Abstract
Didecyldimethylammonium chloride (DDAC) is an antimicrobial dialkyl-quaternary ammonium compound used in industrial and commercial products. Clinical data suggest that DDAC exposure elicits multiple types of hypersensitivity reactions; here, we confirm this observation in a BALB/c murine model. To examine the immunological mechanism behind this mixed-type response and the potential involvement of type 2 innate lymphoid cells (ILC2s), we assessed early immune responses in the skin following topical DDAC exposure (0.125% and 0.5%). DDAC exposure resulted in a rapid and dramatic increase in the Th2-skewing and ILC2 activating cytokine thymic stromal lymphopoietin. Correspondingly, dermal ILC2s were activated 24 hours after DDAC exposure, resulting in increased expression of CD25, ICOS and KLRG1, and decreased CD127 throughout 7 days of exposure. Following ILC2 activation, the Th2 cytokine IL-4 was elevated compared to control mice in total ear protein lysate (0.5% DDAC). Rag2−/− mice were used to determine a functional role for ILC2s in DDAC induced sensitization. ILC2s from Rag2−/− mice were similarly activated by DDAC and, importantly, produced significant levels of IL-4 and IL-5 in the skin (0.5% DDAC). These data indicate that ILC2s contribute to early Th2 immune responses following DDAC exposure. ILC2s have been previously implicated in allergic responses, but to our knowledge have not been thoroughly investigated in chemical sensitization. These results indicate that following DDAC exposure, skin ILC2s become activated and produce Th2 cytokines, providing a possible mechanism for the development of the mixed-type allergic responses commonly observed with chemical sensitizers.
Keywords: chemical, allergy, ILC2s, mixed-type hypersensitivity, skin
Introduction:
Chemical exposure can result in allergic diseases that manifest through a variety of immunological mechanisms. While the most recognized hypersensitivity mechanisms are immediate (IgE mediated/Th2) and delayed (T-cell mediated/Th1), it is increasingly being recognized that a multitude of immunological responses can occur at once; these mixed responses may influence allergic disease outcomes and diagnostics. Low molecular weight (LMW) chemical sensitizers are traditionally classified as either respiratory or dermal sensitizers, based on sensitization site and their induction of Th2 or Th1-dominated immune responses, respectively (Dearman et al., 1996). Primarily, most chemical sensitizers are capable of causing dermal sensitization but are not respiratory sensitizers (Kimber and Dearman, 2005); conversely, many respiratory sensitizers also cause skin sensitization, leading to both allergic contact dermatitis (ACD) and asthma following sensitization.
The classification of chemical allergens, especially LMW chemical allergens, has proven to be difficult as exposure to certain chemicals can result in multiple hypersensitivity pathways (i.e. both ACD and asthma) (De Vooght et al., 2010; Matheson et al., 2005). The quaternary ammonium compound (QAC), didecyldimethylammonium chloride (DDAC) was identified by our lab to be a T-cell mediated/Th1 sensitizer (Anderson et al., 2016) using standard classifications schemes. However, clinical evidence suggests this LMW chemical can induce multiple types of hypersensitivity reactions (Geier et al., 2013; Houtappel et al., 2008; Vandenplas et al., 2013). Discordance between laboratory studies and clinical observations may partially be due to incomplete understanding of the immunological mechanisms of sensitization for LMW chemicals (Palm and Medzhitov, 2009).
Recently our laboratory determined that another QAC, didecyldimethylammonium bromide (DDAB), elicits multiple types of hypersensitivity reactions following topical application (Shane et al., 2017). Phenotypic analyses revealed significant increases in the number of immune cells in the draining lymph nodes (DLN) following 4 and 14 days of exposure with increased activation of CD4+ and CD8+ T-cells at 4-days, and elevated IgE levels only at 14 days post exposure. Additionally, significant increases in the Th2-skewing cytokine thymic stromal lymphopoietin (TSLP) were observed in the ear at both timepoints, with elevated expression observed at 14 days. These results suggest a potential role for unidentified mediators capable of influencing multiple hypersensitivity responses.
The microenvironment plays an important role in the development and regulation of immunological responses. Specifically, early signaling events in the cutaneous environment are thought to provide a bridge between the innate and adaptive immune systems, and are of pivotal importance for the initiation of immune responses in the skin, including those to chemical allergens resulting in sensitization (Ainscough et al., 2013). In general, these signals are required as a supplementary signal for the initiation of an adaptive immune response. For example, the epithelial cell derived cytokine, TSLP, promotes the production of IL-4 from CD4 T cells through the activation of dendritic cells (DCs) (Leyva-Castillo et al., 2013). Previously, our laboratory has shown that TSLP production is strongly induced in the skin following dermal exposure to the chemical adjuvant triclosan, and that TSLP plays an essential role in triclosan-induced Th2 responses (Marshall et al., 2015).
Another potential mechanism through which TSLP can influence the development of allergic diseases is through the activation of innate lymphoid cells (ILCs). ILCs are a recently defined family of lymphocytes that lack rearranged antigen-specific receptors; they are activated by innate signals including cytokines and danger signals (Artis and Spits, 2015). Type 2 ILCs (ILC2s) have been of particular interest in dermal allergic diseases, as they produce the type 2 cytokines IL-4, IL-5, and IL-13 (Roediger et al., 2014) and are found in relative abundance in the skin, even in healthy individuals (Salimi et al., 2013). ILC2s are activated by the cytokines TSLP, IL-33 and IL-25, with TSLP playing a particularly important role in the activation of skin ILC2s (Kim et al., 2013). ILC2s have been shown to modulate allergic responses both directly and indirectly, through DC licensing (Halim et al., 2016).
The specific type of immune responses elicited following chemical exposure may have consequences on long-term disease development, as Th2 cytokine production has been linked to the development of respiratory diseases such as asthma, while Th1 cytokines are associated with the ACD. Therefore, it is important to understand the mechanism(s) through which a chemical induces allergic disease in order to properly predict and evaluate the development of disease. Given the role the skin environment plays in immunological responses, the development of DDAC-induced immune responses at this site were further examined. In this study, the potential for mixed hypersensitivity responses to DDAC were examined and a potential role for ILC2s in DDAC-induced sensitization was identified.
Materials and Methods:
Animals:
Female BALB/c mice and Rag2−/− mice on a BALB/c background (C.129S6(B6)-Rag2tm1Fwa N12) were used for the murine models. The BALB/c mouse strain has a Th2 bias and is commonly used to evaluate IgE-mediated sensitization (Klink and Meade, 2003; Woolhiser et al., 2000). Rag2−/− mice lack the ability to initiate V(D)J rearrangement and therefore do not generate mature T or B cells (Shinkai et al., 1992). Rag2−/− mice have been used to study the role of the innate lymphocytes in the development of allergic diseases (Doherty et al., 2013; Halim et al., 2012). The mice were purchased from Taconic at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to a treatment group, weighed and individually identified (via tail marking) using a permanent marker or tattoo. A preliminary analysis of variance on body weights was performed to ensure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of 5/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water were provided from water bottles, ad libitum. The animal facility temperature was maintained at 68–72 °F and the relative humidity between 36–57%. A light/dark cycle was maintained on 12-hr intervals. For the Rag2−/− studies, a sterile environment was maintained. All animal experiments were performed in the AAALAC International accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Chemicals and Exposures:
Didecyldimethylammonium chloride (DDAC; CAS#7173–51-5) and acetone (CAS#67–64-1) were purchased from Sigma-Aldrich. Concentrations and time points were chosen based on observations from previous studies. The doses were chosen as they did not result in any significant morbidity for up to 14 days of exposure, and both induced sensitization as assessed by the local lymph node assay (LLNA); only the 0.5% dose induced a significant level of irritation (Anderson et al., 2016). For the Mouse Ear Swelling Test (MEST) and IgE analysis, mice were exposed as described below. For all other studies, mice were exposed to the acetone vehicle control (VC) or increasing concentrations of DDAC on the dorsal surface of each ear (50 μl/mouse;25 μl/ear) daily for the number of days indicated. Animals were euthanized by either sodium pentobarbital injection, or CO2 asphyxiation 24 hours following the last exposure.
