Summary
Intercellular contacts are essential for precise organ morphogenesis, function, and maintenance; however, spatiotemporal information of cell-cell contacts or adhesions remains elusive in many systems. We developed a genetically encoded fluorescent indicator for intercellular contacts with optimized intercellular GFP reconstitution using glycosylphosphatidylinositol (GPI) anchor, GRAPHIC (GPI anchored reconstitution-activated proteins highlight intercellular connections), which can be used for an expanded number of cell types. We observed a robust GFP signal specifically at the interface between cultured cells, without disrupting natural cell contact. Application of GRAPHIC to the fish retina specifically delineated cone-bipolar connection sites. Moreover, we showed that GRAPHIC can be used in the mouse central nervous system to delineate synaptic sites in the thalamocortical circuit. Finally, we generated GRAPHIC color variants, enabling detection of multiple convergent contacts simultaneously in cell culture system. We demonstrated that GRAPHIC has high sensitivity and versatility, which will facilitate the analysis of the complex multicellular connections without previous limitations.
Subject Areas: Biological Sciences, Molecular Biology, Cell Biology
Graphical Abstract
Highlights
-
•
Development of GRAPHIC to visualize intercellular contact site
-
•
GPI anchor and different split site provides stronger fluorescent signal
-
•
GRAPHIC can be used to delineate synaptic site in mouse CNS and zebrafish retina
-
•
GRAPHIC color variants for multi–contact site visualization
Biological Sciences; Molecular Biology; Cell Biology
Introduction
In multicellular organisms, intercellular communication controls orchestrated morphogenesis during development and functional cooperation of multiple cells. Long-range intercellular communication via secreted ligands organizes cellular functions extending over multiple tissues and organs (Baes and Denef, 1987, Pires-daSilva and Sommer, 2003), whereas direct cell-cell contact plays a crucial role in tuning more local and specific events, including polarized cell migration (Carmona-Fontaine et al., 2008, Mayor and Carmona-Fontaine, 2010), control of organ mass (McClatchey and Yap, 2012), immune system maturation (Miller and Basten, 1996, van Panhuys, 2016), and formation and plasticity of functional neural circuits (Craig and Kang, 2007, Holland et al., 1998, Varoqueaux et al., 2006). Therefore identification of specific cell-cell contacts and analysis of its physiological significances are important to investigate how each organ acquires and maintains its proper function. However, detection of transient intercellular contacts and isolation of specific interactions within intermingled multicellular networks are difficult to perform.
To address this issue, systems that visualize intercellular contacts via trans-cellular molecular interactions between pairs of receptor-ligand membrane proteins have been reported. The GRASP (green fluorescence protein [GFP] reconstitution across synaptic partners) system developed by the Bargmann lab employed the human T cell protein CD4 (Feinberg et al., 2008). To minimize intracellular interactions, the cytosolic domains of CD4 that interact with signaling molecules were deleted, leaving a seven-amino acid cytosolic tail; the extracellular domain was also truncated to include only one or two of its four immunoglobulin domains. This CD4::split GFP system (i.e., CD4::GFP1-10 + CD4::GFP11) has been extensively used to label many cellular contacts in Drosophila (Gordon and Scott, 2009, Makhijani et al., 2017, Roy et al., 2014) and transient immune synaptic contacts between T cells and antigen-presenting cells (Pasqual et al., 2018). Most of the other probe systems to identify intercellular contacts have been designed to label synaptic connections in neural circuits, based on interactions between synaptogenesis molecules, neurexin-neuroligin. ID-PRIM (interaction-dependent probe incorporation mediated by enzymes) (Liu et al., 2013) and the horseradish peroxidase reconstitution system (Liu et al., 2013, Martell et al., 2016) employ an enzyme-substrate reaction, and in GRASP (Feinberg et al., 2008) and SynView (Tsetsenis et al., 2014) systems, split GFP fragments tethered to pre- and postsynaptic membrane proteins reconstitute a GFP molecule in the synaptic cleft after synapse formation (Scheiffele et al., 2000). These systems are successful in isolating specific neuronal connectivity from highly heterogeneous connections among numerous neurons. However, to use these probes in the mammalian system, specific expression of probes is required in post- or presynaptic cells to reveal specific connections, which seems to be causing low expression level of probes and low signal intensity (Kim et al., 2012). To generate a simpler system, we utilized GPI (glycosylphosphatidylinositol)-anchored membrane-associated domains, which lack a cytoplasmic tail, to permit visualization via the reconstitution of split GFP (N-terminal fragment probe [NT-probe]: 1–7 within its 11 β-sheets, C-terminal fragment probe [CT-probe]: within its 11 β-sheets). Moreover, by utilizing a GFP split site distinct from the previous indicators we could dramatically increase the signal intensity. Additional optimizations of molecular structure achieved higher GFP reconstitution activity at intercellular contact sites.
Our next challenge is to engineer a color variant that will enable us to distinguish different connectivities at the same time. GFP has several color variants (blue fluorescent protein [BFP], cyan fluorescent protein [CFP], yellow fluorescent protein [YFP], etc.), and their fluorescent characteristics depend on specific point mutations (Pakhomov and Martynov, 2008, Shaner et al., 2007). Combination-dependent color variation of a GFP reconstitution system utilizes GFP diversity and is a useful application to obtain multiple data simultaneously (Hu and Kerppola, 2003).
As our probe molecules have no cell type specificity, no directionality, and no specific interacting domain for endogeneous molecules, the GRAPHIC system can be applied to many types of intercellular contacts in organisms. In the present study, we applied this system to visualize neuronal connectivity in mouse brain and zebrafish retina and demonstrated that it provides a strong signal that can specifically highlight synaptic sites. This GFP reconstitution probe will be a powerful tool to analyze specific intercellular contacts, even in highly complicated systems.
