Abstract
IMPORTANCE
In-hospital cardiac arrest is common and associated with a high mortality rate. Despite this, in-hospital cardiac arrest has received little attention compared with other high-risk cardiovascular conditions, such as stroke, myocardial infarction, and out-of-hospital cardiac arrest.
OBSERVATIONS
In-hospital cardiac arrest occurs in over 290 000 adults each year in the United States. Cohort data from the United States indicate that the mean age of patients with in-hospital cardiac arrest is 66 years, 58% are men, and the presenting rhythm is most often (81%) nonshockable (ie, asystole or pulseless electrical activity). The cause of the cardiac arrest is most often cardiac (50%-60%), followed by respiratory insufficiency (15%-40%). Efforts to prevent in-hospital cardiac arrest require both a system for identifying deteriorating patients and an appropriate interventional response (eg, rapid response teams). The key elements of treatment during cardiac arrest include chest compressions, ventilation, early defibrillation, when applicable, and immediate attention to potentially reversible causes, such as hyperkalemia or hypoxia. There is limited evidence to support more advanced treatments. Post–cardiac arrest care is focused on identification and treatment of the underlying cause, hemodynamic and respiratory support, and potentially employing neuroprotective strategies (eg, targeted temperature management). Although multiple individual factors are associated with outcomes (eg, age, initial rhythm, duration of the cardiac arrest), a multifaceted approach considering both potential for neurological recovery and ongoing multiorgan failure is warranted for prognostication and clinical decision-making in the Post–cardiac arrest period. Withdrawal of care in the absence of definite prognostic signs both during and after cardiac arrest should be avoided. Hospitals are encouraged to participate in national quality-improvement initiatives.
CONCLUSIONS AND RELEVANCE
An estimated 290 000 in-hospital cardiac arrests occur each year in the United States. However, there is limited evidence to support clinical decision making. An increased awareness with regard to optimizing clinical care and new research might improve outcomes.
In-hospital cardiac arrest is an acute event that can potentially affect any hospitalized patient. For the purposes of clinical care, research, and guideline development, in-hospital cardiac arrest (as opposed to death without resuscitation) is most commonly defined as the loss of circulation prompting resuscitation with chest compressions, defibrillation, or both.
Traditionally, in-hospital cardiac arrest has been viewed as a condition with such poor outcomes that resuscitation may not even be warranted. Although outcomes remain poor, recent data suggest improvement over the past 2 decades.1,2 One reason for this improvement might be an increased awareness of the influence that clinical management can have on outcomes in patients with in-hospital cardiac arrest and cardiac arrest in general. Despite this increased interest, in-hospital cardiac arrest remains a somewhat neglected condition compared with out-of-hospital cardiac arrest and other cardiovascular conditions, such as stroke and myocardial infarction. For example, in a systematic review of all randomized clinical cardiac arrest trials (n = 92) involving at least 50 patients from 1995 to 2014, only 4 (4%) exclusively involved patients with in-hospital cardiac arrest.3 Although guidelines for in- and out-of-hospital cardiac arrest are almost identical,4,5 there are important differences between the conditions that warrant consideration (Table 1).
Table 1.
Comparison of Out-of-Hospital and In-Hospital Cardiac Arrest
| In-Hospital Cardiac arrest |
Out-of-Hospital Cardiac Arrest |
|
|---|---|---|
| Incidence | 290 000 per year in the United States | 350 000 per year in the United Statesa |
| Patient characteristics | Mean age: 66 y Approximately 60% men | Median age: 65 y Approximately 60% men |
| Presenting rhythm | Often nonshockable (approximately 80%) | Often nonshockable (approximately 80%) |
| Cause | Primarily cardiac and respiratory | Primarily cardiac |
| Prevention | Potentially possible with recognition of deterioration and early intervention | Often impossible given the lack of pre-cardiac arrest monitoring |
| Timing of basic life support | Often instantaneously | Variable depending on bystander involvement |
| Timing of advanced life support drugs | Within 5 to 10 min | On average, approximately 20 min after the onset of cardiac arrest |
| Airway management | Approximately one-third of patients already intubated (eg, intensive care unit patients); often performed by physicians | Often performed by clinicians (eg, paramedics) with variable experience in advanced airway management |
| Drugs | Limited evidence; epinephrine and amiodarone recommended | Some evidence; epinephrine and amiodarone recommended |
| Post-cardiac arrest treatment | Limited evidence; supportive care and targeted temperature management recommended | Some evidence; supportive care and targeted temperature management recommended |
| Prognostication | Limited evidence; focuses on both neurological status and organ failure | Some evidence; focuses on neurological status |
| Survival to discharge | Approximately 25% | 10% to 12% |
Assessed by emergency medical services but not necessarily treated.2
In this review, we discuss adult in-hospital cardiac arrest, including epidemiology, causes, management during and after cardiac arrest, characteristics related to outcomes, prognostication, and quality improvement. There are relatively few randomized clinical trials of patients with in-hospital cardiac arrest (Table 2). Therefore, much of the current knowledge is based on observational studies primarily from large registries, extrapolation of results from trials of out-of-hospital cardiac arrest, and expert opinion.
Table 2.
Randomized Trials Including In-Hospital Cardiac Arrest Patients Between 1988 and 2018
| Study | Country | Sites | Years of Inclusion |
Main Inclusion Criteria |
Main Exclusion Criteria | Patients | Intervention | Control | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Brain Resuscitation Clinical Trial II Study
Group,6 1991a |
Multiple | 24 | 1984-1989 | IHCA, age ≥12 y, ROSC, comatose | Terminal illness, central nervous system disease, atrial fibrillation, women of childbearing age | 184 | Lidoflazine (calcium channel blocker) | Placebo | No difference in survival at 6 mo (12% vs 15%) |
| Stiell et al,7 1992a | Canada | 2 | 1989-1992 | IHCA, age ≥16 y | Terminal illness, acute trauma, in the operating or recovery rooms of hospitals | 315 | High-dose epinephrine (7 mg; max 5 doses) | Standard-dose epinephrine (1 mg) | No difference in ROSC (25% vs 31%) or survival to hospital discharge (5% vs 7%) |
| Sack et al,8 1992 | United States | 1 | 1989-1890 | IHCA, age ≥18 y | Traumatic or respiratory cause, pregnancy, abdominal aortic aneurysm | 103 | Interposed abdominal counterpulsation CPR | Standard CPR | Higher ROSC (60% vs 25%), survival to hospital discharge (25% vs 7%), and survival to hospital discharge neurologically intact (17% vs 6%) in the intervention group |
| Sack et al,9 1992 | United States | 1 | 1990-1991 | IHCA, age ≥18 y, initial nonshockable rhythm | Traumatic or respiratory cause, pregnancy, abdominal aortic aneurysm, recent abdominal surgery, prolonged intubation | 143 | Interposed abdominal counterpulsation CPR | Standard CPR | Higher ROSC (49% vs 28%) and survival at 24 h (33% vs 13%) in the intervention group. Few survived to hospital discharge |
