Abstract
Gliomas are the most frequent brain tumors in the adult population and unfortunately the adjuvant therapies are not effective. Brain tumorigenesis has been related both to the increased levels of free radicals as inductors of severe damages in healthy cells, but also with the reduced response of endogenous enzyme and non-enzymatic antioxidant defenses. In turn, both processes induce the change to malignant cells. In this review, we analyzed the role of the imbalance between free radicals production and antioxidant mechanism in the development and progression of gliomas but also the influence of redox status on the two major distinctive forms of programmed cell death related to cancer: apoptosis and autophagy. These data may be the reference to the development of new pharmacological options based on redox microenvironment for glioma treatment.
Keywords: Glioma, reactive oxygen species, antioxidant systems, autophagy, apoptosis
1. INTRODUCTION
Gliomas, the main common primary brain tumor in the adult population [1, 2], are currently non curable central nervous system neoplasias and unfortunately, there has been little improvement in the efficacy of adjuvant therapies [3, 4]. Therefore, development of novel and effective treatments are essential, but for this, it is essential to know the mechanisms that underlie the carcinogenesis process. Different subtypes of gliomas have been described based on the primitive originating cell line. The WHO classification includes a grading scheme based on the evolution, of the surgical specimen and morphological features such as atypia, mitoses, endothelial proliferation and necrosis [2, 5]. Table 1 shows the characteristics of different subtypes of glioma [2].
Table 1.
WHO classification of gliomas.
| Grade | Characteristics | Types |
|---|---|---|
| I | Slow proliferation | Pediatric age Pilocyticic Astrocytoma Pleomorphic Xanthoastrocytoma Ganglioglioma |
| II | High rate of differentiation Diffuse growth into the normal brain tissue Progression into a malignant phenotype |
Diffuse astrocytome Low grade glioma |
| III | Greater cellular density Strong presence of atypia and mitoses |
Anaplastic astrocytoma Anaplastic oligoastrocytoma Anaplastic oligodendroglioma |
| IV | Microvascular proliferation Necrosis Wide tendency to spread into the brain |
Glioblastoma Gliosarcoma |
Brain tumorigenesis (such as other tumor types) has been associated with oxidative stress that is reflected by an imbalance between free radicals production and antioxidant mechanisms. In fact, the resulting oxidative stress promotes various pathological reactions which contribute to different pathologies including carcinogenesis [6]. In these conditions, free radicals may be generated in excess from endogenous sources (mitochondria and peroxisomes, but also from inflammatory cell activation or neurotransmitter oxidation) and also exogenous sources as environmental agents, drugs, irradiation and chemicals.
Free radicals mainly derived from oxygen (reactive oxygen species, ROS, such as singlet oxygen (1O2), superoxide anion radical (O2•¯), hydrogen peroxide (H2O2) and hydroxyl radical (HO•), peroxyl radical and hypochlorous acid (HOCl)), nitrogen (reactive nitrogen species, RNS, such as nitric oxide (NO), nitric dioxide (NO2), nitroxyl (HNO) and peroxynitrite (ONOO-)) and sulfur (reactive sulfur species, RSS, such as hydrogen sulfide (H2S), persulfides (RSSH) and sulfur oxide (SO2)). All of them serve as important signaling molecules acting in several physiological functions when their amounts are precisely controlled (Table 2) [7].
Table 2.
Physiological functions of free radicals.
| Reactive Species | Formula | Physiological Function |
|---|---|---|
| Reactive oxygen species | ||
| Singlet oxygen | 1O2 | Inflammation Oxidative stress Aging mechanism |
| Superoxide anion radical | O2•¯ | |
| Hydrogen peroxide | H2O2 | |
| Hydroxyl radical | HO• | |
| Hypochlorous acid | HOCl | |
| Peroxyl radical | ROO• | Unknown |
| Alcoxyl radical | RO• | Unknown |
| Reactive nitrogen species | ||
| Nitric oxide | NO | Immune process Cardiovascular modulation Nervous modulation |
| Nitroxyl | HNO | |
| Peroxynitrite | ONOO- | |
| Reactive sulfur species | ||
| Hydrogen sulfide | H2S | Synaptic transmission Cardioprotection |
| Persulfides | RSSH | |
| Sulfur oxide | SO2 | |
However, when the oxidants/antioxidants equilibrium is disrupted, free radicals trigger lipid peroxidation of the cellular membranes, oxidation of proteins and DNA, and also lead to changes in chromosome structure, genetic mutations and/or modulation of cell growth promoting carcinogenesis. However, much attention has been focused on the important role of oxidative stress induced by ROS in cancer. In fact, different studies have been shown that free radicals and especially ROS may be involved in different steps in tumorigenesis including initiation, progression, angiogenesis and metastasis [8-10]. In this way, it has been proved that mitochondria in malignant cells present an overproduction of ROS and structural and functional differences from mitochondria of normal cells [11]. The increased generation of ROS in cancer cells may alter the mitochondrial metabolism [11, 12] and also different cellular signaling pathways mediated through the transcription factors NF-κB and STAT 3, hypoxia-inducible factor-1α, kinases, growth factors, cytokines and other enzymes [8, 10, 13]. All these modifications in signaling pathways have also been described in gliomas [14-21].
Furthermore, ROS can induce cellular DNA damage and DNA methylation [22] resulting in mutations which causes healthy cells to transform in malignant cells [10]. But tumor development involves not only oxidative aggression due to an increased level of ROS but also a reduced response of antioxidant defenses. It is known that during a prolonged oxidative stress, changes in endogenous enzyme and non-enzymatic antioxidant systems have been detected. Both systems act to prevent or decrease damages caused by free radicals in excess. However, they are controlled by polymorphic genes which can be altered by free radicals, leading to dysfunctions [6]. It is known that the modulation of intracellular levels of ROS plays a key role in the maintenance of cellular homeostasis, in fact, different levels of ROS may induce different responses; low and moderate levels of ROS may act as modulator of cellular proliferation and differentiation and also may be involved in the expression of antioxidant genes, but high levels induce severe cellular damages and also cellular death [23].
On other hand, recent studies have considered that oxidative stress also induces the development of antioxidant mechanisms in the cancer cells although many evidences have supported the hypothesis that excess ROS may result in cancer cell death through autophagy [8, 10, 24, 25]. Thus, not only ROS production, but also antioxidant mechanisms regulation may be considered as therapeutic target in the treatment of different types of cancer including gliomas.
In this review, we analyze the knowledge about oxidative stress biomarkers, enzyme and non-enzymatic antioxidant defense system in glioma and how this data may be related to the hypothesis about the role of the increased levels of ROS and the reduced activity of antioxidant systems as possible inductors of carcinogenesis; or about the role of ROS as inductors of the cellular death through autophagy and/or apoptosis as a selective mechanism of defense for cancer cells without affecting normal cells. These data may be the reference to the development of other new pharmacological options in cancer treatment [26].
2. OXIDATIVE STRESS BIOMARKERS
Several reports have described the oxidative stress biomarkers and the role of enzyme and non-enzymatic antioxidant defense systems in gliomas. Between the most widely used biomarkers, lipid peroxidation assayed as thiobarbituric acid reactive substances (TBARS) or malondialdehyde (MDA) production, protein oxidation (assayed as carbonyl groups content) and DNA damage (assayed as the content of 7, 8-dihydro-8-oxoguanine (8-oxo-G) have been described.
2.1. Lipid Peroxidation
It has been extensively described lipid peroxidation as an early biomarker of oxidative damage because of the wider propagation of free radicals associated with it. The elevated oxidative stress in cells can lead to modification of a number of cellular targets and cause cell damage and death and thereby it has been associated with carcinogenesis [27, 28]. The magnitude of this damage depends not only on free radical levels but also on the body’s defense mechanisms against them mediated by various cellular antioxidants. Thus, high levels of oxidative stress result in peroxidation of membrane lipids with the generation of peroxides that can decompose to multiple mutagenic aldehyde products as malondialdehyde (MDA) which is used as a marker of oxidative stress. MDA is a low-molecular weight aldehyde that can be produced from free radical attack on polyunsaturated fatty acids. In practice, lipid peroxidation is determined as thiobarbituric acid reactive substances (TBARS) which are expressed in terms of malondialdehyde equivalents. The levels of TBARS reflect the extent of lipid peroxidation. An enhanced lipid peroxidation is considered to be mutagenic and carcinogenic [29].
