Abstract
Objective. Skin fibrosis is the predominant feature of SSc and arises from excessive extracellular matrix deposition. Glycosaminoglycans are macromolecules of the extracellular matrix, which facilitate Na+ accumulation in the skin. We used 23Na-MRI to quantify Na+ in skin. We hypothesized that skin Na+ might accumulate in SSc and might be a biomarker for skin fibrosis.
Methods. In this observational case–control study, skin Na+ was determined by 23Na-MRI using a Na+ volume coil in 12 patients with diffuse cutaneous SSc and in 21 control subjects. We assessed skin fibrosis by the modified Rodnan skin score prior to 23Na-MRI and on follow-up 12 months later.
Results. 23Na-MRI demonstrated increased Na+ in the fibrotic skin of SSc patients compared with skin from controls [mean (s.d.): 27.2 (5.6) vs 21.4 (5.3) mmol/l, P < 0.01]. Na+ content was higher in fibrotic than in non-fibrotic SSc skin [26.2 (4.8) vs 19.2 (3.4) mmol/l, P < 0.01]. Furthermore, skin Na+ amount was correlated with changes in follow-up modified Rodnan skin score (R2 = 0.68).
Conclusions. 23Na-MRI detected increased Na+ in the fibrotic SSc skin; high Na+ content was associated with progressive skin disease. Our findings provide the first evidence that 23Na-MRI might be a promising tool to assess skin Na+ and thereby predict progression of skin fibrosis in SSc.
Keywords: systemic sclerosis, fibrosis, skin Na+, 23Na-MRI, extracellular matrix
Rheumatology key messages
23Na-MRI detected Na+ accumulation in the fibrotic skin of SSc patients.
Skin Na+ of SSc patients correlated positively with progression of skin fibrosis.
23Na-MRI might be a promising tool to indicate active fibrotic disease in SSc.
Introduction
Skin and organ fibrosis is a leading cause of morbidity and mortality in SSc [1]. Fibrotic tissue arises from the excessive accumulation of extracellular matrix (ECM) released by pathologically activated fibroblasts [2]. Although fibrosis has long been considered a unidirectional process, growing evidence suggests that the fibrotic tissue feeds back to maintain the aberrant activation of fibroblasts and to enhance ECM release further. GAGs are integral parts of the ECM and play a central role in inducing and maintaining the activated state of fibroblasts by direct interactions and/or modulation of cytokine signalling [3]. In SSc, fibroblasts release increased amounts of hyaluronan and other GAGs, which accumulate and may propel fibrosis [4, 5]. GAGs are negatively charged macromolecules, and their polyanionic character allows them to bind cations [6]. We demonstrated earlier that GAGs make the skin into a major Na+ storage depot, which is actively regulated by immune cells [6–8]. To translate these findings clinically, we introduced and validated 23Na-MRI and simultaneously estimated tissue water content by 1H-MRI [9–11].
We hypothesized that Na+ content is increased in the fibrotic SSc skin and that the increased Na+ content can be detected by 23Na-MRI. Moreover, we investigated whether increased Na+ content might be associated with disease progression.