| Cohen et al,10 1993 | United States | 1 | 1992-1993 | IHCA, age ≥18 y, witnessed cardiac arrest | Traumatic cause, inability to achieve endotracheal intubation within 15 min | 62 | Active compression-decompression CPR | Standard CPR | Higher ROSC (62% vs 30%) and survival at 24 h (45% vs 9%) in the intervention group. Few survived to hospital discharge |
| Lipman et al,11 1993 | South Africa | 1 | 1990-1991 | IHCA in the ICU, age ≥18 y, witnessed cardiac arrest, asystole | NA | 37 | High-dose epinephrine (10 mg) | Standard-dose epinephrine (1 mg) | No difference in ROSC (68% vs 66%) or survival at 24 h (21% vs 31%). Few patients survived to hospital discharge |
| Tucker et al,12 1994 | United States | 1 | 1992-1993 | IHCA, age ≥18 y | Traumatic or respiratory cause, pregnancy | 53 | Active compression-decompression CPR | Standard CPR | Higher ROSC (60% vs 32%) and survival at 24 h (48% vs 21%) in the intervention group. No difference in survival to hospital discharge (24% vs 11%) or neurologically intact at hospital discharge (20% vs 11%) |
| Woodhouse et al,13 1995 | Australia | 1 | 1989-1992 | IHCA or OHCA with ongoing CPR in the ED | Noncardiac cause | 194 (109 with IHCA) | Epinephrine (10 mg; max 2 doses) | Placebo (max 2 doses) | No difference in ROSC (10% vs 7%). No patients survived to hospital discharge |
| Patrick et al,14 1995 | Canada | 1 | 1990-1992 | Witnessed IHCA or OHCA with ongoing CPR in the ED | Traumatic cause, severe hypothermia/hemorrhage | 145 (91 with IHCA) | Methoxamine (40 mg per dose) | Epinephrine (2 mg per dose) | No difference in ROSC (42% vs 53%), 24-h survival (30% vs 37%), or survival to hospital discharge (12% vs 15%) |
| Stiell et al,15 1996a | Canada | 5 | 1993-1995 | IHCA, age ≥16 y | Terminal illness; acute trauma or exsanguination; recent sternotomy; arrest in operating, recovery, or delivery room | 773 | Active compression-decompression CPR | Standard CPR | No difference in survival at 1 h (35% vs 35%) or at hospital discharge (10% vs 11%) |
| Thel et al,16 1997 | United States | 1 | 1993-1996 | IHCA, age ≥18 y | Arrest in emergency, operating, or recovery room; clinical indication for magnesium; signs of irreversible death | 156 | Magnesium (2 g bolus + 8 g infusion over 24 h) | Placebo | No difference in ROSC (54% vs 60%), survival at 24 h (43% vs 50%), or at hospital discharge (21% vs 21%) |
| Stiell et al,17 2001 | Canada | 3 | 1997-1998 | IHCA, age ≥16 y | Terminal illness; traumatic injury prior to admission; arrest due to exsanguination; arrest in operating, recovery, or delivery room | 200 | Vasopressin (40 IU; max 1 dose) | Epinephrine (1 mg) | No difference in survival at 1 h (39% vs 35%), 24 h (26% vs 24%), hospital discharge (12% vs 14%), or 30 d (13% vs 14%) |
| Mentzelopoulos et al,18 2009 | Greece | 1 | 2006-2007 | IHCA, age ≥18 y | Terminal illness, exsanguination, treatment with IV corticosteroids prior to the cardiac arrest | 100 | Vasopressin (20 IU; max 5 doses) + methylprednisolone (40 mg) + hydrocortisone (300 mg/ 24 h) if in shock after cardiac arrest | Placebo | Higher ROSC (81% vs 52%) and survival to hospital discharge (19% vs 4%) in the intervention group |
| Weidman et al,19 2010 | United States | 1 | 2007-2008 | IHCA, age ≥18 y | Arrest in emergency or operating room | 98 | Immersive simulation training of residents | Standard training | No difference in CPR quality between groups |
| Pittl et al,20 2013 | Germany | 1 | 2008-2009 | IHCA or OHCA, age ≥18 y, ROSC, cardiac origin, comatose | Pregnancy, coagulation disorder, cardiogenic shock, terminal illness | 80b | Invasive cooling | Surface cooling | No difference in survival to hospital discharge (62% vs 54%) or with a good neurological outcome (36% vs 36%). More bleeding complications with invasive cooling |
| Mentzelopoulos et al,21 2013 | Greece | 3 | 2008-2010 | IHCA, age ≥18 y | Terminal illness, exsanguination, treatment with intravenous corticosteroids prior to the cardiac arrest | 268 | Vasopressin (20 IU; max 5 doses) + methylprednisolone (40 mg) + hydrocortisone (300 mg/ 24 h) if in shock after cardiac arrest | Placebo | Higher ROSC (84% vs 66%) and survival to hospital discharge with a good neurological outcome (14% vs 5%) in the intervention group |
| Vahedian-Azimi et al,22 2016 | Iran | 4 | 2014 | IHCA in the ICU, age ≥18 y | NA | 83 | CPR with feedback devise | Standard CPR | Higher CPR quality and ROSC (72% vs 35%) in the intervention group |
| Eastwood et al,23 2016 | Australia and New Zealand | 4 | 2012-2014 | IHCA or OHCA, age ≥18 y, mechanically ventilated | Traumatic cause, pregnancy, imminent death, raised intracranial pressure/bleeding, severe chronic airflow limitation, severe metabolic acidosis | 86 (16 with IHCA | Mild hypercapnia after cardiac arrest (PaCO2 50-55 mm Hg) for 24 h | Normocapnia after cardiac arrest (PaCO2 35-45 mm Hg) for 24 h | Lower NSE values in the intervention group. No difference in survival to hospital discharge (74% vs 63%) and good neurological outcome at 6 mo (59% vs 46%) |
| Movahedi et al,24 2016 | Iran | 1 | 2014 | IHCA, endotracheal intubation, age 18-85 y, arrest in hospital ward or ED | Traumatic cause, pregnancy, abdominal surgery in the past 2 weeks, history of abdominal aortic aneurysm, coagulopathy, ascites, active gastrointestinal bleeding, pulmonary embolism | 83 | Interposed abdominal counterpulsation CPR | Standard CPR | Higher end-tidal carbon dioxide in the intervention group. No difference in ROSC (60% vs 53%) or survival at 24 h (38% vs 38%) |
| Anantharaman et al,25 2017 | Singapore | 4 | 2005-2008 | IHCA or OHCA with ongoing CPR in the ED, age ≥21 y, initial shockable rhythm | Traumatic cause, pregnancy | 235 (89 with IHCA) | Escalating high-energy shocks (200-300-360 J) | Low-energy shocks (150-150 - 150 J) | No difference in first shock success (67% vs 64%), ROSC (55% vs 55%), or 30-d survival (21% vs 28%) |
| Koster et al,26 2017a | The Netherlands | 1 | 2006-2014 | IHCA, age ≥18 y | Traumatic cause | 199 | Mechanical chest compressions (AutoPulse or LUCAS) | Manuel chest compressions | No difference in serious or life-threatening visceral damage (9% vs 8%) |
| Zhang et al,27 2017 | China | 50 | 2012-2015 | IHCA, age ≥18 y | Pregnancy, malignancy, HIV, arrest caused by brain/liver/lung disease, end-stage heart disease, Shenfu (a traditional Chinese medication including ginseng and aconite) allergy | 978 | Shenfu (200 mL/d for 14 d) | Standard care | Higher 28-d (43% vs 30%) and 90-d survival (40% vs 26%) in the intervention group |
| Look et al28 2018 | Singapore | 1 | 2006-2014 | IHCA or OHCA, age 18-80 y | Traumatic cause, hemodynamically unstable, pregnancy, poor premorbid status | 45 (7 with IHCA) | Invasive cooling | Surface cooling | No difference in 30-d survival (48% vs 32%) or good neurological outcome (30% vs 23%) |
Abbreviations: CPR, cardiopulmonary resuscitation; ED, emergency department; IHCA, in-hospital cardiac arrest; ICU, intensive care unit; NA, not applicable; NSE: neuron-specific enolase; OHCA, out-of-hospital cardiac arrest; PaCO2, partial pressure of carbon dioxide; ROSC, return of spontaneous circulation.
The trial also included patients with OHCA, but those patients are not included in this table.
Number of IHCAs not reported.
Methods
This review was based on a series of informal searches of PubMed addressing each relevant topic, including incidence and outcomes, causes, prevention, treatment, and prognostication of in-hospital cardiac arrest. In addition, we performed a systematic search to identify all randomized trials including patients with in-hospital cardiac arrest published within the past 30 years. Additional details regarding the systematic search are provided in Supplement 1.
