Abstract
Background:
Despite its various side effects, morphine has been widely used in clinics for decades due to its powerful analgesic effect. Morphine tolerance is one of the major side effects, hindering its long-term usage for pain therapy. Currently, the thorough cellular and molecular mechanisms underlying morphine tolerance remain largely uncertain.
Methods
We searched the PubMed database with Medical subject headings (MeSH) including ‘morphine tolerance’, ‘cytokines’, ‘interleukin 1’, ‘interleukin 1 beta’, ‘interleukin 6’, ‘tumor necrosis factor alpha’, ‘interleukin 10’, ‘chemokines’. Manual searching was carried out by reviewing the reference lists of relevant studies obtained from the primary search. The searches covered the period from inception to November 1, 2017.
Results
The expression levels of certain chemokines and pro-inflammatory cytokines were significantly increased in animal models of morphine tolerance. Cytokines and cytokine receptor antagonist showed potent effect of alleviating the development of morphine tolerance.
Conclusion
Cytokines play a fundamental role in the development of morphine tolerance. Therapeutics targeting cytokines may become alternative strategies for the management of morphine tolerance.
Keywords: Morphine tolerance, pro-inflammatory cytokines, chemokines, anti-inflammatory cytokines, pain, glial cells
1. Introduction
Morphine produces powerful analgesic effect mainly through acting on mu opioid receptors (MOR) [1]. It has been widely used in clinics for the treatments of acute pain, postoperative pain and moderate to severe chronic pain [2]. However, chronic usage of morphine unavoidably results in various side effects including drug tolerance and dependence, respiratory depression, nausea, vomiting and sedation, which hinder its clinical application [3-5]. Several mechanisms have been reported to underlie the development of morphine tolerance, including desensitization and internalization of opioid receptor [6, 7], heterodimers of G protein-coupled receptors [8, 9], activation of adenosine 3′,5′-monophosphate (cAMP) pathway [10] and mitogen-activated protein kinase (MAPK) pathway [11, 12] and so on. However, the current therapies for the management of morphine tolerance are still insufficient.
Cytokines are a broad and loose category of small hydrosoluble proteins and peptides (5-140 kDa) produced by a wide range of cells, including immune cells, endothelial cells, and various stromal cells [13, 14]. Based on the nature
of immune response, cytokines can be broadly classified into three groups [14-16]: adaptive immunity which act on cells related to adaptive immune response, such as common γ chain receptor ligands, common β chain (CD131) receptor ligands; pro-inflammatory signaling which promote inflammation, including interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), etc.; anti-inflammatory signaling which inhibit inflammation, including interleukin 4 (IL-4),interleukin 10 (IL-10), interleukin 13 (IL-13), etc. Cytokines could also be divided into interleukins, tumor necrosis factors, interferons, colony stimulating factors, chemokines and others such as TGF-β [16, 17]. As the potent and pivotal mediators in cell signaling, cytokines exert crucial roles in inflammation, immune responses, hematopoiesis, and other critical physiologic processes [13]. It has been reported that the analgesic effect of morphine could be affected by abnormal expressions of pro-inflammatory cytokines, chemokines, and anti-inflammatory cytokines induced by chronic administration of morphine. Here we outline the evidences that cytokines are related to the development of morphine tolerance, providing hope for new medical treatments.
2. Literature search
We searched the PubMed database for English language publications only. Medical subject headings (MeSH) including ‘morphine tolerance’, ‘cytokines’, ‘interleukin 1’, ‘interleukin 1 beta’, ‘interleukin 6’, ‘tumor necrosis factor alpha’, ‘interleukin 10’, ‘chemokines’ were cross-referenced in the search, which was supplemented with a secondary manual search of PubMed. Further manual searching was carried out by reviewing the reference lists of relevant studies obtained from the primary search. The searches covered the period from inception to November 1, 2017.
3. Pro-inflammatory cytokines and morphine tolerance
3.1. IL-1/IL-1β
IL-1 family consists of 11 members, including IL-1α, IL-1β, IL-1ra, IL-18, IL-33, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-37 and IL-1Hy2 [15, 18], which are primarily produced by immune cells such as macrophages and monocytes, as well as non-immune cells such as activated fibroblasts and endothelial cells [17-19]. IL-1β is one of the most important members of IL-1 family. It could be produced and secreted by multiple immune cells, such as macrophages, monocytes, astrocytes, neurons and microglia [13, 20]. As a potent pro-inflammatory cytokine, IL-1β is originally identified as an endogenous pyrogen, which is involved in several features of inflammation,such as the recruitment of white blood cells, fever, acute phase protein release and the increase of permeability of blood vessels [21, 22].
What matters is that IL-1β has been proved to be an angiogenic agent involved in the mechanism of neuropathic pain [13], as well as in the development and maintenance of postoperative pain [23]. Furthermore, it has been demonstrated that IL-1β was one of the first cytokines to be involved in the development of morphine analgesic tolerance. Genetic and pharmacological blockade of IL-1 or IL-1β signaling could significantly prevent the development of tolerance following chronic morphine administration [24, 25]. Therefore, mice lacking IL-1β showed an enhancement of morphine analgesia and prevention of morphine tolerance. Additionally, those mutant strains of mice, such as mice with transgenic over-expression of IL-1ra within the brain and spinal cord (IL-1raTG), mice with targeted deletion of the IL-1 type I receptor (IL-1rKO), and mice with targeted deletion of the IL-1 receptor accessory protein (IL-1rAcPKO), failed to develop mechanical or thermal pain behaviors following morphine tolerance, compared with their respective WT controls [24], suggesting a crucial contribution of IL-1 signaling to morphine tolerance. Pharmacological studies have, however, delineated the role of spinal IL-1β in the development of morphine tolerance. Chronic intrathecal delivery of morphine and various IL-1 blockers (IL-1ra, a-melanocyte-stimulating hormone, or IL-1 tripeptide antagonist) to rats/mice could enhance the acute morphine analgesia and suppress the development of analgesic tolerance [24-26]. Conversely, morphine analgesia could be abolished by administrating a neutral dose of IL-1β in mice. Either chronic or acute exposure to morphine could upregulate the expression of IL-1β in spinal glial cells and neurons [27-29]. IL-1β derived from the activated microglia after chronic morphine exposure has recently been confirmed to be attributed to toll-like receptor 4 (TLR4) [30-32] and P2X4 receptor (P2X4R) pathways. Because the activated TLR4 signaling has been proved to be responsible for morphine-induced neuroinflammation [33, 34]. Thus, chronic morphine exposure led to the activation of endocytosis of TLR4 in microglia and P2X4R up-regulation, then finally inducing IL-1β release [35]. Therefore, the endocytosis of TLR4 contributes to the microglia activation and IL-1β release following the induction of morphine tolerance.
To sum up, genetic and pharmacological studies demonstrated that IL-1/IL-1β is upregulated in activated glial cells after chronic exposure to morphine. The activation of TLR4-P2X4R pathway in microglia modulates the release of IL-1β. IL-1 blocker and IL-1 receptor antagonist can prolong and enhance the analgesic effect of morphine and attenuate the development of morphine tolerance, indicating a pivotal role of IL-1 in morphine tolerance.
3.2. TNF-α
TNF-α is initially called cachectin and always be considered as a potent pro-inflammatory cytokine. It is expressed by macrophages, monocytes, T cells, mast cells, NK cells, keratinocytes, fibroblasts and neurons and glial cells [17, 36]. It could communicate with target cells via high-affinity membrane receptors, which include tumor necrosis factor receptor type 1 (TNFR1 or p55) and type 2 (TNFR2 or p75) [36, 37]. TNF-α could play a critical role in inflammation and immune processes as well as in the process of nociception [38].
A growing body of literatures demonstrated that inhibition of TNF-α signaling could suppress the development of morphine tolerance in morphine-tolerant rats. The study of the role of TNF-α in morphine tolerance has been aided by a number of tools available to pharmacologically and genetically interfere with TNF-α signaling. These include TNF-α biological antagonist etanercept, HSV vectors expressing p55 TNF soluble receptor, and lentiviral vector expressing a dominant-negative TNF peptide. Intrathecal treatment with etanercept could partially restore the analgesia effect of morphine, as well as inhibit spinal proinflammatory cytokines expression and neuroinflammation in the microglia [39, 40]. Moreover, etanercept also could inhibit the downregulated glutamate transporters (GLT-1 and GLAST) and upregulated AMPA receptor and NMDA receptor subunits (GluR1/ GluR2 and NR1/NR2A) in morphine-tolerant rats [40, 41]. Those suggested that etanercept may be a novel therapy for morphine tolerance by suppressing spinal neuroinflammation and attenuation of the glutamatergic transmission. Subcutaneous inoculation of HSV vectors over-expressing of TNFR1 in the hind paw of rats could enhance the acute morphine analgesia and alleviate the development of morphine tolerance [42]. It has been recently demonstrated that TLR4-mediated neuroinflammation in the periaqueductal gray (PAG) drives tolerance. Moreover, PAG delivery of lentiviral vector expressing a dominant-negative TNF peptide eliminate morphine tolerance, suggesting that TNF-α signaling was responsible for TLR4-mediated morphine tolerance [43]. However, Fukagawa et al. [44] recently found that microglial activation involved in morphine tolerance is not mediated by TLR4. They used TLR4 mutation and deletion mice respectively to explore the relationship between TLR4 and morphine tolerance. Compared to wild-type mice, mutation or deletion of the TLR4 gene did not significantly affect the development of morphine tolerance. Besides, their data indicated that microglial activation has nothing to do with TLR4 after morphine treatment.
