Abstract
Recent advances in the field of novel anticancer agents prolong patients’ survival and show a promising future. Tyrosine kinase inhibitors and immunotherapy for lung cancer are the two major areas undergoing rapid development. Although increasing novel anticancer agents were innovated, how to translate and optimize these novel agents into clinical practice remains to be explored. Besides, toxicities and availability of these drugs in specific regions should also be considered during clinical determination. Herein, we summarize emerging agents including tyrosine kinase inhibitors, checkpoint inhibitors, and other potential immunotherapy such as chimeric antigen receptor T cell for non-small cell lung cancer attempting to provide insights and perspectives of the future in anticancer treatment.
Keywords: Tyrosine kinase inhibitors, Checkpoint inhibitors, CAR-T, Bispecific antibodies, Lung cancer
Background
In the past few decades, systemic treatment for lung cancer remained to be cytoxicity agents with platinum-based regimens. ECOG1594 was the first trial comparing four different chemotherapy regimens for advanced non-small cell lung cancer (NSCLC) head to head [1]. All chemotherapy regimens showed almost the same efficacy with objective response rate (ORR) of 19% and 7.9 m median overall survival (OS). The platinum-based doublet chemotherapy seemed to reach the plateau since then. In 2005, the first-ever trial combining small molecular targeted agent known as bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, with doublet chemotherapy, had shown superiority of overall survival with this treatment modality in advanced non-squamous non-small cell lung cancer patients without brain metastasis [2]. Yet, several trials including molecular targeted agents and chemotherapy fail to reach the endpoints [3–5].
Epidermal growth factor receptor, a well-known biomarker for targeted therapy at present, was first brought up with potential clinical responsiveness to tyrosine kinase inhibitor gefitinib in 2004[6]. Since then the era of targeted therapy was uncovered and multiple trials demonstrated the efficacy of tyrosine kinase inhibitor (TKI) in oncogene-driven non-small cell lung cancer patients [7–11]. In these trials, significantly improved progression-free survival (PFS) was observed compared to tradition chemotherapy; however, no overall survival benefit was identified which may be partly due to high crossover rate after disease progression [12–14]. Moreover, resistance to tyrosine kinase inhibitor was inevitable and sequential treatment was warranted [7, 15–17].
Although up to 69% of patients with advanced NSCLC could harbor actionable driver mutations, a number of patients barely got a chance for more effective agents other than chemotherapy [16, 18–21]. Until 2013, immunotherapy was crowned as the first place of scientific breakthroughs [22]. Efficacy of immunotherapy for those without targetable oncogene mutation was proven from second-line treatment [23–27] to first-line treatment [28, 29]. Through long-term follow-up, immunotherapy had also shown itself the greatest potential of long-term clinical benefit [30, 31], even though the efficacy was not that satisfactory [23–30]. Indeed, similar to targeted therapy, patients may eventually develop resistance to immunotherapy [32, 33] and some may even suffer hyperprogression after immunotherapy [34, 35]. The desire of novel agents that showed better efficacy, prolong survival benefit, and overcame resistance promoted the development of potential targets and corresponding drugs. In recent years, we have witnessed the birth of numerous emerging agents and their superior clinical responsiveness. Herein, we summarized the novel agents in tyrosine kinase inhibitors especially for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) inhibitors, checkpoint inhibitors, and other potential immunotherapy aiming to provide a landscape of emerging agents for NSCLC as well as insights and perspectives for the future in anticancer treatment.
Epidermal growth factor receptor and human epidermal growth factor receptor 2 inhibitors
Dacomitinib
Dacomitinib is a selective and irreversible inhibitor for EGFR [36, 37]. In 2014, an official announcement from Pfizer indicated the trial failure of dacomitinib in patients with refractory advanced non-small cell lung cancer. However, based on superior results from phase II single-arm trial (ARCHER 1017) in the first-line setting, ARCHER1050, a phase III randomized control trial (ARCHER 1050) comparing dacomitinib and gefitinib head to head, was set to confirm its clinical efficacy and safety in expanded population. The results were promising, and the median PFS for dacomitinib and gefitinib was 14.7 months and 9.2 months, respectively (HR = 0.59, 95% CI 0.47–0.74) [38]. Similar efficacy was shown between EGFR 19Del and EGFR 21L858R which suggested opposite results compared to the first-generation TKI in previous researches [39, 40]. Further OS results have been recently unleashed, and the median OS was 34.1 m with dacomitinib versus 26.8 m with gefitinib (HR = 0.76, 95% CI 0.58–0.99) [41]. Higher incidence of adverse events compared to the first-generation TKI should be noticed [38, 41]. Currently, updated results of dose reduction in dacomitinib have been released and higher efficacy was found in dose modulation group [42]. Yet, efficacy results in patients with brain metastasis and resistant mechanism to dacomitinib were poorly explored and whether patients who had treatment failure after dacomitinib could still have a great chance of receiving osimertinib has not been answered [41]. Indeed, dacomitinib has been officially approved by the FDA in 2018 due to its superior performance in the first-line setting. Clinically, dacomitinib as a first-line treatment would be an optional choice, and hopefully, the third generation may be a salvage treatment after disease progression. But it would be too early to confirm its significant clinical role in first-line treatment neglecting the striking performance from osimertinib. Further clinical researches were warranted to provide evidence for a better therapeutic scheme.
Osimertinib (AZD9291)
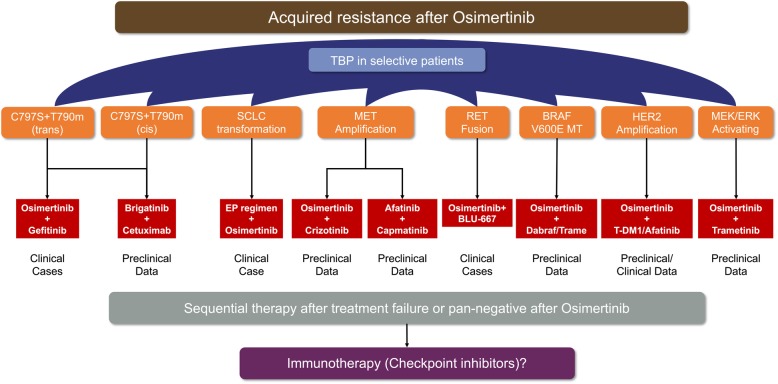
Despite the high response rate to the first-generation TKI, majority of patients would suffer disease progression after 9–13 months of treatment [7, 15–17]. The most common resistant mechanism to the first-generation TKI is p.Thr790Met point mutation (T790 M) with almost 60% [15, 20]. Osimertinib, an irreversible third-generation TKI, was set to overcome resistance to T790M, and sensitive EGFR mutations (19Del and 21L858R) were covered as well [43, 44]. In a phase II single-arm AURA2 study [44], ORR was 70% (95% CI 64–77) among 199 pretreated patients receiving osimertinib and manageable side effect was identified. Extension population-based AURA study showed that 201 pretreated patients harboring T790 M mutation received osimertinib with a median treatment duration of 13.2 months. Objective response rate was 62% (95% CI, 54% to 68%), and median PFS was 12.3 months (95% CI, 9.5 to 13.8) [45]. Treatment-related adverse events were milder compared to previous TKI [7–10, 44, 45]. With the superior performance, the FDA has approved its indications in second-line treatment. To further demonstrate the efficacy of osimertinib in the first-line setting, FLAURA study has been put forward and preliminary results have been released. Osimertinib showed significantly prolonged PFS compared to standard EGFR-TKIs in first-line setting (18.9 months vs. 10.2 months, HR = 0.46, 95% CI 0.37–0.57) [46]. So far, the median overall survival for both osimertinib and standard EGFR-TKI group was not reached. Favorable trend for osimertinib could be identified with a P value of 0.007. Indeed, compared to previous EGFR-TKIs, osimertinib revealed much longer PFS and better efficacy as well as decreased toxicity. And since that, the FDA has approved its first-line setting in the early 2018. Yet, should the winner take it all? Recent studies have provided more evidence and faith of using osimertinib in the first line. The exploratory postprogression outcomes of phase III FLAURA study has been reported showing not reached median second PFS in osimertinib arm while 20 months for standard of care EGFR-TKI arm [47]. Another study found that the continuation of osimertinib after disease progression could lead to a median second PFS of 12.6 months and be associated with longer overall survival compared with discontinuation [48]. Indeed, the mature results of OS are requested to further give a final deposition of this issue. The other focal aspect for osimertinib is the resistant mechanism. Till now, limited studies reported the resistant mechanism of osimertinib and extreme complicated resistant profiles were identified based on current data [49–51]. Fortunately, several preclinical and small size studies have provided potential treatment modalities to overcome the resistance, but an umbrella trial should be designed to address the pending issues [48, 52–63]. On the other hand, considering the rapid development of checkpoint inhibitors (CPIs) in advanced NSCLC, whether CPIs could benefit in patients with pan-negative oncogenes after osimertinib or treatment failure of novel combination modality remained to be explored in prospective trials. (Figure 1). Among patients with advanced lung cancer, brain metastasis was regarded as one of the major factors for poorer prognosis [64–66]. In contrast to the first-generation EGFR-TKI, osimertinib showed much better response rate in brain metastasis which may be due to higher penetration through the blood-brain barrier (BBB) [67, 68]. Collectively, osimertinib would be a more competitive first-line treatment for advanced non-small cell lung cancer patients beyond the first-generation EGFR-TKIs and further OS data of FLAURA study was pending to decipher the order issue.
Fig. 1.
Reported acquired resistance to osimertinib and corresponding potential strategies. Preclinical and clinical data consisted of EGFR-dependent/independent resistant mechanism to osimertinib were included. Additional corresponding TKI with osimertinib may be available in other oncogene-driven resistance, and whether checkpoint inhibitors would be beneficial in pan-negative patients after osimertinib or treatment failure was yet to be answered. TBP, treatment beyond progression
AZD3759
Over 50% of NSCLC patients with EGFR-activating mutations would develop CNS metastasis during treatment [65, 69, 70]. Poor survival was observed in these patients with 16 months for brain metastasis [64] and 4.5–11 months for leptomeningeal metastasis [66]. AZD3759 is an oral EGFR-TKI which was specifically designed to overcome the weak penetration of the blood-brain barrier [71, 72]. This drug contained no substrate for efflux transport [71] and achieved 100% penetration through BBB [69], suggesting superior clinical efficacy in CNS metastasis. The BLOOM study is a phase I, open-label, multicenter trial evaluating the safety and preliminary antitumor efficacy of AZD3759 [69]. Tolerable safety profile was observed in this trial, and high consistent concentration of AZD3759 between CSF and free plasma was observed. However, whether a high concentration of AZD3759 in CSF would be translated into durable CNS response and not inferior efficacy in extracranial target lesions compared to previous EGFR-TKI warrants further clinical results.
