Abstract
Background:
Drug resistance is a challenge for the global control of tuberculosis. We examined mortality in tuberculosis patients from high-burden countries, according to concordance or discordance of results from drug susceptibility testing (DST) done locally and in a reference laboratory.
Methods:
We collected Mycobacterium tuberculosis isolates from adult patients in Côte d’Ivoire, Democratic Republic of the Congo, Kenya, Nigeria, South Africa, Peru, and Thailand, stratified by HIV status and tuberculosis drug resistance. Molecular or phenotypic drug susceptibility testing (DST) was done locally and at the Swiss tuberculosis reference laboratory. We examined mortality during treatment according to DST results and treatment adequacy in logistic regression models adjusting for sex, age, sputum microscopy and HIV status.
Findings:
634 tuberculosis patients were included; median age was 33.2 years, 239 (37.7%) were female, 272 (42.9%) HIV-positive and 69 (10.9%) patients died. Based on the reference laboratory DST, 394 (62.2%) strains were pan-susceptible, 45 (7.1%) mono-resistant, 163 (25.7%) multidrug-resistant (MDR-TB), and 30 (4.7%) had pre-extensive or extensive drug resistance (pre-XDR/XDR-TB). Results of reference and local laboratories were discordant in 121 (19.1%) cases. Overall, sensitivity and specificity to detect any resistance were 90.8% and 84.3%, respectively. Mortality ranged from 6.0% (20/336) in patients with pan-susceptible tuberculosis treated according to WHO guidelines to 57.1% (8/14) in patients with resistant strains who were under treated. In logistic regression, compared to concordant DST results, the adjusted odds ratio of death was 7.33 (95% CI 2.70–19.95) for patients with discordant results potentially leading to under treatment.
Interpretation:
Inaccurate DST by comparison to a reference standard led to under treatment of drug resistant tuberculosis and increased mortality. Rapid molecular DST of first- and second-line drugs at diagnosis is required to improve outcomes in patients with MDR-TB and pre-XDR/XDR-TB.
Keywords: Tuberculosis, drug resistance, MDR-TB, XDR-TB, mortality, treatment success, low- and middle-income countries
INTRODUCTION
Tuberculosis is a global public health concern. In 2016, an estimated 10.4 million individuals developed active tuberculosis worldwide, of whom an estimated 1.0 million (10%) were HIV-positive 1. The scale-up of antiretroviral combination therapy (ART) has substantially improved the prognosis of HIV-positive patients 2,3, and reduced the incidence of tuberculosis in this population 4,5. However, the risk of tuberculosis among HIV-positive patients on ART remains four times higher than among HIV-negative patients 6.
The emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) is another threat to the control of tuberculosis 7–9. In 2016, it was estimated that 4% of the new patients and 19% (up to 48% in Eastern Europe) of previously treated patients had MDR-TB 1. Treatment of MDR-TB and XDR-TB is challenging due to the longer treatment duration, adverse effects and lower efficacy of second-line drugs 10,11. Strategies to prevent drug-resistant tuberculosis include monitoring of the prevalence of MDR-TB, wide-spread drug susceptibility testing (DST) and ensuring rapid initiation and completion of full courses of effective treatment regimens 12,13. Culture-based phenotypic DST is considered the gold-standard, but is time and resource intensive, and too slow to influence decisions on starting treatment 14. Molecular-based resistance testing offers an alternative to culture-based DST 15. Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) detects resistance to rifampicin directly from sputum and provides results within 1.5 hours 16, while line-probe assays (LPAs) from sputum detect resistance to isoniazid, rifampicin, ethambutol, fluoroquinolones, or second-line injectable drugs (amikacin, capreomycin, or kanamycin) and provide results within 1–2 days 15.
We compared the results of resistance testing performed locally in ART and tuberculosis programmes in high tuberculosis burden countries to those from gold standard phenotypic DST performed in the Swiss reference laboratory, and examined mortality in HIV-positive and HIV-negative tuberculosis patients with concordant and discordant test results.
METHODS
This multi-centric cohort study is part of a larger research project on the evolution of drug-resistant Mycobacterium tuberculosis (M. tuberculosis) in the context of HIV co-infection within the International Epidemiology Databases to Evaluate AIDS (IeDEA), a global network of ART programs (see www.iedea.org) 17,18. Isolates and clinical data were collected from tuberculosis patients in seven high-burden countries in sub-Saharan Africa, Asia and Latin America. The sample size was calculated so that the study had adequate power to detect differences in the prevalence of drug resistance between HIV-positive and HIV-negative patients.
Patient recruitment and data collection
We included adult patients aged 16 years or older who were treated for active pulmonary tuberculosis in Côte d’Ivoire, Democratic Republic of the Congo (DRC), Kenya, Nigeria, South Africa, Peru, and Thailand. All seven countries are defined by the World Health Organization (WHO) as high tuberculosis burden countries, and DRC, Kenya, Nigeria South Africa and Thailand are also high MDR-TB burden and high HIV/tuberculosis burden countries 19.
HIV-positive tuberculosis patients were recruited prospectively from ART clinics participating in IeDEA, HIV-negative patients from tuberculosis clinics serving the same population. In South Africa, patients included came from well-documented strain collections held at the University of Cape Town. Sites were asked to contribute pulmonary pre-treatment M. tuberculosis isolates from 25 or more patients within each of the four strata defined by HIV status (positive or negative) and drug resistance (MDR or pan-susceptible), for a total of 100 patients per site. Supplemental Table S1 summarizes the characteristics of participating sites. Patient characteristics were entered online in French or English at baseline, using the Research Electronic Data Capture (REDCap) tool 20, including site, type of TB patient as defined by WHO, age, sex, HIV status, CD4 cell count at start of tuberculosis treatment (if HIV positive), sputum smear microscopy result and risk factors for tuberculosis. Treatment regimens were updated and outcomes entered during regular follow-up visits.
Outcomes
Treatment outcomes were defined according to WHO as cured, treatment completed, treatment failure, death, lost to follow-up, transferred to other clinics, ongoing treatment at the time of evaluation or unknown treatment outcome 21. “Treatment success” included cured patients and patients who completed treatment 21. The main outcome for this study was mortality during tuberculosis treatment. Outcome data received up to March 31, 2018 were included in analyses.