Indeed, the expression of TNF-α in the spinal cord is normal under physiological condition, while it is significantly increased in activated microglias and astrocytes induced by chronic morphine administration [39, 45, 46]. Meanwhile, previous study also showed that TNF-α was barely colocalized with microglia marker OX42 and only merged with astrocyte marker GFAP [47]. It has been reported that the release of TNF-α in activated microglial cells is mediated by MOR-PKCɛ-Akt-ERK1/2 signaling pathway in the development of morphine tolerance [28]. Calcitonin gene-related peptide (CGRP) has been already indicated to be involved in the mechanism of morphine tolerance. The increased expression of CGRP induced by chronic morphine exposure could lead to ERK-dependent up-regulation of TNF-α in spinal astrocyte [46]. Therefore, it could be accepted that TNF-α expression is upregulated in activated microglia mediated by MOR-PKCɛ-Akt-ERK1/2 signaling pathway and in astrocyte mediated by CGRP-ERK pathway after chronic morphine exposure, in addition, inhibiting TNF-α signaling might be an effective strategy for the treatment of morphine tolerance.
3.3. IL-6
IL-6, which was first named B-stimulatory factor 2, is produced by monocytes, macrophages, hepatocytes, eosinophils as well as glial cells [19, 48]. The production of IL-6 can be induced by TNF-α and IL-1 [17]. Like TNF-α and IL-1β, IL-6 has been reported to be involved in morphine tolerance. High level of spinal IL-6 produced by activated glial cells could be detected in the morphine-tolerant rats [29]. Additionally, the CGRP family member adrenomedullin (AM) has been verified to increase proinflammatory cytokines (IL-1β, IL-6 and TNF-α) expression and activate microglia and astrocytes in the spinal dorsal horn, thus contributing to the development of morphine tolerance [45, 49-51]. Moreover, using small interfering RNA (siRNA) to knock down the expression of AM in cultured dorsal root ganglion (DRG) could inhibit morphine-induced increase in IL-1β and IL-6 syntheses [45]. In addition, previous study further illuminated the exact mechanisms of CGRP contributing to morphine tolerance [46]. The up-regulation of IL-6 induced by morphine could be inhibited by blocking p38 activity in microglia. Moreover, CGRP has been confirmed to be related to the development of morphine tolerance by differentially modulating p38-dependent up-regulation of IL-6 in spinal microglia. These results suggested that CGRP-p38 signaling pathway in microglia mediated the synthesis and release of IL-6, finally resulting in morphine tolerance. In another study, repeated intrathecal injection of melanocortin 4 receptor (MC4R) antagonist (HS014) could down-regulate the expression of IL-6 and inhibit the activated astrocyte in morphine tolerant rats [52], suggesting that MC4R might be responsible for the development of morphine tolerance via increasing the expression of IL-6. Additionally, the activation of PKCɛ, Akt and MAPK signaling pathway might be involve in the increase of IL-6 released by activated murine microglial cells in the development of morphine tolerance [28].
In summary, directly or indirectly inhibiting the expression of IL-6 in activated microglial cells and astrocytes might be an alternative approach to manage morphine tolerance.
4. Chemokines and morphine tolerance
Chemokines are a family of small proteins (8-14 kDa) characterized by the presence of three to four conserved cysteine residues [53-55]. Based on the sequence of the N-terminal cysteine residues, chemokines are classified into four groups: CC chemokines, CXC chemokines, XC chemokines and CX3C chemokines [56-58]. There are approximately over 20 chemokine receptors. While some chemokines share the same receptors, certain chemokines can bind to more than one chemokine receptor. It is worth noting that several chemokines have unknown receptors, including CCL18, CXCL14, CXCL15 and CXCL17 [59-62]. Chemokines have been reported to be involved in the recruitment of leukocytes to the site of inflammation. Emerging evidence has demonstrated that chemokines are also responsible for other functions such as fever, modulation of the immune response and inflammatory pain, as well as morphine tolerance [19, 55, 63, 64].
CX3CL1, also known as fractalkine, is claimed to modulate the development of morphine tolerance and mechanical allodynia and thermal hyperalgesia [25]. Co-administration of morphine with neutralizing antibody against the CX3CL1 receptor (CX3CR1) could enhance the acute morphine analgesia and attenuate the development of drug tolerance, hyperalgesia, and allodynia [19]. However, our study found that the expressions of spinal CX3CL1/CX3CR1 were not significantly changed in morphine-tolerant rats. Exogenous CX3CL1 and CX3CR1 inhibitor both could not inhibit the development of morphine tolerance. Additionally, a microarray profiling further confirmed that CX3CL1/CX3CR1 were not up-regulated in morphine-tolerant rats [65]. Therefore, on one hand, we think that this discrepancy might be due to the different experimental protocols including the evaluation of pain threshold. On the other hand, we speculate that the spinal CX3CR1 expressed in neuron may bind to MOR to form into the heterodimer, which at least partly contribute to morphine analgesia or tolerance. In another study, the interaction between CX3CL1/ CX3CR1 and MOR, delta opioid receptors (DOR) or kappa opioid receptors (KOR) in PAG was explored. Pretreatment with CX3CL1 into PAG before injection of DAMGO, DPDPE or dynorphin could significantly abolish the analgesia effect of opioids respectively [66], suggesting that the antinociception effect of mu, delta and kappa opioid agonists could be reduced by activating CX3CR1 in PAG.
Monocyte chemoattractant protein (MCP-1), known as the chemokine (C-C motif) ligand 2 (CCL2), has been demonstrated to play a key role in morphine antinociceptive tolerance. Indeed, the expression of MCP-1 is very low in spinal cord under physiological condition, while it could be significantly up-regulated after chronic morphine administration. Intrathecal injection of MCP-1 neutralizing antibody could suppress the activated spinal microglial cells as well as the development of morphine tolerance [67, 68]. Interestingly, the cellular location of MCP-1 could be various under different conditions. Most studies show MCP-1 was mainly colocalized with small-to-medium-diameter neurons in DRG under physiological condition and several chronic pain conditions [69-71]. Additionally, the expression of MCP-1 has also been found in spinal astrocytes and some fibers after spinal nerve ligation, while the increased spinal MCP-1 induced by chronic morphine administration was verified to be colocalized with neurons, but not astrocytes and microglia [67]. These suggested that spinal neuronal MCP-1 plays a critical role in microglial activation and morphine antinociceptive tolerance, which makes MCP-1 becomes a potential target for the management of morphine tolerance.
CXCL12, also known as stromal cell-derived factor-1 (SDF-1), belongs to CXC chemokine family. Most studies show that pretreatment with chemokines CXCL12 into PAG can attenuate the analgesic effect of morphine or selective DOR agonist DPDPE via activating CXCL12 receptor (CXCR4) [72, 73]. Moreover, it has been verified that the activation of Src family-kinases (SFK) induced by CXCR4 might be involved in the regulation of morphine analgesic effect [74]. CXCL12 could induce SFK phosphorylation in DRG and spinal cord via activating CXCR4 [74, 75]. Pretreatment with CXCR4 antagonist could abrogate those activation and restore the antinociceptive effect of morphine. it is confirmed that phosphorylated-SFK (p-SFK) was expressed in astrocyte in the DRG and in spinal microglial cells [74]. Interestingly, p-SFK and CXCR4 both were found in MOR- and DOR-immunoreactive neurons in DRG and spinal cord. Intrathecal injection of specific SFK inhibitor PP2 could significantly abolish the inhibition of morphine analgesic effect induced by CXCL12/CXCR4 signaling [74], suggesting that SFK signaling pathway might be a novel target for the management of CXCL12-induced loss of acute morphine analgesia. However, whether SFK signaling pathway involved in morphine tolerance after chronic morphine administration is still not clear. Recently, CXCL12/CXCR4 pathway has been reported to be involved in the mechanism of morphine tolerance [76]. It is proved that CXCL12 was significantly increased in cerebrospinal fluid (CSF) of opioid-tolerant patients and in spinal cord of morphine-tolerant rats. Additionally, intrathecal infusion of CXCL12 neutralizing antibody or CXCR4 antagonist AMD 3100 both could maintain the analgesic effects of morphine, indicating that CXCL12/CXCR4 signaling might be a potential therapeutic target for morphine tolerance.
CXCL1, also known as growth-related oncogene [GRO] or keratinocyte-derived chemokine, belongs to CXC chemokine family. A clinical study investigated the CSF samples from 30 opioid tolerant cancer patients and 10 naive control subjects revealed that the expression of CXCL1 is obviously increased in CSF of opioid tolerant cancer patients, while the level of CSF CXCL10, CCL2, and CX3CL1 are not significantly different between opioid-tolerant patients and naive controls [77]. Besides the expression of spinal CXCL1 mRNA has also been reported to be drastically up-regulated in morphine tolerant rats [77]. Furthermore, Intrathecal injection of CXCL1 could significantly alleviate the antinociceptive effect of morphine and promote the development of morphine tolerance. Interestingly, co-injection of CXCL1-neutralizing antibody (CXCL1-Ab) or CXCR2 antagonist -antileukine hexapeptide could partially restore the antinociceptive effect of morphine and inhibit the development of morphine tolerance. Those results suggested that inhibiting CXCL1/CXCR2 signal pathway might effectively attenuate the development of opioid tolerance.