Methods
The study was approved by the local Ethics Committee of the University of Erlangen-Nürnberg (no. 3948) and was conducted according to the principles of the Declaration of Helsinki. All study participants provided written informed consent. In this observational case–control study, we compared skin Na+ intra-individually in SSc patients and with healthy control subjects. Besides, we prospectively followed-up on SSc disease activity within 1 year. We examined 12 consecutive patients with dcSSc, who regularly present to our outpatient university clinic, and 21 age- and sex-matched normal controls. Inclusion criteria were the diagnosis of SSc according to the new ACR/EULAR classification [12] and the affiliation to the diffuse cutaneous subset of the disease according to the 1988 criteria of LeRoy et al. [13]. Furthermore, solely patients with skin involvement of the forearm, according to clinical assessment by the modified Rodnan skin score (mRSS) at the time of 23Na-MRI, were included. Patients with the diffuse cutaneous form of the disease were chosen for this pilot study, because we expected more severe fibrosis at the forearm, the primary site of measurement. Healthy control subjects presented without any history of chronic illness, normal laboratory findings and, except for two (one with hormonal replacement therapy and one with l-thyroxine substitution), did not take any regular medication (see Supplementary Table S1 for further details). The same experienced rheumatologist performed the mRSS on all patients on the day of the 23Na-MRI measurements and at the follow-up 12 months later to determine skin involvement in SSc. To assess changes in skin fibrosis, the initial complete mRSS (from 17 sites) was subtracted from the follow-up mRSS after 12 months and correlated with skin Na+ content from 23Na-MRI analysis. The EULAR Scleroderma Trials and Research Disease Activity Score (EUSTAR DAS) was determined before 23Na-MRI measurements according to the proposed assessment by Valentini et al. [14]. Blood samples were obtained and blood pressure was assessed prior to the MRI measurement.
We introduced 23Na-MRI to quantify tissue Na+ in human skin and muscle [10]. In this study, we measured skin Na+ content in the region of the left dorsal forearm (10 cm proximal of the wrist) and the left dorsal lower leg (at the largest circumference) with a 23Na volume coil (Stark-Contrast, Erlangen, Germany) and a 3.0 T MRI scanner (Magnetom Verio; Siemens Healthcare, Erlangen, Germany). Fibrosis of the lower leg is more rarely observed in dcSSc patients and was therefore selected to serve as an intra-individual control. For analysis, the extremities were placed on a cylindrically shaped surface to avoid deviation in the Z-axis. The extent of the skin measurement sites in the X–Y-axis is indicated by arrows within the figures. According to the resolution specifications (see below), 30 mm of skin Na+ was averaged in the Z-axis. We used a two-dimensional Fast Low-Angle Shot (FLASH) sequence (total acquisition time, TA = 13.7 min; echo time, TE = 2.07 ms; repetition time, TR = 100 ms; flip angle, FA = 90°; 128 averages; resolution, 3 mm × 3 mm × 30 mm). Four tubes containing aqueous solutions with 40, 30, 20 and 10 mmol/l sodium chloride served as calibration standards by relating intensity to a concentration in a linear trend analysis. In parallel, we quantified tissue water content by 1H-MRI, using a fat-saturated inversion recovery sequence with spin density contrast (inversion time, TI = 210 ms; TA = 6.29 min; TE = 12 ms; TR = 3 s; FA1/2 = 90°/180°; resolution, 1.5 mm × 1.5 mm × 5 mm). The 10 mmol/l sodium chloride tube served as a calibration standard for tissue water.
Data are expressed as the mean (s.d.). Data were analysed by Student’s unpaired and paired t test and Pearson’s correlation coefficient, using SPSS software (version 21.0). Values of P <0.05 were considered to indicate statistical significance.
Results
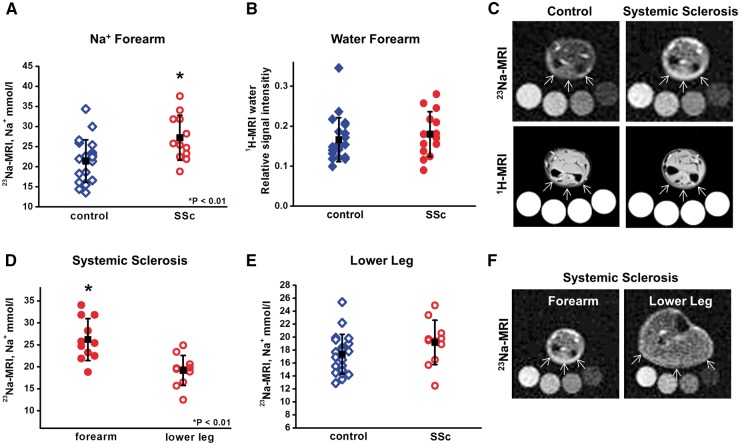
All SSc patients had fibrotic skin at the forearm (first site of 23Na-MRI measurement), and 11 out of 12 showed no clinical skin involvement at the lower leg (second site of measurement). Control subjects had no skin disease. Patients and controls were matched for age and BMI and showed similar blood pressure. Characteristics of the study population are further detailed in Supplementary Table S1. We found significantly higher skin Na+ at the fibrotic forearm of SSc patients compared with the non-fibrotic forearm of control subjects [27.2 (5.6) vs 21.4 (5.3) mmol/l, P < 0.01; Fig. 1A and C]. In line with our clinical assessment, 1H-MRI measurements showed no differences in water content between fibrotic skin and normal skin [0.19 (0.06) vs 0.17 (0.06) relative signal intensity, P = 0.36; Fig. 1B and C], indicating that the observed Na+ deposition in fibrotic skin of SSc patients was not the result of formation of oedema.
