Abstract
Impaired maturation of nerve growth factor precursor (proNGF) and its accumulation has been reported in several neurodegenerative diseases, myocardial infarction and diabetes. To elucidate the direct impact of proNGF accumulation identified the need to create a transgenic model that can express fully mutated cleavage-resistant proNGF. Using Cre-Lox technology, we developed an inducible endothelial-specific proNGF transgenic mouse (proNGFLoxp) that overexpresses GFP-conjugated cleavage-resistant proNGF123 when crossed with VE-cadherin-CreERT2 (Cre). Expression of proNGF, inflammatory mediators, NGF and VEGF was evaluated by PCR, Western blot and immunohistochemistry. EC-proNGF overexpression was confirmed using colocalization of anti-proNGF within retinal vasculature. EC-proNGF did not cause retinal neurotoxicity or marked glial activation at 4-weeks. Microvascular preparation from Cre-proNGF mice showed significant imbalance of proNGF/NGF ratio, enhanced expression of TNF-α and p75NTR, and tendency to impair TrkA phosphorylation compared to controls. EC-proNGF overexpression triggered mRNA expression of p75NTR and inflammatory mediators in both retina and renal cortex compared to controls. EC-proNGF expression induced vascular permeability including breakdown of BRB and albuminuria in the kidney without affecting VEGF level at 4-weeks. Histopathological changes were assessed after 8-weeks and the results showed that EC-proNGF triggered formation of occluded (acellular) capillaries, hall mark of retinal ischemia. EC-proNGF resulted in glomerular enlargement and kidney fibrosis, hall mark of renal dysfunction. We have successfully created an inducible mouse model that can dissect the contribution of autocrine direct action of cleavage-resistant proNGF on systemic microvascular abnormalities in both retina and kidney, major targets for microvascular complication.
Keywords: Neurotrophin, Nerve growth factor, Diabetic retinopathy, Acellular capillary, Apoptosis, Neurodegeneration, Inflammation, Barrier dysfunction
1. Introduction
ProNGF is the precursor form of the mature nerve growth factor (NGF), which is mainly secreted from glia. ProNGF normally undergoes proteolytic cleavage; intracellularly by plasmin and furin and extracellularly by matrix metalloproteinase to produce the mature form [1,2]. Diabetes-induced alteration in NGF levels has been correlated with various microvascular complications including retinopathy, nephropathy, and neuropathy. Diabetes increased serum and kidney levels of NGF in an experimental model of diabetic nephropathy [3]. A clinical study showed positive correlation of serum NGF levels in patients with diabetic retinopathy with the existence of diabetic nephropathy [4]. We discovered that diabetes causes imbalance of NGF and its precursor proNGF ratio in retina and ocular fluids that were putatively correlated with serum levels in diabetic patients [5,6]. This imbalance was associated with increased expression of p75NTR receptor, retinal inflammation [7], vascular permeability, neurodegeneration [5,8] and formation of occluded capillaries [9,10]. In addition to diabetes, impaired maturation of proNGF and its accumulation has been also reported in neurodegenerative diseases such as Alzheimer [11,12], Pick’s disease [13] and diabetic encephalopathy [14].
The main biological function of proNGF is regulating cell death via binding to p75NTR and its co-receptor; sortilin, a member of VPS-10p domain receptor family [2,15–17]. Cell death mechanisms involve direct activation of apoptotic pathways or non-cell autonomous signaling pathways [18]. To further study the role of proNGF away from complex nature of diabetic milieu, we have stably over-expressed a cleavage-resistant isoform of proNGF in rodent retina. Stable overexpression of cleavage-resistant proNGF resulted in neuro- and microvascular degeneration [19]. Interestingly, we and others have shown that proNGF can induce p75NTR/NFkB pathway on glia to sustain its own expression and induce other inflammatory mediators expression including TNF-α and IL-1β resulting in neurodegeneration [7,20]. Retinal overexpression of proNGF plasmid created similar pro-inflammatory milieu that render dissecting the direct action of proNGF on vasculature unachievable goal. Prior attempts to create a transgenic mouse that systemically overexpressed the fully mutant cleavage-resistant proNGF123 construct have proven embryonic lethal [19]. Therefore, we used Cre-Lox technology to generate a conditional transgenic mouse model, where stable expression of a GFP-conjugated cleavage-resistant proNGF123 [21] can be induced in the specific tissue of interest. In the current study, we attempted to create and characterize the impact of endothelial-specific (EC)-overexpression of proNGF in both retina and kidney, major targets of diabetes-mediated microvascular complications. The study also assessed expression of inflammatory mediators and barrier function in both retina and kidney after 4-weeks and major histopathological changes at 8-weeks post EC-proNGF overexpression.
2. Materials and methods
2.1. Animals
All animal experiments were performed in accordance with Association for Research in Vision and Ophthalmology statement for use of animals in ophthalmic and vision research, and Charlie Norwood VA Medical Center Animal Care and Use Committee. All studies were approved by the appropriate institutional animal review board (Charlie Norwood VA Medical Center Animal Care and Use Committee, Augusta; Approval ID ACORP#16–01–088).
2.2. Generation of inducible endothelial-specific (EC)-proNGF transgenic mice
Using Cre-Lox technology, we have developed an inducible endothelial-specific proNGF transgenic mouse. A construct of cleavage-resistant proNGF123 [21], originally obtained from Dr. Kenneth Neet (University of Chicago), was engineered to produce GFP-conjugated-proNGF construct [19] and then used to create proNGFLoxp mice at the Transgenic Mouse Core Facility, University at Albany, NY. Transgenic proNGFLoxp mice were backcrossed with C57BL6J mice from Jackson Laboratories for 5-generations before actual experiments. To drive proNGF expression in endothelial cells, Transgenic proNGFLoxp mice were crossed with Cre-VE-Cadherin mice, which were obtained from Dr. Luisa Iruela-Arispe, University of California Los Angeles [22]. Age-matched groups (6–8 weeks) of Cre or Cre-proNGFLoxp mice received five-consecutive intraperitoneal injections of tamoxifen (2 mg) in sunflower oil to activate proNGF expression. Mice were sacrificed after 4-weeks or 8-weeks of inducing proNGF expression (1-week post first tamoxifen injection). Cre-mice, proNGFLoxp mice and/or WT-mice served as controls.
2.3. Immunohistochemistry
After 4-weeks of proNGF expression, frozen retinal cryo-sections (10-μm thickness) were permealized, blocked in goat serum and incubated with primary antibodies including anti-GFP (Abcam, Rabbit polyclonal, 1:200), anti-proNGF (Alomone, Israel, Rabbit polyclonal, 1:100), anti-GFAP (polyclonal, Affinity Bioreagents, Rockford, IL, USA; 1:200), followed by Oregon-green or Texas-red conjugated goat antirabbit secondary antibody (Invitrogen, Carlsbad, CA, USA; 1:500). Retinal vasculature was labeled with the red fluorescent Alexa Fluor® 594 isolectin GS-IB4 conjugate (Molecular Probes, Life Technology, Grand Island, NY, USA). Mounting solution (Vector laboratories, Burlingame, CA, USA) was added, and the slides were sealed using a cover slip. Images were taken by a microscope (AxioObserver.Z1; Zeiss, Germany) under 20× magnification using AxioVision software.
2.4. Retinal cell death evaluation
ApopTag® Fluorescein In Situ Apoptosis Detection Kit was used to determine cell death in retinal sections according to the manufacturer’s instructions (S7110, Millipore. Darmstadt, Germany). Sections were cover-slipped with Vectashield without DAPI (Vector Laboratories, Burlingame, CA, USA) and images were captured at 20× by fluorescent microscope (AxioObserver.Z1; Zeiss, Jena, Germany). The total and differential number of TUNEL positive cells were determined in the ganglion cell layer (GCL), inner nuclear layer (INL) and outer nuclear layer (ONL).
2.5. Isolation of microvascular preparation
Microvasculature from brain pial blood vessels were isolated as described before [23]. Pial microvasculature share common neurovascular unit characteristics of the retina [24]. Brains from various experimental groups were isolated and placed on ice cold PBS and pial vessels including anterior, middle, posterior cerebral arteries and basilar arteries were isolated and snap frozen. Retinas or microvascular preparation were isolated and homogenized in radioimmunoprecipitation assay (RIPA) buffer as described previously [25].
