Abstract
The stability and variability of older adults’ late-differentiated peripheral blood T and natural killer (NK) cells over time remains incompletely quantified or understood. We examined the variability and change over time in T and NK cell subsets in a longitudinal sample of older adults; the effects of sex, cytomegalovirus (CMV) serostatus, and chronic disease severity on immune levels and trajectories; and interdependencies among T and NK cell subsets. Older adults (N=149, age 64–94 years, 42% male) provided blood every 6 months for 2.5 years (up to 5 waves) to evaluate late-differentiated CD8 T cells (CD28–, CD57+) and CD56dimNK cells (CD57+, NKG2C+, FcεRIγ–). In multilevel models, most of the variance in immune subsets reflected stable differences between people. However, CD56dimNK cell subsets (CD57+ and FcεRIγ–) also increased with age, whereas T cell subsets did not. Independent of age, all subsets examined were higher in CMV-positive older adults. Men had higher levels of CD56dim CD57+ than women. Chronic disease was not associated with any immune subset investigated. T and NK cell subsets correlated within each cell type, but interdependencies differed by CMV serostatus. Our results suggest the accumulation of these stable cell populations may be driven less by chronological aging, even less by chronic disease severity, and more by CMV, which may differentially skew T and NK cell differentiation.
Keywords: aging, cytomegalovirus, immunosenescence, CD57, CD28, NKG2C, FcεRγ, longitudinal
1. Introduction
Age-related immune deterioration is associated with increased morbidity and mortality in older adults (Fülöp et al., 2014; Pawelec, 2017). Normal chronological aging changes the frequency, phenotype, and function of innate and adaptive immune cells (Pera et al., 2015; Solana et al., 2012). Viral infections, particularly cytomegalovirus (CMV), or chronic diseases and their treatments may also drive aspects of immunological aging (Kohanski et al., 2016, Muntasell et al., 2013, Weltevrede et al., 2016). Characteristics of immune aging include the accumulation of late-differentiated peripheral blood CD8 T cells that express maturation marker CD57 or lack co-stimulatory molecule CD28 (Appay et al., 2008; Vallejo, 2005) and of CD56dim natural killer (NK) cells that express CD57 or activating receptor NKG2C (Björkström et al., 2010; Solana et al., 2014). Additionally, a subset of CD56dim NK cells from CMV seropositive donors lack the adaptor protein FcεRIγ (Muntasell et al., 2016, Zhang et al., 2013).
Age-heterogenous cross-sectional studies that describe age differences have been used as a basis for inferring age-related change in late-differentiated immune cells (e.g., Bayard et al., 2016; Campos et al., 2014; Saule et al., 2006; Wertheimer et al., 2014). Although cross-sectional approaches provide useful age-associated information in ways not typically feasible in longitudinal studies (e.g., following an individual from young adulthood through old age), they are not amenable to assessing the within-person dynamics in immune subsets over time – this requires longitudinal designs. A handful of studies have examined longitudinal changes in late-differentiated T and NK cells in adults over time (Apoil et al., 2017; Béziat et al., 2013; Cantisán et al., 2017; Foleyet al., 2012; Gumá et al., 2004; Hadrup et al., 2006; High et al., 2005; Iancu et al., 2009; Lee et al., 2015; Lopez-Vergès et al., 2011). Previous evidence is limited, however, by smaller sample sizes, few repeated assessments within person, statistical approaches that do not account for interdependencies in the data, and a focus on primarily middle-age or transplant recipients. Moreover, the influence of sex, one factor that may affect overall levels and changes in immune subsets with age, is not always considered but should be included in analyses (Al-Attar et al., 2016; Whiting et al., 2015). An improved understanding of the dynamics of late-differentiated T and NK cell subsets in healthy older adults has implications for theory development regarding the temporal stability of age- and viral-associated immune markers and for research design considerations (e.g., how reproducible markers are over time). For example, immunomodulatory intervention efforts in older adults will require knowledge of the typical trajectories of these subsets to inform power calculations and decisions about sampling frequency and over what time frame.
The threefold purpose of this investigation was to (1) characterize the variability between individuals and change over time within individuals in CD8 T cell subsets (CD28-, CD57+) and CD56dim NK cell subsets (NKG2C+, CD57+, and FcεRIγ-) in a longitudinal sample of older adults; (2) examine the main and interacting effects of sex, CMV serostatus, and chronic disease severity on immune levels and trajectories over time (i.e., changes with age); and (3) report interdependencies among CD8 T and NK cell subsets. Previous cross-sectional or longitudinal studies have tended to focus on cells from either the innate or adaptive immune system (e.g., Björkström et al., 2010; Hadrup et al., 2006). Our longitudinal study focused on both of the innate and adaptive lymphocytes that recognize and control viral infections and are associated with elderly health outcomes, NK and CD8 T cells (Pera et al., 2015). Based on previous research, we hypothesized that frequencies and cell counts of late-differentiated CD8 T and NK cell subsets would increase with age; that male sex, CMV+ serostatus, and higher chronic disease severity would be associated with higher levels and/or increases over time in CD8 T and NK cell subsets; and that CD8 T cell subsets would correlate with each other, as would NK cell subsets.
2. Material and methods
2.1. Participants
Participants were 149 community-dwelling older adults (mean age = 77 years; range: 64–92 at the first wave). The majority of the sample was female (58%), white (94%), and the remainder was African American (4%) and Asian American/Pacific Islander (2%). Median household income was $60,000 (range: $9,000 – $400,000), and median education was 16 years (range: 9–22). Exclusion criteria included diseases or disorders affecting the immune system including autoimmune diseases (e.g., Grave’s disease, type I diabetes), cancers (e.g., melanoma, lymphoma, breast cancer), immunosuppressive disorders (e.g., HIV, immunoglobulin deficiency), or chronic, severe infections (e.g., hepatitis); chemotherapy or radiation treatment in the 5 years prior to enrollment; unwillingness to undergo venipuncture; immunomodulatory medications including opioids and steroids; or more than two of the following classes of medications: psychotropics, anti-hypertensives, hormone replacement, or thyroid supplements. These criteria excluded major influences on immunity and allowed reasonably healthy adults to participate in this study.
2.2. Procedures
Participants were recruited from a volunteer subject pool maintained by the University of Kentucky Sanders-Brown Center on Aging. The study was conducted with the approval of the University of Kentucky Institutional Review Board. Prospective participants were contacted and screened by telephone. Those who were interested and eligible were enrolled. Participants were interviewed in their homes every 6 months for 2.5 years (up to 5 waves) between July 2011 and December 2016. Sex and date of birth were self-reported at the first wave. At each completed wave, participants received a US $50 gift card. After each interview, study nurses drew blood in the morning hours to control for potential circadian variation.
