Abstract
Purpose:
Sjögren’s syndrome (SS) is a common autoimmune disease affecting about four million Americans. Although approximately 1 in 10 patients with clinically-significant aqueous deficient dry eye has underlying SS, widespread underappreciation of SS leads to significant underdiagnosis, delays in diagnosis, and consequent morbidity and mortality. The purpose of this article is to illustrate that in addition to dry eye, SS can cause serious, vision-threatening extraglandular ocular manifestations.
Methods:
We conducted a narrative review of studies that have examined the dry eye and extraglandular ocular complications of SS.
Results:
SS-related dry eye is a progressive condition with major negative impact on the quality-of-life of afflicted patients, not only due to symptoms of ocular discomfort but also visual dysfunction. In addition, SS can lead to corneal melt/perforation, uveitis, scleritis, retinal vasculitis, and optic neuritis. A major problem with currently-available SS disease activity measurement instruments is the lack of domains evaluating dry eye-related visual dysfunction. For example, one of the most commonly-used instruments for assessing patient symptoms in SS (the EULAR Sjögren’s Syndrome Patient Reported Index [ESSPRI]) only includes one item (out of three) that addresses the severity of overall dryness, without mention of dry eye symptoms or vision-related quality-of-life. Similarly, no extraglandular ocular complications are included in currently-available SS disease activity instruments (e.g., the EULAR Sjögren’s Syndrome Disease Activity Index [ESSDAI]).
Conclusions:
There needs to be a paradigm shift in which eye care providers and rheumatologists become more familiar with various SS-related extraglandular ocular manifestations. Existing disease activity measurement instruments must incorporate dry eye symptoms, particularly those related to visual dysfunction. An evidence-based screening algorithm for determining which patients with dry eye should be tested for underlying SS may be particularly helpful in preventing delays in diagnosis.
Keywords: Sjögren’s syndrome, dry eye, extraglandular symptoms, underdiagnosis
Sjögren’s syndrome (SS) is a common autoimmune disease that affects approximately 4 million Americans.1 SS manifests as chronic and debilitating inflammation that is mediated by autoantibody production and lymphocytic infiltration and, ultimately, causes permanent destruction of exocrine glands,2 resulting in sicca symptoms, such as dry eye and dry mouth. SS was first described in 1933 by Dr. Henrik Sjögren,3 a Swedish ophthalmologist, who reported 19 women with severe dry eye and dry mouth who also had lymphocytic infiltration of lacrimal and salivary glands, as well as systemic manifestations.
OBSERVATIONS
Although sicca symptoms are the sine qua non and most bothersome symptoms of SS, systemic consequences, such as visceral organ involvement, malignancies, peripheral neuropathy, myelitis, and meningitis, are common as well.4–7 A 2014 systematic review found that compared with the general population, SS was associated with an increased risk of any cancer (relative risk [RR], 1.5; 95% confidence interval [CI], 1.2–1.9), non-Hodgkin lymphoma (RR, 13.8; 95% CI, 8.5–19.0), and thyroid cancer (RR, 2.6; 95% CI, 1.1–4.0).5 Indeed, SS is an independent risk factor and the autoimmune disease most frequently associated with lymphoma.6–13
Dry eye affects approximately 8% of the more than 108 million Americans older than 50 years; approximately 1 in 10 of these patients with dry eye has underlying SS.14 However, underlying SS remains undiagnosed in two-thirds of those patients with dry eye who have SS and are seen in eye clinics.2,15,16 Even in the one-third of patients in whom the diagnosis is made, the average time from onset of dry eye symptoms to diagnosis of SS is about a decade.17,18
Various factors have led to this significant underdiagnosis and delayed diagnosis of SS in patients with dry eye. First, SS is a complex disease with diverse symptoms involving various organs and systems in the body. Second, dry eye is highly prevalent and multifactorial, making it challenging to identify patients with underlying SS. There is a lack of evidence-based, validated screening tools or algorithms to determine which patients with dry eye should be worked up for SS. Third, there is widespread underappreciation of the importance of SS among eye care providers, leading to underreferral for systemic workups. In a recent survey of ophthalmologists, approximately half reported that they refer fewer than 5% of patients for SS workup; approximately 1 in 5 ophthalmologists reported never referring any patients.19 Systemic inflammatory diseases are common in patients with clinically significant aqueous-deficient dry eye, and a majority of those patients have underlying SS with no previous diagnosis.20
SS-related dry eye is a progressive condition that has a major negative impact on the quality of life of afflicted patients. On a daily basis, patients with SS experience significant vision fluctuation with blinking, blurred vision, eye fatigue, and difficulty with reading, despite perfect visual acuity.18,21,22 This difficulty with reading has a negative impact on employment and workplace productivity, particularly in individuals who work in desk jobs. Although patients with SS-related dry eye have generally higher ocular surface staining scores, they have less severe symptoms of ocular discomfort than patients with non–SS-related dry eye.23,24 However, patients with SS-related dry eye have a greater degree of visual difficulty. The reason for this paradox is unknown, but reduced ocular discomfort may be related to reduced corneal sensation in the setting of advanced ocular surface dryness and inflammation.25 Therefore, routine ocular surface evaluations should be performed in all patients with SS-related dry eye, even in the absence of symptoms.