Mouse Ear Swelling Test:
A small area of fur (1” x 1”) was removed using clippers on the dorsal side of the animals prior to dermal exposure with 50 μl of vehicle or sensitizing concentrations of DDAC (0.03%, 0.06%, 0.125%, and 0.25%) for 3 consecutive days. The mice were rested for 4 days, then challenged with 25 μl of acetone or DDAC (0.25% or 0.5%) on the dorsal surface of each ear pinna. This sensitization and challenge scheme resulted in 11 treatment groups; the first concentration listed represents the DDAC sensitization concentration while the second concentration listed represents the DDAC challenge concentration. The groups are identified as follows: 1) 0%, + 0% = Vehicle control (VC), 2) 0%+0.25% = 0.25% irritation control (0.25% IC), 3) 0% + 0.5% = 0.5% IC, 4) 0.03% + 0.25%, 5) 0.06% + 0.25%, 6) 0.125% + 0.25%, 7) 0.25% + 0.25%, 8) 0.03% + 0.5%, 9) 0.06% + 0.5%, 10) 0.125% + 0.5%, and 11) 0.25% + 0.5%. Immediately prior to the challenge, then 24 and 48 hours post-challenge, measurements were taken of ear thickness using a modified engineer’s micrometer (2 measurements per ear), and the average thickness per ear was determined. Additional challenges were performed after allowing the animals to rest for 7 days, following the steps described above. Percent change in ear thickness was calculated following guidelines determined by (Gad, 2014) and each average ear measurement was plotted as a single point for each ear on the graph. The MEST was considered positive if at least one ear of the test animal (n=5 mice/group, 2 ears/mouse) resulted in a post-challenge measurement with a 20% increase from pre-challenge.
IgE Analysis:
To further evaluate type of hypersensitivity response induced by DDAC, IgE was evaluated following dermal exposure. Since we previously identified that exposure duration may influence the development of allergic responses (Shane et al., 2017), IgE levels in mice exposed to DDAC for 14-days were assessed at 28 days post initial exposure. Mice (n=5) were treated for 14-days with DDAC and rested for 14-days. Blood samples were collected via cardiac puncture and sera was then separated by centrifugation and frozen at −20°C for subsequent analysis. To quantify serum IgE levels a standard colorimetric sandwich ELISA was performed according to manufacturer directions (Mouse IgE Ready-Set-Go!, eBioscience). To determine local production of IgE the IgE+B220+ (IgE+ B-cells) population were analyzed by flow cytometry as previously described using IgE-FITC (R35–72) (BD Biosciences) (Shane et al., 2017).
Gene expression analysis:
Ears were mechanically disrupted on a TissueLyser II in Buffer RLT (Qiagen). Total RNA was extracted using Qiagen’s RNeasy mini spin column kits with DNase treatment on a QIAcube workstation. RNA concentrations and purity were analyzed on a NanoDrop spectrophotomer (Thermo Fisher Scientific). The cDNA (1–2 μg) was prepared on an Eppendorf Mastercycler using Applied Biosystems’ High Capacity Reverse Transcription kit. The cDNA was used as template for real-time PCR reactions containing TaqMan PCR Master Mix with gene-specific primers (Applied Biosystems) on a 7500 Real-Time PCR System. Relative fold gene expression changes (2-ΔΔCT) were determined compared to acetone controls unless otherwise indicated and normalized for expression of housekeeping gene Actb. Genes that were evaluated include: tslp, il33, il4, il13, cdh1, ccl17, areg, il6, s100a7a, s100a8, s100a9, tlr4, and bcl2.
Single cell preparation and flow cytometry:
Left and right auricular draining lymph nodes (DLNs; drain the site of chemical application) were collected in 4 mL sterile phosphate-buffered saline (pH 7.4). DLN cell suspensions (2 nodes/animal) were prepared by mechanical disruption of tissues between frosted microscope slides in phosphate buffered saline (PBS) and live cells were counted on a Cellometer (Nexcelom) using acridine orange and propidium iodide solution (Nexcelom). Ear cell suspensions were prepared by splitting ears into ventral and dorsal halves, mincing into ~1 mm x 1 mm pieces followed by an enzymatic digestion for 90 min at 37°C with 0.25mg/ml Liberase-TL Research grade (Roche) in RPMI with 100μg/ml DNase I (Sigma-Aldrich). Following digestion cells were passed through a 70 μm cell strainer to make a single cell solution, washed with RPMI 10% FBS, then live cells were counted on a Cellometer (Nexcelom). For staining, single cell suspensions were resuspended in staining buffer containing α-mouse CD16/32 antibody (Fc Block) (BD Biosciences) then incubated with a cocktail of fluorochrome-conjugated antibodies specific for mouse cell surface antigens: PerCP-Cy5.5-conjugated α-CD19 (eBio1D3), α-CD3e (145–2C11,) α-Ly6-G (RB6–8C5), α-CD11b (M1/70), α-Ter119 (Ter119), α-CD11c (N418), FITC-conjugated α-CD2 (RM2–5), α-CD90.2-APC-eF780 (53–2.1), α-CD25-PE-eF610 (PC61.5), α-CD127-PE (A7R34), α-CD103-eF450 (2E7), α-KLRG1-APC (2F1) (all eBioscience), α-ICOS-PE-Cy7 (C398.4A) (Biolegend), and α-CD45-BV605 (30-F11) (BD Bioscience). Cells were then washed, fixed in Cytofix buffer (BD Biosciences), resuspended in staining buffer, and events (3,000 ILCs) were collected on an LSR II flow cytometer (BD Biosciences) and analyzed using Flowjo software v10.
Ex Vivo Lymph Node Cell Stimulations:
Lymph node cells (5 × 105) were seeded into 96-well u-bottom plates with soluble α-CD3 (BD Pharmingen) (2 μg/ml) and α-CD28 (BD Pharmingen) (2 μg/ml) in RPMI media containing L-glutamine and HEPES (Cellgro) with 10% fetal bovine serum (Hyclone), and Gentamicin solution (Sigma-Aldrich) (cRPMI). Cells were incubated for 24h in a humidified incubator (37°C/5% CO2). Cytokines in the supernatants were measured using the Th1/Th2/Th9/Th17/Th22/Treg Cytokine 17-Plex Mouse ProcartaPlex™ Panel (ThermoFisher) according to manufacturer’s instructions and data was acquired using a Luminex 200 system (Millipore).
