Abstract
Tuberculosis (TB) is a large global health problem, in part because of the long period of coevolution of the pathogen, Mycobacterium tuberculosis, and its human host. A major factor that sustains the global epidemic of TB is the lack of a sufficiently effective vaccine. While basic mechanisms of immunity that protect against TB have been identified, attempts to improve immunity to TB by vaccination have been disappointing. This Review discusses the mechanisms used by M. tuberculosis to evade innate and adaptive immunity and that likely limit the efficacy of vaccines developed to date. Despite multiple mechanisms of immune evasion, recent trials have indicated that effective TB vaccines remain an attainable goal. This Review discusses how knowledge from other systems can inform improvements on current vaccine approaches.
Tuberculosis
Despite the impression that tuberculosis (TB) is a disease of historic (Dubos and Dubos, 1952) or romantic (Lawlor, 2007) interest, TB causes more deaths (1.7 million in 2016) than does HIV (http://www.who.int/tb/en/). Mycobacterium tuberculosis, the bacteria that cause TB, is estimated to have infected 23% of the current human population (Houben and Dodd, 2016) and progressed to cause active disease in 10.4 million people in 2016. TB is potentially curable, but there were an estimated 500,000 new cases of drug-resistant TB in 2016. Cure rates are lower with resistant strains, the drugs are more costly and more toxic, and drug-resistant M. tuberculosis can be transmitted to other individuals (Shah et al., 2017).
Most people that encounter M. tuberculosis do not progress to active TB disease and are considered to have latent TB infection (LTBI). Although only 5%–10% of people progress to active, TB disease, a person with active TB is estimated to transmit TB to an average of 10 other people per year (superspreaders may infect as many as 200), so progression from LTBI to active TB occurs at a rate sufficient to sustain the global epidemic.
The relationship between M. tuberculosis and our ancestors is long standing, going back as many as 3 million years (Gutierrez et al., 2005). M. tuberculosis has no other ecological niche, so all of its evolutionary selection is through interactions with humans. Coevolution has resulted in an infection that induces partial immunity, where the host survives most of the time and so does the pathogen. TB is spread through the air by people with active TB, so mechanisms that promote release of bacteria from the lungs benefit the bacteria, and TB may be unique in its ability to exploit adaptive immune responses (through inflammatory lung tissue damage) to promote its transmission. M. tuberculosis is also unusual, as the vast majority of its antigens do not exhibit sequence diversity (Comas et al., 2010; Coscolla et al., 2015). Although the full implications of antigen conservation remain to be determined, the lack of escape mutations is consistent with partial immunity.
TB Vaccine History
M. tuberculosis was identified as the cause of TB in 1882, and by 1927 the live attenuated bacille Calmette-Guérin (BCG) TB vaccine became available. Approximately 100 million infants receive BCG annually, due to its low cost, stability, and safety. Although BCG reduces the risk of disseminated tuberculosis in childhood (Rodrigues et al., 1993), its efficacy in preventing pulmonary tuberculosis in adults varies widely (Mangtani et al., 2014). The variation is attributed to multiple factors, including the BCG strain, dose, and route of administration; prevalence of nontu-berculous mycobacteria (NTM); host genetics, microbiota, and coinfections; and prevalence of specific M. tuberculosis lineages. Variations in outcomes notwithstanding, BCG has not been sufficiently effective to prevent the growth of global TB.
One mechanism that may limit BCG efficacy is that its antigenic composition is insufficiently related to M. tuberculosis. Analysis of 13 BCG genomes revealed that of the 1,530 known human T cell epitopes in M. tuberculosis, 21%–28% of the epitopes are deleted from BCG (Copin et al., 2014). Besides the deleted antigens, 15 epitopes in 9 antigens differ in sequence compared with M. tuberculosis. Although the evidence is insufficient to conclude that antigen loss and sequence variation account for the limited efficacy of BCG, several immunodominant antigens (ESAT-6, CFP-10, PE35, and PPE68) are lacking from all BCG strains.
The only new TB vaccine examined in phase II trials, MVA85A, lacks efficacy. MVA85A is comprised of the attenuated poxvirus, Modified Vaccinia Ankara (MVA), that expresses the M. tuberculosis antigen 85A (Ag85A). Ag85A is an abundant secreted protein of M. tuberculosis, and during infection, it induces high-frequency T cell responses. After MVA85A was found safe and immunogenic in humans, two groups at high risk of TB were selected for efficacy trials: HIV-infected adults and infants.
One efficacy study was performed in 650 HIV-infected adults that were randomized to receive MVA85A or a control (Ndiaye et al., 2015). MVA85A induced Ag85A-reponsive T cells that produced IFNg, as well as cells that produced the cytokines Tumor necrosis factor (TNF), IL-2, or IL-17; low-magnitude CD8 T cell responses were detected, but fewer than 1% of the recipients produced detectable antibodies to Ag85A. There were 6 cases of active TB among the 320 MVA85A recipients and 9 cases in the 325 controls, and this difference was not significant. MVA85A also failed to prevent new infections, as reflected by Quantiferon-TB (QFT) conversion, which reflects an antigen-specific T cell response that develops several weeks after infection with M. tuberculosis.
The other trial enrolled 2,797 healthy infants who received BCG at birth and were randomized to MVA85A or control (Tameris et al., 2013). MVA85A induced Ag85A-responsive T cells that could produce IFNγ, TNF, and IL-2, as well as IL-17. No Ag85A-responsive CD8 T cells were detected. Thirty-nine MVA85A recipients and 32 controls developed active TB defined by stringent diagnostic criteria, and 178 MVA85A recipients and 171 controls developed evidence of new infection (QFT conversion). Therefore, MVA85A did not confer protection from M. tuberculosis disease or infection.
In the absence of an efficacious vaccine, current TB control consists of identifying people with active TB and treating them effectively. Additionally, close contacts of the patient are also tested for evidence of infection, and those who test positive are administered 3–9 months of preventive chemotherapy. The WHO End TB Strategy program emphasizes the need for new tools, including vaccines, to achieve the goal of 95% reduction in TB deaths and 90% reduction in TB incidence by 2035.