A major problem with currently available SS disease activity measurement instruments is that they do not include any domains specific to dry eye. For example, one of the most commonly used instruments for assessing patient symptoms in SS (the EULAR Sjögren’s Syndrome Patient Reported Index [ESSPRI]26) includes only 1 item (of 3) that addresses the severity of dryness, specifically overall dryness and not ocular dryness. The other 2 items address the severity of fatigue and pain (in the arms or legs). Patients are asked to focus on the previous 2 weeks and rate each of the 3 items on a scale of 0 (absence of the symptom) to 10 (maximal possible severity of the symptom).26 A large survey of patients with dry eye due to SS revealed that they rate dry eye as considerably more important than dryness of the mouth.27 Therefore, there is a critical need for inclusion of items that specifically capture symptoms of ocular dryness, particularly in relation to impairment of visual function.
In addition, SS can lead to serious ocular manifestations, such as decreased vision or even blindness (Table 1). Patients with SS often develop inflammation of the ocular surface, such as chronic conjunctivitis, sterile keratolysis, and nonhealing corneal ulcers (Fig. 1).17,28–33 Inflammation of other parts of the eye, such as uveitis,17,34,35 scleritis (Fig. 2),17,36–38 retinal vasculitis,17 and optic neuritis,17 has also been reported. In a large, tertiary care–based longitudinal cohort study, extraglandular ocular manifestations were present in more than 1 in 3 patients with SS, and 13% had vision-threatening findings.17 In addition, compared with patients without vision-threatening extraglandular ocular findings, patients with such findings were much more likely to develop life-threatening systemic complications.17 Systemic immunosuppressive agents are commonly initiated in patients with systemic complications.39 Because most eye care providers are not sufficiently trained to appropriately prescribe these agents and monitor these patients, prompt referral to rheumatology clinics is essential. Regrettably, none of these extraglandular ocular complications are included in currently available SS disease activity instruments (eg, the EULAR Sjögren’s Syndrome Disease Activity Index [ESSDAI]40). The ESSDAI includes 12 domains representing 12 organ systems (cutaneous, respiratory, renal, articular, muscular, peripheral nervous system, central nervous system, hematologic, glandular, constitutional, lymphadenopathic, and biological), with each domain assessed for different levels of disease activity.40 The ESSDAI has been used commonly when assessing the effectiveness of systemic immunosuppressive or immunomodulatory treatments for systemic complications of SS. However, the use of these agents for ocular complications is much less recognized.39 It is critical that future revisions of the ESSDAI and other disease activity measures include an assessment of ocular complications.
TABLE 1.