Results
Design and Characterization of GRAPHIC Probes
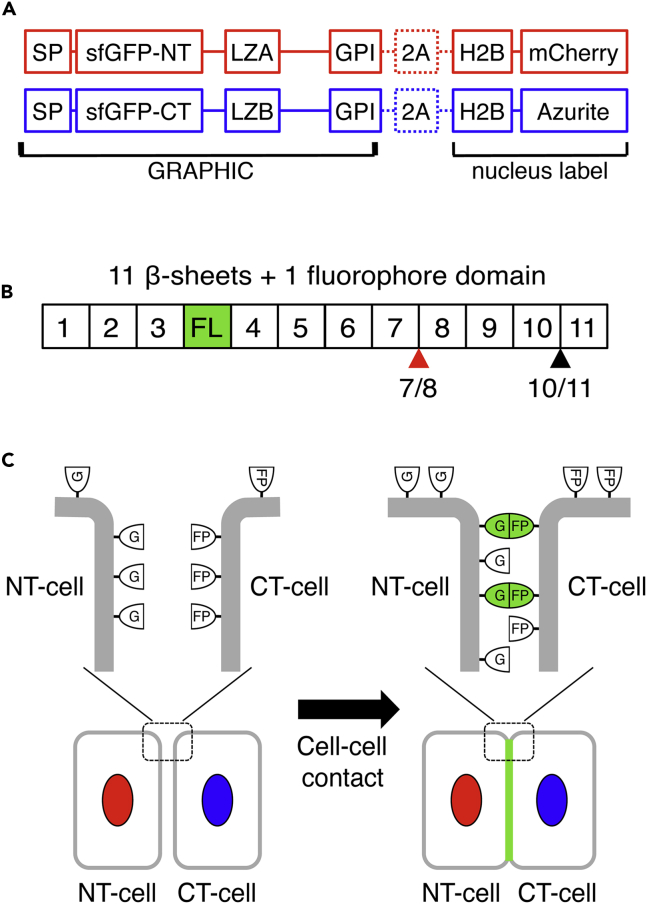
We designed a set of GPI-anchored membrane proteins for effectively displaying two complementary GFP fragments on the plasma membrane (Figure 1A). With this strategy, fluorescent GFP molecules will be reconstituted specifically at the contact area between two cells expressing each fragment (Figure 1C). To identify the cells expressing the GFP N-terminal fragment probe (NT-probe), H2B (histone 2B)-mCherry was attached to the NT-probe with 2A self-cleavable peptide (Figure 1A). For GFP C-terminal fragment probe (CT-probe), H2B-Azurite was attached. To determine the most efficient split site of superfolder GFP (sfGFP) (Cabantous et al., 2005, Pedelacq et al., 2006), we tested the reconstitution activity of two probe pairs containing sfGFP fragments cut at 1-7/8-11 and 1-10/11 within its 11 β-sheets (Figure 1B). The 1-7/8-11 split site is frequently used in the BiFC (bimolecular fluorescence complementation) method (Kerppola, 2008, Shyu and Hu, 2008), whereas the 1-10/11 split site is used for all previous intercellular probes (Feinberg et al., 2008, Kim et al., 2012, Tsetsenis et al., 2014). In this system, we found that the 1-7/8-11 combination possessed higher reconstitution activity than the 1-10/11 combination (Figure S1). Moreover, because there are no endogeneous receptor-ligand molecular interactions in the system, we introduced a leucine zipper domain in both NT- and CT-probes as to facilitate GFP reconstitution (Figure 1A). We fused an acidic leucine zipper domain to the NT-probe and basic leucine zipper domain to the CT-probe, to promote trans-molecular (acidic-basic) over cis-molecular (acidic-acidic or basic-basic) interactions (O'Shea et al., 1993). This probe contains an N-terminal mouse preproacrosin signal peptide (24 amino acids [aa]: SP) followed by a split-GFP fragment, leucine zipper domain, and mouse Thy-1 GPI anchor domain (C-terminal 31 aa) (Figure 1A). Based on the design of the probe, we named it GRAPHIC (GPI anchored reconstitution-activated proteins highlight intercellular connections), with the notation of NT- and CT-probes as n- and c-GRAPHIC, respectively.
Figure 1.
Design of GRAPHIC and Its Signal Pattern
(A) Diagram of GRAPHIC molecular structures. GRAPHIC molecules consist of signal peptide (SP), split sfGFP fragment, leucine zipper domains (LZA or LZB), and GPI anchor domain. To identify and estimate probe expression, H2B-mCherry and H2B-Azurite were co-expressed with NT-probe and CT-probe, respectively.
(B) GFP molecule consists of 11 β-sheets and fluorophore domain (FL). sfGFP split site for GRAPHIC is between seventh and eighth β-sheet (7/8). Other intercellular probe systems utilize 10/11 split site.
(C) Diagram of GRAPHIC labeling intercellular contact. GFP molecules are reconstituted by intercellular interaction of a set of probe molecules at cell-cell contact site.
