1016/j.ejpb.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A.K., Gothwal A., Kesharwani P., Alsaab H., Iyer A.K., Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today. 2017;22(2):314–326. doi: 10.1016/j.drudis.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Undre S.B., Pandya S.R., Kumar V., Singh M. Dendrimers as smart materials for developing the various applications in the field of biomedical sciences. Adv. Mater. Lett. 2016;7(7):502–516. [Google Scholar]
- 20.Shcharbin D., Janaszewska A., Klajnert-Maculewicz B., Ziemba B., Dzmitruk V., Halets I., Loznikova S., Shcharbina N., Milowska K., Ionov M., Shakhbazau A., Bryszewska M. How to study dendrimers and dendriplexes III. Biodistribution, pharmacokinetics and toxicity in vivo. J. Control. Release. 2014;181:40–52. doi: 10.1016/j.jconrel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Aillon K.L., Xie Y., El-Gendy N., Berkland C.J., Forrest M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009;61(6):457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes L.P., Pan J., Torchilin V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401–1422. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciolkowski M., Petersen J.F., Ficker M., Janaszewska A., Christensen J.B., Klajnert B., Bryszewska M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomedicine. 2012;8(6):815–817. doi: 10.1016/j.nano.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Szymanski P., Markowicz M., Mikiciuk-Olasik E. Nanotechnology in pharmaceutical and biomedical applications. Dendrimers Nano Brief Rep. Rev. 2011;6(6):509–539. [Google Scholar]
- 25.Choudhary S., Gupta L., Rani S., Dave K. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017;8(261):1–23. doi: 10.3389/fphar.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ertürk A.S., Gürbüz M.U., Tülü M. The effect of PAMAM dendrimer concentration, generation size and surface functional group on the aqueous solubility of candesartan cilexetil. Pharm. Dev. Technol. 2017;22(1):111–121. doi: 10.1080/10837450.2016.1219372. [DOI] [PubMed] [Google Scholar]
- 27.Singh M.K., Pooja D., Kulhari H., Jain S.K., Sistla R., Chauhan A.S. Poly (amidoamine) dendrimer-mediated hybrid formulation for combination therapy of ramipril and hydrochlorothiazide. Eur. J. Pharm. Sci. 2017;96:84–92. doi: 10.1016/j.ejps.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Soltani F., Ramezani M., Amel Farzad S., Mokhtarzadeh A., Hashemi M. Comparison study of the effect of alkyl-modified and unmodified PAMAM and PPI dendrimers on solubility and antitumor activity of crocetin. Artif. Cells Nanomed. Biotechnol. 2017;45(7):1356–1362. doi: 10.1080/21691401.2016.1236805. [DOI] [PubMed] [Google Scholar]
- 29.Khutale G.V., Casey A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur. J. Pharm. Biopharm. 2017;119:372–380. doi: 10.1016/j.ejpb.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Hu J., Cheng Y., Ma Y., Wu Q., Xu T. Host−Guest chemistry and physicochemical properties of the Dendrimer−Mycophenolic acid complex. J. Phys. Chem. B. 2009;113(1):64–74. doi: 10.1021/jp8078919. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C., Wang Y., Su Y., Zhang H., Ding L., Yan X., Zhao D., Shao N., Ye X., Cheng Y. Inclusion complexes of isoflavones with two commercially available dendrimers: solubility, stability, structures, release behaviors, cytotoxicity, and anti-oxidant activities. Int. J. Pharm. 2011;421(2):301–309. doi: 10.1016/j.ijpharm.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., Wu Q., Li Y., Xu T. External electrostatic interaction versus internal encapsulation between cationic dendrimers and negatively charged drugs: which contributes more to solubility enhancement of the drugs? J. Phys. Chem. B. 2008;112(30):8884–8890. doi: 10.1021/jp801742t. [DOI] [PubMed] [Google Scholar]
- 33.Conda-Sheridan M., Marler L., Park E.J., Kondratyuk T.P., Jermihov K., Mesecar A.D., Pezzuto J.M., Asolkar R.N., Fenical W., Cushman M. Potential chemopreventive agents based on the structure of the lead compound 2-Bromo-1-hydroxyphenazine, isolated from streptomyces species, strain CNS284. J. Med. Chem. 2010;53(24):8688–8699. doi: 10.1021/jm1011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rewcastle G.W., Denny W.A., Baguley B.C. Potential antitumor agents. 51. Synthesis and antitumor activity of substituted phenazine-1-carboxamides. J. Med. Chem. 1987;30(5):843–851. doi: 10.1021/jm00388a017. [DOI] [PubMed] [Google Scholar]