Results
Incidence and Outcomes
The global incidence of in-hospital cardiac arrest in adults has not been well described,29,30 and the majority of data are derived from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) registry31 and the National Cardiac Arrest Audit from the Resuscitation Council (UK) and the Intensive Care National Audit and Research Centre.32 Based on GWTG-R data from 2003 to 2007, the estimated incidence of in-hospital cardiac arrests in the United States was 211 000 annually, or roughly 6 to 7 cardiac arrests per 1000 admissions.30,31 Data from 2008 to 2017 showed the incidence increased to 292 000 annually, or 9 to 10 in-hospital cardiac arrests per 1000 admissions.33 In contrast, an incidence of 1.6 in-hospital cardiac arrests per 1000 admissions in the United Kingdom from 2011-2013 was estimated using data from the UK National Cardiac Arrest Audit.32
Based on data from the GWTG-R registry, the mean age of patients with in-hospital cardiac arrest in the United States is 66 years, 58% are men, and the presenting rhythm is most often (81%) nonshockable (ie, asystole or pulseless electrical activity). Approximately half of in-hospital cardiac arrests occur in wards, with the remaining half occurring in other locations, such as intensive care units and operating rooms.2,32,34
In a review from 2007, survival (most commonly to hospital discharge) varied from 0% to 42% between studies, although most larger studies reported survival around 20%.29 Survival has been increasing over the last 2 decades (Figure 1)1,2 and, in 2017, survival to hospital discharge was 25% in the GWTG-R registry. Among patients alive at hospital discharge, 85% were discharged with a favorable neurological outcome (cerebral performance category 1 or 2).2 Data from 2011 to 2013 indicate 18% survival to hospital discharge in the United Kingdom32 while, in Denmark and Sweden, 30-day survival is approximately 30% in contemporary national registries.35,36 A 2018 systematic review that included more than 1 million in-hospital cardiac arrests and 39 studies from 1992 to 2016 found an overall 1-year survival rate of 13%, with large between-study variability and an increase in 1-year survival over time.37 In elderly (aged ≥65 years) patients with in-hospital cardiac arrest in the United States who survived to hospital discharge, 59% were alive after 1 year and 34% had not been readmitted to a hospital.38
Figure 1. Survival After In-Hospital Cardiac Arrest, 2000 to 2017.
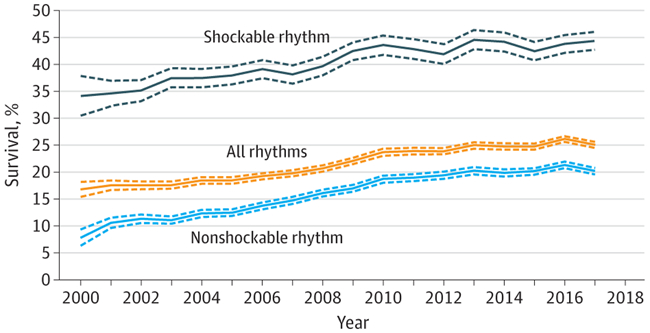
Based on data from the Get With The Guidelines-Resuscitation registry on all adult in-hospital cardiac arrests from 2000 to 2017 The dotted lines represent 95% CIs. Shockable rhythms include ventricular fibrillation and pulseless ventricular tachycardia. Nonshockable rhythms include asystole and pulseless electrical activity. Adapted from Benjamin et al.2
The variability between countries in both incidence of and survival after in-hospital cardiac arrest likely reflects differences in (1) the definitions used to identify in-hospital cardiac arrest, (2) the proportion of cardiac arrests captured by various registries, (3) the patient populations, (4) country-specific culture surrounding cardiopulmonary resuscitation (CPR), do-not-resuscitate orders, and withdrawal of care, and (5) treatment during and after cardiac arrest. Comparisons between countries or registries should therefore be performed carefully.
Causes of Cardiac Arrest
Historically, the etiologies of cardiac arrest have been dichotomized as cardiac or noncardiac. Because patients with no obvious cause are generally classified as cardiac, and because discrepancies often exist between clinical and postmortem diagnoses, the causes of cardiac arrest are often uncertain. In general, cardiac causes of cardiac arrest, such as myocardial infarction, arrhythmia, or heart failure, are most frequent, with a prevalence of approximately 50% to 60%. Respiratory insufficiency is the second most common cause (15%-40%).34,39,40 The median admission duration prior to cardiac arrest is 1 to 2 days, with a higher prevalence of respiratory insufficiency as the cause of cardiac arrest with longer duration of preceding hospitalization.32,36,40 Neurological causes of cardiac arrest are rare in the in-hospital setting.36,41
Identifying the cause of cardiac arrest serves several purposes. During cardiac arrest, resuscitation guidelines emphasize that potential reversible causes should be identified, which are categorized into 4 h’s and t’s (Box).42 Although not all of these categories (eg, hypothermia) are applicable in the in-hospital setting, the majority of in-hospital cardiac arrests can be categorized using this approach.43 Identifying the cause of cardiac arrest could improve outcomes.44 Identification of the cause of cardiac arrest also has implications if return of spontaneous circulation is achieved, because Post–cardiac arrest organ dysfunction is partly dependent on the underlying cause, and post–cardiac arrest treatment should be tailored accordingly (see “Treatment After Cardiac Arrest”). Additional research is needed to create a useful and more precise framework for the classification of causes of cardiac arrest. Such a framework might not only improve research efforts but may eventually improve care provided during and after cardiac arrest.
Box. Potential Reversible Causes of Cardiac Arrest.
| h's |
| Hypokalemia/hyperkalemiaa |
| Hypothermia |
| Hypovolemia |
| Hypoxia |
| t’s |
| Tamponade |
| Tension pneumothorax |
| Thrombosis (coronary or pulmonary) |
| Toxins |
Can include other metabolic alterations such as severe acidosis.
Prevention of cardiac arrest will be best achieved by addressing the mechanisms underlying the cause of the cardiac arrest. For example, the prescription of QT interval–prolonging drugs during hospital admission can lead to arrhythmias, while the prescription of opioids or sedatives may lead to respiratory insufficiency 45,46 Another potentially preventable cause of cardiac arrest is sepsis. The prevalence of preexisting sepsis in patients with in-hospital cardiac arrest varies across studies, ranging from 13% to 27%.34,47 Organ failure from sepsis contributes to multiple potential causes of arrest, including circulatory failure, respiratory insufficiency, and metabolic derangements.
Prevention of Cardiac Arrest
Contrary to the out-of-hospital setting, in-hospital cardiac arrest facilitates observation of a patient’s clinical condition prior to cardiac arrest. Clinical deterioration is common prior to in-hospital cardiac arrest,48 and many in-hospital cardiac arrests are considered preventable or avoidable on retrospective review.49 Prevention has therefore been added as the first link in the Chain of Survival for in-hospital cardiac arrest in the 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.50 Key elements of success include identification of at-risk patients combined with early interventions to prevent deterioration to cardiac arrest. Such identification might occur through the use of early warning systems triggered by specific vital sign abnormalities, a scoring system based on multiple criteria, or by staff concern. However, current prediction models often lack optimal sensitivity and/or specificity to identify at-risk patients and may be limited by differences in the logistical structure of individual hospital systems.51 Further, although interventions in terms of rapid response teams are generally supported by the literature, the evidence is weak because there are few rigorous randomized clinical trials and heterogeneity between hospital systems.52 Despite these limitations, individual hospitals should create a 2-part system for preventing cardiac arrest that includes (1) identifying at-risk or deteriorating patients (requiring relevant education, monitoring, and recognition) and (2) creating appropriate interventional responses (eg, rapid response teams).
Treatment During Cardiac Arrest
Chest compressions, ventilation, and early defibrillation, when applicable, are the cornerstones of cardiac arrest treatment.4,5 Early initiation of CPR is associated with improved outcomes for both out-of-hospital53 and in-hospital54 cardiac arrest. CPR training for all hospital personnel has, therefore, been mandatory in many hospital systems for decades, facilitating the rapid identification and management of cardiac arrest prior to the arrival of the cardiac arrest team. Quality of chest compressions and of CPR in general have been associated with better outcomes in patients with cardiac arrest.55 Optimization of CPR quality is therefore a priority.