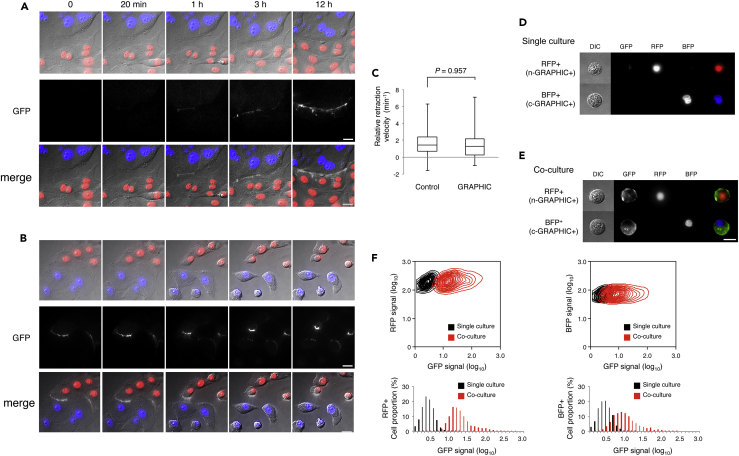
To test the sensitivity of GRAPHIC and to compare with other indicators for mammalian intercellular contacts, we first assessed the GRAPHIC and mGRASP systems (the mammalian variant of the GRASP system) in epithelial cells (LLCPK1) (Figure 2A). All GRAPHIC and mGRASP probes were co-expressed with nucleic fluorescent labels for normalization of probe expression level. GRAPHIC showed a strong signal at the cell-cell contact sites, whereas there was no significant signal observed in mGRASP under identical imaging conditions. To test whether change of GFP split site also affects mGRASP signal intensity, we generated modified mGRASP with 1-7/8-11 sfGFP fragments instead of the original 1-10/11 fragments. Although the modified mGRASP significantly increased signal intensity, it was still much weaker than GRAPHIC (Figure 2). As mGRASP is designed to express most efficiently in neurons, it is possible that the improved GRAPHIC signal is specific to LLCPK1 cells. To test this, we used a mouse neuroblastoma cells line, N2A cells, to express both probes and observed similar results (data not shown). These results demonstrate that the 1-7/8-11 GFP split site, the unique leucine zipper interaction, and GPI anchor tethering of GRAPHIC provides higher signal intensity than the GRASP system, suggesting that GRAPHIC may be a more sensitive indicator for intercellular interactions to visualize cell-cell contact domain.
Figure 2.
Comparison of GRAPHIC with Other Probes
(A) GRAPHIC showed higher signal intensity in LLCPK1 epithelial cell culture. Modification of GFP split site (10/11 to 7/8) in mGRASP system increased its signal intensity. All three culture and image acquisition conditions are same.
(B) Quantification and comparison of GRAPHIC (n = 57) and modified mGRASP (7/8) (n = 66) signal intensity. GFP signals were normalized with co-expressed nuclei label intensities of contacted cells. GFP-NT fragment (post-mGRASP and n-GRAPHIC)-expressing cells are red nuclei (H2B-mCherry, attached with 2A peptide) and GFP-CT fragment (pre-mGRASP and c-GRAPHIC)-expressing cells are blue nuclei (H2B-Azurite, attached with 2A peptide). ∗∗∗p = 1.09 × 10−24; Student's unpaired t test. Scale bars, 20 μm.
Spatiotemporal Dynamics of GRAPHIC Signal
To quantify the speed of GFP reconstitution by GRAPHIC, we performed time-lapse imaging using the LLCPK1 cell line. An initial GRAPHIC signal was detected 1 h after cell-cell contact, and its intensity gradually increased at cell-cell contact sites over 12 h (Figure 3A and Video S1). To determine the GRAPHIC signal stability, we next observed GRAPHIC signal dynamics when intercellular contacts were disrupted by ion chelation (Figure 3B, Video S2, Figure S2). Time-lapse imaging revealed that the GRAPHIC signal was not abolished, but still remained on retracted plasma membrane of n- and c-GRAPHIC-expressing cells for at least 1 h after ion chelation. Although GRAPHIC signal complexes are formed at cell-cell contact sites, its robustness may artificially strengthen cell adhesion. To address this issue, we estimated cell detachment rates of LLCPK1 cells with and without expression of GRAPHIC molecules and could not observe significant effect of GRAPHIC on cell adhesion (Figure 3C). Considering that formation of juxtamembrane complexes and anchoring to the cytoskeleton are necessary for adhesion molecules, such as cadherins, to function effectively (Leshchyns'ka and Sytnyk, 2016, Yonemura, 2017), GRAPHIC is unlikely to generate significant force at cell-cell contact sites due to its lack of intracellular domain. Moreover, fluorescence-activated cell sorting analysis showed that the GRAPHIC signals induced by co-culturing the two cell populations were detectable after complete cell dissociation (Figures 3D–3F). These results indicate that GRAPHIC has specific GFP reconstitution activity at cell-cell contact sites, and its signal complex is stable and can remain on either cell until it is degraded even after dissolution of the intercellular contacts.
Figure 3.
Characteristics of GRAPHIC Signal
(A and B) Time-lapse images of GRAPHIC signal in constructing (A) or disrupting (B) intercellular contact between n-GRAPHIC-expressing LLCPK1 cells (red nuclei) and c-GRAPHIC-expressing LLCPK1 cells (blue nuclei). Upper panels are merged images of bright-field (differential interference contrast), RFP and BFP fluorescence. Bottom panels are GFP fluorescent images. In (A), two cell lines first contacted at time 0. In (B), EDTA was administrated at time 0 (final concentration; 5mM).
(C) Quantification of relative membrane retraction velocity during 10–12 min after EDTA ion chelation. H2B-mCherry-expressing (without GRAPHIC) LLCKP1 cells (n = 51) were used as control. Membrane retraction velocity of GRAPHIC was calculated between n- and c-GRAPHIC-expressing cells (n = 33). Student's unpaired t test.
(D-F) GRAPHIC signal still remains in completely dissociated cells. Dissociated (with 5 mM EDTA, without trypsin) LLCPK1 cells from single culture of RFP+ (n-GRAPHIC expressing) or BFP+ (c-GRAPHIC expressing) cell line and co-culture of both cell lines were subjected to flow cytometry (single cultured RFP+; n = 4751, single cultured BFP+; n = 4851, co-cultured RFP+; n = 6062, co-cultured BFP+; n = 5131). Both microscope observation (D and E) and histogram of GFP intensity (F) of sorted cells showed co-culture dependent GRAPHIC signal in dissociated single cell. Scale bars, 20 μm.