Of the 149 participants, 139 had cryopreserved cells stored for one or more waves; 69 participants had valid data at wave 1, 66 at wave 2, 107 at wave 3, 117 at wave 4, and 109 at wave 5. Of the possible 149 (people) * 5 (waves) = 745 observations, missing data were present because 36 people missed one or more blood draw appointments or had problems with blood withdrawal (47 person-waves missing), 23 people dropped out (57 person-waves missing), 3 people died (6 person-waves missing), and 3 people did not allow their blood to be drawn (9 person-waves missing). Additionally, cells for flow cytometry analyses were collected and cryopreserved beginning in November 2012; therefore 76 participants did not have cells available from earlier waves for analysis (158 person-waves missing). Overall, 468 person-waves were available for analysis. Of the 139 participants with cryopreserved cells, participants had on average 3.4 available immune data points (SD = 1.26, median = 3, range= 1–5).
2.3. Cytomegalovirus (CMV) serostatus
CMV IgG antibody levels were determined by ELISA (DRG International, Inc, Springfield, NJ) according to manufacturer specifications. The dynamic range of the kit is 1.2–18 IU/mL; initial runs were diluted 40-fold. Samples that were above 18 IU/mL were further diluted (80- and 160-fold) and re-run (n=11 samples). The sensitivity, specificity, and accuracy of the test were 100%, 98%, and 99%, respectively. The intra-assay and inter-assay coefficients of variability were 5.1% and 9.9%. Seropositive status was defined as a CMV IgG Index ≥ 1.00 or concentration > 1.2 IU/mL; seronegative status was defined as a CMV IgG Index < 0.90 (concentration < 1.2 IU/mL). Equivocal status (CMV IgG Index between 0.91–0.99) was assigned to 6 person-waves (4 people); using CMV data from each person’s surrounding time points, 2 people were identified as seronegative (each person had 1–2 waves as equivocal status and 3–4 waves as seronegative); and 2 people were identified as seropositive (each person had 1–2 waves as equivocal status and 2–3 waves as seropositive). Additionally, based on CMV IgG Index scores, 4 people converted from seropositive (1–2 waves each) to seronegative (2–4 waves each) during the study; because their seropositive concentrations were low (range: 0.89–1.7 IU/mL), and they were identified as seronegative for the majority of their waves (range: 0.20 – 0.76 IU/mL), we categorized these people as seronegative. Sensitivity analyses that excluded these 4 individuals did not change results and so they were retained.
The majority of our sample (71%, n=106) was CMV seropositive, which aligns with the high prevalence of CMV infection in older adults in the United States (83% – 90.8%) (Staras et al., 2006). Our sample’s slightly lower CMV seroprevalence may be due to participant socioeconomic factors (i.e., higher education and income) that differ from the US population on average and that are inversely related to CMV seropositivity (Bate et al., 2010).
2.4. Chronic disease
At each interview, participants provided a list of their current prescription medications, which were category-coded by a registered nurse. The medication categories were used to derive the Chronic Disease Score (CDS), which quantifies chronic disease severity (Clark et al.,1995). This score was empirically derived in a large (N > 250,000) sample of adults to predict health care utilization; the CDS represents the chronic diseases for which a participant was being treated, weighted by the likelihood that the disease would result in hospitalization or death (30). Participants in the current study (mean CDS = 1067, range: 175–2511) were taking medications for non-excluded conditions common among community-dwelling older adults, including hypertension, hyperlipidemia, type II diabetes, COPD, arthritis, kidney stones, and osteoporosis. Although the CDS does not directly assess the health outcomes, it provides a valid, indirect measure of physical health and maximizes feasibility in studies such as this one in which there are multiple providers and medical records both between and within participants.
2.5. T and NK cell subsets
Selected panels of immunological markers of T and NK cells were evaluated by flow cytometry. Blood (20 mL) was collected by venipuncture into heparinized tubes and processed within 1–2 hours of collection. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (20 min at 670 g, brake off) using Histopaque (Sigma, St. Louis, MO). Buffy coats were washed once, and cells were counted using a hemocytometer. PBMCs were cryopreserved in liquid nitrogen in RPMI-1640 (Lonza) + 10% fetal bovine serum (Hyclone) + 10% DMSO (Fisher). For flow cytometry analysis, 0.5 million cells were thawed, washed, counted, and treated with Fixable Viability Dye eFluor 780 (eBioscience) for 30 minutes at room temperature. After washing the cells with PBS + 10% FBS, samples were incubated with human IgG for 15 min on ice to block surface Fc receptors. Next, samples were stained on ice for 30 min with combinations of fluorescently labelled monoclonal antibodies specific for surface proteins, washed, and then fixed with 2% paraformaldehyde. For some samples, intracellular staining was performed by first permeabilizing the cells with 1× permeabilization buffer (eBioscience) and then staining with antibodies for 20 min at room temperature. Percentages of lymphocytes of PBMCs were determined; T cells were defined as CD3+ cells and NK cells were defined as CD3-CD56+CD16+ within the lymphocyte population. Surface proteins on T cells were identified with anti-CD8, -CD28, and -CD57 antibodies. Although CD28 and CD57 are unable to account for the full extent of age- and viral-related changes in the CD8 T cell compartment, we focused on these markers in particular because they are associated with clinically relevant health outcomes including mortality, frailty, and impaired vaccine response (Effros, 2007; Lu et al., 2016; Wikby et al., 2005). Also, the majority of CD28 negative cells are within the CD8 T EMRA subset, which are comprised of sub-populations expressing markers associated with late-differentiated cells (Larbi & Fulop, 2014) and are strongly represented in older adults (e.g., Saule et al., 2006). Surface and intracellular proteins on NK cells were identified with anti-CD57, -NKG2C, and -FcεRIγ (intracellular) antibodies. Other antibodies that are not the focus of this investigation but were included in the T and NK cell staining tubes were CD4, KIR, and CD14 (see Supplemental Table 1). After washing, cells were analyzed on a LSR-II flow cytometer (BD, Franklin Lakes, NJ). OneComp ebeads (eBioscience) stained with individual fluorochrome-conjugated antibodies electronically compensated for spectral overlap. Data were analyzed using FlowJo v7.6 software.
Illustrative plots of the analyzed T and NK cell subsets are shown in Supplementary Figures 1 and 2. Frequencies of CD8 T cell subsets are expressed as a percentage of total viable, singlet, CD3+CD8+ cells. Frequencies of CD56dim NK cell subsets are expressed as a percentage of total viable, singlet, CD3-CD16+CD56dim cells. Cell counts (per μL) were obtained by multiplying total leukocyte count via hemocytometer by the percentages of gated lymphocytes. Two laboratory scientists completed the standardized staining and flow cytometry protocol and a categorical variable was included in all models to control for any inter-individual differences.
