Abstract
Background
It is now widely established that the devastating effects of prenatal alcohol exposure on the embryo and fetus development cause marked cognitive and neurobiological deficits in the newborns. The negative effects of the gestational alcohol use have been well documented and known for some time. However, also the subtle role of alcohol consumption by fathers prior to mating is drawing special attention.
Objective
Both paternal and maternal alcohol exposure has been shown to affect the neurotrophins' signalling pathways in the brain and in target organs of ethanol intoxication. Neurotrophins, in particular nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), are molecules playing a pivotal role in the survival, development and function of the peripheral and central nervous systems but also in the pathogenesis of developmental defects caused by alcohol exposure.
Methods
New researches from the available literature and experimental data from our laboratory are presented in this review to offer the most recent findings regarding the effects of maternal and paternal prenatal ethanol exposure especially on the neurotrophins' signalling pathways.
Results
NGF and BDNF changes play a subtle role in short- and long-lasting effects of alcohol in ethanol target tissues, including neuronal cell death and severe cognitive and physiological deficits in the newborns.
Conclusion
The review suggests a possible therapeutic intervention based on the use of specific molecules with antioxidant properties in order to induce a potential prevention of the harmful effects of the paternal and/or maternal alcohol exposure.
Keywords: Gestational alcohol, FAS, oxidative stress, FASD, NGF, BDNF
1. INTRODUCTION
Neurotrophic factors regulate growth, proliferation, differentiation, and maturation of cells, but also cell migration, cell metabolism and apoptotic cell death [1, 2]. Neurotrophins are members of the family of neurotrophic factors and include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and NT-4/5. Neurotrophins play a pivotal role in proper brain development and in mediating synaptic plasticity during adulthood [3]. NGF is the first and best characterized neurotrophin, expressed both in the central (CNS) and peripheral nervous system (PNS) [2, 4, 5], while BDNF is more widely distributed in the CNS [6-8]. The biological effects of these two neurotrophins are mediated through the activation of different members of the tropomyosin-related kinase (Trk) family also known as high-affinity receptors: NGF binds to TrkA while BDNF activates TrkB. Once activated, these receptors stimulate several intracellular signalling mechanisms, leading to different cellular responses and promoting survival, growth, differentiation and maintenance of both neuronal and non-neuronal cells. Furthermore, both neurotrophins are able to bind and activate the p75 neurotrophin receptor, p75NTR, (the neurotrophins low-affinity receptor), a member of the tumor necrosis factor receptor superfamily [6-8]. It is known that during central nervous system development, the expression of neurotrophins and their receptors are regulated and mainly localized in the hippocampus and cortex, brain areas involved in neuronal plasticity associated with learning and memory mechanisms [9], including brain-related disease [10]. During fetal growth, abnormalities in NGF and/or BDNF synthesis could deregulate CNS development, and in particular the limbic system development, with long-lasting effects on neuronal connections. These alterations in hippocampus and cortex development and in NGF/BDNF levels could be associated with the vulnerability to neuropsychiatric disorders. There are several epigenetic variables that can modify neuronal activity and, in this context, ethanol exposure certainly exerts an important modulation in the genetic expression of growth factors [11, 12]. Furthermore, it has been suggested that alcohol-neurotrophin interaction during development could contribute to the deleterious effects of prenatal alcohol exposure on neuroplasticity, learning and memory [3].
Several lines of evidence in the last years have highlighted that alcohol exposure during development may impair neurotrophic factors production and the expression of their receptors [13-20]. The most important neurotrophins involved in ethanol-induced toxicity are NGF and BDNF and their levels are known to be disrupted in the brain and periphery during acute/chronic alcohol consumption [13, 14, 21, 22]. Ethanol exposure has been demonstrated to alter the expression of neurotrophins and their receptors as well as the expression of their downstream signalling proteins. In particular, alcohol inhibits the expression of endogenous extracellular signal-regulated kinase (ERK) and the phosphatidylinositol-3-kinase (PI3K) [23-25]. Furthermore, Heberlein and colleagues demonstrated possible links between the epigenetic modulation of NGF and BDNF, the serum levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and the symptomatology of alcohol dependence [26]. In particular, they showed an increase in NGF and IL-6 serum levels following alcohol consumption [27, 28] as well as an association between BDNF, TNF-α serum levels and the history of alcohol consumption [28, 29], suggesting that changes in the methylation of neurotrophins genes may contribute to the development of alcohol dependence by affecting relevant downstream signalling cascades [26].
2. The Pathophysiology of Prenatal Alcohol Exposure
From ancient times it has been reported the possibility that drinking can cause fetal harm. According to a legend, Vulcan was born lame because Jupiter was drunk during conception. Hippocrates argued that parental drunkenness is the cause of the weakness of their children. In Carthage, there was a law that forbade the couple to drink alcohol on the day when they had to consummate the marriage.
Recommendations for women to not drink during pregnancy are already in the Bible, while, in more recent times, this problem began to be mentioned in medical journals, from the seventeenth century onwards. However, only in the last fifty years it has come to some awareness that children of alcoholic women who continue to drink even in pregnancy may have a variety of problems, including birth defects and neurological and cognitive disorders, closely related to a teratogenic action of alcohol.
Children of alcoholics, with particular and recurrent morphological and cognitive features have been described first in France and then in the United States [30, 31]. In 1973, Jones and Smith [32] coined the term Fetal Alcohol Syndrome (FAS) to indicate the complex characteristics of these children, and particularly emphasized the constant facial abnormalities and cognitive and behavioral problems. In 1978, in an important review on the subject, Clarren and Smith asserted that alcohol abuse during pregnancy was probably the leading cause of mental retardation in the Western world, redefining the series of malformations and behavioral alterations that were called Funny Looking Kinds (bizarre-types) and considering the mental retardation as the main neurocognitive problem of these children [33]. The extent of damage induced by ethanol can vary due to the timing, frequency and volume of alcohol consumed, as well as the genetic and metabolic features of the mother, leading to a wide variability in severity symptoms. The disorders resulting from prenatal alcohol exposure, together identified as Fetal Alcohol Spectrum Disorders (FASD) [34-37], include a range of categories, referred by the Institute of Medicine (IOM) [38] as Alcohol Related Neurodevelopmental Disorders (ARND), Alcohol Related Birth Defects (ARBD), the partial Fetal Alcohol Syndrome (pFAS) and finally, the FAS, which is the most severe form.
In adults, the ethanol toxicity has been demonstrated in several organs, but those mostly affected seem to be the brain and the liver. As for the fetal exposure, the primarily affected organ is the brain [39]. At the time, the mechanisms of ethanol toxicity on the fetus have not been fully elucidated and several hypotheses have been proposed. In this regard, in 1981, Henderson and colleagues in their studies on the mechanisms leading to FAS focused on the mutagenicity of ethanol [40]. Ten years later, Michaelis wrote a review that discussed various cellular and molecular mechanisms including the disruption of placental transport of nutrients and the inhibition of protein synthesis on growth and maturation; the development of hypoxia and changes in the control of vascular tone; interactions between hypoxic conditions and neurotransmitter-activated mechanisms in the production of cellular damage in developing neurons as well as abnormalities in calcium-handling mechanisms and their effects on neuronal migration and differentiation [41]. More recently, another research group has sought to shed light on how ethanol affects brain maturation demonstrating the activation of a widespread neuronal death during the development of rat forebrain consequent to N-methyl-D aspartate (NMDA)-glutamate receptor blockage and activation of GABA-A receptors [42]. It should be emphasized that the excitatory amino acids may influence the processes of neuronal differentiation and synaptogenesis, may modulate the organization of neural circuits and may timely regulate biochemical events related to the phenomenon of neuronal plasticity. Therefore, it is conceivable that a reduction of glutamatergic transmission, caused by ethanol exposure at critical stages of development, plays a key role in determining the neurotoxic effects of this substance abuse.