Poziotinib, TAK-788, afatinib, and pyrotinib
In NSCLC, approximately 10–15% of patients harbored EGFR-activating mutations. For those whose tumor has sensitive EGFR mutation including deletion in exon 19 and mutation encoding p.L858R, standard first-generation TKI could probably provide dramatic efficacy [7–11]. However, approximately 10–12% of patients within have an in-frame insertion in exon 20 of their tumors [73–75]. The EGFR exon 20 insertions are generally resistant to most EGFR-TKIs [76, 77] which may be due to the altered drug-binding pocket of exon 20 [76]. Poziotinib has been proven to be a potent inhibitor of both EGFR and HER2 exon 20 insertion mutations through preclinical models and clinical experience [78]. Its preliminary clinical activity has been reported in 2018 WCLC with confirmed ORR of 43% in advanced NSCLC [79]. Another novel agent for EGFR exon 20 insertion, TAK-788, has also been reported in 2018 WCLC [80]. The ORR was approximately 40% in NSCLC patients with EGFR exon 20 insertion. Remarkably, the disease control rate could reach up to 100% in this small group of population. Additionally, for HER2 mutations, afatinib has shown some activity through retrospective researches with limited prospective clinical trials assessing its efficacy in HER2 mutation patients [81–84]. A recent published phase II study (NICHE trial) showed a disease control rate of 53.8% with median PFS of 15.9 weeks and median OS of 56.0 weeks failing to verify the efficacy of afatinib in HER2 mutation patients [85]. On the other hand, pyrotinib is an oral, irreversible pan-HER receptor TKI. Preclinical data indicated effective antitumor activity in vitro and in vivo [86, 87]. A small sample size phase 2 study has investigated its efficacy and safety. Patients who received 400 mg of pyrotinib showed an ORR of 53.3% and a median PFS of 6.4 months regardless of prior treatment lines along with tolerable adverse events [88]. Indeed, further larger sample size evaluation was warranted to verify its clinical value.
Anaplastic lymphoma kinase and ROS1 proto-oncogene receptor kinase inhibitors
Ceritinib
The current standard first-line treatment for advanced non-small cell lung cancer harboring ALK rearrangement is crizotinib [89]. Despite the rapid response to crizotinib, most of the patients would suffer disease progression within 12 months [90, 91]. Approximately, a resistant mechanism in one third of patients with ALK-rearranged NSCLC was owning to ALK-dependent mutation including tyrosine kinase domain or amplification of ALK fusion. Ceritinib is a small molecule, oral tyrosine kinase inhibitor of ALK [92]. In contrast to crizotinib, ceritinib is 20 times more potent as crizotinib for ALK fusion, yet no tumor activity against MET was observed. In a phase I study evaluating the antitumor activity and safety in NSCLC harboring ALK fusion, ceritinib showed 58% ORR in 114 patients with a dose of at least 400 mg. Median progression-free survival, although not that mature enough (38% patients censored), was 7.0 months with a median follow-up time of 9.5 months [93]. Overall adverse events rate of grade 3 or 4 related to ceritinib therapy was 49% (varied from 20–80% among different dose groups), majority of which was gastrointestinal (GI) issues. Based on these superior clinical outcomes, the FDA granted an accelerated approval to ceritinib for the treatment in NSCLC patients harboring ALK rearrangement [94]. In 2016, the updated results of ASCEND-1 was released. Two hundred fifty-five patients who received at least 1 dose of ceritinib 750 mg/day showed an overall response rate of 72% and 56% in treatment-naive and ALK inhibitor-pretreated groups, respectively. Intracranial disease control was reported in 79% of ALK inhibitor-naïve patients and 65% for ALK inhibitor-pretreated patients. However, approximately 81% of patients suffer at least 1 adverse events of grade 3 or 4 [95]. In a phase II trial (ASCEND-2), ceritinib was tested specifically in chemotherapy and ALK inhibitor-pretreated patients. ORR was 38.6% and median PFS was 5.7 months. The intracranial overall response rate was 45.0% with similar and manageable tolerability as previous researches [96]. Moving into the first-line setting, ASCEND-4, a phase III study, showed 72.5% ORR and median PFS of 16.6 months. Yet, the control arm of this study was chemotherapy without providing head to head comparison to crizotinib. Besides, adverse events of grade 3 or 4 (78.0%) were as high as previously reported with ceritinib 750 mg daily [97]. Due to that, ASCEND-8, a phase I study assessing the tolerability of different dose of ceritinib in ALK-positive NSCLC, was initiated [98]. Compared to 750 mg daily, 450 mg with food may be optimal with favorable gastrointestinal tolerability. Further updated analysis suggested consistent efficacy between 450 mg with food and 750 mg along with less GI toxicities [99].
Attributed to a similar molecular structure with ALK fusion, ROS1 fusion may also be potential beneficiaries with ALK-TKI [100]. For advanced NSCLC patients harboring ROS1 fusion, crizotinib was first reported to have an antitumor activity for the treatment of ROS1 fusion. The ORR was 72.0% with a median PFS of 19.2 months. Toxicities were mild with no treatment related to adverse events of grade 4 or 5 [101]. Results were further demonstrated in a larger East Asian population in a phase II study. To be noticed, 13.4% of ROS1-positive patients within the study received a complete response to crizotinib [102]. In a phase II single-arm study, the efficacy and safety of ceritinib were assessed in a small sample size population harboring ROS1 fusion [103]. ORR was 62% with a median PFS of 24 months in overall patients who received at least 2 prior systemic treatment. Grade 3 or 4 toxicities of ceritinib 750 mg daily in ROS1 fusion were much milder than in ALK fusion (37% vs. ~ 80%) which may probably be owing to the diverse interaction between drugs and targets.
Alectinib
Similar to EGFR mutation, patients with ALK rearrangement would be under high risk of brain metastasis [104]. Alectinib is a highly selective inhibitor of anaplastic lymphoma kinase (ALK) which has shown both systemic and central nerve system efficacy in ALK-positive non-small cell lung cancer [70, 105–108]. J-ALEX trial is the first trial comparing alectinib and crizotinib as the first-line setting in advanced non-small cell lung cancer with ALK rearrangement, but only involving the Japanese population [109]. The result of median PFS was rather promising with 20.3 months for alectinib and 10.3 months for crizotinib. The superiority had been duplicated in the upcoming ALEX trial involving a larger amount of population [110]. According to the updated results, the median PFS for alectinib in first-line treatment was 34.8 months which was almost three times longer than the standard first-line treatment for ALK rearrangement [89]. Besides, the phase III ALUR study directly compared alectinib with chemotherapy in crizotinib-pretreated ALK-positive non-small cell lung cancer [111]. Median PFS was 9.6 months with alectinib and 1.4 months with chemotherapy as second-line treatment indicating an absolute clinical role of alectinib as a first-line setting. Besides, in contrast to other ALK-TKI such as crizotinib, alectinib did not have a substrate for efflux transport [104, 112, 113] and penetration through BBB was significantly higher than crizotinib [114, 115]. Recent data revealed high objective response rate of 73.3% with 100% central nerve system (CNS) disease control rate (DCR) in patients with ALK rearrangement and symptomatic or large CNS metastasis [115]. Although the resistant profile of crizotinib has been well described [90, 116, 117], little was known about alectinib and Gly1202Arg (G1202R) remained a hot potato for alectinib [118, 119]. Indeed, considering its tremendous improvement in first-line treatment compared to other ALK TKIs, alectinib should currently be the first option from all aspects for treatment-naive patients with advanced non-small cell lung cancer harboring ALK rearrangement.
Brigatinib (AP26113)
Brigatinib, another second-generation highly potent ALK-TKI, was also designed for a broad range of ALK resistance mutations [120]. Similar to lorlatinib, brigatinib was proved to be efficiently inhibiting all clinically relevant ALK resistance mutations including ALK G1202R through preclinical models [121]. However, another study showed diverse outcome in preclinical models with IC50 of 129.5 nM to brigatinib indicating inferior sensitivity to G1202R [106]. In a multicenter retrospective study, one alectinib-pretreated patient harboring G1202R had a progressive disease as the best response to brigatinib [122]. Whether brigatinib could overcome the resistance to G1202R remained to be explored in a larger sample size trial. Through a phase II trial of brigatinib in patients with crizotinib-refractory ALK-positive NSCLC, brigatinib yielded both substantial systemic and intracranial response and 180 mg once daily was proven to have better efficacy with acceptable safety [123]. Currently, an interim analysis of ALTA-L1 has been reported, showing 51% of progression risk reduced although median PFS was not reached [124]. Although, relevant research suggested the brain accumulation of brigatinib may be restricted by P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) [125]. For patients with brain metastasis at baseline in ALTA trial, brigatinib achieved potent efficacy with a progression risk reduction of 73%. Similar adverse events were identified compared to previous ALK-TKI [89, 97, 126–128]. Most importantly, the final PFS and OS result of brigatinib should be expected to decipher whether brigatinib might be superior to the new standard first-line alectinib.
Lorlatinib
Lorlatinib is a highly potent and brain-penetrant third-generation ALK-TKI in patients with advanced ALK-positive NSCLC [106]. Most ALK-positive patients treated with first- or second-generation ALK-TKI would develop resistance to TKI including ALK Gly1202Arg (G1202R) solvent-front mutation located at the solvent-front region of ALK, and can impair drug binding through steric hindrance [106, 129]. A preclinical data showed lorlatinib was the only ALK inhibitor to potently inhibit wide-range ALK secondary mutations, including ALK G1202R [130]. So far for lorlatinib, only phase 1 and 2 study has been released, and the results were promising [131]. For treatment-naive patients, ORR was 90% and 69.5% for crizotinib-treated patients. Based on a preliminary analysis of paired cerebrospinal fluid and plasma samples, lorlatinib has been demonstrated with a high degree of penetration across the blood-brain barrier [104]. In this phase 1 and 2 trial, the intracranial response for treatment-naive patients was 66.7% while 87% for crizotinib-treated patients. Additionally, a phase I study evaluating the efficacy and safety of lorlatinib in ALK/ROS1-positive NSCLC showed encouraging results in either ALK rearrangement or ROS1 rearrangement patients regardless of treatment lines [132]. For ALK-positive patients, the overall ORR was 46% along with a median PFS of 9.6 months and 50% along with 7.0 months for ROS1-positive patients. Collectively, although PFS result was not that mature enough, considering its wide range profile for resistance to ALK-TKI, lorlatinib would be an optional sequential treatment for patients previously treated with ALK-TKI. A phase III study was being investigated comparing lorlatinib to crizotinib at first-line setting (NCT03052608), and the preliminary results may be presented in 2020.
Ensartinib (X-396)
Ensartinib (X-396) is a novel, aminopyridazine-based small molecule drug that could potently inhibit ALK. Through preclinical study, tenfold more potent than crizotinib inhibiting ALK-positive lung cancer cell lines was observed [133]. Results of a multicenter expansion study had been first reported in 2016 WCLC [134] showing similar response rate and adverse events compared to previous ALK-TKI. Currently, this first-in-human phase I/II multicenter study has revealed the survival benefit of ensartinib with median PFS of 26 months in treatment-naive patients and 9 months for crizotinib-pretreated patients [135]. ORR for treatment-naive patients was 80% and 69% for previously treated patients. Among patients with brain metastasis, intracranial disease control rate could reach up to 92.9%. eXalt3 is a phase 3 randomized trial comparing ensartinib and crizotinib head to head [136]. The preliminary results would be released in 2019 which would further verify and discuss its clinical role in ALK-positive lung cancer patients (Table 1).
Table 1.