This video shows the signal distribution of GRAPHIC and change in its intensity during establishment of the intercellular contact between n-GRAPHIC-expressing LLCPK1 cells (red nuclei) and c-GRAPHIC-expressing LLCPK1 cells (blue nuclei). Length of video, 12 h.
This video shows dynamics in distribution and intensity of GRAPHIC signal when intercellular contact between n--GRAPHIC-expressing LLCPK1 cells (red nuclei) and c--GRAPHIC-expressing LLCPK1 cells (blue nuclei) is disrupted by ion chelation. Length of video, 1 h.
Color Multiplexing of GRAPHIC
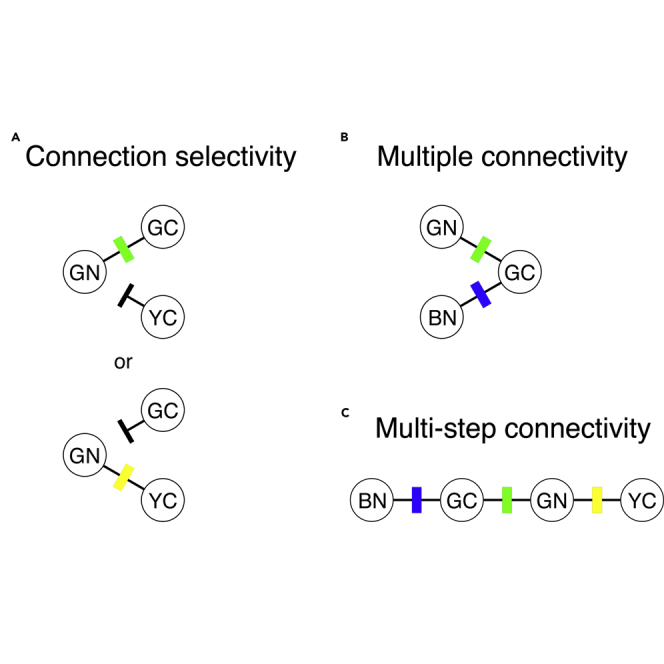
Tissues and organs consist of heterogeneous cell types, therefore, to clarify their developmental and functional mechanisms, identification of contact specificity and selectivity among multiple cells is important. Multicolored labeling is an advantageous strategy to detect multiple contacts simultaneously, and combination dependency of multicolored BiFC method (Hu and Kerppola, 2003) is suitable for identification of contact selectivity, therefore we developed GRAPHIC color variants.
GFP has several color variants (BFP, CFP, YFP, etc.), and their fluorescent characteristics depend on specific point mutations (Pakhomov and Martynov, 2008, Shaner et al., 2007). Combination-dependent color variation of a GFP reconstitution system utilizes GFP diversity and is a useful application to obtain multiple data simultaneously (Hu and Kerppola, 2003). Considering that the critical mutation for BFP is within the GFP 1-7 fragment, and for YFP, within the GFP 8-11 fragment, we designed these color probe molecules (XFP-NT (XN), XFP-CT (XC)) and tested them in LLCPK1 cells. As expected, reconstituted signal between BN-probe-expressing cells and GC-probe-expressing cells showed blue fluorescence, which is distinguishable from GFP reconstitution (Figures 4A–4C). Next, we tested CT-probe-dependent color variant, and the reconstitution signal of GN- and YC-probe clearly showed a fluorescent spectrum shift from GFP to YFP (Figures 4D–4H). Thus, we generated distinguishable GRAPHIC color variants, which can visualize combinations of connected cell types. Also, distinct from previous indicators, GRAPHIC provides NT-probe- or CT-probe-dependent color variant. This enables us to identify which connections have been selected among many prospective candidates by observation of the color of contacted sites.
Figure 4.
Development of Color Variants of GRAPHIC
(A–F) Fluorescent character of BFP is mainly dependent on GFP-NT fragment region (GFP 1-7). Substitution of eight amino acid residues in GFP 1-7, I39N, T65S, Y66H, S72A, K105T, T128V, V150I, D155V altered the fluorescent character of reconstituted signal, GFP to BFP. GFP-type combination (GN + GC) signal at cell-cell contact sites of LLCPK1 could be detected with microscope filter set for GFP detection (excitation 465-485 nm, emission 502-534 nm) (C), but not for BFP (excitation 355-405 nm, emission 420-480 nm) (B), whereas BFP-type combination (BN + GC) signal could be detected with microscope filter set for BFP (E), but not for GFP (F).
(G and H) Fluorescent character of YFP is mainly dependent on T203I amino acid substitution of GFP, which is within GFP-CT fragment region (GFP 8-11). (H) Comparing fluorescent spectrums at cell-cell contact sites of GFP- (GN + GC) and YFP-type combination (GN + YC), T203I substitution in GFP 8-11 domain of c-GRAPHIC shifted reconstituted signal character to YFP-like longer wavelength. Error bars, ± SD.
(I–N) Co-culture of three LLCPK1 cell lines (GN cells express n-GRAPHIC and H2B-mCherry, GC cells express only c-GRAPHIC, and YC cells express YFP type c-GRAPHIC and H2B-Azurite) showed that the GRAPHIC system simultaneously detected multiple connectivity in one cell. (I) GN* cell contacts with both GC cell and YC cell. (J) Fluorescent spectra at points 1 and 2 showed GFP-like and YFP-like characters, respectively. (K) Ratiometric image of reconstituted signal intensities at 510 nm (gated 505–515 nm) (L) and 525 nm (gated 520–530 nm) (M). GN* cell contacts with both GC cell and YC cell, and each contact region can be separated by its fluorescent character (K and N). Scale bars, 20 μm.