In animal models, increased levels of lipid peroxidation have been found in the serum of animals with C6 glioma implanted in the subcutaneous region when compared with non-tumor healthy control animals [6]. However, using transplacental ethylnitrosourea (ENU)-induced glioma, no changes in TBARS levels were observed in plasma of animals with glioma in comparison with healthy controls. On the contrary, significant increases were detected in tumor tissue [30]. Although it has been reported that lipid peroxidation products generated in the central nervous system may diffuse across the blood-brain barrier, these results may indicate that the oxidative changes detected in tumor tissue are not reflected in plasma until they reach a certain levels. Also, in accordance with data obtained from human patients, Zengin and col [31] observed increased levels of TBARS in tumor tissue samples when compared with peritumoral areas, which could be attributed to increased formation or inadequate clearance of free radicals by the cellular antioxidants. In other types of cancer such as astrocytoma and meningioma, TBARS levels were significantly higher when compared with their corresponding peritumoral adjacent tissue. In the same way, it was clearly seen that lipid peroxidation was significantly higher in high-grade tumor. In any case, elevated levels of lipid peroxidation products support the hypothesis that the tumoral cells produce large amounts of free radicals demonstrating a relationship between free radical activity and carcinogenesis.
Other human studies have also demonstrated that lipid peroxidation state depends on the tumoral area studied, however, the microenvironment of each area may play a key role. Cirak and col [32] also studied lipid peroxidation levels in serum as well as in the tissue samples of patients with high and low-grade glial tumors, showing that patients with high-grade tumor had higher MDA levels both in sera and tissue samples than those with low grade tumor and controls.
2.2. Protein Oxidation
Carbonyl groups content has been used as the measurement of protein oxidation, a process occurred as a strong result of the existence of oxidative stress and as the next step of damage after lipid peroxidation. In animal models, high levels of carbonyl groups content were found in serum of animals with C6 xerograph when compared with healthy control animals. However, in animals with ENU-induced glioma, a significant increase of carbonyl groups content was found in tumor tissue but no changes were observed in plasma [33]. As occurred for lipid peroxidation products, the differences observed between systemic and tissue levels may be due to the fact that not enough time has elapsed for the oxidative changes observed at the cerebral level to be reflected at the peripheral level. Therefore, the possibility that the measurements of parameters related to lipid peroxidation and protein oxidation in serum/plasma could be used as a marker of oxidative stress associated with the carcinogenesis process of brain glioma does not seem enough useful.
2.3. DNA Oxidation
Oxidative damage to nucleic acids has been also found to be associated with carcinogenesis. Guanine is the base that is most prone to oxidation, and 8-hydroxy-2’-deoxyguanosine (from DNA) is the form of oxidized guanine that is most commonly studied. In recent years, it has become increasingly clear that the both DNA and RNA are damaged by oxidation in disease states, and that the repair processes that are initiated to correct this damage release multiple oxidized guanine species, including the ribose-free base (8-oxo-guanine or 8-hydroxyguanine), the nucleoside from RNA (8-oxo-guanosine or 8-hydroxyguanosine), and the deoxynucleoside from DNA (8-oxo-deoxyguanosine or 8-hydroxy-2’-deoxyguanosine). While 8-hydroxy-2’- deoxyguanosine is the form most researchers are familiar with, other published studies have reported that the base (8-hydroxyguanine) is a better marker in some cancer patients [34]. However, little is known about these DNA/RNA biomarkers in glioma cells, animal models or patients. Lian and col [35] using immunohistochemical staining described that the number of 8-hydroxydeoxyguanosine positive cells was higher in high grade glioma samples. These results were in agreement with other previous studies which demonstrated that oxidative DNA damage was high in glioblastoma cell lines and glioblastoma tumor [36-38].
3. GLUTATHIONE AS THE MAIN NON-ENZYMATIC ANTIOXIDANT DEFENCE SYSTEM
The existence of oxidative stress is promoted by the imbalance between free radicals production and antioxidant defense mechanisms. Under physiological conditions, the endogenous antioxidant systems protect the cell against toxic levels of free radicals. At this point, it is important to highlight that the brain is an organ that needs maximal efficient redox-maintaining mechanisms due to its high oxygen consumption and hence a high oxidative metabolism. Glutathione (GSH) is the most abundant intracellular non-enzymatic antioxidant involved in the protection of cells against oxidative damage and in various detoxification mechanisms [39, 40]. During the oxidative stress, its oxidized form (glutathione disulfide, GSSG) may accumulate, leading to deleterious consequences for metabolic regulation, cellular integrity and homeostasis [41].
Therefore, while the decrease in GSH or the GSH/GSSG ratio leads to an increased susceptibility to oxidative stress and to carcinogenesis, elevated GSH levels increase the antioxidant capacity of many cancer cells enhancing their resistance to oxidative stress [23, 42]. Furthermore, many studies demonstrated that high levels of GSH in cells are related to apoptosis resistance [43]. On the contrary, depletion of intracellular GSH levels results in oxidative stress, which is known as an inducer of the transcription of specific genes involved in cell death [44]. This demonstrates the importance of the antioxidants in favoring tumor progression [45]. In the animal model with C6 glioma xenograph, it has been described a significant GSH decrease and GSSG increase in serum when compared with healthy animals. A significant decrease of GSH and increased GSSG content were also observed in brain tissue of rats with ENU-induced glioma and also at a systemic level. The depletion in GSH correlated with increased lipid peroxidation and protein oxidation. Increased GSSG levels correlated with increased H2O2 production by the tumor, as well as to the changes in the activity of the GSH-related antioxidant enzymes [6]. These results are in accordance with those obtained in patients, where a significant depletion of GSH levels in astrocytoma, meningioma, metastatic and other types of brain tumors was also found by Navarro and col [46] when compared with their peritumoral tissues. These results confirm that changes in GSH status in blood and in cancer cells were associated with tumor growth in vivo.
4. ENZYMATIC ANTIOXIDANT DEFENCE SYSTEMS
Several enzyme systems that catalyze reactions to neutralize free radicals constitute the body’s endogenous defense mechanisms. They protect against free radical-induced cell damage. Between them, superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) are the most studied. SOD eliminates superoxide radical (O2-) catalyzing a reaction of dismutation. CAT and GPx are responsible for the disintegration of H2O2 and in this way protect the cell against the formation of the most reactive hydroxyl radical in the Fenton or Haber-Weiss reaction [47]. During prolonged oxidative stress, changes appear in these antioxidant enzymatic activities.
4.1. Superoxide Dismutases
SOD is present in all aerobic living cells probably because O2- is a common product of oxygen metabolic reactions. In mammals, there are three distinctive SODs: the copper/zinc SOD or SOD1, the manganese SOD or SOD2 and the extracellular SOD or SOD3. Recently, many evidences have supported the role of SODs in different aspects of human cancer. Taking into account the different cellular localizations of SODs, the role of each one in cancer may be different. SOD1 is closely linked to cancer. On one hand, loss of SOD1 increases ROS levels which cause oxidative DNA damage and promotes carcinogenesis, and on the other hand, it is also well known that cancer cells have higher ROS content and become increasingly dependent on activated antioxidants such as SOD1 to prevent excessive cellular damage and apoptosis during tumor progression. Although SOD2 has long been considered as a tumor suppressor because early studies showed that SOD2 expression was decreased in tumors [48], recently, it has been reported that this activity shows a considerable heterogeneity, suggesting that SOD2 expression may be stage/tumor type dependent [49, 50]. Finally, the role of SOD3 in cancer is less well understood and probably it effects in cancer are mediated through the tumor microenvironment due to it extracellular localization [51]. In animals with C6 implanted-gliomas it has been described significant increased levels of serum SOD compared with the healthy control group [6]. However, the results described for other authors in human patients show a great heterogeneity; thus, lower levels of SOD have been described in brain tumor cases when compared with controls [52, 53], as well as a proportionate decrease of SOD activity with increasing grades of malignancy in brain tumors [54]. Lower SOD activity in astrocytomas, meningiomas, metastatic tumors and other types of tumors was observed when compared with their peritumoral tissues [31], and also it has been reported that human glioma cells generally have relatively higher SOD activity compared with other tumor types in contrast with the general observation of low SOD activity in tumor cells [55]. This data may be explained by the fact that the brain is well known as an organ with high levels of oxygen consumption. There is a high production of superoxides during normal aerobic metabolism in the brain cells. Thus, relatively high levels of SOD and other antioxidant enzymes are required to remove high levels of free radicals, in order to protect against damage to brain tissues. In any case, most of the studies present a significant reduction in SOD activity in several brain tumors [6]. In fact, it has been also observed lower SOD activity in brain tissue of animals with ENU-induced glioma. This decrease in brain SOD activity may be interpreted from two perspectives. Firstly, the levels of ROS are higher as a consequence of tumor, and the availability of SOD is limited; and secondly, the limited availability of SOD is the responsibility of the higher levels of ROS. However, the study of mRNA expression and protein level of SOD1 did not show changes. These data may indicate that the generation of SOD is not altered but probably its efficacy is reduced and the ROS may be the responsible for this alteration. In any case, although the levels of SOD are important to protect against oxidative damage, a balance of antioxidant enzymes is probably more important, as well as their levels, which may influence intracellular oxidative states.