In addition, our previous study has demonstrated that the expression of CXCL10 was increased by a single morphine administration in spinal neuron and the analgesic effect of morphine could be enhanced by blocking spinal CXCL10/CXCR3 signaling in bone cancer pain rats [78]. Recently, our lab found that CXCL10/CXCR3 signaling also contributes to morphine tolerance in PAG by neuron-microglia interaction. Repeated morphine administration could up-regulate the expressions of CXCR3 and CXCL10 in PAG. CXCR3 was proved to express in neuron, while CXCL10 was found in microglia [79]. CXCL11, which shares the same receptor CXCR3 with CXCL10 (IP-10) and CXCL9 (Mig), exhibits higher affinity to CXCR3 and greater efficacy in activating the receptor than other two ligands [80, 81]. We have found that the expression of spinal CXCL11 was upregulated in the development of morphine tolerance in cancer-induced bone pain (CIBP) rats and normal rats. Administration of CXCL11 neutralizing antibody could significantly attenuate the development of morphine tolerance and inhibit the activation of astrocytes. Besides, CXCL11 was confirmed to express in astrocytes and neurons in spinal cord [82]. Thus, those findings may have significant therapeutic implications in inhibiting the development of morphine tolerance. Moreover, our microarray profiling results further verified that CXCL10, CXCL11, CXCL12 and CCL2 were upregulated in spinal cord after chronic morphine administration, indicating the potential contribution of chemokines to the development of morphine tolerance [65].
5. Anti-inflammatory cytokines and morphine tolerance
The anti-inflammatory cytokines are a series of immunoregulatory molecules which could be responsible for the proinflammatory cytokine response. IL-10 is an 18-kDa non-glycosylated peptide secreted by numerous cell types including monocytes, macrophage, activated lymphocytes and mast cells [19, 20]. As the first described anti-inflammatory cytokine, IL-10 is vital to the regulation of immune responses. Similar to interferon receptors, IL-10 receptor (IL-10R) belongs to the class II cytokine receptor family [21, 83]. When IL-10 specifically binds to IL-10R, Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway could be activated which results in the anti-inflammatory effect [84]. Previous study has demonstrated that IL-10 could inhibit the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and stimulate the endogenic expression of anti-inflammatory cytokines [22, 85, 86]. In addition, IL-10 could enhance the proliferation of mastocytes and hinder the production of IFN-γ from natural killer cells. Several cytokines, such as IL-4, IL-13, and IFN-γ, could suppress the expression of IL-10, and be inhibited by its own auto-regulation [87-89].
Previous studies which aimed to explore the relationship between anti-inflammatory cytokines and morphine tolerance mainly focus on IL-10. Morphine could suppress the host innate and adaptive immune response [90, 91]. The effects of morphine on anti-inflammatory cytokines related to immune response are complex, which depends on the patterns of morphine administration. The in vivo and in vitro experiments have confirmed that the extent of inhibiting IL-10 expression is related to the dosage of morphine [92, 93]. Interestingly, the inhibition of IL-10 expression induced by acute morphine treatment could be abolished by chronic morphine administration [92, 93]. Besides, it is confirmed that spinal IL-10 expression could be down-regulated in chronic morphine-treated rats [94]. Moreover, up-regulating IL-10 expression by intrathecal infusion of adenoviral vector encoding for IL-10 (AD-IL10) or administration of recombinant rat IL-10 (rrIL-10) could significantly maintain the antinociceptive effect of morphine after repeated injection, as well as attenuate the development of morphine tolerance [25, 95]. The increased expressions of spinal pro-inflammatory cytokine TNF-α, IL-1β, and IL-6 in chronic morphine-treated rats were also markedly inhibited by rrIL-10 [95]. Several studies have reported that some drugs such as amitriptyline, naloxone, and gabapentin can attenuate the development of morphine tolerance by increasing the expression of IL-10 [94-97]. The effects of these drugs on the morphine-tolerant rats could be abolished by anti-IL-10 antibody [94-96]. And for amitriptyline, its effect to maintain morphine’s analgesia could be inhibited by p38 MAPK inhibitor. Both anti-IL-10 antibody and p38 MAPK inhibitor could down-regulate the expressions of p38 MAPK and heme oxygenase-1 (HO-1) [96]. Taken together, it is suggested that amitriptyline could restore the anti-nociceptive and anti-inflammation effects of morphine by up-regulating the production of IL-10 via p38 MAPK-HO-1 signaling pathway, which provided a novel target for alleviating morphine tolerance.
As a potent immune-regulatory cytokine, IL-10 exhibits critical impact on modulating inflammation and directing adaptive immune response [98]. Spinal MOR, which has been proved to be involved in the development of morphine tolerance, is related to the regulation of serum anti-inflammatory cytokine IL-10 in adjuvant arthritis (AA) morphine-tolerant rats [99]. The expressions of spinal MOR and serum IL-10 were both increased in AA morphine-tolerant rats. The development of morphine tolerance in AA rats occurred markedly later than control group. Additionally, daily administration of anti-IL-10 antibody could significantly enhance the thermal hyperalgesia, decrease the spinal MOR expression and accelerate the development of morphine tolerance in AA rats [99]. These results suggested that the up-regulation of serum IL-10 might inhibit the development of morphine tolerance via modulating spinal MOR expression in AA rats.
Conclusion
In this review, we discussed the relationship between cytokines and morphine tolerance. Accumulated evidences have indicated that certain chemokines and pro-inflammatory cytokines are up-regulated, while the expression of anti-inflammatory cytokines are decreased in various sites (such as spinal cord, DRG and PAG, etc.) under morphine-tolerant states (Table 1). Additionally, most studies paid more attention to study the mechanisms between cytokines and morphine tolerance in spinal cord (Fig. 1). The inhibition of the analgesic effect of morphine by chemokines and pro-inflammatory cytokines may due to directly affecting the activation of MOR or indirectly offsetting morphine analgesia by inducing hyperalgesia. heterologous desensitization and internalization/endocytosis is rapid between MOR and inflammatory substances via shared G-protein-coupled systems [73] or other secondary messenger systems within neurons and glia, such as MAP kinases and calcium [28, 46]. glutamatergic signal transmission was proved to be involved in morphine tolerance, while inflammatory substances released by glial cells after chronic morphine treatment can activate glutamatergic transmission to mediate neuroplastic changes [100-102]. Other substances released by activated glial cells also contribute to analgesic tolerance in diverse exaggerated pain states (e.g., NO, ATP, excitatory amino acids, prostaglandins, dynorphin) [103-105]. Meanwhile, the anti-inflammatory cytokines, such as IL-10, exert its impact on morphine analgesia via inhibiting the production of pro-inflammatory cytokines, and then suppress the activation of glial cells, thus contributing to the consolidation of morphine tolerance. Interestingly, amitriptyline, naloxone, and gabapentin have been confirmed to attenuate morphine tolerance through increasing the expression of anti-inflammatory cytokine IL-10 and decreasing the level of pro-inflammatory cytokines in spinal cord. Novel treatment options that may modulate morphine tolerance are summarized in Table 2. It is promising to investigate the effects of these drugs for the management of morphine tolerance in the future. However, further studies investigating the detailed mechanisms of how cytokines affect the development of morphine tolerance are warranted.
Table 1. Cytokines alteration in morphine tolerance.
| Cytokine/chemokine | Sites | Cell Type | Changes in Morphine Tolerance | Refs. |
|---|---|---|---|---|
| IL-1/IL-1β | spinal cord lumbosacral CSF (rats) DRG |
microglia, astrocytes NR |
↑ ↑ ↑ |
[56, 73, 87] [36, 40] [96] |
| TNF-α | spinal cord PAG |
microglia, astrocytes NR |
↑ ↑ |
[73, 87] [25] |
| IL-6 | spinal cord DRG |
microglia, astrocytes NR |
↑ ↑ |
[73, 87] [96] |
| CX3CL1/ fractalkine | spinal cord PAG CSF(human) |
neuron,glial cell NR |
→ NR → |
[63] [11] [48] |
| CCL2/MCP-1 | spinal cord CSF(human) |
neuron | ↑ → |
[100] [48] |
| CXCL1/ GRO | spinal cord CSF(human) |
NR | ↑ ↑ |
[48] [48] |
| CXCL10 | spinal cord CSF(human) PAG |
neuron microglia |
↑ → ↑ |
[94] [48] [86] |
| CXCL11 | spinal cord | astrocytes, neuron | ↑ | [34] |
| CXCL12/ SDF-1 | spinal cord CSF(human) PAG DRG |
NR NR neuron |
↑ ↑ NR ↑ |
[49] [48] [77] [92] |
| IL-10 | spinal cord serum |
NR | ↓ ↓ |
[5, 40, 51] [95] |
↑ increase; ↓ decrease; → no signifcant change; CSF: cerebrospinal fluid; DRG: dorsal root ganglion; NR: not report; PAG: periaqueductal gray.