- 35.Gao X., Lu Y., Xing Y., Ma Y., Lu J., Bao W., Wang Y., Xi T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012;167(10):616–622. doi: 10.1016/j.micres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Cerecetto H., González M., Lavaggi M.L., Azqueta A., López de Cerain A., Monge A. Phenazine 5,10-dioxide derivatives as hypoxic selective cytotoxins. J. Med. Chem. 2005;48(1):21–23. doi: 10.1021/jm0492150. [DOI] [PubMed] [Google Scholar]
- 37.Cerecetto H., González M., Lavaggi M.L. Development of hypoxia selective cytotoxins for cancer treatment: an update. Med. Chem. 2006;2(3):315–327. doi: 10.2174/157340606776930808. [DOI] [PubMed] [Google Scholar]
- 38.Lavaggi M.L., Cabrera M., Pintos C., Arredondo C., Pachón G., Rodriguez J., Raymondo S., Pacheco J., Cascante M., Olea-Azar C., Lopez de Ceráin A., Monge A., Cerecetto H., González M. Novel phenazine 5,10-dioxides release •OH in simulated hypoxia and induce reduction of tumour volume in vivo. ISRN Pharmacol. 2011 doi: 10.5402/2011/314209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerecetto H., González M., Lavaggi M.L., Aravena M.A., Rigol C., Olea-Azar C., Azqueta A., López de Cerain A., Monge A., Bruno A.M. Phenazine 5,10-dioxide derivatives as hypoxic selective cytotoxins: part II. Structure-activity relationship studies. Med. Chem. 2006;2(5):511–521. doi: 10.2174/157340606778250207. [DOI] [PubMed] [Google Scholar]
- 40.Fernández L., Sigal E., Otero L., Silber J.J., Santo M. Solubility improvement of an anthelmintic benzimidazole carbamate by association with dendrimers. Braz. J. Chem. Eng. 2011;28(4):679–689. [Google Scholar]
- 41.Fernández L., Santo M., Silber J.J., Cerecetto H., Gonzalez M. Solubilization and release properties of dendrimers. Evaluation as prospective drug delivery systems. Supramol. Chem. 2006;18(8):633–643. [Google Scholar]
- 42.Fernandez L., Calderón M., Martinelli M., Strumia M., J Silber J., Santo M. Evaluation of a new dendrimeric structure as prospective drugs carrier for intravenous administration of antichagasic active compounds. J. Phys. Org. Chem. 2008;21(12):1079–1085. [Google Scholar]
- 43.Dib N., Fernández L., Gonzalez M., Cerecetto H., Durantini E., Otero L., Santo M. Evaluation of different PAMAM dendrimers as molecular vehicle of 1,2,4-triazine N-oxide derivative with potential antitumor activity. J. Incl. Phenom. Macrocycl. Chem. 2014;79(1):55–73. [Google Scholar]
- 44.Dib N., Fernández L., Otero L., Santo M., Calderón M., Martinelli M., Strumia M. First generation newkome-type dendrimer as solubility enhancer of antitumor benzimidazole carbamate. J. Incl. Phenom. Macrocycl. Chem. 2015;82(3–4):351–359. [Google Scholar]
- 45.Mekuria S.L., Debele T.A., Tsai H.C. PAMAM dendrimer based targeted nano-carrier for bio-imaging and therapeutic agents. RSC Adv. 2016;6(68):63761–63772. [Google Scholar]
- 46.Duran M., Canbaz M.C. pKa determination of newly synthesized N-(benzothiazole-2-yl)-2-(4,5-dimethyl-1-(phenylamino)-1H-imidazol-2-ylthio)acetamide derivatives. Ind. Eng. Chem. Res. 2013;52(25):8355–8360. [Google Scholar]
- 47.Nan G., Shi J., Huang Y., Sun J., Lv J., Yang G., Li Y. Dissociation constants and solubilities of daidzein and genistein in different solvents. J. Chem. Eng. Data. 2014;59(4):1304–1311. [Google Scholar]
- 48.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weenerc J.W., Meijerc E.W., Paulusd W., Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 49.Rivero R., Alustiza F., Capella V., Liaudat C., Rodriguez N., Bosch P., Barbero C., Rivarola C. Physicochemical properties of ionic and non-ionic biocompatible hydrogels in water and cell culture conditions: relation with type of morphologies of bovine fetal fibroblasts in contact with the surfaces. Colloids Surf. B Biointerfaces. 2017;158:488–497. doi: 10.1016/j.colsurfb.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 50.K Jain N., Prajapati R.N., Agarwal A., Gupta U., Asthana A. Dendrimers – reflections on host-guest interaction mechanism towards solubility enhancement. Asian J. Pharm. 2009;3(3):188–196. [Google Scholar]
