Abstract
Background and Objective:
A new 20-gauge (G) biopsy needle with a core-trap technology has been developed with a large core size and enhanced flexibility. The aim of this multicenter study was to determine the feasibility, efficacy, and safety of EUS-guided fine-needle biopsy (EUS-FNB) with the new 20G needle in diagnosing subepithelial lesions (SELs).
Materials and Methods:
Retrospectively collected data from consecutive patients with SELs undergoing EUS-FNB with the 20G needle at five centers were analyzed.
Results:
A total of 50 SELs were included. The mean lesion size was 43.1 ± 17.5 mm. The lesion locations were esophagus (n = 1), stomach (n = 37), distal duodenum (n = 5), rectum (n = 6), and colon (n = 1). The procedure was technically feasible in all patients. Definitive diagnosis with full histological assessment including immunohistochemistry was obtained in 88% (44/50) of the patients. Considering malignant versus benign lesions, the sensitivity, specificity, positive predictive value, and negative predictive value were 85% (95% confidence interval [CI] 70.2–94.3), 100% (95% CI 58.7%–100%), 100% (95% CI 85.1%–100%), and 62.5 (95% CI 27.7–84.8), respectively. No major complications requiring additional care have been observed.
Conclusions:
In this multicenter study, we found that EUS-FNB with the new 20G core needle is an effective and safe method for the diagnosis of SELs with a high rate of producing adequate histological material and high diagnostic accuracy even from difficult-to-approach anatomical locations.
Keywords: Endoscopic ultrasound, fine-needle aspiration, gastrointestinal stromal tumor, subepithelial lesion, submucosal mass
INTRODUCTION
Gastrointestinal (GI) subepithelial lesions (SELs) are usually incidental findings detected during endoscopy and encompass a wide variety of neoplastic and nonneoplastic lesions.[1] Neoplastic SELs include benign (leiomyoma, schwannoma, and lipoma), malignant (metastases, SEL-like cancer), and potentially malignant (GI stromal tumor [GIST]) lesions. A definitive diagnosis of incidental SELs is crucial to ensure correct management. Although EUS morphological evaluation can provide useful information about SELs, tissue acquisition for histological examinations and immunohistochemical (IHC) stains is often required.[2]
EUS-FNA is the primary modality in the diagnosis of SELs, but still has limited accuracy with a diagnostic rate ranging from 34% to 79%.[3,4,5,6] A recent meta-analysis about EUS-guided tissue acquisition for the diagnosis of SELs reported a pooled diagnostic rate of 59.9%.[7] The major limitation of FNA is the frequently reported nondiagnostic and indeterminate cytological results. The inability to obtain a core tissue specimen with preserved architecture for histological examination and IHC studies can make definitive diagnosis challenging for certain SELs, such as GI mesenchymal tumors.[8] To overcome these limitations, EUS-guided fine-needle biopsy (EUS-FNB) with larger-bore 19-gauge (G) needles have been performed. However, both Tru-Cut biopsy needle (TCB) and 19G ProCore needle have encountered several technical difficulties in the upper GI tract (i.e., gastric antrum and duodenum). For these reasons, very recently, a new 20G biopsy needle with enhanced flexibility and a core-trap technology (EchoTip ProCore®, Cook Medical) has been developed [Figure 1].
Figure 1.

Detailed image of the new 20-gauge biopsy needle tip with a core trap cut-out (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)
The aim of this multicenter study was to determine the feasibility, efficacy, and safety of EUS-FNB with the new 20G needle in diagnosing SELs.
MATERIALS AND METHODS
Patients and endoscopic ultrasound-guided procedure
Data retrieved from a prospectively collected database at five Italian medical centers (“Augusto Murri” Hospital, Fermo; “Fondazione IRCCS Istituto Nazionale Tumori,” Milan; “Asst Rhodense,” Garbagnate Milanese; “Bellaria-Maggiore” Hospital, Bologna; “Maggiore della Carità” Hospital, Novara) were analyzed and all consecutive patients with SELs undergoing EUS-FNB with the 20G needle were included in the present study. All SELs were previously diagnosed by endoscopy, and conventional biopsies on the overlying mucosa resulted inconclusive.