To clarify the patterns of highly heterogeneous connections, the simultaneous detection of multiple convergent connections upon single cells is crucial. For multicolored display of multiple intercellular connections in one cell, we co-cultured cells transfected with GN-, GC-, and YC-probe into red (H2B-mCherry), colorless, and blue (H2B-Azurite) nuclei LLCPK1 cells, respectively. At the junctions of these three cell types, reconstituted signals showed GFP fluorescent characteristics at GN-GC contact site and YFP characteristics at GN-YC contact site (Figures 4I–4M). Even in one cell, GRAPHIC color variants clearly showed individual contact sites with different colors suggesting that multiple types of intercellular connections, such as multistep connections and multiple convergent connections, may be separated and identified simultaneously (Figure 4N).
Taken together, further development of color variant GRAPHIC has the possibility of being a powerful tool to investigate multiple heterogeneous connections in multicellular organisms.
GRAPHIC Enables the Visualization of Neuronal Connectivity
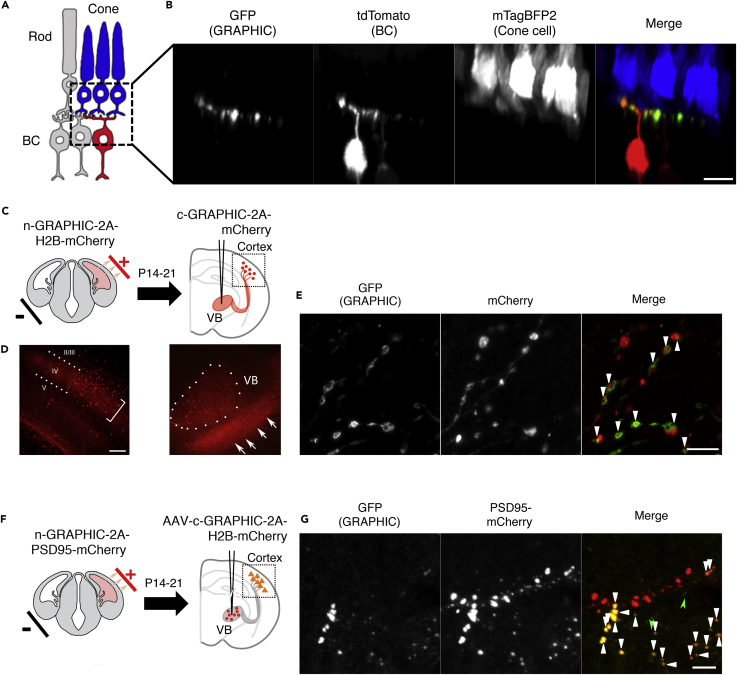
We have shown that GRAPHIC delineates cell-cell contact site efficiently and precisely in vitro. To further investigate whether GRAPHIC can be used in specific intercellular contacts in vivo, we first tested GRAPHIC in cone-bipolar cell connections in the zebrafish retina, whose connectivity is well known (Figure 5A). n-GRAPHIC and membrane-targeted tdTomato were co-expressed in bipolar cells (vsx1 promoter, Randlett et al., 2013), whereas c-GRAPHIC and mTagBFP2 were co-expressed in cone cells (gnat2 gene promoter, Kennedy et al., 2007) (Figure 5A). In 5 days postfertilization retina, GRAPHIC fluorescence was only observed within the synaptic region where the dendritic tips of tdTomato-positive bipolar cells associated with mTagBFP-positive cone pedicles (Figure 5B). Next, we applied GRAPHIC to mouse central nervous system (CNS) to test if GRAPHIC can detect specific synaptic site in more complicated neuronal circuit. Considering in highly myelinated neural circuits, neuron-neuron contact areas are known to be mostly limited to synapses. Therefore it will be a good system to test the specificity of GRAPHIC in CNS to detect synapses without carrying synaptic localization signal. First, we tested localization of GRAPHIC signal in the synapses of the mouse thalamocortical circuit. The primary somatosensory cortex (S1) layer IV neurons receive dense innervation of thalamocortical axons (TCAs) from the ventrobasal (VB) thalamus and form synaptic connections (Lopez-Bendito and Molnar, 2003, Wu et al., 2011), making this an ideal circuit to assess the ability of GRAPHIC to delineate synaptic connections. To label cortical and thalamocortical neurons, in utero electroporation (IUE) and Adeno-associated Virus (AAV) injection were performed, respectively (Figure 5C). Cortical layer neurons are transfected n-GRAPHIC-2A-H2B-mCherry by IUE at embryonic day (E) 13.5. At 2–3 weeks after birth electroporated mice were stereotaxically injected with c-GRAPHIC-2A-mCherry encoding AAV into the VB thalamus. Transfected neurons, visualized by nuclear-localized mCherry, were distributed in cortex, mostly in layer IV (Figure 5D left). Transfected neurons are seen in the VB thalamus by mCherry signal, and TCAs were detected by a diffuse mCherry signal in the thalamus (Figure 5D right, arrows) and cortical layer IV (Figure 5D left, bracket). Confocal images of cortical layer IV showed scattered GFP signal along mCherry-positive TCAs surrounding mCherry-positive bouton-like structures (Figure 5E, arrowheads). Interestingly there are no synaptic boutons in layer V and no GRAPHIC signal on mCherry-positive TCA in layerV, which suggest synapse specific GFP reconstitution by GRAPHIC. These results suggest that GFP reconstitution only occurs in specific regions, most likely postsynaptic sites. To test this more directly we replaced H2B-mCherry with PSD95-mCherry in n-GRAPHIC to label the postsynaptic density. Cortical neurons were transfected n-GRAPHIC-2A-PSD95-mCherry by IUE at E13.5. To distinguish mCherry-labeled TCA terminals and PSD95-mCherry signal in cortex, VB neurons were labeled by c-GRAPHIC-2A-H2B-mCherry encoding AAV for this experiment (Figure 5F). In confocal images of cortical layer IV we observed a punctate GRAPHIC GFP signal, which largely overlapped with the PSD95-mCherry labeled postsynaptic sites (83.1% ± SD 4.5%, 12 regions of 2 animals) (Figure 5G). The GFP puncta, which did not co-localize with PSD-95, were smaller in size than those that overlapped (Figure 5G, green arrow heads), suggesting these are possibly immature synapses or residual GRAPHIC signal of disrupted synapses. Finally, to test whether GRAPHIC has preference for pre- or postsynaptic neurons, we swapped the position of n-GRAPHIC (VB: presynaptic) and c-GRAPHIC (cortical neurons: postsynaptic) (Figure 5C) and observed staining patterns identical to those seen in Figure 5E (data not shown). These experiments demonstrate that GRAPHIC shows strong GFP reconstitution activity without any orientation preference for pre- or postsynaptic neurons.