4.2. Catalase
Catalase is a peroxisomal enzyme that converts hydrogen peroxide into water and oxygen. Inhibition of catalase can result in the increase in ROS and oxidative damage. Catalase shows a protective and anti-apoptotic role in most cases by eliminating ROS [56]. Several studies have investigated the relationship between catalase and cancer but the results obtained have been very variable and even contradictory. In animal models of C6 glioma xenograph, no differences were observed in serum catalase activity between non-tumor healthy control animals and animals with glioma. On the contrary, lower levels of CAT activity were found in brain tissue of animals with ENU-induced glioma. Several other authors have described that catalase activity was significantly higher for different brain tumors [52, 57]. In patients with brain tumors, it has been described that serum catalase activity is higher but no differences have been observed between meningiomas and gliomas [57]. However, gliomas appear to constitutively overexpress catalase when compared with normal astrocytes [58, 59]. These results may suggest that tumor induction blocks the role of catalase in converting H2O2 to H2O and O2 and therefore its antioxidant effect would be compromised, but these result could also indicate that due to the lower SOD activity observed, H2O2 production could be decreased and therefore a lower catalase activity may be necessary to catalyze the reaction from H2O2 to H2O and O2. The study of protein level and mRNA expression showed significant increases of CAT in brain glioma of rats with ENU-induced gliomas. Therefore, although the enzyme production is increased, its effectiveness is diminished as a consequence of tumor process, but it could be also considered as a compensatory mechanism.
4.3. Glutathione Peroxidases
GPx are another group of enzymes capable of reducing hydroperoxides, using GSH as a substrate and generating GSSG which is, once again reduced by the enzyme glutathione reductase (GR) [23]. Therefore, the effective detoxification of peroxides by GPx requires an intact GSH/GSSG system that maintains GSH which is the electron donor in the reaction catalyzed by GPx in excess of GSSG [60, 61]. This GSH/GSSG ratio is furthermore determined by the GR. A correlation between low levels of total GPx and GR activities and high levels of protein oxidation suggestive of oxidative damage has been reported in samples of human glioma tissues by Tanriverdi et al. [62]. On other hand, the presence of an active GPx is an essential element that determines the sensitivity to oxidative stress. A decreased or lack of detectable expression of GPx correlated with a high rate of cell death induced by ROS despite the presence of catalase activity in the cells, indicating their dependency of GPx for free radicals detoxification [61].
In C6 xenograph animal model of glioma, GPx activity was also decreased when compared to the control group. These data also explain the lower antioxidant capacity of the antioxidant system against free radicals, as well as the lower levels of GSH and the higher values of GSSG/GSH index. Other authors have found diminished levels of GPx in brain tumors [63]; and also have described a relation with the histopathology of tumor, showing significant lower GPx activity as the tumor became more malignant [53].
5. THE DELICATE EQUILIBRIUM BETWEEN OXIDANTS AND ANTIOXIDANTS
In general, the increased oxidative damage found in brain gliomas could be the result of the lower levels of antioxidant defenses, which indeed aggravated the oxidative damage increasing the chance of developing cancer in a sequential reaction. Therefore antioxidants´ role in prevention and the role of oxidative injury in the causation of cancer is a self-powered process. Therefore, it seems that the ability of scavenging oxygen free radicals was impaired in glioma tumors because of the lowered levels of antioxidants which predispose towards the progression of cancer.
5.1. Free Radicals as Inductors of Cellular Death: Apoptosis and Autophagy
There are two major distinctive forms of programmed cell death related to cancer: apoptosis and autophagy. Apoptosis is related to a family of proteases called caspases. It is known that defective apoptosis represents a major causative factor in the development and progression of cancer [64]. Autophagy is a caspase-independent mode of cell death in which different cellular components are engulfed by double membrane vesicles, which are then directed to lysosomes for massive degradation [65, 66]. Recently, much attention has been focused in autophagy because it could be both as a cell survival and cell death mechanism and it has been suggested that there is a significant and complex cross-talk between apoptosis and autophagy [67]. In this way, ROS may be a linker between these processes because it is well known that ROS participate in the activation of apoptosis and autophagy [68] and therefore, the knowledge of how ROS can modulate these processes of programmed cell death can be the key to establish possible therapeutic strategies.
As it is known, mitochondria play an important role in different cellular functions such as generation of energy and maintenance of intracellular calcium homeostasis. They are responsible for the formation of ROS and also are implicated in triggering the apoptosis [69, 70]. Therefore, mitochondria may be involved in the carcinogenesis not only by the generation of ROS but also through alteration in cell death pathways [71]. In fact, high levels of ROS take part in the interruption of the cellular programmed death and therefore contribute to the development of tumor cells, but if the levels of ROS are increased over those considered compatible with the survival of the cell, they can exert cytotoxic effects that lead to the death of tumoral cells and therefore limit the cancer progression [70]. All these data have allowed the development of antitumoral therapies called “oxidative therapies” that act at mitochondrial level with the aim to increase the production of ROS, specially H2O2 and O2-, in order to promote the death of the tumor cells by apoptosis although in some cases, the high levels of ROS generated may inhibit apoptosis at caspase level and them the cell death is redirected towards necrosis [72]. Thereby, death by apoptosis is preferred because produces less damage in nearby tissues [70]. On other hand, many evidences have associated higher ROS levels with metastatic capacity in most tumors [70, 73]. Therefore, the drugs used in oxidative therapy may act on the mitochondria and cause an increase of ROS levels and cell death across two possible ways: Increasing the levels of ROS by induction of their generation and secondly by inhibiting the antioxidant system of tumor cells as SOD, catalase and GPx which are the first defenses against ROS [74]. In these two ways, drugs trigger the cell death (apoptosis/necrosis) but also can act sensitizing cancer cells to initial treatment although toxicity and side effects must be considered [2]. Specifically, in glioma, many molecules have been considered to modify ROS production but it is very complicated their therapeutic use considering the different signalling pathways involved. Rinaldi and col. [2] in an interesting review, analyzed several strategies to modify redox status in glioma cells. These new therapeutic agents have general difficulties to cross the blood-brain-barrier and access to the intracranial compartment and also the brain tissues are highly sensitive, so only limited doses can be used. All these limitations may be avoided by the application of nanotechnology.
Autophagy is a multistep process involved in cellular homeostasis by the degradation and recycling of long-lived proteins, intracellular aggregates and damaged organelles. Many evidences support the role of ROS in the regulation of autophagy. In fact, a caspase inhibition induces autophagy by the degradation of catalase and therefore by the accumulation of ROS [75]. The endogenous antioxidant systems may act as regulators of ROS-induced autophagy. Enzymatic (SOD, catalase and GPx) and non-enzymatic (vitamin C and E and glutathione) antioxidant systems reduce ROS levels and autophagy [76, 77]. Autophagy has been related with different pathologies including cancer [78], although its effect is complex and according to the stage of the tumor, the type of cell and tissue it may be positive or negative for the growth of the tumor [79, 80]. These positive and negative effects seem to be directly correlated with different stressors, such as ROS accumulation [81]. In fact, different authors have described that autophagy protects against the production of ROS and therefore inhibits its effects on DNA mutation which is related to the induction of tumorigenesis [82, 83], however, autophagy may be considered as a tumor suppressor by the elimination of damaged mitochondria and therefore preventing ROS accumulation [81, 84], but also may be considered as a tumor suppressor by regulating the chronic inflammation with also leads to the release of some soluble molecules as ROS. Nevertheless autophagy can also induce cancer cells survival during transformation-induced metabolism stress [85]. It has been described that autophagy plays a key role in the survival of cancer cells under hypoxic stress before the neovascularization of the tumor [82]. In fact, many evidences have suggested that autophagy is activated by hypoxia and ROS to promote survival of cancer cells [81]. A pro-metastasis role also has been described for autophagy, moreover, increased autophagy is associated with metastasis and poor prognosis in different human cancer [86]. The stress-induced autophagy in tumor cells can lead to treatment resistance and tumor re-growth [87, 88]. However, the inhibition of autophagy may be considered as a novel therapeutic strategy because tumor cells may be killed and trigger apoptotic cell death [89, 90]. Furthermore, different studies both in vivo and in vitro have shown a greater suppressed tumor growth and cell death using a combination of autophagy inhibitors and chemotherapy than chemotherapy alone [88]. But autophagy has also been referred as a pro-death, especially in apoptosis-defective cells [88, 91]. Therefore, in the treatment of cancer, the knowledge of the cellular characteristics in each tumor is absolutely necessary to regulate the three key factors: ROS-level, autophagy and apoptosis [25, 81].