Drug susceptibility testing
DST was performed locally using liquid or solid cultures or molecular methods: Xpert MTB/RIF or LPAs, such as Genotype MTBDRplus or MTBDRsl tests (Hain Lifesciences, Germany). The reference laboratory of the Swiss National Center for Mycobacteria, Zurich, Switzerland performed DST using the Mycobacteria Growth Indicator Tube liquid medium system (MGIT, Becton Dickinson, USA) with the following drug concentrations: 0.1 mg/L for isoniazid, 1.0 mg/L for rifampicin, 100.0 mg/L for pyrazinamide, 5.0 mg/L for ethambutol, 1.0 mg/L for amikacin and 0.25 mg/L for moxifloxacin, in line with the critical concentrations recently published by WHO 22.
WHO defines mono-resistance as resistance to one first-line anti-tuberculosis drug (isoniazid, rifampicin, pyrazinamide, or ethambutol); MDR as resistance to isoniazid and rifampicin; pre-XDR as MDR with additional resistance to any fluoroquinolone or one of the second-line injectable drugs (amikacin, capreomycin, or kanamycin); XDR as MDR with additional resistances to any fluoroquinolone and at least one of the second-line injectable drugs 21. The category “other” drug resistance included any other combination. We defined “pan-susceptible” tuberculosis as no resistance against the six drugs tested at the reference laboratory and any resistance as resistance against at least one of the tested drugs. First-line regimens (standard treatment) included first-line anti-tuberculosis drugs (isoniazid, rifampicin, pyrazinamide, and ethambutol) and second-line regimens included a combination of first-line and second-line drugs 21,23.
Exposure definition and data analysis
We calculated test accuracy statistics for the diagnosis of any drug resistance. We further classified comparisons between the phenotypic and molecular DST results obtained in the local laboratories and the reference laboratory as follow: concordant results, discordance potentially leading to under treatment, discordance potentially leading to over treatment, and other discordant results. We defined drug regimens received by patients as compatible with the WHO guidelines in place during the study period, as under treatment or as over treatment, based on the reference DST results. First-line regimens for pan-susceptible tuberculosis, first or second line-regimens prescribed to isoniazid mono-resistant patients, second line-regimens prescribed to rifampicin mono-resistant patients, MDR-TB and pre-XDR/XDR-TB patients were classified as in accordance with WHO guidelines. Under treatment included first-line regimens given to rifampicin mono-resistant patients, MDR-TB and pre-XDR/XDR-TB patients, and over treatment second-line regimens given to pan-susceptible tuberculosis patients. Supplemental Table S2 shows the classification of regimens.
We used descriptive statistics to describe patient characteristics by levels of drug resistance based on DST performed at the reference laboratory and by HIV status. We examined determinants of mortality in multivariate logistic regression models. Patients with unknown or missing treatment outcome, ongoing treatment, missing treatment regimen, missing sputum microscopy and “other” drug-resistant tuberculosis were excluded from logistic regression analyses. Logistic models were adjusted for age, sex, sputum microscopy result and HIV status. We stratified models by study site by including an indicator variable for all sites except South Africa (the reference group). We calculated the population attributable fraction of mortality due to discordant DST results based on the adjusted model as described by Greenland and Drescher 24.
Other variables, for example smoking history, diabetes, substance abuse and contact to other tuberculosis patients worsened the fit of the model. For HIV-positive individuals, models were additionally adjusted for CD4 cell count at tuberculosis treatment start. All analyses were done using STATA version 15 (Stata Corporation, College Station, Texas, USA).
Ethical statement
Local institutional review boards or ethics committees approved the study at all participating sites. Informed consent was obtained where requested per local regulations. The study was also approved by the Cantonal Ethics Committee in Bern, Switzerland.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
We obtained M. tuberculosis isolates from 871 patients diagnosed between 2013 and 2016. We excluded 237 patients from analyses of the accuracy of DST, mainly because isolates were contaminated or not viable, and a further 61 patients from analyses of mortality, mainly because treatment was ongoing or outcomes unknown at the time of closing the database (supplementary Figure S1). Excluded patients were similar in terms of age, sex, HIV status, site of tuberculosis, but had lower CD4 counts and were more likely to be patients with recurrent tuberculosis and treatment after failure or default (supplementary Table S3).
Characteristics of patients and isolates
The median age of the 634 included TB patients was 33.2 years (interquartile range [IQR] 26.9–42.5 years); 239 (37.7%) were female. The reference laboratory identified 394 (62.1%) pan-susceptible M. tuberculosis strains, 45 (7.1%) mono-resistant strains, 163 (25.7%) MDR strains, 30 (4.7%) pre-XDR/XDR strains, and 2 (0.3%) strains with other drug resistance profiles (Table 1). Among the 163 patients with MDR-TB, 85 (52.1%) had resistance to rifampicin and isoniazid only, while the remaining patients were additionally resistant to pyrazinamide and/or ethambutol. Among the 24 patients with pre-XDR-TB, resistance to moxifloxacin (n=15) was more frequent than resistance to amikacin (n=9; Table 2). Patients with resistant strains were more likely to receive second-line tuberculosis treatment, and to experience unfavourable treatment outcomes than patients with pan-susceptible strains (Table 1).
Table 1:
Patient characteristics by phenotypic drug resistance profiles obtained at the Swiss National Center for Mycobacteria.