Mechanisms of Immunity that Control M. tuberculosis
T cells are essential to control M. tuberculosis. In mice, CD4 and CD8 T cells both contribute to immunity (Mogues et al., 2001). In humans, a role for CD4 T cells has been revealed by the impact of HIV-mediated CD4 T cell depletion on reduced TB immunity (Kwan and Ernst, 2011). However, the contributions of CD8 T cells remain unclear, although CD8 T cell depletion worsened the course of TB in nonhuman primates after a high inoculum (3,000 cfu) challenge (Chen et al., 2009). It has been widely believed that IFNγ production by T cells is a major determinant of TB immunity. However, in mice, CD4 T cells can contribute to control without expressing IFNγ (Gallegos et al., 2011; Sakai et al., 2016), and transfer of CD4 T cells engineered to produce larger amounts of IFNγ resulted in worse outcomes (Sakai et al., 2016). These results in mice are consistent with those of the MVA85A trials, in which increases in IFNγ-producing CD4 T cells did not provide protection. They are also consistent with results in rhesus macaques, in which aerosol vaccination with an adenovirus expressing Ag85A, along with the immunodominant antigens Ag85B and Tb10.4, induced IFNγ-producing CD4 and CD8 T cells but did not protect from M. tuberculosis (Darrah et al., 2014). Therefore, although both CD4 T cells and IFNγ are essential for control of M. tuberculosis, IFNγ production is not the sole effector mechanism that contributes to the protective immunity mediated by CD4 T cells. Indeed, there is emerging evidence that production of the pro-inflammatory cytokine IL-17 as well as IL-17 responsiveness are important for control of M. tuberculosis in mice (Freches et al., 2013), and there is also evidence that induction of IL-17 responses can contribute to efficacy of an adjuvanted subunit TB vaccine (Khader et al., 2007). Since CD4 T cells that produce both IL-17 and IFNγ have been detected in other contexts (Zielinski et al., 2012), additional investigation of the roles of IL-17-producing T cells in natural and vaccine-induced protective immunity to TB is warranted.
The multifunctional proinflammatory cytokine TNF is essential for control of M. tuberculosis in mice (Flynn et al., 1995), nonhuman primates (Lin et al., 2010), and humans (Keane et al., 2001), and is produced by myeloid cells as well as by T lymphocytes. In mice, TNF produced by T cells contributes to control of M. tuberculosis (Allie et al., 2013; Sakai et al., 2016; Saunders et al., 2004) although another study found that CD4 T cells specific for the secreted protein ESAT-6 improved control without producing TNF (Gallegos et al., 2011). While studies in humans cannot determine the importance of distinct cellular sources of TNF, the frequency of CD4 T cells that produce only TNF is higher in subjects with active TB than in those with LTBI and correlate with bacterial burdens (Day et al., 2011), indicating that TNF-producing T cells correlate inversely with control of M. tuberculosis.
Other mechanisms contribute to M. tuberculosis innate immunity, including pattern recognition molecules and adaptors (Stamm et al., 2015), neutrophils (Blomgran et al., 2012; Blom-gran and Ernst, 2011), reactive oxygen (Köister et al., 2017) and nitrogen (Mishra et al., 2017), autophagy (Ouimet et al., 2016), and apoptosis (Martin et al., 2012). For each of these, M. tuberculosis has evolved the ability to inhibit or resist these defense mechanisms (Cambier et al., 2014; Goldberg et al., 2014). There is also increasing knowledge of “unconventional” or donor-unrestricted T cells (e.g., those with TCRs that interact with nonpolymorphic antigen-presenting molecules), including mycobacterial lipid antigen-specific T cells restricted by CD1a, CD1b, or CD1c (Van Rhijn and Moody, 2015), mucosal-associated invariant T cells (MAITs) (Gold et al., 2015), and γδ T cells. Although these may bear on TB vaccine development, there is less known regarding their contributions to protection. Similarly, antibodies are produced in response to M. tuberculosis infection. Until recently, TB vaccine strategies to target antibodies have been largely dismissed, and new information on the potential roles of antibodies in protective immunity to M. tuberculosis is just emerging (Li et al., 2017; Lu et al., 2016).
Since T cells that recognize peptides bound to MHC class I or II have been the focus of most research and TB vaccine efforts, this Review focuses on mechanisms that limit the efficacy of conventional T cells, especially CD4 T cells, and that contribute to challenges in developing effective TB vaccines.
Mechanisms of T Cell Evasion in TB
M. tuberculosis possesses multiple mechanisms to perturb innate immunity (Cambier et al., 2014). By disrupting innate responses, such as phagosome maturation (Mehra et al., 2013), apoptosis (Velmurugan et al., 2007), and autophagy (Ouimet et al., 2016), or by inducing detrimental type I interferon secretion (Mayer-Barber et al., 2011) or excessive TNF (Roca and Ramakrishnan, 2013), M. tuberculosis optimizes its early survival and modulates adaptive immunity to its own advantage. Development of attenuated mycobacterial vaccines and selection of adjuvants for subunit vaccines depends on understanding how M. tuberculosis uses specific innate responses to bias or attenuate beneficial T cell responses. Other mechanisms that act early in infection, including impaired or misregulated dendritic cell maturation (Hanekom et al., 2003) and delayed priming of CD4 T cells (Wolf et al., 2008) should be bypassed by vaccination, although their characterization may shed light on mechanisms that limit T cell containment of infection early after exposure.
Most knowledge of initiation and regulation of T cell responses is based on studies of antigen administration followed by restimulation of cells harvested from blood or lymphoid tissues. However, protective immunity to tuberculosis requires T cells to traffic to infected tissues where they must recognize and respond to pathogen antigens (Figure 1). Even if antigen-specific T cells are generated by vaccination, they will not contribute to protection if they are not activated through antigen recognition at the site of infection. Recent studies have revealed mechanisms whereby M. tuberculosis interferes with activation of antigen-specific T cells, and these mechanisms likely contribute to the limitations of existing TB vaccines. Because of the tractability and accessibility of studies in mice, most of the mechanisms described below have been discovered and validated in mice. When similar findings have been described in nonhuman primate or human studies, they are noted.