Extraglandular Ocular Complications of Sjögren Syndrome
| Type of Complication | Cumulative Incidence (or No. Cases) | References | Study Design |
|---|---|---|---|
| Conjunctival inflammation | |||
| Papillary conjunctivitis | 1.4% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Follicular conjunctivitis | 1.4% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Cicatrizing conjunctivitis | 1.4% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Corneal inflammation | |||
| Haze/scarring | 1.4% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Sterile corneal ulcer/infiltration | 0.7% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Corneal melt/perforation | 1.4% of 163 patients | Akpek et al,17 2015 | Cohort study |
| 5 cases | Gottsch,29 2000 | Case series | |
| 2 cases | Cohen,28 1982 | Case series | |
| 2 cases | Shan,32 2009 | Case series | |
| 1 case | Murtagh,30 2018 | Case study | |
| 1 case | Ou,31 2007 | Case study | |
| 1 case | Vivino,33 2001 | Case study | |
| Other inflammation | |||
| Uveitis | 2.0% of 163 patients | Akpek et al,17 2015 | Cohort study |
| 8 cases | Rosenbaum,35 1987 | Case series | |
| 1 case | Bridges,34 1992 | Case report | |
| Scleritis/episcleritis | 2.0% of 163 patients | Akpek et al,17 2015 | Cohort study |
| 1 case | Ahmadi-Simab,36 2005 | Case study | |
| 1 case | Bamrolia,37 2012 | Case study | |
| 1 case | Choi,38 2012 | Case study | |
| Optic neuritis | 2.0% of 163 patients | Akpek et al,17 2015 | Cohort study |
| Retinal vasculitis | 0.7% of 163 patients | Akpek et al,17 2015 | Cohort study |
FIGURE 1.
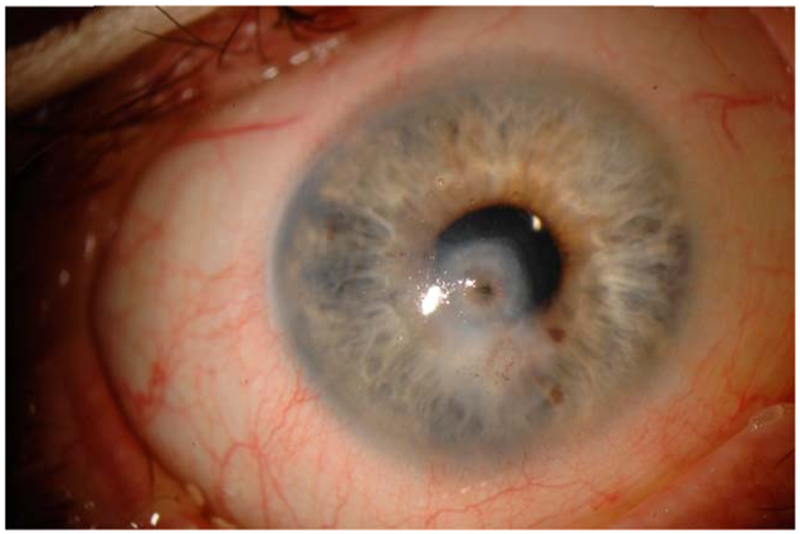
Slit-lamp photograph of a patient with Sjögren syndrome who presented with sterile corneal melt and perforation.
FIGURE 2.
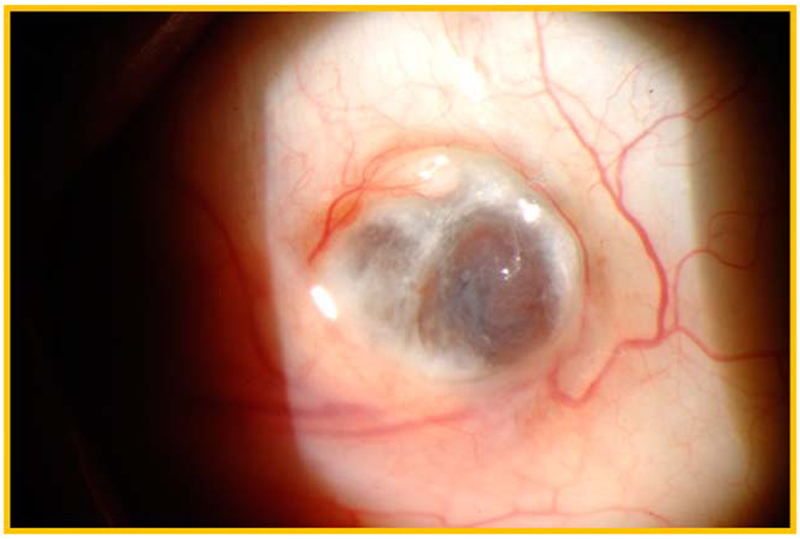
Slit-lamp photograph of a patient with necrotizing scleritis due to primary Sjögren syndrome.