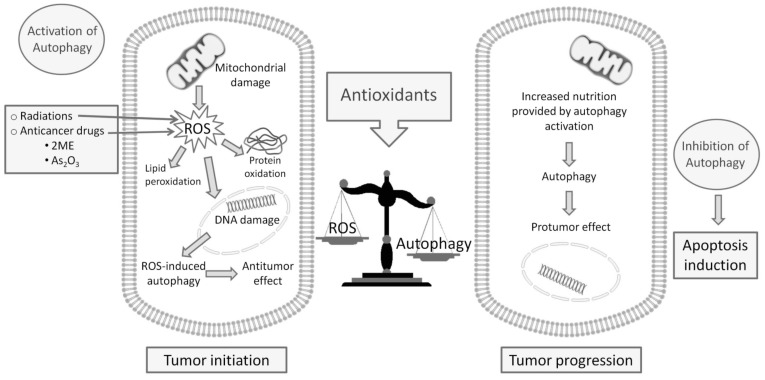
Some data have suggested that autophagy may be involved in high-grade glioma prognosis and response to therapy [92-94]. It has been demonstrated that autophagy was involved in the modulation of viability and survival effects in human glioma cell lines after ionizing radiation combined or not with chemotherapy drug [95]. The mechanisms underlying this neoplastic glial cell growth inhibition are unknown, however, in vivo and in vitro studies have identified autophagy as the main mechanism of non-apoptotic cellular death after ionizing radiation alone or combined with chemotherapy drugs [94, 96]. On the other hand, it has been described that gliomas are mostly resistant to apoptotic cell death, but are less resistant to therapies related with autophagy [97, 98]. In fact, while apoptosis has not been detected after ionizing radiation in different glioma cell lines, autophagy has been described. Therefore, drugs involved in regulation of autophagy may be attractive as novel anti-cancer therapies close to the habitual radiotherapy treatments. However, the results showed by different studies are heterogeneous, in part due to the double role of autophagy as tumor-suppressor and tumor-inductor, so many authors support the use of inhibitors of autophagy [99-101] but other consider the possible therapeutic effects of drugs that stimulate the cellular death of autophagy [66, 102, 103]. In fact, pharmacologically, mitochondrial-targeted drugs can promote autophagy through stimulation of bioenergetic stress, increased ROS formation, AMPk activation and mTOR inhibition [104]. Several cancer treatments used cause cellular damage through the generation of ROS and the induction of autophagy, i.e. ionizing radiations and some radiosensitizing agents [105]. Many conventional anticancer drugs as 2-ME and As2O3 induced oxidative stress leading to autophagic cell death [106-109]. The balance between ROS level and ROS-induced autophagy may be critical for tumor progression or regression. Many authors have been reported that antioxidants may be linked to the cytoprotective or cytotoxic mechanism of autophagy [110] (Fig. 1). In any case, it is necessary to know the specific type of cellular death (apoptosis/autophagy) in each tumor type and also in every patient to design the most specific and effective treatment. In this context, the ROS and antioxidant levels are involved, so the knowledge of tumor redox microenvironment may be essential in the development of future new pharmacological strategies.
Fig. (1).
The double role of autophagy as tumor-suppressor and tumor-inductor and the treatment strategies exploit ROS and autophagy.
CONCLUSION
Glioma has been associated with the unbalance between the production of free radicals and antioxidant mechanisms mediated by several enzyme and non- enzyme defense systems, which are modified in different degree and could, additionally, be used as biomarkers. However, free radicals play a key role in apoptosis and autophagy, the two forms of programmed cell death related to cancer. Both phenomena may be considered, depending on their particular conditions, as inductors or inhibitors of the tumoral process. Therefore, the use of substances that modulate the production and/or the effects of free radicals (antioxidants and pro-oxidants) may be considered a new therapeutic strategy in the treatment of gliomas. In any case, it is necessary to know the specific characteristics of cellular death for each tumor type in every patient to design the most specific and effective treatment. The knowledge of the tumor redox microenvironment may be essential in the development of future new pharmaceutical strategies.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
This study was supported by Junta de Andalucía (PAIDI group BIO296 (Currently CTS1039), Consejería de Innovación, Ciencia y Empresa through Proyecto de Excelencia Motriz (grant CVI2009-4957M) and University of Jaén through Proyectos de Fortalecimiento.
REFERENCES
- 1.Caruso G., Caffo M. Antisense oligonucleotides in the treatment of cerebral gliomas. Review of concerning patents. Recent Patents CNS Drug Discov. 2014;9(1):2–12. doi: 10.2174/1574889809666140307113439. [http://dx.doi.org/10.2174/1574889809666140307113439]. [PMID: 24605941]. [DOI] [PubMed] [Google Scholar]
- 2.Rinaldi M., Caffo M., Minutoli L., Marini H., Abbritti R.V., Squadrito F., Trichilo V., Valenti A., Barresi V., Altavilla D., Passalacqua M., Caruso G. ROS and brain gliomas: An overview of potential and innovative therapeutic strategies. Int. J. Mol. Sci. 2016;17(6):E984. doi: 10.3390/ijms17060984. [http://dx.doi.org/10.3390/ijms17060984]. [PMID: 27338365]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondy M.L., Scheurer M.E., Malmer B., Barnholtz-Sloan J.S., Davis F.G., Il’yasova D., Kruchko C., McCarthy B.J., Rajaraman P., Schwartzbaum J.A., Sadetzki S., Schlehofer B., Tihan T., Wiemels J.L., Wrensch M., Buffler P.A. Brain tumor epidemiology, C. brain tumor epidemiology: Consensus from the brain tumor epidemiology consortium. Cancer. 2008;113(7) Suppl.:1953–1968. doi: 10.1002/cncr.23741. [http://dx.doi.org/10.1002/cncr.23741]. [PMID: 18798534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis F.G., Malmer B.S., Aldape K., Barnholtz-Sloan J.S., Bondy M.L., Brännström T., Bruner J.M., Burger P.C., Collins V.P., Inskip P.D., Kruchko C., McCarthy B.J., McLendon R.E., Sadetzki S., Tihan T., Wrensch M.R., Buffler P.A. Issues of diagnostic review in brain tumor studies: From the Brain Tumor Epidemiology Consortium. Cancer Epidemiol. Biomarkers Prev. 2008;17(3):484–489. doi: 10.1158/1055-9965.EPI-07-0725. [http://dx.doi.org/10.1158/1055-9965.EPI-07-0725]. [PMID: 18349266]. [DOI] [PubMed] [Google Scholar]
- 5.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [http://dx.doi.org/10.1007/s00401-007-0243-4]. [PMID: 17618441]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illán-Cabeza N.A., García-García A.R., Martínez-Martos J.M., Ramírez-Expósito M.J., Peña-Ruiz T., Moreno-Carretero M.N. A potential antitumor agent, (6-amino-1-methyl-5-nitrosouracilato-N3)-triphenylphosphine-gold(I): Structural studies and in vivo biological effects against experimental glioma. Eur. J. Med. Chem. 2013;64:260–272. doi: 10.1016/j.ejmech.2013.03.067. [http://dx.doi.org/10.1016/j.ejmech.2013.03.067]. [PMID: 23644209]. [DOI] [PubMed] [Google Scholar]
- 7.Deng Z., Hu J., Liu S. Reactive oxygen, nitrogen, and sulfur species (RONSS)-responsive polymersomes for triggered drug release. Macromol. Rapid Commun. 2017;38(11) doi: 10.1002/marc.201600685. [http://dx.doi.org/10.1002/marc.201600685]. [PMID: 28240442]. [DOI] [PubMed] [Google Scholar]
- 8.Wu C.C., Bratton S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013;19(6):546–558. doi: 10.1089/ars.2012.4905. [http://dx.doi.org/10.1089/ars.2012.4905]. [PMID: 22978471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosa V., Moliné T., Somoza R., Paciucci R., Kondoh H. LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013;12(1):376–390. doi: 10.1016/j.arr.2012.10.004. [http://dx.doi.org/10.1016/j.arr.2012.10.004]. [PMID: 23123177]. [DOI] [PubMed] [Google Scholar]