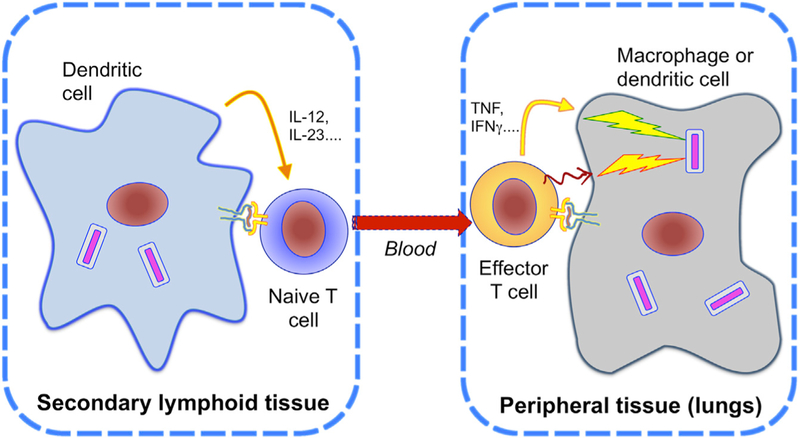
Figure 1. T Cells Must Be Activated in Distinct Compartments for Protection.
Naive T cells are primed by their cognate antigens in secondary lymphoid tissues. Priming initiates the clonal expansion of T cells and is influenced by cytokines produced by dendritic cells, leading to their differentiation into effector or memory T cell subsets, and egress from the lymph node via the blood. To accomplish effector functions, T cells must traffic to the site of infection and be activated by recognizing their cognate epitope presented by infected cells, to secrete cytokines (curved solid arrow in figure) and express surface ligands for activating receptors (squiggly arrow in figure) that activate intracellular microbicidal mechanisms. If T cells cannot be activated at the site of infection, they will not contribute to control of the infection.
Mechanisms Directed at Antigen-Presenting Cells
Since M. tuberculosis infects professional antigen-presenting cells, the bacteria are ideally located to perturb the functions of these cells. One target is the MHC class II antigen presentation pathway (hereafter termed the MHC II pathway), which is essential for antigen activation of CD4 T cells. Initially described as sequestration of antigens produced by intramacrophage mycobacteria from human CD4 T cells (Pancholi et al., 1993), multiple mechanisms contribute to reduced recognition of M. tuberculosis-infected cells by antigen-specific CD4 T cells.
M. tuberculosis inhibits MHC II expression by blocking IFNγ-mediated induction of class II transactivator (CIITA) (Kincaid and Ernst, 2003; Pai et al., 2003), which controls genes in the MHC II pathway in mice and humans. One mechanism that blocks IFNγ induction ofMHC II expression is by an incompletely characterized mechanism that involves prolonged engagement of the innate sensor TLR2 by mycobacterial lipoproteins and lipoglycans (Pai et al., 2003), although there is also evidence for TLR2-independent mechanisms (Fortune et al., 2004).
Mycobacteria also perturb the MHC II pathway by interfering with intracellular trafficking of MHC II. During MHC II assembly and trafficking, the class II invariant chain is processed by cathepsin S and then occupies the MHC II peptide-binding groove to maintain complex stability. M. tuberculosis targets the invariant chain and MHC II via inducing IL-10, which reduces cathepsin S expression (Sendide et al., 2005). Although interference with invariant chain processing due to IL-10 suppression of cathepsin S has not been directly examined in vivo, support for the relevance of this mechanism is provided by the finding that IL-10-deffcient mice have accelerated CD4 T cell responses and lower lung bacterial burdens when infected with M. tuberculosis (Redford et al., 2010).
Two recent studies have identified M. tuberculosis proteins that perturb MHC II antigen presentation and activation of CD4 T cells. A genome-wide screen revealed inhibition of MHC II antigen presentation by PE_PGRS47, a member of a large M. tuberculosis multigene family (Saini et al., 2016). Targeted deletion of PE_PGRS47 attenuated M. tuberculosis, and PE_PGRS47 blocked presentation of multiple M. tuberculosis antigens to CD4 T cells in vitro and in vivo. PE_PGRS47 was also found to impair autophagy in infected macrophages, providing evidence for a role of autophagy in MHC class II presentation of mycobacterial antigens.
Another recent study revealed interference with MHC II antigen presentation by the M. tuberculosis secreted effector, EsxH. EsxH directly interacts with Hrs, a component of the endo-somal sorting complex required for transport (ESCRT) machinery in human and murine cells, which contributes to phagosome maturation and antigen processing (Portal-Celhay et al., 2016). EsxH overexpression diminished activation of M. tuberculosis antigen-specific CD4 T cells in vitro and in vivo, and deletion of EsxH increased activation of CD4 T cells in vitro and in vivo in ESCRT-replete, but not ESCRT-deficient, cells. EsxH-deficient mycobacteria were attenuated in vivo, but much of the attenuation was lost in CD4 T cell-deficient mice, indicating that EsxH interferes with CD4 T cell activation.
In addition to inhibition of MHC class II antigen presentation by PE_PGRS47 and by EsxH, M. tuberculosis diverts secreted mycobacterial antigens from the MHC II pathway to the extracellular space (Srivastava and Ernst, 2014; Srivastava et al., 2016). This export of M. tuberculosis antigens from infected cells involves vesicular transport. Depletion of the motor protein, kine-sin-2, involved in this vesicular movement, revealed that not only was antigen export decreased, but antigen presentation to Ag85B- or ESAT-6-specific CD4 T cells by M. tuberculosis-infected cells was increased. Blockade of antigen export from infected cells improved T cell-dependent restriction or killing of intracellular M. tuberculosis in vitro and enhanced control of M. tuberculosis in vivo (Srivastava et al., 2016).
Unlike other mechanisms of M. tuberculosis perturbation of antigen presentation that decrease CD4 T cell priming, antigen export enhances priming of naive CD4 T cells by allowing uptake, processing, and presentation of exported antigens by uninfected cells in lymph nodes (Srivastava and Ernst, 2014). However, antigen export can be detrimental to CD4 T cell control of M. tuberculosis in the lungs through at least two mechanisms. First, since direct recognition of infected cells by CD4 T cells is essential for control of M. tuberculosis in vivo (Srivastava and Ernst, 2013), diversion of bacterial antigens from the MHC II pathway decreases antigen presentation by infected cells, thereby decreasing their recognition by CD4 T cells (Srivastava et al., 2016). Second, uptake and presentation of exported antigens by uninfected cells (which outnumber infected cells in the lungs [Wolf et al., 2007]) allows antigen-specific T cells to be engaged by uninfected cells instead of infected cells.