Figure 5.
GRAPHIC Visualizes Synaptic Connection in Neuronal Networks
(A and B) GRAPHIC labels synaptic sites in zebrafish retina. (A) In the zebrafish retina, typical structures, ribbon synapses, are formed between cone photoreceptor axon terminals, namely, pedicles and dendrites of bipolar cells. n-GRAPHIC and membrane-targeted tdTomato were co-expressed in a subpopulation of bipolar cells using the vsx1 promoter (vsx1: memtdTomato-2A-n-GRAPHIC), and c-GRAPHIC and TagBFP2 were co-expressed in cone cells using the guanine nucleotide-binding protein G(t) subunit alpha-2 (gnat2) gene promoter (gnat2: TagBFP2-2A-c-GRAPHIC). Both expression vectors were co-injected into one-cell-stage zebrafish embryos and then fixed at 5 days postfertilization. (B) Reconstituted GFP signals were only detected at the dendritic tips of tdTomato-positive bipolar cell closely associated with TagBFP2-expressing cone pedicle. Scale bar, 5 μm.
(C–E) GRAPHIC signals in S1 layer IV neurons are merged with thalamocortical axons of VB neurons in mouse brain. (C) In utero electroporation (IUE) was performed at E13.5–14.5 to express n-GRAPHIC-2A-H2B-mCherry in cortical layer IV. After the electroporated mice had grown up to P14-21, c-GRAPHIC-2A-mCherry encoding AAV was stereotaxically injected into VB. After 30–40 days, the injected brains were sectioned and observed. (D) Certain number of cortical layer IV neurons (bracket) expressed n-GRAPHIC (indicated by co-expressed red nuclei label, dot line) and many mCherry labeled fibers of c-GRAPHIC expressing VB neurons reached to cortical layer IV (white arrows). (E) Higher magnification of confocal images of layer IV showed many GFP puncta largely overlapped with mCherry-positive bouton-like structures. GFP puncta that co-localize with mCherry-positive axons are indicated by arrowheads. Scale bars, 200 μm in (D) and 5 μm in (E).
(F) IUE was performed at E13.5 to express n-GRAPHIC-2A-PSD95-mCherry in cortical layer IV. After the electroporated mice had grown to adult (about 2-month-old), c-GRAPHIC-2A-H2B-mCherry encoding AAV was stereotaxically injected into thalamus VB. After about 4 weeks, the injected brains were sectioned and observed.
(G) Distribution of GRAPHIC signals in cortical layer IV neurons. GFP (GRAPHIC) signals indicate contacted sites between n-GRAPHIC-expressing layer IV neurons and c-GRAPHIC-expressing VB neurons. PSD95-mCherry signals indicate postsynaptic sites of electroporated layer IV neurons. GFP puncta that co-localize with PSD95-mCherry puncta are indicated by arrowheads. Scale bar, 5 μm.
Taken together, GRAPHIC is a robust approach to delineate the synaptic connections in various neural circuits of various vertebrates, and thus GRAPHIC will be a useful tool to analyze the complex connectivity in vivo.
Discussion
Here we have generated a fluorescent probe system, GRAPHIC, which highlights specific intercellular interactions based on contact-dependent GFP reconstitution. The GRAPHIC system does not utilize cell-type-specific molecular interactions, and its unique and optimized molecular structure showed high GFP reconstitution activity. Moreover, color variant GRAPHICs revealed specific junctions between different pairs of cells depending on probe combinations. In this study, we demonstrated that GRAPHIC could be applied to the visualization of synaptic connectivity in neural circuits of different vertebrates. We anticipate that this will enable the identification of specific intercellular contacts in highly heterogeneous multicellular interactions of various tissues and animals.
The molecular structure of GRAPHIC is distinct from previous probes designed for detecting vertebrate intercellular contact with GFP reconstitution. GRAPHIC utilized 1-7/8-11 split sfGFP, whereas all other trans-synaptic GFP reconstitution probes utilize 1-10/11 split site (Feinberg et al., 2008, Kim et al., 2012, Tsetsenis et al., 2014). Our present study revealed that the 1-7/8-11 split pair in the mGRASP system (mGRASP(7/8)) showed higher reconstitution activity than 1-10/11 split pair. In the detection of intracellular molecular interactions, 1-7/8-11 is the most popular split site owing to its higher reconstitution activity, although it may increase non-specific molecular interactions (Kerppola, 2008, Shyu and Hu, 2008). To detect intercellular contacts, however, GFP is theoretically not reconstituted unless the cell-cell distance becomes close enough for intercellular molecular interaction. Therefore higher reconstitution activity is desirable for the visualization of intercellular connectivity, especially when its contact area is small or its contact duration is short.