2.6. Data Analysis
All T and NK cell immune subsets examined were natural log transformed, with the exceptions of frequencies of CD8 T cell subsets (CD28– and CD57+) and one CD56dim NK cell subset (CD57+), which were normally distributed and used in their raw form. Longitudinal data were analyzed in multilevel models that accounted for repeated immune measurements (Level 1, time-varying) within persons (Level 2, time invariant) using the lme (linear mixed-effects) function from the nlme package (version 3.1.118) in R software (version 3.0.3). Standard linear models (e.g., regression) typically should not be used to analyze longitudinal data because they assume each observation is independent of the others. However, given repeated immune measurements over time within a person, we expect that measurements within a person might be more similar to each other than measurements from different persons. This assumption can be modeled using multilevel models, reducing the probability of committing Type I and Type II errors, which could happen if we incorrectly treated observations as independent and conducted a standard linear model. Additional information on all multilevel models conducted (including statistical equations) is provided in Supplementary Material.
2.6.1. Null model.
Data analyses proceeded in several steps. First, to characterize the variability in each immune subset over time, null models with no predictors (see Supplementary Material for equations) were used to estimate the intraclass correlation coefficients (ICC) for each immune subset. The ICC indicates the amount of variability between people; therefore, higher ICC values indicate more variability is attributable to people being different from each other (Level 2) rather than fluctuations over time within persons (Level 1). The ICC also summarizes the size of the residual autocorrelation, or the average correlation between any pair of composite residuals (e.g., between waves 1 and 2, or 2 and 3, or 1 and 5) (Singer & Willett, 2003); thus, the ICC can also be interpreted as the reliability of repeated measures.
2.6.2. Growth curve model.
Next, to test they hypothesis that late-differentiated T and NK cells would increase with age, growth curve models (see Supplementary Material for equations) estimated how each immune subset changed in a linear and quadratic fashion with respect to age (represented by the predictors age and age2). These models provide an overall estimate of the intercept (i.e., the initial level of each T and NK cell subset at age 64, the youngest age at the first wave), as well as the linear slope (i.e., the effect of age) and quadratic slope (i.e., the effect of age2) of each immune subset over time. Random effects of linear (age) and quadratic age (age2) were tested and retained if they improved model fit. In addition, an autoregressive (AR(1)) covariance structure for the residuals was retained if it improved model fit. Residual plots were used to check model assumptions.
2.6.3. Growth curve model with moderators.
To test the hypotheses that male sex, CMV+ serostatus, and higher chronic disease severity would be associated with higher levels and/or increases over time in T and NK cell subsets, these demographic and health variables were added to the growth curve models (see Supplementary Material for equations) to examine their main and interacting effects on the intercept (initial levels) and change over time in each of the five immune subsets. Therefore, these models provide estimates of the intercept and slope (as explained above), as well as estimates of the effect of sex (male versus female), CMV serostatus (seropositive versus seronegative), and chronic disease severity on the initial level of each immune subset (intercept) and its change over time (linear or quadratic slope).
All multilevel results are reported as gamma weights with their standard errors and associated t-tests. Gamma weights are similar to unstandardized B weights in regression. Final models are reported using restricted maximum likelihood (REML) estimation to provide unbiased variance estimates. Standard deviation in the intercept and slope (i.e., between-person variation in initial levels and change over time not accounted for by the predictors) and residuals (i.e., within-person variation not accounted for by predictors) are reported for each model. Full model results are provided in Supplementary Material.
2.6.4. Correlation analysis.
We also conducted a correlational analysis to examine interdependencies among T and NK cell subsets to test the hypothesis that T cell subsets would correlate with each other and that NK cell subsets would correlate with each other. The longitudinal design of the study allowed us to partition the immune subsets into their between-person (Level 2) and within-person (Level 1) components and present correlations at both levels. Between-person correlations depict associations among average levels of immune subsets across all waves, whereas within-person correlations depict how persons’ deviations from their own immune subsets’ averages over time are associated.
3. Results
Descriptive statistics for all study variables are provided in Table 1.
Table 1.
Descriptive Statistics for All Study Variables
| Mean (SD) | Range | ICC | |
|---|---|---|---|
| Age at first wave (years) | 77.8 (5.4) | 64.7 – 92.8 | -- |
| Sex (%) | 42% men | -- | |
| CMV serostatus | 71% seropositive | -- | |
| Mean CMV IgG levels (IU/mL) | 19.2 (21.92) | 0.12 – 111.4 | -- |
| Mean Chronic Disease Score | 1067 (476) | 175 – 2511 | -- |
| Cell Frequencies (%) | |||
| CD8+ CD28- | 56.3 (23.2) | 0.78 – 94.5 | 0.91 |
| CD8+ CD57+ | 48.3 (20.5) | 3.09 – 88.1 | 0.94 |
| CD56dim CD57+ | 70.1 (13.5) | 21.8 – 93.1 | 0.93 |
| CD56dim NKG2C+ | 9.7 (13.3) | 0.06 – 79.7 | 0.96 |
| ln(CD56dim NKG2C+) | 1.5 (1.2) | −2.9 – 4.4 | 0.94 |
| CD56dim FcεRIγ- | 12.0 (17.0) | 0.01 – 83.9 | 0.96 |
| ln(CD56dim FcεRIγ-) | 1.6 (1.4) | −4.6 – 4.4 | 0.92 |
| Cell Counts (counts/μL) | |||
| CD8+ CD28- | 112.2 (117.4) | 0.77 – 781.7 | 0.77 |
| ln(CD8+ CD28-) | 4.1 (1.24) | −0.26 – 6.7 | 0.86 |
| CD8+ CD57+ | 94.5 (97.0) | 1.3 – 566.9 | 0.78 |
| ln(CD8+ CD57+) | 4.0 (1.21) | 0.26 – 6.34 | 0.88 |
| CD56dim CD57+ | 98.2 (77.1) | 3.6 – 497.2 | 0.64 |
| ln(CD56dim CD57+) | 4.3 (0.81) | 1.3 – 6.2 | 0.67 |
| CD56dim NKG2C+ | 14.6 (28.1) | 0.04 – 337.9 | 0.76 |
| ln(CD56dim NKG2C+) | 1.6 (1.5) | −3.2 – 5.8 | 0.86 |
| CD56dim FcεRIγ- | 19.6 (37.6) | 0.01 – 356.1 | 0.78 |
| ln(CD56dim FcεRIγ-) | 1.6 (1.7) | −4.3 – 5.8 | 0.88 |
Note. ln() indicates natural log transformed variable. CMV = cytomegalovirus; ICC = intraclass correlation coefficient; SD = standard deviation.