2.6. Quantitative real-time PCR
All samples were analyzed after 4-weeks of proNGF overexpression. One-step quantitative RT-PCR kit (Invitrogen) was used to amplify 10 ng retinal mRNA as described previously [25]. PCR primers (listed in Table 2) were purchased from Integrated DNA Technologies (Coralville, IA, USA). Quantitative PCR was performed StepOnePlus qPCR system (Applied BioSystems, Life Technologies). The expression of various genes was normalized to 18S.
Table 2.
List of antibodies used for protein of interest and internal controls.
| Primary antibodies | Source | Catalog # | Dilution |
|---|---|---|---|
| NGF Pro-NGF |
Alomon Labs (Jerusalem, Israel) | AN-240 ANT-005 |
1:500 1:500 |
| GAPDH | Cell Signaling (Danvers, MA, USA) | 2118L | 1:1000 |
| polyclonal anti-p75NTR | Bruce Carter | 1:3000 | |
| TrkA pTrkA |
Abcam (Cambridge, MA, USA) | Ab 76291 Ab-1445 (Y490) |
1:1000 1:500 |
| VEGF | Millipore (Billerica, MA, USA) | ABS82 | 1:1000 |
| Cleaved-Caspase-1 | Enzo Life sciences Farmingdale, NY, USA |
ALX-210–804-C100 | 1:500 |
| Cleaved-IL-1b | Abcam, Cambridge, MA, USA | ab82558 | 1:500 |
| TNF-α | Novus Biologicals, Littleton, CO, | NBP1–19532 | 1:500 |
| Actin | Sigma | A5060 | 1:1000 |
2.7. Western blot analysis
Samples (20–30 μg protein) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and electro-blotted to a nitrocellulose membrane. Membranes were probed with the primary antibodies (see list in Table 1) then reprobed with GAPDH to confirm equal loading. The primary antibody was detected using a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (EMD, La Jolla, CA) and enhanced chemiluminescence. The films were scanned and the band intensity was quantified using Image J densitometry software version and expressed as optical density (OD).
Table 1.
List of PCR primers used for amplification of gene of interest and internal controls.
| Gene | Forward | Reverse |
|---|---|---|
| 18S | CGCGGTTCTATTTTGTTGGT | AGTCGGCATCGTTTATGGTC |
| GAPDH | GCCAAAAGGGTCATCATCTC | CACACCCATCACAAACATGG |
| NGF | AGGCCCATGGTACAATCCCTTTCA | ATCTCCAACCCACACACTGACACT |
| proNGF | TTTGATCGGCGTACAGGCAG | AGTTTAGTCCAGTGGGCTTCAGGG |
| P75NTR | GCAGCTCCAAGCCTGTAGTG | TAGGCCACAAGGCCCACAAC |
| IL-1β | GCAACTGTTCCTGAACTCACCT | ATCTTTTGGGGTCCGTCAACT |
| TNFα | GATCGGTCCCCAAAGGGATG | GTGGTTTGTGAGTGTGAGGGT |
2.8. Assessment of blood-retinal barrier (BRB) breakdown
To assess BRB breakdown, mice were sacrificed 20 min after receiving jugular vein injections of 100 μl of 5 mg/ml BSA-conjugated Alexa-Fluor 555 (Invitrogen, Thermo Scientific, USA). Retinas were snap-frozen in liquid nitrogen, and blood was collected from abdominal inferior vena cava. Fluorescence of retinal lysate was normalized to that of the serum. Fluorescent plate reader (BioTek Synergy2, Winooski, VT, USA) was used for measuring BSA-conjugated fluorescein (excitation 555 nm, emission 565 nm) and a non-injected control retina served as a blank. The average retinal fluorescence intensity was calculated and normalized first to milligram of retinal protein and serum fluorescence intensity for each animal as described previously by our group [5].
2.9. Quantification of retina ganglion cells (RGC)
Retinal sections containing the optic nerve were fixed using 2% paraformaldehyde and incubated overnight in Brn3A antibody (1:25 dilution, Santa Cruz, CA) at 4 °C. Sections were blocked for 1 h with 20% goat serum in PBS containing 0.3%Triton X-100 and incubated for 2 h with secondary antibody (1:500) in PBS-containing 0.3% Triton X-100 and 1% BSA. Sections were cover-slipped with Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) and images were captured by fluorescent microscope (AxioObserver.Z1; Zeiss, Jena, Germany). The total number cells in the ganglion cell layer (GCL) were counted as DAPI-positive and retinal ganglion cells were counted as both Brn3A and DAPI-positive from one periphery of one Ora-Serrata to the other as described before [19].
2.10. Measurement of urinary albumin excretion rate
Excretion of urinary albumin was determined after 4-weeks of proNGF overexpression using albumin-to creatinine ratio in 24-hour urine collections [26]. Twenty-four-hour urine was collected using Nalgene metabolic cage system (Rochester, NY), which allows efficient separation of urine and feces from a single mouse. The level of albumin in urine sample was quantified by ELISA (Bethyl Laboratories, Inc., Montgomery, TX), and the level of urine creatinine was determined using creatinine assay kit (Diazyme Laboratories, Poway, CA).
2.11. Trypsin digest and determination of the number of occluded (acellular) capillaries
Histopathological changes in retinal capillaries were assessed after 4 and 8-weeks of EC-proNGF overexpression. Freshly enucleated eyes were fixed overnight in 2% paraformaldehyde. Dissected retinal cups were incubated in 3% Difco-trypsin 250 (BD Biosciences, San Jose, CA, in 25 mmol/l Tris buffer, pH 7.4) at 37 °C till full digestion, which was confirmed by the removal of the vitreous and nonvascular cells. Several washes of 0.5% Triton X-100 were used to remove the neuronal retina. Periodic acid-Schiff and hematoxylin (PASH) was used to stain the trypsin-digested retinas. Numbers of acellular capillaries were quantified in five different areas of the mid-retina at 20× in a blinded manner. Acellular capillaries were identified as capillary-sized blood vessel tubes with no nuclei along their length [9].
2.12. Assessment of renal histopathological changes
Histopathological changes in the kidney were assessed after 8-weeks of EC-proNGF overexpression. Kidneys were fixed in 10% buffered formalin overnight at room temperature, transferred to 70% ethanol for 24 h, and paraffin embedded. The kidneys were sectioned at a thickness of 5 μm onto Superfrost plus slides and processed. For assessment of injury, 5 μM sections were stained with periodic acid-Schiff followed by hematoxylin (PASH) as described previously [26]. Fibrosis was determined with Mason’s trichrome staining. Ten fields of 40× magnifications were examined and averaged. The individual scoring of the slides was blinded to the genotype of the animals. Glomerular size was calculated as described before [25]. Briefly, glomerular size was determined using ImageJ analysis software (US National Institutes of Health, Bethesda, MD, USA http://imagej.nih.gov/ij). The largest area in these sections was measured in four different mice per group with at least ten glomeruli of each mouse.
2.13. Determination of renal RhoA kinase activity using pull down assay
RhoA kinase activity was assessed by pull-down assay as previously described previously [27]. In brief, kidneys were homogenized in assay buffer (50 mM Tris, pH 7.2; 1% Triton X-100; 0.5% sodium deoxycholate; 0.1% SDS; 500 mM NaCl; 10 mM MgCl2 and protease inhibitors). Next, 100 g of renal homogenate was incubated with 10 g of agarose (GST)-TRBD beads at 4 °C for 60 min. The beads were washed four times with the assay buffer. Bound Rho proteins were then detected by Western blot by using a monoclonal antibody against RhoA (Millipore).
2.14. Statistical analysis
Results are expressed as mean ± SD and the data were evaluated for normality and appropriate transformations were used when necessary. Data was processed for statistical analysis with One-Way ANOVA followed by Bonferroni post-hoc multiple comparisons to assess significant differences between groups (Graphpad-Ver.6). Significance was defined at probability p < 0.05.