While the selection of the GFP split site is a major factor for the improved signal intensity in GRAPHIC, other optimizations contribute to its increased sensitivity. The GPI anchor domain promotes effective membrane display of GRAPHIC molecules, and the leucine zipper domain facilitates GFP reconstitution from split fragments. In the comparison of reconstitution activity with modified mGRASP (7/8), these unique molecular structures showed their superiority for intercellular GFP reconstitution.
For labeling synaptic connections, all other probes utilize a pair of pre- and postsynaptic membrane proteins, neurexin and neuroligin (Feinberg et al., 2008, Kim et al., 2012, Liu et al., 2013, Martell et al., 2016, Tsetsenis et al., 2014), which clearly label precise synaptic sites (Choi et al., 2018). However, utilization of intact cytoplasmic domains of neurexin and neuroligin may generate artificial pre- and postsynaptic complexes and affect synaptic morphogenesis (Biederer and Sudhof, 2001, Craig and Kang, 2007, Irie et al., 1997). In the GRAPHIC system, the lack of a cytoplasmic domain prevents such complex formation, whereas its extracellular region is designed specifically for interactions between GRAPHIC molecules, minimizing disruption of intercellular signaling. Furthermore, GRAPHIC has no preferential cell types, allowing us to label not only neuron-neuron interactions but also potentially neuron-glia or glia-glia interactions. Furthermore, GRAPHIC could be applied to the detection of intercellular contacts in other systems, such as thymic education of T cells or cell adhesion changes in carcinogenesis. In addition, the remaining GRAPHIC signal after cell dissociation (Figures 3B and 5F) may be utilized to record past transient intercellular interactions. Thus the unique molecular structure of GRAPHIC enables its broad versatility in the investigation of diverse intercellular contacts.
In this study, we showed that the GRAPHIC system visualized synaptic connections in different species and organs with various gene expression systems. Although this system has no synaptic localization signal, in myelinated neural circuits, GRAPHIC delineated intercellular connections between neurons. In the zebrafish retina, GRAPHIC signal specifically accumulated within the synaptic region between cone and bipolar cells. Furthermore, in mouse brain, a large population of GRAPHIC signal co-localized with synaptic sites in S1 layer IV (Figures 5C–5G). These results indicate that GRAPHIC can be a widely effective method to investigate in vivo connectivity of vertebrate nervous systems.
GRAPHIC is also capable of analyzing higher-order connectivity consisting of more than three cell types with its combination-dependent color variation. We generated both NT-probe-dependent variant (BFP) and CT-probe-dependent variant (YFP). Considering that the essential amino acid substitutions for some GFP variants (e.g., YFP, Sapphire, Ehrig et al., 1995) are in 10th β-sheet, the 1-7/8-11 split site allows us to generate CT-probe-dependent color variants. This multicolor combinatorial variety makes it possible to identify contact selectivity and detect multiple convergent connections and multistep connectivity. These GRAPHIC technical features will be important to clarify the heterogeneous complex cell-cell connections. Together with higher sensitivity and successful visualization of in vivo neural circuit connectivity, GRAPHIC will be a useful tool to analyze various intercellular contacts, especially within highly complicated systems, such as the nervous system.
Limitations of Study
As GRAPHIC does not have synapse localization signal, we do not know if it can be used in developing brains to visualize newly formed synapses.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank G. Kondoh (Kyoto University) for cDNAs of mouse preproacrosin signal peptide and mouse Thy-1 GPI-anchored domain, T. Kanda (Aichi Cancer Center Research Institute) for cDNA of human histone H2B, R. Tsien (UC San Diego) for cDNA of mCherry, S. Yonemura (RIKEN CDB) for LLCPK1 cell, and H. Miyoshi (RIKEN BRC) for lentiviral vectors. We thank T.R. Young for comments on this paper, S.S. Kikuchi for animal care, and A.C. Yoshida for technical assistance. This work was supported by the RIKEN CBS (to T.J.M., A.M., and T.S.) and OIST (to I.M.). This work was supported by ERATO-JST (to A.M.), Grant-in-Aid for Scientific Research on Innovative Areas “Dynamic regulation of brain function by Scrap &Build system,” 16H06459 (T.S.), and Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K14329 (to N.K.).
Author Contributions
N.K. designed GRAPHIC components and performed cell biological assays, animal surgery, imaging, and data analysis. A.J.Y.H. and T.J.M. developed AAV and performed stereotaxic injections. I.H.K. and S.H.S. performed contralateral S1 connection experiment. S.C.S. and I.M. performed zebrafish retina experiment. N.K., A.M., and T.S. wrote the paper. All authors discussed and commented on the manuscript.
Declaration of Interests
The authors declare no competing financial interest.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.013.