EUS examinations were performed by 5 experienced endosonographers all with advanced EUS training and >100 EUS-FNA/FNB per year. All procedures were performed using a linear array echoendoscope (Olympus UCT140 or UCT180; Olympus America Corp, Center Valley, PA; Pentax Europe, GmbH, Hamburg, Germany) with patients in the left lateral decubitus position under conscious or deep sedation. After exclusion of the presence of intervening vasculature with EUS color Doppler evaluation, SELs were sampled using PC20 needle. Number of passes and tissue acquisition techniques such as the capillary sampling method or syringe suction technique were performed according to the endosonographer's preference. Fanning technique was applied in all participant centers. Any technical failure or needle malfunction was recorded during the procedure.
Written informed consent was obtained from all patients. The study was approved by the ethical committee of the coordinating center (“Augusto Murri” Hospital, Fermo, Italy). The study was conducted in accordance with the human and ethical principles of research set forth in the Declaration of Helsinki.
Preparation of specimen for histological and immunohistochemical analysis
During the procedure, there was no on-site cytopathologist. The content of the needle after EUS-FNB was directly placed into formalin for histologic examination. The formalin-fixed specimens were subsequently embedded in paraffin and stained with hematoxylin and eosin for morphological evaluation and IHC staining when required.
Pathologic results were categorized as diagnostic or nondiagnostic. A diagnostic result was defined when adequate samples were obtained to allow for the architectural details and IHC studies (i.e., stains for CD34, c-kit, smooth muscle actin, and S100). A diagnosis of GIST was made when microscopy revealed epithelioid or spindle cells that were positive for c-kit with or without positive CD34. A tumor with a negative reaction to c-kit, CD34, and smooth muscle actin and positive reaction for S-100 was diagnosed as a neurogenic tumor (schwannoma). A tumor with a negative reaction to c-kit, CD34, and S-100 and a positive reaction for smooth muscle actin was diagnosed as a myogenic tumor (leiomyoma).
DEFINITION OF STANDARD REFERENCE
When available, histological diagnosis based on surgically resected specimens was considered as the reference standard. Alternatively, when surgery was not indicated, other criteria were used. When histological examination obtained through EUS-FNB was diagnostic for malignancy or for benign disease, this was considered to be the definitive diagnosis. In case of an inconclusive diagnosis at EUS-FNB and no surgical specimen was available, the presence of malignancy was based on the results of other diagnostic investigation or clinical follow-up, including repeated EUS-guided sampling. For this purpose, all patients were followed for at least 6 months after the EUS procedure. An inadequate sample was defined as a specimen from which the pathologist could not make a definitive diagnosis due to inadequate quality or quantity of tissue.
Outcome measures
The primary outcome measure was the diagnostic adequacy, defined as the rate of cases in which a tissue specimen for histological examination was achieved. Secondary outcome measures were feasibility, safety, and diagnostic accuracy of EUS–FNB using the 20G in patients with GI-SELs. Early (≤72 h since the EUS procedure) and late (>72 h) complications were recorded.
Data analysis
Frequencies, percentages, and means (± standard deviation) were used, as appropriate, for descriptive analysis. Sensitivity, specificity, positive, and negative likelihood ratios were calculated. Definitive diagnoses were divided into malignant and benign lesions. Inadequate samples for histological evaluation or technical failures were considered as false-negative cases. Statistical analysis was performed using Statistica software version 10 (StatSoft Inc., Tulsa, Oklahoma, USA).
RESULTS
Between January 2016 and October 2016, a total of 50 patients with 50 SELs underwent EUS-FNB with the 20G needle. Clinicopathological characteristics of the lesions are summarized in Table 1. The mean lesion size was 43.1 mm ± 17.5 mm. The lesion locations were esophagus (n = 1), stomach (n = 37), distal duodenum (n = 5), rectum (n = 6), and transverse colon (n = 1).