3. Results
3.1. Characterization of EC-proNGF overexpressing transgenic mice and localization of GFP conjugated-proNGF expression within retina vasculature
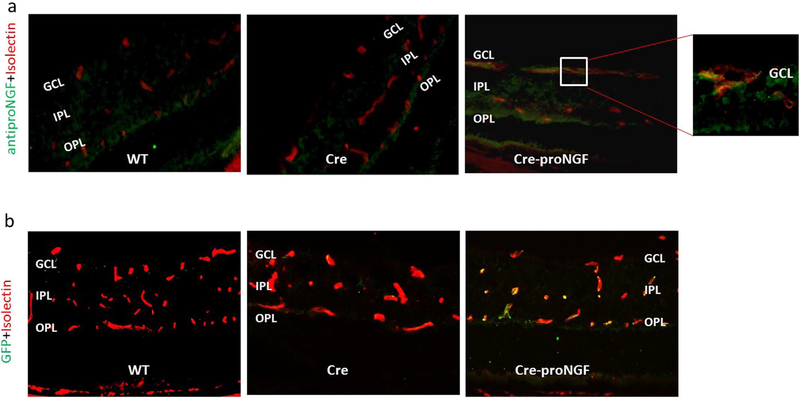
To avoid the detrimental action of proNGF during embryonic development and to dissect the direct action of proNGF on vasculature apart from other cells, an inducible and tissue-specific mouse model was needed. We utilized the Cre-Lox technology to create a tamoxifen-inducible and endothelial-specific overexpression of GFP-cleavage resistance proNGF123 animal model (EC-proNGF). The main studied groups were Cre-VE-Cadherin abbreviated as (Cre), and the Cre-VE-Cadherin-proNGFloxp abbreviated as (Cre-proNGF). Results were compared to additional controls including the proNGFloxp abbreviated as (proNGF), and/or wild type mice abbreviated as (WT). There was no significant difference in body weight or blood glucose levels among studied groups (Supplementary Fig. 1a and b). Next, we examined the co-localization of proNGF or GFP within retina vasculature. As shown in Fig. 1a, vasculature within retinas from various groups was stained red with isolectin-B4, a vascular marker. Co-localization with anti-proNGF antibody (green) confirmed expression of proNGF within vasculature (yellow) in Cre-proNGF mice retina compared to Cre- or WT-control mice. Expression of proNGF was prominent especially in the superficial capillaries (see insert in Fig. 1a) within the ganglion cell layer (GCL) and also within deep retina capillaries in inner plexiform layer (IPL). Similarly, staining retinal section with isolectin-B4 (red) and anti-GFP antibody (green) showed co-localization (yellow) only where GFP-conjugated proNGF plasmid is expressed within retinal vasculature in Cre-proNGF mice (Fig. 1b).
Fig. 1.
Characterization of proNGF and GFP expression within retinal vasculature. (a) Representative images from WT, Cre and Cre-proNGF frozen retinal sections showing vasculature stained with isolectin B-4 (red) and anti-proNGF (green). Note the colocalization (yellow) of proNGF is prominent in the superficial capillaries at the ganglion cell layer (GCL) and deep retina vessels at the inner plexiform layer (IPL) of Cre-proNGF group, but not other groups. (b) Representative images from WT, Cre and Cre-proNGF showing vasculature stained with isolectin B-4 (red) and anti-GFP-protein (green). Note co-localization (yellow) of GFP staining within retinal capillaries in Cre-proNGF group, but not in WT or Cre-control.
3.2. EC-proNGF overexpression does not cause neuronal cell toxicity or Muller glial activation
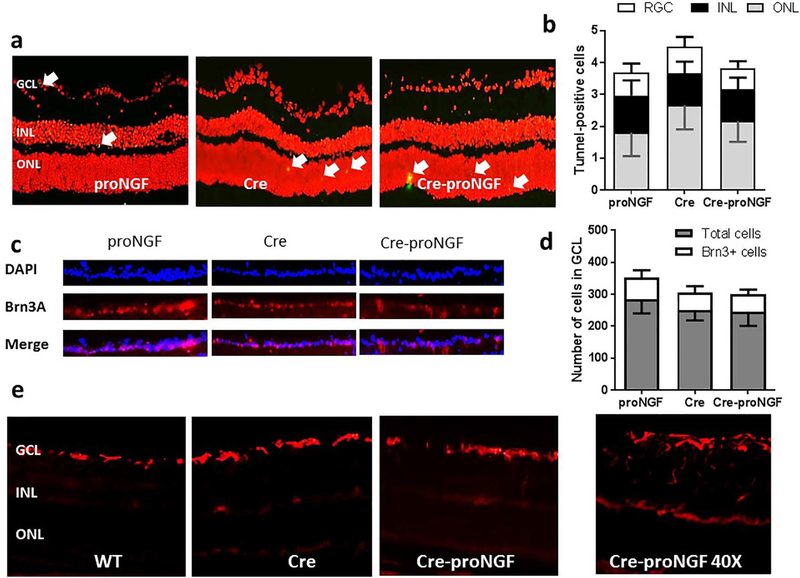
Overexpression of proNGF and diabetes has been shown to induce glial inflammation and neurodegeneration in retina [7]. TUNEL-staining was performed to explore whether EC-specific proNGF expression has any paracrine toxic effects on retinal neuronal cell death. As shown in Fig. 2a and b, overexpression of EC-proNGF did not cause cellular toxicity evident by no significant difference in the number of TUNEL-positive cells (arrows) among various layers of the retina including ganglion cell layer (GCL), inner nuclear layer (INL) and outer nuclear layer (ONL) in Cre-proNGF compared to Cre- or proNGF controls. Furthermore, immunostaining with Brn-3a, a specific RGC marker confirmed that EC-proNGF overexpression did not adversely alter the total number of neuronal cells (Fig. 2c & d) or the number of RGC (Fig. 2f). Next, glial Muller cell activation was assessed by immunostaining of the glial fibrillary acidic protein (GFAP). As shown in Fig. 2e, retinas from examined groups showed consistent activation of astrocytes at the GCL with minimal GFAP radial staining of Muller cells. As shown in the insert, EC-proNGF expression was associated with some scattered and solitary batches of activated Muller cells compared to WT- or Cre-controls. These results suggest that EC-proNGF overexpression did not exert marked paracrine effect on glial activation after 4-weeks.
Fig. 2.
EC-proNGF overexpression did not cause neuronal toxicity or Muller cell activation. (a & b) Representative images and quantitative analysis of the number of TUNEL-positive cells (green, arrows) in retina sections counterstained with Propedium Iodide (red). There were no significant changes in TUNEL-positive cells among examined groups including Cre-proNGF, Cre and proNGF, (n = 4). GCL; ganglion cell layer, INL; inner nuclear layer and ONL; outer nuclear layer. (c) Representative images of ganglion cell layer showing total number of cells stained with DAPI (blue), retinal ganglion cells (RGC) stained with Brn3A (Red) and the merge. (d) One way ANOVA Statistical analysis showed no significant change in the total number of neuronal cells or RGC count in GCL among all groups (n = 4–5). (e) Representative images of retinal sections stained with GFAP, marker for glial Muller activation showed no marked glial activation in Cre-proNGF compared to Cre or WT mice. (For interpretation of the references to color in this figure legend, the reader is referred to the online version of this chapter.)
3.3. EC-proNGF overexpression triggers proNGF and p75NTR expression in microvasculature
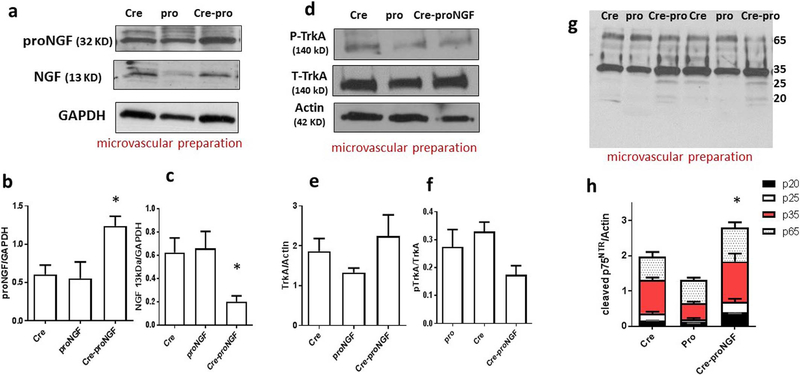
Isolating retinal microvascular preparation would require combining retinas from 6 to 8 mice [28], which is not feasible given the challenges of breeding of transgenic mice. Alternatively, we isolated microvasculature from brain pial blood vessels they share common neurovascular unit characteristics of the retina [24]. As shown in Fig. 3a & b, Western blot analysis showed significant increase (2-fold) in vascular proNGF compared to Cre- or proNGF-controls. Whereas vascular NGF level is significantly reduced (60%) compared to Cre-or proNGF-controls (Fig. 3a & c). Of note, the ratio between vascular proNGF to NGF was significantly higher (4-fold) in vessel preparation from Cre-proNGF mice compared to Cre- or proNGF controls (Supplementary Fig. 2). Next, we assessed the protein expression and activation of the survival receptor TrkA among studied groups. As shown in Fig. 3d & e, vascular TrkA protein expression was not altered among various groups. The results showed also that EC-proNGF overexpression tended to reduce activation of Y490-TrkA but it did not reach statistical significance compared to Cre- and proNGF-controls (Fig. 3d & f). We and others have shown that proNGF has high affinity towards the receptor p75NTR activating cell death signaling pathway (reviewed in [17,29]). Next, we examined the impact of EC-proNGF overexpression on vascular p75NTR. Western Blot of microvascular preparation showed significant increase in p75NTR protein expression compared to Cre- and proNGF-controls (Fig. 3g & h). Analysis of cleaved-fragments of p75NTR receptor revealed receptor shedding in Cre-proNGF compared to Cre and proNGF-controls (Fig. 3g).