Ear protein Analysis:
Ears (1 per mouse) were mechanically disrupted on a TissueLyser II (Qiagen) in 0.75 ml T-PER protein extraction reagent (Pierce) and soluble proteins were quantified by BCA protein assay (Pierce). Cytokines were measured using the Th1/Th2/Th9/Th17/Th22/Treg Cytokine 17-Plex Mouse ProcartaPlex™ Panel (ThermoFisher) according to manufacturer’s instructions and data was acquired using a Luminex 200 system (Millipore). TSLP, IL-4, and IL-5 were assessed by ELISA according to manufacturers’ instructions using Mouse TSLP ELISA MAX Deluxe™ Set (BioLegend), Mouse IL-4 ELISA Ready-SET-Go! (eBioscience), and BD OptEIA™ Set Mouse IL-5 (BD Biosciences), respectively.
Statistical Analyses:
For analysis, data were first tested for homogeneity using Bartlett’s Chi Square test. If homogeneous, a one-way ANOVA was conducted for single timepoint studies. If the ANOVA showed significance at p < 0.05, Dunnett’s multiple range t-test was used to compare treatment groups with the VC. For the kinetics experiments (gene expression and ILC phenotyping) a two-way ANOVA was conducted, followed by Bonferroni post-tests to compare each treatment group to its respective acetone control. For the IgE analysis in Figure 1 an unpaired Student’s t-test was used. Statistical analysis was performed using Prism v.5.0 (GraphPad Software). Statistical significance is designated by * p < 0.05, ** p < 0.01 and *** p < 0.001.
Figure 1. Exposure to DDAC indicates a mixed allergic response.
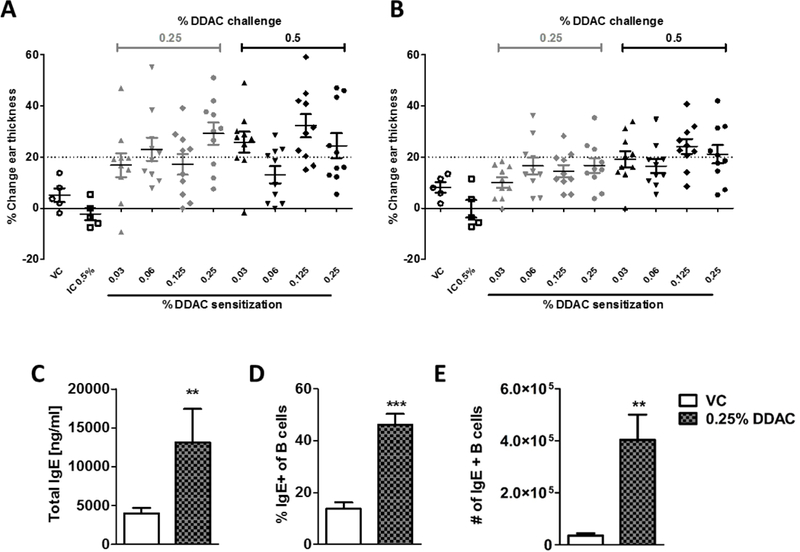
A+B) Mice were sensitized on the shaved dorsum with acetone, for the vehicle control (VC) and the irritation control (IC) or increasing concentrations of DDAC (0.03% - 0.25%), as indicated at the bottom of the x-axis. Following sensitization, mice were challenged with either acetone (VC), 0.25% DDAC (gray points) or 0.5% DDAC (black points) on the ear pinna. Each point represents the change in ear thickness (average of 2 measurements; 5 mice/group) from a pre-challenge reading measured at 24 hr (A) or 48 hr (B) post the third challenge. VC = vehicle control, IC = 0.5% DDAC irritation control. A positive response was identified by at least one subject per group exhibiting >20% swelling. C-E) Mice were exposed to DDAC daily for 2 weeks, then total IgE was measured in the serum by ELISA (C) and localized IgE production was determined in the DLNs by analyzing B220+IgE+ cells by flow cytometry (D+E). n = 5 mice/group; data was analyzed using an unpaired student’s t-test ** = p< 0.01, *** = p <0.001.
Results:
Topical exposure to DDAC induces a mixed-type allergic response
Due to varying clinical manifestations observed following DDAC exposure, the immunological mechanisms induced by topical DDAC sensitization were further assessed. To first confirm our previous finding that DDAC is a T-cell mediated contact sensitizer (Anderson et al., 2016), we performed a MEST to evaluate contact sensitization by quantitatively measuring changes in mouse ear thickness following a topical sensitization and challenge (Gad, 2014). There was no significant swelling in the VC or IC (0.25% or 0.5% DDAC). Only mild reactions (< 20% increase) were observed in the sensitized animals at 24 hours post the 0.5% challenge (Supplemental Figure 1A) and the swelling resolved by 48 hours (Supplemental Figure 1B). Upon the second (Supplemental Figure 1C and D) and third (Figure 1A and B) challenges, mice that were sensitized to even the lowest dose of DDAC (0.03%) were positive for the MEST, indicated by at least one of the mice having a >20% change in ear thickness at both 24 and 48 hours post-challenge, supporting a Th1 mediated hypersensitivity response.
To further characterize the immune response to DDAC, mice were dermally exposed to 0.125% or 0.5% DDAC for 7 consecutive days. Following 7 days of topical exposure to DDAC, DLN were collected, cells were isolated, and T cells were stimulated ex-vivo in order to assess DDAC-induced cytokine production. Similar to the results reported in our previous study, DLN cellularity was increased following exposure to all concentrations of DDAC (data not shown). All cytokines examined were significantly elevated for at least one of the DDAC concentrations following exposure with the majority being elevated following exposure to both concentrations (Table 1). DLN cells isolated from mice exposed to DDAC (0.125% and 0.5%) produced significantly higher levels of both Th1 and Th2 cytokines compared to VC. Interestingly, while the Th1 cytokines IL-2, IL-12, IFN-γ, and TNF-α overall had a higher level of induction compared to the Th2 cytokines IL-4, IL-5, and IL-13, both T-cell subsets were induced, further supporting a mixed-type allergic response to DDAC.
Table 1.