3.1. Intraclass Correlation Coefficients (ICC)
ICCs for each T and NK cell immune subset (Table 1) were dually interpreted as the amount of variability within versus between people and as the within-person correlation between immune levels at any two random points (to evaluate reliability). ICCs for late-differentiated CD8 T cell and CD56dim NK cell subsets were high (range: 0.91–0.94). Thus, in older adults, 6–8% of the total variability in late-differentiated T and NK cell immune subsets was within individuals (i.e., fluctuations over time within a person) whereas 91–94% of the total variability was due to people being different (i.e., between-person differences). The high ICCs also suggest there was considerable reliability in each immune subset. In other words, the repeated measures of frequencies/counts of immune subsets were highly dependent (i.e., nested) within individuals, and there was very low within-person variability.
3.2. Initial Levels and Change Over Time
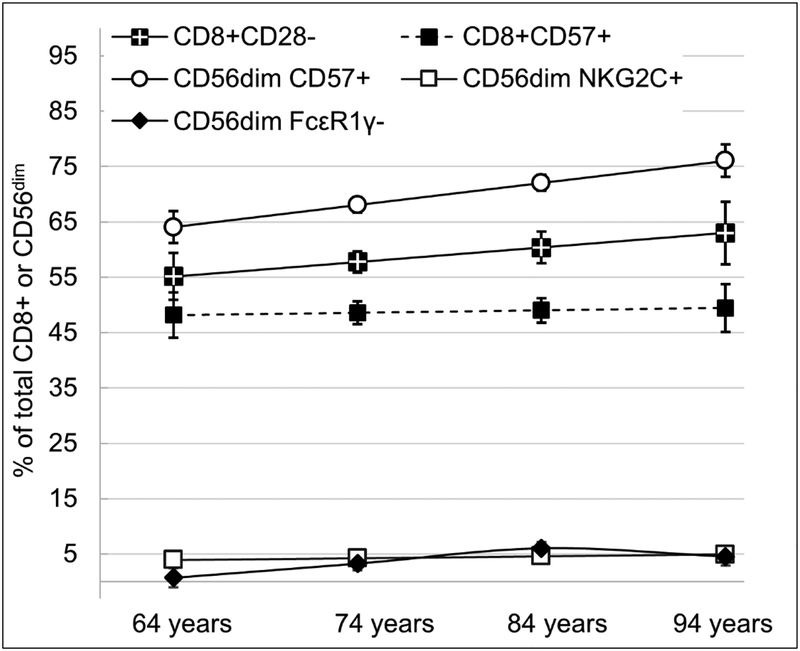
Initial levels and change over time (i.e., the effect of age) in the immune subsets were assessed by estimating the intercepts at age 64 years and the linear and quadratic slopes (age and age2) from that age onward (see Table 2). Figure 1 visually depicts the model-estimated intercepts and age trajectories for the five late-differentiated immune subsets.
Table 2.
Multilevel Model Results for Basic Longitudinal Models of CD8 T cell subsets and CD56dim NK cell subsets
| Fixed effects | CD8+ CD28- | CD8+ CD57+ | CD56dim CD57+ | CD56dim NKG2C+ | CD56dim FcεRIγ- | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | counts | % | counts | % | counts | % | counts | % | counts | |
| γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | |
| Intercept | 55.35** (4.05) |
4.03** (0.26) |
48.19** (3.98) |
3.89** (0.25) |
64.35** (2.71) |
3.63** (0.16) |
1.38** (0.26) |
0.98* (0.32) |
−0.19 (0.52) |
0.022 (0.33) |
| Age (slope) | 0.26 (0.31) |
0.010 (0.017) |
0.043 (0.26) |
0.004 (0.017) |
0.39* (0.18) |
0.037** (0.011) |
0.007 (0.017) |
0.031 (0.021) |
0.19* (0.067) |
0.099** (0.024) |
| Age2 (slope) | NSa | NSa | NSa | NSa | NSa | NSa | NSa | NSa | −0.004* (0.002) |
NSa |
| Random effects |
||||||||||
| Level 2 | ||||||||||
| Intercept SD | 11.69 | 1.08 | 19.43 | 1.09 | 12.40 | 0.61 | 1.16 | 1.31 | 1.35 | 1.03 |
| Age SD | 0.88 | NSb | NSb | NSb | NSb | NSb | NSb | NSb | NSb | 0.06 |
| Level 1 | ||||||||||
| Residual SD | 6.48 | 0.50 | 4.94 | 0.45 | 3.75 | 0.48 | 0.33 | 0.59 | 0.40 | 0.56 |
| Autocorrelation | 0.04 | 0.14 | NSb | 0.09 | 0.11 | 0.07 | 0.17 | 0.14 | NSb | NSb |
Note. All analyses adjusted for lab scientist. All immune subsets were natural log transformed, with the exceptions of % CD8+CD28–, % CD8+CD57+, and % CD56dim CD57+, which were used in their raw form. SD=standard deviation; SE=standard error.
Age2 was removed from covariate-adjusted models when not significant (NS) to accurately estimate the linear age effect.
Random effect did not significantly (NS) improve model fit and was therefore not included.
p<.05;
p<.001.
Figure 1.
Longitudinal age trajectories of frequencies of CD8 T cell subsets (CD28–, CD57+) and CD56dim NK cell subsets (CD57+, NKG2C+, FcεRIγ–). Estimates are from multilevel models and have been back-transformed into original units where appropriate. Error bars represent standard errors of the estimates.
For all immune subsets, the intercepts were significantly different from zero, indicating that for the average older adult aged 64 years, the frequencies and counts of the immune subsets were non-zero. Frequencies and counts of CD56dim NK cell subsets CD57+ (%: γ=0.40, SE=.18, t(314)=2.26, p=.025, counts: γ=0.04, SE=.011, t(300)=3.44, p<.001) and FcεRIγ– (%: γ=0.19, SE=.067, t(295)=2.81, p=.005; counts: γ= 0.10, SE=.024, t(281)= 4.12, p< .0001) significantly increased with age. Additionally, a significant quadratic effect of age (age2) on frequencies of CD56dim NK cell subset FcεRIγ– (γ= −0.004, SE=.002, t(295)=−2.12, p=.035) indicated that the positive slope of age leveled off at older ages. T cell subsets (CD28- and CD57+) and one CD56dim NK cell subset (NKG2C+) did not show significant linear or quadratic change with age.
3.3. Between-Person Variability
Between-person differences in initial levels and change over time in immune subsets were evaluated using variance estimates of the intercepts and age-related slopes. The best-fitting model for most immune subsets included only a random intercept but no random slope; thus, people significantly varied in their initial levels of each immune subset but did not significantly vary in their trajectories over time. There were two exceptions, however: specifying a random slope significantly improved the model fit for CD8 T cell subset CD28– (%) and CD56dim NK cell subset FcεRIγ– (counts) by log likelihood comparisons. Therefore, people significantly varied in their age trajectories of CD8+CD28– and CD56dim FcεRIγ–, but had similar trajectories for all other immune subsets.
3.4. Main and Interacting Effects of Demographic and Health Variables
We tested main and interacting effects of sex, CMV serostatus, and CDS on initial levels and change over time in immune variables. Full model results are presented in Supplementary Material.