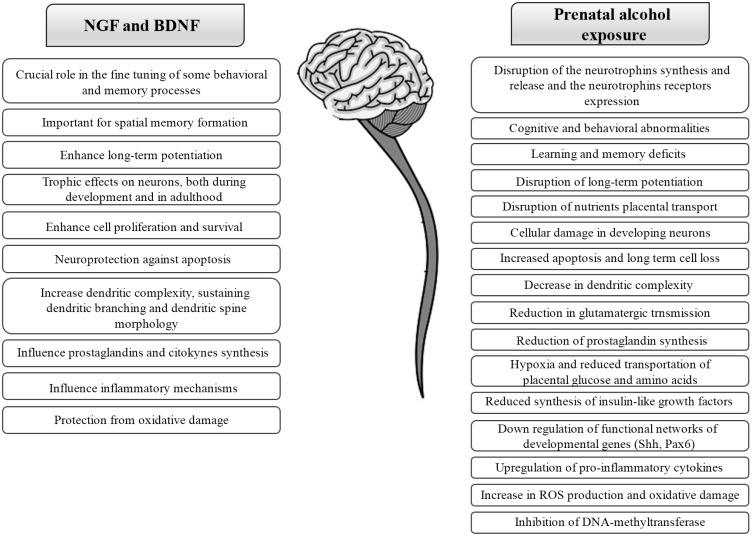
Other alcohol teratogenic mechanisms proposed in recent years are reduced prostaglandin synthesis, reduced transportation of placental glucose, amino acids and hypoxia, reduced synthesis of insulin-like growth factor, alterations of IL-1 mediated cellular transmission, down regulation of functional networks of developmental genes (Shh, Pax6), inhibition of DNA-methyltransferase and alterations at the neurotrophins level (Fig. 1). Furthermore, during the last 20 years, among the mechanisms responsible for the alcohol toxicity great interest it has been given to oxidative stress [43] and in those compounds having antioxidant properties [39, 44-47]. The ethanol-induced cell damage is probably the sum of all the mechanisms described above and probably also of others that have not yet been uncovered.
Fig. (1).
Schematic representation of the main roles of NGF and BDNF in the nervous system and the overlapping effects of the prenatal alcohol exposure.
It seems now widely recognized that the adverse effects of alcohol are not only produced by the ethanol molecule itself, but may depend also on the action of ethanol metabolites/products within the central nervous system (CNS) [48-51]. The first metabolite product of alcohol is the acetaldehyde, an extremely toxic molecule that is produced by the partial oxidation of ethanol through several non-enzymatic and enzymatic mechanisms, including alcohol dehydrogenase (ADH), cytochrome P4502E1 (CYP2E1) and the catalase H2O2 system [50]. As for FAS, it is not well known whether alcohol itself or one or more of its metabolites is the primary cause of its onset [40]. Because of the kinetics of amniotic fluid circulation and because of the absence of enzymes necessary for ethanol biotransformation in the embryo, the amniotic fluid acts as a reservoir for unchanged alcohol and acetaldehyde. Indeed, ethanol and its teratogenic metabolite acetaldehyde freely cross the placenta, and accumulate in fetal blood at concentrations similar to those found in maternal blood [52-54]. Thus, the fetus is exposed to both compounds long after they have been metabolized from the maternal organism [55].
An important contribution in the study of prenatal ethanol toxicity has been given by findings from animal models, in order to clarify the cellular and biochemical mechanisms as well as the consequences of early developmental ethanol exposure [13, 16, 56].
3. Maternal Alcohol Exposure
A number of studies from our and other groups showed that ethanol exposure in mice during pregnancy considerably affects NGF and BDNF synthesis and release [13, 14, 16, 25, 57-59]. The main element that exposes the fetus to the teratogenic risk of alcohol is the reduced ability of women to metabolize this substance. Women, at the same alcohol amounts ingested, have a blood alcohol concentration (BAC) of up to 3-4 times higher than that of men. Moreover, women were more likely than men to report drink problems at the same level of alcohol consumption. Compared to men, over the same timeframe and with comparable alcohol use, women have a 50 to 100 percent higher death rate due to suicides, accidents, and health problems [60] (web site: https://pubs.niaaa.nih.gov/publications/brochurewomen/Woman_English.pdf), experience a higher incidence of cardiomyopathy and liver diseases [61, 62] and show enhanced motor and cognitive impairments following exposure to alcohol [63-65].
This gender difference, leading to potentiated women vulnerability to the alcohol effects, is physiological and it is thought to be mainly due to differences in body water content, enzymatic activity of ADH and sex hormone, between men and women [66-69]. As for the enzymatic activity of ADH, it has been demonstrated that it varies with ages, in particular, in normal male drinkers it is highest between 20 and 40 years and then decreases until be halved between 61 and 80 years. By contrast, in women the gastric ADH activity is very low between 20 and 40 years, reaches its peak between 40 and 60 and then decreases similarly to what happens in men [70]. Therefore, the critical period corresponds to the age group between 20 and 40 years, when the gender differences are more significant, women are metabolically more exposed to the alcohol effects and above all they are of childbearing age, so, the risk of exposure and fetal damage increases. The increased concentration of ethanol in the blood stream leads to the saturation of the enzymes involved in its metabolism. Ethanol quickly reaches the mother's bloodstream and, by facilitated diffusion, crosses the placental barrier and, from there, is distributed to fetal circulatory system. Within minutes after taking an alcoholic drink, the alcohol concentration in the blood of the fetus and in the amniotic fluid is similar to that of the mother [71, 72]. Unlike the adult, however, the fetus cannot metabolize alcohol because devoid of enzymes responsible for this purpose. In this context, is also necessary to consider the presence, at an individual level, of genetic variants affecting genes involved in alcohol metabolism (alcohol dehydrogenase and aldehyde dehydrogenase) [73].
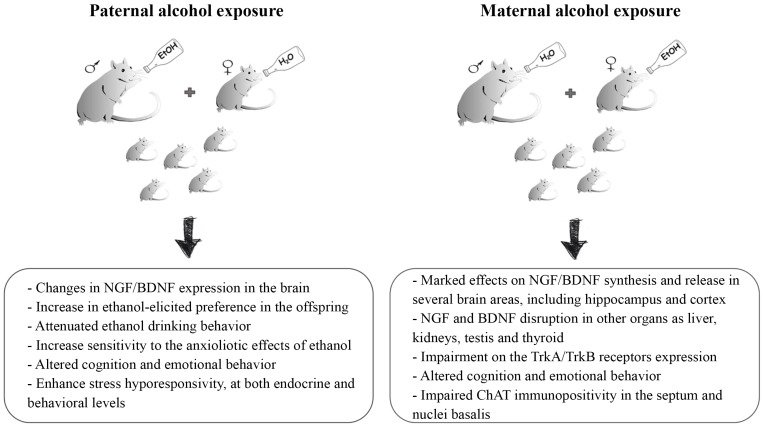
A still growing number of studies from animal models demonstrated that ethanol exposure in mice during pregnancy has marked effects on the NGF and BDNF synthesis and release [15, 74-76]. These findings suggest that the abnormal development of the central and peripheral nervous system may be due, at least partly, to growth factors deregulation (Fig. 2). Studies have provided evidence that BDNF and other neurotrophins appear to be crucial for the neurogenesis in the hippocampus and other neuronal plasticity systems [77, 78] and that alcohol exposure can affect NGF and BDNF expression in several brain areas [79-83]. Developmental models have demonstrated that prenatal ethanol exposure significantly potentiate NGF levels in cortex/striatum of one day old rats, while levels return to baseline after 10 days [84, 85]. Moreover, prenatal ethanol exposure induces an increase in TrkA receptor levels in the cortex of male and female pups on one day old rats [86]. Prenatal alcohol exposure has also been shown to alter BDNF signalling in several brain regions, for example decreasing the levels of BDNF protein in the medial prefrontal cortex [79]. It was also demonstrated that ethanol exposure from gestational days 5 to 20 induces a reduction in BDNF protein and mRNA in the rat cortex and hippocampus, when assessed on postnatal days 7-8 [58]. Prenatal ethanol exposure alters also TrkB receptor levels, inhibiting the phosphorylation of TrkB and leaving the total TrkB level unchanged [58]. Another study showed that alcohol exposure in utero produces a decrease in TrkB levels in the hippocampus of male rat pups on postnatal day 1, an increase in TrkB in the cortex, while no changes in TrkB levels in the septum and cerebellum were found [86]. The same authors have shown a different pattern of changes in female pups, demonstrating a decrease in TrkB levels in the septum, a consistent elevation in the cortex and no changes in hippocampus [86]. In general, the variety of changes in BDNF signalling highlighted by several studies is related to brain region, sex of animal and time point [3].
Fig. (2).
Representative illustration describing the prenatal alcohol exposure mice models and the main effects that both maternal and paternal alcohol exposure induce in the offspring.
Our research group, in a longitudinal analysis using a mouse model of prenatal ethanol exposure, investigated the short- and long-term consequences of prenatal alcohol consumption in the offspring during adolescence, adulthood and aging [13-16]. We analyzed cognitive and behavioral parameters, the levels of NGF and BDNF in the offspring’s brain, including hippocampus and cortex, and the choline acetyltransferase (ChAT) immunopositivity. Data showed that early administration of ethanol induces profound alterations in the levels of NGF and BDNF in the hippocampus and other brain areas of the offspring, associated to altered cognition and emotional behavior and with impaired ChAT immunopositivity in the septum and nuclei basalis. The biggest changes have been reported in adult mice (90 days). NGF and BDNF disruptions were also discovered in other organs as the liver, kidneys, testis and thyroid.