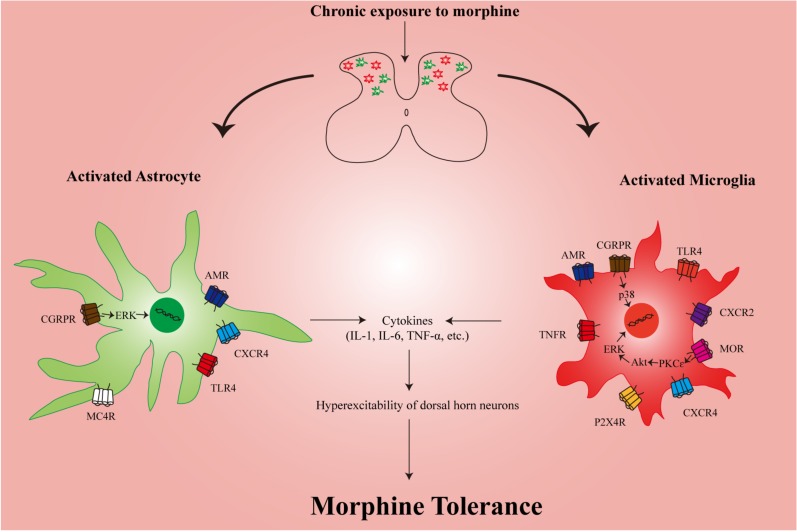
Fig. (1).
Schematic representation of the possible mechanisms by which cytokines mediate morphine tolerance. After chronic exposure to morphine, the glial cells in the spinal cord are activated, leading to release of proinflammatory cytokines such as IL-1β, IL-6, and TNFα, etc. These proinflammatory cytokines contribute to the hyperexcitability of dorsal horn neurons, which may account for the development of morphine tolerance. Activation of CXCR4, TLR4, MC4R, AMR and CGRPR in the spinal astrocyte have been shown to induce production of proinflammatory cytokines. Similarly, several receptors in the spinal microglia also play an important role in the release of proinflammatory cytokines. AMR: adrenomedullin receptor; Akt: protein kinase B; CGRPR: Calcitonin gene-related peptide receptor; CXCR4: C-X-C chemokine receptor type 4; ERK: extracellular signal-regulated kinases; IL-1: interleukin 1; IL-6: interleukin 6; MC4R: melanocortin 4 receptor; MOR: mu opioid receptor; P2X4R: P2X4 receptor; PKCɛ: Protein kinase C ɛ; TLR4: toll-like receptor 4; TNFR: tumor necrosis factor receptor; TNF-α: tumor necrosis factor alpha.
Table 2. Novel treatment options that may modulate morphine tolerance.
| Compound | Mechanisms of Action | Status | Potential Uses | Refs. |
|---|---|---|---|---|
| IL-1ra; A-melanocyte-stimulating hormone; IL-1 tripeptide antagonist | IL-1 receptor antagonist and IL-1 blockers | Prolong and enhance the analgesic effect of morphine; Attenuate the development of morphine tolerance | [24-26] | |
| Etanercept | TNF-α receptor antagonist | Approved for rheumatoid arthritis, plaque psoriasis, ankylosing spondylitis | Restore the analgesic effect of morphine; Attenuate the development of morphine tolerance; Anti-neuroinflammatory | [39-41] |
| Cytokine synthesis inhibitors | Prevent proinflammatory cytokine production | p38 MAPK PKCɛ-Akt-ERK | Anti-neuroinflammatory; Attenuate the development of morphine tolerance | [28, 46] |
| AM22–52 | Selective AM1 receptor antagonist | Antinociceptive; Anti-neuroinflammatory; Improve the analgesic potency of morphine; May reverse the development of morphine tolerance | [45, 49-51, 106, 107] | |
| Amitriptyline | Tricyclic antidepressant | Approved for major depressive disorder, anxiety disorder, migraine, fibromyalgia, postherpetic neuralgia | Anti-neuroinflammatory; Antineuropathic; Neuroprotection; Preserve the analgesic effect of morphine; May attenuate the development of morphine tolerance | [96, 108-114] |
| Naloxone | Opioid receptors antagonist | Used to block the effects of opioids, especially in overdose | Anti-neuroinflammatory; Enhance the analgesic effect of morphine; May attenuate the development of morphine tolerance | [95, 115-120] |
| Gabapentin | Derivative of GABA, a anticonvulsant and analgesic drug | Used to treat epilepsy, neuropathic pain, hot flashes, and restless leg syndrome | Anti-neuroinflammatory; Antineuropathic; Neuroprotection; Enhance the analgesic effect of morphine; May attenuate the development of morphine tolerance | [94, 97, 121-125] |
| Gene therapies | Upregulate anti-inflammatory cytokine (IL-10) production; Downregulate proinflammatory cytokine production | Analgesic; antineuropathic; Neuroprotective; May prevent the development of morphine tolerance | [24, 25, 42, 45] |
Abbreviations: AM: adrenomedullin; Akt: protein kinase B; ERK: extracellular signal-regulated kinases; GABA: Gamma-aminobutyric acid; IL-1ra: interleukin 1 receptor antagonist; IL-10: interleukin 10; MAPK: Mitogen-activated protein kinase; PKCɛ: Protein kinase C ɛ; TNF-α: Tumor necrosis factor alpha.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China 81771191, 81471143, 81171259.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Bao Y., Gao Y., Yang L., Kong X., Yu J., Hou W., Hua B. The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence. Channels (Austin) 2015;9(5):235–243. doi: 10.1080/19336950.2015.1069450. [http://dx.doi.org/10.1080/19336950.2015.1069450]. [PMID: 26176938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King T., Ossipov M.H., Vanderah T.W., Porreca F., Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14(4):194–205. doi: 10.1159/000087658. [http://dx.doi.org/10.1159/000087658]. [PMID: 16215302]. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol. Neurobiol. 2009;40(2):101–107. doi: 10.1007/s12035-009-8074-z. [http://dx.doi.org/10.1007/s12035-009-8074-z]. [PMID: 19468867]. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Bao L., Li S. Opioid receptor trafficking and interaction in nociceptors. Br. J. Pharmacol. 2015;172(2):364–374. doi: 10.1111/bph.12653. [http://dx.doi.org/10.1111/bph.12653]. [PMID: 24611685]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda H. Locus-specific involvement of anti-opioid systems in morphine tolerance and dependence. Ann. N. Y. Acad. Sci. 2004;1025:376–382. doi: 10.1196/annals.1307.046. [http://dx.doi.org/10.1196/annals.1307.046]. [PMID: 15542739]. [DOI] [PubMed] [Google Scholar]
- 6.Narita M., Suzuki M., Narita M., Niikura K., Nakamura A., Miyatake M., Yajima Y., Suzuki T. mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience. 2006;138(2):609–619. doi: 10.1016/j.neuroscience.2005.11.046. [http://dx.doi.org/10.1016/j.neuroscience.2005.11.046]. [PMID: 16417975]. [DOI] [PubMed] [Google Scholar]
- 7.Martini L., Whistler J.L. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr. Opin. Neurobiol. 2007;17(5):556–564. doi: 10.1016/j.conb.2007.10.004. [http://dx.doi.org/10.1016/j.conb.2007.10.004]. [PMID: 18068348]. [DOI] [PubMed] [Google Scholar]
- 8.Pasternak G.W., Pan Y.X. Mix and match: heterodimers and opioid tolerance. Neuron. 2011;69(1):6–8. doi: 10.1016/j.neuron.2010.12.030. [http://dx.doi.org/10.1016/j.neuron.2010.12.030]. [PMID: 21318174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szentirmay A.K., Király K.P., Lenkey N., Lackó E., Al-Khrasani M., Friedmann T., Timár J., Gyarmati S., Tóth G., Fürst S., Riba P. Spinal interaction between the highly selective μ agonist DAMGO and several δ opioid receptor ligands in naive and morphine-tolerant mice. Brain Res. Bull. 2013;90:66–71. doi: 10.1016/j.brainresbull.2012.09.006. [http://dx.doi.org/10.1016/j.brainresbull.2012.09.006]. [PMID: 22995282]. [DOI] [PubMed] [Google Scholar]
- 10.Nestler E.J., Aghajanian G.K. Molecular and cellular basis of addiction. Science. 1997;278(5335):58–63. doi: 10.1126/science.278.5335.58. [http://dx.doi.org/10.1126/science.278.5335.58]. [PMID: 9311927]. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y., Chen Y., Zhi J.L., Guo R.X., Feng J.Q., Chen P.X. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069(1):235–243. doi: 10.1016/j.brainres.2005.11.066. [http://dx.doi.org/10.1016/j.brainres.2005.11.066]. [PMID: 16403466]. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Geis C., Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J. Neurosci. 2008;28(22):5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.4170-07.2008]. [PMID: 18509045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark A.K., Old E.A., Malcangio M. Neuropathic pain and cytokines: current perspectives. J. Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [PMID: 24294006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang T.L., Schaeffer A.J. The role of cytokines in prostatitis. World J. Urol. 2003;21(2):95–99. doi: 10.1007/s00345-003-0335-2. [http://dx.doi.org/10.1007/s00345-003-0335-2]. [PMID: 12783173]. [DOI] [PubMed] [Google Scholar]
- 15.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [http://dx.doi.org/10.1016/j.bbamcr.2014.05.014]. [PMID: 24892271]. [DOI] [PubMed] [Google Scholar]
- 16.Strouse T.B. The relationship between cytokines and pain/depression: a review and current status. Curr. Pain Headache Rep. 2007;11(2):98–103. doi: 10.1007/s11916-007-0005-y. [http://dx.doi.org/10.1007/s11916-007-0005-y]. [PMID: 17367587]. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira C.M., Sakata R.K., Issy A.M., Gerola L.R., Salomao R. Cytokines and pain.Revista brasileira de anestesiologia. Cytokines Pain. 2011. [DOI] [PubMed]
- 18.Boraschi D., Lucchesi D., Hainzl S., Leitner M., Maier E., Mangelberger D., Oostingh G.J., Pfaller T., Pixner C., Posselt G., Italiani P., Nold M.F., Nold-Petry C.A., Bufler P., Dinarello C.A. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur. Cytokine Netw. 2011;22(3):127–147. doi: 10.1684/ecn.2011.0288. [PMID: 22047735]. [DOI] [PubMed] [Google Scholar]