- 10.Hung Y.C., Pan T.L., Hu W.L. Roles of reactive oxygen species in anticancer therapy with Salvia miltiorrhiza Bunge. Oxid. Med. Cell. Longev. 2016;2016:5293284. doi: 10.1155/2016/5293284. [http://dx.doi.org/10.1155/2016/5293284]. [PMID: 27579153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Karakhanova S., Hartwig W., D’Haese J.G., Philippov P.P., Werner J., Bazhin A.V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell. Physiol. 2016;231(12):2570–2581. doi: 10.1002/jcp.25349. [http://dx.doi.org/10.1002/jcp.25349]. [PMID: 26895995]. [DOI] [PubMed] [Google Scholar]
- 12.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014;14(11):709–721. doi: 10.1038/nrc3803. [http://dx.doi.org/10.1038/nrc3803]. [PMID: 25342630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. 2014;34(4):1467–1482. [PMID: 24692674]. [PubMed] [Google Scholar]
- 14.Martín V., Herrera F., García-Santos G., Antolín I., Rodriguez-Blanco J., Rodriguez C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic. Biol. Med. 2007;42(11):1715–1722. doi: 10.1016/j.freeradbiomed.2007.02.028. [http://dx.doi.org/10.1016/j.freeradbiomed.2007.02.028]. [PMID: 17462539]. [DOI] [PubMed] [Google Scholar]
- 15.Anderson K.N., Bejcek B.E. Parthenolide induces apoptosis in glioblastomas without affecting NF-kappaB. J. Pharmacol. Sci. 2008;106(2):318–320. doi: 10.1254/jphs.sc0060164. [http://dx.doi.org/10.1254/jphs.SC0060164]. [PMID: 18277052]. [DOI] [PubMed] [Google Scholar]
- 16.Jackson C., Ruzevick J., Amin A.G., Lim M. Potential role for STAT3 inhibitors in glioblastoma. Neurosurg. Clin. N. Am. 2012;23(3):379–389. doi: 10.1016/j.nec.2012.04.002. [http://dx.doi.org/10.1016/j.nec.2012.04.002]. [PMID: 22748651]. [DOI] [PubMed] [Google Scholar]
- 17.Yu M.O., Park K.J., Park D.H., Chung Y.G., Chi S.G., Kang S.H. Reactive oxygen species production has a critical role in hypoxia-induced Stat3 activation and angiogenesis in human glioblastoma. J. Neurooncol. 2015;125(1):55–63. doi: 10.1007/s11060-015-1889-8. [http://dx.doi.org/10.1007/s11060-015-1889-8]. [PMID: 26297045]. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh C.H., Lee C.H., Liang J.A., Yu C.Y., Shyu W.C. Cycling hypoxia increases U87 glioma cell radioresistance via ROS induced higher and long-term HIF-1 signal transduction activity. Oncol. Rep. 2010;24(6):1629–1636. doi: 10.3892/or_00001027. [http://dx.doi.org/10.3892/or_00001027]. [PMID: 21042761]. [DOI] [PubMed] [Google Scholar]
- 19.Wang P., Wan W., Xiong S., Wang J., Zou D., Lan C., Yu S., Liao B., Feng H., Wu N. HIF1α regulates glioma chemosensitivity through the transformation between differentiation and dedifferentiation in various oxygen levels. Sci. Rep. 2017;7(1):7965. doi: 10.1038/s41598-017-06086-2. [http://dx.doi.org/10.1038/s41598-017-06086-2]. [PMID: 28801626]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad F., Ghosh S., Sinha S., Joshi S.D., Mehta V.S., Sen E. TGF-β-induced hCG-β regulates redox homeostasis in glioma cells. Mol. Cell. Biochem. 2015;399(1-2):105–112. doi: 10.1007/s11010-014-2237-6. [http://dx.doi.org/10.1007/s11010-014-2237-6]. [PMID: 25300619]. [DOI] [PubMed] [Google Scholar]
- 21.Park E.J., Park K. Induction of oxidative stress and inflammatory cytokines by manganese chloride in cultured T98G cells, human brain glioblastoma cell line. Toxicol. In Vitro. 2010;24(2):472–479. doi: 10.1016/j.tiv.2009.09.022. [http://dx.doi.org/10.1016/j.tiv.2009.09.022]. [PMID: 19815061]. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q., Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets. 2015;16(1):13–19. doi: 10.2174/1389450116666150113121054. [http://dx.doi.org/10.2174/1389450116666150113121054]. [PMID: 25585126]. [DOI] [PubMed] [Google Scholar]
- 23.Marengo B., Nitti M., Furfaro A.L., Colla R., Ciucis C.D., Marinari U.M., Pronzato M.A., Traverso N., Domenicotti C. Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev. 2016;2016:6235641. doi: 10.1155/2016/6235641. [http://dx.doi.org/10.1155/2016/6235641]. [PMID: 27418953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewaele M., Maes H., Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Cancer Metastasis Rev. 2006;25:669–705. doi: 10.4161/auto.6.7.12113. [PMID: 17160556]. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Karakhanova S., Werner J., Bazhin A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013;20(30):3677–3692. doi: 10.2174/0929867311320999165. [http://dx.doi.org/10.2174/0929867311320999165]. [PMID: 23862622]. [DOI] [PubMed] [Google Scholar]
- 26.Carrera M.P., Ramírez-Expósito M.J., Martínez-Martos J.M. Actual and potential agents and biomarkers in the treatment of cancer. Anticancer. Agents Med. Chem. 2009;9(5):500–516. doi: 10.2174/187152009788451824. [http://dx.doi.org/10.2174/187152009788451824]. [PMID: 19519292]. [DOI] [PubMed] [Google Scholar]
- 27.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [http://dx.doi.org/10.1002/ijc.23192]. [PMID: 17893868]. [DOI] [PubMed] [Google Scholar]
- 28.Nair U., Bartsch H., Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic. Biol. Med. 2007;43(8):1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [http://dx.doi.org/10.1016/j.freeradbiomed.2007.07.012]. [PMID: 17854706]. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Chen S.Y., Hsu T., Santella R.M. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis. 2002;23(1):207–211. doi: 10.1093/carcin/23.1.207. [http://dx.doi.org/10.1093/carcin/23.1.207]. [PMID: 11756243]. [DOI] [PubMed] [Google Scholar]
- 30.M.J. Mayas, M.D.; Carrera, M.P.; Cobo, M.P.; García, M.J.; martínez-Martos, J.M. Oxidative stress parameters in rat with gliomas induced by transplacental N-ethyl-N-nitrosourea exposure. Eur. J. Neurol. 2012;19:772–772. [Google Scholar]
- 31.Zengin E., Atukeren P., Kokoglu E., Gumustas M.K., Zengin U. Alterations in lipid peroxidation and antioxidant status in different types of intracranial tumors within their relative peritumoral tissues. Clin. Neurol. Neurosurg. 2009;111(4):345–351. doi: 10.1016/j.clineuro.2008.11.008. [http://dx.doi.org/10.1016/j.clineuro.2008.11.008]. [PMID: 19117666]. [DOI] [PubMed] [Google Scholar]
- 32.Cirak B., Inci S., Palaoglu S., Bertan V. Lipid peroxidation in cerebral tumors. Clin. Chim. Acta. 2003;327(1-2):103–107. doi: 10.1016/s0009-8981(02)00334-0. [http://dx.doi.org/10.1016/S0009-8981(02)00334-0]. [PMID: 12482624]. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez-Exposito M.J., Carrera M.P., Mayas M.D., Martínez-Martos J.M. Redox status in transplacental ethyl-nitrosourea-induced experimental glioma.; 9th FENS Forum of Neuroscience; Milan (Italy). 2014. p. 423. [Google Scholar]
- 34.Roszkowski K., Olinski R. Urinary 8-oxoguanine as a predictor of survival in patients undergoing radiotherapy. Cancer Epidemiol. Biomarkers Prev. 2012;21(4):629–634. doi: 10.1158/1055-9965.EPI-11-0981. [http://dx.doi.org/10.1158/1055-9965.EPI-11-0981]. [PMID: 22301827]. [DOI] [PubMed] [Google Scholar]