An interesting aspect of our work was the comparison between the effects of prenatal exposure to ethanol per se compared to red wine (at the same ethanol concentration). Data obtained in this investigation showed that animals early exposed to red wine, although the amount of ethanol was comparable, did not display the same alteration in the brain morphology and function and in cognitive performance of those mice exposed to ethanol only, suggesting that compounds contained into the red wine could counteract some deleterious effects of alcohol, acting as “protective” molecules. However, it should be also noted that no protection was observed for other ethanol target organs as liver, testis and adrenal glands.
Nevertheless, data emerged by our studies comparing the consequences of early exposure to ethanol and red wine (at the same ethanol concentration) during different stages of development in animal models should not be interpreted as encouraging women to consume red wine, before or during pregnancy and/or lactation. Among these “protective” compounds, we suggested that the main candidates could be antioxidant compounds as the polyphenols [44-47, 87]. The potential protective effect of the polyphenolic content of the red wine in our mouse model is in agreement with animal studies showing that the administration of antioxidants may contribute to reducing ethanol-induced fetal damage [88]. Particular attention is now focused by the scientific community on these molecules present in plants and plant-derived products due to their several effects in various pathological states [89]. Indeed, they are able to modulate and control oxidative stress, inflammation, apoptosis, mitochondrial dysfunction, all mechanisms which contribute to the etiology of neurodegenerative diseases [90], including ethanol-induced brain diseases [47]. Moreover, they demonstrated to potentiate neurotrophins’ action and this is probably an additional protective mechanism of these compounds [87]. Quite interestingly, red wine drinking associated with an enriched diet with fresh vegetables and fruits, known to contain high amounts of antioxidant polyphenols, by women during pregnancy could explain the low incidence of FAS-related problems in some mediterranean areas, although, with the contemporary presence of FASD children [91, 92] if compared to other countries [93]. Further findings showed that in humans affected by alcohol use disorders olive polyphenols may modulate and counteract BDNF serum elevation, but not NGF, due to alcohol withdrawal [21, 94].
4. Paternal Alcohol Exposure
Also the role of the paternal alcohol exposure prior to mating received special attention (Fig. 2). Previous data reported that about 75% of children with FASD had heavy drinkers or alcoholic biological fathers [95], suggesting that the anomalies in the offspring, generally attributed to the teratogenic effect of maternal drinking, may also be due to or exacerbated by the paternal alcohol consumption. Data showed that offspring sired by alcoholic fathers exhibit difficulty in a variety of learning tasks, as passive avoidance [96], and that paternal alcohol exposure seems to induce an increase in major malformations or average fetal or birth weights in animal offspring [95]. A number of studies from rodent models showed that paternal alcohol exposure prior to mating might induce developmental abnormalities, as changes in organs weight, including brain [97, 98], reduction in testosterone levels [99] and thickening of cortical layers [100], and also behavioral abnormalities as altered spatial learning performance [101] and disrupted immobility in a forced swim test [102]. In the context of these studies, our research group investigated whether or not paternal alcohol exposure may disrupt NGF and/or BDNF affecting EtOH preference/rewarding properties in the male offspring. We found changes in NGF and BDNF expression in the brain and an increase in ethanol-elicited preference in the offspring [103]. In another study it was found that male offspring sired by adult male mice exposed to chronic intermittent vapour ethanol prior to mating show attenuated ethanol drinking behavior, increased sensitivity to the anxiolytic effects of ethanol, and increased BDNF gene expression in the ventral tegmental area [104]. More recently, the same research group, in a study on adult male mice exposed to chronic intermittent vapour ethanol or control conditions for 5 weeks before being mated with ethanol-naïve females to produce ethanol- and control-sired offspring, investigated whether paternal ethanol exposure could impact stress responsivity in the offspring. They demonstrated that paternal ethanol exposure confers stress hypo-responsivity to male offspring, at both endocrine and behavioral levels [105]. In particular, in this study, authors showed reduced plasma Corticosterone in response to acute restraint stress and resistance to stress-induced excessive fluid intake. Indeed, using a stress-evoked ethanol-drinking assay, authors found increased total fluid intake in response to stress in the control-sired male offspring while ethanol-sired offspring seemed to be resistant to this stress-induced phenotype [105]. Liang and colleagues hypothesized also a possible alteration, induced by chronic paternal ethanol exposure, on the methylation of imprinted genes in sire spermatozoa that could also be inherited by the offspring, giving rise to developmental disorders [106].
Recently, in addition to animal studies strongly supporting the contribution of paternal alcoholic habits, robust evidence in humans are becoming increasingly available. Zuccolo and colleagues, using data from 68,244 mothers, fathers and children from the Norwegian Mother and Child Cohort Study (MoBa), investigated the association of maternal and paternal alcohol drinking before and early in pregnancy with infant head circumference. Researchers demonstrated higher probability of microcephaly at birth for higher paternal alcohol consumption before pregnancy, but not for maternal consumption [107, 108].
5. NEUROTROPHINS IN THE Strategies for FAS/FASD intervention and treatment
Alcohol consumption before conception and during pregnancy and lactation is among the most common preventable causes of mental retardation in the Western world. However, despite this, women and men continue to drink before and during pregnancy, making necessary the study of the mechanisms that contribute to alcohol’s harmful fetal effects and then the development of effective treatments and interventions for FAS and FASD as well as of reliable preventive and diagnostic systems [109].
Since, at the present time, a safe threshold of consumption can not be estimated, the only feasible recommendation for pregnant women is the total abstention from alcohol use before and during pregnancy [110, 111]. In addition, it is now known that fathers should also refrain from drinking before conception, being currently known a direct paternal impact on changes affecting the progeny, probably due to epigenetic mechanisms [103]. In addition to the preventive approach, it may be useful to identify prognostic markers of prenatal alcohol exposure in order to provide effective early intervention systems. The difficulty of establishing a correct estimation of FASD incidence is demonstrated by several studies reporting discordant epidemiological data. Popova and colleagues [112] reviewing international literature from 1984, tried to establish an estimation of the global, regional, and national prevalence of alcohol consumption causing FASD. According to these authors, Italy is among the five countries worldwide with the highest prevalence of FAS per 10,000 people. By contrast, Pichini and colleagues seem to disagree with this estimation [113], underlining that Italy is the country with the lowest annual consumption of alcohol per capita, lower percentage of women with alcohol use disorders, dependence and the highest number of female lifetime abstainers. Published data from an observational, cross-sectional case-control study [92], on 976 children from an area of small towns in central Italy, show a FAS rate between 4 and 12 per 1000 people in 2011. The large number of studies reflects the currently unreliable estimation of prevalence of FAS/FASD also due to the presence of several factors that could lead to overestimation or underestimation of the data [114].
CONCLUSION
Unfortunately, establishing the mother’s alcohol consumption is rather difficult as the admission of alcohol use before and during pregnancy may be accompanied by heavy guilt feelings [110, 115]. This means that for assessing the real mother’s alcohol consumption is necessary an approach based not only on specific screening questionaries but also on objective measurements of the real presence of alcohol in the body. The use of direct and non-direct biomarkers of alcohol use as Carbohydrate Deficient Transferrin, Ethylglucuronide, Fatty Acid Ethyl Esters, Ethylsulfate and Phosphatidylethanol can help to overcome this problem [109, 116, 117]. In particular, the measurement of EtG in several biological matrices, including neonatal and maternal hair, neonatal meconium and maternal urine, is currently receiving increasing interest. Furthermore, since NGF and BDNF levels are known to be disrupted in the brain and periphery during acute/chronic and prenatal alcohol consumption [15, 21, 83, 94], it could be predicted that their changes in the meconium, cord blood or in the mother/infant serum could be proposed as additional markers of FASD and/or other alcohol-related diseases and current investigations are in progress. Early detection of prenatal ethanol exposure would allow early detection of neonatal outcomes and the possible occurrence of FASD as well as early intervention with an appropriate therapeutic approach.