Overview of novel agents regarding TKI in advanced NSCLC including systemic and intracranial efficacy
| Identifier | Trials | Agents | Phase | Indication | Population | ORR* | mPFS | mOS | iRR | imPFS/iDOR | Toxcicty (≧ grade3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EGFR | |||||||||||
| NCT00818441 | ARCHER1070 | Dacomitinib | II | First-line | Advanced lung cancer with clinical (never-smoker or former light smokers) or molecular selected | 75.6% | 18.2 months | 40.2 months | NA | NA | NA |
| NCT01774721 | ARCHER1050 | Dacomitinib | III | First-line | Advanced NSCLC with one EGFR mutation (19Del or L858R) | 75.0% | 14.7 months | 34.1 months | NA | NA | 51% |
| NCT01802632 | AURA2 | Osimertinib | I/II | ≧ Second-line | Advanced NSCLC progressed after at least one prior treatment involving EGFR-TKI | 62.0% | 12.3 months | NR | 64.0% | 7.1 months | NA |
| NCT02151981 | AURA3 | Osimertinib | III | Second-line | Advanced NSCLC presented with EGFR sensitive mutations and T790m after first-line EGFR-TKI | 71.0% | 10.1 months | NR | 70.0% | 8.9 months | NA |
| NCT02296125 | FLAURA | Osimertinib | III | First-line | Untreated advanced NSCLC harbored sensitive EGFR mutations | 80.0% | 18.9 months | NR | 76.0% | 15.2 months | 22% |
| NCT02228369 | BLOOM | AZD3759 | I | ≧ Second-line | Advanced EGFR-mutant NSCLC with brain/leptomeningeal metastases progressed after EGFR-TKI and chemotherapy | 65.0% | NR | NA | 83.0% | NR | 17% (200 mg), 40% (300 mg) |
| NCT03066206 | / | Poziotinib | II | Unlimited | Advanced NSCLC bearing mutations/insertions of EGFR or HER2 exon 20 regardless of prior treatment | 43.0% | 5.6 months | NA | NA | NA | 60% |
| ALK | |||||||||||
| NCT01283516 | ASCEND-1 | Ceritinib | I | Unlimited | Locally advanced or metastatic cancer harboring genetic alterations in ALK (NSCLC) | 72.0% (TN) 56.0% (PT) |
18.4 months (TN) 6.9 months (PT) |
NA | 79.0% (TN)# 65.0% (PT)# |
8.2 months (TN) 11.1 months (PT) |
81% |
| NCT01685060 | ASCEND-2 | Ceritinib | II | > Second-line | Advanced ALK-rearranged NSCLC with asymptomatic or neurologically stable baseline brain metastasis | 38.6% | 5.7 months | NA | 45.0% | NA | 71.4% |
| NCT01828099 | ASCEND-4 | Ceritinib | III | First-line | Untreated metastatic non-squamous NSCLC harboring ALK rearrangement | 72.5% | 16.6 months | NR | 72.7% | 16.6 months | 78.0% |
| NCT01828112 | ASCEND-5 | Ceritinib | III | ≧ Second-line | Advanced ALK-positive NSCLC with at least two prior systemic treatment including crizotinib | 38.1% | 5.4 months | 18.1 months | 35.0% | 6.9 months | NA |
| NCT01801111 | / | Alectinib | II | Second-line | Advanced NSCLC with ALK rearrangement after disease progression of prior crizotinib | 50.0% | 8.9 months | NR | 57.0% | 10.3 months | NA |
| JapicCTI-132316 | J-ALEX | Alectinib (300 mg) | III | First-line | Advanced ALK-positive NSCLC with either chemotherapy naive or one previous chemotherapy | 92.0% | 25.9 months | NR | NA | NA | 32% |
| NCT0207584 | ALEX | Alectinib (600 mg) | III | First-line | Untreated advanced NSCLC harbored ALK rearrangment | 82.9% | 34.8 months | NR | 81.0% | 17.3 months | 41% |
| NCT03052608 | / | Loraltinib | I | Unlimited | Advanced NSCLC harbored ALK/ROS1 rearrangement regardless of prior treatments | 46.0% | 9.6 months | NA | 42.0% | NA | NA |
| NCT01970865 | / | Loraltinib | II | First-line | Untreated advanced NSCLC bearing ALK/ROS1-positive | 90.0% | NR | NR | 66.7% | NR | NA |
| Second-line | Advanced ALK/ROS1 positive NSCLC with previous crizotinib | 69.5% | NR | NR | 87.0% | NR | NA | ||||
| NCT01449461 | / | Brigatinib | I/II | First-line | Treatment naive advanced ALK/EGFR positive NSCLC | 100.0% | NR | NA | 53.0% | 15.6 months | 36% |
| Second-line | Advanced ALK/EGFR positive NSCLC with refractory disease after crizotinib | 62.0% | 14.5 months | NA | NA | NA | |||||
| NCT02737501 | ATLA-L1 | Brigatinib | III | First-line | Advanced ALK-positive NSCLC without previous ALK TKI treatment | 71.0% | NR | NR | 78.0% | NR | 61% |
| ROS1 | |||||||||||
| NCT00585195 | / | Crizotinib | I | Unlimited | Advanced NSCLC with a ROS1 rearrangement | 72.0% | 19.2 months | NR | NA | NA | NA |
| NCT01945021 | / | Crizotinib | II | Unlimited | Advanced NSCLC with a ROS1 rearrangement | 71.7% | 15.9 months | 32.5 months | 73.9% | NA | 25.2% |
| NCT00585195 | / | Ceritinib | II | ≧ Second-line | Advanced ROS1-positive NSCLC progressed after at least one prior systemic treatment | 62.0% | 9.3 months (CT) 19.3 months (CN) |
24.0 months | 25.0%# | NA | 37.0% |
| NCT03052608 | / | Lorlatinib | I | Unlimited | Advanced NSCLC harbored ALK/ROS1 rearrangement regardless of prior treatments | 50.0% | 7.0 months | NA | 60.0% | NA | NA |
ORR objective response rate, mPFS median progression-free survival, mOS median overall survival, iRR intracranial response rate, imPFS intracranial progression-free survival, iDOR intracranial duration of response, TN treatment naive, PT pretreated, NR not reach, NA not applicable
*ORR for EGFR-TKI was based on results regarding sensitive EGFR-activating mutations
#The disease control rate of intracranial response
Other novel tyrosine kinase inhibitor
Entrectinib (RXDX-101), larotrectinib (LOXO-101), and LOXO-195
Recurrent gene fusions are one of the essential oncogenic drivers to promote tumor growth among varied malignancies [137]. Similar to ALK and ROS1 rearrangement, fusions of NTRK1, NTRK2, and NTRK3 are actionable drivers of tumor growth. The incidence of NTRK fusion in solid tumor was reported as 0.1% [138]. Entrectinib and larotrectinib were both inhibitors targeting NTRK fusions [139, 140]. Unlike larotrectinib, entrectinib also showed efficacy in ROS1 and ALK rearrangement [139]. Two phase I study (ALKA-372-001 and STARTRK-1) assessing entrectinib in NTRK-, ROS1-, and ALK-positive solid tumor showed promising efficacy and durable clinical benefit of 32 months in a ROS1-positive lung cancer patients [139]. Besides, promising intracranial efficacy was observed indicating high penetration through BBB.
Although limited data of larotrectinib was shown, a recent study containing three phase I/II clinical trials was published and splendid responsiveness was revealed in pan-solid tumor including lung cancer harboring NTRK fusion [140]. Responsiveness was observed regardless of tumor type, and the overall response rate was 80%. Based on its superb outcome, the FDA has approved its application in patients of solid malignancies harboring NTRK fusion. Despite the preliminary clinical results, primary and acquired resistance has already been characterized in several studies [141, 142]. To overcome the resistance mediated by acquired kinase domain mutations, LOXO-195, a selective TRK-TKI, was designed and preclinically proven to be highly potent in vitro [143]. Two patients whose tumor developed an acquired resistance to larotrectinib were treated with LOXO-195 and showed potential efficacy, but relevant data was warranted specifically in lung cancer patients for the future.
Repotrectinib (TPX-0005)
Similar to entrectinib and larotrectinib, repotrectinib is a next-generation TKI developed to inhibit clinically recalcitrant solvent front substitutions involving TRK, ROS1, and ALK. In a preclinical study, among common the acquired resistance to ALK, ROS1, and TRK including ALK G1202R, ROS1 G2032R, and TRKB G639R, repotrectinib showed high efficacy in vitro compared to other ALK/ROS1/TRK inhibitors. For patients with brain metastasis, a significant clinical challenge, this next-generation TKI showed superior efficacy compared to crizotinib in patients with CNS metastasis. This may be partly due to its smaller molecule structure compared to previous TKI drugs [144]. The current phase I/II clinical trial investigating the efficacy and safety of repotrectinib is still ongoing (NCT03093116), and further results should be expected.
RXDX-105, LOXO-292, and BLU-667
RET fusion is a well-established driver oncogene in a variety of malignancies. In lung cancer, RET fusion was found in 1–2% unselected cases [145]. RXDX-105 is an orally, VEGFR-sparing, multikinase inhibitor with activity against RET. Compared to other RET inhibitors including cabozantinib, vandetanib, and lenvatinib, RXDX-105 showed high preclinical activity [146–149]. In a phase I/Ib trial [150], treatment-naive NSCLC patients with RET fusion showed 19% ORR to RXDX-105 while 0% ORR for TKI-pretreated patients. Specifically looking into different upstream partners for RET, only non-KIF5B RET fusion showed satisfactory clinical efficacy to RXDX-105 which is similar to other RET inhibitors.
Unlike multikinase inhibitors such as RXDX-105 which may be under substantial “off-targets” hindering their clinical efficacy, LOXO-292 is a novel RET inhibitor with high selectivity [147, 149, 151, 152]. Through preclinical research and clinical experience with LOXO-292, it showed both high selectivity and responsiveness to RET fusion cell lines. Besides, LOXO-292 revealed high efficacy in KIF5B-RET fusion engineered cells which were different from previous RET inhibitors [151]. Another similar small molecule specifically targeting RET is BLU-667 which covered both RET fusion and RET-activating mutations as well [153]. Compared to RXDX-105, cabozantinib, and vandetanib, BLU-667 showed a broad range of efficacy in RET fusion and activating mutation with high selectivity in KIF5B-RET preclinically. A phase I, first-in-human study (NCT03037385) which tried to define the maximum tolerated dose and evaluate the safety along with antitumor activity is still ongoing, and clinical potentials of such high selective RET inhibitors will be elucidated in the future.
Capmatinib (INC280)
Capmatinib is a highly potent MET inhibitor [154], and its single-agent activity has been observed in preclinical models with strong MET amplification, overexpression, and mutations. MET amplification could be accounted for 5–26% in patients resistant to previous EGFR-TKI [20, 155–159]. Preclinical research suggested INC280 could restore sensitivity to erlotinib and promote apoptosis in EGFR-mutant NSCLC models [160]. As a clinical rationale for the combination of capmatinib and EGFR-TKI, a phase Ib/II single-arm trial evaluated the combination of INC280 and gefitinib in EGFR-TKI-pretreated patients [161]. Overall response rate across phase Ib/II regardless of MET copy number was 27%. In patients with high MET amplification (copy number ≥ 6), the ORR was 47% with acceptable adverse events. However, the survival data was not mature enough and the resistant mechanism of capmatinib has not been released yet. A study established MET-amplified NSCLC cell lines which showed an acquired resistance to capmantinib. With further examination, they found that the combined treatment of EGFR or PIK3CA would dramatically suppress cell proliferation and downstream signals [162]. This may partly suggest an alternative therapeutic strategy to overcome the resistance to capmatinib, but further clinical researches were required to elucidate.