3.4.1. Sex.
There was a significant main effect of sex on frequencies of CD56dim NK cell subset CD57+ (γ= −5.01, SE=2.17, t(136)= −2.31, p=.022) such that men had significantly higher frequencies (68%) than women (63%). This association remained significant after adjusting for CMV serostatus (γ= −5.31, SE=2.14, t(134)= −2.49, p=.014). In addition, there was a significant sex by age interaction for CD56dim NK cell subset FcεRIγ– (γ= −0.10, SE=.04, t(294)=−2.51, p=.013); men had a steeper increase in FcεRIγ– with age (simple slope: b=0.24, SE=.07, t(294)=3.30, p=.001) compared with women (simple slope: b=0.15, SE=0.07, t(294)=2.13, p=.034). This effect decreased only partially in magnitude but was no longer statistically significant when adjusting for CMV serostatus (γ= −0.07, SE=.03, t(293)=−1.94, p=.054).
3.4.2. CMV.
There were significant main effects of CMV serostatus on all CD8 T cell and CD56dim NK cell subsets examined. Specifically, CMV+ serostatus was associated with higher frequencies and counts of CD8+CD28– (%: γ= 23.62, SE=3.30, t(136)=7.16, p<.0001; counts: γ= 1.43, SE=0.18, t(136)=8.12, p<.0001); CD8+CD57+ (%: γ= 21.21, SE=3.26, t(136)=6.51, p<.0001; counts: γ=1.42, SE=0.18, t(136)=8.09, p<.0001); CD56dim CD57+ (%: γ=5.70, SE=2.36, t(135)=2.41, p=.017; counts: γ=0.25, SE=0.13, t(135)=1.93, p=.055); CD56dim NKG2C+ (%: γ=1.04, SE=0.21, t(132)=4.96, p<.0001; counts: γ=1.20, SE=0.24, t(132)=4.97, p<.0001) and CD56dim FcεRIγ– (%: γ=1.67, SE=0.22, t(132)=7.60, p<.0001; counts: γ=1.78, SE=0.25, t(132)=7.24, p<.0001). There were no significant interactions between CMV serostatus and age or age2 for any immune subset examined. Therefore, CMV serostatus affected initial levels of immune subsets, but not changes over time.
3.4.3. CDS.
There were no significant main or interacting effects of CDS on any immune subset. Higher CDS scores tended to be associated with higher frequencies of CD56dim NK cell subset CD57+, but this effect was not statistically significant (γ=0.004, SE=0.002, t(136)=1.81, p=.072).
3.5. Interdependencies among CD8 T cell and CD56dim NK cell Subsets
Table 3 depicts bivariate associations among the frequencies and counts of late-differentiated T cell and NK cell subsets. Given the substantial effect of CMV infection on cell subsets, we stratified the sample and present correlations among immune subsets for CMV seropositive (N=106) and seronegative (N=41) older adults.
Table 3.
Interdependencies among CD8 T cell subsets and CD56dim NK cell subsets in CMV seropositive (N=106) and seronegative (N=41) older adults
| CD8+CD28- | CD8+CD57+ | CD56dim CD57+ | CD56dim NKG2C+ | CD56dim FcεRIγ- | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | counts | % | counts | % | counts | % | counts | % | counts | |
| CMV seropositive | ||||||||||
| CD8+CD28- | -- | -- | 0.77 | 0.98 | 0.12 | 0.49 | 0.11 | 0.23 | 0.06 | 0.24 |
| CD8+CD57+ | 0.91* | 0.98* | -- | -- | 0.16 | 0.51 | 0.10 | 0.24 | 0.04 | 0.26 |
| CD56dim CD57+ | −0.06 | −0.06 | −0.05 | −0.06 | -- | -- | 0.28 | 0.58 | 0.36 | 0.68 |
| CD56dim NKG2C+ | 0.11 | 0.01 | 0.08 | 0.03 | 0.29* | 0.52* | -- | -- | 0.63 | 0.82 |
| CD56dim FcεRIγ- | 0.14 | 0.01 | 0.12 | 0.03 | 0.41* | 0.62* | 0.52* | 0.69* | -- | -- |
| CMV seronegative | ||||||||||
| CD8+CD28- | -- | -- | 0.66 | 0.93 | 0.09 | 0.23 | 0.22 | 0.27 | 0.18 | 0.23 |
| CD8+CD57+ | 0.93* | 0.94* | -- | -- | 0.19 | 0.23 | 0.30 | 0.26 | 0.23 | 0.21 |
| CD56dim CD57+ | −0.14 | −0.01 | 0.01 | 0.10 | -- | -- | 0.38 | 0.36 | 0.39 | 0.26 |
| CD56dim NKG2C+ | 0.10 | 0.47* | 0.21 | 0.60* | −0.12 | 0.34* | -- | -- | 0.95 | 0.97 |
| CD56dim FcεRIγ- | 0.33* | 0.47* | 0.37* | 0.58* | −0.07 | 0.24 | 0.76* | 0.92* | -- | -- |
Note. The sample was stratified according to the presence (upper block) or absence (bottom block) of latent CMV infection. Between-person (Level 2) correlations are shown below the diagonal. Within-person (Level 1) correlations are shown above the diagonal. No statistical significance indication for within-person correlations is provided because doing so may be biased when the data are not independent observations. Correlations are shown only for the purpose of illustrating effect sizes.
p<.05
3.5.1. T cell subsets.
In both CMV-positive and CMV-negative older adults, higher frequencies and counts of CD8 T cell subsets CD28– and CD57+ were strongly correlated across the entire study period (r’s = .91–.93 for % and .94–.98 for counts). Additionally, changes in CD28– cells within-person were associated with changes in CD57+ in the same direction (r’s = .66–.77 for % and .93–.98 for counts).
3.5.2. NK cell subsets.
In CMV-positive older adults, the CD56dim NK cell subsets were moderately correlated with each other both between people (r’s = .29–.52 for % and .52–.69 for counts) and within-person (r’s=.28–.63 for % and .58–.82 for counts); the strongest associations were between FcεRIγ– and NKG2C+ subsets. In CMV-negative older adults, the NK cell subsets were correlated with each other at the within-person level (r’s = .38–.95 for % and .26–.97 for counts), but CD57+ was not as strongly associated with NKG2C+ or FcεRIγ– between people (r’s = −.07– −.12 for % and .24–34 for counts).
3.5.3. T cell and NK cell subsets.
In CMV-positive older adults, there were no associations among the T cell and NK cell subsets at the between-person level (r’s = −.06–.14 for % and −.06–.03 for counts). Similarly, at the within-person level, T cell and NK cell frequencies were not associated (r’s = .04–.16) but cell counts were moderately associated (r’s=.23–.51). In CMV seronegative older adults, higher frequencies of CD56dim NK cell subset FcεRIγ– across the study period were associated with higher CD8 T cell subsets (CD28– and CD57+) (r’s = .33–.37 for % and .47–.58 for counts) and changes in CD56dim NKG2C+ within-person were moderately associated with changes in CD8+CD57+ (r’s=.30 for % and .26 for counts).