Supplemental Information
References
- Baes M., Denef C. Evidence that stimulation of growth hormone release by epinephrine and vasoactive intestinal peptide is based on cell-to-cell communication in the pituitary. Endocrinology. 1987;120:280–290. doi: 10.1210/endo-120-1-280. [DOI] [PubMed] [Google Scholar]
- Biederer T., Sudhof T.C. CASK and protein 4.1 support F-actin nucleation on neurexins. J. Biol. Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- Cabantous S., Terwilliger T.C., Waldo G.S. Protein tagging and detection with engineered self-assembling fragments of green fluorescent protein. Nat. Biotechnol. 2005;23:102–107. doi: 10.1038/nbt1044. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Matthews H.K., Kuriyama S., Moreno M., Dunn G.A., Parsons M., Stern C.D., Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Sim S.E., Kim J.I., Choi D.I., Oh J., Ye S., Lee J., Kim T., Ko H.G., Lim C.S. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–435. doi: 10.1126/science.aas9204. [DOI] [PubMed] [Google Scholar]
- Craig A.M., Kang Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig T., O'Kane D.J., Prendergast F.G. Green-fluorescent protein mutants with altered fluorescence excitation spectra. FEBS Lett. 1995;367:163–166. doi: 10.1016/0014-5793(95)00557-p. [DOI] [PubMed] [Google Scholar]
- Feinberg E.H., Vanhoven M.K., Bendesky A., Wang G., Fetter R.D., Shen K., Bargmann C.I. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Gordon M.D., Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S.J., Peles E., Pawson T., Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase β. Curr. Opin. Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- Hu C.D., Kerppola T.K. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat. Biotechnol. 2003;21:539–545. doi: 10.1038/nbt816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M., Hata Y., Takeuchi M., Ichtchenko K., Toyoda A., Hirao K., Takai Y., Rosahl T.W., Sudhof T.C. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Kennedy B.N., Alvarez Y., Brockerhoff S.E., Stearns G.W., Sapetto-Rebow B., Taylor M.R., Hurley J.B. Identification of a zebrafish cone photoreceptor-specific promoter and genetic rescue of achromatopsia in the nof mutant. Invest. Ophthalmol. Vis. Sci. 2007;48:522–529. doi: 10.1167/iovs.06-0975. [DOI] [PubMed] [Google Scholar]
- Kerppola T.K. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol. 2008;85:431–470. doi: 10.1016/S0091-679X(08)85019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zhao T., Petralia R.S., Yu Y., Peng H., Myers E., Magee J.C. mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat. Methods. 2012;9:96–102. doi: 10.1038/nmeth.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchyns'ka I., Sytnyk V. Reciprocal interactions between cell adhesion molecules of the immunoglobulin superfamily and the cytoskeleton in neurons. Front. Cell Dev. Biol. 2016;4:9. doi: 10.3389/fcell.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.S., Loh K.H., Lam S.S., White K.A., Ting A.Y. Imaging trans-cellular neurexin-neuroligin interactions by enzymatic probe ligation. PLoS One. 2013;8:e52823. doi: 10.1371/journal.pone.0052823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G., Molnar Z. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Makhijani K., Alexander B., Rao D., Petraki S., Herboso L., Kukar K., Batool I., Wachner S., Gold K.S., Wong C. Regulation of Drosophila hematopoietic sites by Activin-beta from active sensory neurons. Nat. Commun. 2017;8:15990. doi: 10.1038/ncomms15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell J.D., Yamagata M., Deerinck T.J., Phan S., Kwa C.G., Ellisman M.H., Sanes J.R., Ting A.Y. A split horseradish peroxidase for the detection of intercellular protein-protein interactions and sensitive visualization of synapses. Nat. Biotechnol. 2016;34:774–780. doi: 10.1038/nbt.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R., Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319–328. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A.I., Yap A.S. Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 2012;24:685–694. doi: 10.1016/j.ceb.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Miller J.F.A.P., Basten A. Mechanisms of tolerance to self. Curr. Opin. Immunol. 1996;8:815–821. doi: 10.1016/s0952-7915(96)80010-0. [DOI] [PubMed] [Google Scholar]
- O'Shea E.K., Lumb K.J., Kim P.S. Peptide 'Velcro': design of a heterodimeric coiled coil. Curr. Biol. 1993;3:658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- Pakhomov A.A., Martynov V.I. GFP family: structural insights into spectral tuning. Chem. Biol. 2008;15:755–764. doi: 10.1016/j.chembiol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Pasqual G., Chudnovskiy A., Tas J.M.J., Agudelo M., Schweitzer L.D., Cui A., Hacohen N., Victora G.D. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature. 2018;553:496–500. doi: 10.1038/nature25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedelacq J.D., Cabantous S., Tran T., Terwilliger T.C., Waldo G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- Pires-daSilva A., Sommer R.J. The evolution of signalling pathways in animal development. Nat. Rev. Genet. 2003;4:39. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- Randlett O., MacDonald R.B., Yoshimatsu T., Almeida A.D., Suzuki S.C., Wong R.O., Harris W.A. Cellular requirements for building a retinal neuropil. Cell Rep. 2013;3:282–290. doi: 10.1016/j.celrep.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Huang H., Liu S.M., Kornberg T.B. Cytoneme-mediated contact-dependent transport of the drosophila decapentaplegic signaling protein. Science. 2014;343:1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P., Fan J.H., Choih J., Fetter R., Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shaner N.C., Patterson G.H., Davidson M.W. Advances in fluorescent protein technology. J. Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- Shyu Y.J., Hu C.D. Fluorescence complementation: an emerging tool for biological research. Trends Biotechnol. 2008;26:622–630. doi: 10.1016/j.tibtech.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T., Boucard A.A., Arac D., Brunger A.T., Sudhof T.C. Direct visualization of trans-synaptic neurexin-neuroligin interactions during synapse formation. J. Neurosci. 2014;34:15083–15096. doi: 10.1523/JNEUROSCI.0348-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuys N. TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation. Front. Immunol. 2016;7:6. doi: 10.3389/fimmu.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F., Aramuni G., Rawson R.L., Mohrmann R., Missler M., Gottmann K., Zhang W., Südhof T.C., Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wu C.S., Ballester Rosado C.J., Lu H.C. What can we get from 'barrels': the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur. J. Neurosci. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S. Actin filament association at adherens junctions. J. Med. Invest. 2017;64:14–19. doi: 10.2152/jmi.64.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows the signal distribution of GRAPHIC and change in its intensity during establishment of the intercellular contact between n-GRAPHIC-expressing LLCPK1 cells (red nuclei) and c-GRAPHIC-expressing LLCPK1 cells (blue nuclei). Length of video, 12 h.
This video shows dynamics in distribution and intensity of GRAPHIC signal when intercellular contact between n--GRAPHIC-expressing LLCPK1 cells (red nuclei) and c--GRAPHIC-expressing LLCPK1 cells (blue nuclei) is disrupted by ion chelation. Length of video, 1 h.