Dabrafenib and trametinib
BRAF mutations occurred in about 2–4% of lung adenocarcinoma, and approximately 50% of them were BRAF V600E mutations [163, 164]. BRAF V600E mutations were reported to have shorter overall survival, and limited patients responded to chemotherapy compared to wild-type BRAF [165, 166]. Vemurafenib was the first BRAF V600E inhibitor assessed in a basket trial which indicated a 42% overall response rate within BRAF V600E mutation NSCLC [167]. Dabrafenib was a highly potent adenosine triphosphate-competitive inhibitor of BRAF kinase selective for the BRAF V600E mutations [168]. In a phase II non-randomized trial, the disease control rate (DCR) was 53% with a median PFS of 5.5 months. Serious adverse events were reported in 42% of patients [169]. Through preclinical study, dabrafenib plus trametinib had shown high antitumor activity in BRAF V600E mutation cell lines, and clinically, BRAF plus MEK inhibitors revealed improved clinical outcome in patients with BRAF V600E mutant metastatic melanoma [170, 171]. In two phase II non-randomized trials, consistent overall response rate was observed with 63.2% and 64.0% in previously treated and untreated patients, respectively. Similar median PFS was found as well in previously treated and untreated patients with 8.6 months and 10.9 months, respectively [172, 173]. Within previously treated patients, grades 3 and 4 events occurred in 49% of patients while almost 73% for untreated patients. So far, considering limited choices in BRAF mutation especially V600E mutations, dabrafenib plus trametinib should be the first option in these group of patients.
Anlotinib
Anlotinib is a novel, small molecule receptor tyrosine kinases (RTKs) and inhibits both tumor proliferation and angiogenesis [174–176]. Preclinical studies have shown that anlotinib has emerged much stronger anti-angiogenic activity than other anti-angiogenesis agents [177]. Clinically, the efficacy and safety of anlotinib was first demonstrated in a randomized phase II study as a third-line therapy in advanced NSCLC [178]. Patients in the anlotinib group showed a significantly longer PFS than the placebo group (4.8 months vs. 1.2 months). Although no statistical significance was shown in OS, favor trend of survival benefit was identified in the anlotinib group (9.3 months vs. 6.3 months). Final results of an expanded population phase III randomized trial (ALTER 0303) has been released last year in ASCO meeting and showed both prolonged PFS and OS in the anlotinib group with well-tolerable adverse events indicating anlotinib as a potential third-line treatment in advanced NSCLC patients.
Checkpoint inhibitors
Pembrolizumab, nivolumab, and atezolizumab
With the rapid growth of immunotherapy these years, tradition chemotherapy in pan-negative advanced non-small cell lung cancer has been challenged from second-line treatment to first-line treatment by single-agent checkpoint inhibitors [23, 24, 27, 28]. For checkpoint inhibitors, the expression of PD-L1 has been considered as a major predictive factor for immunotherapy so far [179]. Given that significant discrepancy results of Keynote-024 and Checkmate-026[29, 180], only highly selective patients should be available for a single agent in the first-line setting. Back to the era of targeted therapy, combination strategies have achieved great success, and theoretically, this may probably work out in checkpoint inhibitors [181–183]. Keynote-021 first reported preliminary results of checkpoint inhibitors combined with chemotherapy [180]. Superior response rate and progression-free survival were observed with minor increased toxicities. Identical results were duplicated in phase III study Keynote-189 and Keynote-407 for lung adenocarcinoma and squamous cell carcinoma, respectively [184, 185]. To be noticed, the component of combination immunotherapy seemed to significantly influence the incidence of adverse events. Cisplatin or paclitaxel showed much better tolerance than carboplatin or nab-paclitaxel as combination components. In Keynote-042 (2018 ASCO meeting), single-agent pembrolizumab has broadened its indication to a larger population with PD-L1 positive. Yet, patients with high expression PD-L1 in both trials showed a similar clinical outcome. Considering the cost-effectiveness and toxicities, it would be optimal to provide single-agent pembrolizumab in patients with PD-L1 high expression while combination regimens for low or negative PD-L1 expression. As for nivolumab, even post hoc analysis with stratification of tumor mutation burden showed statistical significance, and it is still a negative trial showing no significant improvement between single-agent nivolumab and platinum-based chemotherapy in PD-L1-positive patients based on the study design probably due to high crossover rate and non-highly selective patients. Checkmate-227 was the first reported combination trial involving nivolumab in advanced NSCLC. Compared to standard platinum-based chemotherapy, nivolumab plus ipilimumab revealed a significantly improved ORR (45.3% vs. 26.9%) and prolonged mPFS (7.2 months vs. 5.5 months) in patients with high mutation burden regardless of PD-L1 expression [186]. Indeed, OS was not mature enough to present preliminary data and comparison between other arms as well as subgroup analysis was not released yet. Additionally, the three phase III trials of combination regimens involving atezolizumab (IMpower 150, IMpower131(2018 ASCO meeting), IMpower132(2018 WCLC meeting)) have all shown superiority in clinical outcome with tolerable adverse events in either non-squamous or squamous NSCLC [187]. Details of all posted trials with combination regimens are summarized in Table 2.
Table 2.
Posted results of combination regimen trials for pembrolizumab, nivolumab, and atezolizumab in advanced non-small cell lung cancer
| Identifier | Trials | Phase | Indication | Population | Intervention | mORR | mPFS | 1-year PFS rate | mOS | 1-year OS rate | Any cause of AE rates ≥grade 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT02578680 | Keynote-189 | III | First-line | Treatment-naive metastatic non-squamous NSCLC without EGFR/ALK alteration | Platinum-based CT | 18.9% | 4.9 months | 17.3% | 11.3 months | 49.4% | 65.8% |
| Platinum-based CT plus pembrolizumab | 47.6% | 8.8 months | 34.1% | NR | 69.2% | 67.2% | |||||
| NCT02775435 | Keynote-407 | III | First-line | Untreated metastatic squamous NSCLC without EGFR/ALK alteration | Carboplatin-based CT | 38.4% | 4.8 months | NA | 11.3 months | 48.3% | 68.2% |
| Carboplatin-based CT plus pembrolizumab | 57.9% | 6.4 months | NA | 15.9 months | 65.2% | 69.8% | |||||
| NCT02477826 | Checkmate-227 | III | First-line | Untreated recurrent NSCLC of high mutation burden without EGFR/ALK alteration | Platinum-based CT | 26.9% | 5.5 months | 13.2% | NA | NA | 36.0%* |
| Nivolumab plus Ipilimumab | 45.3% | 7.2 months | 42.6% | NA | NA | 37.0%* | |||||
| NCT02366143 | IMpower150 | III | First-line | Untreated metastatic non-squamous NSCLC (wild type group) | Bevacizumab plus carboplatin plus paclitaxel (BCP) | 48.0% | 6.8 months | 18.0% | 14.7 months# | 60.6%# | 50.0%+ |
| Atezolizumab plus BCP | 63.5% | 8.3 months | 36.5% | 19.2 months# | 67.3%# | 58.5%+ | |||||
| NCT02657434 | IMpower131 | III | First-line | Untreated metastatic squamous cell NSCLC without EGFR/ALK alteration | Carboplatin-based CT | 41.0% | 5.6 months | 12.0% | 13.9 months# | 56.9%# | 58.0%* |
| Carboplatin-based CT plus atezolizumab | 49.0% | 6.3 months | 24.7% | 14.0 months# | 55.6%# | 69.0%* | |||||
| NCT02657434 | IMpower132 | III | First-line | Untreated metastatic non-squamous NSCLC without EGFR/ALK alteration | Platinum-based CT | 32.0% | 5.2 months | 17.0% | 13.6 months# | 55.4%# | 59.0% |
| Platinum-based CT plus atezolizumab | 47.0% | 7.6 months | 33.7% | 18.1 months# | 59.6%# | 69.0% |
CT chemotherapy, PFS progression-free survival, OS overall survival, ORR objective response rate, AE adverse event
*Treatment-related adverse events
+Treatment-related adverse events (grade 3–4)
#Interim analysis results
Avelumab
Avelumab is a fully human immunoglobulin G1 (IgG1) monoclonal antibody [188]. Beyond pembrolizumab, nivolumab, and atezolizumab, it is one of the last PD-L1 inhibitors along with durvalumab to access the market. Avelumab had been first approved in the USA for the treatment of metastatic Merkel cell carcinoma. In contrary to other PD-1/PD-L1 drugs, the binding of avelumab to the surface of tumor cell via PD-L1 could induce natural killer cell-mediated antibody-dependent cellular cytotoxicity (ADCC) which may enhance its clinical efficacy [189, 190]. In a phase Ib, multicenter trial (JAVELIN Solid Tumor), patients with advanced, platinum-treated NSCLC were given a single-agent avelumab [188]. Acceptable safety profile was observed, and 50% of patients achieved disease control. Similar median progression-free survival and overall survival compared to previous PD-1/PD-L1 were observed with 17.6 weeks and 8.4 months, respectively [23, 26, 27]. Clinical efficacy was consistent with the level of PD-L1 expression, and higher expression of PD-L1 may be translated into a longer survival benefit. Recent results from a randomized phase 3 trial (JAVELIN Lung 200) also investigating the efficacy and safety of avelumab in platinum-treated patients with advanced NSCLC have been released [191]. In PD-L1-positive (≥ 1%) patients, no significant survival benefit was observed between the avelumab and docetaxel groups (11.4 months vs. 10.3 months) except the high PD-L1 expression groups (≥ 50% cutoff and ≥ 80% cutoff). Increased ORR was consistent with the higher expression of PD-L1 in avelumab group instead of docetaxel group indicating PD-L1 as an essential predictive biomarker for avelumab. However, according to the primary endpoint this trial set up initially, it is a negative study even with numerical significance in survival. Other relevant trials including JAVELIN Lung 100, JAVELIN Lung 101, and JAVELIN Medley were still ongoing (Table 3), and the closest report of JAVELIN Lung 100 will be released in 2019.
Table 3.