Although only approximately 20% of patients with in-hospital cardiac arrest have an initial shockable rhythm, rapid defibrillation is associated with improved outcomes for these patients.56 It is not clear whether automated (compared with manual) external defibrillators, which have been associated with markedly improved outcomes in the out-of-hospital setting,57 are of any benefit in the in-hospital setting.58
Data supporting the efficacy of medications during in-hospital cardiac arrest are sparse. Current guidelines recommend the use of epinephrine and amiodarone, both of which improve short-term outcomes in out-of-hospital cardiac arrest, but there is limited evidence supporting substantial neurological improvement when these medications are used.59,60 Given the differences between in-hospital and out-of-hospital cardiac arrest (Table 1), especially the much earlier administration of drugs in the in-hospital setting, it is unclear whether findings from studies of out-of-hospital cardiac arrest apply to in-hospital cardiac arrest. For in-hospital events, early administration of epinephrine in patients with a nonshockable rhythm is associated with better outcomes.61 In contrast, early epinephrine for patients with shockable rhythms is associated with worse outcomes.62 The combination of vasopressin and methylprednisolone during in-hospital cardiac arrest has been tested in 2 small randomized clinical trials, with promising results.18,21 This combination of drugs is not recommended in the US or European guidelines because of insufficient evidence to support their use (see eTable 1 in Supplement 1 for an overview of ongoing randomized clinical trials).42,63
Airway management is a key component of advanced life support during cardiac arrest. Endotracheal intubation has traditionally been considered the preferred approach to ensure adequate ventilation and oxygenation, but emerging evidence in both out-of-hospital64-66 and in-hospital67 cardiac arrest suggests that alternative approaches, such as bag-valve-mask ventilation or supraglottic airways, may be equally or even more effective. How to optimize ventilation and oxygenation likely depends on the specific clinical conditions of the patient with cardiac arrest.
Extracorporeal circulation during CPR is being assessed for use in cardiac arrest and it may be useful for in-hospital events and in certain patient populations, such as in patients who have recently undergone cardiac surgery. Some reports have shown good outcomes from extracorporeal circulation during CPR but, overall, the evidence of benefit is scarce and limited by the observational nature of the studies.68 Extracorporeal circulation during CPR may help carefully selected patients, but balance must be achieved between efficient resource use and clinical benefits.
Treatment After Cardiac Arrest
Management in the Post–cardiac arrest period generally focuses on the precipitating cause, hemodynamic and respiratory support, and neuroprotective care. Conditions before and during cardiac arrest determine the severity of the Post–cardiac arrest syndrome (see “Characteristics Related to Outcomes”) and the need for various interventions. For example, a patient admitted with acute coronary syndrome who develops ventricular fibrillation that is rapidly treated with defibrillation may be cognitively intact and require treatment specific for the cardiac condition without need of neuroprotection. Conversely, a patient who has a prolonged cardiac arrest and significant ischemia-reperfusion injury may have multiorgan injury and require numerous treatments. One of the more distinguishing components of in-hospital cardiac arrest is that the event may result from progressively worsening underlying disease, whereas out-of-hospital arrest is often sudden and unpredictable.
Despite some controversy, targeted temperature management at 32°C to 36°C for at least 24 hours after cardiac arrest remains the primary neuroprotective approach following out-of-hospital cardiac arrest.69,70 To our knowledge, no randomized trials have evaluated targeted temperature management following in-hospital cardiac arrest. Outcomes data are limited to observational studies and extrapolation from the out-of-hospital investigations. The largest observational study of targeted temperature management in patients with in-hospital cardiac arrest (N = 26 183) found that it was associated with worse overall outcomes.71 However, this study did not consider coma status, which could have biased the results against the intervention because targeted temperature management is only used in comatose patients.72 While future studies are needed to better evaluate targeted temperature management for patients with in-hospital cardiac arrest (eTable 1 in Supplement 1), the current American Heart Association recommendation is to provide targeted temperature management for at least 24 hours.70
Other proposed neuroprotective strategies for in-hospital cardiac arrest supported by indirect observational data include minimizing supplemental oxygen therapy when oxygen levels are adequate and maintaining normal carbon dioxide levels.73,74 Observational studies that examined the association between the maintenance of low tidal-volume ventilation and outcomes in patients with out-of-hospital cardiac arrest75 and in-hospital cardiac arrest76 have shown conflicting results. While further studies are necessary, one important element to note is that patients who experienced cardiac arrest rarely die from refractory respiratory failure,77,78 potentially limiting the effectiveness of ventilator-based interventions.
Hemodynamic management is essential, but no specific differences have been defined in the management of patients after cardiac arrest compared with other critically ill patients. Preexisting disease, underlying diagnoses, and myocardial stunning from ischemia reperfusion all contribute to the hemodynamic profile of patients after cardiac arrest. Targeted temperature management appears to have some effects on hemodynamics and data from a small 2002 trial suggested that patients receiving targeted temperature management had lower heart rates, increased systematic vascular resistance, and slightly decreased cardiac outputs.79 A 2015 analysis of patients cooled to 33°C vs 36°C reported higher vasopressor dosages and lactate levels for patients maintained at 33°C.80 Consequently, patients can be maintained with higher temperatures (ie, 36°C) if there is a concern that targeted temperature management negatively influences hemodynamics.81 Targeted temperature management at 33°C should probably be avoided in patients with sepsis and septic shock82 or bacterial meningitis,83 because recent trials suggest worse outcomes in these patient groups with targeted temperature management.
Characteristics Related to Outcomes
Many patient and event characteristics are associated with the clinical outcomes of in-hospital cardiac arrest. Some of these characteristics cannot be modified, such as age, gender, and preexisting conditions, while other characteristics, such as time to drug administration and monitoring, are modifiable and the subject of quality-improvement efforts. While many risk factors, such as advanced age, are predictive of clinical outcomes, none can individually be used to estimate prognosis following cardiac arrest (see “Prognostication”).
Increased age is associated with decreased survival following cardiac arrest in most studies, especially for patients older than 70 years.84 The association with outcomes of other demographic features, such as sex and race, is less clear. Although the incidence of in-hospital cardiac arrest is higher among men, men and women have similar clinical outcomes,85 although women of child-bearing age (15 to 44 years) may have better outcomes compared with men of the same age.86 Studies that investigated the relationship between race and outcomes have found black and Hispanic patients to have lower rates of neurological recovery and survival following in-hospital cardiac arrest compared with white patients.47,87 Data from the GWTG-R registry have shown that racial disparities in outcomes have narrowed over time, with a reported absolute survival difference between black and white patients of 4.5% in 2000 and 1.8% in 2014.87 Differing distribution of risk factors88 or variability in patient- and hospital-level care during and after cardiac arrest may explain why these racial differences exist.
The presence of preexisting medical and surgical conditions is strongly associated with outcomes following in-hospital cardiac arrest. For example, malignancy, sepsis, poor functional status prior to the cardiac arrest, pneumonia, hypotension, renal dysfunction, and hepatic dysfunction have been identified as significant predictors of poor survival.84,89 Conversely, acute myocardial infarction causing an in-hospital cardiac arrest is associated with increased survival compared with cardiac arrest not caused by myocardial infarction.90
Factors related to early detection of cardiac arrest, such as the event being witnessed47,91 or occurring in a monitored location,34,47,84 are associated with improved outcomes. However, the association between monitoring/location and outcomes34,47,84,91 is complex given the different case-mix of patients in various locations.
Two of the factors most strongly associated with outcomes are the presenting rhythm and the duration of the cardiac arrest.84,92,93 Patients with a shockable rhythm have 2 to 3 times higher survival to hospital discharge compared with patients with a nonshockable rhythm (Figure 1).2,92 While this difference might reflect the potential for more effective treatment in the shockable group (ie, defibrillation), part of the difference is likely also explained by differences in patient characteristics and preexisting conditions, which may influence the presenting rhythm. The chance of 30-day survival markedly decreases with increasing duration of CPR.93
Prognostication
During Cardiac Arrest
Deciding when to stop CPR during cardiac arrest remains challenging, with limited guidance in contemporary guidelines.94,95 Although longer duration of resuscitation is associated with worse outcomes, survival with good neurological outcome is possible with prolonged CPR.93 Additionally, Goldberger et al96 found that hospitals where CPR is performed for longer durations have better outcomes, suggesting that average CPR duration may be too short at some hospitals.
Several observational studies have reported that cardiac standstill on point-of-care cardiac ultrasonography is associated with very low likelihood of survival.97 However, there are significant concerns with interrater variability in image interpretation and potential interference of ultrasonography with CPR.98 End-tidal carbon dioxide values less than 10 mm Hg after 20 minutes of CPR are also strongly associated with poor outcomes.99 However, studies regarding point-of-care cardiac ultrasonography and end-tidal carbon dioxide should be interpreted carefully because blinding was rarely performed and the findings described might reflect a self-fulfilling prophecy where resuscitation is terminated based on these specific findings. Neither cardiac ultrasonography nor end-tidal carbon dioxide should be used in isolation, but might be considered together with other factors, such as the initial rhythm and the duration of the cardiac arrest (see “Characteristics Related to Outcomes”).