| Pan- susceptible |
Any resistance |
P- value |
Mono-resistance |
Poly-resistance |
|||||
|---|---|---|---|---|---|---|---|---|---|
| INH | RIF | PZA | MDR | Pre- XDR/XDR |
Other | ||||
| Total | 394 (100) | 240 (100) | 29 (100) | 14 (100) | 2 (100) | 163 (100) | 30 (100) | 2 (100) | |
| Sex | |||||||||
| Female | 150 (38.1) | 89 (37.1) | 0.80 | 6 (20.7) | 3 (21.4) | 0 | 65 (39.9) | 14 (46.7) | 1 (50.0) |
| Male | 244 (61.9) | 151 (62.9) | 23 (79.3) | 11 (78.6) | 2 (100) | 98 (60.1) | 16 (53.3) | 1 (50.0) | |
| Age (year) | 34.6 (27.8–44.6) |
31.5 (25.3–40.2) |
0.003 | 34.3 (26.5–43.2) |
27.1 (24.9–35.5) |
26.1 (23.3–28.9) |
31.5 (25.4–41.4) |
30.3 (24.2–37.5) |
27.3 (24.4–30.2) |
| HIV status | |||||||||
| Negative | 200 (50.8) | 162 (67.5) | <0.001 | 20 (69.0) | 8 (57.1) | 1 (50.0) | 114 (69.9) | 18 (60.0) | 1 (50.0) |
| Positive | 194 (49.2) | 78 (32.5) | 9 (31.0) | 6 (42.9) | 1 (50.0) | 49 (30.1) | 12 (40.0) | 1 (50.0) | |
| CD4 count at baselinecells/μl | 215 (85–369) |
161 (61–369) |
0.79 | 92.5 (55–161) |
63.5 (43–81) |
43 | 259 (151–528) |
32 (5–105) |
213 |
| No. of observations (%) | 155 (39.3) | 45 (18.9) | 6 (20.7) | 6 (42.9) | 1 (50.0) | 24 (14.7) | 7 (23.3) | 1 (50.0) | |
| Treatment regimen | |||||||||
| First line | 369 (93.7) | 46 (19.2) | <0.001 | 27 (93.1) | 0 | 2 (5.4) | 14 (9.2) | 2 (6.7) | 1 (50.0) |
| Second line | 25 (6.3) | 188 (78.3) | 2 (6.9) | 14 (100) | 0 | 143 (85.3) | 28 (93.3) | 1 (50.0) | |
| Unknown | 0 | 6 (2.5) | 0 | 0 | 0 | 6 (5.5) | 0 | 0 | |
| Treatment outcomes | |||||||||
| Success | 287 (72.8) | 124 (51.7) | <0.001 | 15 (51.7) | 7 (50.0) | 0 | 88 (54.0) | 13 (43.3) | 1 (50.0) |
| Mortality | 24 (6.1) | 45 (18.8) | 7 (24.1) | 2 (14.3) | 1 (50.0) | 24 (14.7) | 10 (33.3) | 1 (50.0) | |
| Treatment failure | 12 (3.0) | 10 (4.2) | 0 | 0 | 1 (50.0) | 5 (3.1) | 4 (13.3) | 0 | |
| Lost to follow-up | 29 (7.4) | 30 (12.5) | 1 (3.5) | 3 (21.4) | 0 | 26 (16.0) | 0 | 0 | |
| Transfer | 15 (3.8) | 14 (5.8) | 0 | 2 (14.3) | 0 | 9 (5.5) | 3 (10.0) | 0 | |
| Ongoing treatment / unknown | 27 (6.9) | 17 (7.1) | 6 (20.7) | 0 | 0 | 11 (6.7) | 0 | 0 | |
| Country | |||||||||
| Côte d’Ivoire | 48 (12.2) | 51 (21.3) | <0.001 | 3 (10.3) | 0 | 0 | 44 (27.0) | 4 (13.3) | 0 |
| Democratic Republic of the Congo | 33 (8.4) | 29 (12.1) | 0 | 1 (7.1) | 0 | 19 (11.7) | 9 (30.0) | 0 | |
| Kenya | 24 (6.1) | 11 (4.6) | 2 (6.9) | 1 (7.1) | 0 | 8 (4.9) | 0 | 0 | |
| Nigeria | 20 (5.1) | 36 (15.0) | 1 (3.5) | 5 (35.7) | 0 | 26 (16.0) | 4 (13.3) | 0 | |
| Peru | 66 (16.8) | 38 (15.8) | 8 (27.6) | 0 | 0 | 27 (16.6) | 3 (10.0) | 0 | |
| South Africa | 130 (33.0) | 57 (23.8) | 6 (20.7) | 7 (50.0) | 1 (50.0) | 32 (15.5) | 10 (33.3) | 1 (50.0) | |
| Thailand | 73 (18.5) | 18 (7.5) | 9 (31.0) | 0 | 1 (50.0) | 7 (4.3) | 0 | 1 (50.0) | |
Analysis based on 634 patients (see supplementary Figure S1). Numbers (%) or median (interquartile range) are shown.
INH, isoniazid; MDR, multidrug resistant; PZA, pyrazinamide; RIF, rifampicin; XDR, extensively drug resistant.
Table 2:
Drug resistance profiles identified at the Swiss National Center for Mycobacteria.
| Resistance profiles | No. of patients (n=634) |
|---|---|
| Pan-susceptible | 394 (62.2%) |
| Mono-resistance | 45 (7.1%) |
| INH mono-resistance | 29 |
| RIF mono-resistance | 14 |
| PZA mono-resistance | 2 |
| MDR | 163 (25.7%) |
| INH+RIF | 85 |
| INH+RIF+EMB | 11 |
| INH+RIF+PZA | 47 |
| INH+RIF+EMB+PZA | 20 |
| Pre-XDR | 24 (3.2%) |
| INH+RIF +MOX+EMB+PZA | 8 |
| INH+RIF +MOX+EMB | 1 |
| INH+RIF +MOX+PZA | 4 |
| INH+RIF +MOX | 2 |
| INH+RIF +AMK+PZA+EMB | 4 |
| INH+RIF +AMK+PZA | 4 |
| INH+RIF +AMK | 1 |
| XDR | 6 (0.8%) |
| INH+RIF +AMK+MOX+EMB | 3 |
| INH+RIF +AMK+MOX+PZA | 2 |
| INH+RIF +AMK+MOX | 1 |
| Other | 2 (0.3%) |
| INH+MOX | 1 |
| INH+PZA | 1 |
Analysis based on 634 patients (see supplementary Figure S1).
AMK, amikacin; EMB, ethambutol; INH, isoniazid; MDR, multidrug resistant; MOX, moxifloxacin; PZA, pyrazinamide; RIF, rifampicin; XDR, extensively drug resistant.
A total of 272 (42.9%) tuberculosis patients were HIV-positive, with a median CD4 cell count at the start of tuberculosis treatment of 192 cells/μl (IQR 77.5–369 cells/μl). Among them, 175 (64.3%) were either on ART at the start of tuberculosis treatment or initiated ART within 3 months; the ART status of the remaining patients was unknown. Compared to HIV-negative individuals, HIV-positive patients were more likely to be female, more likely to have both pulmonary and extrapulmonary disease, and more likely to be patients with recurrent tuberculosis (supplemental Table S4). HIV-positive patients were also more likely to have a negative sputum smear microscopy result and more likely to have a pan-susceptible M. tuberculosis infection than HIV-negative patients.
Drug susceptibility testing and treatments
Local laboratories used the Xpert MTB/RIF system, culture, LPAs, or a combination of these methods to diagnose drug-resistant infections and inform treatment regimens (Table 3, supplemental Table S2). Among the 27 isolates assessed by a combination of tests, Xpert MTB/RIF and LPA were used in 17 (63.0%) isolates, Xpert MTB/RIF and culture in 8 (29.6%), culture and LPA in one, and Xpert MTB/RIF, culture and LPA in another isolate.