The significance of impairing antigen presentation by M. tuberculosis was revealed by comparing CD4 T cell responses to Ag85B presented by cells infected with M. tuberculosis (which persists in vivo) or M. bovis BCG (which is eliminated by T cells) (Grace and Ernst, 2016). Ag85B-specific CD4 T cell responses after M. tuberculosis infection were more delayed and required more antigen-producing bacteria to prime Ag85B-specific CD4 T cells than after aerosol BCG infection. When the numbers of bacteria in infected cells were equivalent, M. tuberculosis-infected cells were poorer stimuli than BCG-infected cells for activating Ag85B-specific CD4 T cells in vivo and in vitro. The number of CD4 T cells recruited to the lungs was greater after M. tuberculosis infection than after BCG infection, indicating that M. tuberculosis persistence is due to poor CD4 T cell efficacy, rather than to deficient T cell recruitment. These results indicate that M. tuberculosis interference with antigen presentation to CD4 T cells contributes to its persistence and imply that interference with antigen presentation contributes to evasion of vaccine-induced T cells.
M. tuberculosis also modulates its gene expression to limit T cell efficacy. Expression of Ag85B is highest during early growth of M. tuberculosis in the lungs and then decreases, leading to decreased activation of Ag85B-specific CD4 T cells. This mechanism is functionally important, since an M. tuberculosis strain engineered to express Ag85B throughout infection is attenuated in mice, but only in the presence of CD4 T cells (Bold et al., 2011). However, the findings with Ag85B cannot be extrapolated to all M. tuberculosis antigens, since expression of ESAT-6 is maintained throughout infection, and ESAT-6-spe-cific CD4 T cells can be activated at comparable levels during early and later stages of infection (Moguche et al., 2017). These results emphasize that vaccine antigens must be selected with specific knowledge of their expression dynamics.
Mechanisms that Directly Affect T Cells
The mycobacterial glycopliids lipomannan and mannosylated lipoarabinomannan (man-LAM) are components of the bacterial cell wall that can inhibit T cell activation through reduced phosphorylation of ZAP-70, Lck, and LAT downstream of TCR triggering (Mahon et al., 2012; Sande et al., 2016). Whether this mechanism contributes to the inability of T cell responses to eliminate M. tuberculosis in vivo has not been established, but man-LAM and other mycobacterial lipoglycans can be acquired by T cells via bacterial membrane vesicles, and man-LAM can be detected on T cells isolated from lungs of M. tuberculosis-infected mice (Athman et al., 2017).
M. tuberculosis-specific T cells have been characterized for their maturation states that affect their functions and antimycobacterial efficacy. Chronic M. tuberculosis infection in mice drives ESAT-6-specific CD4 T cells to terminal differentiation (Reiley et al., 2010) and retention in the pulmonary vasculature (Moguche et al., 2015; Sakai et al., 2014). Terminally differentiated (KLRG1 hiCX3CR1 hiT-bethi) CD4 T cells confer little protection when adoptively transferred, despite producing larger quantities of IFNγ than less-differentiated (CXCR3+CX3CR1− PD-1 hiCD69hi) CD4 T cells that traffic to the lung parenchyma and provide protection (Sakai et al., 2014). Terminal differentiation is antigen dependent: ESAT-6-specific CD4 T cells exhibit this property, but Ag85B-specific CD4 T cells do not, since Ag85B expression is reduced in chronic infection (Moguche et al., 2017). T cells with similar properties are present in humans: a vaccine that contains ESAT-6 was found to expand CD4 T cells with limited functional capacities when administered to subjects with LTBI (Moguche et al., 2017). This finding has important implications, since TB vaccines administered to those that already have LTBI will have little efficacy if they expand poorly functional antigen-specific T cells.
Chronic M. tuberculosis in mice is also associated with T cells whose phenotype and functions are characteristic of exhaustion. Lung T cells that express exhaustion markers (TIM3, PD-1, LAG3, 2B4) and transcriptional signatures are less able to produce multiple cytokines (Jayaraman et al., 2016). Although human studies have indicated that T cell exhaustion may develop at certain stages of infection or disease, more investigation is warranted. This is particularly important for T cells with distinct antigen specificities, since the data will be important for design of TB vaccines administered to those with LTBI or active TB.
Other mechanisms limit effector T cells and may impact TB vaccine efficacy. Regulatory T cells (Treg) are induced early after infection in mice and retard priming, expansion, and recruitment of effector T cells to the lungs. Treg contract later and do not accumulate in the lungs of M. tuberculosis-infected mice (Shafiani et al., 2013). Likewise, the immunosuppressive cytokine IL-10 is expressed by myeloid cells and T cells during M. tuberculosis infection, and T cell-derived IL-10 limits control of bacteria in the lungs (Moreira-Teixeira et al., 2017). Blockade of IL-10 receptor signaling during BCG vaccination of mice enhances IL-17 and IFNγ responses by T cells and innate lymphoid cells and improves control of M. tuberculosis (Pitt et al., 2012). These results indicate that blockade of IL-10 at the time of vaccination may improve the protection obtained by TB vaccines.