After Cardiac Arrest
Current guidelines on prognostication are based on literature describing out-of-hospital cardiac arrest and focus exclusively on neurological status.70 While two-thirds of patients with out-of-hospital cardiac arrest who survive to intensive care unit admission die of neurological causes, neurologic death only occurs in one-fourth of patients with in-hospital cardiac arrest, for whom multiorgan dysfunction drives mortality.78 Because in-hospital cardiac arrest is most often witnessed, times from cardiac arrest to initiation of CPR and to return of spontaneous circulation are shorter than in out-of-hospital cardiac arrest, which may contribute to the lower rate of neurological injury. These differences leave clinicians without clear guidance regarding how to best prognosticate outcomes (ie, survival and survival with good neurological/functional recovery) following in-hospital cardiac arrest.
Chan et al attempted to address this issue by developing a scoring system for estimation of survival to hospital discharge with a favorable neurological outcome in patients with return of spontaneous circulation after in-hospital cardiac arrest (Figure 2; Supplement 2).84 The final score, which has been externally validated,100 is based on 11 parameters available immediately after the cardiac arrest. The score successfully categorized patients with varying chances of hospital survival.84 Such a scoring system can inform conversations about goals of care when patients survive the initial cardiac arrest but remain seriously ill. However, the score is not able to accurately identify many patients with a very low/no chance of survival84 and should therefore not be used in isolation for decisions regarding withdrawal of care.
Figure 2. The Cardiac Arrest Survival Postresuscitation In-hospital (CASPRI) Score.
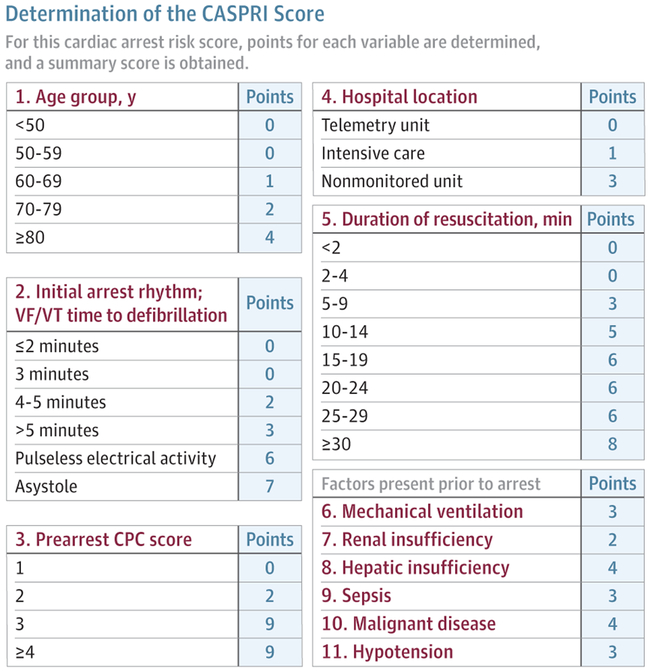
Reprinted from Chan et al,84 where a detailed description of the score's interpretation is presented. Scores of 0-4 are associated with 83% survival, 15-19 are associated with 23% survival, and 30-34 are associated with 2% survival. CPC indicates cerebral performance score; VF/VT, ventricular fibrillation or ventricular tachycardia.
Treatment recommendations for patients with in-hospital cardiac arrest with presumed severe neurological impairment following the arrest are based on studies of patients with out-of-hospital cardiac arrest.70 A key recommendation is that neuroprognostication should not be performed too early, especially when targeted temperature management is used, because slower metabolism of sedatives and neuromuscular blockade may occur. Several studies have found that delayed awakening (>48 hours after cessation of sedation) is common in patients after cardiac arrest.101 In a study of patients still in a coma 7 days after cardiac arrest, 22% obtained a favorable neurological outcome at 6 months.102 The American Heart Association recommends that neuroprognostication based on physical examination findings should be deferred until at least 72 hours after return of spontaneous circulation or rewarming (if targeted temperature management is used), and often longer if effects of sedation or neuromuscular blockade may still be present.70
The clinical examination findings most predictive of poor outcomes are absent pupillary light reflexes and absent corneal reflexes after 72 hours, as well as status myoclonus (continuous, prolonged, and generalized myoclonus) within 72 to 120 hours of the cardiac arrest. Extensor motor responses and intermittent myoclonus are less predictive of outcomes and should not be relied upon. On electroencephalography, persistent burst suppression after rewarming and nonreactivity predict poor outcomes, as does the absence of somatosensory evoked potentials at 72 hours. Cerebral edema on early head computed tomographic imaging and restricted diffusion on magnetic resonance imaging at 2 to 6 days after return of spontaneous circulation are potentially helpful in predicting outcomes, but cannot preclude a favorable neurological outcome. Biomarkers for the degree of neurological injury (eg, neuron-specific enolase and S100 calcium-binding protein B) do not adequately predict outcomes by themselves, but may be helpful when considered with other clinical features that predict outcomes.70,103
Little evidence exists for any one tool for prognostication after in-hospital cardiac arrest. A multifaceted approach that assesses the neurological prognosis combined with ongoing organ failure is prefered.102,104-106 Information regarding neurological prognosis and organ failure can be considered along with patient and cardiac arrest characteristics to support clinical decision making.
Quality Improvement
Organizations have proposed strategies and quality-improvement initiatives to improve outcomes after in-hospital cardiac arrest. For example, the GWTG-R registry currently tracks a number of quality-improvement measures, including the proportion of cardiac arrests that are monitored or witnessed, the time to relevant intervention (defibrillation for shockable rhythms and epinephrine for nonshockable rhythms), and confirmation of correct airway placement.107 Observational studies have shown that longer duration of participation in the GWTG-R registry is associated with both improved quality of care108 and increased return of spontaneous circulation.109 Specific adherence to performance measures is also associated with improvement in outcomes.110 Other aspects of cardiac arrest care, such as training, monitoring, and cardiac arrest team composition, are also potential targets for quality improvement. To monitor and improve quality of care for patients with in-hospital cardiac arrest, hospitals are encouraged to participate in national registries (eTable 2 in Supplement 1).
Limitations
The aim of this review was to provide an overview of in-hospital cardiac arrest. As such, many topics are only briefly discussed and others have been omitted. We did not perform comprehensive systematic reviews to address all topics and, therefore, relevant studies might have inadvertently been omitted. The data and recommendations provided herein are limited by the available data and, in some cases, reflect expert opinion.
Conclusions
An estimated 290 000 in-hospital cardiac arrests occur each year in the United States. However, there is limited evidence to support clinical decision making. An increased awareness with regard to optimizing clinical care and new research might improve outcomes.
Supplementary Material
Acknowledgments
Conflict of Interest Disclosures: Dr Donnino reported receiving grants from the National Institutes of Health (RO1HL136705 and K24HL127101). No other disclosures were reported.
Footnotes
Disclaimer: Dr Andersen, a paid statistical reviewer for JAMA, was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Contributor Information
Lars W. Andersen, Research Center for Emergency Medicine, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; Center for Resuscitation Science, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Department of Intensive Care Medicine, Randers Regional Hospital, Randers, Denmark.
Mathias J. Holmberg, Research Center for Emergency Medicine, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; Center for Resuscitation Science, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Katherine M. Berg, Center for Resuscitation Science, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Michael W. Donnino, Center for Resuscitation Science, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Asger Granfeldt, Department of Intensive Care, Aarhus University Hospital, Aarhus, Denmark.