- 19.de Miguel M., Kraychete D.C., Meyer Nascimento R.J. Chronic pain: cytokines, lymphocytes and chemokines. Inflamm. Allergy Drug Targets. 2014;13(5):339–349. doi: 10.2174/1871528114666150114170004. [http://dx.doi.org/10.2174/1871528114666150114170004]. [PMID: 25587846]. [DOI] [PubMed] [Google Scholar]
- 20.Verri W.A., Jr, Cunha T.M., Parada C.A., Poole S., Cunha F.Q., Ferreira S.H. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [http://dx.doi.org/10.1016/j.pharmthera.2006.04.001]. [PMID: 16730375]. [DOI] [PubMed] [Google Scholar]
- 21.Lin E., Calvano S.E., Lowry S.F. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127(2):117–126. doi: 10.1067/msy.2000.101584. [http://dx.doi.org/10.1067/msy.2000.101584]. [PMID: 10686974]. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [http://dx.doi.org/10.1097/AIA.0b013e318034194e]. [PMID: 17426506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf G., Livshits D., Beilin B., Yirmiya R., Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav. Immun. 2008;22(7):1072–1077. doi: 10.1016/j.bbi.2008.03.005. [http://dx.doi.org/10.1016/j.bbi.2008.03.005]. [PMID: 18442892]. [DOI] [PubMed] [Google Scholar]
- 24.Shavit Y., Wolf G., Goshen I., Livshits D., Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115(1-2):50–59. doi: 10.1016/j.pain.2005.02.003. [http://dx.doi.org/10.1016/j.pain.2005.02.003]. [PMID: 15836969]. [DOI] [PubMed] [Google Scholar]
- 25.Johnston I.N., Milligan E.D., Wieseler-Frank J., Frank M.G., Zapata V., Campisi J., Langer S., Martin D., Green P., Fleshner M., Leinwand L., Maier S.F., Watkins L.R. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004;24(33):7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [http://dx.doi.org/10.1523/JNEUROSCI.1850-04.2004]. [PMID: 15317861]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson M.R., Coats B.D., Lewis S.S., Zhang Y., Sprunger D.B., Rezvani N., Baker E.M., Jekich B.M., Wieseler J.L., Somogyi A.A., Martin D., Poole S., Judd C.M., Maier S.F., Watkins L.R. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 2008;22(8):1178–1189. doi: 10.1016/j.bbi.2008.05.004. [http://dx.doi.org/10.1016/j.bbi.2008.05.004]. [PMID: 18599265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berta T., Liu T., Liu Y.C., Xu Z.Z., Ji R.R. Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9. Mol. Pain. 2012;8:18. doi: 10.1186/1744-8069-8-18. [http://dx.doi.org/10.1186/1744-8069-8-18]. [PMID: 22439811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merighi S., Gessi S., Varani K., Fazzi D., Stefanelli A., Borea P.A. Morphine mediates a proinflammatory phenotype via μ-opioid receptor-PKCɛ-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem. Pharmacol. 2013;86(4):487–496. doi: 10.1016/j.bcp.2013.05.027. [http://dx.doi.org/10.1016/j.bcp.2013.05.027]. [PMID: 23796752]. [DOI] [PubMed] [Google Scholar]
- 29.Raghavendra V., Rutkowski M.D., DeLeo J.A. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 2002;22(22):9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [http://dx.doi.org/10.1523/JNEUROSCI.22-22-09980.2002]. [PMID: 12427855]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson M.R., Lewis S.S., Coats B.D., Rezvani N., Zhang Y., Wieseler J.L., Somogyi A.A., Yin H., Maier S.F., Rice K.C., Watkins L.R. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167(3):880–893. doi: 10.1016/j.neuroscience.2010.02.011. [http://dx.doi.org/10.1016/j.neuroscience.2010.02.011]. [PMID: 20178837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace P.M., Ramos K.M., Rodgers K.M., Wang X., Hutchinson M.R., Lewis M.T., Morgan K.N., Kroll J.L., Taylor F.R., Strand K.A., Zhang Y., Berkelhammer D., Huey M.G., Greene L.I., Cochran T.A., Yin H., Barth D.S., Johnson K.W., Rice K.C., Maier S.F., Watkins L.R. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience. 2014;280:299–317. doi: 10.1016/j.neuroscience.2014.09.020. [http://dx.doi.org/10.1016/j.neuroscience.2014.09.020]. [PMID: 25241065]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grace P.M., Maier S.F., Watkins L.R. Opioid-induced central immune signaling: implications for opioid analgesia. Headache. 2015;55(4):475–489. doi: 10.1111/head.12552. [http://dx.doi.org/10.1111/head.12552]. [PMID: 25833219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson M.R., Zhang Y., Shridhar M., Evans J.H., Buchanan M.M., Zhao T.X., Slivka P.F., Coats B.D., Rezvani N., Wieseler J., Hughes T.S., Landgraf K.E., Chan S., Fong S., Phipps S., Falke J.J., Leinwand L.A., Maier S.F., Yin H., Rice K.C., Watkins L.R. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. [http://dx.doi.org/10.1016/j.bbi.2009.08.004]. [PMID: 19679181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eidson L.N., Murphy A.Z. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J. Neurosci. 2013;33(40):15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013. [http://dx.doi.org/10.1523/JNEUROSCI.1609-13.2013]. [PMID: 24089500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang Y., Chu H., Jiang Y., Yuan L. Morphine enhances IL-1beta release through toll-like receptor 4-mediated endocytic pathway in microglia. Purinergic Signal. 2016;12(4):637–645. doi: 10.1007/s11302-016-9525-4. [http://dx.doi.org/10.1007/s11302-016-9525-4]. [PMID: 27506813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tracey D., Klareskog L., Sasso E.H., Salfeld J.G., Tak P.P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [http://dx.doi.org/10.1016/j.pharmthera.2007.10.001]. [PMID: 18155297]. [DOI] [PubMed] [Google Scholar]
- 37.Schaible H.G., von Banchet G.S., Boettger M.K., Bräuer R., Gajda M., Richter F., Hensellek S., Brenn D., Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. N. Y. Acad. Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [http://dx.doi.org/10.1111/j.1749-6632.2009.05301.x]. [PMID: 20398009]. [DOI] [PubMed] [Google Scholar]
- 38.Lobito A.A., Gabriel T.L., Medema J.P., Kimberley F.C. Disease causing mutations in the TNF and TNFR superfamilies: Focus on molecular mechanisms driving disease. Trends Mol. Med. 2011;17(9):494–505. doi: 10.1016/j.molmed.2011.05.006. [http://dx.doi.org/10.1016/j.molmed.2011.05.006]. [PMID: 21724465]. [DOI] [PubMed] [Google Scholar]
- 39.Shen C.H., Tsai R.Y., Shih M.S., Lin S.L., Tai Y.H., Chien C.C., Wong C.S. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth. Analg. 2011;112(2):454–459. doi: 10.1213/ANE.0b013e3182025b15. [http://dx.doi.org/10.1213/ANE.0b013e3182025b15]. [PMID: 21081778]. [DOI] [PubMed] [Google Scholar]
- 40.Shen C.H., Tsai R.Y., Tai Y.H., Lin S.L., Chien C.C., Wong C.S. Intrathecal etanercept partially restores morphine’s antinociception in morphine-tolerant rats via attenuation of the glutamatergic transmission. Anesth. Analg. 2011;113(1):184–190. doi: 10.1213/ANE.0b013e318217f7eb. [http://dx.doi.org/10.1213/ANE.0b013e318217f7eb]. [PMID: 21490086]. [DOI] [PubMed] [Google Scholar]
- 41.Shen C.H., Tsai R.Y., Wong C.S. Role of neuroinflammation in morphine tolerance: effect of tumor necrosis factor-α. Acta Anaesthesiol. Taiwan. 2012;50(4):178–182. doi: 10.1016/j.aat.2012.12.004. [http://dx.doi.org/10.1016/j.aat.2012.12.004]. [PMID: 23385041]. [DOI] [PubMed] [Google Scholar]
- 42.Sun J., Liu S., Mata M., Fink D.J., Hao S. Transgene-mediated expression of tumor necrosis factor soluble receptor attenuates morphine tolerance in rats. Gene Ther. 2012;19(1):101–108. doi: 10.1038/gt.2011.76. [http://dx.doi.org/10.1038/gt.2011.76]. [PMID: 21614028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eidson L.N., Inoue K., Young L.J., Tansey M.G., Murphy A.Z. Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology. 2017;42(3):661–670. doi: 10.1038/npp.2016.131. [http://dx.doi.org/10.1038/npp.2016.131]. [PMID: 27461080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukagawa H., Koyama T., Kakuyama M., Fukuda K. Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J. Anesth. 2013;27(1):93–97. doi: 10.1007/s00540-012-1469-4. [http://dx.doi.org/10.1007/s00540-012-1469-4]. [PMID: 22926420]. [DOI] [PubMed] [Google Scholar]
- 45.Zeng X., Lin M.Y., Wang D., Zhang Y., Hong Y. Involvement of adrenomedullin in spinal glial activation following chronic administration of morphine in rats. Eur. J. Pain. 2014;18(9):1323–1332. doi: 10.1002/j.1532-2149.2014.493.x. [http://dx.doi.org/10.1002/j.1532-2149.2014.493.x]. [PMID: 24664661]. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Ma W., Chabot J.G., Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–2586. doi: 10.1096/fj.08-128348. [http://dx.doi.org/10.1096/fj.08-128348]. [PMID: 19299480]. [DOI] [PubMed] [Google Scholar]