Cytokines produced by DLN cells confirm a mixed-type allergic response following 7 days of DDAC exposure
| Cytokine | Th designation | Vehicle control (pg/ml) |
DDAC (0.125%) (pg/ml) |
DDAC (0.5%) (pg/ml) |
|---|---|---|---|---|
| IL-4 | Th2 | 32.00 ± 5.32 | 94.15 ± 14.42 * | 98.00 ± 19.99 * |
| IL-5 | Th2 | 35.27 ± 13.03 | 89.42 ± 11.79 * | 58.75 ± 12.72 |
| IL-13 | Th2 | 19.77 ± 5.74 | 125.35 ± 19.39 * | 152.66 ± 33.90 ** |
| IFN-γ | Th1 | 375.40 ± 81.15 | 2037.37 ± 59.52 ** | 1532.85 ± 169.33 *** |
| IL-12p70 | Th1 | 1.54 ± 0.41 | 5.17 ± 0.33 *** | 4.86 ± 0.67 *** |
| IL-18 | Th1 | 475.06 ± 42.94 | 967.60 ± 48.48 ** | 962.30 ± 77.90 ** |
| IL-17A | Th17 | 16.70 ± 6.60 | 675.83 ± 83.02 *** | 199.19 ± 45.85 |
| IL-22 | Th17 | 1.35 ± 1.21 | 420.95 ± 92.69 ** | 130.92 ± 26.11 |
| IL-23 | Th17 | 5.05 ± 1.25 | 22.99 ± 3.11 *** | 14.62 ± 1.69 * |
| GM-CSF | Th1/Th17 | 5.19 ± 1.03 | 20.71 ± 2.09 *** | 19.65 ± 2.41 *** |
| TNF-α | Th1/Th17 | 91.65 ± 15.30 | 223.28 ± 14.51 *** | 195.92 ± 9.55 *** |
| IL-27 | Th1/Treg | 0.00 ± 0.00 | 1.48 ± 0.27 *** | 0.35 ± 0.21 |
| IL-2 | Th1/Th2/Treg | 544.45 ± 67.99 | 1095.81 ± 76.42 *** | 1104.28 ± 70.00 *** |
| IL-1β | Th1/Th2/Th17 | 0.82 ± 0.15 | 3.36 ± 0.23 *** | 3.15 ± 0.25 *** |
| IL-6 | Th1/Th2/Th17 | 44.26 ± 6.83 | 147.75 ± 14.72 *** | 182.19 ± 13.24 *** |
| IL-9 | Th9/Th2/Th17 | 36.91 ± 13.26 | 459.63 ± 32.30 *** | 249.95 ± 25.75 *** |
| IL-10 | Th1/Th2/Th17/Treg | 2.83 ± 0.57 | 19.23 ± 2.05 ** | 20.03 ± 4.42 ** |
N= 5 mice per group; statistical significance was determined using an one-way ANOVA with a Dunnett’s post-test where
= p< 0.05
= p< 0.01
= p <0.001
Since a potential Th2 involvement was suggested by the cytokine data, IgE levels were evaluated to better characterize the DDAC hypersensitivity response. Prolonged exposure to DDAC (0.25%) resulted in significant increases in total IgE measured in the serum (Figure 1C), and significant increases in the percent (Figure 1D) of and number (Figure 1E) of IgE+ B-cells in the DLNs, supporting a Th2-mediated response.
Gene expression profile indicates a role of ILC2s in DDAC sensitization
In an attempt to examine the mechanism(s) driving the mixed hypersensitivity response following DDAC exposure, early immunological responses to DDAC were evaluated in the skin, focusing on markers that indicate the involvement of ILC2s. Kinetic gene expression analysis of mRNA isolated from the ears from 0.5 to 7 days post-exposure (dpe) revealed that the dermal ILC2 activating cytokines tslp and il-33 (Kim et al., 2013; Salimi et al., 2013) were significantly elevated following DDAC exposure (Figure 2) with early elevations (0.5–1 dpe) following 0.125% DDAC exposure. Expression of tslp was strongly induced, with increases of more than 400-fold for the 0.5% DDAC treated animals and over 300-fold in the 0.125% treated animals. tslp gene expression peaked at 2–4 days post exposure in the 0.125% DDAC group and at day 4 in the 0.5% DDAC group. In addition, cdh1 (cadherin 1/e-cadherin) which is important for epidermal barrier function and is an inhibitor of ILC2 cytokine production (Salimi et al., 2013) was downregulated at 4 and 7 dpe (Figure 2). Increased expression of ccl17, il-13, and il-4, cytokines either directly or indirectly produced by ILC2s (Halim et al., 2016; Noval Rivas et al., 2016), was also observed at various times throughout the 7 day timecourse, as well as Areg (Figure 2), the gene encoding the immunoregulatory protein amphiregulin, produced by ILC2s (Monticelli et al., 2011). Together, this gene expression signature implicates ILC2s are involved early in the immune response to DDAC in the skin. In addition to ILC2s related genes, a number of immunomodulary genes were also elevated in the ear following DDAC exposure, including il6, s100a7a, s100a8, s100a9, tlr4, bcl2 (Supplemental Figure 2).
Figure 2. Gene expression kinetics indicate ILC2s may be involved in the response to DDAC.
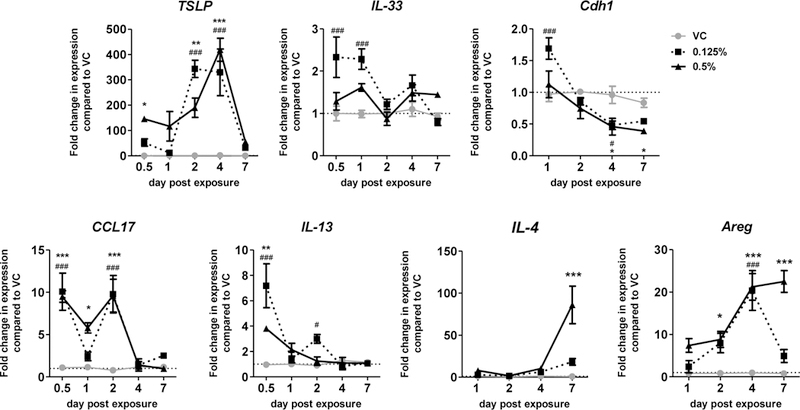
Gene expression analysis was performed on ear tissue over time following exposure to DDAC for up to 7 days. Each point represents the level of the indicated gene’s mRNA expression relative to acetone (VC), and normalized to β-actin as an endogenous control. Each dot represents mean (± SEM) of 5 mice per group. Significance was determined using a two-way ANOVA and Bonferroni post-tests to compare each treatment group to its respective acetone control. P values are represented by #s (0.125% DDAC) and *s (0.5% DDAC) where #/* = p< 0.05, ##/** = p< 0.01, and ###/*** = p <0.001.
DDAC activated type 2 innate lymphoid cells at the site of topical application
To directly assess if ILC2s are activated by DDAC as the gene expression signature suggests, cellular populations and phenotypes in the skin were evaluated by flow cytometry (Figure 3A). Treatment with DDAC resulted in a significant increase of CD45+ immune cells in the ears of mice compared to VC treated mice, indicative of cellular proliferation or infiltration at the site of chemical application (Figure 3B). ILC2s (CD45+, CD90+, Lin-, CD2-, CD25+,CD127+, ICOS+) were a clear population in the skin, making up approximately 20% of the CD90+ lymphocyte population and > 95% of the total ILC population (CD45+, CD90+, Lin-, CD2-) in the VC treated ear (Figure 3A). Although the numbers were not significantly changed at 7 days post DDAC treatment (Figure 3C), there was a decreasing trend of total ILC2s with increasing DDAC exposure. Next, ILC2s were evaluated for the expression of the activation markers CD25, ICOS and CD127, kinetically, over the course of the 7 day treatment. On a per cell basis, as assessed by median fluorescence intensity (MFI), treatment with both 0.125% and 0.5% DDAC significantly increased the expression of the IL-2 receptor alpha chain (CD25) (Figure 3D), inducible T-cell costimulatory (ICOS) (Figure 3E), and reduced expression of the IL-7 receptor alpha chain (CD127) (Figure 3F) throughout the course of DDAC exposure. Significant upregulation of CD25 was seen as early as 24 hour post exposure in the 0.5% DDAC group (Figure 3D), and in both groups between 2–7 days (Figure 3D). ICOS was significantly upregulated in ILC2 populations by 2 dpe in both treatment groups through the course of exposure (Figure 3E). CD127 was downregulated by 1 dpe in the 0.5% DDAC group, and while it was still significantly decreased at 7 dpe for the 0.5% group, the 0.25% DDAC treatment group showed a limited downregulation (days 2–4), which resolved by day 7. In addition, ILC2s upregulated KLRG1 by 7 dpe, and showed reduced levels of intergrin alpha E (CD103) (Supplemental Figure 3 A and B). Together, these data show that ILC2s are located at the site of DDAC exposure and become activated rapidly following topical treatment with DDAC, suggesting that ILC2s may play a role in the development of the mixed-type response observed following DDAC exposure.