Table 3:
Concordance and discordance of drug susceptibility results obtained from reference and local laboratories.
| Concordance/ discordance of DST results |
DST results by laboratory |
Total |
Test used at local laboratories |
||||
|---|---|---|---|---|---|---|---|
| Reference laboratory (phenotypic) |
Local laboratories | (n=634) | Xpert MTB/RIFa |
Culture | LPA | Combination of tests |
|
| Concordance | Pan-susceptible | Pan-susceptible | 332 (64.7) | 167 (77.3) | 101 (65.6) | 1 (9.1) | 5 (6.8) |
| RIF mono-resistance | RIF mono-resistance | 8 (1.6) | 0 | 0 | 0 | 7 (9.6) | |
| INH mono-resistance | INH mono-resistance | 8 (1.6) | 0 | 8 (5.2) | 0 | 0 | |
| MDR | MDR | 153 (29.8) | 49 (22.7) | 44 (28.6) | 8 (72.7) | 52 (71.2) | |
| Pre-XDR and XDR | Pre-XDR and XDR | 12 (2.3) | 0 | 1 (0.6) | 2 (18.2) | 9 (12.3) | |
| Total | 513 (100) | 216 (100) | 154 (100) | 11 (100) | 73 (100) | ||
|
Discordance potentially leading to under treatment |
MDR | Pan-susceptible | 5 (21.7) | 2 (25.0) | 2 (22.2) | 0 | 1 (16.7) |
| Pre-XDR and XDR | MDR | 18 (78.3) | 6 (75.0) | 7 (77.8) | 0 | 5 (83.3) | |
| Total | 23 (100) | 8 (100) | 9 (100) | 0 | 6 (100) | ||
|
Discordance potentially leading to over treatment |
Pan-susceptible | RIF mono-resistance | 14 (20.9) | 0 | 0 | 3 (100) | 10 (71.4) |
| Pan-susceptible | MDR | 14 (20.9) | 3 (60.0) | 8 (18.2) | 0 | 3 (21.4) | |
| Pan-susceptible | Other mono-resistanceb | 33 (49.3) | 2 (40.0) | 31 (70.5) | 0 | 0 | |
| Other mono-resistancec | MDR | 5 (7.5) | 0 | 5 (11.4) | 0 | 0 | |
| MDR | Pre-XDR or XDR | 1 (1.5) | 0 | 0 | 0 | 1 (7.1) | |
| Total | 67 (100) | 5 (100) | 44 (100) | 3 (100) | 14 (100) | ||
|
Other
discordance |
Pan-susceptible | EMB, SM | 1 (3.2) | 0 | 1 (16.7) | 0 | 0 |
| RIF mono-resistance | MDR | 7 (22.6) | 2 (12.5) | 0 | 0 | 5 (28.6) | |
| Other mono-resistanced | Pan-susceptible | 17 (54.8) | 13 (81.3) | 3 (50.0) | 0 | 0 | |
| INH, MOX | Mono-resistance | 1 (3.2) | 0 | 1 (16.7) | 0 | 0 | |
| IHN, PZA | MDR | 1 (3.2) | 0 | 1 (16.7) | 0 | 0 | |
| MDR | RIF mono-resistance | 3 (9.7) | 0 | 0 | 1 (100) | 2 (71.4) | |
| MDR | EMB, SM | 1 (3.2) | 1 (6.2) | 0 | 0 | 0 | |
| Total | 31 (100 | 16 (100) | 6 (100) | 1 (100) | 7 (100) | ||
Analysis based on 634 patients (see supplementary Figure S1). Number of patients (%) are shown.
DST, drug susceptibility testing; EMB, ethambutol; INH, isoniazid; LPA, line probe assay; MDR, multidrug resistance; PZA, pyrazinamide; RIF, rifampicin; SM, streptomycin; XDR, extensively drug resistant.
In some patients the test used to diagnose drug-resistant infection at the local laboratories was unknown. Therefore, numbers do not always add up to the row totals.
RIF resistance diagnosed with Xpert MTB/RIF was classified as MDR
Twenty-one strains were resistant to EMB, ten to SM and two INH.
Five strains were resistant to INH.
Fifteen strains were resistant to INH, two to PZA
Comparing local with reference laboratory results for any resistance, there were 218 true and 62 false positives and 332 true and 22 false negatives, for an overall sensitivity and specificity of 90.8% (95% CI 87.2–94.5) and 84.3% (80.7–87.9), respectively. Sensitivities and specificities were 79.5% (68.4–88.0) and 97.1% (93.4–99.1) for Xpert MTB/RIF, 93.1% (84.5–97.7) and 71.6% (63.4–78.9) for culture, 100% (71.5–100) and 25.0% (0.63–80.6) for LPA and 98.8% (93.4–99.9) and 27.8% (9.7–53.5%) for combinations of tests. Considering four categories of drug resistance (rifampicin mono-resistance, isoniazid mono-resistance, MDR, pre-XDR/XDR), results from the reference laboratory and local laboratories were concordant for 513 of 634 (80.9%) and discordant for 121 of 634 (19.1%) patients. The proportions with concordant test results were 88.2% (216 of 245), 72.3% (154 of 213), 73.3% (11 of 15) and 73.0% (73 of 100) for Xpert MTB/RIF, culture, LPA, or a combination of tests, respectively (P<0.001).
There were 23 of 634 (3.6%) discrepancies potentially leading to under treatment, 67 of 634 (10.6%) discordant results potentially leading to over treatment, and 31 of 634 (4.9%) other discordances (Table 3, supplementary Table S2). When analysing the treatments received, they were compatible with WHO guidelines in 491 of 507 (96.8%) patients with concordant DST results compared to 94 of 121 patients (77.7%) with discordant results (P<0.001).