Details of ongoing clinical trials for avelumab in early-stage and advanced-stage lung cancer
| Objectives | Identifier | Title | Phase | Intervention | Study design | Population | Primary endpoint | Secondary endpoint | Status | Primary completion |
|---|---|---|---|---|---|---|---|---|---|---|
| Early-stage | NCT03050554 | Phase I/II study of the safety, tolerability, and efficacy of stereotactic body radiation therapy (SBRT) combined with concurrent and adjuvant avelumab for definitive management of early stage non-small cell lung cancer (NSCLC) | I/II | Avelumab+SBRT | Single-arm trial | Stage I NSCLC with tumor(s) less than 5 cm in diameter or 250 cm3 in volume | Safety and tolerability, RFS | Locoregional control, OS | Recruiting | Oct 2020 |
| Advanced-stage | NCT02576574 | A Phase III, open-label, multicenter trial of avelumab (MSB0010718C) versus platinum-based doublet as a first line treatment of recurrent or stage IV PD-L1+non-small cell lung cancer | III | Avelumab | Randomized control trial | Metastatic or recurrent NSCLC without EGFR or ALK | PFS, OS | Best overall response, DOR, EQ-5D-5L | Active, not recruiting | 2019 Oct |
| NCT03472560 | A phase II, open-label study to evaluate safety and clinical activity of avelumab in combination with axitinib in patients with advanced or metastatic previously treated non-small cell lung cancer or treatment naive cisplatin-ineligble urothelial cancer (JAVELIN MEDLEY VEGF) | II | Avelumab+axitinib | Single-arm trial | Pretreated advanced NSCLC with no more than 2 prior lines and EGFR/ALK/ROS1 negative | ORR | TTR, DOR, PFS | Recruiting | Sep 2020 | |
| NCT03717155 | A phase IIa, single-arm, multicenter study to investigate the clinical activity and safety of avelumab in combination with cetuximab plus gemcitabine and cisplatinin participants with advanced squamous non-small-cell lung cancer | II | Avelumab+cetuximab+gemcitabine+cisplatin | Single-arm trial | Advanced lung squamous carcinoma without EGFR mutation, ALK rearrangementand brain metastasis | Best overall response | Occurrence of treatment-emergent adverse events, PFS, DOR | Recruiting | Jan 2021 | |
| NCT03568097 | Phased avelumab combined with chemotherapy as first-line treatment for patients with advanced small-cell lung cancer (SCLC) | II | Avelumab+cisplatin (carboplatin)+etoposide | Single-arm trial | Treatment-naive advanced SCLC with untreated stable brain metastasis | 1-year PFS | OS, best overall response, ORR | Recruiting | Nov 2020 | |
| NCT02584634 | A phase 1B/2, open-label, dose-finding study to evaluate safety, efficacy, pharmacokinetics and pharmacodynamics of avelumab in combination with either crizotinib or PF-06463922 in patients with advanced or metastatic non-small cell lung cancer | II | Group A: avelumab+crizotinib; group B: avelumab+PF-06463922 | Non-randomized trial | Pretreated advanced NSCLC without ALK rearrangement for group A and unlimited prior lines with ALK positive for group B | DLT, overall response rate | DCR, OS | Active, not recruiting | Feb 2019 | |
| NCT03268057 | Phase 1b/study of VX15/2503 in combination with avelumab in advanced non-small cell lung cancer | I/II | VX15/2503+Avelumab | Single-arm trial | No prior immunotherapy treated NSCLC | DLT, AEs | ORR, DOR, PFS | Recruiting | May 2020 | |
| NCT03317496 | A multicenter, open-label, phase 1B/2 study to evaluate safety and efficacy of avelumab in combination with chemotherapy with or without other anti-cancer immunotherapies as first-line treatment in patients with advanced malignancies | II | Avelumab+pemetrexed/carboplatin | Non-randomized parallel trial | Untreated advanced non-squamous NSCLC without EGFR mutations or ALK rearrangement | DLT, ORR | PFS, DOR, TTR | Recruiting | Oct 2020 | |
| NCT03158883 | A pilot study of avelumab and stereotactic ablative radiotherapy in non-responding and progressing NSCLC patients previously treated with a PD-1 inhibitor | I | Avelumab+Stereotactic ablative radiotherapy | Non-randomized parallel trial | Immunotherapy pre-treated advanced NSCLC without EGFR mutations or ALK rearrangement | Overall response rate | OS, PFS, DCR | Recruiting | Jun 2020 | |
| NCT02554812 | A phase 1B/2, open-label study to evaluate safety, clinical activity, pharmacokinetics and pharmacodynamics of avelumab in combination with other cancer immunotherapies in patients with advanced maligancies | II | Avelumab+Utomilumab | Randomized parallel trial | Advanced NSCLC without EGFR mutation or ALK/ROS1 rearrangement regardless of prior lines treatment | DLT, ORR | TTR, DOR, PFS | Recruiting | Dec 2020 |
PFS progression-free survival, OS overall survival, ORR objective response rate, DLT dose-limiting toxicities, DOR duration of response, TTR time to response
Durvalumab
Durvalumab is a selective, high affinity human IgG1 monoclonal antibody which impedes PD-L1 from binding to PD-1 and CD 80 [192, 193]. Its clinical efficacy was first reported at the 2014 ASCO meeting through a phase I study, showing limited toxicity and potential response rate. On account of previous single-agent checkpoint inhibitor success in second-line treatment [23, 24, 26, 27], several phase trials of combination regimens involving durvalumab were initiated [194, 195]. Preliminary results of these studies showed no significant difference to other checkpoint inhibitors. One should notice that combination regimens involving durvalumab in both TATTON and CAURAL study revealed an extremely high risk of developing interstitial pneumonia, leading to the termination of both trials. The phase II ATLANTIC trial (NCT02087423) evaluated the efficacy of durvalumab as a third-line treatment in advanced NSCLC [196]. The ORR was 7.5%, 16.4%, and 30.9% in patients with PD-L1 expression of < 25%, > 25% and > 90%, respectively. PFS in patients with high PD-L1 and low/negative PD-L1 expression was 3.3 and 1.9 month. To be noticed, cohort 1 in this trial receiving single-agent durvalumab included advanced NSCLC patients with EGFR-sensitive mutations or ALK rearrangement. The ORR in this group was not remarkable even in PD-L1 high expression population. Two well-known phase III trials with diverse ending were unleashed last year. The MYSTIC trial (NCT02453282) assessing durvalumab plus tremelimumab or durvalumab monotherapy versus platinum-based chemotherapy showed both combination and single-agent regimen which failed to reach the primary endpoint without PFS benefit. The PACIFIC trial (NCT02125461), on the other hand, achieved great success and led to a treatment paradigm shift for unresectable locally advanced NSCLC [197, 198]. Additionally, several phase III trials of durvalumab are pending or ongoing, and hopefully, more optional treatment would be provided (Table 4).
Table 4.
Ongoing phase III trials for durvalumab in early-stage and advanced-stage lung cancer
| Objectives | Identifier | Title | Intervention | Study design | Population | Primary endpoint | Secondary endpoint | Status | Primary completion |
|---|---|---|---|---|---|---|---|---|---|
| Early-stage | NCT03703297 | A phase III, randomized, double-blind, placebo-controlled, multi-center, international study of durvalumab or durvalumab and tremelimumab as consolidation treatment for patients with stagei-iii limited disease small-cell lung cancer who have not progressed following concurrent chemoradiation therapy (ADRIATIC) | • Durvalumab+placebo • Durvalumab tremelimuab, placebo+placebo |
Randomized parallel trial | Limited-stage SCLC without progression after definitive concurrent chemoradiation | PFS, OS | ORR, TTD/TTM, PFS2 | Recruiting | Jun 2021 |
| NCT03800134 | A phase III, double-blind, placebo-controlled, multi-center international study of neoadjuvant/adjuvant durvalumab for the treatment of patients with resectable stages II and III non-small cell lung cancer (AEGEAN) | • Durvalumab+platinum-based chemotherapy • Placebo+platinum-based chemotherapy |
Randomized parallel trial | Resectable stage IIA-IIIB NSCLC | MPR | pCR, OS, DFS | Recruiting | Jul 2020 | |
| NCT03519971 | A phase III, randomized, placebo-controlled, double-blind, multi-center, international study of durvalumab given concurrently with platinum-based chemoradiation therapy in patients with locally advanced, unresectable non-small cell lung cancer (StageIII) (PACIFIC2) | • Durvalumab+platinum-based chemotherapy and radiation • Placebo+platinum-based chemotherapy and radiation |
Randomized parallel trial | Unresectable locally advanced stage III NSCLC | PFS, ORR | OS, DOR, PFS2 | Recruiting | Sep 2020 | |
| NCT02273375 | A phase III prospective double blind placebo controlled randomized study of adjuvant MEDI4736 in completely resected non-small cell lung cancer | • Durvalumab • Placebo |
Randomized parallel trial | Stage IB (> 4 cm) to IIIA NSCLC after complete surgical resection | DFS | OS, LCSS | Recruiting | Jan 2023 | |
| NCT03706690 | A phase III, randomized,double-blind,placebo-controlled,study of durvalumab as consolidation therapy in patients with locally advanced, unresectable NSCLC, who have not progressed following definitive, platinum-based chemoradiation therapy (PACIFIC5) | • Durvalumab • Placebo |
Randomized parallel trial | Unresectable locally advanced stage III NSCLC | PFS | OS, ORR, DOR | Recruiting | Mar 2021 | |
| Advanced-stage | NCT03164616 | A phase III, randomized, multi-center, open-label, comparative global study to determine the efficacy of durvalumab or durvalumab and tremelimumab in combination with platinum-based chemotherapy for first-line treatment in patients with metastatic non small-cell lung cancer (NSCLC) (POSEIDON) | • Durvalumab+tremelimumab • Durvalumab monotherapy+SoC • SoC chemotherapy alone |
Randomized parallel trial | Untreated advanced NSCLC without activating EGFR mutation or ALK fusions | PFS, OS | ORR, DOR, PFS2 | Recruiting | Sep 2019 |
| NCT03003962 | A phase III randomized, open-label, multi-center study of durvalumab (MEDI4736) versus standard of care (SoC) platinum-based chemotherapy as first line treatment in patients with PD-L1-high expression advanced non small-cell lung cancer | • Durvalumab • SoC chemotherapy |
Randomized parallel trial | Untreated advanced PD-L1 positive NSCLC without EGFR mutation and ALK rearrangement | OS | ORR, DOR, PFS | Recruiting | Sep 2019 | |
| NCT02453282 | A phase III randomized, open-label, multi-center, global study of MEDI4736 in combination with tremelimumab therapy or MEDI4736 monotherapy versus standard of careplatinum-based chemotherapy in first line treatment of patients with advanced or metastatic non small-cell lung cancer (MYSTIC) | • Durvalumab • Durvalumab+tremelimumab • SoC chemotherapy |
Randomized parallel trial | Untreated advanced NSCLC without activating EGFR mutation or ALK fusions | OS, PFS | ORR | Active, not recruiting | Oct 2018 | |
| NCT03043872 | A phase III, randomized, multicenter,open-label, comparative study to determine the efficacy of durvalumab or durvalumab and tremelimumab in combination with platinum-based chemotherapy for the first-line treatment in patients with extensive disease small-cell lung cancer (SCLC) (CASPIAN) | • Durvalumab+tremelimumab+EP • Durvalumab+EP • EP |
Randomized parallel trial | Untreated extensive stage SCLC | OS, PFS | ORR, EORTC QLQ-C30 | Active, not recruiting | Sep 2019 | |
| NCT02542293 | A phase III randomized, open-label, multi-center, global study of medi4736 in combination with tremelimumab therapy versus standard of care platinum-based chemotherapy in first-line treatment of patients with advanced or metastatic non small-cell lung cancer (NSCLC) | • Durvalumab+tremelimumab • SoC chemotherapy |
Randomized parallel trial | Untreated advanced NSCLC without activating EGFR mutation or ALK fusions | OS | PFS, ORR, DOR | Active, not recruiting | Mar 2019 | |
| NCT02352948 | A phase III, open label, randomized, multi-centre, international study of MEDI4736, given as monotherapy or in combination with tremelimumab determinedby PD-L1 expression versus standard of care in patients with locally advanced or metastatic non-small cell lung cancer (stage IIIB-IV) who have received at least two prior systemic treatment regimens including one platinum based chemotherapy regimen and do not have known EGFR activating mutations or ALK rearrangements (ARCTIC) | • Durvalumab • Durvalumab+tremelimumab • Tremelimumab • Vinorelbine/gemcitabine/erlotinib |
Randomized parallel trial | Advanced NSCLC without EGFR mutations and ALK rearrangment after progression of chemotherapy and at least one prior regimens treatment | OS, PFS | ORR, DOR | Active, not recruiting | Feb 2018 |
DCR disease control rate, LCSS lung cancer-specific survival, PFS2 time from randomization to second progression, TTD/TTM time to death/time to distant metastasis, MPR major pathological response, pCR pathological complete response, DFS disease-free survival
Potential novel treatment modalities for lung cancer
Chimeric antigen receptor T cell and bispecific antibodies
Beyond the field of checkpoint inhibitors, another immunotherapy such as adoptive cellular immunotherapy has emerged as a remarkable treatment modality in the past decades [199, 200]. Unlike checkpoint inhibitors, which induce antitumor activity through blocking the barrier between effective T cells and tumor cells [201–203], adoptive cellular immunotherapy is a novel approach providing “artificial” effective T cells to specifically target tumor cells directly regardless of tumor types [204–206]. With that being said, adoptive cellular immunotherapy may bring broad range effect on different tumors compared to checkpoint inhibitors, which have been reported diverse response among a variety of tumors [207–213]. So far, significant advances in chimeric antigen receptor T cell (CAR-T) have been accelerated in hematological malignancies especially for CD19-targeted CAR-T-cell therapy in leukemia [214–218]. However, in solid tumors, it is tough to design CAR-T because no such surface antigen as unique as CD19 has yet been identified [219]. Indeed, numerous clinical trials of CAR-T regarding lung cancer have been initiated including tumor-associated antigen (TAA) of EGFR (NCT02862028, NCT01869166), HER2 (NCT00889954, NCT01935843), carcinoembryonic antigen (CEA) (NCT01723306, NCT02349724), and mesothelin (MSLN) (NCT01583686, NCT03054298). Gladly, several trials focusing on NSCLC were initiated and ongoing in China [220]. One clinical trial of EGFR-specific CAR-T regarding non-small cell lung cancer (NCT01869166) had reported its preliminary results. 45.5% (5/11) of advanced NSCLC patients achieved stable disease, and 2 achieved partial response (PR). Treatment-related adverse events were manageable indicating its potentials in NSCLC. Yet, several aspects regarding the application of CAR-T in solid tumors should be noticed. First, the “off-target” effect is one of the major causes that lead to increased toxicity and less efficacy. In hematological malignancies, for example, the B cell acute lymphoblastic leukemia (B-ALL), well tolerance could be observed during the treatment of CD19-targeted CAR-T cells [221] due to the ubiquitous expression of CD19 on differentiated B cells instead of hematopoietic stem cells. On the contrary, target antigen in a solid tumor may be additionally expressed in other tissue or organs which may lead to unexpected treatment-related toxicity. Besides, it is much more difficult to select candidate antigen in solid tumors with higher antigen heterogeneity [222–224]. Second, microenvironment in solid tumors was relatively immunosuppressive-preferred compared to hematological malignancies leading to less efficient CAR-T therapy in solid tumors. Indeed, ongoing trials regarding solid tumors may further decipher the uncertainty in the future.