Spatial Restriction of T cells
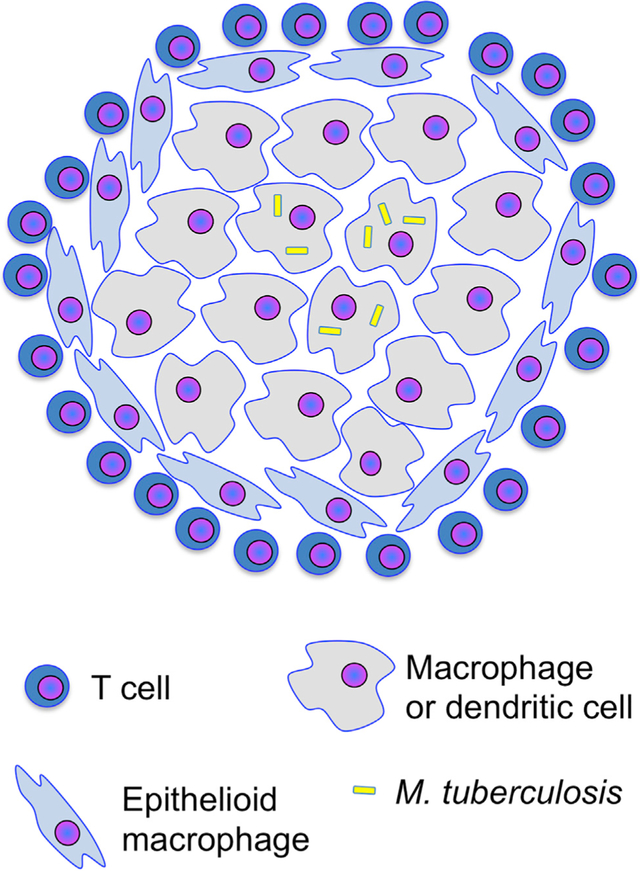
The characteristic tissue lesion in TB is the granuloma, an aggregate of macrophages, dendritic cells, and neutrophils, with variable frequencies of B and T lymphocytes. The architecture of (Marakalala et al., 2016) and animals (Gideon et al., 2015; Smith et al., 2016), and in a common form of TB granulomas, T cells are confined to the peripheral regions, while infected cells are in the central core (Figure 2) (Ernst et al., 2018; Kauffman et al., 2018). Since CD4 T cells must directly recognize and contact infected cells to control intracellular M. tuberculosis (Srivastava and Ernst, 2013), the architecture of TB granulomas may limit the efficacy of T cells in TB. One promising finding is that in M. tuberculosis-infected rhesus macaques, inhibition of indoleamine-2,3-dioxygenase (IDO; a tryptophan-catalyzing enzyme whose products can modulate T cell differentiation and activation) at the onset of the adaptive immune response resulted in enhanced T cell responses, positioning more T cells in the central regions of granulomas, and reducing the number of bacteria in lesions (Gautam et al., 2018). If IDO inhibition is effective when administered after establishment of granulomas, this may be a promising adjunct to TB chemotherapy or vaccination.
Figure 2. Positional Limitation of T Cell Efficacy.
Granulomas form by aggregation of infected and uninfected macrophages, followed by epithelioid transformation of macrophages surrounding the initial aggregate. T cells are recruited to granulomas but often concentrate at the granuloma periphery and do not contact infected cells in the central region. Mechanisms that restrict contact between T cells and infected cells in granulomas may include: (1)failure to produce or respond to chemoattractants; (2) signals that repel T cells from infected cells; (3) failure of T cells to recognize infected cells and stop migrating; (4) killing of T cells in proximity to infected cells; (5) arrest of T cells when they contact uninfected cells that have acquired antigen; and (6) mechanical barriers that prevent T cells from penetrating to the granuloma interior.
Resistance to T Cell Effector Mechanisms
An additional mechanism that may limit the efficacy of T cells is resistance to their effectors. One example is that M. tuberculosis infection of human (Ting et al., 1999) or murine (Fortune et al., 2004) macrophages inhibits responses to IFNγ. This effect of M. tuberculosis operates at a late step in transcription initiation and does not inhibit transcription of all IFNγ-responsive genes. Among the genes most repressed in M. tuberculosis-infected macrophages is the transcription factor CIITA, the master regulator of MHC II genes (LeibundGut-Landmann et al., 2004). There is currently no information whether M. tuberculosis infection inhibits macrophage responses to IFNγ in vivo. However, the observation that M. tuberculosis can interfere with cytokine signaling potentially explains why IFNγ production is not a correlate of protection in TB; if the IFNγ produced cannot act optimally due to disrupted signaling, then the quantities will not correlate with outcomes (Figure 3). It is reasonable to hypothesize that resistance to other effector mechanisms limits the efficacy of T cell control of TB.
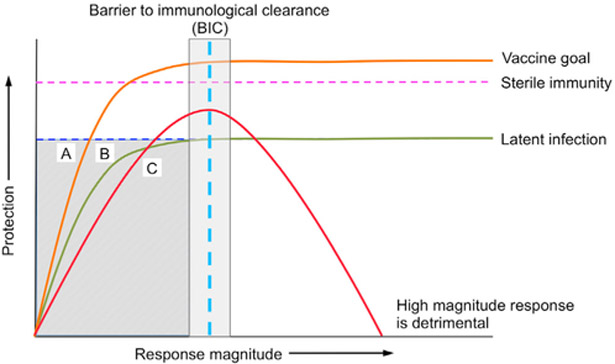
Figure 3. Models for Incomplete Immunity.
The graph plots the magnitude of hypothetical immunological responses versus the extent of protection, with distinct responses required to establish latent infection and to achieve sterile immunity. The “Barrierto immunological clearance” (BIC) represents any process (such as evasion of T cells)hat constrains the extent of protection provided by an immune response, such that increasing the magnitude of that response does not increase protection.
Mechanism A (orange line), if great enough in magnitude, can provide sterile immunity without being limited by the BIC;this is an ideal mechanism to induce by vaccination. Mechanism B (green line) is constrained by the BIC and cannot provide sterile immunity regardless of the magnitude of the response. Mechanism C represents immunological mechanisms that, when excessive, cause pathological inflammation and tissue damage. CD4 T cell production of IFNγ conforms to the pattern of Mechanism C.
The shaded region indicates the relationship between response magnitudes and protection that cannot inform identification of correlates of immunity: Mechanisms A, B, and C all exhibit inadequate protection if the response is insufficient in magnitude, even though the individual mechanisms differ markedly in their potential for protection. Studies of vaccines that induce responses in the range shown in the shaded box will not reveal correlates of immunity, even if the specific mechanisms have the potential to provide sterilizing immunity.
Recent Progress
Despite the plethora of mechanisms that interfere with immune responses to M. tuberculosis, recent results raise optimism for successful TB vaccine development.
A rhesus cytomegalovirus (rhCMV) vaccine containing M. tuberculosis antigens provided protection not previously achieved by a TB vaccine (Hansen et al., 2018). RhCMV-TB induced IFNγ- and TNF-producing CD4 and CD8 memory (effector > central memory) T cells. Additionally, and when challenged with a low inoculum (10–25 cfu) of M. tuberculosis Erdman, a strain that is especially virulent in nonhuman primates, 14 of the 34 RhCMV-TB vaccinated animals had no evidence of TB disease, and 10 of the 14 had no viable M. tuberculosis recovered. Although RhCMV-TB induced memory T cells, the magnitude did not correlate with the outcomes of infectious challenge. Instead, a durable whole-blood transcriptional signature correlated with protection and included genes involved in innate immunity, including neutrophil degranulation. The durability of the innate immune signature is thought to relate to persistence of the RhCMV vector. The possibility that the vaccine induced lung-resident memory T cells was not examined but should be the subject of future studies, since the lungs are a major site of human CMV persistence (Gordon et al., 2017), and the rapid onset of protection after bacterial challenge suggests that vaccine-induced immunity was present in the lungs. While it is notable that protection was achieved by a vaccine that did not induce detectable antibodies, the findings do not exclude a potential role for antibodies in immunity to TB.