Mortality
After excluding 61 of 634 (9.6%) patients with unknown treatment outcomes, missing data or “other” drug resistance (supplementary Figure S1), mortality ranged from 5.6% (17 of 302) among patients with pan-susceptible strains and concordant DST results to 44.4% (8 of 18) among patients with pre-XDR/XDR tuberculosis and discordant DST results (Table 4). It ranged from 9.8% (6 of 61) in patients with discordant results potentially leading to over treatment to 40.9% (9 of 22) in in patients with discordant results potentially leading to under treatment (Figure 1, Table 5). Mortality ranged from 6.4% (23 of 359) in patients with pan-susceptible strains to 34.5% (10 of 29) in patients with pre-XDR/XDR tuberculosis. Mortality was higher in patients with isoniazid mono-resistant strains (7 of 23, 30.4%) than in patients with rifampicin mono-resistant strains (2 of 14, 14.3%) but the difference was not statistically significant (P=0.38, Table 4) and the two categories were combined in further analyses. Finally, mortality ranged from 6.0% (20 of 336) in patients with pan-susceptible tuberculosis treated according to WHO guidelines to 57.1% (8 of 14) in patients with resistant strains who were under treated (Figure 1, Table 5).
Table 4:
Mortality by phenotypic drug resistance profiles obtained at the Swiss National Centre for Mycobacteria and by concordance with local results.
| Concordant results |
Discordant results |
Total | |
|---|---|---|---|
| Pan-susceptible | 17/302 (5.6%) | 6/57 (10.5%) | 23/359 (6.4%) |
| Any resistance | 29/164 (17.7%) | 15/50 (30.0%) | 44/214 (20.6%) |
| Mono-resistance | |||
| INH | 5/8 (62.5%) | 2/15 (13.3%) | 7/23 (30.4%) |
| RIF | 0/7 (0%) | 2/7(28.6%) | 2/14 (14.3%) |
| PZA | - | 1/2 (50.0%) | 1/2 (50.0%) |
| Poly-resistance | |||
| MDR | 22/138 (15.9%) | 2/8 (25.0%) | 24/146 (14.4%) |
| Pre-XDR/XDR | 2/11 (18.2%) | 8/18 (44.4%) | 10/29 (34.5%) |
| Total | 46/466 (9.9%) | 21/107 (19.6%) | 67/573 (11.7%) |
Analysis based on 573 patients with complete data (see supplementary Figure S1).
Figure 1: Mortality according to drug resistance, to concordance or discordance of drug susceptibility testing (DST) results and to treatment adequacy.
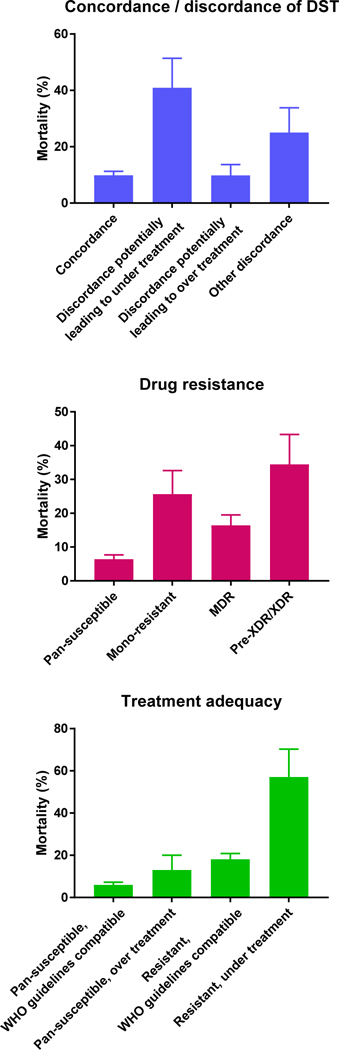
Error bars are standard errors. All P values <0.001 for difference in mortality across categories. Analysis based on 573 patients with complete data.
Table 5.
Results from logistic regression models of the probability of death during tuberculosis treatment.
| No. of patients |
No. of deaths (%) |
Model 1 aOR (95% CI) |
Model 2 aOR (95% CI) |
Model 3 aOR (95% CI) |
|
|---|---|---|---|---|---|
|
Concordance / discordance
of DST results | |||||
| Concordance | 466 | 46 (9.9) | 1 | ||
| Discordance potentially leading to under treatment |
22 | 9 (40.9) | 7.33 (2.70–19.95) | ||
| Discordance potentially leading to over treatment |
61 | 6 (9.8) | 0.81 (0.31–2.11) | ||
| Other discordance | 24 | 6 (25.0) | 4.92 (1.69–14.33) | ||
| Drug resistance a | |||||
| Pan-susceptible | 359 | 23 (6.4) | 1 | ||
| Mono-resistance | 39 | 10 (25.6) | 6.05 (2.36–15.56) | ||
| MDR | 146 | 24 (16.4) | 3.83 (1.88–7.81) | ||
| Pre-XDR/XDR | 29 | 10 (34.5) | 15.19 (5.45–42.36) | ||
|
Treatment adequacy by drug resistance |
|||||
| Pan-susceptible, compatible with WHO guidelines |
336 | 20 (6.0) | 1 | ||
| Pan-susceptible, over treatment |
23 | 3 (13.0) | 3.31 (0.82–13.45) | ||
| Any resistance, compatible with WHO guidelines |
200 | 36 (18.0) | 4.66 (2.16–9.14) | ||
| Any resistance, under treatment |
14 | 8 (57.1) | 19.32 (5.59–66.73) | ||
| Sex | |||||
| Female | 219 | 20 (9.1) | 1 | 1 | 1 |
| Male | 354 | 47 (13.3) | 1.47 (0.81–2.67) | 1.42 (0.78–2.60) | 1.46 (0.80–2.70) |
| Age (per 1 year increase) | 573 | 67 (11.7) | 1.04 (1.01–1.06) | 1.04 (1.01–1.06) | 1.04 (1.01–1.06) |
| Sputum microscopy | |||||
| Negative | 111 | 10 (9.0) | 1 | 1 | 1 |
| Positive | 462 | 57 (12.3) | 1.14 (0.51–2.56) | 1.03 (0.45 −2.37) | 0.90 (0.40–2.07) |
| HIV status | |||||
| Negative | 337 | 43 (12.8) | 1 | 1 | 1 |
| Positive | 236 | 24 (10.2) | 0.90 (0.50–1.61) | 1.19 (0.65–2.20) | 1.19 (0.65–2.20) |
Models based on 573 patients with complete data for all variables shown (see supplementary Figure S1).
Model 1 was adjusted for concordance / discordance of DST results, sex, age, sputum microscopy and HIV status; model 2 was adjusted for drug resistance, sex, age, sputum microscopy and HIV status; model 3 was adjusted for treatment adequacy, sex, age, sputum microscopy and HIV status.