As for the induction of alcohol-related deficits, it has been suggested an important link between alcohol and neurotrophins, thus neuropharmacological approaches based on the increase in neurotrophin availability promoting neuroprotection against alcohol toxicity could provide a possible support. Indeed, some therapeutic interventions naturally enhancing neurotrophin brain expression may further represent a valuable system to make the brain potentially self healing [3]. Among these interventions learning experiences, exercise, environmental stimulation, dietary restriction [118-121] and polyphenol-rich diet [46, 122] can support the brain to potentiate its own defences.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Barde Y.A. Neurotrophic factors: an evolutionary perspective. J. Neurobiol. 1994;25(11):1329–1333. doi: 10.1002/neu.480251102. [http://dx.doi.org/10.1002/neu.480251102]. [PMID: 7852988]. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–1162. doi: 10.1126/science.3306916. [http://dx.doi.org/10.1126/science.3306916]. [PMID: 3306916]. [DOI] [PubMed] [Google Scholar]
- 3.Boschen K.E., Klintsova A.Y. Neurotrophins in the brain: Interaction with alcohol exposure during development. Vitam. Horm. 2017;104:197–242. doi: 10.1016/bs.vh.2016.10.008. [http://dx.doi.org/10.1016/bs.vh.2016.10.008]. [PMID: 28215296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebendal T. NGF in CNS: Experimental data and clinical implications. Prog. Growth Factor Res. 1989;1(3):143–159. doi: 10.1016/0955-2235(89)90008-2. [http://dx.doi.org/10.1016/0955-2235(89)90008-2]. [PMID: 2562358]. [DOI] [PubMed] [Google Scholar]
- 5.Fiore M., Chaldakov G.N., Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009;20(2):133–145. doi: 10.1515/revneuro.2009.20.2.133. [http://dx.doi.org/10.1515/REVNEURO.2009.20.2.133]. [PMID: 19774790]. [DOI] [PubMed] [Google Scholar]
- 6.Barde Y.A. The nerve growth factor family. Prog. Growth Factor Res. 1990;2(4):237–248. doi: 10.1016/0955-2235(90)90021-b. [http://dx.doi.org/10.1016/0955-2235(90)90021-B]. [PMID: 2133291]. [DOI] [PubMed] [Google Scholar]
- 7.Barde Y.A. Neurotrophins: A family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 1994;390:45–56. [PMID: 7724649]. [PubMed] [Google Scholar]
- 8.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [http://dx.doi.org/10.1016/0166-2236(91)90097-E]. [PMID: 1713715]. [DOI] [PubMed] [Google Scholar]
- 9.Chao M.V., Rajagopal R., Lee F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006;110(2):167–173. doi: 10.1042/CS20050163. [http://dx.doi.org/10.1042/CS20050163]. [PMID: 16411893]. [DOI] [PubMed] [Google Scholar]
- 10.Fiore M., Angelucci F., Aloe L., Iannitelli A., Korf J. Nerve Growth Factor and Brain-Derived Neurotrophic Factor in schizophrenia and depression: Findings in humans and in animal models. Curr. Neuropharmacol. 2003;1:109–123. [http://dx.doi.org/10.2174/1570159033477206]. [Google Scholar]
- 11.Aloe L., Alleva E., Fiore M. Stress and nerve growth factor: Findings in animal models and humans. Pharmacol. Biochem. Behav. 2002;73(1):159–166. doi: 10.1016/s0091-3057(02)00757-8. [http://dx.doi.org/10.1016/S0091-3057(02)00757-8]. [PMID: 12076735]. [DOI] [PubMed] [Google Scholar]
- 12.Cirulli F., Francia N., Berry A., Aloe L., Alleva E., Suomi S.J. Early life stress as a risk factor for mental health: Role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 2009;33(4):573–585. doi: 10.1016/j.neubiorev.2008.09.001. [PMID: 18817811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceccanti M., Mancinelli R., Tirassa P., Laviola G., Rossi S., Romeo M., Fiore M. Early exposure to ethanol or red wine and long-lasting effects in aged mice. A study on nerve growth factor, brain-derived neurotrophic factor, hepatocyte growth factor, and vascular endothelial growth factor. Neurobiol. Aging. 2012;33(2):359–367. doi: 10.1016/j.neurobiolaging.2010.03.005. [http://dx.doi.org/10.1016/j.neurobiolaging.2010.03.005]. [PMID: 20382450]. [DOI] [PubMed] [Google Scholar]
- 14.Ceccanti M., De Nicolò S., Mancinelli R., Chaldakov G., Carito V., Ceccanti M., Laviola G., Tirassa P., Fiore M. NGF and BDNF long-term variations in the thyroid, testis and adrenal glands of a mouse model of fetal alcohol spectrum disorders. Ann. Ist. Super. Sanita. 2013;49(4):383–390. doi: 10.4415/ANN_13_04_11. [http://dx.doi.org/10.4415/ann_13_04_11]. [PMID: 24334784]. [DOI] [PubMed] [Google Scholar]
- 15.Fiore M., Laviola G., Aloe L., di Fausto V., Mancinelli R., Ceccanti M. Early exposure to ethanol but not red wine at the same alcohol concentration induces behavioral and brain neurotrophin alterations in young and adult mice. Neurotoxicology. 2009;30(1):59–71. doi: 10.1016/j.neuro.2008.11.009. [http://dx.doi.org/10.1016/j.neuro.2008.11.009]. [PMID: 19100286]. [DOI] [PubMed] [Google Scholar]
- 16.Fiore M., Mancinelli R., Aloe L., Laviola G., Sornelli F., Vitali M., Ceccanti M. Hepatocyte growth factor, vascular endothelial growth factor, glial cell-derived neurotrophic factor and nerve growth factor are differentially affected by early chronic ethanol or red wine intake. Toxicol. Lett. 2009;188(3):208–213. doi: 10.1016/j.toxlet.2009.04.013. [http://dx.doi.org/10.1016/j.toxlet.2009.04.013]. [PMID: 19397965]. [DOI] [PubMed] [Google Scholar]
- 17.Heaton M.B., Paiva M., Madorsky I., Mayer J., Moore D.B. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: relationship to periods of vulnerability. Brain Res. Dev. Brain Res. 2003;140(2):237–252. doi: 10.1016/s0165-3806(02)00610-7. [http://dx.doi.org/10.1016/S0165-3806(02)00610-7]. [PMID: 12586429]. [DOI] [PubMed] [Google Scholar]
- 18.Heaton M.B., Moore D.B., Paiva M., Madorsky I., Mayer J., Shaw G. The role of neurotrophic factors, apoptosis-related proteins, and endogenous antioxidants in the differential temporal vulnerability of neonatal cerebellum to ethanol. Alcohol. Clin. Exp. Res. 2003;27(4):657–669. doi: 10.1097/01.ALC.0000060527.55252.71. [http://dx.doi.org/10.1111/j.1530-0277.2003.tb04402.x]. [PMID: 12711928]. [DOI] [PubMed] [Google Scholar]
- 19.Idrus N.M., Thomas J.D. Fetal alcohol spectrum disorders: experimental treatments and strategies for intervention. Alcohol Res. Health. 2011;34(1):76–85. [PMID: 23580044]. [PMC free article] [PubMed] [Google Scholar]
- 20.Light K.E., Ge Y., Belcher S.M. Early postnatal ethanol exposure selectively decreases BDNF and truncated TrkB-T2 receptor mRNA expression in the rat cerebellum. Brain Res. Mol. Brain Res. 2001;93(1):46–55. doi: 10.1016/s0169-328x(01)00182-6. [http://dx.doi.org/10.1016/S0169-328X(01)00182-6]. [PMID: 11532337]. [DOI] [PubMed] [Google Scholar]
- 21.Ceccanti M., Carito V., Vitali M., Iannuzzi S., Tarani L., De Nicolò S. Serum BDNF and NGF modulation by olive polyphenols in alcoholics during withdrawal. J. Alcohol. Drug Depend. 2015;3:1–6. [http://dx.doi.org/10.4172/2329-6488.1000214]. [Google Scholar]