REFERENCES
- 1.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS; American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920. doi: 10.1056/NEJMoa1109148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 3.Sinha SS, Sukul D, Lazarus JJ, et al. Identifying important gaps in randomized controlled trials of adult cardiac arrest treatments: a systematic review of the published literature. Circ Cardiovasc Qual Outcomes. 2016;9(6):749–756. doi: 10.1161/CIRCOUTCOMES.116.002916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumar RW, Shuster M, Callaway CW, et al. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18)(suppl 2):S315–S367. doi: 10.1161/CIR.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 5.Monsieurs KG, Nolan JP, Bossaert LL, et al. ; ERC Guidelines 2015 Writing Group. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation. 2015;95:1–80. doi: 10.1016/j.resuscitation.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 6.Brain Resuscitation Clinical Trial II Study Group. A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324 (18):1225–1231. doi: 10.1056/NEJM199105023241801 [DOI] [PubMed] [Google Scholar]
- 7.Stiell IG, Hebert PC, Weitzman BN, et al. High-dose epinephrine in adult cardiac arrest. N Engl J Med 1992;327(15):1045–1050. doi: 10.1056/NEJM199210083271502 [DOI] [PubMed] [Google Scholar]
- 8.Sack JB, Kesselbrenner MB, Bregman D. Survival from in-hospital cardiac arrest with interposed abdominal counterpulsation during cardiopulmonary resuscitation. JAMA. 1992;267(3): 379–385. doi: 10.1001/jama.1992.03480030057037 [DOI] [PubMed] [Google Scholar]
- 9.Sack JB, Kesselbrenner MB, Jarrad A. Interposed abdominal compression-cardiopulmonary resuscitation and resuscitation outcome during asystole and electromechanical dissociation. Circulation. 1992;86(6):1692–1700. doi: 10.1161/01.CIR.86.6.1692 [DOI] [PubMed] [Google Scholar]
- 10.Cohen TJ, Goldner BG, Maccaro PC, et al. A comparison of active compression-decompression cardiopulmonary resuscitation with standard cardiopulmonary resuscitation for cardiac arrests occurring in the hospital. N Engl J Med. 1993;329(26):1918–1921. doi: 10.1056/NEJM199312233292603 [DOI] [PubMed] [Google Scholar]
- 11.Lipman J, Wilson W, Kobilski S, et al. High-dose adrenaline in adult in-hospital asystolic cardiopulmonary resuscitation: a double-blind randomised trial. Anaesth Intensive Care. 1993;21(2):192–196. [DOI] [PubMed] [Google Scholar]
- 12.Tucker KJ, Galli F, Savitt MA, Kahsai D, Bresnahan L, Redberg RF. Active compression-decompression resuscitation: effect on resuscitation success after in-hospital cardiac arrest. J Am Coll Cardiol. 1994;24(1):201–209. doi: 10.1016/0735-1097(94)90564-9 [DOI] [PubMed] [Google Scholar]
- 13.Woodhouse SP, Cox S, Boyd P, Case C, Weber M. High dose and standard dose adrenaline do not alter survival, compared with placebo, in cardiac arrest. Resuscitation. 1995;30(3):243–249. doi: 10.1016/0300-9572(95)00890-X [DOI] [PubMed] [Google Scholar]
- 14.Patrick WD, Freedman J, McEwen T, Light RB, Ludwig L, Roberts D. A randomized, double-blind comparison of methoxamine and epinephrine in human cardiopulmonary arrest. Am J Respir Crit Care Med. 1995;152(2):519–523. doi: 10.1164/ajrccm.152.2.7633701 [DOI] [PubMed] [Google Scholar]
- 15.Stiell IG, Hébert PC, Wells GA, et al. The Ontario trial of active compression-decompression cardiopulmonary resuscitation for in-hospital and prehospital cardiac arrest. JAMA. 1996;275(18): 1417–1423. doi: 10.1001/jama.1996.03530420045034 [DOI] [PubMed] [Google Scholar]
- 16.Thel MC, Armstrong AL, McNulty SE, Califf RM, O’Connor CM; Duke Internal Medicine Housestaff. Randomised trial of magnesium in in-hospital cardiac arrest. Lancet. 1997;350(9087):1272–1276. doi: 10.1016/S0140-6736(97)05048-4 [DOI] [PubMed] [Google Scholar]
- 17.Stiell IG, Hébert PC, Wells GA, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105–109. doi: 10.1016/S0140-6736(01)05328-4 [DOI] [PubMed] [Google Scholar]
- 18.Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169(1):15–24. doi: 10.1001/archinternmed.2008.509 [DOI] [PubMed] [Google Scholar]
- 19.Weidman EK, Bell G, Walsh D, Small S, Edelson DP. Assessing the impact of immersive simulation on clinical performance during actual in-hospital cardiac arrest with CPR-sensing technology: a randomized feasibility study. Resuscitation. 2010;81(11):1556–1561. doi: 10.1016/j.resuscitation.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 20.Pittl U, Schratter A, Desch S, et al. Invasive versus non-invasive cooling after in- and out-of-hospital cardiac arrest: a randomized trial. Clin Res Cardiol. 2013;102(8):607–614. doi: 10.1007/s00392-013-0572-3 [DOI] [PubMed] [Google Scholar]
- 21.Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310(3):270–279. doi: 10.1001/jama.2013.7832 [DOI] [PubMed] [Google Scholar]
- 22.Vahedian-Azimi A, Hajiesmaeili M, Amirsavadkouhi A, et al. Effect of the Cardio First Angel device on CPR indices: a randomized controlled clinical trial. Crit Care. 2016;20(1):147. doi: 10.1186/s13054-016-1296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastwood GM, Schneider AG, Suzuki S, et al. Targeted therapeutic mild hypercapnia after cardiac arrest: A phase II multi-centre randomised controlled trial (the CCC trial). Resuscitation. 2016;104:83–90. doi: 10.1016/j.resuscitation.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 24.Movahedi A, Mirhafez SR, Behnam-Voshani H, et al. A comparison of the effect of interposed abdominal compression cardiopulmonary resuscitation and standard cardiopulmonary resuscitation methods on end-tidal CO2 and the return of spontaneous circulation following cardiac arrest: a clinical trial. Acad Emerg Med. 2016;23(4): 448–454. doi: 10.1111/acem.12903 [DOI] [PubMed] [Google Scholar]
- 25.Anantharaman V, Tay SY, Manning PG, et al. A multicenter prospective randomized study comparing the efficacy of escalating higher biphasic versus low biphasic energy defibrillations in patients presenting with cardiac arrest in the in-hospital environment. Open Access Emerg Med. 2017;9:9–17. doi: 10.2147/OAEM.S109339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster RW, Beenen LF, van der Boom EB, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J. 2017;38(40):3006–3013. doi: 10.1093/eurheartj/ehx318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Li C, Shao F, Zhao L, Wang M, Fang Y. Efficacy and safety of combination therapy of shenfu injection and postresuscitation bundle in patients with return of spontaneous circulation after in-hospital cardiac arrest: a randomized, assessor-blinded, controlled trial. Crit Care Med. 2017;45(10):1587–1595. doi: 10.1097/CCM.0000000000002570 [DOI] [PubMed] [Google Scholar]
- 28.Look X, Li H, Ng M, et al. Randomized controlled trial of internal and external targeted temperature management methods in post- cardiac arrest patients. Am J Emerg Med. 2018;36(1):66–72. doi: 10.1016/j.ajem.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 29.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33(2):237–245. doi: 10.1007/s00134-006-0326-z [DOI] [PubMed] [Google Scholar]
- 30.Morrison LJ, Neumar RW, Zimmerman JL, et al. ; American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on P. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127(14):1538–1563. doi: 10.1161/CIR.0b013e31828b2770 [DOI] [PubMed] [Google Scholar]
- 31.Merchant RM, Yang L, Becker LB, et al. ; American Heart Association Get With The Guidelines-Resuscitation Investigators. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406. doi: 10.1097/CCM.0b013e3182257459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan JP, Soar J, Smith GB, et al. ; National Cardiac Arrest Audit. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation. 2014;85(8):987–992. doi: 10.1016/j.resuscitation.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 33.Holmberg M, Ross C, Chan P, et al. Incidence of adult in-hospital cardiac arrest in the United States. Abstract presented at: American Heart Association Resuscitation Science Symposium; November 10-11, 2018; Chicago, IL. [Google Scholar]
- 34.Perman SM, Stanton E, Soar J, et al. ; American Heart Association’s Get With the Guidelines®— Resuscitation Investigators. Location of in-hospital cardiac arrest in the United States: variability in event rate and outcomes. J Am Heart Assoc. 2016;5(10):e003638. doi: 10.1161/JAHA.116.003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DANARREST Steering Commitee. DANARREST: Registrering af hjertestop på hospital. Årsrapport 2015 [Danish]. https://www.sundhed.dk/content/cms/83/70283_danarrest-%C3%A5rsrapport-2015.pdf. Published June 30, 2016. Accessed June 5, 2017.