Abbreviations: DST, drug susceptibility testing; MDR, multidrug resistant; XDR, extensively drug-resistant
Results from the Swiss National Reference Center for Mycobacteria
In multivariable logistic models adjusted for sex, age, sputum microscopy result and HIV status, discordant DST results continued to be associated with increased mortality compared to concordant DST results (Table 5). Compared to concordant DST results, the adjusted odds ratio (aOR) of death was 7.33 (95% CI 2.70–19.95) for patients with discordant results potentially leading to under treatment. The population attributable fraction associated with any type of discordance obtained from the logistic model was 15.15% (95% CI 2·08–26%).
Drug resistance was associated with higher mortality compared to pan-susceptible tuberculosis. The aOR was 5.06 (95% Cl 2.74–9.35) for any type of drug resistance, and 15.19 (95% 5.54–42.36) for pre-XDR/XDR (Table 5). Finally, compared to patients treated according to WHO guidelines with pan-susceptible strains, the aOR for death was 4.66 (95% CI 2.38–9.14) for adequately treated patients with resistant strains and 19.32 (95% CI 5.59–66.73) for patients with resistant strains receiving inadequate regimens (Table 5). Of note, patients with pan-susceptible tuberculosis who were over treated also had an increased risk of death: the aOR compared to patients with pan-susceptible tuberculosis treated according to WHO guidelines was 3.31 (0.82–13.45, P=0.10). Sex, positive sputum smear microscopy and HIV status were not associated with the odds of death. The results from univariable models were similar to the aOR from multivariable models (Table S5). When restricting the analysis to HIV-positive patients, mortality was higher among patients with CD4 cell counts <50 cells/μL: the aOR was 6.89 (95% CI 1.57–30.26) compared to patients with higher CD4 counts at tuberculosis treatment start.
DISCUSSION
This study of patients treated for drug-resistant or drug-susceptible tuberculosis in seven high tuberculosis burden countries showed that the accuracy of DST testing in routine care was moderate, with discordant results from local DST compared to phenotypic DST in a reference laboratory in about 20 percent of patients. Discordant results led to inadequate treatment and contributed to the excess mortality associated with drug-resistant tuberculosis. As expected, mortality was highest in patients with pre-XDR/XDR tuberculosis and higher in patients who were under treated. Interestingly, patients with pan-susceptible tuberculosis who were over treated also had higher mortality, although the difference failed to reach conventional levels of statistical significance. It is possible that over treated patients had worse adherence and were at higher risk of adverse drug effects. To our knowledge, this is the first study to assess the accuracy of DST in real world, routine settings and to examine the impact of inaccurate results on mortality. Our findings support the recent call for a precision medicine approach to the treatment of drug-resistant tuberculosis, guided by detailed molecular DST done locally, to replace the standardised, empirical combination regimens used in many high tuberculosis burden low- and middle-income countries 25.
At present, WHO recommends that “Xpert MTB/RIF should be used as the initial diagnostic test in individuals suspected of having MDR-TB or HIV-associated tuberculosis” 26, based on a Cochrane review of test accuracy studies in adults with suspected rifampicin-resistance or MDR-TB 27. In line with this recommendation, Xpert MTB/RIF was the most commonly used test in our study sites. The Cochrane review reported a pooled sensitivity of 95%, based on 17 studies and 555 patients with rifampicin-resistant strains 27. The pooled specificity was 98%. We examined accuracy of DST strategies at the level of the local laboratories in high-burden countries, in routine care settings, rather than by evaluating a single test. Our estimates of sensitivity and specificity, for the detection of any drug resistance, were lower overall (90.8% and 84.3%, respectively), and lower for Xpert MTB/RIF (79.5% and 97.1%) and for culture (93.1% and 71.6%), indicating that DST is less accurate in routine settings than in test accuracy studies 27.
There are concerns both about false-negative and false-positive Xpert MTB/RIF test results, and a policy of confirmatory testing has been introduced in South Africa and Brazil 28,29. The discordant DST results that potentially led to under treatment of drug-resistant tuberculosis (false negative for resistance) were mainly based on locally performed cultures, Xpert MTB/RIF tests, or a combination of the two. Of note, the recently developed Xpert MTB/RIF Ultra assay has been shown to improve detection of rifampicin resistance 30. Culture-based tests dominated discordance that potentially led to over treatment, while Xpert MTB/RIF dominated in the category of discordance with unclear clinical significance. We acknowledge that some discordance could be explained by mixed infections, heteroresistance, or minority resistant populations 31,32.
LPAs were rarely used in our study, possibly because they have been widely replaced by Xpert MTB/RIF, which is easier to use and provides results in a shorter time. In addition, LPA suffer from suboptimal accuracy for isoniazid resistance, and WHO recommends that culture-based DST for isoniazid should still be used, particularly in patients with suspected MDR-TB where the LPA result does not detect isoniazid resistance 33. In one case, the local laboratory detected resistance to ethambutol but this could not be confirmed in the reference laboratory: DST is challenging for ethambutol and less reproducible 34.
Data on treatment outcomes in drug-resistant tuberculosis are scarce, particularly for sub-Saharan Africa. A recent systematic review of treatment outcomes in MDR-TB included data on mortality among adults from seven studies from sub-Saharan Africa, six from South Africa and one from Lesotho 35. In these studies, mortality during tuberculosis treatment ranged from 12.4% in patients with MDR-TB treated in a referral hospital in the Western Cape, South Africa 36, to 45.8% in a study of XDR-TB patients from three South African provinces 37. Our results extend these data to other countries in the region, and add further data for Peru and Thailand.
Our study confirms the poor outcome in patients with isoniazid mono-resistant tuberculosis who are treated with first-line regimens (as recommended by WHO during the study period 38), in line with a study from Durban, South Africa 39 and a recent systemic review and meta-analysis 40. Mortality in mono-resistant tuberculosis patients was higher than in MDR-TB patients, especially in isoniazid mono-resistant tuberculosis. This might be due to the treatment of almost all isoniazid mono-resistant tuberculosis patients with first-line regimens, whereas most MDR-TB patients received second-line treatment. Of note, WHO recently updated its guidelines recommending the inclusion of fluoroquinolones in the treatment of isoniazid mono-resistant tuberculosis 41. Chance is another explanation: there were only few patients with mono-resistant tuberculosis and the analysis of mortality, the confidence intervals of the odds ratios for mono-resistant and MDR tuberculosis overlapped widely (Table 5).