- 22.Kulkarny V.V., Wiest N.E., Marquez C.P., Nixon S.C., Valenzuela C.F., Perrone-Bizzozero N.I. Opposite effects of acute ethanol exposure on GAP-43 and BDNF expression in the hippocampus versus the cerebellum of juvenile rats. Alcohol. 2011;45(5):461–471. doi: 10.1016/j.alcohol.2010.12.004. [http://dx.doi.org/10.1016/j.alcohol.2010.12.004]. [PMID: 21367572]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Ding M., Thiele C.J., Luo J. Ethanol inhibits brain-derived neurotrophic factor-mediated intracellular signaling and activator protein-1 activation in cerebellar granule neurons. Neuroscience. 2004;126(1):149–162. doi: 10.1016/j.neuroscience.2004.03.028. [http://dx.doi.org/10.1016/j.neuroscience.2004.03.028]. [PMID: 15145081]. [DOI] [PubMed] [Google Scholar]
- 24.Miller M.W., Mooney S.M. Chronic exposure to ethanol alters neurotrophin content in the basal forebrain-cortex system in the mature rat: effects on autocrine-paracrine mechanisms. J. Neurobiol. 2004;60(4):490–498. doi: 10.1002/neu.20059. [http://dx.doi.org/10.1002/neu.20059]. [PMID: 15307153]. [DOI] [PubMed] [Google Scholar]
- 25.Mooney S.M., Miller M.W. Nerve growth factor neuroprotection of ethanol-induced neuronal death in rat cerebral cortex is age dependent. Neuroscience. 2007;149(2):372–381. doi: 10.1016/j.neuroscience.2007.08.012. [http://dx.doi.org/10.1016/j.neuroscience.2007.08.012]. [PMID: 17869443]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heberlein A., Schuster R., Kleimann A., Groh A., Kordon A., Opfermann B., Lichtinghagen R., Gröschl M., Kornhuber J., Bleich S., Frieling H., Hillemacher T. Joint effects of the epigenetic alteration of neurotrophins and cytokine signaling: A possible exploratory model of affective symptoms in alcohol-dependent patients? Alcohol Alcohol. 2017;52(3):277–281. doi: 10.1093/alcalc/agw100. [http://dx.doi.org/10.1093/alcalc/agw100]. [PMID: 28430931]. [DOI] [PubMed] [Google Scholar]
- 27.Heberlein A., Muschler M., Frieling H., Behr M., Eberlein C., Wilhelm J., Gröschl M., Kornhuber J., Bleich S., Hillemacher T. Epigenetic down regulation of nerve growth factor during alcohol withdrawal. Addict. Biol. 2013;18(3):508–510. doi: 10.1111/j.1369-1600.2010.00307.x. [http://dx.doi.org/10.1111/j.1369-1600.2010.00307.x]. [PMID: 21392176]. [DOI] [PubMed] [Google Scholar]
- 28.Heberlein A., Käser M., Lichtinghagen R., Rhein M., Lenz B., Kornhuber J., Bleich S., Hillemacher T. TNF-α and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol. 2014;48(7):671–676. doi: 10.1016/j.alcohol.2014.08.003. [http://dx.doi.org/10.1016/j.alcohol.2014.08.003]. [PMID: 25262503]. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer F., Jahn H., Schick M., Wiedemann K. Alcohol intake, tumour necrosis factor-alpha, leptin and craving: factors of a possibly vicious circle? Alcohol Alcohol. 2002;37(4):401–404. doi: 10.1093/alcalc/37.4.401. [http://dx.doi.org/10.1093/alcalc/37.4.401]. [PMID: 12107045]. [DOI] [PubMed] [Google Scholar]
- 30.Jones K.L. The fetal alcohol syndrome. Addict. Dis. 1975;2(1-2):79–88. [PMID: 1163375]. [PubMed] [Google Scholar]
- 31.Lemoine P., Harousseau H., Borteyru J.P., Menuet J.C. Les enfants de parents alcooliques: Anomalies observees a propos de 127 cas. Ouest Med. 1968;21:2. [Google Scholar]
- 32.Jones K.L., Smith D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [http://dx.doi.org/10.1016/S0140-6736(73)91092-1]. [PMID: 4127281]. [DOI] [PubMed] [Google Scholar]
- 33.Clarren S.K., Smith D.W. The fetal alcohol syndrome. N. Engl. J. Med. 1978;298(19):1063–1067. doi: 10.1056/NEJM197805112981906. [http://dx.doi.org/10.1056/NEJM197805112981906]. [PMID: 347295]. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand J., Floyd L.L., Weber M.K. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm. Rep. 2005;54(RR-11):1–14. [PMID: 16251866]. [PubMed] [Google Scholar]
- 35.Caley L.M., Kramer C., Robinson L.K. Fetal alcohol spectrum disorder. J. Sch. Nurs. 2005;21(3):139–146. doi: 10.1177/10598405050210030301. [http://dx.doi.org/10.1177/10598405050210030301]. [PMID: 15898848]. [DOI] [PubMed] [Google Scholar]
- 36.Hoyme H.E., May P.A., Kalberg W.O., Kodituwakku P., Gossage J.P., Trujillo P.M., Buckley D.G., Miller J.H., Aragon A.S., Khaole N., Viljoen D.L., Jones K.L., Robinson L.K. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [http://dx.doi.org/10.1542/peds.2004-0259]. [PMID: 15629980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol R.J., Abel E.L. Alcohol-related birth defects: Outlining current research opportunities. Neurotoxicol. Teratol. 1988;10(3):183–186. doi: 10.1016/0892-0362(88)90015-3. [http://dx.doi.org/10.1016/0892-0362(88)90015-3]. [PMID: 3062355]. [DOI] [PubMed] [Google Scholar]
- 38.Stratton K., Howe C., Battaglia F.C., editors. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. National Academies Press; 1996. https://www.nap.edu/read/4991/chapter/1 [Google Scholar]
- 39.Cohen-Kerem R., Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol. Teratol. 2003;25(1):1–9. doi: 10.1016/s0892-0362(02)00324-0. [http://dx.doi.org/10.1016/S0892-0362(02)00324-0]. [PMID: 12633732]. [DOI] [PubMed] [Google Scholar]
- 40.Henderson G.I., Patwardhan R.V., Hoyumpa A.M., Jr, Schenker S. Fetal alcohol syndrome: Overview of pathogenesis. Neurobehav. Toxicol. Teratol. 1981;3(2):73–80. [PMID: 6114444]. [PubMed] [Google Scholar]
- 41.Michaelis E.K. Fetal alcohol exposure: cellular toxicity and molecular events involved in toxicity. Alcohol. Clin. Exp. Res. 1990;14(6):819–826. doi: 10.1111/j.1530-0277.1990.tb01821.x. [http://dx.doi.org/10.1111/j.1530-0277.1990.tb01821.x]. [PMID: 1982397]. [DOI] [PubMed] [Google Scholar]
- 42.Ikonomidou C., Bittigau P., Ishimaru M.J., Wozniak D.F., Koch C., Genz K., Price M.T., Stefovska V., Hörster F., Tenkova T., Dikranian K., Olney J.W. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–1060. doi: 10.1126/science.287.5455.1056. [http://dx.doi.org/10.1126/science.287.5455.1056]. [PMID: 10669420]. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran V., Watts L.T., Maffi S.K., Chen J., Schenker S., Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J. Neurosci. Res. 2003;74(4):577–588. doi: 10.1002/jnr.10767. [http://dx.doi.org/10.1002/jnr.10767]. [PMID: 14598302]. [DOI] [PubMed] [Google Scholar]
- 44.Carito V., Venditti A., Bianco A., Ceccanti M., Serrilli A.M., Chaldakov G., Tarani L., De Nicolò S., Fiore M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014;28(22):1970–1984. doi: 10.1080/14786419.2014.918977. [http://dx.doi.org/10.1080/14786419.2014.918977]. [PMID: 24865115]. [DOI] [PubMed] [Google Scholar]
- 45.Carito V., Ceccanti M., Chaldakov G., Tarani L., De Nicolò S., Ciafrè S. 2015. [Google Scholar]
- 46.Carito V., Ceccanti M., Tarani L., Ferraguti G., Chaldakov G.N., Fiore M. Neurotrophins’ modulation by olive polyphenols. Curr. Med. Chem. 2016;23(28):3189–3197. doi: 10.2174/0929867323666160627104022. [http://dx.doi.org/10.2174/0929867323666160627104022]. [PMID: 27356540]. [DOI] [PubMed] [Google Scholar]
- 47.Carito V., Ceccanti M., Cestari V., Natella F., Bello C., Coccurello R., Mancinelli R., Fiore M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition. 2017;33:65–69. doi: 10.1016/j.nut.2016.08.014. [http://dx.doi.org/10.1016/j.nut.2016.08.014]. [PMID: 27908553]. [DOI] [PubMed] [Google Scholar]
- 48.Correa M., Salamone J.D., Segovia K.N., Pardo M., Longoni R., Spina L., Peana A.T., Vinci S., Acquas E. Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neurosci. Biobehav. Rev. 2012;36(1):404–430. doi: 10.1016/j.neubiorev.2011.07.009. [http://dx.doi.org/10.1016/j.neubiorev.2011.07.009]. [PMID: 21824493]. [DOI] [PubMed] [Google Scholar]