- 35.Lian M., Zhang X., Wang H., Liu H., Chen W., Guo S. Increased 8-hydroxydeoxyguanosine in high-grade gliomas is associated with activation of autophagy. Int. J. Neurosci. 2014;124(12):926–934. doi: 10.3109/00207454.2014.891998. [http://dx.doi.org/10.3109/00207454.2014.891998]. [PMID: 24617962]. [DOI] [PubMed] [Google Scholar]
- 36.Chang S.M., Parney I.F., Huang W., Anderson F.A., Jr, Asher A.L., Bernstein M., Lillehei K.O., Brem H., Berger M.S., Laws E.R. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. doi: 10.1001/jama.293.5.557. [http://dx.doi.org/10.1001/jama.293.5.557]. [PMID: 15687310]. [DOI] [PubMed] [Google Scholar]
- 37.Tuzgen S., Hanimoglu H., Tanriverdi T., Kacira T., Sanus G.Z., Atukeren P., Dashti R., Gumustas K., Canbaz B., Kaynar M.Y. Relationship between DNA damage and total antioxidant capacity in patients with glioblastoma multiforme. Clin. Oncol. (R. Coll. Radiol.) 2007;19(3):177–181. doi: 10.1016/j.clon.2006.11.012. [http://dx.doi.org/10.1016/j.clon.2006.11.012]. [PMID: 17359903]. [DOI] [PubMed] [Google Scholar]
- 38.lida, T.; A., F.; Kawashima, M. Accumulation of 8-oxo-2´-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro-oncol. 2011;3:73–81. doi: 10.1093/neuonc/3.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang J.I., Chang T.Y., Liu H.S. Glutathione depletion-induced apoptosis of Ha-ras-transformed NIH3T3 cells can be prevented by melatonin. Oncogene. 2003;22(9):1349–1357. doi: 10.1038/sj.onc.1206289. [http://dx.doi.org/10.1038/sj.onc.1206289]. [PMID: 12618760]. [DOI] [PubMed] [Google Scholar]
- 40.Guha P., Dey A., Sen R., Chatterjee M., Chattopadhyay S., Bandyopadhyay S.K. Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J. Pharmacol. Exp. Ther. 2011;336(1):206–214. doi: 10.1124/jpet.110.171983. [http://dx.doi.org/10.1124/jpet.110.171983]. [PMID: 20876229]. [DOI] [PubMed] [Google Scholar]
- 41.Salazar-Ramiro A., Ramírez-Ortega D., Pérez de la Cruz V., Hérnandez-Pedro N.Y., González-Esquivel D.F., Sotelo J., Pineda B. Role of redox status in development of glioblastoma. Front. Immunol. 2016;7:156. doi: 10.3389/fimmu.2016.00156. [http://dx.doi.org/10.3389/fimmu.2016.00156]. [PMID: 27199982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traverso N., Ricciarelli R., Nitti M., Marengo B., Furfaro A.L., Pronzato M.A., Marinari U.M., Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013;2013:972913. doi: 10.1155/2013/972913. [http://dx.doi.org/10.1155/2013/972913]. [PMID: 23766865]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [http://dx.doi.org/10.1038/nrc706]. [PMID: 11902585]. [DOI] [PubMed] [Google Scholar]
- 44.Kretz-Remy C., Arrigo A.P. Gene expression and thiol redox state. Methods Enzymol. 2002;348:200–215. doi: 10.1016/s0076-6879(02)48639-9. [http://dx.doi.org/10.1016/S0076-6879(02)48639-9]. [PMID: 11885273]. [DOI] [PubMed] [Google Scholar]
- 45.Harris I.S., Treloar A.E., Inoue S., Sasaki M., Gorrini C., Lee K.C., Yung K.Y., Brenner D., Knobbe-Thomsen C.B., Cox M.A., Elia A., Berger T., Cescon D.W., Adeoye A., Brüstle A., Molyneux S.D., Mason J.M., Li W.Y., Yamamoto K., Wakeham A., Berman H.K., Khokha R., Done S.J., Kavanagh T.J., Lam C.W., Mak T.W. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27(2):211–222. doi: 10.1016/j.ccell.2014.11.019. [http://dx.doi.org/10.1016/j.ccell.2014.11.019]. [PMID: 25620030]. [DOI] [PubMed] [Google Scholar]
- 46.Navarro J., Obrador E., Carretero J., Petschen I., Aviñó J., Perez P., Estrela J.M. Changes in glutathione status and the antioxidant system in blood and in cancer cells associate with tumour growth in vivo. Free Radic. Biol. Med. 1999;26(3-4):410–418. doi: 10.1016/s0891-5849(98)00213-5. [http://dx.doi.org/10.1016/S0891-5849(98)00213-5]. [PMID: 9895233]. [DOI] [PubMed] [Google Scholar]
- 47.Woźniak B., Woźniak A., Kasprzak H.A., Drewa G., Mila-Kierzenkowska C., Drewa T., Planutis G. Lipid peroxidation and activity of some antioxidant enzymes in patients with glioblastoma and astrocytoma. J. Neurooncol. 2007;81(1):21–26. doi: 10.1007/s11060-006-9202-5. [http://dx.doi.org/10.1007/s11060-006-9202-5]. [PMID: 16773213]. [DOI] [PubMed] [Google Scholar]
- 48.Oberley L.W., Buettner G.R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39(4):1141–1149. [PMID: 217531]. [PubMed] [Google Scholar]
- 49.Hempel N., Carrico P.M., Melendez J.A. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer. Agents Med. Chem. 2011;11(2):191–201. doi: 10.2174/187152011795255911. [http://dx.doi.org/10.2174/187152011795255911]. [PMID: 21434856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhar S.K., St Clair D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012;52(11-12):2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [http://dx.doi.org/10.1016/j.freeradbiomed.2012.03.009]. [PMID: 22561706]. [DOI] [PubMed] [Google Scholar]
- 51.Che M., Wang R., Li X., Wang H.Y., Zheng X.F.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today. 2016;21(1):143–149. doi: 10.1016/j.drudis.2015.10.001. [http://dx.doi.org/10.1016/j.drudis.2015.10.001]. [PMID: 26475962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popov B., Gadjeva V., Valkanov P., Popova S., Tolekova A. Lipid peroxidation, superoxide dismutase and catalase activities in brain tumor tissues. Arch. Physiol. Biochem. 2003;111(5):455–459. doi: 10.3109/13813450312331342328. [http://dx.doi.org/10.3109/13813450312331342328]. [PMID: 16026034]. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal S., Subberwal M., Kumar S., Sharma M. Brain tumor and role of beta-carotene, a-tocopherol, superoxide dismutase and glutathione peroxidase. J. Cancer Res. Ther. 2006;2(1):24–27. doi: 10.4103/0973-1482.19771. [http://dx.doi.org/10.4103/0973-1482.19771]. [PMID: 17998669]. [DOI] [PubMed] [Google Scholar]
- 54.Gönenç A., Ozkan Y., Torun M., Simşek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001;26(2):141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [http://dx.doi.org/10.1046/j.1365-2710.2001.00334.x]. [PMID: 11350537]. [DOI] [PubMed] [Google Scholar]
- 55.Del Maestro R.F., McDonald W.R. A. Oxy radicals and their scavenger systems. 1983;2:28–35. [Google Scholar]
- 56.Jeong C.H., Joo S.H. Downregulation of reactive oxygen species in apoptosis. J. Cancer Prev. 2016;21(1):13–20. doi: 10.15430/JCP.2016.21.1.13. [http://dx.doi.org/10.15430/JCP.2016.21.1.13]. [PMID: 27051644]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yilmaz N., Dulger H., Kiymaz N., Yilmaz C., Bayram I., Ragip B., Oğer M. Lipid peroxidation in patients with brain tumor. Int. J. Neurosci. 2006;116(8):937–943. doi: 10.1080/00207450600553141. [http://dx.doi.org/10.1080/00207450600553141]. [PMID: 16861159]. [DOI] [PubMed] [Google Scholar]
- 58.Preuss M., Girnun G.D., Darby C.J., Khoo N., Spector A.A., Robbins M.E. Role of antioxidant enzyme expression in the selective cytotoxic response of glioma cells to gamma-linolenic acid supplementation. Free Radic. Biol. Med. 2000;28(7):1143–1156. doi: 10.1016/s0891-5849(00)00210-0. [http://dx.doi.org/10.1016/S0891-5849(00)00210-0]. [PMID: 10832077]. [DOI] [PubMed] [Google Scholar]