Fig. 1.
23Na-MRI detects increased Na+ in fibrotic SSc skin and discriminates between affected and non-affected skin
(A–C) Skin Na+ (A) and water content (B) of SSc patients (n = 12) and age-matched controls (n = 21) quantified by 23Na-MRI and 1H-MRI. (C) Representative23Na-MRI detects increased Na+ in fibrotic SSc skin and discriminates between affected and non-affected skin. 23Na- and 1H-magnetic resonance images of healthy (left) and fibrotic skin (right). Calibration tubes are placed below the forearm. Arrows indicate the site of measurement. (D) Skin Na+ content in fibrotic forearm vs non-involved lower leg in the same patients (n = 11). (E) Skin Na+ content of non-affected SSc skin vs healthy control patients (both lower leg). (F) Representative 23Na-magnetic resonance images of the forearm vs lower leg of an SSc patient.
We next tested the hypothesis that unaffected skin of the lower leg showed normal Na+ content in SSc patients. We found that Na+ content was higher in lesional skin of the forearm compared with the non-lesional skin of the same patients’ lower limb [26.2 (4.8) vs 19.2 (3.4) mmol/l, P < 0.01; Fig. 1D and F). Na+ content in the non-fibrotic skin of SSc patients were not different from skin Na+ content in our healthy control subjects [17.4 (3.0) vs 19.2 (3.4) mmol/l, P = 0.16; Fig. 1E].
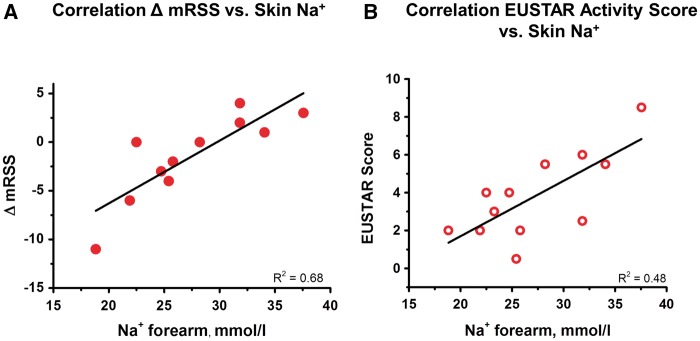
The mRSS is the clinical standard method to assess skin fibrosis in SSc. The mRSS does not only assess skin thickening but, at least to some degree, also fibrotic skin tethering. Skin Na+ content was poorly correlated with the local mRSS (at the site of measurement, 0–3, R2 = 0.02) or the total mRSS (17 sites, 0–51, R2 = 0.12). In contrast, however, we found a steep direct relationship between tissue Na+ accumulation and the change of the total mRSS within 12 months (R2 = 0.68; Fig. 2A). Likewise, the EUSTAR DAS, developed to detect disease activity in clinical studies [14], and skin Na+ were directly correlated (R2 = 0.48; Fig. 2B).
Fig. 2.