In patients co-infected by HIV, the treatment of drug-resistant tuberculosis is challenging for several reasons, including the poorer absorption of drugs 42, the risk of the immune reconstitution inflammatory syndrome (IRIS) 43, or interactions between antiretroviral and second-line tuberculosis drugs 44–46. In contrast to previous studies from South Africa, which reported higher mortality at end of treatment in HIV-positive patients with MDR-TB compared to HIV-negative MDR-TB patients 36,47, we found no association with HIV infection, although confidence intervals were wide. The median CD4 cell count of HIV-positive patients was considerably higher in our study (192 cells/μL) than in the South African studies 36,47, which may explain the discrepant results. A study from Lesotho 48 also found little evidence for a difference in mortality between HIV-positive patients (median CD4 cell count 185 cells/ μL) and HIV-negative patients. Finally, for patients with XDR-TB, treatment outcomes have been uniformly poor in previous studies, irrespective of HIV status 37.
Our study has several limitations. We sampled eligible patients within strata defined by drug resistance and HIV infection, and therefore could not estimate the incidence or prevalence of drug-resistant tuberculosis in HIV-positive or HIV-negative patients. In previous studies, HIV infection has not been consistently associated with drug resistance 28, but it is clear that in regions with a high-burden of HIV, the majority of patients with MDR-TB will be co-infected with HIV 28. Although we initially exceeded the planned sample size, about a quarter of patients had to be excluded from analyses of drug susceptibility, mainly due to lack of growth or contamination of cultures, and about a third was excluded from the analysis of mortality outcomes, mainly because vital status was unknown at database closure. The reference laboratory tested resistance against six drugs, and we will have missed resistance against other drugs used, for example kanamycin, ethionamide or levofloxacin. Further, the presence of different subpopulations of M. tuberculosis in isolates tested at the local sites vs reference laboratory might have introduced variability in phenotypic or molecular DST testing 49.
In conclusion, our study shows that the accuracy of DST testing in routine care in high-burden countries was limited and that inaccurate results led to inadequate treatment and contributed to the excess mortality associated with drug-resistant tuberculosis. Our results support the notion that access to rapid molecular DST of first- and second-line drugs at treatment initiation is required to improve outcomes in patients with MDR-TB and pre-XDR/XDR-TB 28. Whole genome sequencing is the most promising approach to reach this goal, but much work remains to be done to make this approach feasible and affordable in low- and middle-income countries 28. In particular, direct testing of sputum samples should become routine to circumvent lengthy mycobacterial cultures 40. A standardised approach for the interpretation of drug resistance conferring mutations has recently been developed 50. In the meantime, the capacity for the phenotypic and molecular DST testing recommended by WHO should be increased to ensure the most adequate treatment of drug-resistant tuberculosis in these settings.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are serious threats to the World Health Organization’s End-TB strategy, due to limited access to rapid drug resistance identification and appropriate treatment for patients with MDR-TB or XDR-TB in many high tuberculosis burden countries. We searched PubMed for systematic reviews and original research articles published in any language up to March 31, 2018. We combined terms for “tuberculosis”, “drug resistance testing”, and “mortality”. Several individual studies and systematic reviews have documented the poor outcomes of MDR-TB and pre-XDR/XDR-TB in high-burden countries. Two Cochrane reviews evaluated the accuracy of molecular tests detecting specific mutations associated with resistance, for example the Xpert MTB/RIF, which is recommended by the World Health Organization to detect rifampicin resistance directly from sputum.
Added value of this study
To our knowledge, this is the first multi-country study assessing the accuracy of drug susceptibility testing (DST) in routine settings in high-burden countries by comparing local DST results with those from a tuberculosis reference laboratory, and assessing the impact on mortality. The study showed that the accuracy of local DST to detect any resistance in high-burden countries was moderate (sensitivity 90.8%, specificity 84.3%). Results from the reference and local laboratories were discordant in about 20% of patients. Mortality during treatment was increased almost two-fold in patients with discordant DST results compared to patients with concordant results. Mortality ranged from 6.0% in adequately treated patients with pan-susceptible strains to 53.3% in inadequately treated patients with drug-resistant strains. In multivariable analyses, associations with mortality changed little after adjustment for sex, age, sputum microscopy result and HIV status. Of note, HIV infection was not associated with mortality during tuberculosis treatment.
Implications of all the available evidence
Drug-resistant tuberculosis is difficult to diagnose and to treat, particularly in high-burden settings, where resources are limited. In these settings, inaccurate DST leading to inappropriate treatment contributes to the high mortality associated with drug-resistant tuberculosis. Local access to accurate and rapid DST of first- and second-line drugs is required to improve outcomes in patients with MDR-TB and pre-XDR/XDR-TB. Whole genome sequencing is the most promising approach to reach this goal, but much work remains to be done to make this approach feasible and affordable in high-burden countries.
ACKNOWLEDGEMENTS
We thank all sites who participated in this survey and the patients whose data were used in this study. We are grateful to the Tuberculosis Working Group of IeDEA for helpful discussions, and to four reviewers for thoughtful comments. We also would like to thank all regional data centers, who contributed to the coordination of the study. RJW is supported by the Francis Crick Institute (10218), which is funded by the Wellcome Trust, Cancer Research UK, and Research Councils UK. He also receives support from the Wellcome Trust (104803, 203135). HC is supported by a Wellcome Trust fellowship and reports grants from UK Medical Research Council and the National Research Foundation of South Africa. SG is supported by the Swiss National Science Foundation (310030_166687, IZRJZ3_164171, IZLSZ3_170834 and CRSII5_177163 to SG), the European Research Council (309540-EVODRTB to SG) and SystemsX.ch. ME is supported by by special project funding (Grant No. 174281) from the Swiss National Science Foundation. KK was supported by the National Research University Program, Office of the Higher Education Commission, Bangkok, Thailand, and is no financial supported from Tuberculosis Research Unit, Chulalongkorn University, Thailand, during the conduct of the study.
Funding: National Institutes of Allergy and Infectious Diseases, Swiss National Science Foundation, Swiss National Center for Mycobacteria.
Sources of support
This research was supported by the Swiss National Science Foundation (grant numbers 153442, 310030_166687 and 174281), the National Institutes of Allergy and Infectious Diseases (NIAID) under award numbers U01 AI096299, U01 AI069919, U01 AI069924, U01 AI069911, U01 AI069907, U01 AI096186, and U01 AI069923, and Swiss National Center for Mycobacteria, University of Zurich, Switzerland.