- 49.Deitrich R.A. Acetaldehyde: déjà vu du jour. J. Stud. Alcohol. 2004;65(5):557–572. doi: 10.15288/jsa.2004.65.557. [http://dx.doi.org/10.15288/jsa.2004.65.557]. [PMID: 15536764]. [DOI] [PubMed] [Google Scholar]
- 50.Muggironi G., Fois G.R., Diana M. Ethanol-derived acetaldehyde: pleasure and pain of alcohol mechanism of action. Front. Behav. Neurosci. 2013;7:87. doi: 10.3389/fnbeh.2013.00087. [http://dx.doi.org/10.3389/fnbeh.2013.00087]. [PMID: 23882197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quertemont E., Tambour S., Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: A comprehensive review of animal studies. Prog. Neurobiol. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [http://dx.doi.org/10.1016/j.pneurobio.2005.03.003]. [DOI] [PubMed] [Google Scholar]
- 52.Burd L., Blair J., Dropps K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J. Perinatol. 2012;32(9):652–659. doi: 10.1038/jp.2012.57. [http://dx.doi.org/10.1038/jp.2012.57]. [PMID: 22595965]. [DOI] [PubMed] [Google Scholar]
- 53.Espinet C., Argilés J.M. Ethanol and acetaldehyde concentrations in the rat foeto-maternal system after an acute ethanol administration given to the mother. Arch. Int. Physiol. Biochim. 1984;92(5):339–344. doi: 10.3109/13813458409080609. [http://dx.doi.org/10.3109/13813458409080609]. [PMID: 6085549]. [DOI] [PubMed] [Google Scholar]
- 54.Guerri C., Sanchis R. Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Alcohol. 1985;2(2):267–270. doi: 10.1016/0741-8329(85)90057-6. [http://dx.doi.org/10.1016/0741-8329(85)90057-6]. [PMID: 4040377]. [DOI] [PubMed] [Google Scholar]
- 55.Spagnolo A. Teratogenesis of alcohol. Ann. Ist. Super. Sanita. 1993;29(1):89–96. [PMID: 8129276]. [PubMed] [Google Scholar]
- 56.Sulik K.K., Pfefferbaum A. Fetal alcohol spectrum disorder: Pathogenesis and mechanisms. Handb. Clin. Neurol. 2014;125:463–475. doi: 10.1016/B978-0-444-62619-6.00026-4. [http://dx.doi.org/10.1016/B978-0-444-62619-6.00026-4]. [PMID: 25307590]. [DOI] [PubMed] [Google Scholar]
- 57.Aloe L. Alcohol intake during prenatal life affects neuroimmune mediators and brain neurogenesis. Ann. Ist. Super. Sanita. 2006;42(1):17–21. [PMID: 16801721]. [PubMed] [Google Scholar]
- 58.Feng M.J., Yan S.E., Yan Q.S. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring. Brain Res. 2005;1042(2):125–132. doi: 10.1016/j.brainres.2005.02.017. [http://dx.doi.org/10.1016/j.brainres.2005.02.017]. [PMID: 15854584]. [DOI] [PubMed] [Google Scholar]
- 59.Moore D.B., Madorsky I., Paiva M., Barrow Heaton M. Ethanol exposure alters neurotrophin receptor expression in the rat central nervous system: Effects of neonatal exposure. J. Neurobiol. 2004;60(1):114–126. doi: 10.1002/neu.20010. [http://dx.doi.org/10.1002/neu.20010]. [PMID: 15188277]. [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health National Institute on Alcohol Abuse and Alcoholism. 2003 https://www.acog.org/-/media/Departments/Tobacco-Alcohol-and-Substance-Abuse/AlcoholA-Womans-Health-Issue.pdf
- 61.Fernández-Solà J., Estruch R., Nicolás J.M., Paré J.C., Sacanella E., Antúnez E., Urbano-Márquez A. Comparison of alcoholic cardiomyopathy in women versus men. Am. J. Cardiol. 1997;80(4):481–485. doi: 10.1016/s0002-9149(97)00399-8. [http://dx.doi.org/10.1016/S0002-9149(97)00399-8]. [PMID: 9285662]. [DOI] [PubMed] [Google Scholar]
- 62.Thurman R.G. 2000.
- 63.Ceylan-Isik A.F., McBride S.M., Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sci. 2010;87(5-6):133–138. doi: 10.1016/j.lfs.2010.06.002. [http://dx.doi.org/10.1016/j.lfs.2010.06.002]. [PMID: 20598716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nixon S.J., Tivis R., Ceballos N., Varner J.L., Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(5):919–927. doi: 10.1016/s0278-5846(02)00206-3. [http://dx.doi.org/10.1016/S0278-5846(02)00206-3]. [PMID: 12369267]. [DOI] [PubMed] [Google Scholar]
- 65.Retson T.A., Sterling R.C., Van Bockstaele E.J. Alcohol-induced dysregulation of stress-related circuitry: The search for novel targets and implications for interventions across the sexes. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:252–259. doi: 10.1016/j.pnpbp.2015.05.009. [http://dx.doi.org/10.1016/j.pnpbp.2015.05.009]. [PMID: 26006055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ely M., Hardy R., Longford N.T., Wadsworth M.E. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol Alcohol. 1999;34(6):894–902. doi: 10.1093/alcalc/34.6.894. [http://dx.doi.org/10.1093/alcalc/34.6.894]. [PMID: 10659726]. [DOI] [PubMed] [Google Scholar]
- 67.Mancinelli R. Gender differences in alcohol-related impairment: A critical review. OA Alcohol. 2013;1:1–6. [Google Scholar]
- 68.Mancinelli R., Barlocci E., Ciprotti M., Senofonte O., Fidente R.M., Draisci R., Attilia M.L., Vitali M., Fiore M., Ceccanti M. Blood thiamine, zinc, selenium, lead and oxidative stress in a population of male and female alcoholics: Clinical evidence and gender differences. Ann. Ist. Super. Sanita. 2013;49(1):65–72. doi: 10.4415/ANN_13_01_11. [http://dx.doi.org/10.4415/ann_13_01_11]. [PMID: 23535132]. [DOI] [PubMed] [Google Scholar]
- 69.Pascale E., Ferraguti G., Codazzo C., Passarelli F., Mancinelli R., Bonvicini C., Bruno S.M., Lucarelli M., Ceccanti M. Alcohol dependence and serotonin transporter functional polymorphisms 5-HTTLPR and rs25531 in an Italian population. Alcohol Alcohol. 2015;50(3):259–265. doi: 10.1093/alcalc/agv014. [http://dx.doi.org/10.1093/alcalc/agv014]. [PMID: 25770138]. [DOI] [PubMed] [Google Scholar]
- 70.Lex B.W. 1994. Women and Substance Abuse. [Google Scholar]
- 71.van Faassen E., Niemelä O., editors. Biochemistry of Prenatal Alcohol Exposure. Nova Biomedical Books; 2011. [Google Scholar]
- 72.Nava-Ocampo A.A., Velázquez-Armenta Y., Brien J.F., Koren G. Elimination kinetics of ethanol in pregnant women. Reprod. Toxicol. 2004;18(4):613–617. doi: 10.1016/j.reprotox.2004.02.012. [http://dx.doi.org/10.1016/j.reprotox.2004.02.012]. [PMID: 15135856]. [DOI] [PubMed] [Google Scholar]
- 73.Ferraguti G., Pascale E., Lucarelli M. Alcohol addiction: A molecular biology perspective. Curr. Med. Chem. 2015;22(6):670–684. doi: 10.2174/0929867321666141229103158. [http://dx.doi.org/10.2174/0929867321666141229103158]. [PMID: 25544474]. [DOI] [PubMed] [Google Scholar]
- 74.Aloe L., Tirassa P. The effect of long-term alcohol intake on brain NGF-target cells of aged rats. Alcohol. 1992;9(4):299–304. doi: 10.1016/0741-8329(92)90070-q. [http://dx.doi.org/10.1016/0741-8329(92)90070-Q]. [PMID: 1322141]. [DOI] [PubMed] [Google Scholar]
- 75.Jeanblanc J., Coune F., Botia B., Naassila M. Brain-derived neurotrophic factor mediates the suppression of alcohol self-administration by memantine. Addict. Biol. 2014;19(5):758–769. doi: 10.1111/adb.12039. [http://dx.doi.org/10.1111/adb.12039]. [PMID: 23414063]. [DOI] [PubMed] [Google Scholar]