The concept of bispecific antibody for oncogene was based on the simultaneous activation of different pathways driving tumor proliferation and growth [225–227]. So far, the bispecific antibody for lung cancer is still under initial researches. It is indeed an encouraging agent for lung cancer in the future according to the preliminary results. The novel EGFR/cMet bispecific antibody (JNJ-61186372), a fully humanized IgG1 antibody, was first put up in 2016, and its preliminary results in human were reported in 2018 WCLC [228, 229]. Objective response was shown in various activating EGFR mutations including T790m and exon 20 insertion. It seemed like the two separate targets combined may broaden its antitumor activity compared to single-target inhibition. However, the efficacy and duration of response along with adverse events are warranted to further clarify its clinical application.
Discussion
Novel agents for lung cancer have been booming these years. Since the post era of IPASS and 2013 when immunotherapy has been crowned as one of the breakthroughs, remarkable clinical results from both tyrosine kinase inhibitors and checkpoint inhibitors for lung cancer have generated a great number of potential agents which significantly improved patients’ survival beyond the era of chemotherapy. The current advances have been undoubtedly shifting the clinical paradigm for advanced lung cancer. So far, numerous potential agents including TKIs, CPIs, and underlying treatment modalities other than what we have mentioned above are under preclinical researches or early phase trials. Compared to previous standard treatment regarding TKIs, novel agents showed significant improvement in several aspects including improvement of BBB penetration, broadened target profiles, overcoming resistant mechanism, prolonged survival, and lower toxicity. In EGFR-TKIs, osimertinib has been undoubtedly claiming its essential clinical role in the first-line setting. However, whether sequential treatment following combination modalities (NEJ009 and JO25567 presented in 2018 ASCO meeting) or even novel second-generation TKI dacomitinib would be translated into better clinical outcome remained unknown. With potentially manageable acquired resistance, osimertinib would be temporally the best first option for untreated EGFR-mutant NSCLC patients. For ALK rearrangement, on the other hand, numerous highly potent targeted drugs were innovated and approved these years. Extremely complex mutational profiles were observed after the treatment of ALK-TKIs. Through chord diagram (Fig. 2) regarding sensitive and resistant mutational profiles of corresponding ALK-TKIs integrated from previous researches [106, 230–242], it is easy to tell that novel ALK-TKIs could cover more ALK-dependent mutations other than ALK fusion and meanwhile show antitumor activity in more resistant subtypes. Concurrent ALK-resistant mutations remained to be unyielding fields at the moment, and combination of different ALK-TKIs might be worthy to try in the future. For CPIs, numerous similar checkpoint inhibitors targeting PD-1/PD-L1 including CPIs developed by Chinese pharmaceutical companies [243] have been generated since the heat of immunotherapy. The clinical paradigm for wild-type NSCLC has been undoubtedly shifted with so many approved single or combination regimens involving CPIs in the first- and second-line setting. Some other phase III trials of novel checkpoint inhibitors were pending, and hopefully, more choices would be available in the first-line setting (Fig. 3). Indeed, due to first-mover advantage and limited understanding of how immunotherapy works within microenvironment, novel CPIs would be much tougher to compete as a single agent with previous CPIs. Combination treatment modality and clinical unmet needs, being the two major aspects, were ideal resolutions for the development of novel CPIs. To be noticed, challenges to immunotherapy remained to be unsolved including hyperprogression [244, 245], immune-related toxicities [246, 247], and primary/adaptive resistance to immunotherapy [248]. Moreover, potential novel treatment modalities have aroused great interest and their preliminary performances revealed remarkable prospects for development in lung cancer. Yet, most of the novel agents were still under the early stage of birth and further results should be expected. Whether these novel immunotherapy modalities may take place after the treatment failure of first-line checkpoint inhibitors in the future would be worth looking forward to (Fig. 4). At this moment, we are facing the condition of numerous novel agents developed for lung cancer. Improvement of clinical trials accelerating the application of novel drugs in clinical practice and discovery of novel effective targets along with much more precise biomarkers would be so much essential for anticancer treatment in the future.
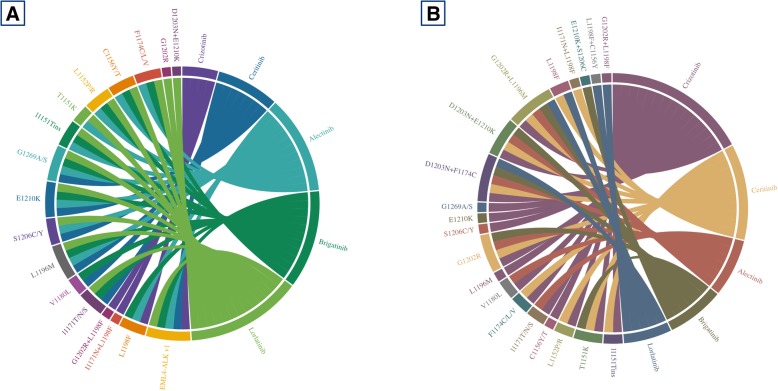
Fig. 2.
Chord diagrams for sensitive and resistant mutations regarding ALK-TKIs. Both preclinical data and clinical reported cases (preferred) were enrolled to determine the efficacy of ALK-TKIs to different ALK-dependent mutations. Crizotinib had smallest sensitive mutation profiles compared to lorlatinib while opposite in resistant profiles. a Mutation profiles showed responsiveness to different ALK-TKIs. b Mutation profiles reported to be resistant to different ALK-TKIs
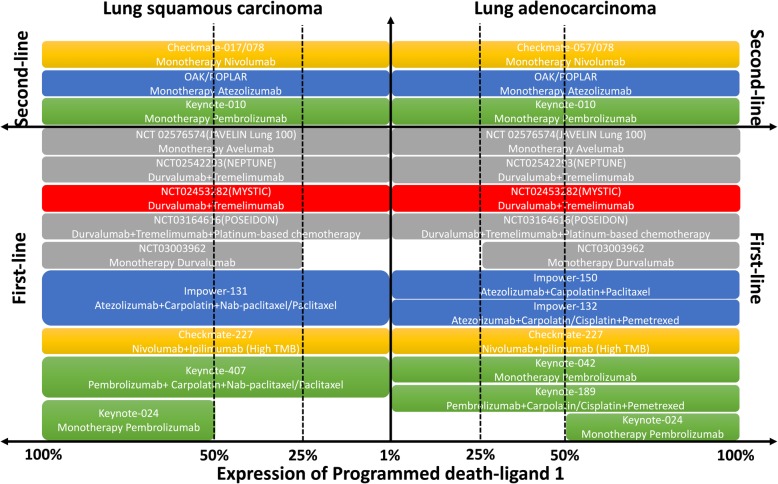
Fig. 3.
Results of posted and pending trials of PD-1/PD-L1 inhibitors between lung adenocarcinoma and squamous carcinoma regarding different PD-L1 expression. All posted and pending trials were stratified based on the indication for different expression of PD-L1 and treatment lines. Only PD-1/PD-L1 inhibitors of pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab were included except for checkpoint inhibitors from Chinese pharmaceutical companies due to early phase trials of these checkpoint inhibitors for lung cancer. Checkmate-227, although regardless of PD-L1 expression, required high tumor mutation burden (TMB)
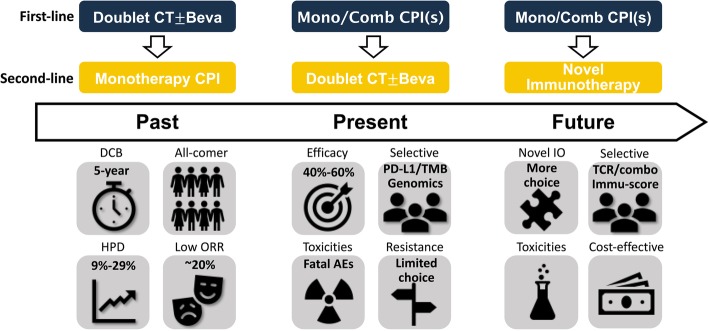
Fig. 4.