- 36.Tran S, Deacon N, Minokadeh A, et al. Frequency and survival pattern of in-hospital cardiac arrests: the impacts of etiology and timing. Resuscitation. 2016;107:13–18. doi: 10.1016/j.resuscitation.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schluep M, Gravesteijn BY, Stolker RJ, Endeman H, Hoeks SE. One-year survival after in-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2018;132:90–100. doi: 10.1016/j.resuscitation.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 38.Chan PS, Nallamothu BK, Krumholz HM, et al. ; American Heart Association Get with the Guidelines–Resuscitation Investigators. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368(11):1019–1026. doi: 10.1056/NEJMoa1200657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallmuller C, Meron G, Kurkciyan I, Schober A, Stratil P, Sterz F. Causes of in-hospital cardiac arrest and influence on outcome. Resuscitation. 2012;83(10):1206–1211. doi: 10.1016/j.resuscitation.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 40.Radeschi G, Mina A, Berta G, et al. ; Piedmont IHCA Registry Initiative. Incidence and outcome of in-hospital cardiac arrest in Italy: a multicentre observational study in the Piedmont Region. Resuscitation. 2017;119:48–55. doi: 10.1016/j.resuscitation.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 41.Legriel S, Bougouin W, Chocron R, et al. ; for Paris-SDEC investigators. Early in-hospital management of cardiac arrest from neurological cause: diagnostic pitfalls and treatment issues. Resuscitation. 2018;132:147–155. doi: 10.1016/j.resuscitation.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 42.Soar J, Nolan JP, Böttiger BW, et al. ; Adult advanced life support section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 43.Tirkkonen J, Hellevuo H, Olkkola KT, Hoppu S. Aetiology of in-hospital cardiac arrest on general wards. Resuscitation. 2016;107:19–24. doi: 10.1016/j.resuscitation.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 44.Bergum D, Haugen BO, Nordseth T, Mjølstad OC, Skogvoll E. Recognizing the causes of in-hospital cardiac arrest—a survival benefit. Resuscitation. 2015;97:91–96. doi: 10.1016/j.resuscitation.2015.09.395 [DOI] [PubMed] [Google Scholar]
- 45.De Bruin ML, Langendijk PN, Koopmans RP, Wilde AA, Leufkens HG, Hoes AW. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc-prolonging drugs. Br J Clin Pharmacol. 2007;63(2):216–223. doi: 10.1111/j.1365-2125.2006.02722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. doi: 10.1371/journal.pone.0150214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larkin GL, Copes WS, Nathanson BH, Kaye W. Pre-resuscitation factors associated with mortality in 49,130 cases of in-hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81(3):302–311. doi: 10.1016/j.resuscitation.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 48.Andersen LW, Kim WY, Chase M, et al. ; American Heart Association’s Get With the Guidelines(®) – Resuscitation Investigators. The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation. 2016;98:112–117. doi: 10.1016/j.resuscitation.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galhotra S, DeVita MA, Simmons RL, Dew MA; Members of the Medical Emergency Response Improvement Team (MERIT) Committee. Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Qual Saf Health Care. 2007;16(4):260–265. doi: 10.1136/qshc.2007.022210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronick SL, Kurz MC, Lin S, et al. Part 4: Systems of Care and Continuous Quality Improvement: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18)(suppl 2):S397–S413. doi: 10.1161/CIR.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 51.Smith ME, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454–1465. doi: 10.1513/AnnalsATS.201403-102OC [DOI] [PubMed] [Google Scholar]
- 52.Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. doi: 10.1186/s13054-015-0973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasselqvist-Ax I, Riva G, Herlitz J, et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2015;372(24):2307–2315. doi: 10.1056/NEJMoa1405796 [DOI] [PubMed] [Google Scholar]
- 54.Bircher NG, Chan PS, Xu Y; American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Delays in cardiopulmonary resuscitation, defibrillation, and epinephrine administration all decrease survival in in-hospital cardiac arrest. Anesthesiology. 2019;130:414–422. doi: 10.1097/ALN.0000000000002563 [DOI] [PubMed] [Google Scholar]
- 55.Wallace SK, Abella BS, Becker LB. Quantifying the effect of cardiopulmonary resuscitation quality on cardiac arrest outcome: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2013;6(2):148–156. doi: 10.1161/CIRCOUTCOMES.111.000041 [DOI] [PubMed] [Google Scholar]
- 56.Chan PS, Krumholz HM, Nichol G, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. doi: 10.1056/NEJMoa0706467 [DOI] [PubMed] [Google Scholar]
- 57.Andersen LW, Holmberg MJ, Granfeldt A, et al. ; CARES Surveillance Group. Neighborhood characteristics, bystander automated external defibrillator use, and patient outcomes in public out-of-hospital cardiac arrest. Resuscitation. 2018;126:72–79. doi: 10.1016/j.resuscitation.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 58.Chan PS, Krumholz HM, Spertus JA, et al. ; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Automated external defibrillators and survival after in-hospital cardiac arrest. JAMA. 2010;304(19):2129–2136. doi: 10.1001/jama.2010.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kudenchuk PJ, Brown SP, Daya M, et al. ; Resuscitation Outcomes Consortium Investigators. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374(18):1711–1722. doi: 10.1056/NEJMoa1514204 [DOI] [PubMed] [Google Scholar]
- 60.Perkins GD, Ji C, Deakin CD, et al. ; PARAMEDIC2 Collaborators. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–721. doi: 10.1056/NEJMoa1806842 [DOI] [PubMed] [Google Scholar]
- 61.Donnino MW, Salciccioli JD, Howell MD, et al. ; American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028. doi: 10.1136/bmj.g3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersen LW, Kurth T, Chase M, et al. ; American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Early administration of epinephrine (adrenaline) in patients with cardiac arrest with initial shockable rhythm in hospital: propensity score matched analysis. BMJ. 2016;353:i1577. doi: 10.1136/bmj.i1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18)(suppl 2):S444–S464. doi: 10.1161/CIR.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 64.Jabre P, Penaloza A, Pinero D, et al. Effect of bag-mask ventilation vs endotracheal intubation during cardiopulmonary resuscitation on neurological outcome after out-of-hospital cardiorespiratory arrest: a randomized clinical trial. JAMA. 2018;319(8):779–787. doi: 10.1001/jama.2018.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang HE, Schmicker RH, Daya MR, et al. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2018;320(8):769–778. doi: 10.1001/jama.2018.7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benger JR, Kirby K, Black S, et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. JAMA. 2018;320(8):779–791. doi: 10.1001/jama.2018.11597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersen LW, Granfeldt A, Callaway CW, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Association between tracheal intubation during adult in-hospital cardiac arrest and survival. JAMA. 2017;317(5):494–506. doi: 10.1001/jama.2016.20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmberg MJ, Geri G, Wiberg S, et al. ; International Liaison Committee on Resuscitation’s (ILCOR) Advanced Life Support and Pediatric Task Forces. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: A systematic review. Resuscitation. 2018;131:91–100. doi: 10.1016/j.resuscitation.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soar J, Callaway CW, Aibiki M, et al. ; Advanced Life Support Chapter Collaborators. Part 4: Advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2015;95:e71–e120. doi: 10.1016/j.resuscitation.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 70.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18)(suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan PS, Berg RA, Tang Y, Curtis LH, Spertus JA; American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association between therapeutic hypothermia and survival after in-hospital cardiac arrest. JAMA. 2016;316(13):1375–1382. doi: 10.1001/jama.2016.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berg KM, Grossestreuer AV, Uber A, Patel PV, Donnino MW. Intubation is not a marker for coma after in-hospital cardiac arrest: a retrospective study. Resuscitation. 2017;119:18–20. doi: 10.1016/j.resuscitation.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts BW, Kilgannon JH, Hunter BR, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation. 2018;137(20):2114–2124. doi: 10.1161/CIRCULATIONAHA.117.032054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with Post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–2113. doi: 10.1161/CIRCULATIONAHA.112.000168 [DOI] [PubMed] [Google Scholar]