Fig. 3.
EC-proNGF triggers proNGF expression and shedding of p75NTR in microvascular preparation. (a) Representative Western blots of proNGF, NGF and GAPDH in microvascular preparation. (b & c) Statistical analysis showed upregulation in the protein expression of proNGF (n = 5–8), and reduction of NGF in microvascular preparation isolated from the EC-proNGF transgenic mice (n = 5–7). (d) Representative Western blot of total TrkA, phosphorylated TrkA-Y490 and actin in microvascular preparation. (e & f) Statistical analysis showed no significant change in the expression or activation of TrkA receptor (n = 6–7). (g) Representative Western blot of p75NTR receptor in microvascular preparation. (h) One-Way ANOVA Statistical analysis showed significant increase in overall levels of the cleaved isoforms of p75NTR receptor including p65, p35, p25, p20 in Cre-proNGF compared to controls. (*p < 0.05 vs Cre and proNGF, n = 6–7).
3.4. EC-proNGF overexpression triggers proNGF andp75NTR mRNA expression in the retina
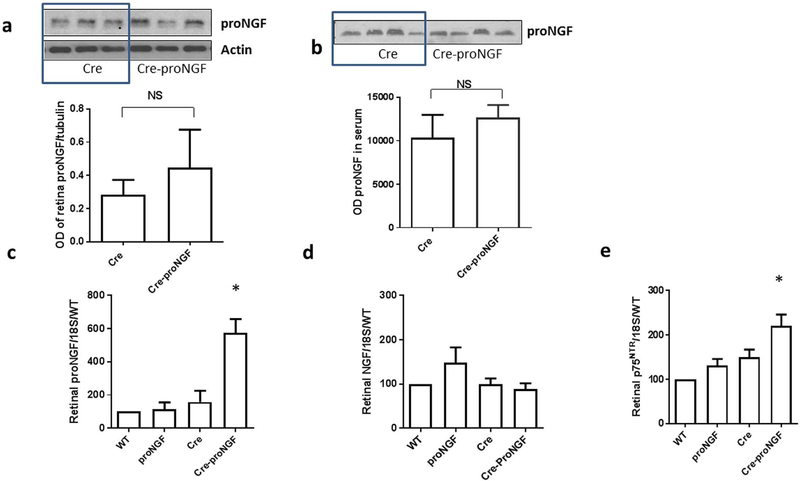
As shown in Fig. 4a, Western blot of retina lysate showed a strong trend of increased proNGF protein expression (1.5-fold) in Cre-proNGF compared to Cre-controls, but it did not reach statistical significance (p = 0.08). Similar trend was observed in proNGF protein levels detected in serum samples from Cre-proNGF and Cre-controls (Fig. 4b). Total retina lysates were used to assess expression of proNGF and NGF at mRNA. Overexpression of proNGF resulted in significant increases in mRNA expression of proNGF (~4–6 fold), but not in NGF in retinas from Cre-proNGF mice compared to control groups; WT, Cre and proNGF alone (Fig. 4c & d). In parallel, retinas from Cre-proNGF transgenic animals showed significant increase in p75NTR mRNA expression (Fig. 4e).
Fig. 4.
EC-proNGF overexpression triggers expression of p75NTR and inflammatory mediators in the retina. (a) Representative of Western Blot and statistical analysis of proNGF and actin from retina lysate showed a trend of increased proNGF but no significant difference in Cre-proNGF compared to Cre-controls (n = 3–4). (b) Representative of Western Blot and statistical analysis of systemic proNGF from serum samples showing no significant difference between Cre and Cre-proNGF animals (n = 4). (c & d) Quantitative real-time PCR showed a significant increase in retinal proNGF mRNA levels in Cre-proNGF group, while there is no change in retinal NGF levels among all groups. (*p < 0.05 vs WT, proNGF and Cre, n = 6–10). (e) Quantitative real-time PCR showing a significant increase of retinal p75NTR receptor mRNA level compared to controls (*p < 0.05 vs Cre and proNGF, n = 6–7).
3.5. EC-proNGF overexpression triggers retinal mRNA expression of inflammatory mediators
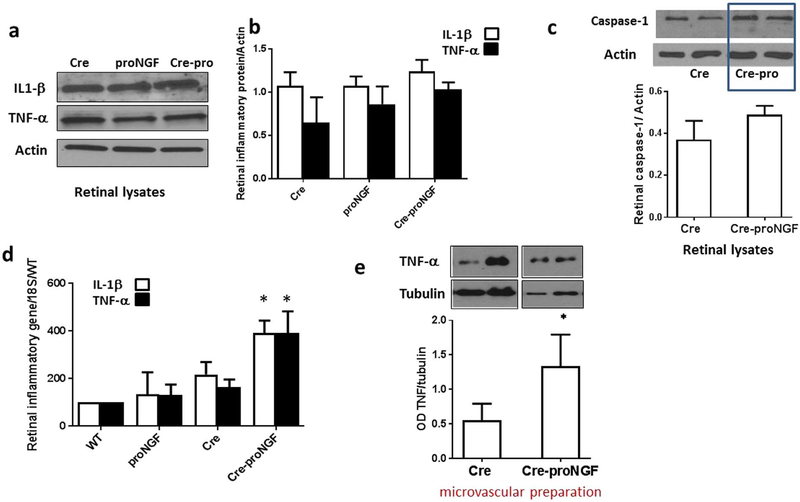
Previous studies showed pro-inflammatory action of proNGF in Müller glial cells [7,20]. To study whether EC-proNGF overexpression can induce retinal inflammation, we measured the classical inflammatory cytokines TNF-α and IL-1β in total retinal lysate. As shown in Fig. 5a and b, there was no significant difference in TNF-α and IL-1β among Cre-proNGF, Cre- and proNGF-controls. We also assessed the levels of cleaved caspase-1, a protease involved in maturation and cleavage of IL-1β and there was no significant difference between Cre-proNGF and Cre-controls (Fig. 5c). At the mRNA level, expression of both TNF-α and IL-1β mRNA was significantly increased in Cre-proNGF compared to Cre- or proNGF-controls (Fig. 5d). Next, Western blot analysis showed significant increase (2-fold) in vascular TNF-α compared to Cre- controls (Fig. 5d). These results demonstrate that EC-specific proNGF overexpression can contribute to vascular inflammation.
Fig. 5.
EC- proNGF overexpression induces expression of inflammatory markers. (a & b) Representative Western blots of TNFα, IL1β and actin in isolated retina. (d & e) Statistical analysis showed no significant change in cleaved-IL1β expression (17 kDa) or membrane-bound TNFα (26 kDa) expression among different animal groups (n = 5). (c) Representative image and statistical analysis of Western blots of retinal lysates showing no significant difference in cleaved caspase-1 (22 kDa) expression between Cre-proNGF and Cre-controls (n = 4). (d) Real-time PCR showed a significant increase in mRNA of IL1β (n = 5–6) and TNFα (n = 5–10) in retina isolated from Cre-proNGF mice compared to controls (*p < 0.05 vs WT, Cre and proNGF). (f) Representative of Western Blot and statistical analysis of membrane-bound TNF-α (26 kDa) expression in microvascular preparation showing significant (2-fold) increase in TNF-α from Cre-proNGF compared to Cre-controls(*p < 0.05 vs Cre, n = 4).