Underappreciation of the significance of eye disease in SS is further demonstrated by examining the classification criteria for SS. For example, in the 2012 American College of Rheumatology (ACR) SS classification criteria, the ocular staining score was assigned equal weight as the other 2 criteria (positive labial salivary gland biopsy and serology).41 However, more recently, the ACR and EULAR put forth a new set of SS classification criteria.42 In this set of criteria, the presence of a positive labial salivary gland biopsy and a positive SS antibody are each weighted 3 times as heavily as the ocular examination findings (ocular surface staining or Schirmer test).42 Given the absence of available evidence-based screening tools for patients with dry eye, clinicians often use these classification criteria as a guide when deciding which patients should be referred for systemic workups. Because the ocular criteria are weighted less than the other criteria, clinicians may underestimate the importance of the presence of these ocular findings, thereby continuing to contribute to the patterns of underreferral for systemic workups for SS.
CONCLUSIONS
Because patients with undiagnosed SS often present with symptoms of dry eye, eye care providers have the opportunity and responsibility to significantly reduce delays in diagnosis and improve the quality of life of patients by timely referral for systemic workups. There needs to be a paradigm shift in which eye care providers and rheumatologists become more familiar with the various ocular manifestations of SS. There is an urgent need for development of evidence-based screening algorithms for determining which patients with dry eye should be assessed for underlying SS to prevent delays in diagnosis. Existing SS disease activity measurement instruments need to be updated so that they effectively capture information about symptoms of visual dysfunction due to dry eye. Future research focused on the development of effective screening and treatment algorithms is needed to improve visual function and quality of life of patients.
Footnotes
The authors have no funding to disclose.
E. K. Akpek is an uncompensated member of the Board of Directors of the Sjögren’s Syndrome Foundation, Bethesda, MD. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum. 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 2.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275–1284. [DOI] [PubMed] [Google Scholar]
- 3.Sjögren H On knowledge of kerataconjunctivitis sicca: keratitis filiformis due to lacrimal gland hypofunction. Acta Opthalmol. 1933;1: 1–151. [Google Scholar]
- 4.Brito-Zeron P, Ramos-Casals M. Advances in the understanding and treatment of systemic complications in Sjogren’s syndrome. Curr Opin Rheumatol. 2014;26:520–527. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Yang Z, Qin B, et al. Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis. 2014;73:1151–1156. [DOI] [PubMed] [Google Scholar]
- 6.Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. [DOI] [PubMed] [Google Scholar]
- 7.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165:2337–2344. [DOI] [PubMed] [Google Scholar]
- 8.Baldini C, Pepe P, Luciano N, et al. A clinical prediction rule for lymphoma development in primary Sjögren’s syndrome. J Rheumatol. 2012;39:804–808. [DOI] [PubMed] [Google Scholar]
- 9.Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk for lymphoma development in Sjogren syndrome: an easy tool for clinical use. Medicine (Baltimore). 2016;95:e3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassan SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978;89:888–892. [DOI] [PubMed] [Google Scholar]
- 11.Kleinstern G, Averbuch M, Abu Seir R, et al. Presence of autoimmune disease affects not only risk but also survival in patients with B-cell non-Hodgkin lymphoma. Hematol Oncol. 2018;36:457–462. [DOI] [PubMed] [Google Scholar]
- 12.Tzioufas AG, Voulgarelis M. Update on Sjögren’s syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol. 2007;21:989–1010. [DOI] [PubMed] [Google Scholar]
- 13.Voulgarelis M, Skopouli FN. Clinical, immunologic, and molecular factors predicting lymphoma development in Sjogren’s syndrome patients. Clin Rev Allergy Immunol. 2007;32:265–274. [DOI] [PubMed] [Google Scholar]
- 14.Akpek EK, Klimava A, Thorne JE, et al. Evaluation of patients with dry eye for presence of underlying Sjögren syndrome. Cornea. 2009;28:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruszka P, O’Brian RJ. Diagnosis and management of Sjögren syndrome. Am Fam Physician. 2009;79:465–470. [PubMed] [Google Scholar]