- 75.Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med. 2017;195(9):1198–1206. doi: 10.1164/rccm.201609-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moskowitz A, Grossestreuer AV, Berg KM, et al. ; Center for Resuscitation Science. The association between tidal volume and neurological outcome following in-hospital cardiac arrest. Resuscitation. 2018;124:106–111. doi: 10.1016/j.resuscitation.2017.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z [DOI] [PubMed] [Google Scholar]
- 78.Witten L, Gardner R, Holmberg MJ, et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation. 2019;136:93–99. doi: 10.1016/j.resuscitation.2019.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289 [DOI] [PubMed] [Google Scholar]
- 80.Bro-Jeppesen J, Annborn M, Hassager C, et al. ; TTM Investigators. Hemodynamics and vasopressor support during targeted temperature management at 33°C versus 36°C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial. Crit Care Med. 2015;43(2):318–327. doi: 10.1097/CCM.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 81.Annborn M, Bro-Jeppesen J, Nielsen N, et al. ; TTM-trial investigators. The association of targeted temperature management at 33 and 36 °C with outcome in patients with moderate shock on admission after out-of-hospital cardiac arrest: a post hoc analysis of the Target Temperature Management trial. Intensive Care Med. 2014;40(9):1210–1219. doi: 10.1007/s00134-014-3375-8 [DOI] [PubMed] [Google Scholar]
- 82.Itenov TS, Johansen ME, Bestle M, et al. ; Cooling and Surviving Septic Shock (CASS) Trial Collaboration. Induced hypothermia in patients with septic shock and respiratory failure (CASS): a randomised, controlled, open-label trial. Lancet Respir Med. 2018;6(3):183–192. doi: 10.1016/S2213-2600(18)30004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mourvillier B, Tubach F, van de Beek D, et al. Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA. 2013;310(20):2174–2183. doi: 10.1001/jama.2013.280506 [DOI] [PubMed] [Google Scholar]
- 84.Chan PS, Spertus JA, Krumholz HM, et al. ; Get With the Guidelines-Resuscitation Registry Investigators. A validated prediction tool for initial survivors of in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947–953. doi: 10.1001/archinternmed.2012.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison DA, Patel K, Nixon E, et al. ; National Cardiac Arrest Audit. Development and validation of risk models to predict outcomes following in-hospital cardiac arrest attended by a hospital-based resuscitation team. Resuscitation. 2014;85(8):993–1000. doi: 10.1016/j.resuscitation.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Topjian AA, Localio AR, Berg RA, et al. ; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Women of child-bearing age have better inhospital cardiac arrest survival outcomes than do equal-aged men. Crit Care Med. 2010;38(5):1254–1260. doi: 10.1097/CCM.0b013e3181d8ca43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joseph L, Chan PS, Bradley SM, et al. ; American Heart Association Get With the Guidelines–Resuscitation Investigators. Temporal changes in the racial gap in survival after in-hospital cardiac arrest. JAMA Cardiol. 2017;2(9):976–984. doi: 10.1001/jamacardio.2017.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galea S, Blaney S, Nandi A, et al. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007;166(5):534–543. doi: 10.1093/aje/kwm102 [DOI] [PubMed] [Google Scholar]
- 89.Ebell MH, Afonso AM. Pre-arrest predictors of failure to survive after in-hospital cardiopulmonary resuscitation: a meta-analysis. Fam Pract. 2011;28(5):505–515. doi: 10.1093/fampra/cmr023 [DOI] [PubMed] [Google Scholar]
- 90.Ebell MH. Prearrest predictors of survival following in-hospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract. 1992;34(5):551–558. [PubMed] [Google Scholar]
- 91.Brady WJ, Gurka KK, Mehring B, Peberdy MA, O’Connor RE; American Heart Association’s Get with the Guidelines (formerly, NRCPR) Investigators. In-hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82(7):845–852. doi: 10.1016/j.resuscitation.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 92.Nadkarni VM, Larkin GL, Peberdy MA, et al. ; National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50 [DOI] [PubMed] [Google Scholar]
- 93.Rohlin O, Taeri T, Netzereab S, Ullemark E, Djärv T. Duration of CPR and impact on 30-day survival after ROSC for in-hospital cardiac arrest: a Swedish cohort study. Resuscitation. 2018;132:1–5. doi: 10.1016/j.resuscitation.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 94.Mancini ME, Diekema DS, Hoadley TA, et al. Part 3: Ethical Issues: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18)(suppl 2):S383–S396. doi: 10.1161/CIR.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 95.Bossaert LL, Perkins GD, Askitopoulou H, et al. ; ethics of resuscitation and end-of-life decisions section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015: Section 11. The ethics of resuscitation and end-of-life decisions. Resuscitation. 2015;95:302–311. doi: 10.1016/j.resuscitation.2015.07.033 [DOI] [PubMed] [Google Scholar]
- 96.Goldberger ZD, Chan PS, Berg RA, et al. ; American Heart Association Get With The Guidelines—Resuscitation (formerly National Registry of Cardiopulmonary Resuscitation) Investigators. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380(9852):1473–1481. doi: 10.1016/S0140-6736(12)60862-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu C, Zheng Z, Jiang L, et al. The predictive value of bedside ultrasound to restore spontaneous circulation in patients with pulseless electrical activity: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0191636. doi: 10.1371/journal.pone.0191636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu K, Gupta N, Teran F, Saul T, Nelson BP, Andrus P. Variability in interpretation of cardiac standstill among physician sonographers. Ann Emerg Med. 2018;71(2):193–198. doi: 10.1016/j.annemergmed.2017.07.476 [DOI] [PubMed] [Google Scholar]
- 99.Paiva EF, Paxton JH, O’Neil BJ. The use of end-tidal carbon dioxide (ETCO2) measurement to guide management of cardiac arrest: a systematic review. Resuscitation. 2018;123:1–7. doi: 10.1016/j.resuscitation.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 100.Wang CH, Chang WT, Huang CH, et al. Validation of the Cardiac Arrest Survival Postresuscitation In-hospital (CASPRI) score in an East Asian population. PLoS One. 2018;13(8):e0202938. doi: 10.1371/journal.pone.0202938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paul M, Bougouin W, Geri G, et al. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42(7):1128–1136. doi: 10.1007/s00134-016-4349-9 [DOI] [PubMed] [Google Scholar]
- 102.Velly L, Perlbarg V, Boulier T, et al. ; MRI-COMA Investigators. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol 2018;17(4):317–326. doi: 10.1016/S1474-4422(18)30027-9 [DOI] [PubMed] [Google Scholar]
- 103.Sandroni C, D’Arrigo S, Nolan JP. Prognostication after cardiac arrest. Crit Care. 2018;22(1):150. doi: 10.1186/s13054-018-2060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Youn CS, Callaway CW, Rittenberger JC; Post Cardiac Arrest Service. Combination of initial neurologic examination and continuous EEG to predict survival after cardiac arrest. Resuscitation. 2015;94:73–79. doi: 10.1016/j.resuscitation.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 105.Youn CS, Callaway CW, Rittenberger JC; Post Cardiac Arrest Service. Combination of initial neurologic examination, quantitative brain imaging and electroencephalography to predict outcome after cardiac arrest. Resuscitation. 2017;110:120–125. doi: 10.1016/j.resuscitation.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coppler PJ, Elmer J, Calderon L, et al. ; Post cardiac arrest Service. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Get With the Guidelines-Resuscitation Registry. Resuscitation Fact Sheet. American Heart Association website; 2014. http://www.heart.org/idc/groups/heart-public/@private/@wcm/@hcm/@gwtg/documents/downloadable/ucm_434082.pdf. Accessed February 25, 2019. [Google Scholar]
- 108.Starks MA, Dai D, Nichol G, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators. The association of duration of participation in get with the guidelines-resuscitation with quality of care for in-hospital cardiac arrest. Am Heart J. 2018;204:156–162. doi: 10.1016/j.ahj.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 109.Bradley SM, Huszti E, Warren SA, Merchant RM, Sayre MR, Nichol G. Duration of hospital participation in Get With the Guidelines-Resuscitation and survival of in-hospital cardiac arrest. Resuscitation. 2012;83(11):1349–1357. doi: 10.1016/j.resuscitation.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anderson ML, Nichol G, Dai D, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association between hospital process composite performance and patient outcomes after in-hospital cardiac arrest care. JAMA Cardiol. 2016;1(1):37–45. doi: 10.1001/jamacardio.2015.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