Table 1.
Baseline characteristics of patients and gastrointestinal subepithelial lesions
| Characteristics | Population (n=50) |
|---|---|
| Sex, n (%) | |
| Male | 22 (44) |
| Female | 28 (56) |
| Age, median (range), year | 61.5 (23-86) |
| Size of lesion on EUS, median (range), mm | 43.1 (20-90) |
| Location of the lesion, n (%) | |
| Esophagus | 1 (2) |
| Gastric cardia | 4 (8) |
| Gastric fundus | 14 (28) |
| Gastric body | 16 (32) |
| Gastric antrum | 3 (6) |
| Distal duodenum | 5 (10) |
| Rectum | 6 (12) |
| Colon (transverse) | 1 (2) |
| Layer of origin on EUS, n (%) | |
| Third | 3 (6) |
| Fourth | 47 (94) |
EUS: Endoscopic ultrasound
The procedure was technically feasible in all patients, and no needle malfunction was recorded in any cases. Mean number of passes required to reach a diagnosis was 2.2 (range 1–4). No major complications requiring additional care have been observed. In few cases, we have observed minor and self-limiting bleeding in the site of the puncture. The final diagnosis of SELs is presented in Table 2. GIST was the most common diagnosis occurring in 35 (70%) patients.
Table 2.
Final diagnosis of gastrointestinal subepithelial lesions
| Lesion | n (%) | Correct diagnosis by EUS-FNB, n (%) |
|---|---|---|
| Malignant | ||
| GISTs | 35 (70) | 33 (66) |
| Metastasis (breast cancer) | 1 (2) | 1 (2) |
| Leiomyosarcoma | 1 (2) | 1 (2) |
| Carcinoid | 1 (2) | 1 (2) |
| SEL-like adenocarcinoma | 1 (2) | 1 (2) |
| Benign | ||
| Leiomyomas | 5 (10) | 3 (6) |
| Schwannomas | 4 (8) | 4 (8) |
| Lipomas | 2 (4) | 1 (2) |
EUS: Endoscopic ultrasound, FNB: Fine-needle biopsy, SEL: Subepithelial lesions, GISTs: Gastrointestinal stromal tumors
EUS-FNB using 20G needle has allowed definitive diagnosis in 88% (44/50) of the patients [Table 3]. IHC was feasible in all these adequate specimens. The diagnosis of EUS-FNB showed 36 (72%) malignant SELs (32 GISTs, 1 metastasis from breast cancer, 1 leiomyosarcoma, 1 carcinoid, and 1 SEL-like adenocarcinoma) and 8 (16%) benign SELs (3 leiomyomas, 4 schwannomas, and 1 lipoma). In six patients (12%), scanty or no tissue was obtained during EUS-FNB and the sample was considered to be inadequate for histological examination. Three (50%) of these six indeterminate patients were diagnosed on surgery (2 GISTs and 1 leiomyoma), and three (50%) of them by repeated EUS-guided sampling (1 GIST, 1 leiomyoma, and 1 lipoma). Surgical resection was performed in 30 out of 32 patients with a diagnosis of GIST at EUS-FNB and in the patient with SEL-like adenocarcinoma, with confirmation of the diagnosis in all of them. One patient with duodenal carcinoid of 27 mm and one patient with gastric GIST refused surgery.
Table 3.
Diagnostic performance of endoscopic ultrasound-guided fine-needle biopsy
| Parameter | Outcome |
|---|---|
| Diagnostic accuracy (95% CI) | 88 (75.7-95.5) |
| Sensitivity (95% CI) | 85 (70.2-94.3) |
| Specificity (95% CI) | 100 (58.7-100) |
| Complication rate, n (%) | Nil |
| Technical failure | Nil |
CI: Confidence interval
Macroscopically visible core evaluated by endosonographers and defined as whitish or yellowish piece of tissue with an apparent bulk[9] [Figure 2], was obtained in all diagnostic cases.