3.6. EC-proNGF triggers expression of p75NTR and inflammatory mediators in the kidney cortex but not medulla
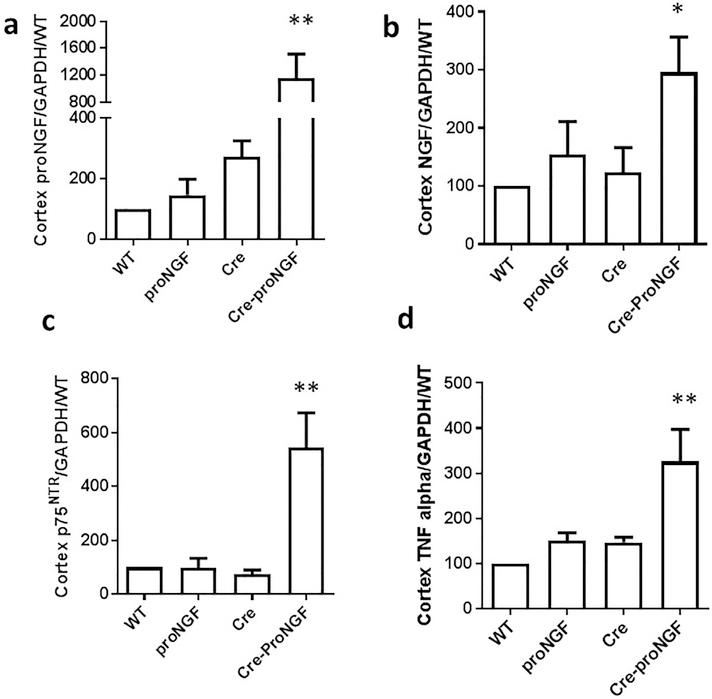
Since proNGF is globally overexpressed in endothelial cells, we investigated the pattern and impact of EC-proNGF on the kidney. As shown in Fig. 6a, proNGF gene expression was increased 10-fold compared to controls in the cortex while there was no significant difference in the medulla isolated from various groups (Supplementary Fig. 3a). In parallel, cortex levels of NGF and p75NTR mRNA expression were increased (6- and 3-fold), respectively in Cre-proNGF mice compared to Cre- or proNGF-controls (Fig. 6b & c). Whereas, NGF and p75NTR levels were comparable in the medulla from various groups studied (Supplementary Fig. 3b & c). Consistent with the above, cortex, but not medulla from Cre-proNGF mice showed significant increase (3-fold) in TNF-α expression compared to WT-, Cre- or proNGF-controls (Fig. 6d).
Fig. 6.
EC-proNGF overexpression induces expression of p75NTR and inflammatory markers in renal cortex. (a) Renal cortex from Cre-proNGF showed significant and dramatic (10-fold) increase in proNGF mRNA compared to controls. (b) Renal cortex from Cre-proNGF showed significant 3-fold increase in NGF mRNA compared to controls. (c) Renal cortex from Cre-proNGF showed significant 5-fold increase in p75NTR mRNA compared to controls. (d) Renal cortex from Cre-proNGF showed significant 3-fold increase in TNF-α mRNA compared to controls. (**p < 0.01, *p < 0.05 vs WT, pro and Cre-controls, n = 5–7).
3.7. EC-proNGF overexpression triggers retinal vascular permeability without altering VEGF expression
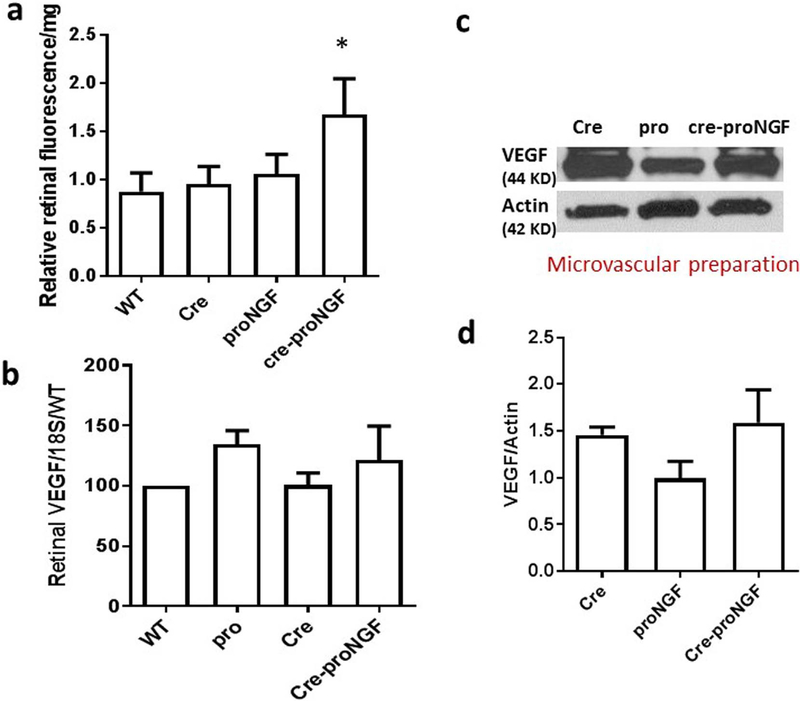
Diabetes-induced retinal barrier breakdown (BRB) coincided with increased proNGF expression [5,7]. Nevertheless, there is no direct evidence that proNGF can directly trigger retinal vascular permeability. Since Cre-proNGF mice also express GFP, vascular permeability was assessed by extravasation of BSA-FITC conjugated with Alexa-Fluor 555 red dye, which does not overlap with GFP-fluorescence. As shown in Fig. 7a, EC-specific proNGF overexpression significantly triggered BRB breakdown evident by 1.6-fold increase in retinal fluorescence in Cre-proNGF compared to WT, Cre- or proNGF controls. Vascular endothelial growth factor (VEGF) expression is known to induce vascular permeability in the retinas [30]. Furthermore, to rule out the involvement of VEGF in increasing vascular permeability, we measured the VEGF expression in the retina. As shown in Fig. 7b, overexpression of EC-proNGF did not alter VEGF mRNA in the retina compared to Cre- or proNGF-controls. EC-proNGF overexpression did not alter vascular protein expression in Cre-proNGF compared to other controls (Fig. 7c & d). These results suggest that the observed vascular permeability is likely due to increased expression of proNGF and other inflammatory mediators in EC rather than direct involvement of VEGF.
Fig. 7.
EC-proNGF overexpression induces retinal vascular permeability without affecting VEGF expression. (a) Retinal vascular permeability was assessed by measuring extravasation of Rhodamine-BSA in retinal lysate normalized to serum fluorescence and the protein content of each sample. Statistical analysis using One-Way ANOVA analysis showed that significant increase in retinal vascular permeability in Cre-proNGF mice compared to Cre, proNGF or WT-mice (*p < 0.05, 5–7). (b) Statistical analysis showed no change in retinal VEGF mRNA levels assessed by real-time PCR among all groups (n = 5). (c) Representative image of Western blots and (d) statistical analysis of protein expression of VEGF and actin in microvascular preparation. There was no significant difference in VEGF protein expression among all groups (n = 4).
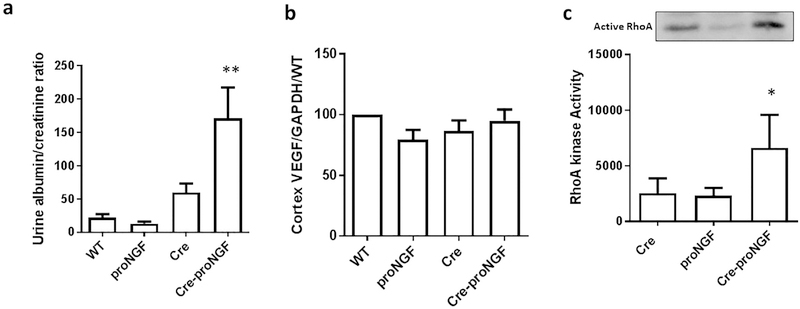
3.8. EC-proNGF overexpression triggers renal barrier dysfunction and RhoA activation without altering VEGF expression
Overexpression of EC-proNGF induced 6-fold increase in the ratio of albumin to creatinine ratio as compared to WT-, proNGF and Cre-controls (Fig. 8a). Similar to the observation in the retina, overexpression of EC-proNGF did not alter gene expression of VEGF mRNA in cortex isolated from various studied groups (Fig. 8b). As shown in Fig. 8c, pull down assay was performed on the kidney lysates and the results showed that overexpression of proNGF significantly increased RhoA kinase activation compared to Cre-control and proNGF-control animals. These results are in agreement with our prior observation that proNGF triggered active RhoA in the retina using proNGF plasmid overexpression [31].
Fig. 8.