- 76.Zucca S., Valenzuela C.F. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J. Neurosci. 2010;30(19):6776–6781. doi: 10.1523/JNEUROSCI.5405-09.2010. [http://dx.doi.org/10.1523/JNEUROSCI.5405-09.2010]. [PMID: 20463239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D’Sa C., Duman R.S. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4(3):183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [http://dx.doi.org/10.1034/j.1399-5618.2002.01203.x]. [PMID: 12180273]. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt H.D., Duman R.S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 2007;18(5-6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [http://dx.doi.org/10.1097/FBP.0b013e3282ee2aa8]. [PMID: 17762509]. [DOI] [PubMed] [Google Scholar]
- 79.Caldwell K.K., Sheema S., Paz R.D., Samudio-Ruiz S.L., Laughlin M.H., Spence N.E., Roehlk M.J., Alcon S.N., Allan A.M. Fetal alcohol spectrum disorder-associated depression: Evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol. Biochem. Behav. 2008;90(4):614–624. doi: 10.1016/j.pbb.2008.05.004. [http://dx.doi.org/10.1016/j.pbb.2008.05.004]. [PMID: 18558427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tapia-Arancibia L., Rage F., Givalois L., Dingeon P., Arancibia S., Beaugé F. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J. Neurosci. Res. 2001;63(2):200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [http://dx.doi.org/10.1002/1097-4547(20010115)63:2<200:AID-JNR1012>3.0.CO;2-Q]. [PMID: 11169630]. [DOI] [PubMed] [Google Scholar]
- 81.MacLennan A.J., Lee N., Walker D.W. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci. Lett. 1995;197(2):105–108. doi: 10.1016/0304-3940(95)11922-j. [http://dx.doi.org/10.1016/0304-3940(95)11922-J]. [PMID: 8552271]. [DOI] [PubMed] [Google Scholar]
- 82.Heaton M.B., Paiva M., Swanson D.J., Walker D.W. Responsiveness of cultured septal and hippocampal neurons to ethanol and neurotrophic substances. J. Neurosci. Res. 1994;39(3):305–318. doi: 10.1002/jnr.490390308. [http://dx.doi.org/10.1002/jnr.490390308]. [PMID: 7869423]. [DOI] [PubMed] [Google Scholar]
- 83.Aloe L., Bracci-Laudiero L., Tirassa P. The effect of chronic ethanol intake on brain NGF level and on NGF-target tissues of adult mice. Drug Alcohol Depend. 1993;31(2):159–167. doi: 10.1016/0376-8716(93)90068-2. [http://dx.doi.org/10.1016/0376-8716(93)90068-2]. [PMID: 8436061]. [DOI] [PubMed] [Google Scholar]
- 84.Heaton M.B., Mitchell J.J., Paiva M., Walker D.W. Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Brain Res. Dev. Brain Res. 2000;121(1):97–107. doi: 10.1016/s0165-3806(00)00032-8. [http://dx.doi.org/10.1016/S0165-3806(00)00032-8]. [PMID: 10837897]. [DOI] [PubMed] [Google Scholar]
- 85.Heaton M.B., Mitchell J.J., Paiva M. Overexpression of NGF ameliorates ethanol neurotoxicity in the developing cerebellum. J. Neurobiol. 2000;45(2):95–104. [http://dx.doi.org/10.1002/1097-4695(20001105)45:2<95:AID-NEU4>3.0.CO;2-Y]. [PMID: 11018771]. [PubMed] [Google Scholar]
- 86.Moore D.B., Madorsky I., Paiva M., Barrow H.M. Ethanol exposure alters neurotrophin receptor expression in the rat central nervous system: Effects of prenatal exposure. J. Neurobiol. 2004;60(1):101–113. doi: 10.1002/neu.20009. [http://dx.doi.org/10.1002/neu.20009]. [PMID: 15188276]. [DOI] [PubMed] [Google Scholar]
- 87.De Nicoló S., Tarani L., Ceccanti M., Maldini M., Natella F., Vania A., Chaldakov G.N., Fiore M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition. 2013;29(4):681–687. doi: 10.1016/j.nut.2012.11.007. [http://dx.doi.org/10.1016/j.nut.2012.11.007]. [PMID: 23466052]. [DOI] [PubMed] [Google Scholar]
- 88.Chen J.H., Tipoe G.L., Liong E.C., So H.S., Leung K.M., Tom W.M., Fung P.C., Nanji A.A. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am. J. Clin. Nutr. 2004;80(3):742–751. doi: 10.1093/ajcn/80.3.742. [http://dx.doi.org/10.1093/ajcn/80.3.742]. [PMID: 15321817]. [DOI] [PubMed] [Google Scholar]
- 89.Carito V., Ciafrè S., Tarani L., Ceccanti M., Natella F., Iannitelli A., Tirassa P., Chaldakov G.N., Ceccanti M., Boccardo C., Fiore M. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super. Sanita. 2015;51(4):382–386. doi: 10.4415/ANN_15_04_21. [http://dx.doi.org/10.4415/ANN_15_04_21]. [PMID: 26783228]. [DOI] [PubMed] [Google Scholar]
- 90.Bhullar K.S., Rupasinghe H.P.V. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013;2013:891748. doi: 10.1155/2013/891748. [http://dx.doi.org/10.1155/2013/891748]. [PMID: 23840922]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ceccanti M., Alessandra S.P., Tarani L., Luisa A.M., Chessa L., Mancinelli R., Stegagno M., Francesco Sasso G., Romeo M., Jones K.L., Robinson L.K., Del Campo M., Phillip Gossage J., May P.A., Eugene Hoyme H. Clinical delineation of fetal alcohol spectrum disorders (FASD) in Italian children: Comparison and contrast with other racial/ethnic groups and implications for diagnosis and prevention. Neurosci. Biobehav. Rev. 2007;31(2):270–277. doi: 10.1016/j.neubiorev.2006.06.024. [http://dx.doi.org/10.1016/j.neubiorev.2006.06.024]. [PMID: 17215042]. [DOI] [PubMed] [Google Scholar]
- 92.May P.A., Fiorentino D., Phillip Gossage J., Kalberg W.O., Eugene Hoyme H., Robinson L.K., Coriale G., Jones K.L., del Campo M., Tarani L., Romeo M., Kodituwakku P.W., Deiana L., Buckley D., Ceccanti M. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol. Clin. Exp. Res. 2006;30(9):1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [http://dx.doi.org/10.1111/j.1530-0277.2006.00188.x]. [PMID: 16930219]. [DOI] [PubMed] [Google Scholar]
- 93.May P.A., Gossage J.P., Kalberg W.O., Robinson L.K., Buckley D., Manning M., Hoyme H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [http://dx.doi.org/10.1002/ddrr.68]. [PMID: 19731384]. [DOI] [PubMed] [Google Scholar]
- 94.Aloe L., Tuveri M.A., Guerra G., Pinna L., Tirassa P., Micera A., Alleva E. Changes in human plasma nerve growth factor level after chronic alcohol consumption and withdrawal. Alcohol. Clin. Exp. Res. 1996;20(3):462–465. doi: 10.1111/j.1530-0277.1996.tb01076.x. [http://dx.doi.org/10.1111/j.1530-0277.1996.tb01076.x]. [PMID: 8727238]. [DOI] [PubMed] [Google Scholar]
- 95.Abel E. Paternal contribution to fetal alcohol syndrome. 2004. [DOI] [PubMed]
- 96.Abel E.L., Dintcheff B.A. Effects of prenatal alcohol exposure on behavior of aged rats. Drug Alcohol Depend. 1986;16(4):321–330. doi: 10.1016/0376-8716(86)90066-9. [http://dx.doi.org/10.1016/0376-8716(86)90066-9]. [PMID: 3698812]. [DOI] [PubMed] [Google Scholar]
- 97.Abel E.L. Rat offspring sired by males treated with alcohol. Alcohol. 1993;10(3):237–242. doi: 10.1016/0741-8329(93)90042-m. [http://dx.doi.org/10.1016/0741-8329(93)90042-M]. [PMID: 8507394]. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka H., Suzuki N., Arima M. Experimental studies on the influence of male alcoholism on fetal development. Brain Dev. 1982;4(1):1–6. [http://dx.doi.org/10.1016/S0387-7604(82)80094-6]. [PMID: 7039389]. [PubMed] [Google Scholar]
- 99.Abel E.L. Paternal and maternal alcohol consumption: Effects on offspring in two strains of rats. Alcohol. Clin. Exp. Res. 1989;13(4):533–541. doi: 10.1111/j.1530-0277.1989.tb00373.x. [http://dx.doi.org/10.1111/j.1530-0277.1989.tb00373.x]. [PMID: 2679211]. [DOI] [PubMed] [Google Scholar]
- 100.Jamerson P.A., Wulser M.J., Kimler B.F. Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Res. Dev. Brain Res. 2004;149(2):103–111. doi: 10.1016/j.devbrainres.2003.12.010. [http://dx.doi.org/10.1016/j.devbrainres.2003.12.010]. [PMID: 15063090]. [DOI] [PubMed] [Google Scholar]