4. Discussion
Longitudinal studies provide a powerful means to examine within- and between-person changes in immunological aging markers while the within-person element simultaneously avoids potential confounds due to between-person differences (because people act as their own controls over time). To our knowledge, this longitudinal study is the first and largest, with the most immune assessments (up to 5 waves per person), to explicitly address key methodological questions of late-differentiated immune subsets and their stability, change, and interdependencies over time.
Frequencies and cell counts of late-differentiated CD8 T cell (CD28– and CD57+) and CD56dim NK cell subsets (CD57+, NKG2C+, FcεRIγ–) were stable within people over time (up to 2.5 years) and reliable. However, there was substantial variability between older adults (up to 94%) in immune subsets, reflecting differences in levels of immune subsets more than differences in aging trajectories. Our findings corroborate previous studies that report CD28 expression on CD8 T cells is relatively stable across 14 days to 5 months in older adults without acute illness (High et al., 2005) and that terminally differentiated T cell subsets are relatively stable over 22 months in healthy adults (Apoil et al., 2017). Additionally, our NK cell findings parallel others suggesting that NK cell subsets, particularly those found in CMV infected people, are stable across 6 months to 4 years in healthy donors (Béziat et al., 2013; Lee et al., 2015). After infection with a pathogen such as CMV, epigenetic remodeling of the innate and adaptive immune system may promote the stability of these immune populations over time (Lee et al., 2015; Tu & Rao, 2016), and our findings suggest this stability persists into older adulthood. The purpose of this stability is to maintain lymphocytes’ memory-like properties to effectively control CMV, which likely represents an adaptation of the aging immune system that confers survival advantages (Pawelec, 2012).
Frequencies and counts of two CD56dim NK cell subsets, CD57+ and FcεRIγ–, increased with age. Furthermore, increases in FcεRIγ– leveled off at older ages (i.e., a significant quadratic effect). Cross-sectional evidence suggests proportions of CD56dim CD57+ cells are higher in older ages (Le Garff‐Tavernier et al., 2010; Segerstrom et al., 2012), and our longitudinal study confirms this finding using repeated assessments within people. To our knowledge, there are no previous reports of CD56dim FcεRIγ– changing with age. Instead, NK cells lacking this adaptor protein are primarily examined in the context of CMV (Zhang et al., 2013). The impact of age versus the impact of CMV on late-differentiated T and NK cell subsets is an ongoing area of investigation (Bayard et al., 2016; Campos et al., 2014; Wertheimer et al., 2014). Our results of the effects of age on CD56dim NK cell subsets CD57+ and FcεRIγ– over time are independent of CMV serostatus. Moreover, the lack of significant interaction between CMV and age on these NK cell subsets suggests their effects are additive and not interactive.
Contrary to our hypothesis, neither frequencies nor counts of CD56dim NK cell subset NKG2C+ and CD8 T cell subsets (CD28–, CD57+) increased with age. Previous cross-sectional studies have documented age differences (over multiple decades) in these NK cell and T cell populations (e.g., Bayard et al., 2016; Saule et al., 2006). In the current study, there was variability in the frequencies and counts of these immune populations, so restriction of range was not an issue, and the older adult sample had a large age range (64–94 years). Ultimately, these cell subsets may not be driven by chronological aging during older age, but rather, aging effects may occur through young and middle age. Another possibility is that independent of aging, CMV infection occurs before or in midlife (e.g., at least 25 years prior to enrolling in a study involving older adults, aged 64+), which causes an expansion of T and NK cell populations that become late-differentiated and persist into older adulthood (Nikolich-Zugich et al., 2017). Indeed, even younger cross-sectional cohorts with congenital CMV infection display high frequencies of late-differentiated NK and CD8 T EMRA cells (Goodier et al., 2014; Noyola et al., 2012; Saghafian-Hedengren et al., 2013), possibly reflecting a larger impact of early CMV acquisition rather than age-dependent effects.
Previous studies have consistently identified an association between CMV and late-differentiated T and NK cell subsets (reviewed in Muntasell et al., 2013 and Weltevrede et al., 2016), but evidence of the moderating role of CMV on change over time in subsets is limited. Our previous work has demonstrated that CMV concentrations (i.e., the ability to control CMV) was inversely associated with late-differentiated T and NK cell composites (Reed et al., 2018). However, CMV infection forever alters the host immune system, and so in the current study we focused on CMV serostatus, or the categorical difference between those whose immune systems have versus have not been modified by CMV. In our sample, CMV serostatus had the most consistent and prominent effect between people on all immune subsets, as compared to other demographic and health variables. CMV+ serostatus was associated with significantly higher initial levels of all five immune subsets examined. However, CMV serostatus did not moderate age trajectories for any cell subset, suggesting CMV+ serostatus affects overall levels but does not accelerate the accumulation of late-differentiated immune subsets during older age. This contrasts in part with a cross-sectional study that reported a significant CMV by age category (but not CMV by linear chronological age) interaction, such that CMV was associated with significant individual differences in CD8 effector memory cells and these differences were especially pronounced for older adults (65+ years), compared with middle-aged (50–64 years) and younger adults (20–40 years) (Wertheimer et al., 2017). If our study had also assessed older adults earlier in their development (e.g., middle age), we may have recapitulated these results in a longitudinal design; however, this is one instance where comparisons of cross-sectional and longitudinal findings yield different conclusions about the pattern of CMV and age on T cell subsets.
There were sex differences in CD56dim NK cell but not CD8 T cell subsets. Across all waves, men had higher percentages of CD56dimCD57+ than women. Men also had steeper increases in CD56dimFcεRIγ– with age than women, but this effect was diminished and in part explained by CMV. The CD56bright to CD56dim NK cell ratio is lower in elderly men than in elderly women (Al-Attar et al., 2016), and this lower ratio for men may be due in part to expansion of long-lived memory-like NK cells that accumulate over time and with chronic infection exposure, such as CMV. Although differences in sex hormones decrease with age (Decaroli & Vincenzo, 2017), sex differences in NK cell maturation and differentiation may persist into older adulthood. Our findings may in part explain sex-specific health disparities in major causes of death, including infectious diseases and cancer in which the (innate) immune system is invoked (Austad & Fischer, 2016). In contrast with NK cells, we found no sex differences in initial levels or changes over time in CD8 T cell subsets (CD28–, CD57+). Our T cell findings are similar to one previous report in a similar aged cohort (70–90 years old) (Al-Attar et al., 2016), but contrast with others who reported women had lower levels of terminally differentiated CD8+ T cells in a sample aged 19–67 years (Apoil et al., 2017) and higher levels of less differentiated CD8+CD27+ T cells in a sample aged 40–97 years (Whiting et al., 2015). Including sex in investigations of immune subsets will continue to be an important future consideration.