Figure 3: Type 2 innate lymphoid cells are rapidly activated by DDAC.
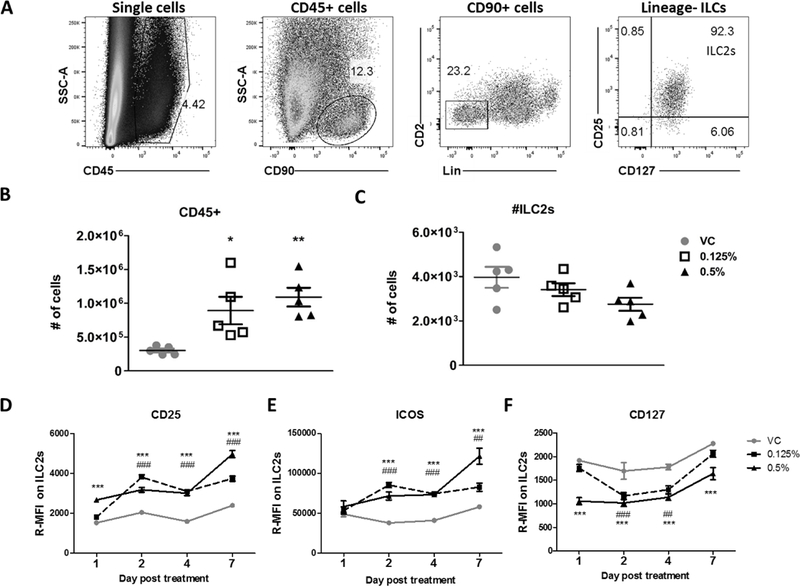
Identification and analysis of dermal innate lymphoid cells. A) Representative flow plots showing the identification of type 2 innate lymphoid cells from ear tissue from a control mouse. Doublet exclusion gating was performed prior the gating steps shown. Cells were first gated on CD45+ cells, followed by gating on CD90+ lymphocytes, CD45+CD90+Lineage-lymphocytes were then gated on cells that were positive for both CD127 and CD25, and defined as ILC2s. Total numbers of CD45+ cells (B) and ILC2s (C) isolated from the ear following treatment with the indicated concentrations of DDAC or vehicle control were quantified at day 7 post DDAC exposure. Median fluorescent intensity (relative to unstained controls) was determined for the expression of CD25 (D), ICOS (E), and CD127 (F) on ILC2s at 1, 2, 4, and 7 days post exposure. For panels B and C, statistical analysis was determined using a one-way ANOVA with a Dunnett’s post-test. For the kinetics experiments (D-F) significance was determined using a two-way ANOVA and Bonferroni post-tests to compare each treatment group to its respective acetone control. P values are represented by #s (0.125% DDAC) and *s (0.5% DDAC) where #/* = p< 0.05, ##/** = p< 0.01, and ###/*** = p <0.001.
ILC2s produce Th2 cytokines following activation by DDAC
In order to prescribe a functional role to ILC2s in the mixed-type allergic response to DDAC, we utilized a Rag2−/− mouse model. Like BALB/c mice, treatment of Rag2−/− mice for 7 days with 0.125 and 0.5% DDAC, did not result in any significant morbidity as assessed by body weight (data not shown), and increased ear thickness indicative of irritation was only observed for the 0.5% concentration (data not shown). Additionally, a similar increase of total CD45+ cells was observed, indicating that the majority of cells in the ear at 7 dpe are of the innate immune system (Figure 4B). Numbers and the activation profile of ILC2s were assessed at 7 days post exposure of DDAC, as CD25 and ICOS expression peaked at this time-point in the BALB/c mouse model (Figure 3D &E). Due to the lack of T and B cells, ILCs were identified to make up a greater proportion of Rag2−/− ear lymphocytes compared to BALB/c mice (~65% compared to ~20%, respectively), while a similar proportion of ILCs were ILC2s (Figure 3A and Figure 4A). Overall, the number of ILC2s isolated from Rag2−/− mice were higher than BALB/c mice (Figure 3C and Figure 4C) and significantly higher numbers of ILC2s were observed in the ears of Rag2−/− mice treated with 0.125% DDAC compared to the VC. However, there was no significant difference in number of ILC2 with the 0.5% treatment (Figure 4C). Similar to BALB/c mice, treatment with DDAC induced an increase in the expression of activation markers CD25 (Figure 4D) and ICOS (Figure 4E).There was no significant change in CD127 expression at the timepoints assessed (Figure 4F). Additionally, similar to the BALB/c mice, ILC2s from Rag2−/− mice showed increased levels of KLRG1, and decreased expression of CD103 (Supplemental Figure 3C &D).
Figure 4: Type 2 innate lymphoid cells are rapidly activated by DDAC in Rag2−/− mice.
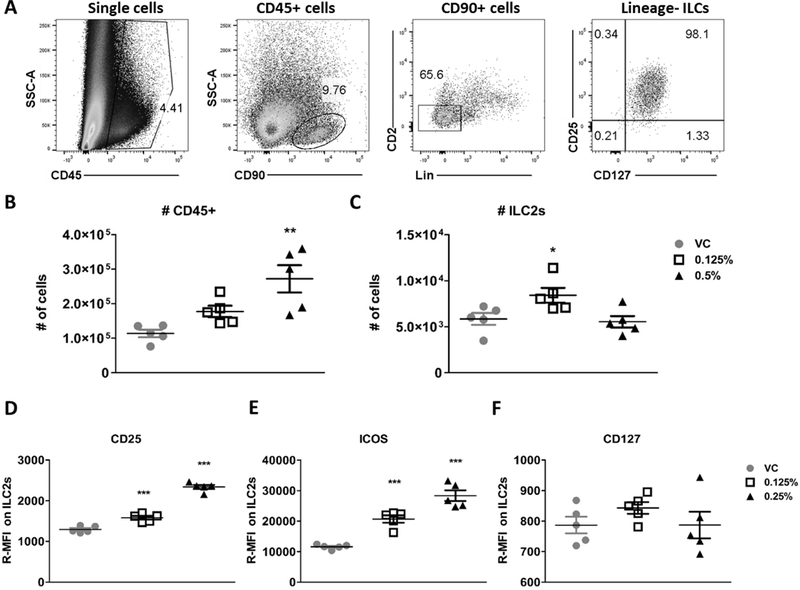
Identification and analysis of dermal innate lymphoid cells. A) Representative flow plots showing the identification of ILC2s from ear tissue from a control mouse. Doublet exclusion gating was performed prior the gating steps shown. Cells were first gated on CD45+ cells, followed by gating on CD90+ lymphocytes, CD45+CD90+Lineage-lymphocytes were then gated on cells that were positive for both CD127 and CD25, and defined as ILC2s. Total numbers of CD45+ cells (B) and ILC2s (C) isolated from the ear following treatment with the indicated concentrations of DDAC or vehicle control were quantified at day 7 post DDAC exposure. Median fluorescent intensity (relative to unstained controls) was determined for the expression of CD25 (D), ICOS (E), and CD127 (F) on ILC2s at 7 days post exposure. For panels B and C, statistical analysis was determined using an one-way ANOVA with a Dunnett’s post-test. For the kinetics experiments (D-F) significance was determined using a two-way ANOVA and Bonferroni post-tests to compare each treatment group to its respective acetone control. P values are represented by #s (0.125% DDAC) and *s (0.5% DDAC) where #/* = p< 0.05, ##/** = p< 0.01, and ###/*** = p <0.001.