Figure 2.

Macroscopically visible core with a whitish piece of tissue expressed onto a glass slide
Considering malignant versus benign lesions, the sensitivity, specificity, positive predictive value, and negative predictive value were 85% (95% confidence interval [CI] 70.2–94.3), 100% (95% CI 58.7%–100%), 100% (95% CI 85.1%–100%), and 62.5 (95% CI 27.7–84.8), respectively.
DISCUSSION
In the current study, we explored the technical performance and the safety profile of EUS-FNB with a newly available 20G needle in diagnosing GI-SELs. Adequate samples for histologic evaluation were found in 88% of patients and in all of them, we were able to establish a final diagnosis with full IHC studies. No complications or technical problems were encountered in any of the EUS-FNB procedures.
SELs can occur everywhere in the GI tract and are commonly identified during endoscopic examinations. Asymptomatic SELs can be a challenge because they include several neoplastic and nonneoplastic lesions and most of them require IHC staining for definitive diagnosis. For this reason, the current standard of practice for the characterization of SELs is EUS-guided sampling with large-bore needles with the intent to obtain a tissue core for histological examination. 19G Tru-Cut biopsy (TCB) needle was the first device developed to increase diagnostic accuracy by delivering a histological specimen.[10] However, its diagnostic yield in gastric SELs was very low (55%–63%)[11,12] and limited by technical difficulties, mostly related to the increased needle stiffness that made its passage through the scope cumbersome, especially in angulated position (e.g., duodenum). To overcome these limitations, a 19G needle with reverse-bevel technology was introduced (ProCore needle, Cook). In a prospective multicenter study of miscellaneous lesions, final diagnosis was obtained in 81% of 11 SELs.[13] However, technical problems were reported in 19% of the cases, especially when the needle was deployed out of the echoendoscope in the duodenum.[13] Thereafter, a more flexible 19G needle (Expect 19-G Flex; Boston Scientific) was developed to obtain better performance in angulated position. Preliminary results suggested that the flexible 19G needle can be used for procuring histologic specimens even by the transduodenal route with a diagnostic accuracy of 95%.[14] However, very few patients with SELs were included in the study by Varadarajulu et al. (6 cases: 5 in the stomach and 1 in the rectum), and none of them were located in the duodenum. Thus, more consistent data on the outcome of this needle for the characterization of GI-SELs are awaited. 22G needles have been used to circumvent the technical limitations encountered by larger-bore needles. However, even if retrospective studies with 22G needles showed yields ranging from 64% to 100%, the two largest studies reported a lower diagnostic accuracy (43%–62%) when “suspected lesions” were excluded.[4,15] Furthermore, a recent meta-analysis reported that neither the different needle type (FNA, FNB, and TCB) nor the different needle size (25G, 22G, and 19G) seems to have an impact on the final diagnostic rate of SELs.[7]
In our study, EUS-FNB with a novel 20G needle was technically feasible in all lesions, irrespective of their locations. In particular, in 5 duodenal SELs, the diagnosis was achieved in 100% of the cases. The improved flexibility of the needle allows to pull the needle out of the scope and puncture the lesion, even in angulated position. Furthermore, no frictioning to back-and-forth movement was registered during the puncture within the lesion.
Concern exists when large-bore needles are used in necrotic SELs. This because, although severe septic complications are rarely described during EUS-FNB of SELs, most of them occurred in large and/or necrotic lesions sampled with 19G needles.[7] In our study, no major complications requiring additional care have been reported.
This study has some limitations. First, this is a retrospective analysis of prospectively collected databases; therefore, there might be biases in data collection or patient selection. Second, the lack of a control group was a significant limitation of this study. However, in this study, our intent was to explore the outcomes of EUS-FNB with the 20G needle diagnosing SELs, rather than to affirm its superiority to other needles. Third, a predetermined maximum number of needle passes has not been established, but the choice was at endosographer's discretion. However, our results showed that a limited number of passes (mean 2.2) are required to reach a diagnosis, with a maximum of four passes.