Skin Na+ correlates with change of the modified Rodnan skin score and EULAR Scleroderma Trials and Research Disease Activity Score
(A) The mRSS was assessed at the time of 23Na-MRI and 12 months later at a follow-up visit in 11 out of 12 patients. The initial mRSS was substracted from the follow-up mRSS (ΔmRSS) to describe the course of the fibrotic skin disease. (B) The EUSTAR DAS was determined at the first visit together with 23Na-MRI in all SSc patients. The amount of Na+ in the forearm skin assessed by 23Na-MRI was positively correlated with the EUSTAR DAS. EUSTAR: EULAR Scleroderma Trials and Research; mRSS: modified Rodnan skin score.
Discussion
Using 23Na-MRI, our study demonstrates increased Na+ content in the involved skin of patients with SSc as well as a correlation between Na+ content and progression of skin fibrosis. We previously introduced 23Na-MRI as a non-invasive technique to quantify the Na+ content of tissues. By using calibration standards, 23Na-MRI can be easily standardized to produce very objective and reliable results. Importantly, 23Na-MRI does not require gadolinium or other contrast dyes [9–11], which are associated with the development of nephrogenic systemic fibrosis. Using 23Na-MRI, we discovered earlier that hypertensive patients store larger amounts of Na+ in tissues than normotensive healthy controls [9, 10]. In addition, 23Na-MRI revealed that cutaneous infections can trigger local salt accumulation in humans. Local high-salt conditions in skin boost pro-inflammatory macrophage function, thereby helping to ward off infections [15].
Previously, we demonstrated that skin is the major Na+ storage depot of the human body. Negatively charged hyaluronans and other GAGs serve as the binding sites for Na+ in the skin. GAGs have been associated with important regulatory roles during physiological and pathological fibrogenesis. Although increased levels of these ECM molecules promote fibroblast activation and proliferation during early stages of wound healing [3], a high and persistent expression in fibrotic diseases might have deleterious consequences, resulting in a vicious circle of persistent fibroblast activation and uncontrolled ECM deposition. Additionally, recent evidence indicates that a high Na+ concentration itself promotes fibrosis in the skin via the epithelial Na+ channel in keratinocytes [16]. In line with these pathophysiological considerations, we observed in our study that skin Na+ was increased in patients with active and progressive disease, who were characterized by an increasing mRSS. In contrast, low skin Na+ amount was associated with a regression of skin fibrosis. Although additional studies are needed to investigate whether Na+ itself is involved in the disease process or if it is a secondary phenomenon attributable to increased ECM levels, our data suggest that skin Na+ might evolve as a sensitive biomarker for disease activity of SSc skin fibrosis.
Outcome measures to assess skin fibrosis objectively are lacking in clinical practice. The semi-quantitative mRSS performs fairly well, with low intra- and inter-observer variability when performed by experienced examiners. Nevertheless, even then standardization of the scoring is difficult [17, 18]. In our study, 23Na-MRI reliably discriminated fibrotic from non-fibrotic and normal skin. Although the same experienced examiner assessed the mRSS in all patients at all visits, the correlation between lesional Na+ accumulation and the local mRSS was poor. This finding may not be surprising, because the mRSS does not solely measure skin thickness but is also susceptible to skin tethering, even when done by experienced examiners. Furthermore, the local assessment of the mRSS is coarse, with only four grades to scale the extent of skin fibrosis. Importantly, studies with other imaging techniques, such as ultrasonography, had also produced poor correlations of skin thickness and the local mRSS, which supports our interpretation [19, 20].
We are aware of several shortcomings. First and foremost, as a result of the low incidence of SSc and our focus on the diffuse cutaneous subset, the patient cohort is small and does not reflect the complete spectrum of SSc skin disease. Future studies are needed to investigate Na+ content in a larger number of patients, different ethnicities and varying disease subtypes. Second, follow-up measurements by 23Na-MRI should explore whether Na+ as a surrogate of skin fibrosis is sensitive to change and could therefore be used to assess the disease-modifying efficacy of novel therapies. In contrast to the mRSS and other imaging modalities, the 23Na-MRI scans could be evaluated by central reference centres during clinical trials to ensure highly consistent readings. Finally, to gain a better understanding of the close correlation of skin Na+ content with the change of the mRSS, molecular studies including skin biopsies are needed to strengthen our knowledge on the increased amount of Na+ in the fibrotic tissue.