EC-proNGF overexpression induces albuminuria and RhoA activation. (a) Statistical analysis using One-Way ANOVA of Urine Albumin to Creatinine ratio showed significant increases in Cre-proNGF animals compared to controls (n = 5, *p < 0.05 vs WT and proNGF, #p < 0.05 vs Cre). (b) Real-time PCR of VEGF mRNA level from renal cortex showed no difference in VEGF levels among all groups (n = 5). (c) Pull down assay from renal lysate from Cre-proNGF showed significant increase in RhoA kinase activity in Cre-proNGF compared to Cre and proNGF alone. (*p < 0.05 vs Cre and proNGF controls, n = 4).
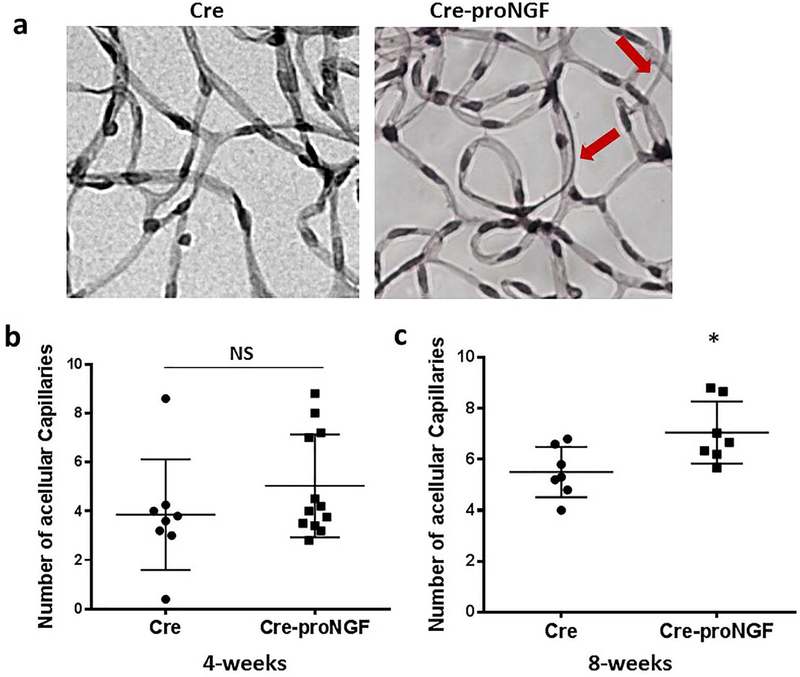
3.9. EC-proNGF overexpression stimulated occluded (acellular) capillaries formation
proNGF overexpression induced acellular capillaries formation in the retina, a surrogate marker for retinal ischemia [9]. To dissect out the direct contribution of EC-proNGF overexpression on acellular capillaries formation, we determined the number of occluded (acellular) capillaries in retinal vasculature. Trypsin-digested followed by periodic acid-Schiff and hematoxylin (PASH) stained and flat-mounted retinas showed a significant increase in the number of occluded (acellular) capillaries (Fig. 9a, c) at 8-week compared to Cre control. However, we did not see statistical significance in retinas from Cre-proNGF compared to Cre- controls at 4 week (Fig. 9b).
Fig. 9.
EC-proNGF overexpression induces development of occluded (acellular) capillaries In the retina. (a) Representative Images of trypsinized retina stained with PASH to Identify healthy and occluded (acellular) capillaries (arrows) from Cre-proNGF and Cre-controls. (b) EC-proNGF overexpression tended to increase number of acellular capillaries but it did not reach statistical significance compared to Cre mice at 4-weeks (n = 8–12) (c) EC-proNGF overexpression significantly increased the number of acellular capillaries in Cre-proNGF compared to Cre mice at 8-weeks (*p < 0.05, n = 7).
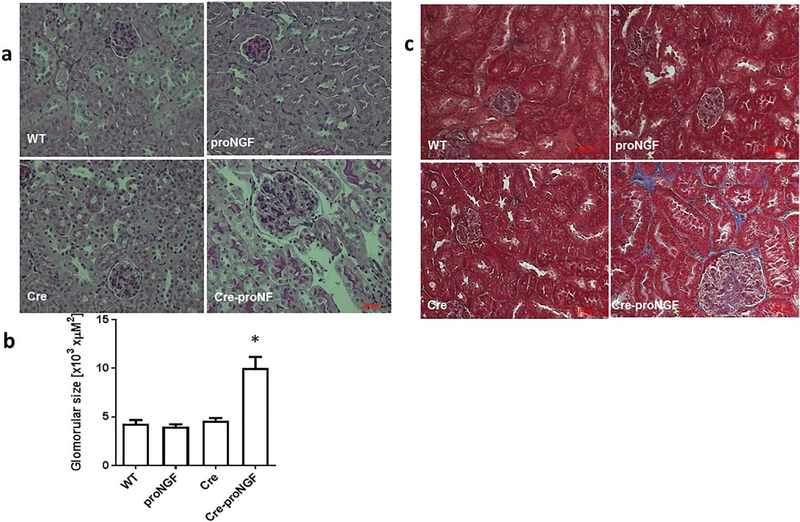
3.10. EC-proNGF overexpression increased glomerular size and fibrosis in kidney
Glomerulosclerosis and interstitial fibrosis are hallmarks of diabetic kidney diseases. As shown in Fig. 10a & b, EC-proNGF overexpression induced a significant increase (2-fold) in glomerular area compared to Cre, WT and proNGF mice. Consistence with this, increased fibrosis of glomeruli and the interstitial was observed in kidneys from EC-proNGF mice as evident by increased trichrome staining compared to WT-, Cre- or proNGF-controls (Fig. 10c).
Fig. 10.
EC-proNGF overexpression induces glomerular enlargement and fibrosis. Histopathological changes Including renal fibrosis and glomerular sclerosis was assessed by Masson’s trichrome staining and PASH staining after 8-weeks of EC-proNGF overexpression. (a & b) Representative and statistical analysis using One-Way ANOVA of PASH-stained kidney sections from Cre-proNGF mice showed glomerular expansion and mesangial expansion compared to control groups (*p < 0.001 versus control, n = 4). (c) Masson Trichrome staining showed increased glomeruli enlargement and the interstitial fibrosis (blue) in kidney sections from Cre-proNGF mice compared to other controls (n = 4). Scale bar = 100 μM.
4. Discussion
The main findings of this study are: a) EC-proNGF overexpression alters NGF homeostasis in microvasculature by enhancing proNGF/p75NTR and depleting endogenous neurotrophic support evident by significant reduction of NGF level and a trend of impaired TrkA activation (Fig. 3). b) EC-proNGF overexpression triggered mRNA expression of p75NTR and inflammatory mediators in both retina and renal cortex (Figs. 4, 5, 6). c) EC-proNGF expression induced vascular permeability including breakdown of BRB and albuminuria in the kidney without affecting VEGF level (Figs. 7, 8). d) After 8-weeks, EC-proNGF triggered histopathological changes including formation of acellular capillaries in the retina and glomerular enlargement and kidney fibrosis (Figs. 9, 10). We believe that this is the first report dissecting the unique contribution of EC-proNGF overexpression to microvascular abnormalities in retina and kidney.
NGF is a secreted protein that belongs to class of growth factors, is important regulator of neuronal differentiation, development, maturation, survival and regulation of cell death of retinal neurons [2]. Initially NGF is synthesized as proform; proNGF which undergoes proteolytic cleavage intracellularly by plasmin and furin and extracellularly by matrix metalloproteinase to produce the mature form of neurotrophins [1]. We previously reported that due to oxidative stress, processing and maturation of proNGF is impaired in the diabetic milieu, resulting in increased proNGF and decreased NGF levels in serum and ocular fluids from patients with proliferative diabetic retinopathy [5,6]. Furthermore, accumulation of proNGF has been reported in neurodegenerative diseases including Alzheimer’s Disease [11], Down’s syndrome [32] and myocardial infarction [33] as well as models of neuronal ischemic injury [34,35]. Therefore, there is a pressing need of rodent models that stably express a non-cleavable form of proNGF to better understand the impact of its accumulation in various tissues.
Our prior studies showed that a global overexpression of cleavage-resistant proNGF was embryonic lethal and we successfully developed a stable expression of proNGF locally in rodent retina [19]. Accumulated proNGF has deleterious effect on retinal vasculature by increasing acellular capillaries formation, a surrogate marker for ischemia, and vascular permeability in both proNGF overexpression and diabetic animal model [9]. However, till now no studies has explored the direct action of proNGF on retinal vasculature. To address this issue, we successfully created endothelial-proNGF animal model using Cre-Lox technology. Colocalization with a vascular marker revealed that proNGF is mainly localized in vasculature (Fig. 1). These effects coincided with lack of neurotoxicity evident by similar number of TUNEL-positive cells in various retina layers as well as similar number of RGCs (Brn-3a-positive) among investigated groups. Moreover, there was no significant activation of Muller cell activation assessed by GFAP immunostaining among groups at 4-weeks.