Despite these limitations, our study presents several strengths. This is the first study specifically evaluating the utility of the new 20G EUS biopsy needle in the diagnosis of GI-SELs. Second, the multicenter setting can eliminate single operator's bias, thus allowing the external validity of our findings in different setting. Third, only histological specimens have been evaluated, and only patients with full definitive diagnosis have been considered [Figure 3]. No “suspected” lesions have been included in the positive FNB results. Fourth, EUS-FNB procedures were performed in different GI tract sites (from esophagus to colon), thus allowing a broad evaluation of the technical performance of the needle in diagnosing different SELs.
Figure 3.
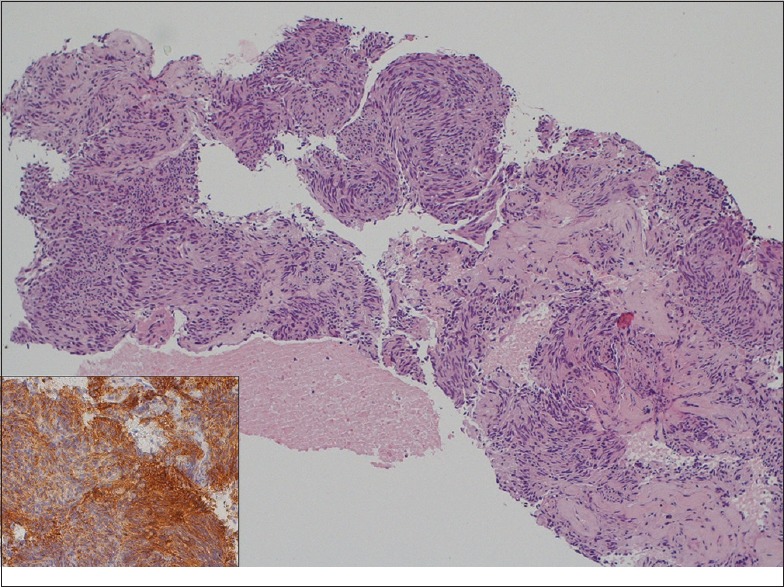
Histological diagnosis of a gastrointestinal stromal tumor case obtained with 20-gauge biopsy needle. Tissue fragments showing a group of spindled-shaped cells (H and E, ×10). Diffuse positive staining for DOG1 (inset, ×20)
CONCLUSIONS
The current multicenter study showed that EUS-FNB with the new 20G core needle is an effective and safe method for the diagnosis of GI-SELs with a high rate of producing adequate histological material and high diagnostic accuracy even from difficult-to-approach anatomical locations. Comparative studies with different needle sizes are needed to further validate these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute. American gastroenterological association institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217–28. doi: 10.1053/j.gastro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol. 2009;23:679–701. doi: 10.1016/j.bpg.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–5. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt AJ, Jenssen C. Current endoscopic ultrasound-guided approach to incidental subepithelial lesions: Optimal or optional? Ann Gastroenterol. 2015;28:160–72. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg Endosc. 2016;30:2431–41. doi: 10.1007/s00464-015-4494-1. [DOI] [PubMed] [Google Scholar]
- 8.Fuccio L, Larghi A. Endoscopic ultrasound-guided fine needle aspiration: How to obtain a core biopsy? Endosc Ultrasound. 2014;3:71–81. doi: 10.4103/2303-9027.123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seicean A, Gheorghiu M, Zaharia T, et al. Performance of the standard 22G needle for endoscopic ultrasound-guided tissue core biopsy in pancreatic cancer. J Gastrointestin Liver Dis. 2016;25:213–8. doi: 10.15403/jgld.2014.1121.252.ugg. [DOI] [PubMed] [Google Scholar]
- 10.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–8. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 11.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: A prospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–43. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 15.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]