Based on evidence that chronic diseases may drive aspects of biological aging (Kohanski et al., 2016), we expected that chronic disease prevalence and severity would associate with higher initial levels or increases over time in immune subsets. We found no such associations. One consideration is the study’s exclusion criteria, which may have resulted in restriction of range of chronic disease severity by excluding the very people who may significantly differ in accumulation of late-differentiated immune cells (e.g., those with cancer or HIV). However, in the OCTO Immune Study, there were no morbidity-related differences in the T cell phenotypes of their sample (aged 86, 90, or 94 at baseline), the majority of which were considered “frail” but also included “very healthy” and “moderately healthy” people (Nilsson et al., 2003). Therefore, late-differentiated T and NK cells subsets may be independent of individuals’ current health status.
There were interdependencies among T and NK cell subsets, mainly within each immune cell compartment. In both CMV seropositive and seronegative older adults, CD8 T cell subsets (CD28–, CD57+) were strongly correlated. Our results corroborate a similar cross-sectional correlation between these subsets in 50 donors (r=.91) (Apoil et al., 2017). In future research, these subsets could be combined to form a reliable composite of late-differentiated T cell aging (e.g., Reed et al., 2018). Within the CD56dim NK cell compartment, NKG2C+ and FcεRIγ– correlated more strongly with each other than with CD57+; however, they too might form a reliable composite. In the present study, we were unable to examine NK cell subsets that co-expressed NKG2C+ and CD57+; however, we expect that these subsets would display similar associations as did their individual markers. Correlations among T cell and NK cell subsets differed based on CMV serostatus. In CMV-positive older adults, T and NK cell subsets were not associated between people. However, in CMV-negative older adults, some NK cell subsets correlated with late-differentiated T cell subsets. CMV may have divergent effects on T and NK cell differentiation (Bayard et al., 2016; Bengnér et al., 2014; Bigley et al., 2016). For example, in CMV-positive young and middle-age adults, those with good viral control had either high T cell or high NK cell markers of late differentiation, but not both, suggesting people with CMV may contain the virus through either an NK cell or T cell mediated response (Bigley et al., 2016). This interpretation may extend to older adults, however, we cannot make this conclusion because we found no significant inverse correlations between NK and T cell subsets in CMV-infected older adults.
There are few longitudinal studies that explicitly examine the dynamics of immunological aging markers over time, despite implications for theory development and research design. In the present investigation we examined changes in immune subsets over an intermediate time frame (e.g., months) and found evidence that these subsets are stable within people and reproducible on the time scale of months to 2.5 years. Future longitudinal studies should strive to focus on the populations (e.g., clinically diverse cohorts), age ranges, and environmental conditions (e.g., life stressors that may accelerate differentiation) with high probability for being the most dynamic with respect to the age- and viral-associated phenotypes of interest. Additionally, utilizing the longest possible study duration (e.g, on the order of 5–10 years) with a lower sampling frequency (e.g., annually) could increase the possibility of capturing change within person over time.
Limitations of the present study include a sample drawn from a WEIRD (i.e., Western, Educated, Industrialized, Rich, Democratic) society–so to the extent that larger social institutions may affect health, these were generally healthier older adults. Thus, the results may not extend to older adult samples from other populations. For practical reasons, immune cells from the peripheral blood were collected, but these may not exhibit the same patterns of cell subset distribution as tissue compartments (e.g., see Dock et al., 2017). Additionally, future research on the molecular mechanisms regulating receptor expression and stability in NK and T cells is warranted to complement our current understanding of the dynamics of T and NK cell markers of late differentiation. Despite any study limitations, this is, to our knowledge, the largest longitudinal study to date to rigorously examine within-person stability over time in these immune subsets and therefore improves upon previous cross-sectional studies in which longitudinal inferences are made from independent observations.
5. Conclusion
Late-differentiated T and NK cell subsets were stable within persons on the order of months to 2.5 years, but exhibited considerable heterogeneity between people. Some CD56dim NK cell subsets (CD57+ and FcεRIγ–) increased with age, whereas CD56dim NKG2C+ and CD8 T cell subsets (CD28– and CD57+) did not. Independent of age, all subsets examined were significantly higher in CMV+ older adults (~21–24% higher in T cell subsets, and ~3–6% higher in NK cell subsets). Therefore, the accumulation of these late-differentiated populations may be driven less by chronological aging and even less by chronic disease severity and more by latent viral infections, particularly CMV. This viral infection, however, may differentially affect the T and NK cell populations such that if an individual has high levels in one immune cell compartment, they may not necessarily have high levels in the other. Ultimately, our findings and others dictate the next step is to test the predictive value of these immune parameters for clinical outcomes (e.g., response to vaccines, frailty, and morbidity) to determine whether these markers reflect functional “immunosenescence” (Pawelec, 2017).
Supplementary Material
Highlights.
Late-differentiated T and NK cell subsets were stable over 2.5 years in older adults
Independent of age, all subsets examined were higher in CMV-positive adults
Men had higher levels of CD56dim CD57+ than women
Chronic disease severity was not associated with any immune subset investigated
Interdependencies among T and NK cell subsets differed by CMV serostatus
Acknowledgements
The UK Flow Cytometry & Cell Sorting core facility is supported in part by the Office of the Vice President for Research, the Markey Cancer Center and an NCI Center Core Support Grant (P30 CA177558) to the University of Kentucky Markey Cancer Center.