Another recent study revealed the potential for protection against TB by trained innate immunity (Kaufmann et al., 2018). Intravenous M. bovis BCG stimulated expansion of hematopoietic stem cells (HSC), associated with epigenetic changes transmitted to their progeny, including multipotent progenitors and bone marrow-derived macrophages (Kaufmann et al., 2018). In independent experiments using bone marrow chimeras, parabionts with CCR2−/− partners (to minimize circulation of untrained monocytes), and transfer into Rag1−/− mice lacking T cells, BCG “training” of cells enhanced control of M. tuberculosis. Training of bone marrow cells depended on IFNγ responsiveness but did not involve infection of HSC themselves, or the presence of T cells. Although BCG training was insufficient to fully control a high inoculum M. tuberculosis challenge, the results are promising, as they indicate that the cells that harbor M. tuberculosis can be modulated to improve their control of infection. This is significant for at least three reasons: (1) the results may relate to the observation that certain humans exposed to TB remain uninfected, an effect attributed to alveolar macrophage elimination of M. tuberculosis without a T cell response; (2) the protection observed in the RhCMV-TB studies correlated with an innate immune signature—it will be interesting to determine whether there are common features between that signature and that of BCG-trained cells; and (3) training HSC and their progeny may circumvent mechanisms of immune evasion that limit the efficacy of T cell responses.
It’s Hard to Make TB Vaccines that Work, but It’s Not Hopeless
As studies of immunity and pathogenesis generate insight into the complexity and diversity of TB, they provide principles for making effective TB vaccines. Advances in disciplines such as cancer immunotherapy can also inform TB vaccine discovery. Two general principles are important to note. First, efforts and resources for TB vaccine development must acknowledge the vast amount of information that is currently unknown and that is needed to guide TB vaccine development. Second, it is unlikely that one TB vaccine will eliminate TB in all populations. In considering TB vaccine development, a useful framework is the “Tumor Immunogram,” meant to optimize cancer immunotherapies (Blank et al., 2016). A “TB Immunogram” to illustrate major gaps is in Table 1.
Table 1.
The TB Immunogram
| Challenge | Strategies |
|---|---|
| Different vaccine approaches needed for distinct populations | Optimize animal models to test vaccines for naive, BCG-vaccinated, LTBI, and active TB. |
| Optimal vaccine antigens not known | Consider different antigens for distinct target populations.Examine antigen properties beyond frequency or magnitude of T cell responses.Prioritize antigens presented by HLA alleles prevalent in regions with high TB burdens. |
| Vaccine-induced T cells require a combination of properties | Assay vaccines for induction of T cells with appropriate state of differentiation, residence in lung tissue compartments, and expression of effector mechanisms beyond cytokine secretion. |
| M. tuberculosis-infected cells evade T cell recognition | Identify antigens less impacted by evasion mechanisms (nonsecreted antigens, antigens with evidence of selection pressure from T cell recognition); develop pharmacological interventions to overcome specific evasion mechanisms. Exploit trained innate immunity for T cell-independent protection. |
| M. tuberculosis occupies diverse intracellular compartments | Identify mechanisms for elimination of bacteria in immature phagosomes, cytoplasm, autophagosomes, and mature phagolysosomes. |
| M. tuberculosis can be extracellular | Determine the bacterial population fraction that is extracellular during distinct stages of infection; optimize antibodies and other humoral mediators. |
| Range of inoculum size unknown in humans | Examine vaccines efficacious against low-dose challenges for efficacy against higher inocula and more virulent bacterial strains. |
| Correlates of immunity not identified | Develop methods for analysis of cells in human tissues; apply analyses that account for nonlinear relationships between response and protection. |
TB vaccine development is hindered by technical gaps, including suboptimal animal models. A pipeline of concepts and vaccine candidates depends on tractable and economical animal models for initial discovery, and TB vaccine efforts need to improve models to predict effects in humans. An additional gap is a lack of knowledge of the M. tuberculosis epitopes presented by infected cells; certain vaccines induce T cells that recognize epitopes not presented by M. tuberculosis-infected cells (Billeskov et al., 2010; Nyendak et al., 2016). This mismatch may be due to vaccines targeted to antigen-presenting cells whose processing machinery differs from that in M. tuberculosis-infected cells. Knowledge of the repertoire of peptides presented by M. tuberculosis-infected cells would provide a basis for vaccine antigen selection, but there are technical challenges, including: the need to obtain infected cells from humans with TB (or humanized animal models), methods for sample preparation to minimize peptide loss, improved mass spectrometry sensitivity, and the facilities and equipment to perform the necessary procedures with appropriate biosafety.
Recent advances in microscopy, especially two-photon intravital microscopy, provide unprecedented insight into interactions of lymphocytes and antigen-presenting cells during immune responses, but biosafety considerations and cost have prevented the TB field from benefiting from this technology. Since recent studies indicate that defective interactions between T cells and M. tuberculosis-infected cells contribute to the failure of T cells to eliminate infection, the ability to image vaccine-induced T cells and their real-time interactions with infected cells in tissues is likely to guide design and selection of TB vaccines.
Other techniques are less impacted by biosafety requirements, and expanding their use will likely inform TB vaccine development. These include high-content flow cytometry; tran-scriptomic analyses on specific cell subsets and single cells; studies of T cell antigen receptor (TCR) diversity and specificity after vaccination; and studies to determine whether certain individuals and populations are more susceptible to certain bacterial strains. While these technologies and studies are expensive, TB is an expensive problem. In addition to the cost of diagnosis and treatment of millions of cases of drug-susceptible TB each year, treatment of one case of multi-drug resistant (MDR)-TB in South Africa costs $6,800 (Pooran et al., 2013). With 19,000 incident cases of MDR-TB, this translates to ~$130 million for South Africa alone. This number pales when considering that India and China together are estimated to have 220,000 incident cases of MDR-TB (WHO Global TB Report, 2017, http://www.who.int/tb/publications/global_report/en/), that treatment of extensively drug-resistant (XDR)-TB costs even more than treatment of MDR-TB, and that MDR- and XDR-TB are readily transmitted to others with the potential for further expansion of the problem (Shah etal., 2017).