- 47.Di Cesare Mannelli L., Corti F., Micheli L., Zanardelli M., Ghelardini C. Delay of morphine tolerance by palmitoylethanolamide. BioMed Res. Int. 2015;2015:894732. doi: 10.1155/2015/894732. [http://dx.doi.org/10.1155/2015/894732]. [PMID: 25874232]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jücker M., Abts H., Li W., Schindler R., Merz H., Günther A., von Kalle C., Schaadt M., Diamantstein T., Feller A.C. Expression of interleukin-6 and interleukin-6 receptor in Hodgkin’s disease. Blood. 1991;77(11):2413–2418. [PMID: 1710152]. [PubMed] [Google Scholar]
- 49.Hong Y., Wang D., Chabot J.G., Ma W., Chen P., Quirion R. A role for protein kinase C-dependent upregulation of adrenomedullin in the development of morphine tolerance in male rats. J. Neurosci. 2010;30(37):12508–12516. doi: 10.1523/JNEUROSCI.0306-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.0306-10.2010]. [PMID: 20844145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., Chen P., Li Q., Quirion R., Hong Y. Blockade of adrenomedullin receptors reverses morphine tolerance and its neurochemical mechanisms. Behav. Brain Res. 2011;221(1):83–90. doi: 10.1016/j.bbr.2011.02.046. [http://dx.doi.org/10.1016/j.bbr.2011.02.046]. [PMID: 21382419]. [DOI] [PubMed] [Google Scholar]
- 51.Wang D., Li J., Chen P., Hong Y. Upregulation of pronociceptive mediators and downregulation of opioid peptide by adrenomedullin following chronic exposure to morphine in rats. Neuroscience. 2014;280:31–39. doi: 10.1016/j.neuroscience.2014.08.048. [http://dx.doi.org/10.1016/j.neuroscience.2014.08.048]. [PMID: 25218960]. [DOI] [PubMed] [Google Scholar]
- 52.Niu Z., Ma J., Chu H., Zhao Y., Feng W., Cheng Y. Melanocortin 4 receptor antagonists attenuates morphine antinociceptive tolerance, astroglial activation and cytokines expression in the spinal cord of rat. Neurosci. Lett. 2012;529(2):112–117. doi: 10.1016/j.neulet.2012.09.034. [http://dx.doi.org/10.1016/j.neulet.2012.09.034]. [PMID: 23022502]. [DOI] [PubMed] [Google Scholar]
- 53.Bajetto A., Bonavia R., Barbero S., Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J. Neurochem. 2002;82(6):1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [http://dx.doi.org/10.1046/j.1471-4159.2002.01091.x]. [PMID: 12354279]. [DOI] [PubMed] [Google Scholar]
- 54.Kim C.H. Chemokine-chemokine receptor network in immune cell trafficking. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2004;4(4):343–361. doi: 10.2174/1568008043339712. [http://dx.doi.org/10.2174/1568008043339712]. [PMID: 15578986]. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez E.J., Arms L., Vizzard M.A. The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction. BioMed Res. Int. 2014;2014:120525. doi: 10.1155/2014/120525. [http://dx.doi.org/10.1155/2014/120525]. [PMID: 24738044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White F.A., Bhangoo S.K., Miller R.J. Chemokines: integrators of pain and inflammation. Nat. Rev. Drug Discov. 2005;4(10):834–844. doi: 10.1038/nrd1852. [http://dx.doi.org/10.1038/nrd1852]. [PMID: 16224455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbadie C., Bhangoo S., De Koninck Y., Malcangio M., Melik-Parsadaniantz S., White F.A. Chemokines and pain mechanisms. Brain Res. Brain Res. Rev. 2009;60(1):125–134. doi: 10.1016/j.brainresrev.2008.12.002. [http://dx.doi.org/10.1016/j.brainresrev.2008.12.002]. [PMID: 19146875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curfs J.H., Meis J.F., Hoogkamp-Korstanje J.A. A primer on cytokines: sources, receptors, effects, and inducers. Clin. Microbiol. Rev. 1997;10(4):742–780. doi: 10.1128/cmr.10.4.742. [http://dx.doi.org/10.1128/CMR.10.4.742]. [PMID: 9336671]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bacon K., Baggiolini M., Broxmeyer H., Horuk R., Lindley I., Mantovani A., Maysushima K., Murphy P., Nomiyama H., Oppenheim J. Chemokine/chemokine receptor nomenclature. J. Soc. Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 60.Ransohoff R.M. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [http://dx.doi.org/10.1016/j.immuni.2009.09.010]. [PMID: 19836265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy I., Evans D.B., Dwinell M.B. Chemokines and chemokine receptors: update on utility and challenges for the clinician. Surgery. 2014;155(6):961–973. doi: 10.1016/j.surg.2014.02.006. [http://dx.doi.org/10.1016/j.surg.2014.02.006]. [PMID: 24856117]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazennec G., Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [http://dx.doi.org/10.1016/j.molmed.2010.01.003]. [PMID: 20163989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rutkowski M.D., DeLeo J.A. The role of cytokines in the initiation and maintenance of chronic pain. Drug News Perspect. 2002;15(10):626–632. [http://dx.doi.org/10.1358/dnp.2002.15.10.740239]. [PMID: 12677247]. [PubMed] [Google Scholar]
- 64.Constantin G., Majeed M., Giagulli C., Piccio L., Kim J.Y., Butcher E.C., Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13(6):759–769. doi: 10.1016/s1074-7613(00)00074-1. [http://dx.doi.org/10.1016/S1074-7613(00)00074-1]. [PMID: 11163192]. [DOI] [PubMed] [Google Scholar]
- 65.Peng Y., Guo G., Shu B., Liu D., Su P., Zhang X., Gao F. Spinal CX3CL1/CX3CR1 may not directly participate in the development of morphine tolerance in rats. Neurochem. Res. 2017;42(11):3254–3267. doi: 10.1007/s11064-017-2364-z. [http://dx.doi.org/10.1007/s11064-017-2364-z]. [PMID: 28776289]. [DOI] [PubMed] [Google Scholar]
- 66.Chen X., Geller E.B., Rogers T.J., Adler M.W. The chemokine CX3CL1/fractalkine interferes with the antinociceptive effect induced by opioid agonists in the periaqueductal grey of rats. Brain Res. 2007;1153:52–57. doi: 10.1016/j.brainres.2007.03.066. [http://dx.doi.org/10.1016/j.brainres.2007.03.066]. [PMID: 17459345]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao C.M., Guo R.X., Hu F., Meng J.L., Mo L.Q., Chen P.X., Liao X.X., Cui Y., Feng J.Q. Spinal MCP-1 contributes to the development of morphine antinociceptive tolerance in rats. Am. J. Med. Sci. 2012;344(6):473–479. doi: 10.1097/MAJ.0b013e31826a82ce. [http://dx.doi.org/10.1097/MAJ.0b013e31826a82ce]. [PMID: 23187120]. [DOI] [PubMed] [Google Scholar]
- 68.Liu L., Gao X.J., Ren C.G., Hu J.H., Liu X.W., Zhang P., Zhang Z.W., Fu Z.J. Monocyte chemoattractant protein-1 contributes to morphine tolerance in rats with cancer-induced bone pain. Exp. Ther. Med. 2017;13(2):461–466. doi: 10.3892/etm.2016.3979. [http://dx.doi.org/10.3892/etm.2016.3979]. [PMID: 28352316]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka T., Minami M., Nakagawa T., Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci. Res. 2004;48(4):463–469. doi: 10.1016/j.neures.2004.01.004. [http://dx.doi.org/10.1016/j.neures.2004.01.004]. [PMID: 15041200]. [DOI] [PubMed] [Google Scholar]
- 70.White F.A., Sun J., Waters S.M., Ma C., Ren D., Ripsch M., Steflik J., Cortright D.N., Lamotte R.H., Miller R.J. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. USA. 2005;102(39):14092–14097. doi: 10.1073/pnas.0503496102. [http://dx.doi.org/10.1073/pnas.0503496102]. [PMID: 16174730]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J., De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006;97(3):772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.03746.x]. [PMID: 16524371]. [DOI] [PubMed] [Google Scholar]
- 72.Chen X., Geller E.B., Rogers T.J., Adler M.W. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88(1):36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [http://dx.doi.org/10.1016/j.drugalcdep.2006.09.010]. [PMID: 17049756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szabo I., Chen X.H., Xin L., Adler M.W., Howard O.M., Oppenheim J.J., Rogers T.J. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc. Natl. Acad. Sci. USA. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [http://dx.doi.org/10.1073/pnas.102327699]. [PMID: 12130663]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivat C., Sebaihi S., Van Steenwinckel J., Fouquet S., Kitabgi P., Pohl M., Melik Parsadaniantz S., Reaux-Le Goazigo A. Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain Behav. Immun. 2014;38:38–52. doi: 10.1016/j.bbi.2013.11.010. [http://dx.doi.org/10.1016/j.bbi.2013.11.010]. [PMID: 24263070]. [DOI] [PubMed] [Google Scholar]