Footnotes
CONFLICTS OF INTEREST
AA has received honoraria fees from Jensen-Cilag, Gilead and Bristol-Myers Squibb. All other authors have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2017. Geneva, 2017. DOI:WHO/HTM/TB/2017.23. [Google Scholar]
- 2.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ 1997; 315: 1194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet 2010; 376: 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy in Low-Income Countries Collaboration of the International epidemiological Databases to Evaluate AIDS (IeDEA),ART Cohort Collaboration, Brinkhof MWG, et al. Tuberculosis after Initiation of Antiretroviral Therapy in Low-Income and High-Income Countries. Clin Infect Dis 2007; 45: 1518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 2010; 10: 489–98. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012; 7: e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariandyshev A, Eliseev P. Drug-resistant tuberculosis threatens WHO’s End-TB strategy. Lancet Infect Dis 2017; 17: 674–5. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006; 368: 1575–80. [DOI] [PubMed] [Google Scholar]
- 9.Klopper M, Warren RM, Hayes C, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis 2013; 19: 449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange C, Abubakar I, Alffenaar JW, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 2014; 44: 23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jr Horsburgh CR. Barry CE, Lange C Treatment of Tuberculosis. N Engl J Med 2015; 373: 2149–60. [DOI] [PubMed] [Google Scholar]
- 12.Wright A, Zignol M, Van Deun A, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 2009; 373: 1861–73. [DOI] [PubMed] [Google Scholar]
- 13.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38: 516–28. [DOI] [PubMed] [Google Scholar]
- 14.Köser CU, Bryant JM, Becq J, et al. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis. N Engl J Med 2013; 369: 290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schon T, Miotto P, Koser CU, Viveiros M, Bottger E, Cambau E. Mycobacterium tuberculosis drug-resistance testing: challenges, recent developments and perspectives. Clin Microbiol Infect 2017; 23: 154–60. [DOI] [PubMed] [Google Scholar]
- 16.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. NEJM 2010; 363 DOI: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Ekouevi DKD, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41: 1256–1264. PMCID: PMC3465765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 2007; 36: 969–76. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Use of high burden country lists for TB by WHO in the post-2015 era. Geneva, 2015. DOI:http://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf.
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, 2014. DOI:WHO/HTM/TB/2014.11. [PubMed] [Google Scholar]
- 22.Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. Geneva, 2018. http://www.who.int/tb/publications/2018/WHO_technical_report_concentrations_TB_drug_susceptibility/en/.
- 23.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision (updated December 2014). 2014. [Google Scholar]
- 24.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993; 49: 865–72. [PubMed] [Google Scholar]
- 25.Cox H, Hughes J, Black J, Nicol MP. Precision medicine for drug-resistant tuberculosis in high-burden countries: is individualised treatment desirable and feasible? Lancet Infect Dis 2018; 3099: 11–6. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Res... - PubMed - NCBI. Geneva, 2011. https://www.ncbi.nlm.nih.gov/pubmed/26158191 (accessed Jan 6, 2018). [PubMed]
- 27.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane database Syst Rev 2014; 1: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 2017. DOI: 10.1016/s2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 29.Sanker P, Ambika AP, Santhosh VT, et al. Are WHO approved nucleic acid amplification tests causing large-scale ‘false identification’ of rifampicin-resistant tuberculosis?: Programmatic experience from south india. Int J Mycobacteriology 2017; 6: 21–6. [DOI] [PubMed] [Google Scholar]
- 30.Chakravorty S, Simmons AM, Rowneki M, et al. The new Xpert MTB/RIF ultra: Improving detection of Mycobacterium tuberculosis and resistance to Rifampin in an assay suitable for point-of-care testing. MBio 2017; 8 DOI: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinder H, Mieskes KT, Löscher T. Heteroresistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2001; 5: 339–45. [PubMed] [Google Scholar]
- 32.Cohen T, van Helden PD, Wilson D, et al. Mixed-strain mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbiol Rev 2012; 25: 708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Policy update. The use of molecular line probe assays for the detection of resistance to isoniazid and rifampicin. Geneva, 2016. http://www.who.int/tb/publications/molecular-test-resistance/en/.
- 34.Kim SJ. Drug-susceptibility testing in tuberculosis: Methods and reliability of results. Eur Respir J 2005; 25: 564–9. [DOI] [PubMed] [Google Scholar]
- 35.Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur. Respir. J. 2017; 49: 1600803. [DOI] [PubMed] [Google Scholar]
- 36.Mugabo P, Adewumi AO, Theron D, Burger A, Van ZL. Do HIV infection and antiretroviral therapy influence multidrug-resistant tuberculosis treatment outcomes? African J Pharm Pharmacol 2015; 9: 875–80. [Google Scholar]
- 37.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: A cohort study. Lancet 2014; 383: 1230–9. [DOI] [PubMed] [Google Scholar]
- 38.Seung K, Satti H. Management of MDR-TB : A field guide. A companion document to Guidelines for the programmatic management of drug-resistant tuberculosis. 2010. [PubMed] [Google Scholar]
- 39.van der Heijden YF, Karim F, Mufamadi G, et al. Isoniazid-monoresistant tuberculosis is associated with poor treatment outcomes in Durban, South Africa. Int J Tuberc Lung Dis 2017; 21: 670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17: 223–34. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. WHO treatment guidelines for isoniazid-resistant tuberculosis. Supplement to the WHO treatment guidelines for drug-resistant tuberculosis. Geneva, 2018. http://apps.who.int/iris/bitstream/handle/10665/260494/9789241550079-eng.pdf?sequence=1. [PubMed]
- 42.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, et al. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin Infect Dis 2004; 38: 280–3. [DOI] [PubMed] [Google Scholar]
- 43.Muller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burman WJ, Gallicano K, Peloquin C. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus-related tuberculosis. Clin Infect Dis 1999; 28: 419–29; quiz 430. [DOI] [PubMed] [Google Scholar]
- 45.Gopalan N, Chandrasekaran P, Swaminathan S, Tripathy S. Current trends and intricacies in the management of HIV-associated pulmonary tuberculosis. AIDS Res. Ther. 2016; 13: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meintjes G Management of drug-resistant TB in patients with HIV co-infection. J Int AIDS Soc 2014; 17: 19508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi NR, Andrews JR, Brust JCM, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis 2012; 16: 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in southern Africa. PLoS One 2009; 4: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merker M, Kohl TA, Roetzer A, et al. Whole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patients. PLoS One 2013; 8: e82551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miotto P, Tessema B, Tagliani E, et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J 2017; 50 DOI: 10.1183/13993003.01354-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