- 59.Smith P.S., Zhao W., Spitz D.R., Robbins M.E. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radic. Biol. Med. 2007;42(6):787–797. doi: 10.1016/j.freeradbiomed.2006.11.032. [http://dx.doi.org/10.1016/j.freeradbiomed.2006.11.032]. [PMID: 17320761]. [DOI] [PubMed] [Google Scholar]
- 60.Hirrlinger J., Dringen R. The cytosolic redox state of astrocytes: Maintenance, regulation and functional implications for metabolite trafficking. Brain Res. Brain Res. Rev. 2010;63(1-2):177–188. doi: 10.1016/j.brainresrev.2009.10.003. [http://dx.doi.org/10.1016/j.brainresrev.2009.10.003]. [PMID: 19883686]. [DOI] [PubMed] [Google Scholar]
- 61.Dokic I., Hartmann C., Herold-Mende C., Régnier-Vigouroux A. Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress. Glia. 2012;60(11):1785–1800. doi: 10.1002/glia.22397. [http://dx.doi.org/10.1002/glia.22397]. [PMID: 22951908]. [DOI] [PubMed] [Google Scholar]
- 62.Tanriverdi T., Hanimoglu H., Kacira T., Sanus G.Z., Kemerdere R., Atukeren P., Gumustas K., Canbaz B., Kaynar M.Y. Glutathione peroxidase, glutathione reductase and protein oxidation in patients with glioblastoma multiforme and transitional meningioma. J. Cancer Res. Clin. Oncol. 2007;133(9):627–633. doi: 10.1007/s00432-007-0212-2. [http://dx.doi.org/10.1007/s00432-007-0212-2]. [PMID: 17457608]. [DOI] [PubMed] [Google Scholar]
- 63.Rao G.M., Rao A.V., Raja A., Rao S., Rao A. Role of antioxidant enzymes in brain tumours. Clin. Chim. Acta. 2000;296(1-2):203–212. doi: 10.1016/s0009-8981(00)00219-9. [http://dx.doi.org/10.1016/S0009-8981(00)00219-9]. [PMID: 10807983]. [DOI] [PubMed] [Google Scholar]
- 64.Kasibhatla S., Tseng B. Why target apoptosis in cancer treatment? Mol. Cancer Ther. 2003;2(6):573–580. [PMID: 12813137]. [PubMed] [Google Scholar]
- 65.Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [http://dx.doi.org/10.1016/S1534-5807(04)00099-1]. [PMID: 15068787]. [DOI] [PubMed] [Google Scholar]
- 66.Pallichankandy S., Rahman A., Thayyullathil F., Galadari S. ROS-dependent activation of autophagy is a critical mechanism for the induction of anti-glioma effect of sanguinarine. Free Radic. Biol. Med. 2015;89:708–720. doi: 10.1016/j.freeradbiomed.2015.10.404. [http://dx.doi.org/10.1016/j.freeradbiomed.2015.10.404]. [PMID: 26472194]. [DOI] [PubMed] [Google Scholar]
- 67.Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., Deszcz L., Hartl R., Schraml E., Criollo A., Megalou E., Weiskopf D., Laun P., Heeren G., Breitenbach M., Grubeck-Loebenstein B., Herker E., Fahrenkrog B., Fröhlich K.U., Sinner F., Tavernarakis N., Minois N., Kroemer G., Madeo F. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [http://dx.doi.org/10.1038/ncb1975]. [PMID: 19801973]. [DOI] [PubMed] [Google Scholar]
- 68.Kaminskyy V.O., Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014;21(1):86–102. doi: 10.1089/ars.2013.5746. [http://dx.doi.org/10.1089/ars.2013.5746]. [PMID: 24359220]. [DOI] [PubMed] [Google Scholar]
- 69.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [http://dx.doi.org/10.1113/jphysiol.2003.049478]. [PMID: 14561818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Miguel M., Cordero M.D. Oxidative therapy against cancer, oxidative stress and diseases.In Teach. 2012 [Google Scholar]
- 71.Pilkington G.J., Parker K., Murray S.A. Approaches to mitochondrially mediated cancer therapy. Semin. Cancer Biol. 2008;18(3):226–235. doi: 10.1016/j.semcancer.2007.12.006. [http://dx.doi.org/10.1016/j.semcancer.2007.12.006]. [PMID: 18203619]. [DOI] [PubMed] [Google Scholar]
- 72.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29(3-4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [http://dx.doi.org/10.1016/S0891-5849(00)00302-6]. [PMID: 11035261]. [DOI] [PubMed] [Google Scholar]
- 73.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320(5876):661–664. doi: 10.1126/science.1156906. [http://dx.doi.org/10.1126/science.1156906]. [PMID: 18388260]. [DOI] [PubMed] [Google Scholar]
- 74.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [http://dx.doi.org/10.1038/nrd2803]. [PMID: 19478820]. [DOI] [PubMed] [Google Scholar]
- 75.Yu L., Wan F., Dutta S., Welsh S., Liu Z., Freundt E., Baehrecke E.H., Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. USA. 2006;103(13):4952–4957. doi: 10.1073/pnas.0511288103. [http://dx.doi.org/10.1073/pnas.0511288103]. [PMID: 16547133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trachootham D., Lu W., Ogasawara M.A., Nilsa R.D., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [http://dx.doi.org/10.1089/ars.2007.1957]. [PMID: 18522489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36(1):30–38. doi: 10.1016/j.tibs.2010.07.007. [http://dx.doi.org/10.1016/j.tibs.2010.07.007]. [PMID: 20728362]. [DOI] [PubMed] [Google Scholar]
- 78.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368(19):1845–1846. doi: 10.1056/NEJMc1303158. [http://dx.doi.org/10.1056/NEJMc1303158]. [PMID: 23656658]. [DOI] [PubMed] [Google Scholar]
- 79.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer. 2012;12(6):401–410. doi: 10.1038/nrc3262. [http://dx.doi.org/10.1038/nrc3262]. [PMID: 22534666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenific C.M., Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015;25(1):37–45. doi: 10.1016/j.tcb.2014.09.001. [http://dx.doi.org/10.1016/j.tcb.2014.09.001]. [PMID: 25278333]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poillet-Perez L., Despouy G., Delage-Mourroux R., Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–192. doi: 10.1016/j.redox.2014.12.003. [http://dx.doi.org/10.1016/j.redox.2014.12.003]. [PMID: 25590798]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morselli E., Galluzzi L., Kepp O., Vicencio J.M., Criollo A., Maiuri M.C., Kroemer G. Anti- and pro-tumor functions of autophagy. Biochim. Biophys. Acta. 2009;1793(9):1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [http://dx.doi.org/10.1016/j.bbamcr.2009.01.006]. [PMID: 19371598]. [DOI] [PubMed] [Google Scholar]
- 83.Debnath J. The multifaceted roles of autophagy in tumors-implications for breast cancer. J. Mammary Gland Biol. Neoplasia. 2011;16(3):173–187. doi: 10.1007/s10911-011-9223-3. [http://dx.doi.org/10.1007/s10911-011-9223-3]. [PMID: 21779879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morselli E., Galluzzi L., Kepp O., Mariño G., Michaud M., Vitale I., Maiuri M.C., Kroemer G. Oncosuppressive functions of autophagy. Antioxid. Redox Signal. 2011;14(11):2251–2269. doi: 10.1089/ars.2010.3478. [http://dx.doi.org/10.1089/ars.2010.3478]. [PMID: 20712403]. [DOI] [PubMed] [Google Scholar]
- 85.Brahimi-Horn M.C., Bellot G., Pouysségur J. Hypoxia and energetic tumour metabolism. Curr. Opin. Genet. Dev. 2011;21(1):67–72. doi: 10.1016/j.gde.2010.10.006. [http://dx.doi.org/10.1016/j.gde.2010.10.006]. [PMID: 21074987]. [DOI] [PubMed] [Google Scholar]
