Abstract
Recent metabolic and genetic research has demonstrated that risk for specific histological types of lung cancer varies in relation to cigarette smoking and obesity. This study investigated the spatial and temporal distribution of lung cancer histological types in Kentucky, a largely rural state with high rates of smoking and obesity, to discern population-level trends that might reflect variation in these and other risk factors. The Kentucky Cancer Registry provided residential geographic coordinates for lung cancer cases diagnosed from 1995 through 2014. We used multinomial and discrete Poisson spatiotemporal scan statistics, adjusted for age, gender, and race, to characterize risk for specific histological types—small cell, adenocarcinoma, squamous cell, and other types—throughout Kentucky and compared to maps of risk factors. Toward the end of the study period, adenocarcinoma was more common among all population subgroups in north-central Kentucky, where smoking and obesity are less prevalent. During the same time frame, squamous cell, small cell, and other types were more common in rural Appalachia, where smoking and obesity are more prevalent, and in some high poverty urban areas. Spatial and temporal patterns in the distribution of histological types of lung cancer are likely related to regional variation in multiple risk factors. High smoking and obesity rates in the Appalachian region, and likely in high poverty urban areas, appeared to coincide with high rates of squamous cell and small cell lung cancer. In north-central Kentucky, environmental exposures might have resulted in higher risk for adenocarcinoma specifically.
Keywords: lung cancer, Appalachia, obesity, smoking, spatial analysis, histology, BRFSS
Introduction
In the United States, lung cancer has the second highest incidence rate and the highest mortality rate of all cancers. Siegel and colleagues recently estimated that the nation can expect about 224 000 new cases and 158 000 deaths annually.1 Lung cancer is generally divided into 2 categories: small cell and non-small cell lung cancer. Small cell lung cancer accounts for about 13% of cases, while non-small cell cancer accounts for most of the remaining of cases. Non-small cell lung cancer can be further subdivided into subtypes: adenocarcinoma (47.9%), squamous cell carcinoma (23.2%), and other less common subtypes (15.9% combined).2
The incidence rates of these histological types has shifted over the past several decades. Briefly speaking, adenocarcinoma incidence rates have increased, and squamous cell carcinoma rates have decreased.3-5 Evidence from the literature suggests that these changes in histology coincide with changes in cigarette design, including the introduction of filtered and “light” cigarettes, that might be related to smoking behaviors (eg, deeper inhalation).6-9 It is also possible that declining smoking rates are contributing to a shift in the relative distribution of lung cancer histologies. Although all histological types are positively associated with smoking, the risk of adenocarcinoma is less elevated among smokers than risk for squamous or small cell histological types.10 This suggests that a higher proportion of adenocarcinomas should be observable in populations with lower smoking rates or, perhaps, with less intense smoking behaviors or preferences. Since 2000, smoking rates in the Appalachian region of Kentucky have decreased only slightly, while the remainder of the state and the United States have experienced more rapid declines.11 Smoking rates in Kentucky are already the second highest (23.8%) in the United States,12 and Kentucky has the highest lung cancer incidence and mortality rates—about 4900 new cases and 3500 deaths are expected annually in a population of just over 4 million.1 These rates are further elevated in Kentucky’s Appalachian region, where lung cancer incidence is 94% higher than overall Surveillance, Epidemiology, and End Results (SEER) rates (111.1 vs 57.4 per 100 000, respectively).13 Previous research has also demonstrated that survival rates for lung cancer are poorer among patients from Appalachia, which could be due to higher smoking intensity, or even continued smoking after lung cancer diagnosis, though other factors that influence access to care, such as distance to treatment and education, may also play a role.14-17
Recent genetic epidemiology has also revealed interesting associations between body mass index (BMI) and risk for specific histological types of lung cancer. Carreras-Torres and colleagues demonstrated that risk for small cell and squamous cell cancers was significantly elevated among the obese, while risk for adenocarcinoma and other less common types was not.18 In this and other work, researchers have suggested this association could be related to insulin resistance, or even higher cigarette consumption among those who are obese.18-20 Regardless, it seems likely that the relative distribution of histological types would be skewed toward more small cell and squamous cell cases in regions with higher rates of obesity. Within Kentucky, there is wide variation in both lung cancer and obesity rates,21 yielding an opportunity to examine the relationship between them. Furthermore, previous studies have shown that lung cancer incidence and mortality rates in eastern Kentucky—the Appalachian region with a history of coal mining—are higher than expected after adjustment for other important risk factors for lung cancer.22,23 These studies did not address which histological types, if any, were particularly elevated. It is quite possible, however, that risk for lung cancer in this region is elevated due to high rates of obesity. According to the 2016 Behavioral Risk Factor Surveillance System (BRFSS), approximately 39.0% of adults in Appalachian Kentucky were obese, compared to 32.7% in the rest of Kentucky, and 30.1% in Jefferson and Fayette counties—the 2 most populous in the state—combined.21
The purpose of this study was to examine spatial and temporal patterns in the distribution of the major histological types of lung cancer across Kentucky and to compare these with patterns of smoking, obesity, and rurality. Given the results of previous research, we expected to find higher proportions of small cell and squamous cell lung cancers in the Appalachian region, where smoking and obesity are more common. Similarly, we expected to find a higher proportion of adenocarcinomas among patients with lung cancer in areas with lower rates of smoking and obesity, due to lower rates of squamous and small cell cancers.