To further evaluate a role for ILC2s in chemical sensitization, cytokine production was compared between BALB/c and Rag2−/− mice following 7 days of dermal DDAC exposure. Treatment with 0.5% DDAC increased the total amount of protein in the ears of both BALB/c and Rag2−/− mice, while 0.125% DDAC significantly increased protein levels only in the Rag2−/− mice (Figure 5A). TSLP protein levels in the ears were significantly increased in both BALB/c and Rag2−/− mice but only for the 0.5% treatment groups (Figure 5B) and had non-significant increases in the 0.125% treatment group. In the ears by 7 days post DDAC treatment there was an increase in Th2 cytokine expression. IL-5 was elevated, but not significantly, while IL-4 was significantly increased in the BALB/c mice when assessed by both Luminex (Figure 5C and D) and ELISA (data not shown). Despite the lack of CD4+ T cells, IL-5 was significantly increased in the ears of the Rag2−/− mice, assessed by Luminex (Figure 5C), and also by ELISA (data not shown). Additionally, IL-4 went from undetectable in the VC treated Rag2−/− mice to an average of (29.34 ± 18.43 pg/ml) in the 0.5% DDAC treated group, detectable in each animal tested. Low levels of IL-4 were detected in 2/4 of the 0.125% treated Rag2−/− mice (Figure 5D). Other cytokines found to be significantly increased in the ears include GM-CSF, IL-17A, IL-18, IL-22, and TNF-α in the BALB/c mice (Supplemental Table 1) and GM-CSF, IFN-γ, IL-1β, IL-9, IL-10, IL-17A, IL-18, IL-22, IL-27 and TNF-α in the Rag2−/− mice (Supplemental Table 2).
Figure 5. ILC2s are the likely producers of Type 2 cytokines in DDAC-treated skin.
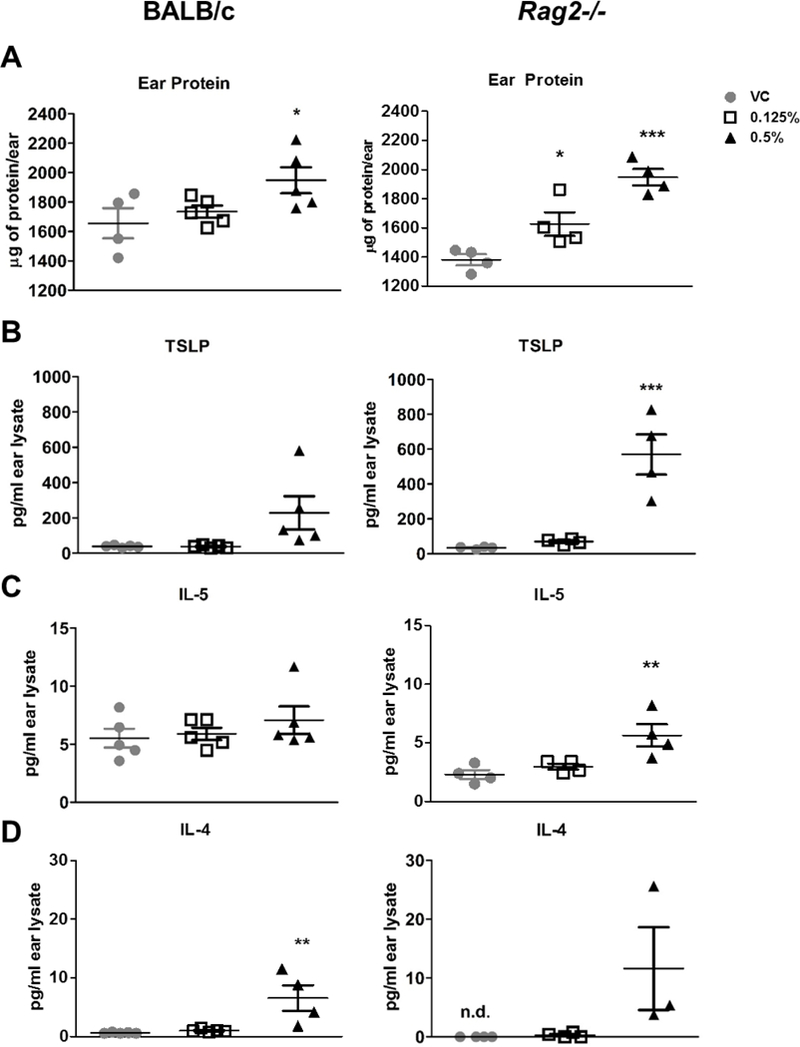
BALB/c and Rag2−/− mice were treated with acetone (VC), 0.125% DDAC or 0.5% DDAC topic, daily for 7 days, total protein was extracted from ears A) Total protein levels were assessed in ears by BCA assay for BALB/c (left) and Rag2−/− mice (right) B) TSLP levels were assessed in protein lysates by ELISA and expressed as pg/ear. C+D) IL-4 and IL-5 were assessed in ear protein lysates via Luminex assay and ear expressed as pg/ear. N= 4–5 mice per group, statistical significance was determined using an one-way ANOVA with a Dunnett’s post-test where * = p< 0.05, ** = p< 0.01, and *** = p <0.001.
Discussion:
Numerous studies have shown that exposure to chemicals can drive the development of allergic diseases, either directly, or indirectly. QACs, generally regarded as safe, display broad-range antimicrobial activity, making them a frequent choice for surface disinfection in clinical settings. The QAC DDAC is a broad-spectrum bactericide, virucide, and fungicide that is used in a wide array of applications, including industrial processes, sanitization in health care settings, food-handling and storage, wood treatment, swimming pool treatments, and as an antimicrobial in numerous commercial products (Ohnuma et al., 2011). Several case reports have implicated DDAC in the development of ACD (Dejobert et al., 1997; Mowitz and Ponten, 2015). In line with these clinical observations, our laboratory has previously identified DDAC as a strong T-cell mediated sensitizer (Anderson et al., 2016). However, other case reports have shown that DDAC can cause IgE-mediated allergy (Houtappel et al., 2008) and the development of respiratory disease in occupations where they are heavily used, such as health care settings (Gonzalez et al., 2014).