Perspectives for the evolving modalities of immunotherapy in NSCLC. Treatment modalities involving immunotherapy in NSCLC had evolved from second-line setting to first-line setting. Prior immunotherapy, highly selective patients, and combination strategies had raised significant efficacy improvement but increased toxicities as well. Novel immunotherapy in the future combined with multiple novel biomarkers may infinitely consolidate the clinical role of immunotherapy in advanced NSCLC
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation (grant no.81673031 and 81872510, to WZ Zhong).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- ALK
Anaplastic lymphoma kinase
- B-ALL
B cell acute lymphoblastic leukemia
- BBB
Blood-brain barrier
- CAR-T
Chimeric antigen receptor T cell
- CEA
Carcinoembryonic antigen
- CNS
Central nerve system
- CPI
Checkpoint inhibitor
- DCR
Disease control rate
- EGFR
Epidermal growth factor receptor
- IgG1
Immunoglobulin G1
- MSLN
Mesothelin
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PD-1/PD-L1
Programmed cell death protein-1/programmed cell death protein ligand-1
- PFS
Progression-free survival
- PR
Partial response
- RTK
Receptor tyrosine kinases
- TAA
Tumor-associated antigen
- TKI
Tyrosine kinase inhibitor
Authors’ contributions
WZZ and CZ designed the outline and drafted the manuscript. CZ designed the figures and tables. YLW and NBL offered professional suggestions to the manuscript. NBL provided the language editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schiller JH, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1416–1423. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94(5):1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 4.Leighl NB, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol. 2005;23(12):2831–2839. doi: 10.1200/JCO.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24(9):1428–1434. doi: 10.1200/JCO.2005.04.3299. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Maemondo M, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 9.Fukuoka M, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 10.Han JY, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–9. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SV, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105(9):595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 14.Haaland B, et al. Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol. 2014;9(6):805–811. doi: 10.1097/JTO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxnard GR, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 17.Sequist LV, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 18.Wong DW, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 19.Gainor JF, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu HA, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao AS, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11(5):613–638. doi: 10.1016/j.jtho.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 23.Borghaei H, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehrenbacher L, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 27.Rittmeyer A, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 29.Carbone DP, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger S, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209–003 study. J Clin Oncol. 2018;36(17):1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 31.von Pawel J, et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–132. doi: 10.1016/j.ejca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–9. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champiat S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 35.Ledford H. Promising cancer drugs may speed tumours in some patients. Nature. 2017;544(7648):13–14. doi: 10.1038/nature.2017.21755. [DOI] [PubMed] [Google Scholar]
- 36.Engelman JA, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 37.Gonzales AJ, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7(7):1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 38.Wu YL, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 39.Yu JY, et al. Clinical outcomes of EGFR-TKI treatment and genetic heterogeneity in lung adenocarcinoma patients with EGFR mutations on exons 19 and 21. Chin J Cancer. 2016;35:30. doi: 10.1186/s40880-016-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W, et al. Postoperative survival of EGFR-TKI-targeted therapy in non-small cell lung cancer patients with EGFR 19 or 21 mutations: a retrospective study. World J Surg Oncol. 2017;15(1):197. doi: 10.1186/s12957-017-1251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mok TS, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 42.Mok T, et al. MA26.11 effects of dose modifications on the safety and efficacy of dacomitinib for EGFR mutation-positive NSCLC. J Thorac Oncol. 2018;13(10):S454. doi: 10.1016/j.jtho.2018.08.546. [DOI] [PubMed] [Google Scholar]
- 43.Cross DA, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goss G, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17(12):1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 45.Yang JC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension Component. J Clin Oncol. 2017;35(12):1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 46.Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 47.Planchard David, Boyer Michael J., Lee Jong-Seok, Dechaphunkul Arunee, Cheema Parneet K., Takahashi Toshiaki, Gray Jhanelle E., Tiseo Marcello, Ramalingam Suresh S., Todd Alexander, McKeown Astrid, Rukazenkov Yuri, Ohe Yuichiro. Postprogression Outcomes for Osimertinib versus Standard-of-Care EGFR-TKI in Patients with Previously Untreated EGFR-mutated Advanced Non–Small Cell Lung Cancer. Clinical Cancer Research. 2019;25(7):2058–2063. doi: 10.1158/1078-0432.CCR-18-3325. [DOI] [PubMed] [Google Scholar]
- 48.Le X, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24(24):6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin CC, et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med. 2018;6(2):107–116. doi: 10.1016/S2213-2600(17)30480-0. [DOI] [PubMed] [Google Scholar]
- 50.Oxnard GR, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527–1534. doi: 10.1001/jamaoncol.2018.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramalingam SS, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(9):841–849. doi: 10.1200/JCO.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, et al. Combined therapy with osimertinib and afatinib in a lung adenocarcinoma patient with EGFR T790M mutation and multiple HER2 alterations after resistance to icotinib: a case report. Thorac Cancer. 2018;9(12):1774–1777. doi: 10.1111/1759-7714.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.York ER, et al. Tolerable and effective combination of full-dose crizotinib and osimertinib targeting met amplification sequentially emerging after T790M positivity in EGFR-mutant non-small cell lung cancer. J Thorac Oncol. 2017;12(7):e85–e88. doi: 10.1016/j.jtho.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Uchibori K, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun. 2017;8:14768. doi: 10.1038/ncomms14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi P, et al. Overcoming acquired resistance to AZD9291, a third-generation EGFR inhibitor, through modulation of MEK/ERK-dependent Bim and Mcl-1 degradation. Clin Cancer Res. 2017;23(21):6567–6579. doi: 10.1158/1078-0432.CCR-17-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piotrowska Z, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant nsclc and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired ret fusion. Cancer Discov. 2018;8(12):1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakatani K, et al. KRAS and EGFR amplifications mediate resistance to rociletinib and osimertinib in acquired afatinib-resistant NSCLC harboring exon 19 deletion/T790M in EGFR. Mol Cancer Ther. 2019;18(1):112–126. doi: 10.1158/1535-7163.MCT-18-0591. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Marti A, et al. Dual MET and ERBB inhibition overcomes intratumor plasticity in osimertinib-resistant-advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2017;28(10):2451–2457. doi: 10.1093/annonc/mdx396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L, et al. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: a case report. Oncotarget. 2017;8(11):18609–18614. doi: 10.18632/oncotarget.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.La Monica S, et al. Trastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell lines. J Exp Clin Cancer Res. 2017;36(1):174. doi: 10.1186/s13046-017-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia Y, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534(7605):129–132. doi: 10.1038/nature17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho CC, et al. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol. 2017;12(3):567–572. doi: 10.1016/j.jtho.2016.11.2231. [DOI] [PubMed] [Google Scholar]
- 63.Arulananda S, et al. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(11):1728–1732. doi: 10.1016/j.jtho.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Umemura S, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77(1):134–139. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Palma JA, et al. Leptomeningeal carcinomatosis: prognostic value of clinical, cerebrospinal fluid, and neuroimaging features. Clin Neurol Neurosurg. 2013;115(1):19–25. doi: 10.1016/j.clineuro.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 66.Liao BC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10(12):1754–1761. doi: 10.1097/JTO.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 67.Ballard P, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 68.Nanjo S, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118(1):32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahn Myung-Ju, Kim Dong-Wan, Cho Byoung Chul, Kim Sang-We, Lee Jong Seok, Ahn Jin-Seok, Kim Tae Min, Lin Chia-Chi, Kim Hye Ryun, John Thomas, Kao Steven, Goldman Jonathan W, Su Wu-Chou, Natale Ronald, Rabbie Sarit, Harrop Bryony, Overend Philip, Yang Zhenfan, Yang James Chih-Hsin. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. The Lancet Respiratory Medicine. 2017;5(11):891–902. doi: 10.1016/S2213-2600(17)30378-8. [DOI] [PubMed] [Google Scholar]
- 70.Rangachari D, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng Q, et al. Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem. 2015;58(20):8200–8215. doi: 10.1021/acs.jmedchem.5b01073. [DOI] [PubMed] [Google Scholar]
- 72.Yang Z, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8(368):368ra172. doi: 10.1126/scitranslmed.aag0976. [DOI] [PubMed] [Google Scholar]
- 73.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98(12):1817–1824. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oxnard GR, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–184. doi: 10.1097/JTO.0b013e3182779d18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yasuda H, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5(216):216ra177. doi: 10.1126/scitranslmed.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 77.Arcila ME, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220–229. doi: 10.1158/1535-7163.MCT-12-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robichaux JP, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24(5):638–646. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heymach J, et al. OA02.06 a phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC) J Thorac Oncol. 2018;13(10):S323–S324. doi: 10.1016/j.jtho.2018.08.243. [DOI] [Google Scholar]
- 80.Neal J, et al. P1.13-44 safety, pk, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK-788 in NSCLC. J Thorac Oncol. 2018;13(10):S599. doi: 10.1016/j.jtho.2018.08.901. [DOI] [Google Scholar]
- 81.Peters S, et al. Activity of afatinib in heavily pretreated patients with ERBB2 mutation-positive advanced nsclc: findings from a global named patient use program. J Thorac Oncol. 2018;13(12):1897–1905. doi: 10.1016/j.jtho.2018.07.093. [DOI] [PubMed] [Google Scholar]
- 82.Mazieres J, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 83.Goss GD, et al. Association of ERBB mutations with clinical outcomes of afatinib- or erlotinib-treated patients with lung squamous cell carcinoma: secondary analysis of the LUX-lung 8 randomized clinical trial. JAMA Oncol. 2018;4(9):1189–1197. doi: 10.1001/jamaoncol.2018.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Greve J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76(1):123–127. doi: 10.1016/j.lungcan.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Dziadziuszko R, et al. Afatinib in non-small cell lung cancer with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol. 2019. 10.1016/j.jtho.2019.02.017. [DOI] [PubMed]
- 86.Ma F, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35(27):3105–3112. doi: 10.1200/JCO.2016.69.6179. [DOI] [PubMed] [Google Scholar]
- 87.Li X, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. doi: 10.1016/j.ejps.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, Rivard C, Gao G, Ng T L, Tu M M, Yu H, Ji H, Zhou C, Ren S, Zhang J, Bunn P, Doebele R C, Camidge D R, Hirsch F R. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Annals of Oncology. 2018;30(3):447–455. doi: 10.1093/annonc/mdy542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon BJ, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 90.Katayama R, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doebele RC, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marsilje TH, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56(14):5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 93.Shaw AT, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khozin S, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2436–2439. doi: 10.1158/1078-0432.CCR-14-3157. [DOI] [PubMed] [Google Scholar]
- 95.Kim DW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crino L, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34(24):2866–2873. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 97.Soria JC, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 98.Cho BC, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC) J Thorac Oncol. 2017;12(9):1357–1367. doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Cho BC, et al. Efficacy and safety of ceritinib (450 mg/day or 600 mg/day) with food vs 750 mg/day fasted in patients with ALK-positive NSCLC: primary efficacy results from ASCEND-8 study. J Thorac Oncol. 2019. 10.1016/j.jtho.2019.03.002. [DOI] [PubMed]
- 100.Huber KV, et al. Stereospecific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature. 2014;508(7495):222–227. doi: 10.1038/nature13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shaw AT, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu YL, et al. Phase II study of crizotinib in east asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 103.Lim SM, et al. Open-Label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 104.Zhang I, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16(13):e510–e521. doi: 10.1016/S1470-2045(15)00013-3. [DOI] [PubMed] [Google Scholar]
- 105.Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12(7):429–439. [PMC free article] [PubMed] [Google Scholar]
- 106.Gainor JF, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21(10):2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seto T, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14(7):590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 109.Hida T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 110.Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 111.Novello S, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kodama T, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74(5):1023–1028. doi: 10.1007/s00280-014-2578-6. [DOI] [PubMed] [Google Scholar]