EC-proNGF triggered significant mRNA expression of proNGF, p75NTR and pro-inflammatory mediators in both retina and renal cortex but not in medulla. Renal cortex is more vascularized than the medulla, hence it is more conceivable that EC-proNGF will be more prominent in cortex but not medulla. Protein expression from retinal lysate showed modest increase in proNGF or pro-inflammatory mediators that did not reach statistical significance in Cre-proNGF compared to controls. This could be attributed to the very small percentage of vasculature within a whole tissue such as retina. While Western blot failed to detect these differences, quantitative PCR was sensitive enough to detect significant difference in expression of these targets. Therefore, we needed to assess protein expression in a microvascular preparation rather than whole tissue lysate. We isolated brain pial blood vessels as microvascular preparation, which offers reasonably sized tissue sample that shares neurovascular characteristics with retinal vasculature [24]. Indeed, microvascular preparation showed significant increases in TNF-α, proNGF and decreases in mature form; NGF in the microvasculature isolated from Cre-proNGF mice compared to controls. This lead to imbalance of proNGF/NGF ratio similar to previously observed in experimental diabetes, and in ocular fluids from diabetic and proliferative DR patients [5–7].
The accumulated proNGF exert its independent biological activity by binding p75NTR and interacting with sortilin, a member of VPS-10p domain receptor family to activate cell death pathway, whereas NGF mediates cell survival by binding to TrkA (reviewed in [17,29]). EC-proNGF overexpression tended to impair TrkA activation at tyrosine 490, but it did not alter TrkA levels compared to controls. Of interest, Western Blot of pial microvascular preparation showed marked shedding and cleavage of p75NTR in EC-proNGF compared to controls. Ligand-induced cleavage of p75NTR is a critical event to mediate p75NTR signaling [36]. Activated p75NTR can mediate apoptosis by translocating p75NTR and the DNA binding protein, neurotrophin receptor interacting factor into nucleus and forming a complex [9,36]. The observation that EC-proNGF activates vascular p75NTR and impaired TrkA activation lend further support to prior reports showing activation of p75NTR with impairment of the TrkA activation [8,9,37,38].
EC-proNGF overexpression significantly increased NGF mRNA expression in kidney, but not retinal lysates. These results came in agreement with prior studies showing increased NGF expression in a rat model of diabetic nephropathy as well as clinical samples from patients with kidney diseases [3,39,40]. These studies identified positive staining for NGF and TrkA in tubular and glomerular cells and p75NTR in the interstitial and mesangial cells [3,39,40]. These observations suggest an autocrine regulation of NGF signaling that might have protective role in kidney cells. The exact contribution of NGF to kidney disease remain to be fully elucidated.
Several studies reported that proNGF could induce glial inflammation that can contribute to microvascular dysfunction [7,20,38]. Indeed, EC-proNGF triggered expression of pro-inflammatory cytokines TNF-α and IL-1β but not VEGF at the mRNA level in the retina and renal cortex as compared to the respective controls. These effects coincided with hallmarks of vascular dysfunction in both retina and kidney. Our results showed that EC-proNGF induced retinal and renal vascular permeability evident by a significant 1.6-fold increase in extravasation of fluorescent BSA in the retina and a significant increase (6-fold) in urine albumin/creatinine ratio in Cre-proNGF animals compared to controls. IL-1β and TNF-α are known regulators of vascular permeability in many diseases including diabetic retinopathy [41–44] as well as diabetic nephropathy [45,46]. After 8-weeks, EC-proNGF expression triggered glomerular enlargement and interstitial fibrosis (Fig. 10), hallmarks of renal dysfunction as well as significant development of acellular capillaries, hall mark of retinal ischemia (Fig. 9). These findings lend further support to our recent work using ocular overexpression of proNGF-plasmid [9] and findings from another transgenic mouse model expressing a mutant NGF allele with impaired processing of proNGF [33]. The later proNGF-expressing mouse model exhibited cardiac microvascular EC activation, a decrease in pericyte process length, and increased vascular permeability [33]. In addition to its known pro-inflammatory action, proNGF is a recognized activator of RhoA kinase family through p75NTR [31,47]. We and others reported that proNGF-mediated RhoA activation coincided with retinal vascular permeability, neurodegeneration [5,48,49]. Activated RhoA kinase through TGFβ siganling is involved in glomerular endothelial-to-mesenchymal transition, which has been shown to worsen albuminuria and kidney fibrosis [50,51] and also involved in development liver fibrosis [52]. Our findings that EC-proNGF overexpression significantly triggered activation of RhoA in renal lysates support the postulated role of proNGF-RhoA kinase signaling pathway in mediating renal fibrosis. Long term studies are warranted that will characterize long-term effects of EC-proNGF as a novel therapeutic target for microvascular diabetic complication.
5. Conclusion
We have successfully established an animal model where expression of a cleavage-resistant form of proNGF can be induced in the specific tissue of interest. Here, we demonstrated that proNGF can be successfully expressed in EC resulting in hallmarks of vascular dysfunction in both retina and kidney, vulnerable targets for diabetes-medicated microvascular complications. Further, this model will help to dissect out autocrine effects of proNGF on vasculature apart from its paracrine effects on glia. We believe that creating the proNGFloxp mouse model will provide powerful molecular tool to better understand the multifaceted signaling of proNGF pathways within the tissue of interest.
Supplementary Material
Acknowledgements
This work was supported by grant from NEI (EY-0022408), and AHA-pre-doctoral fellowship (15PRE2283030019) to SLE. Authors are grateful for Dr. Bruce Carter and Dr. Moses Chao for providing p75NTR antibodies. Authors are grateful for Ms. Megan Bartasis and Dr. Barbara Mysona for helping with genotyping and establishing ProNGFLoxP colony.