Conclusion
TB is a complex disease, and there are major barriers and challenges to developing effective TB vaccines; the magnitude of the problem demands improved tools for bending the curve of TB prevalence steeply downward. Recent progress in TB vaccine development and advances in technology and conceptual understanding provide optimism that sustained and creative efforts will lead to successful vaccines and other modes of immunotherapy for this major global health problem.
ACKNOWLEDGMENTS
Supported by grants from the National Institutes of Health (AI051242, AI124471, and AI111211). I apologize to the numerous investigators whose valuable work could not be cited due to length restrictions.
REFERENCES
- Allie N, Grivennikov SI, Keeton R, Hsu NJ, Bourigault ML, Court N, Fremond C, Yeremeev V, Shebzukhov Y, Ryffel B, et al. (2013). Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobac-terium tuberculosis infection. Sci. Rep. 3, 1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman JJ, Sande OJ, Groft SG, Reba SM, Nagy N, Wearsch PA, Richardson ET, Rojas R, Boom WH, Shukla S, and Harding CV (2017). Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. 198, 2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeskov R, Grandal MV, Poulsen C, Christensen JP, Winther N, Vingsbo-Lundberg C, Hoang TT, van Deurs B, Song YH, Aagaard C, et al. (2010). Difference in TB10.4 T-cell epitope recognition following immunization with recombinant TB10.4, BCG or infection with Mycobacterium tuberculosis. Eur. J. Immunol. 40, 1342–1354. [DOI] [PubMed] [Google Scholar]
- Blank CU, Haanen JB, Ribas A, and Schumacher TN (2016). CANCER IMMUNOLOGY. The “cancer immunogram”. Science 352, 658–660. [DOI] [PubMed] [Google Scholar]
- Blomgran R, and Ernst JD (2011). Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 186, 7110–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V, and Ernst JD (2012). Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe 11, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold TD, Banaei N, Wolf AJ, and Ernst JD (2011). Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 7, e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier CJ, Falkow S, and Ramakrishnan L (2014). Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 159, 1497–1509. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halli-day L, Fortman J, McAllister M, et al. (2009). A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 5, e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, and Gagneux S (2010). Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin R, Coscollá M, Efstathiadis E, Gagneux S, and Ernst JD (2014). Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG). Vaccine 32, 5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscolla M, Copin R, Sutherland J, Gehre F, de Jong B, Owolabi O, Mbayo G, Giardina F, Ernst JD, and Gagneux S (2015). M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe 18, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, Blanchard JL, Didier PJ, Roy CJ, Rao SS, et al. (2014). Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J. Immunol. 193, 1799–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O’rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, et al. (2011). Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 187,2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos RJ, and Dubos J (1952). The White Plague: Tuberculosis, Man, and Society (Rutgers University Press; ). [Google Scholar]
- Ernst JD, Cornelius A, Desvignes L, Tavs J, and Norris BA (2018). Limited antimycobacterial efficacy of epitope peptide administration despite enhanced antigen-specific CD4 T cell activation. J. Infect. Dis. Published online March 14, 2018. 10.1093/infdis/jiy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, and Bloom BR (1995). Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572. [DOI] [PubMed] [Google Scholar]
- Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ,and Ernst JD (2004). Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 172, 6272–6280. [DOI] [PubMed] [Google Scholar]
- Freches D, Korf H, Denis O, Havaux X, Huygen K, and Romano M (2013). Mice genetically inactivated in interleukin-17A receptor are defective in long-term control of Mycobacterium tuberculosis infection. Immunology 140,220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, and Glickman MS (2011). A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 7, e1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam US, Foreman TW, Bucsan AN, Veatch AV, Alvarez X, Ade-kambi T, Golden NA, Gentry KM, Doyle-Meyers LA, Russell-Lodrigue KE, et al. (2018). In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 115, E62–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, et al. (2015). Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 11,e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MC, Napier RJ, and Lewinsohn DM (2015). MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol. Rev. 264, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MF, Saini NK, and Porcelli SA (2014). Evasion of innate and adaptive immunity by Mycobacterium tuberculosis. Microbiol. Spectr. 2, 2. [DOI] [PubMed] [Google Scholar]
- Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA, Igarashi S, Granot T, Lerner H, Goodrum F, and Farber DL (2017). Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J. Exp. Med. 214, 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PS, and Ernst JD (2016). Suboptimal antigen presentation contributes to virulence of Mycobacterium tuberculosis in vivo. J. Immunol. 196, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MC, Brisse S, Brosch R, Fabre M, Omaїs B, Marmiesse M, Supply P, and Vincent V (2005). Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 1, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanekom WA, Mendillo M, Manca C, Haslett PA, Siddiqui MR, Barry C 3rd, and Kaplan G (2003). Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 188, 257–266. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, Gilbride RM, Hughes CM, Ventura AB, Ainslie E, et al. (2018). Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 24, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben RM, and Dodd PJ (2016). The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 13, e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, Anderson AC, Kuchroo VK, and Behar SM (2016). TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 12, e1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman KD, Sallin MA, Sakai S, Kamenyeva O, Kabat J, Weiner D, Sutphin M, Schimel D, Via L, Barry CE 3rd, et al. (2018). Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal. Immunol. 11, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, et al. (2018). BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwie-terman WD, Siegel JN, and Braun MM (2001). Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345, 1098–1104. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. (2007). IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377. [DOI] [PubMed] [Google Scholar]
- Kincaid EZ, and Ernst JD (2003). Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-gamma without inhibiting STAT1 function. J. Immunol. 171, 2042–2049. [DOI] [PubMed] [Google Scholar]
- Köster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, Grigsby SJ, Mittal E, Park HS, Jones V, et al. (2017). Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. USA 114, E8711–E8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CK, and Ernst JD (2011). HIV and tuberculosis: a deadly human syn-demic. Clin. Microbiol. Rev. 24, 351–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor C (2007). Consumption and Literature: The Making of the Romantic Disease (Palgrave Macmillan; ). [Google Scholar]
- LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, and Reith W (2004). Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 34, 1513–1525. [DOI] [PubMed] [Google Scholar]