- 113.Sakamoto H, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Costa DB, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 115.Lin Jessica J., Jiang Ginger Y., Joshipura Nencyben, Ackil Jennifer, Digumarthy Subba R., Rincon Sandra P., Yeap Beow Y., Gainor Justin F., Shaw Alice T. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. Journal of Thoracic Oncology. 2019;14(4):683–690. doi: 10.1016/j.jtho.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi YL, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 117.Heuckmann JM, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res. 2011;17(23):7394–7401. doi: 10.1158/1078-0432.CCR-11-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Friboulet L, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ignatius Ou SH, et al. Next-generation sequencing reveals a Novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol. 2014;9(4):549–553. doi: 10.1097/JTO.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 120.Huang WS, et al. Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59(10):4948–4964. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 121.Zhang S, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22(22):5527–5538. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 122.Lin JJ, et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol. 2018;13(10):1530–1538. doi: 10.1016/j.jtho.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim DW, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–2498. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 124.Camidge DR, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 125.Zhou J, et al. Crizotinib in patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer versus chemotherapy as a first-line treatment. BMC Cancer. 2018;18(1):10. doi: 10.1186/s12885-017-3720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nishio M, et al. Crizotinib versus chemotherapy in asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat. 2018;50(3):691–700. doi: 10.4143/crt.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 128.Shaw AT, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 129.Johnson TW, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno) pyrazolo [4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 130.Redaelli S, et al. Lorlatinib treatment elicits multiple on- and off-target mechanisms of resistance in ALK-driven cancer. Cancer Res. 2018;78(24):6866–6880. doi: 10.1158/0008-5472.CAN-18-1867. [DOI] [PubMed] [Google Scholar]
- 131.Solomon BJ, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 132.Shaw AT, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lovly CM, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71(14):4920–4931. doi: 10.1158/0008-5472.CAN-10-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Horn L, et al. MINI01.02: response and plasma genotyping from phase I/II trial of ensartinib (X-396) in patients (pts) with ALK+NSCLC: topic: medical oncology. J Thorac Oncol. 2016;11(11):S256–S257. doi: 10.1016/j.jtho.2016.09.017. [DOI] [Google Scholar]
- 135.Horn L, et al. Ensartinib (X-396) in ALK-positive non-small cell lung cancer: results from a first-in-human phase I/II, multicenter Study. Clin Cancer Res. 2018;24(12):2771–2779. doi: 10.1158/1078-0432.CCR-17-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horn L, et al. P1.13–02 eXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase positive non-small cell lung cancer patients. J Thorac Oncol. 2018;13(10):S582. doi: 10.1016/j.jtho.2018.08.858. [DOI] [Google Scholar]
- 137.Stransky N, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaishnavi A, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469–1472. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drilon A, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7(4):400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drilon A, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27(5):920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Russo M, et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 2016;6(1):36–44. doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- 143.Drilon A, et al. A Next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7(9):963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Drilon A, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 2018;8(10):1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 145.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14(3):173–186. doi: 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- 146.Lee SH, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol. 2017;28(2):292–297. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 147.Kato S, et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 Patients. Clin Cancer Res. 2017;23(8):1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 148.Li GG, et al. Antitumor activity of RXDX-105 in multiple cancer types with RET rearrangements or mutations. Clin Cancer Res. 2017;23(12):2981–2990. doi: 10.1158/1078-0432.CCR-16-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Drilon A, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drilon A, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019;9(3):384–395. doi: 10.1158/2159-8290.CD-18-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Subbiah V, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29(8):1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kurzrock R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Subbiah V, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 154.Liu X, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17(22):7127–7138. doi: 10.1158/1078-0432.CCR-11-1157. [DOI] [PubMed] [Google Scholar]
- 155.Frampton GM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 156.Chen HJ, et al. Clinicopathologic and molecular features of epidermal growth factor receptor T790M mutation and c-MET amplification in tyrosine kinase inhibitor-resistant Chinese non-small cell lung cancer. Pathol Oncol Res. 2009;15(4):651–658. doi: 10.1007/s12253-009-9167-8. [DOI] [PubMed] [Google Scholar]
- 157.Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 160.Lara MS, et al. Preclinical evaluation of MET Inhibitor INC-280 with or without the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung cancer. Clin Lung Cancer. 2017;18(3):281–285. doi: 10.1016/j.cllc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu YL, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol. 2018;36(31):3101–3109. doi: 10.1200/JCO.2018.77.7326. [DOI] [PubMed] [Google Scholar]
- 162.Kim S, et al. Acquired resistance of MET-amplified non-small cell lung cancer cells to the MET inhibitor capmatinib. Cancer Res Treat. 2018. 10.4143/crt.2018.052. [DOI] [PMC free article] [PubMed]
- 163.Paik PK, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kris MG, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marchetti A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29(26):3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 166.Cardarella S, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19(16):4532–4540. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hyman DM, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.King AJ, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013;8(7):e67583. doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Planchard D, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(5):642–650. doi: 10.1016/S1470-2045(16)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Larkin J, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 171.Flaherty KT, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Planchard D, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 173.Planchard D, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie C, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi: 10.1111/cas.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taurin S, et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer. 2018;28(1):152–160. doi: 10.1097/IGC.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shen G, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi: 10.1186/s13045-018-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin B, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. 2018;654:77–86. doi: 10.1016/j.gene.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 178.Han B, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302) Br J Cancer. 2018;118(5):654–661. doi: 10.1038/bjc.2017.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Diggs LP, Hsueh EC. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res. 2017;5:12. doi: 10.1186/s40364-017-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Langer CJ, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seto T, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 182.Rosell R, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med. 2017;5(5):435–444. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 183.Han B, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer. 2017;141(6):1249–1256. doi: 10.1002/ijc.30806. [DOI] [PubMed] [Google Scholar]
- 184.Paz-Ares L, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 185.Gandhi L, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 186.Hellmann MD, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Socinski MA, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 188.Gulley JL, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fujii R, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7(23):33498–33511. doi: 10.18632/oncotarget.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boyerinas B, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3(10):1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barlesi F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 192.Stewart R, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 193.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Planchard D, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232–236.e1. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 195.Antonia S, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Garassino MC, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Antonia SJ, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 198.Antonia SJ, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 199.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marin-Acevedo JA, et al. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 202.Iafolla MAJ, Juergens RA. Update on programmed death-1 and programmed death-ligand 1 inhibition in the treatment of advanced or metastatic non-small cell lung cancer. Front Oncol. 2017;7:67. doi: 10.3389/fonc.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johnson LA, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Beatty GL, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adusumilli PS, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 209.Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 210.Massard C, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 212.Dirix LY, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Turtle CJ, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li J, et al. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11(1):22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu B, Song Y, Liu D. Clinical trials of CAR-T cells in China. J Hematol Oncol. 2017;10(1):166. doi: 10.1186/s13045-017-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mueller KT, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805(1):105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 224.Feng K, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59(5):468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 225.Planchard D, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol. 2015;26(10):2073–2078. doi: 10.1093/annonc/mdv319. [DOI] [PubMed] [Google Scholar]
- 226.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jiang SX, et al. EGFR genetic heterogeneity of nonsmall cell lung cancers contributing to acquired gefitinib resistance. Int J Cancer. 2008;123(11):2480–2486. doi: 10.1002/ijc.23868. [DOI] [PubMed] [Google Scholar]
- 228.Park K, et al. OA10.06 a first-in-human phase 1 trial of the EGFR-cMET bispecific antibody JNJ-61186372 in patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2018;13(10):S344–S345. doi: 10.1016/j.jtho.2018.08.292. [DOI] [Google Scholar]
- 229.Moores SL, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76(13):3942–3953. doi: 10.1158/0008-5472.CAN-15-2833. [DOI] [PubMed] [Google Scholar]
- 230.Childress MA, et al. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018;16(11):1724–1736. doi: 10.1158/1541-7786.MCR-18-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Esfahani K, Agulnik JS, Cohen V. A systemic review of resistance mechanisms and ongoing clinical trials in ALK-rearranged non-small cell lung cancer. Front Oncol. 2014;4:174. doi: 10.3389/fonc.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Katayama R, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20(22):5686–5696. doi: 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7(2):137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Muller IB, et al. Overcoming crizotinib resistance in ALK-rearranged NSCLC with the second-generation ALK-inhibitor ceritinib. Expert Rev Anticancer Ther. 2016;16(2):147–157. doi: 10.1586/14737140.2016.1131612. [DOI] [PubMed] [Google Scholar]
- 235.Okada Koutaroh, Araki Mitsugu, Sakashita Takuya, Ma Biao, Kanada Ryo, Yanagitani Noriko, Horiike Atsushi, Koike Sumie, Oh-hara Tomoko, Watanabe Kana, Tamai Keiichi, Maemondo Makoto, Nishio Makoto, Ishikawa Takeshi, Okuno Yasushi, Fujita Naoya, Katayama Ryohei. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine. 2019;41:105–119. doi: 10.1016/j.ebiom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ou SH, et al. I1171 missense mutation (particularly I1171N) is a common resistance mutation in ALK-positive NSCLC patients who have progressive disease while on alectinib and is sensitive to ceritinib. Lung Cancer. 2015;88(2):231–234. doi: 10.1016/j.lungcan.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 237.Ou SH, et al. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer. 2016;91:70–72. doi: 10.1016/j.lungcan.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 238.Sabari JK, et al. The activity, safety, and evolving role of brigatinib in patients with ALK-rearranged non-small cell lung cancers. Onco Targets Ther. 2017;10:1983–1992. doi: 10.2147/OTT.S109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yoda S, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8(6):714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhu VW, et al. Identification of a novel T1151K ALK mutation in a patient with ALK-rearranged NSCLC with prior exposure to crizotinib and ceritinib. Lung Cancer. 2017;110:32–34. doi: 10.1016/j.lungcan.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 241.Zhu VW, et al. Dramatic response to alectinib in a lung cancer patient with a novel VKORC1L1-ALK fusion and an acquired ALK T1151K mutation. Lung Cancer (Auckl) 2018;9:111–116. doi: 10.2147/LCTT.S186804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zou HY, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28(1):70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu SY, Wu YL. Ongoing clinical trials of PD-1 and PD-L1 inhibitors for lung cancer in China. J Hematol Oncol. 2017;10(1):136. doi: 10.1186/s13045-017-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fuentes-Antras J, Provencio M, Diaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev. 2018;70:16–21. doi: 10.1016/j.ctrv.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 245.Kurman JS, Murgu SD. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thorac Dis. 2018;10(2):1124–1128. doi: 10.21037/jtd.2018.01.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Johnson DB, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kanai O, et al. Concurrence of nivolumab-induced interstitial lung disease and cancer invasion. Respirol Case Rep. 2017;5(6):e00257. doi: 10.1002/rcr2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marin-Acevedo JA, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.