LMW chemicals are typically recognized as T-cell or IgE mediated sensitizers. T-cell mediated sensitizers can be identified using the MEST assay, where elicitation responses are measured through the induction of swelling at the site of chemical challenge; maximum responses are typically observed 24–48 hours post challenge, congruent with T-cell mediated or delayed-type hypersensitivity responses. Confirming our previous finding, DDAC tested positive in the MEST assay (Figure 1A and B) indicating a T-cell mediated mechanism. However, further characterization of the DDAC-induced immune response showed that cells isolated from the DLNs of DDAC treated mice produce both Th1 and Th2 cytokines (Table 1). Additionally, an extended exposure to DDAC (2 weeks) resulted in significant increases of systemic and local total IgE production (Figure 1C–E). Together, this data indicates that DDAC induces a mixed-type immune response, which may partially explain the spectrum of clinical symptoms associated with exposure to DDAC.
Traditionally, hypersensitivity responses have been defined as either IgE or T-cell mediated following the paradigm defined by Gell and Coombs (1963). However, as our understanding on immunology continues to evolve, along with more advanced and sensitive techniques to evaluate immune responses, many chemicals are falling within the realm of “mixed- type responders”. The need to re-classify the categorization scheme to be more encompassing for non-traditional antigens and mixed-type responses is being recognized (Descotes and Choquet-Kastylevsky, 2001; Rajan, 2003).
Due to their known role in allergic disease (Roediger et al., 2014), ILC2s were explored as a potential mediator of the mixed hypersensitivity responses observed following DDAC exposure. ILC2s produce Th2 cytokines and are enriched in mucosal sites such as the gut, the lungs, and the skin. There is some discrepancy in the literature with what cytokines are the most potent activators of ILC2s, but the majority of research suggest that IL-33 and IL-25 play a more prominent role in the activation of lung ILC2s while TSLP plays a major role in the skin (Kim et al., 2013). In the present study, following DDAC exposure there was an early, but significant, increase in il33 mRNA; however, no increase in il25 mRNA was detected. As early as 12 hours post exposure, there was a dramatic increase in tslp expression, which peaked between 2–4 days, supporting an important role for this cytokine in DDAC-induced sensitization. E-cadherin is a cellular adhesion molecule highly expressed in the skin and associated with ILC2s. E-cadherin has been shown to suppress Th2 cytokine production by ILCs through ligation with KLRG1 and is associated with inflammatory skin diseases such as atopic dermatitis (Trautmann et al., 2001). While the cause of DDAC-induced e-cadherin downregulation described in this manuscript is unknown, it could be partially responsible for the observed increased ILC2 activation. Along with ILC2 activating factors, the observed increases in ccl17, il13, il4, and Areg demonstrated that products of ILC2 activation were also increased following DDAC treatment. The gene expression data of DDAC treated skin resulted in multiple lines of evidence supporting a role for ILC2s in the immune response to DDAC.
Further investigations identified and evaluated ILC2s in the skin via flow cytometry. As skin ILC2s proliferate slowly and are typically observed after longer treatment scenarios in vivo, the absence of increases in ILC2 numbers was not unexpected (Roediger et al., 2013). Of note, ILC2 populations were increased in Rag2−/− mice compared to BALB/c mice, and did not decrease with DDAC treatment. One reason for this may be due to an increase in bioavailability of cytokines aiding in survival, as these would not be consumed by T cells in the Rag2−/− mice. Despite no significant changes in the number of ILCs, DDAC treatment induced activation of ILC2s in BALB/c and Rag2−/− mice, indicated by the increased expression of CD25, ICOS, and KLRG1, coupled with decreased CD127 expression. While ILC2s constitutively express these markers, the changing levels of expression of these surface proteins has been used to assess the activation status of ILC2s in numerous studies (Huang et al., 2015; Moro et al., 2016; Roediger et al., 2013). Temporal downregulation of CD127 is a hallmark feature of activated T lymphocytes (Schluns et al., 2000). While much less is known about CD127 expression dynamics through the course of ILC2 activation, several groups have reported ILC2 CD127 downregulation in activated ILC2s (Moro et al., 2016; Poposki et al., 2017). Together these data confirm the activation status of ILC2s in the skin following exposure to DDAC. In addition to helping identify activated ILCs, the regulation of these proteins has functional consequence, for example, CD25 and ICOS play in an important role in the proliferation and survival of ILCs (Maazi et al., 2015; Mirchandani et al., 2014). The functional consequences of KLRG1 and CD127 up and down-regulation, respectively, are less well understood.
Dermal DDAC exposure elevated Th2 cytokines including IL-4 and IL5 in both BALB/c and Rag2−/− mice. ILC2s have been shown to promote the development of Th2 cytokine responses in CD4 T cells through co-stimulation and IL-4 production, in a mouse model of allergic asthma (Drake et al., 2014). Additionally, in a helminth infection model, IL-4 produced by ILC2s was shown to be essential for Th2 differentiation (Pelly et al., 2016), and therefore may be essential for the development of the mixed-type allergic response induced by DDAC exposure. As CD4+ T cells are the other major producers of type 2 cytokines during sensitization, we compared cytokine responses in BALB/c and Rag2−/− mice, which lack mature B and T cells. Analysis of protein production in BALB/c and Rag2−/− mice confirmed that ILC2s are likely playing a role in the mixed type immune response that develops following exposure to DDAC. Total protein, TSLP, IL-4 and IL-5 were identified to be elevated in the ear following DDAC exposure in both BALB/c and Rag2−/− mice supporting a role for ILC2 in DDAC sensitization. Although mast cells have been shown to produce IL-4, thus making them a potential source for IL-4 production, the majority of the research surrounding mast cell cytokine production has been studied in the context of IgE mediated activation (Mukai et al., 2018). The early expression of IL-4 in the skin supports the Th2 cytokines derived during the sensitization period having an ILC2 origin.
Although not directly investigated in this paper, DDAC treatment also led to increased levels of IL-17, IL-22 and IL-9 in the DLNs (Table 1) and in the ears (Supplemental Table 1 and 2). While these cytokines are produced by Th17 cells, Th22 cells, and subsets of ILCs, the increased production of these cytokines observed in the Rag2−/− mice (Supplemental Table 2) indicate that ILCs contribute to their production. ILC2s have been shown to produce IL-9, which plays a role in the dampening of immune responses in chronic models of inflammation (Rauber et al., 2017) and sepsis (Lai et al., 2018). Interestingly, ILCs are not phenotypically or functionally stable, meaning that in certain inflammatory conditions, they are able to differentiate into other ILC subsets, and may have different functional roles. As the Th17 cytokines have been shown to play a role in a variety of inflammatory skin diseases, this data supports a role for Th17 cytokines in driving DDAC-induced sensitization and warrants further investigation.
The development of chemical allergy is immunologically complex, and our understanding of the mechanisms driving these responses continues to evolve. Dosage, exposure duration, and the route of exposure may all influence or alter the developing immune response. Adding to the complexity of defining immune responses is the increased understanding that the development of hypersensitivity responses are not as divergent nor categorical as once thought. In this manuscript, we highlight the development of the mixed-type immune response that develops following topical exposure to DDAC and we identify the innate lymphocytes, ILC2s, as potential early mediators of Th2-responses in the skin. These findings add insight into the immunological development of mixed-type immune responses in chemical allergy and highlight the need for continued examination of allergic mechanisms, especially in the context of non-traditional sensitizers.
Supplementary Material
Acknowledgments
Funding Information: This work was supported by internal funds from the Health Effects Laboratory Division of the National Institute for Occupational Safety and Health.