- 101.Wozniak D.F., Cicero T.J., Kettinger L., III, Meyer E.R. Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology (Berl.) 1991;105(2):289–302. doi: 10.1007/BF02244324. [http://dx.doi.org/10.1007/BF02244324]. [PMID: 1796134]. [DOI] [PubMed] [Google Scholar]
- 102.Abel E.L., Bilitzke P. Paternal alcohol exposure: Paradoxical effect in mice and rats. Psychopharmacology (Berl.) 1990;100(2):159–164. doi: 10.1007/BF02244399. [http://dx.doi.org/10.1007/BF02244399]. [PMID: 2305005]. [DOI] [PubMed] [Google Scholar]
- 103.Ceccanti M., Coccurello R., Carito V., Ciafrè S., Ferraguti G., Giacovazzo G., Mancinelli R., Tirassa P., Chaldakov G.N., Pascale E., Ceccanti M., Codazzo C., Fiore M. Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addict. Biol. 2016;21(4):776–787. doi: 10.1111/adb.12255. [http://dx.doi.org/10.1111/adb.12255]. [PMID: 25940002]. [DOI] [PubMed] [Google Scholar]
- 104.Finegersh A., Homanics G.E. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One. 2014;9(6):e99078. doi: 10.1371/journal.pone.0099078. [http://dx.doi.org/10.1371/journal.pone.0099078]. [PMID: 24896617]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rompala G.R., Finegersh A., Homanics G.E. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol Fayettev N. 2016;53:19–25. doi: 10.1016/j.alcohol.2016.03.006. [http://dx.doi.org/10.1016/j.alcohol.2016.03.006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang F., Diao L., Jiang N., Zhang J., Wang H-J., Zhou W-H., Huang G.Y., Ma D. Chronic exposure to ethanol in male mice may be associated with hearing loss in offspring. Asian J. Androl. 2015;17(6):985–990. doi: 10.4103/1008-682X.160267. [http://dx.doi.org/10.4103/1008-682X.160267]. [PMID: 26262775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zuccolo L., DeRoo L.A., Wills A.K., Davey S.G., Suren P., Roth C., Stoltenberg C., Magnus P. Pre-conception and prenatal alcohol exposure from mothers and fathers drinking and head circumference: Results from the Norwegian mother-child study (MoBa). Sci. Rep. 2016;7:39535. doi: 10.1038/srep39535. [http://dx.doi.org/10.1038/srep39535]. [PMID: 28008975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zuccolo L., DeRoo L.A., Wills A.K., Smith G.D., Suren P., Roth C., Stoltenberg C., Magnus P. Erratum: Pre-conception and prenatal alcohol exposure from mothers and fathers drinking and head circumference: results from the Norwegian Mother-Child Study (MoBa). Sci. Rep. 2017;7:45877. doi: 10.1038/srep45877. [http://dx.doi.org/10.1038/srep45877]. [PMID: 28436988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferraguti G., Ciolli P., Carito V., Battagliese G., Mancinelli R., Ciafrè S., Tirassa P., Ciccarelli R., Cipriani A., Messina M.P., Fiore M., Ceccanti M. Ethylglucuronide in the urine as a marker of alcohol consumption during pregnancy: Comparison with four alcohol screening questionnaires. Toxicol. Lett. 2017;275:49–56. doi: 10.1016/j.toxlet.2017.04.016. [http://dx.doi.org/10.1016/j.toxlet.2017.04.016]. [PMID: 28455000]. [DOI] [PubMed] [Google Scholar]
- 110.Coriale G., Fiorentino D., Di Lauro F., Marchitelli R., Scalese B., Fiore M., Maviglia M., Ceccanti M. Fetal Alcohol Spectrum Disorder (FASD): Neurobehavioral profile, indications for diagnosis and treatment. Riv. Psichiatr. 2013;48(5):359–369. doi: 10.1708/1356.15062. [http://dx.doi.org/10.1708/1356.15062]. [PMID: 24326748]. [DOI] [PubMed] [Google Scholar]
- 111.Kodituwakku P.W., Kodituwakku E.L. From research to practice: An integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychol. Rev. 2011;21(2):204–223. doi: 10.1007/s11065-011-9170-1. [http://dx.doi.org/10.1007/s11065-011-9170-1]. [PMID: 21544706]. [DOI] [PubMed] [Google Scholar]
- 112.Popova S., Lange S., Probst C., Gmel G., Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5(3):e290–e299. doi: 10.1016/S2214-109X(17)30021-9. [http://dx.doi.org/10.1016/S2214-109X(17)30021-9]. [PMID: 28089487]. [DOI] [PubMed] [Google Scholar]
- 113.Pichini S., Busardò F.P., Ceccanti M., Tarani L., Pacifici R. Unreliable estimation of prevalence of fetal alcohol syndrome. Lancet Glob. Health. 2017;5(6):e574. doi: 10.1016/S2214-109X(17)30173-0. [http://dx.doi.org/10.1016/S2214-109X(17)30173-0]. [PMID: 28495260]. [DOI] [PubMed] [Google Scholar]
- 114.Popova S., Lange S., Probst C., Gmel G., Rehm J. Unreliable estimation of prevalence of fetal alcohol syndrome - Authors’ reply. Lancet Glob. Health. 2017;5(6):e575–e576. doi: 10.1016/S2214-109X(17)30174-2. [http://dx.doi.org/10.1016/S2214-109X(17)30174-2]. [PMID: 28495261]. [DOI] [PubMed] [Google Scholar]
- 115.Roozen S., Black D., Peters G.Y., Kok G., Townend D., Nijhuis J.G., Koek G.H., Curfs L.M. Fetal Alcohol Spectrum Disorders (FASD): an Approach to Effective Prevention. Curr. Dev. Disord. Rep. 2016;3(4):229–234. doi: 10.1007/s40474-016-0101-y. [http://dx.doi.org/10.1007/s40474-016-0101-y]. [PMID: 27891300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morini L., Marchei E., Tarani L., Trivelli M., Rapisardi G., Elicio M.R., Ramis J., Garcia-Algar O., Memo L., Pacifici R., Groppi A., Danesino P., Pichini S. Testing ethylglucuronide in maternal hair and nails for the assessment of fetal exposure to alcohol: Comparison with meconium testing. Ther. Drug Monit. 2013;35(3):402–407. doi: 10.1097/FTD.0b013e318283f719. [http://dx.doi.org/10.1097/FTD.0b013e318283f719]. [PMID: 23666568]. [DOI] [PubMed] [Google Scholar]
- 117.Pichini S., Marchei E., Vagnarelli F., Tarani L., Raimondi F., Maffucci R. Assessment of prenatal exposure to ethanol by meconium analysis: Results of an Italian multicenter study. Alcohol. Clin. Exp. Res. 2012;36:417–424. doi: 10.1111/j.1530-0277.2011.01647.x. [http://dx.doi.org/10.1111/j.1530-0277.2011.01647.x]. [DOI] [PubMed] [Google Scholar]
- 118.Bekinschtein P., Oomen C.A., Saksida L.M., Bussey T.J. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin. Cell Dev. Biol. 2011;22(5):536–542. doi: 10.1016/j.semcdb.2011.07.002. [http://dx.doi.org/10.1016/j.semcdb.2011.07.002]. [PMID: 21767656]. [DOI] [PubMed] [Google Scholar]
- 119.Fiore M., Amendola T., Triaca V., Tirassa P., Alleva E., Aloe L. Agonistic encounters in aged male mouse potentiate the expression of endogenous brain NGF and BDNF: Possible implication for brain progenitor cells’ activation. Eur. J. Neurosci. 2003;17(7):1455–1464. doi: 10.1046/j.1460-9568.2003.02573.x. [http://dx.doi.org/10.1046/j.1460-9568.2003.02573.x]. [PMID: 12713648]. [DOI] [PubMed] [Google Scholar]
- 120.Lee J., Seroogy K.B., Mattson M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [http://dx.doi.org/10.1046/j.0022-3042.2001.00747.x]. [PMID: 11905999]. [DOI] [PubMed] [Google Scholar]
- 121.Russo-Neustadt A., Beard R.C., Cotman C.W. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsycho-pharmacol. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [http://dx.doi.org/10.1016/S0893-133X(99)00059-7]. [DOI] [PubMed] [Google Scholar]
- 122.Cimini A., Gentile R., D’Angelo B., Benedetti E., Cristiano L., Avantaggiati M.L., Giordano A., Ferri C., Desideri G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013;114(10):2209–2220. doi: 10.1002/jcb.24548. [http://dx.doi.org/10.1002/jcb.24548]. [PMID: 23554028]. [DOI] [PMC free article] [PubMed] [Google Scholar]