- 51.Boas U., Christensen J.B., Heegaard P.M. Dendrimers: design, synthesis and chemical properties. J. Mater. Chem. 2006;16(38):3785–3798. [Google Scholar]
- 52.D'Emanuele A., Attwood D. Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 2005;57(15):2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Devarakonda B., Hill R.A., de Villiers M.M. The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int. J. Pharm. 2004;284(1-2):133–140. doi: 10.1016/j.ijpharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Milhem O.M., Myles C., McKeown N.B., Attwood D., D'Emanuele A. Polyamidoamine Starburst® dendrimers as solubility enhancers. Int. J. Pharm. 2000;197(1–2):239–241. doi: 10.1016/s0378-5173(99)00463-9. [DOI] [PubMed] [Google Scholar]
- 55.Ma M.L., Cheng Y.Y., Xu Z.H., Xu P., Qu H.O., Fang Y.J., Xu T.W., Wen L.P. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug. Eur. J. Med. Chem. 2007;42(1):93–98. doi: 10.1016/j.ejmech.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Devarakonda B., Hill R.A., Liebenberg W., Brits M., De Villiers M.M. Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int. J. Pharm. 2005;304(1–2):193–209. doi: 10.1016/j.ijpharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 57.Cheng Y., Li Y., Wu Q., Zhang J., Xu T. Generation-dependent encapsulation/electrostatic attachment of phenobarbital molecules by poly(amidoamine) dendrimers: evidence from 2D-NOESY investigations. Eur. J. Med. Chem. 2009;44(5):2219–2223. doi: 10.1016/j.ejmech.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 58.Shao N., Su Y., Hu J., Zhang J., Zhang H., Cheng Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int. J. Nanomed. 2011;6:3361–3372. doi: 10.2147/IJN.S27028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kabanov V.A., Zezin A.B., Rogacheva V.B., Gulyaeva Z.G., Zansochova M.F., Joosten J.G.H., Brackman J. Polyelectrolyte behavior of astramol poly(propyleneimine) dendrimers. Macromolecules. 1998;31(15):5142–5144. doi: 10.1021/ma971643a. [DOI] [PubMed] [Google Scholar]
- 60.Diallo M.S., Christie S., Swaminathan P., Balogh L., Shi X., Um W., Papelis C., Goddard W.A., III, Johnson J.H. Dendritic chelating agents. 1. Cu(II) binding to ethylene diamine core poly(amidoamine) dendrimers in aqueous solutions. Langmuir. 2004;20(7):2640–2651. doi: 10.1021/la036108k. [DOI] [PubMed] [Google Scholar]
- 61.Vohs J.K., J Brege J., Raymond J.E., Brown A.E., Williams G.L., Fahlman B.D. Low-temperature growth of carbon nanotubes from the catalytic decomposition of carbon tetrachloride. J. Am. Chem. Soc. 2004;126(32):9936–9937. doi: 10.1021/ja0478227. [DOI] [PubMed] [Google Scholar]
- 62.Tomalia D.A. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005;30(3):294–324. [Google Scholar]
- 63.Wang F., Shao N., Cheng Y. Paramagnetic NMR investigation of dendrimer-based host-guest interactions. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0064722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu J., Xu T., Cheng Y. NMR insights into dendrimer-based host–guest systems. Chem. Rev. 2012;112(7):3856–3891. doi: 10.1021/cr200333h. [DOI] [PubMed] [Google Scholar]
- 65.Santo M., Fox M.A. Hydrogen bonding interactions between Starburst dendrimers and several molecules of biological interest. J. Phys. Org. Chem. 1999;12(4):293–307. [Google Scholar]
- 66.Hu J., Fang M., Cheng Y., Zhang J., Wu Q., Xu T. Host−Guest chemistry of Dendrimer−Drug complexes. 4. An in-depth look into the binding/encapsulation of guanosine monophosphate by dendrimers. J. Phys. Chem. B. 2010;114(21):7148–7157. doi: 10.1021/jp1007889. [DOI] [PubMed] [Google Scholar]
- 67.Wang M., Gong X., Hu J., Yu Y., Chen Q., Cheng Y. Understanding the binding interactions between dendrimer and 18 common amino acids by NMR techniques. J. Phys. Chem. B. 2011;115:12728–12735. doi: 10.1021/jp207817f. [DOI] [PubMed] [Google Scholar]
- 68.Klajnert B., Stnisłauska L., Bryszewska M., Pałecz B. Interactions between PAMAM dendrimers and bovine serum albumin. Biochim. Biophys. Acta. 2003;1648(1):115–126. doi: 10.1016/s1570-9639(03)00117-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.