Footnotes
Conflicts of interest
The Authors declare no conflict of interest.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbadis.2017.12.023.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- [1].Hempstead BL, Commentary: regulating proNGF action: multiple targets for therapeutic intervention, Neurotox. Res 16 (2009) 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee R, Kermani P, Teng KK, Hempstead BL, Regulation of cell survival by secreted proneurotrophins, Science 294 (2001) 1945–1948. [DOI] [PubMed] [Google Scholar]
- [3].Aloe L, Rossi S, Manni L, Altered expression of nerve growth factor and its receptors in the kidneys of diabetic rats, J. Nephrol 24 (2011) 798–805. [DOI] [PubMed] [Google Scholar]
- [4].Park KS, Kim SS, Kim JC, Kim HC, Im YS, Ahn CW, Lee HK, Serum and tear levels of nerve growth factor in diabetic retinopathy patients, Am J. Ophthalmol 145 (2008) 432–437. [DOI] [PubMed] [Google Scholar]
- [5].Ali TK, Al-Gayyar MM, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, El-Remessy AB, Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury, Diabetologia 54 (2011) 657–668. [DOI] [PubMed] [Google Scholar]
- [6].Mysona BA, Matragoon S, Stephens M, Biomed. Res. Int 2015 (2015) 571456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB, Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats, Diabetologia 56 (2013) 2329–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ali TK, Matragoon S, Pillai BA, Diabetes 57 (2008) 889. [DOI] [PubMed] [Google Scholar]
- [9].Shanab AY, Mysona BA, Matragoon S, El-Remessy AB, Silencing p75(NTR) prevents proNGF-induced endothelial cell death and development of acellular capillaries in rat retina, Mol. Ther. Methods Clin. Dev 2 (2015) 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mohamed R, Shanab AY, El-Remessy AB, Deletion of the neurotrophin receptor p75NTR prevents diabetes-induced retinal acellular capillaries in streptozotocin-induced mouse diabetic model, J. Diabetes Metab. Disord. Control (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Counts SE, He B, Prout JG, Michalski B, Farotti L, Fahnestock M, Mufson EJ, Cerebrospinal fluid proNGF: a putative biomarker for early Alzheimer’s disease, Curr. Alzheimer Res 13 (2016) 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tiveron C, Fasulo L, Capsoni S, Malerba F, Marinelli S, Paoletti F, Piccinin S, Scardigli R, Amato G, Brandi R, Capelli P, D’Aguanno S, Florenzano F, La Regina F, Lecci A, Manca A, Meli G, Pistillo L, Berretta N, Nistico R, Pavone F, Cattaneo A, ProNGF\NGF imbalance triggers learning and memory deficits, neurodegeneration and spontaneous epileptic-like discharges in transgenic mice, Cell Death Differ. 20 (2013) 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Belrose JC, Masoudi R, Michalski B, Fahnestock M, Increased pro-nerve growth factor and decreased brain-derived neurotrophic factor in non-Alzheimer’s disease tauopathies, Neurobiol. Aging 35 (2014) 926–933. [DOI] [PubMed] [Google Scholar]
- [14].Soligo M, Protto V, Florenzano F, Bracci-Laudiero L, De Benedetti F, Chiaretti A, Manni L, The mature/pro nerve growth factor ratio is decreased in the brain of diabetic rats: analysis by ELISA methods, Brain Res. 1624 (2015) 455–468. [DOI] [PubMed] [Google Scholar]
- [15].Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K, Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration, Neuron 26 (2000) 533–541. [DOI] [PubMed] [Google Scholar]
- [16].Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM, Sortilin is essential for proNGF-induced neuronal cell death, Nature 427 (2004) 843–848. [DOI] [PubMed] [Google Scholar]
- [17].Elshaer SL, El-Remessy AB, Implication of the neurotrophin receptor p75NTR in vascular diseases: beyond the eye, Expert Rev. Ophthalmol 12 (2017) 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, Friedman WJ, Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures, J. Neurosci 28 (2008) 9870–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matragoon S, Al-Gayyar MM, Mysona BA, Abdelsaid MA, Pillai BA, Neet KE, Fagan SC, El-Remessy AB, Electroporation-mediated gene delivery of cleavage-resistant pro-nerve growth factor causes retinal neuro- and vascular degeneration, Mol. Vis 18 (2012) 2993–3003. [PMC free article] [PubMed] [Google Scholar]
- [20].Lebrun-Julien F, Bertrand MJ, De Backer O, Stellwagen D, Morales CR, Di Polo A, Barker PA, ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 3817–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pagadala PC, Dvorak LA, Neet KE, Construction of a mutated pro-nerve growth factor resistant to degradation and suitable for biophysical and cellular utilization, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 17939–17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML, VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium, Dev. Dyn 235 (2006) 3413–3422. [DOI] [PubMed] [Google Scholar]
- [23].Coucha M, Li W, Johnson MH, Fagan SC, Ergul A, Protein nitration impairs the myogenic tone of rat middle cerebral arteries in both ischemic and nonischemic hemispheres after ischemic stroke, Am. J. Physiol. Heart Circ. Physiol 305 (2013) H1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B, Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures, J. Anat 206 (2005) 319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mohamed R, Ranganathan P, Jayakumar C, Nauta FL, Gansevoort RT, Weintraub NL, Brands M, Ramesh G, Urinary semaphorin 3A correlates with diabetic proteinuria and mediates diabetic nephropathy and associated inflammation in mice, J. Mol. Med. (Berl) 92 (2014) 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mohamed R, Jayakumar C, Chen F, Fulton D, Stepp D, Gansevoort RT, Ramesh G, Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis, J. Am. Soc. Nephrol 27 (2016) 745–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW, Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation, Exp. Diabetes Res 2010 (2010) 247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanwar M, Kowluru RA, Diabetes regulates small molecular weight G-protein, H-Ras, in the microvasculature of the retina: implication in the development of retinopathy, Microvasc. Res 76 (2008) 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mohamed R, El-Remessy AB, Imbalance of the nerve growth factor and its precursor: implication in diabetic retinopathy, J. Clin. Exp. Ophthalmol 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP, Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1), Am. J. Pathol 156 (2000) 1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al-Gayyar MM, Mysona BA, Matragoon S, Abdelsaid MA, El-Azab MF, Shanab AY, Ha Y, Smith SB, Bollinger KE, El-Remessy AB, Diabetes and overexpression of proNGF cause retinal neurodegeneration via activation of RhoA pathway, PLoS One 8 (2013) e54692. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [32].Iulita MF, Cuello AC, Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome, Trends Pharmacol. Sci 35 (2014) 338–348. [DOI] [PubMed] [Google Scholar]
- [33].Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL, ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation, J. Exp. Med 209 (2012) 2291–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Irmady K, Jackman KA, Padow VA, Shahani N, Martin LA, Cerchietti L, Unsicker K, Iadecola C, Hempstead BL, Mir-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury, J. Neurosci 34 (2014) 3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Duan L, Chen BY, Sun XL, Luo ZJ, Rao ZR, Wang JJ, Chen LW , LPS-induced proNGF synthesis and release in the N9 and BV2 microglial cells: a new pathway underling microglial toxicity in neuroinflammation, PLoS One 8 (2013) e73768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD, Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons, Neuron 50 (2006) 219–232. [DOI] [PubMed] [Google Scholar]
- [37].Barcelona PF, Saragovi HU, A pro-nerve growth factor (proNGF) and NGF binding protein, alpha2-macroglobulin, differentially regulates p75 and TrkA receptors and is relevant to neurodegeneration ex vivo and in vivo, Mol. Cell. Biol 35 (2015) 3396–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lebrun-Julien F, Duplan L, Pernet V, J. Neurosci 29 (2009) 5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bonofiglio R, Antonucci MT, Papalia T, Romeo F, Capocasale G, Caroleo MC, Di Fausto V, Aloe L, Nerve growth factor (NGF) and NGF-receptor expression in diseased human kidneys, J. Nephrol 20 (2007) 186–195. [PubMed] [Google Scholar]
- [40].Alpers CE, Hudkins KL, Ferguson M, Johnson RJ, Schatteman GC, Bothwell M, Nerve growth factor receptor expression in fetal, mature, and diseased human kidneys, Lab. Investig 69 (1993) 703–713. [PubMed] [Google Scholar]
- [41].Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S, Silver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells, J. Nanobiotechnol 7 (2009) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kowluru RA, Odenbach S, Role of interleukin-1beta in the pathogenesis of diabetic retinopathy, Br. J. Ophthalmol 88 (2004) 1343–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA, TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis, Invest. Ophthalmol. Vis. Sci 52 (2011) 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].van der Wijk AE, Vogels IMC, van Noorden CJF, Klaassen I, Schlingemann RO, TNFalpha-induced disruption of the blood-retinal barrier in vitro is regulated by intracellular 3′,5′-cyclic adenosine monophosphate levels, Invest. Ophthalmol. Vis. Sci 58 (2017) 3496–3505. [DOI] [PubMed] [Google Scholar]
- [45].Chen L, Yang S, Zumbrun EE, Guan H, Nagarkatti PS, Nagarkatti M, Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages, Mol. Nutr. Food Res 59 (2015) 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li X, Wu TT, Chen J, Qiu W, Elevated expression levels of serum insulin-like growth factor-1, tumor necrosis factor-alpha and vascular endothelial growth factor 165 might exacerbate type 2 diabetic nephropathy, J. Diabetes Investig 8 (2017) 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Caporali A, Meloni M, Nailor A, Mitic T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia, Nat. Commun 6 (2015) 8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mysona BA, Rodenbeck D, Shanab AY, Matragoon S, El-Remessy AB, ProNGF causes retinal endothelial cell permeability via activation of p75NTR/RhoA pathway, Invest. Ophthalmol. Vis. Sci 55 (2014) (5827–5827). [Google Scholar]
- [49].Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, Oh YJ, Markelonis GJ, Oh TH, Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury, J. Neurosci 27 (2007) 7751–7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vizza D, Perri A, Toteda G, Lupinacci S, Leone F, Gigliotti P, Lofaro D, La Russa A, Bonofiglio R, Nerve growth factor exposure promotes tubular epithelial-mesenchymal transition via TGF-beta1 signaling activation, Growth Factors 33 (2015) 169–180. [DOI] [PubMed] [Google Scholar]
- [51].Peng H, Li Y, Wang C, Zhang J, Chen Y, Chen W, Cao J, Wang Y, Hu Z, Lou T, ROCK1 induces endothelial-to-mesenchymal transition in glomeruli to aggravate albuminuria in diabetic nephropathy, Sci. Rep 6 (2016) 20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zvibel I, Atias D, Phillips A, Halpern Z, Oren R, Thyroid hormones induce activation of rat hepatic stellate cells through increased expression of p75 neurotrophin receptor and direct activation of Rho, Lab. Investig 90 (2010) 674–684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.