- 75.Wilson N.M., Jung H., Ripsch M.S., Miller R.J., White F.A. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav. Immun. 2011;25(3):565–573. doi: 10.1016/j.bbi.2010.12.014. [http://dx.doi.org/10.1016/j.bbi.2010.12.014]. [PMID: 21193025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin C.P., Kang K.H., Tu H.J., Wu M.Y., Lin T.H., Liou H.C., Sun W.Z., Fu W.M. CXCL12/CXCR4 signaling contributes to the pathogenesis of opioid tolerance: A translational study. Anesth. Analg. 2017;124(3):972–979. doi: 10.1213/ANE.0000000000001480. [http://dx.doi.org/10.1213/ANE.0000000000001480]. [PMID: 28212183]. [DOI] [PubMed] [Google Scholar]
- 77.Lin C.P., Kang K.H., Lin T.H., Wu M.Y., Liou H.C., Chuang W.J., Sun W.Z., Fu W.M. Role of spinal CXCL1 (GROα) in opioid tolerance: a human-to-rodent translational study. Anesthesiology. 2015;122(3):666–676. doi: 10.1097/ALN.0000000000000523. [http://dx.doi.org/10.1097/ALN.0000000000000523]. [PMID: 25383568]. [DOI] [PubMed] [Google Scholar]
- 78.Ye D., Bu H., Guo G., Shu B., Wang W., Guan X., Yang H., Tian X., Xiang H., Gao F. Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: in-volvement of Gi protein. J. Mol. Neurosci. 2014;53:571–579. doi: 10.1007/s12031-013-0223-1. [DOI] [PubMed] [Google Scholar]
- 79.Wang W., Peng Y., Yang H., Bu H., Guo G., Liu D., Shu B., Tian X., Luo A., Zhang X., Gao F. Potential role of CXCL10/CXCR3 signaling in the development of morphine tolerance in periaqueductal gray. Neuropeptides. 2017;65:120–127. doi: 10.1016/j.npep.2017.07.004. [http://dx.doi.org/10.1016/j.npep.2017.07.004]. [PMID: 28755808]. [DOI] [PubMed] [Google Scholar]
- 80.Booth V., Clark-Lewis I., Sykes B.D. NMR structure of CXCR3 binding chemokine CXCL11 (ITAC). Protein Sci. Soc. 2004;13:2022–2028. doi: 10.1110/ps.04791404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cole K.E., Strick C.A., Paradis T.J., Ogborne K.T., Loetscher M., Gladue R.P., Lin W., Boyd J.G., Moser B., Wood D.E., Sahagan B.G., Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998;187(12):2009–2021. doi: 10.1084/jem.187.12.2009. [http://dx.doi.org/10.1084/jem.187.12.2009]. [PMID: 9625760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo G., Peng Y., Xiong B., Liu D., Bu H., Tian X., Yang H., Wu Z., Cao F., Gao F. Involvement of chemokine CXCL11 in the development of morphine tolerance in rats with cancer-induced bone pain. J. Neurochem. 2017;141(4):553–564. doi: 10.1111/jnc.13919. [http://dx.doi.org/10.1111/jnc.13919]. [PMID: 27926984]. [DOI] [PubMed] [Google Scholar]
- 83.Raeburn C.D., Sheppard F., Barsness K.A., Arya J., Harken A.H. Cytokines for surgeons. Am. J. Surg. 2002;183(3):268–273. doi: 10.1016/s0002-9610(02)00781-x. [http://dx.doi.org/10.1016/S0002-9610(02)00781-X]. [PMID: 11943124]. [DOI] [PubMed] [Google Scholar]
- 84.Mosser D.M., Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [http://dx.doi.org/10.1111/j.1600-065X.2008.00706.x]. [PMID: 19161426]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [http://dx.doi.org/10.1146/annurev.immunol.19.1.683]. [PMID: 11244051]. [DOI] [PubMed] [Google Scholar]
- 86.Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147(11):3815–3822. [PMID: 1940369]. [PubMed] [Google Scholar]
- 87.Cunha F.Q., Poole S., Lorenzetti B.B., Veiga F.H., Ferreira S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br. J. Pharmacol. 1999;126(1):45–50. doi: 10.1038/sj.bjp.0702266. [http://dx.doi.org/10.1038/sj.bjp.0702266]. [PMID: 10051119]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiorentino D.F., Zlotnik A., Vieira P., Mosmann T.R., Howard M., Moore K.W., O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146(10):3444–3451. [PMID: 1827484]. [PubMed] [Google Scholar]
- 89.Kasama T., Strieter R.M., Lukacs N.W., Lincoln P.M., Burdick M.D., Kunkel S.L. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J. Clin. Invest. 1995;95(6):2868–2876. doi: 10.1172/JCI117993. [http://dx.doi.org/10.1172/JCI117993]. [PMID: 7769128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J., Barke R.A., Charboneau R., Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J. Immunol. 2005;174(1):426–434. doi: 10.4049/jimmunol.174.1.426. [http://dx.doi.org/10.4049/jimmunol.174.1.426]. [PMID: 15611267]. [DOI] [PubMed] [Google Scholar]
- 91.Lysle D.T., Coussons M.E., Watts V.J., Bennett E.H., Dykstra L.A. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J. Pharmacol. Exp. Ther. 1993;265(3):1071–1078. [PMID: 7685383]. [PubMed] [Google Scholar]
- 92.Limiroli E., Gaspani L., Panerai A.E., Sacerdote P. Differential morphine tolerance development in the modulation of macrophage cytokine production in mice. J. Leukoc. Biol. 2002;72(1):43–48. [PMID: 12101261]. [PubMed] [Google Scholar]
- 93.Sacerdote P. Effects of in vitro and in vivo opioids on the production of IL-12 and IL-10 by murine macrophages. Ann. N. Y. Acad. Sci. 2003;992:129–140. doi: 10.1111/j.1749-6632.2003.tb03144.x. [http://dx.doi.org/10.1111/j.1749-6632.2003.tb03144.x]. [PMID: 12794053]. [DOI] [PubMed] [Google Scholar]
- 94.Bao Y.H., Zhou Q.H., Chen R., Xu H., Zeng L., Zhang X., Jiang W., Du D. Gabapentin attenuates morphine tolerance through interleukin-10. Neuroreport. 2014;25(2):71–76. doi: 10.1097/WNR.0b013e328363fde8. [http://dx.doi.org/10.1097/WNR.0b013e328363fde8]. [PMID: 24247277]. [DOI] [PubMed] [Google Scholar]
- 95.Lin S.L., Tsai R.Y., Tai Y.H., Cherng C.H., Wu C.T., Yeh C.C., Wong C.S. Ultra-low dose naloxone upregulates interleukin-10 expression and suppresses neuroinflammation in morphine-tolerant rat spinal cords. Behav. Brain Res. 2010;207(1):30–36. doi: 10.1016/j.bbr.2009.09.034. [http://dx.doi.org/10.1016/j.bbr.2009.09.034]. [PMID: 19799935]. [DOI] [PubMed] [Google Scholar]
- 96.Tai Y.H., Tsai R.Y., Lin S.L., Yeh C.C., Wang J.J., Tao P.L., Wong C.S. Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology. 2009;110(6):1379–1389. doi: 10.1097/ALN.0b013e31819fccd5. [http://dx.doi.org/10.1097/ALN.0b013e31819fccd5]. [PMID: 19417613]. [DOI] [PubMed] [Google Scholar]
- 97.Bao Y.H., Zhou Q.H., Chen R., Xu H., Zeng L.L., Zhang X., Jiang W., Du D.P. Gabapentin enhances the morphine anti-nociceptive effect in neuropathic pain via the interleukin-10-heme oxygenase-1 signalling pathway in rats. J. Mol. Neurosci. 2014;54:137–146. doi: 10.1007/s12031-014-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [http://dx.doi.org/10.1056/NEJM200103223441207]. [PMID: 11259725]. [DOI] [PubMed] [Google Scholar]
- 99.Zaringhalam J., Hormozi A., Tekieh E., Razavi J., Khanmohammad R., Golabi S. Serum IL-10 involved in morphine tolerance development during adjuvant-induced arthritis. J. Physiol. Biochem. 2014;70(2):497–507. doi: 10.1007/s13105-014-0330-7. [http://dx.doi.org/10.1007/s13105-014-0330-7]. [PMID: 24643510]. [DOI] [PubMed] [Google Scholar]
- 100.Fairbanks C.A., Wilcox G.L. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J. Pharmacol. Exp. Ther. 1997;282(3):1408–1417. [PMID: 9316854]. [PubMed] [Google Scholar]
- 101.Célèrier E., Laulin J., Larcher A., Le Moal M., Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847(1):18–25. doi: 10.1016/s0006-8993(99)01998-8. [http://dx.doi.org/10.1016/S0006-8993(99)01998-8]. [PMID: 10564731]. [DOI] [PubMed] [Google Scholar]
- 102.Ma J.Y., Zhao Z.Q. The involvement of glia in long-term plasticity in the spinal dorsal horn of the rat. Neuroreport. 2002;13(14):1781–1784. doi: 10.1097/00001756-200210070-00017. [http://dx.doi.org/10.1097/00001756-200210070-00017]. [PMID: 12395122]. [DOI] [PubMed] [Google Scholar]
- 103.Watkins L.R., Milligan E.D., Maier S.F. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PMID: 12617561]. [PubMed] [Google Scholar]
- 104.Watkins L.R., Hutchinson M.R., Johnston I.N., Maier S.F. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28(12):661–669. doi: 10.1016/j.tins.2005.10.001. [http://dx.doi.org/10.1016/j.tins.2005.10.001]. [PMID: 16246435]. [DOI] [PubMed] [Google Scholar]
- 105.Hutchinson M.R., Bland S.T., Johnson K.W., Rice K.C., Maier S.F., Watkins L.R. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [http://dx.doi.org/10.1100/tsw.2007.230]. [PMID: 17982582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang D., Huo Y., Quirion R., Hong Y. Involvement of adrenomedullin in the attenuation of acute morphine-induced analgesia in rats. Peptides. 2014;54:67–70. doi: 10.1016/j.peptides.2014.01.009. [http://dx.doi.org/10.1016/j.peptides.2014.01.009]. [PMID: 24468549]. [DOI] [PubMed] [Google Scholar]
- 107.Huang B.Q., Hong Y. 2015.