Funding
This work was supported by the National Institute on Aging (K99-AG056635 [RGR], R01-AG026307 [SCS], K02-AG033629 [SCS], P30-AG028383).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Al-Attar A, Presnell SR, Peterson CA, Thomas DT, Lutz CT. The effect of sex on immune cells in healthy aging: elderly women have more robust natural killer lymphocytes than do elderly men. Mech Ageing Dev. 2016;156:25–33. doi: 10.1016/j.mad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, et al. Influence of age, sex and HCMV-serostatus on blood lymphocyte subpopulations in healthy adults. Cell Immunol. 2017;314:42–53. doi: 10.1016/j.cellimm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard C, Lepetitcorps H, Roux A, et al. Coordinated expansion of both memory T cells and NK cells in response to CMV infection in humans. Eur J Immunol. 2016;46:1168–1179. doi: 10.1002/eji.201546179. [DOI] [PubMed] [Google Scholar]
- Bengnér M, Béziat V, Ernerudh J, et al. Independent skewing of the T cell and NK cell compartments associated with cytomegalovirus infection suggests division of labor between innate and adaptive immunity. Age. 2014;36:571–582. doi: 10.1007/s11357-013-9587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat V, Liu L, Malmberg JA, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013:121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley AB, Spielmann G, Agha N, O’Connor DP, Simpson RJ. Dichotomous effects of latent CMV infection on the phenotype and functional properties of CD8+ T-cells and NK-cells. Cell Immunol. 2016;300:26–32. doi: 10.1016/j.cellimm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK cell differentiation uncoupled from NK cell education. Blood. 2010:116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- Campos C, Pera A, Sanchez-Correa B, et al. Effect of age and CMV on NK cell subpopulations. Exp Gerontol. 2014;54:130–137. doi: 10.1016/j.exger.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Cantisán S, Páez-Vega A, Santos F, et al. Impact of age and cytomegalovirus on CD8+ T-cell compartment remodeling after solid organ transplantation: a one-year follow-up study. Exp Gerontol. 2017;95:98–106. doi: 10.1016/j.exger.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Clark DO, Von Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–795. [DOI] [PubMed] [Google Scholar]
- Decaroli MC, Vincenzo R. Aging and sex hormones in males. Virulence. 2017;8:545–570. doi: 10.1080/21505594.2016.1259053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dock J, Ramirez CM, Hultin L, et al. Distinct aging profiles of CD8+ T cells in blood versus gastrointestinal mucosal compartments. PloS One. 2017; 12:e0182498. doi: 10.1371/journal.pone.0182498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032 [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–138. [DOI] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C+ NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Witkowski JM, Pawelec G, Alan C, Larbi A. On the immunological theory of aging. Interdiscip Top Gerontol. 2014;39:163–176. 10.1159/000358904. [DOI] [PubMed] [Google Scholar]
- Goodier MR, White MJ, Darboe A, Nielsen CM, Goncalves A, Bottomley C, Moore SE, Riley EM. Rapid NK cell differentiation in a population with near-universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood. 2014;124:2213–22. doi: 10.1182/blood-2014-05-576124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Køllgaard T, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006; 176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- High KP, Trader M, Pahor M, Loeb M. Intraindividual variability and the effect of acute illness on immune senescence markers. J Am Geriatr Soc. 2005;53:1761–1766. doi: 10.1111/j.1532-5415.2005.53526.x. [DOI] [PubMed] [Google Scholar]
- Iancu EM, Corthesy P, Baumgaertner P, et al. Clonotype selection and composition of human CD8 T cells specific for persistent herpes viruses varies with differentiation but is stable over time. J Immunol. 2009;183:319–331. doi: 10.4049/jimmunol.0803647. [DOI] [PubMed] [Google Scholar]
- Kohanski RA, Deeks SG, Gravekamp C, et al. Reverse geroscience: how does exposure to early diseases accelerate the age‐related decline in health?. Ann N Y Acad Sci. 2016;1386:30–44. doi: 10.1111/nyas.13297. [DOI] [PubMed] [Google Scholar]
- Larbi A, Fulop T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytometry Part A. 2014;85:25–35. doi: 10.1002/cyto.a.22351 [DOI] [PubMed] [Google Scholar]
- Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garff‐Tavernier M, Béziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tan CTY, Nyunt MSZ, Mok EWH, Camous X, Kared H, … & Larbi A. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget. 2016;7:28783–95. doi: 10.18632/oncotarget.8939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Pupuleku A, Cisneros E, et al. Relationship of NKG2C copy number with the distribution of distinct cytomegalovirus-induced adaptive NK cell subsets. J Immunol. 2016;196:3818–3827. doi: 10.4049/jimmunol.1502438. [DOI] [PubMed] [Google Scholar]
- Muntasell A, Vilches C, Angulo A, López‐Botet M. Adaptive reconfiguration of the human NK‐cell compartment in response to cytomegalovirus: A different perspective of the host‐pathogen interaction. Eur J Immunol. 2013;43:1133–1141. doi: 10.1002/eji.201243117. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Goodrum F, Knox K, Smithey MJ. Known unknowns: how might the persistent herpesvirome shape immunity and aging?. Curr Opin Immunol. 2017;48:23–30. doi: 10.1016/j.coi.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson BO, Ernerudh J, Johansson B, et al. Morbidity does not influence the T-cell immune risk phenotype in the elderly: findings in the Swedish NONA Immune Study using sample selection protocols. Mech Ageing Dev. 2003;124:469–476. doi: 10.1016/S0047-6374(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Noyola DE, Fortuny C, Muntasell A, Noguera‐Julian A, Muñoz‐Almagro C, Alarcón A, Juncosa T, Moraru M, Vilches C, López‐Botet M. Influence of congenital human cytomegalovirus infection and the NKG2C genotype on NK‐cell subset distribution in children. Eur J Immunol. 2012;42:3256–66.doi: 10.1002/eji.201242752 [DOI] [PubMed] [Google Scholar]
- Pawelec G Does the human immune system ever really become “senescent”?. F1000Res. 2017;6: F1000.Faculty Rev-1323. doi: 10.12688/f1000research.11297.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing. 2012;9:15. doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G Immune parameters associated with mortality in the elderly are context-dependent: lessons from Sweden, Holland and Belgium. Biogerontology. 2017;28:1–9. doi: 10.1007/s10522-017-9739-z. [DOI] [PubMed] [Google Scholar]
- Pera A, Campos C, López N, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Reed RG, Presnell SR, Al-Attar A, Lutz CT, Segerstrom SC. Perceived stress, cytomegalovirus titers, and late-differentiated T and NK cells: Between- and within-person associations in a longitudinal study of older adults. Manuscript submitted for publication, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghafian-Hedengren S, Sohlberg E, Theorell J, Carvalho-Queiroz C, Nagy N, Persson JO, Nilsson C, Bryceson YT, Sverremark-Ekström E. Epstein-Barr virus coinfection in children boosts cytomegalovirus-induced differentiation of natural killer cells. J Virol. 2013; 87:13446–55. doi: 10.1128/JVI.02382-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4+ versus effector memory and terminally differentiated memory cells in CD8+ compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Al-Attar A, Lutz CT. Psychosocial resources, aging, and natural killer cell terminal maturity. Psychol Aging. 2012;27:892–902. doi: 10.1037/a0029093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. 2014;29:56–61. doi: 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. 10.1016/j.smim.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–51. doi: 10.1086/508173 [DOI] [PubMed] [Google Scholar]
- Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T‐cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x [DOI] [PubMed] [Google Scholar]
- Weltevrede M, Eilers R, de Melker HE, van Baarle D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: a systematic review. Exp Gerontol. 2016;77:87–95. doi: 10.1016/j.exger.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Wertheimer AM, Bennett MS, Park B, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014:192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting CC, Siebert J, Newman AM, et al. Large-scale and comprehensive immune profiling and functional analysis of normal human aging. PloS One. 2015;10:e0133627. doi: 10.1371/journal.pone.0133627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–65. 10.1093/gerona/60.5.556 [DOI] [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013:190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.