- Li H, Wang XX, Wang B, Fu L, Liu G, Lu Y, Cao M, Huang H, and Javid B (2017). Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 114, 5023–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV, et al. (2010). Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, et al. (2016). Afunctional role for antibodies in tuberculosis. Cell 167, 433–443.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon RN, Sande OJ, Rojas RE, Levine AD, Harding CV, and Boom WH (2012). Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell. Immunol. 275, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, and Sterne JA (2014). Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 58, 470–480. [DOI] [PubMed] [Google Scholar]
- Marakalala MJ, Raju RM, Sharma K, Zhang YJ, Eugenin EA, Prideaux B, Daudelin IB, Chen PY, Booty MG, Kim JH, et al. (2016). Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med. 22, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, Fortune SM, Remold HG, and Behar SM (2012). Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, and Sher A (2011). Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Köster S, Penberthy K, Kubota Y, Dricot A, et al. (2013). Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 9, e1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E, Smith CM, Phuah JY, Long JE, Dubuke ML, et al. (2017). Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat. Microbiol. 2, 17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguche AO, Shafiani S, Clemons C, Larson RP, Dinh C, Higdon LE, Cambier CJ, Sissons JR, Gallegos AM, Fink PJ, and Urdahl KB (2015). ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med. 212, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguche AO, Musvosvi M, Penn-Nicholson A, Plumlee CR, Mearns H, Geldenhuys H, Smit E, Abrahams D, Rozot V, Dintwe O, et al. (2017).An-tigen availability shapes t cell differentiation and function during tuberculosis. Cell Host Microbe 21, 695–706.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogues T, Goodrich ME, Ryan L, LaCourse R, and North RJ (2001). The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Teixeira L, Redford PS, Stavropoulos E, Ghilardi N, Maynard CL, Weaver CT, Freitas do Rosário AP, Wu X, Langhorne J, and O’Garra A (2017). T cell-derived IL-10 impairs host resistance to Mycobacterium tuberculosis infection. J. Immunol. 199, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dièye S, Dieye TN, Esmail H, Goliath R, Huygen K, et al. ; MVA85A 030 trial investigators(2015). Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 3, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyendak M, Swarbrick GM, Duncan A, Cansler M, Huff EW, Hokey D, Evans T, Barker L, Blatner G, Sadoff J, et al. (2016). Adenovirally-induced polyfunctional T cells do not necessarily recognize the infected target: lessons from a phase I trial of the AERAS-402 vaccine. Sci. Rep. 6, 36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Old-ebeken S, Karunakaran D, Portal-Celhay C, Sheedy FJ, Ray TD, et al. (2016). Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 17, 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai RK, Convery M, Hamilton TA, Boom WH, and Harding CV (2003). Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171, 175–184. [DOI] [PubMed] [Google Scholar]
- Pancholi P, Mirza A, Bhardwaj N, and Steinman RM (1993). Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260, 984–986. [DOI] [PubMed] [Google Scholar]
- Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, and O’Garra A (2012). Blockade of IL-10 signaling during bacillus Calm-ette-Guérin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-γ and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J. Immunol. 189, 4079–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooran A, Pieterson E, Davids M, Theron G, and Dheda K (2013). What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 8, e54587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portal-Celhay C, Tufariello JM, Srivastava S, Zahra A, Klevorn T, Grace PS, Mehra A, Park HS, Ernst JD, Jacobs WR Jr., and Philips JA (2016). Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation. Nat. Microbiol. 2, 16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, and O’Garra A (2010). Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 40, 2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, and Woodland DL (2010). Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 107, 19408–19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca FJ, and Ramakrishnan L (2013). TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LC, Diwan VK, and Wheeler JG (1993). Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Saini NK, Baena A, Ng TW, Venkataswamy MM, Kennedy SC, Kun-nath-Velayudhan S, Carrerno LJ, Xu J, Chan J, Larsen MH, et al. (2016). Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 1, 16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, and Barber DL (2014). Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 192, 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov V, and Barber DL (2016). CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 12, e1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande OJ, Karim AF, Li Q, Ding X, Harding CV, Rojas RE, and Boom WH (2016). Mannose-capped Lipoarabinomannan from Mycobacterium tuberculosis induces CD4+T cell Anergy via GRAIL. J. Immunol. 196,691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BM, Briscoe H, and Britton WJ (2004). T cell-derived tumour necrosis factor is essential, but not sufficient, for protection against Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 137, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendide K, Deghmane AE, Pechkovsky D, Av-Gay Y, Talal A, and Hmama Z (2005). Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J. Immunol. 175, 5324–5332. [DOI] [PubMed] [Google Scholar]
- Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, Sharma P, Campbell DJ, Way SS, and Urdahl KB (2013). Pathogen-specific Treg cells expand early during Mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity 38,1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NS, Auld SC, Brust JC, Mathema B, Ismail N, Moodley P, Mlisana K, Allana S, Campbell A, Mthiyane T, et al. (2017). Transmission of extensively drug-resistant tuberculosis in South Africa. N. Engl. J. Med. 376, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Proulx MK, Olive AJ, Laddy D, Mishra BB, Moss C, Gutierrez NM, Bellerose MM, Barreira-Silva P, Phuah JY, et al. (2016). Tuberculosis susceptibility and vaccine protection are independently controlled by host genotype. MBio 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, and Ernst JD (2013). Cutting edge: direct recognition of infected cells by CD4 T cells is required for control of intracellular Mycobacterium tuberculosis in vivo. J. Immunol. 191, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, and Ernst JD (2014). Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 15, 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Grace PS, and Ernst JD (2016). Antigen export reduces antigen presentation and limits T cell control of M. tuberculosis. Cell Host Microbe 19, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm CE, Collins AC, and Shiloh MU (2015). Sensing of Mycobacterium tuberculosis and consequences to both host and bacillus. Immunol. Rev. 264, 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. ; MVA85A 020 Trial Study Team (2013). Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LM, Kim AC, Cattamanchi A, and Ernst JD (1999). Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163, 3898–3906. [PubMed] [Google Scholar]
- Van Rhijn I, and Moody DB (2015). Donor unrestricted T cells: a shared human T cell response. J. Immunol. 195, 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR Jr., Porcelli SA, and Briken V (2007). Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 3, e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Linas B, Trevejo-Nurñez GJ, Kincaid E, Tamura T, Takatsu K, and Ernst JD (2007). Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179,2509–2519. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, and Ernst JD (2008). Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gat-torno M, Monticelli S, Lanzavecchia A, and Sallusto F (2012). Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484,514–518. [DOI] [PubMed] [Google Scholar]