- 108.Tai Y.H., Wang Y.H., Wang J.J., Tao P.L., Tung C.S., Wong C.S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124(1-2):77–86. doi: 10.1016/j.pain.2006.03.018. [http://dx.doi.org/10.1016/j.pain.2006.03.018]. [PMID: 16697108]. [DOI] [PubMed] [Google Scholar]
- 109.Liu S.J., Wang R.I. Increased sensitivity of the central nervous system to morphine analgesia by amitriptyline in naive and morphine-tolerant rats. Biochem. Pharmacol. 1981;30(15):2103–2109. doi: 10.1016/0006-2952(81)90229-x. [http://dx.doi.org/10.1016/0006-2952(81)90229-X]. [PMID: 7295330]. [DOI] [PubMed] [Google Scholar]
- 110.Sivagnanam G., Adithan C., Raveendran R., Bapna J.S. Amitriptyline analgesia: tolerance & cross tolerance pattern. Indian J. Med. Res. 1986;84:200–203. [PMID: 3759175]. [PubMed] [Google Scholar]
- 111.Tai Y.H., Tsai R.Y., Wang Y.H., Cherng C.H., Tao P.L., Liu T.M., Wong C.S. Amitriptyline induces nuclear transcription factor-kappaB-dependent glutamate transporter upregulation in chronic morphine-infused rats. Neuroscience. 2008;153(3):823–831. doi: 10.1016/j.neuroscience.2008.02.055. [http://dx.doi.org/10.1016/j.neuroscience.2008.02.055]. [PMID: 18400403]. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y.N., Tsai R.Y., Lin S.L., Chien C.C., Cherng C.H., Wu C.T., Yeh C.C., Wong C.S. Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKCγ signaling pathway. Behav. Brain Res. 2012;229(2):401–411. doi: 10.1016/j.bbr.2012.01.044. [http://dx.doi.org/10.1016/j.bbr.2012.01.044]. [PMID: 22309983]. [DOI] [PubMed] [Google Scholar]
- 113.Tai Y.H., Wang Y.H., Tsai R.Y., Wang J.J., Tao P.L., Liu T.M., Wang Y.C., Wong C.S. Amitriptyline preserves morphine’s antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain. 2007;129(3):343–354. doi: 10.1016/j.pain.2007.01.031. [http://dx.doi.org/10.1016/j.pain.2007.01.031]. [PMID: 17346885]. [DOI] [PubMed] [Google Scholar]
- 114.Habibi-Asl B., Vaez H., Najafi M., Bidaghi A., Ghanbarzadeh S. Attenuation of morphine-induced dependence and tolerance by ceftriaxone and amitriptyline in mice. Acta Anaesthesiol. Taiwan. 2014;52(4):163–168. doi: 10.1016/j.aat.2014.11.001. [http://dx.doi.org/10.1016/j.aat.2014.11.001]. [PMID: 25557842]. [DOI] [PubMed] [Google Scholar]
- 115.Shen K.F., Crain S.M. Ultra-low doses of naltrexone or etorphine increase morphine’s antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757(2):176–190. doi: 10.1016/s0006-8993(97)00197-2. [http://dx.doi.org/10.1016/S0006-8993(97)00197-2]. [PMID: 9200746]. [DOI] [PubMed] [Google Scholar]
- 116.Crain S.M., Shen K.F. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84(2-3):121–131. doi: 10.1016/s0304-3959(99)00223-7. [http://dx.doi.org/10.1016/S0304-3959(99)00223-7]. [PMID: 10666516]. [DOI] [PubMed] [Google Scholar]
- 117.Singh V.P., Patil C.S., Jain N.K., Singh A., Kulkarni S.K. Paradoxical effects of opioid antagonist naloxone on SSRI-induced analgesia and tolerance in mice. Pharmacology. 2003;69(3):115–122. doi: 10.1159/000072662. [http://dx.doi.org/10.1159/000072662]. [PMID: 14512696]. [DOI] [PubMed] [Google Scholar]
- 118.Lin Y.S., Tsai R.Y., Shen C.H., Chien C.C., Tsai W.Y., Guo S.L., Wong C.S. Ultra-low dose naloxone restores the antinocicepitve effect of morphine in PTX-treated rats: association of IL-10 upregulation in the spinal cord. Life Sci. 2012;91(5-6):213–220. doi: 10.1016/j.lfs.2012.07.005. [http://dx.doi.org/10.1016/j.lfs.2012.07.005]. [PMID: 22820166]. [DOI] [PubMed] [Google Scholar]
- 119.Tsai R.Y., Tai Y.H., Tzeng J.I., Cherng C.H., Yeh C.C., Wong C.S. Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats by reversing the coupling of mu-opioid receptors from Gs-protein to coupling to Gi-protein. Neuroscience. 2009;164(2):435–443. doi: 10.1016/j.neuroscience.2009.08.015. [http://dx.doi.org/10.1016/j.neuroscience.2009.08.015]. [PMID: 19682558]. [DOI] [PubMed] [Google Scholar]
- 120.Tsai R.Y., Jang F.L., Tai Y.H., Lin S.L., Shen C.H., Wong C.S. Ultra-low-dose naloxone restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in PTX-treated rats. Neuropsychopharmacology. 2008;33:2772–2782. doi: 10.1038/sj.npp.1301672. [DOI] [PubMed] [Google Scholar]
- 121.Shimoyama M., Shimoyama N., Inturrisi C.E., Elliott K.J. Gabapentin enhances the antinociceptive effects of spinal morphine in the rat tail-flick test. Pain. 1997;72(3):375–382. doi: 10.1016/s0304-3959(97)00065-1. [http://dx.doi.org/10.1016/S0304-3959(97)00065-1]. [PMID: 9313278]. [DOI] [PubMed] [Google Scholar]
- 122.Hansen C., Gilron I., Hong M. The effects of intrathecal gabapentin on spinal morphine tolerance in the rat tail-flick and paw pressure tests. Anesth. Analg. 2004;99(4):1180–1184. doi: 10.1213/01.ANE.0000130383.87438.A9. [http://dx.doi.org/10.1213/01.ANE.0000130383.87438.A9]. [PMID: 15385372]. [DOI] [PubMed] [Google Scholar]
- 123.Wei X., Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J. Anesth. 2012;26(2):236–241. doi: 10.1007/s00540-011-1272-7. [http://dx.doi.org/10.1007/s00540-011-1272-7]. [PMID: 22048285]. [DOI] [PubMed] [Google Scholar]
- 124.Field M.J., Oles R.J., Lewis A.S., McCleary S., Hughes J., Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br. J. Pharmacol. 1997;121(8):1513–1522. doi: 10.1038/sj.bjp.0701320. [http://dx.doi.org/10.1038/sj.bjp.0701320]. [PMID: 9283683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smiley M.M., Lu Y., Vera-Portocarrero L.P., Zidan A., Westlund K.N. Intrathecal gabapentin enhances the analgesic effects of subtherapeutic dose morphine in a rat experimental pancreatitis model. Anesthesiology. 2004;101(3):759–765. doi: 10.1097/00000542-200409000-00026. [http://dx.doi.org/10.1097/00000542-200409000-00026]. [PMID: 15329602]. [DOI] [PMC free article] [PubMed] [Google Scholar]