- 16.Manthorpe R, Asmussen K, Oxholm P. Primary Sjögren’s syndrome: diagnostic criteria, clinical features, and disease activity. J Rheumatol Suppl. 1997;50:8–11. [PubMed] [Google Scholar]
- 17.Akpek EK, Mathews P, Hahn S, et al. Ocular and systemic morbidity in a longitudinal cohort of Sjogren’s syndrome. Ophthalmology. 2015;122: 56–61. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, et al. Primary Sjogren syndrome. BMJ. 2012;344:e3821. [DOI] [PubMed] [Google Scholar]
- 19.Bunya VY, Fernandez KB, Ying GS, et al. Survey of ophthalmologists regarding practice patterns for dry eye and Sjogren syndrome. Eye Contact Lens. 2018;44(suppl 2):S196–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrich CF, Ramulu PY, Akpek EK. Association of dry eye and inflammatory systemic diseases in a tertiary care-based sample. Cornea. 2014;33:819–825. [DOI] [PubMed] [Google Scholar]
- 21.Segal B, Bowman SJ, Fox PC, et al. Primary Sjögren’s Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf 2015; 13:118–132. [DOI] [PubMed] [Google Scholar]
- 24.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. [DOI] [PubMed] [Google Scholar]
- 25.Adatia FA, Michaeli-Cohen A, Naor J, et al. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren’s syndrome. Can J Ophthalmol. 2004;39:767–771. [DOI] [PubMed] [Google Scholar]
- 26.Seror R, Ravaud P, Mariette X, et al. EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren’s syndrome. Ann Rheum Dis. 2011;70:968–972. [DOI] [PubMed] [Google Scholar]
- 27.Saldanha IJ, Petris R, Han G, et al. Research questions and outcomes prioritized by patients with dry eye. JAMA Ophthalmol. 2018;136:1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen KL. Sterile corneal perforation after cataract surgery in Sjogren’s syndrome. Br J Ophthal. 1982;66:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottsch JD, Akpek EK. Topical cyclosporin stimulates neovascularization in resolving sterile rheumatoid central corneal ulcers. Trans Am Ophthalmol Soc. 2000;98:81–87; discussion 87–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Murtagh P, Comer R, Fahy G. Corneal perforation in undiagnosed Sjögren’s syndrome following topical NSAID and steroid drops post routine cataract extraction. BMJ Case Rep. 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou JI, Manche EE. Corneal perforation after conductive keratoplasty in a patient with previously undiagnosed Sjogren syndrome. Arch Ophthalmol. 2007;125:1131–1132. [DOI] [PubMed] [Google Scholar]
- 32.Shan SJ, Wu EI, Akpek EK. Sterile corneal melt after descemet stripping endothelial keratoplasty in patients with previously undiagnosed Sjogren syndrome. Arch Ophthalmol. 2009;127:219–220. [DOI] [PubMed] [Google Scholar]
- 33.Vivino FB, Minerva P, Huang CH, et al. Corneal melt as the initial presentation of primary Sjögren’s syndrome. J Rheumatol. 2001;28:379–382. [PubMed] [Google Scholar]
- 34.Bridges AJ, Burns RP. Acute iritis associated with primary Sjögren’s syndrome and high-titer anti-SS-A/Ro and anti-SS-B/La antibodies. Treatment with combination immunosuppressive therapy. Arthritis Rheum. 1992;35:560–563. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum JT, Bennett RM. Chronic anterior and posterior uveitis and primary Sjögren’s syndrome. Am J Ophthalmol. 1987;104:346–352. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadi-Simab K, Lamprecht P, Nolle B, et al. Successful treatment of refractory anterior scleritis in primary Sjogren’s syndrome with rituximab. Ann Rheum Dis. 2005;64:1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamrolia NR, Arora R, Yadava U. Unusual presentation of a case of Sjogren’s syndrome with neurological and ocular manifestation. Cont Lens Anterior Eye. 2012;35:85–88. [DOI] [PubMed] [Google Scholar]
- 38.Choi W, Lee SS, Park YG, et al. A case of necrotizing keratoscleritis in primary Sjogren’s syndrome. Korean J Ophthalmol. 2011;25:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordero-Coma M, Anzaar F, Sobrin L, et al. Systemic immunomodulatory therapy in severe dry eye secondary to inflammation. Ocul Immunol Inflamm. 2007;15:99–104. [DOI] [PubMed] [Google Scholar]
- 40.Seror R, Bowman SJ, Brito-Zeron P, et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015;1:e000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012;64:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League against Rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16. [DOI] [PubMed] [Google Scholar]