Footnotes
Disclosure:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References:
- Ainscough JS, Frank Gerberick G, Dearman RJ, and Kimber I (2013). Danger, intracellular signaling, and the orchestration of dendritic cell function in skin sensitization. J Immunotoxicol 10(3), 223–34. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Shane H, Long C, Lukomska E, Meade BJ, and Marshall NB (2016). Evaluation of the irritancy and hypersensitivity potential following topical application of didecyldimethylammonium chloride. J Immunotoxicol 13(4), 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, and Spits H (2015). The biology of innate lymphoid cells. Nature 517(7534), 293–301. [DOI] [PubMed] [Google Scholar]
- De Vooght V, Cruz MJ, Haenen S, Wijnhoven K, Munoz X, Hoet PH, Morell F, Nemery B, and Vanoirbeek JA (2010). Ammonium persulfate can initiate an asthmatic response in mice. Thorax 65(3), 252–7. [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Basketter DA, and Kimber I (1996). Characterization of chemical allergens as a function of divergent cytokine secretion profiles induced in mice. Toxicol Appl Pharmacol 138(2), 308–16. [DOI] [PubMed] [Google Scholar]
- Dejobert Y, Martin P, Piette F, Thomas P, and Bergoend H (1997). Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact dermatitis 37(2), 95–6. [DOI] [PubMed] [Google Scholar]
- Descotes J, and Choquet-Kastylevsky G (2001). Gell and Coombs’s classification: is it still valid? Toxicology 158(1–2), 43–9. [DOI] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, and Broide DH (2013). Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 132(1), 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake LY, Iijima K, and Kita H (2014). Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 69(10), 1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad SC (2014). Mouse Ear Swelling Test In Encyclopedia of Immunotoxicology (Vohr H-W, Ed.) doi: 10.1007/978-3-642-27786-3_1026-2Second Edition ed. Springer-Verlag, Berlin, Heidelberg. [DOI] [Google Scholar]
- Geier J, Lessmann H, Krautheim A, and Fuchs T (2013). Airborne allergic contact dermatitis caused by didecyldimethylammonium chloride in a geriatric nurse. Contact dermatitis 68(2), 123–5. [DOI] [PubMed] [Google Scholar]
- Gell PGH and Coombs RRA (1963). The classification of allergic reactions underlying disease In Clinical Aspects of Immunology (Coombs RRA and Gell PGH, eds) Blackwell Science. [Google Scholar]
- Gonzalez M, Jegu J, Kopferschmitt MC, Donnay C, Hedelin G, Matzinger F, Velten M, Guilloux L, Cantineau A, and de Blay F (2014). Asthma among workers in healthcare settings: role of disinfection with quaternary ammonium compounds. Clin Exp Allergy 44(3), 393–406. [DOI] [PubMed] [Google Scholar]
- Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, and McKenzie AN (2016). Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 17(1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Krauss RH, Sun AC, and Takei F (2012). Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36(3), 451–63. [DOI] [PubMed] [Google Scholar]
- Houtappel M, Bruijnzeel-Koomen CA, and Rockmann H (2008). Immediate-type allergy by occupational exposure to didecyl dimethyl ammonium chloride. Contact dermatitis 59(2), 116–7. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF Jr., and Paul WE (2015). IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol 16(2), 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, and Artis D (2013). TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 5(170), 170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber I, and Dearman RJ (2005). What makes a chemical a respiratory sensitizer? Curr Opin Allergy Clin Immunol 5(2), 119–24. [DOI] [PubMed] [Google Scholar]
- Klink KJ, and Meade BJ (2003). Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicological sciences: an official journal of the Society of Toxicology 75(1), 89–98. [DOI] [PubMed] [Google Scholar]
- Lai D, Tang J, Chen L, Fan EK, Scott MJ, Li Y, Billiar TR, Wilson MA, Fang X, Shu Q, et al. (2018). Group 2 innate lymphoid cells protect lung endothelial cells from pyroptosis in sepsis. Cell Death Dis 9(3), 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, and Li M (2013). Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun 4, 2847. [DOI] [PubMed] [Google Scholar]
- Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, and Akbari O (2015). ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42(3), 538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Long CM, Kashon ML, Sharpnack DD, Nayak AP, Anderson KL, Jean Meade B, and Anderson SE (2015). Triclosan Induces Thymic Stromal Lymphopoietin in Skin Promoting Th2 Allergic Responses. Toxicological sciences : an official journal of the Society of Toxicology 147(1), 127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson JM, Johnson VJ, and Luster MI (2005). Immune mediators in a murine model for occupational asthma: studies with toluene diisocyanate. Toxicological sciences: an official journal of the Society of Toxicology 84(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, and Liew FY (2014). Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol 192(5), 2442–8. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. (2011). Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12(11), 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, and Koyasu S (2016). Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 17(1), 76–86. [DOI] [PubMed] [Google Scholar]
- Mowitz M, and Ponten A (2015). Foot dermatitis caused by didecyldimethylammonium chloride in a shoe refresher spray. Contact dermatitis doi: 10.1111/cod.12456. [DOI] [PubMed] [Google Scholar]
- Mukai K, Tsai M, Saito H, and Galli SJ (2018). Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 282(1), 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M, Burton OT, Oettgen HC, and Chatila T (2016). IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol 138(3), 801–811 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma A, Yoshida T, Horiuchi H, Fukumori J, Tomita M, Kojima S, Takahashi N, Fukuyama T, Hayashi K, Yamaguchi S, et al. (2011). Altered pulmonary defense system in lung injury induced by didecyldimethylammonium chloride in mice. Inhalation toxicology 23(8), 476–85. [DOI] [PubMed] [Google Scholar]
- Palm NW, and Medzhitov R (2009). Immunostimulatory activity of haptenated proteins. Proc Natl Acad Sci U S A 106(12), 4782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Ruckerl D, Seddon B, MacDonald AS, McKenzie A, and Wilson MS (2016). IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol 9(6), 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, Hulse KE, Stevens WW, Peters AT, Grammer LC, et al. (2017). Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis 5(3), 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan TV (2003). The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol 24(7), 376–9. [DOI] [PubMed] [Google Scholar]
- Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, et al. (2017). Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 23(8), 938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Le Gros G, and Weninger W (2014). Dermal group 2 innate lymphoid cells in atopic dermatitis and allergy. Curr Opin Immunol 31, 108–14. [DOI] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. (2013). Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 14(6), 564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. (2013). A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 210(13), 2939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, and Lefrancois L (2000). Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1(5), 426–32. [DOI] [PubMed] [Google Scholar]
- Shane HL, Lukomska E, Stefaniak AB, and Anderson SE (2017). Divergent hypersensitivity responses following topical application of the quaternary ammonium compound, didecyldimethylammonium bromide. J Immunotoxicol 14(1), 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. (1992). RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68(5), 855–67. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Altznauer F, Akdis M, Simon HU, Disch R, Brocker EB, Blaser K, and Akdis CA (2001). The differential fate of cadherins during T-cell-induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Dermatol 117(4), 927–34. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, D’Alpaos V, Evrard G, Jamart J, Thimpont J, Huaux F, and Renauld JC (2013). Asthma related to cleaning agents: a clinical insight. BMJ Open 3(9), e003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, and Meade BJ (2000). Comparison of mouse strains using the local lymph node assay. Toxicology 146(2–3), 221–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