- 86.Lazova R., Camp R.L., Klump V., Siddiqui S.F., Amaravadi R.K., Pawelek J.M. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin. Cancer Res. 2012;18(2):370–379. doi: 10.1158/1078-0432.CCR-11-1282. [http://dx.doi.org/10.1158/1078-0432.CCR-11-1282]. [PMID: 22080440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu Z., Luo R.Z., Lu Y., Zhang X., Yu Q., Khare S., Kondo S., Kondo Y., Yu Y., Mills G.B., Liao W.S., Bast R.C., Jr The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Invest. 2008;118(12):3917–3929. doi: 10.1172/JCI35512. [PMID: 19033662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 2011;10(9):1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [http://dx.doi.org/10.1158/1535-7163.MCT-11-0047]. [PMID: 21878654]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y., Nelson D.A., Jin S., White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [http://dx.doi.org/10.1016/j.ccr.2006.06.001]. [PMID: 16843265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White E., DiPaola R.S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009;15(17):5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [http://dx.doi.org/10.1158/1078-0432.CCR-07-5023]. [PMID: 19706824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239. [http://dx.doi.org/10.1038/nrm2239]. [PMID: 17717517]. [DOI] [PubMed] [Google Scholar]
- 92.Miracco C., Cosci E., Oliveri G., Luzi P., Pacenti L., Monciatti I., Mannucci S., De Nisi M.C., Toscano M., Malagnino V., Falzarano S.M., Pirtoli L., Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int. J. Oncol. 2007;30(2):429–436. [PMID: 17203225]. [PubMed] [Google Scholar]
- 93.Pirtoli L., Cevenini G., Tini P., Vannini M., Oliveri G., Marsili S., Mourmouras V., Rubino G., Miracco C. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy. 2009;5(7):930–936. doi: 10.4161/auto.5.7.9227. [http://dx.doi.org/10.4161/auto.5.7.9227]. [PMID: 19556884]. [DOI] [PubMed] [Google Scholar]
- 94.Palumbo S., Comincini S. Autophagy and ionizing radiation in tumors: the “survive or not survive” dilemma. J. Cell. Physiol. 2013;228(1):1–8. doi: 10.1002/jcp.24118. [http://dx.doi.org/10.1002/jcp.24118]. [PMID: 22585676]. [DOI] [PubMed] [Google Scholar]
- 95.Palumbo S., Pirtoli L., Tini P., Cevenini G., Calderaro F., Toscano M., Miracco C., Comincini S. Different involvement of autophagy in human malignant glioma cell lines undergoing irradiation and temozolomide combined treatments. J. Cell. Biochem. 2012;113(7):2308–2318. doi: 10.1002/jcb.24102. [http://dx.doi.org/10.1002/jcb.24102]. [PMID: 22345070]. [DOI] [PubMed] [Google Scholar]
- 96.Zhuang W., Qin Z., Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim. Biophys. Sin. (Shanghai) 2009;41(5):341–351. doi: 10.1093/abbs/gmp028. [http://dx.doi.org/10.1093/abbs/gmp028]. [PMID: 19430698]. [DOI] [PubMed] [Google Scholar]
- 97.Zhuang W., Li B., Long L., Chen L., Huang Q., Liang Z. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int. J. Cancer. 2011;129(11):2720–2731. doi: 10.1002/ijc.25975. [http://dx.doi.org/10.1002/ijc.25975]. [PMID: 21384342]. [DOI] [PubMed] [Google Scholar]
- 98.Kaza N., Kohli L., Roth K.A. Autophagy in brain tumors: a new target for therapeutic intervention. Brain Pathol. 2012;22(1):89–98. doi: 10.1111/j.1750-3639.2011.00544.x. [http://dx.doi.org/10.1111/j.1750-3639.2011.00544.x]. [PMID: 22150924]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Isakovic A.M., Dulovic M., Markovic I., Kravic-Stevovic T., Bumbasirevic V., Trajkovic V., Isakovic A. Autophagy suppression sensitizes glioma cells to IMP dehydrogenase inhibition-induced apoptotic death. Exp. Cell Res. 2017;350(1):32–40. doi: 10.1016/j.yexcr.2016.11.001. [http://dx.doi.org/10.1016/j.yexcr.2016.11.001]. [PMID: 27818246]. [DOI] [PubMed] [Google Scholar]
- 100.Gammoh N., Fraser J., Puente C., Syred H.M., Kang H., Ozawa T., Lam D., Acosta J.C., Finch A.J., Holland E., Jiang X. Suppression of autophagy impedes glioblastoma development and induces senescence. Autophagy. 2016;12(9):1431–1439. doi: 10.1080/15548627.2016.1190053. [http://dx.doi.org/10.1080/15548627.2016.1190053]. [PMID: 27304681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bi Y., Shen C., Li C., Liu Y., Gao D., Shi C., Peng F., Liu Z., Zhao B., Zheng Z., Wang X., Hou X., Liu H., Wu J., Zou H., Wang K., Zhong C., Zhang J., Shi C., Zhao S. Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumour Biol. 2016;37(3):3549–3560. doi: 10.1007/s13277-015-4125-4. [http://dx.doi.org/10.1007/s13277-015-4125-4]. [PMID: 26454746]. [DOI] [PubMed] [Google Scholar]
- 102.Li C., Liu Y., Liu H., Zhang W., Shen C., Cho K., Chen X., Peng F., Bi Y., Hou X., Yang Z., Zheng Z., Wang K., Wang X., Zhang J., Zhong C., Zou H., Zhang X., Zhao S. Impact of autophagy inhibition at different stages on cytotoxic effect of autophagy inducer in glioblastoma cells. Cell. Physiol. Biochem. 2015;35(4):1303–1316. doi: 10.1159/000373952. [http://dx.doi.org/10.1159/000373952]. [PMID: 25721868]. [DOI] [PubMed] [Google Scholar]
- 103.Wang M.C., Liang X., Liu Z.Y., Cui J., Liu Y., Jing L., Jiang L.L., Ma J.Q., Han L.L., Guo Q.Q., Yang C.C., Wang J., Wu T., Nan K.J., Yao Y. In vitro synergistic antitumor efficacy of sequentially combined chemotherapy/icotinib in non-small cell lung cancer cell lines. Oncol. Rep. 2015;33(1):239–249. doi: 10.3892/or.2014.3583. [http://dx.doi.org/10.3892/or.2014.3583]. [PMID: 25370413]. [DOI] [PubMed] [Google Scholar]
- 104.Hu D., Cao S., Zhang G., Xiao Y., Liu S., Shang Y. Florfenicol-induced mitochondrial dysfunction suppresses cell proliferation and autophagy in fibroblasts. Sci. Rep. 2017;7(1):13554. doi: 10.1038/s41598-017-13860-9. [http://dx.doi.org/10.1038/s41598-017-13860-9]. [PMID: 29051574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agostinelli E., Seiler N. Non-irradiation-derived reactive oxygen species (ROS) and cancer: therapeutic implications. Amino Acids. 2006;31(3):341–355. doi: 10.1007/s00726-005-0271-8. [http://dx.doi.org/10.1007/s00726-005-0271-8]. [PMID: 16680401]. [DOI] [PubMed] [Google Scholar]
- 106.Kanzawa T., Kondo Y., Ito H., Kondo S., Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63(9):2103–2108. [PMID: 12727826]. [PubMed] [Google Scholar]
- 107.Kondo Y., Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2(2):85–90. doi: 10.4161/auto.2.2.2463. [http://dx.doi.org/10.4161/auto.2.2.2463]. [PMID: 16874083]. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y., McMillan-Ward E., Kong J., Israels S.J., Gibson S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15(1):171–182. doi: 10.1038/sj.cdd.4402233. [http://dx.doi.org/10.1038/sj.cdd.4402233]. [PMID: 17917680]. [DOI] [PubMed] [Google Scholar]
- 109.Gibson S.B. A matter of balance between life and death: targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy. Autophagy. 2010;6(7):835–837. doi: 10.4161/auto.6.7.13335. [http://dx.doi.org/10.4161/auto.6.7.13335]. [PMID: 20818163]. [DOI] [PubMed] [Google Scholar]
- 110.Kalyanaraman B., Cheng G., Hardy M., Ouari O., Bennett B., Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018;15:347–362. doi: 10.1016/j.redox.2017.12.012. [http://dx.doi.org/10.1016/j.redox.2017.12.012]. [PMID: 29306792]. [DOI] [PMC free article] [PubMed] [Google Scholar]