We present the first evidence that skin Na+ homeostasis is altered in a human skin disease. Skin Na+ amount assessed by non-invasive 23Na-MRI may serve as indicator of active fibrotic disease. Future studies are necessary to validate and expand these findings.
Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
We thank the Imaging Science Institute (Erlangen, Germany) for providing us with measurement time at the 3 T MRI scanner and for the technical support.
Funding: This work was supported by grants from the Interdisciplinary Center for Clinical Research (IZKF), Erlangen (IZKF Junior Research group 2 and IZKF Junior Project), the German Federal Ministry of Economics Technology (50WB1218), the National Institutes of Health (R01 HL118579-01) and the Deutsche Forschungsgemeinschaft (SFB643/TP B16 and 5088/1-1).
Disclosure statement: M.U. is a member of the speakers’ bureau of Siemens, Bracco, Bayer, Medtronic and has received research support from Bayer. All other authors have declared no conflicts of interest.
References
- 1. Tyndall AJ, Bannert B, Vonk M. et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 2. Varga J, Abraham D.. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 2007;117:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wight TN, Potter-Perigo S.. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol 2011;301:G950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Søndergaard K, Heickendorff L, Risteli L. et al. Increased levels of type I and III collagen and hyaluronan in scleroderma skin. Br J Dermatol 1997;136:47–53. [PubMed] [Google Scholar]
- 5. Bashey RI, Millan A, Jimenez SA.. Increased biosynthesis of glycosaminoglycans by scleroderma fibroblasts in culture. Arthritis Rheum 1984;27:1040–5. [DOI] [PubMed] [Google Scholar]
- 6. Titze J, Shakibaei M, Schafflhuber M. et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol 2004;287:H203–8. [DOI] [PubMed] [Google Scholar]
- 7. Schafflhuber M, Volpi N, Dahlmann A. et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol 2007;292:F1490–500. [DOI] [PubMed] [Google Scholar]
- 8. Machnik A, Neuhofer W, Jantsch J. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 2009;15:545–52. [DOI] [PubMed] [Google Scholar]
- 9. Kopp C, Linz P, Dahlmann A. et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 2013;61:635–40. [DOI] [PubMed] [Google Scholar]
- 10. Kopp C, Linz P, Wachsmuth L. et al. 23Na magnetic resonance imaging of tissue sodium. Hypertension 2012;59:167–72. [DOI] [PubMed] [Google Scholar]
- 11. Dahlmann A, Dörfelt K, Eicher F. et al. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int 2015;87:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 13. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 14. Valentini G, Bencivelli W, Bombardieri S. et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis 2003;62:901–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jantsch J, Schatz V, Friedrich D. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metabol 2015;21:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu W, Hong SJ, Zeitchek M. et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J Invest Dermatol 2015;135:796–806. [DOI] [PubMed] [Google Scholar]
- 17. Ionescu R, Rednic S, Damjanov N. et al. Repeated teaching courses of the modified Rodnan skin score in systemic sclerosis. Clin Exp Rheumatol 2010;28(2 Suppl 58): S37–41. [PubMed] [Google Scholar]
- 18. Czirják L, Nagy Z, Aringer M. et al. The EUSTAR model for teaching and implementing the modified Rodnan skin score in systemic sclerosis. Ann Rheum Dis 2007;66:966–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hesselstrand R, Scheja A, Wildt M, Akesson A.. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology 2008;47:84–7. [DOI] [PubMed] [Google Scholar]
- 20. Brocks K, Stender I, Karlsmark T. et al. Ultrasonic measurement of skin thickness in patients with systemic sclerosis. Acta Dermatol Venereol 2000;80:59–60. [DOI] [PubMed] [Google Scholar]