Materials and Methods
The University of Kentucky Medical institutional review board reviewed this study and determined it to be exempt, since it relied on existing data gathered as part of routine public health surveillance, and all records had been de-identified.
Data Sources
We obtained the primary data set for this study from the Kentucky Cancer Registry (KCR), a member registry of the National Cancer Institute’s SEER cancer surveillance system. This registry has consistently met or exceeded the highest national standards for complete, accurate, and timely data, as certified by the North American Association of Central Cancer Registries, since certification standards were established.24 We included information on each case of lung cancer diagnosed in the 20-year period from 1995 through 2014. For the primary analysis, we categorized histology using the following ICD-O-3 invasive histology codes5:
Adenocarcinoma: 8015, 8050, 8140, 8141, 8143-8145, 8147, 8190, 8201, 8211, 8250-8255, 8260, 8290, 8310, 8320, 8323, 8333, 8401, 8440, 8470, 8471, 8480, 8481, 8490, 8503, 8507, 8550, 8570, 8571, 8572, 8574, 8576
Squamous cell: 8051, 8052, 8070-8078, 8083, 8084, 8090, 8094, 8120, 8123
Small cell: 8002, 8041 to 8045
Other types: 8000, 8001, 8003, 8004, 8010 to 8014, 8020 to 8022, 8030 to 8035, 8046, 8082, 8200, 8230, 8240, 8241, 8243 to 8246, 8249, 8430, 8525, 8560, 8562, 8575
The KCR also provided spatial reference data. For this analysis, we used the US Census tract (2010) of residence at the time of diagnosis as the spatial reference for each lung cancer case. We limited our data set to black and white Kentucky residents because there were not a sufficient number of lung cancer cases in other race/ethnicity categories for robust analysis of histological types by age and gender at the scale of census tracts.
We accessed SEER 13 data from SEER*Stat (version 8.2.1) for comparison with both Kentucky statewide and its Appalachian region. We used SEER 13 data because they were available for the same time period, 1995 to 2014, as the data presented here.
To examine spatial and temporal trends in lung cancer histology in relation to cigarette smoking and obesity, we calculated prevalence estimates for current smoking and obesity (BMI 30.0+) using data from BRFSS surveys conducted during 5 years, 1996 to 2000, toward the beginning of the study period. Because Kentucky has 120 counties, most with a limited number of BRFSS survey respondents, we calculated smoking and obesity prevalence rates for Kentucky’s 15 Area Development Districts (ADDs) to obtain reasonably precise estimates.25 Public health researchers in Kentucky often use these multicounty regions to map the prevalence of health behaviors and outcomes from the BRFSS. We characterized ADDs as high smoking or high obesity if they were in the top tertile among all ADDs for percent of residents who are current smokers or percent who are obese.
We obtained 5-year estimates from the 2010 American Communities Survey of the US Census to identify census tracts with high poverty rates in Kentucky that were located within lung cancer clusters. Since cigarette smoking and obesity are both generally more common among those of low socioeconomic status, high poverty census tracts could indicate small areas with higher smoking rates and obesity rates than suggested by BRFSS estimates for the larger ADD regions.26,27 We characterized census tracts as high poverty if they were in the top tertile among all census tracts in the state.
Analysis
One multinomial spatiotemporal scan statistic and 4 discrete Poisson spatiotemporal scan statistics implemented in SaTScan (version 9.4.2) comprised our primary analysis. (SaTScan is a trademark of Martin Kulldorff. The SaTScan software was developed under the joint auspices of Martin Kulldorff, the National Cancer Institute, and Farzad Mostashari of the New York City Department of Health and Mental Hygiene.) Examples of spatial and spatiotemporal scan statistics appear frequently in research studies focused on identifying clusters of disease or related conditions or behaviors.11,22,28-31 Briefly, spatial scan statistics compare the rate of an event within a large number of candidate clusters, which are determined by drawing concentric circles around a specified set of event locations (aggregating events) or regular grid points, to the corresponding rate outside each candidate cluster.32 Often, the event of interest is a binary condition expressed as a count or rate among the population, in which case a Poisson model is usually appropriate. In this study, however, we first employed a multinomial model33 to discern whether there were regional differences across Kentucky in the relative proportions of the 4 lung cancer histology categories defined above. Given the large number of cases, we conducted the analysis at the census tract level, using 2010 US Census tract population data and geographic data files. We adjusted this and all subsequent spatiotemporal scan statistics for race/ethnicity (white/black), gender (male/female), and age (<55, 55-74, 75+) to eliminate the likelihood of identifying spurious clusters that merely reflect shifts in these demographic factors. After conducting the multinomial scan statistic, we additionally produced 4 Poisson-based spatiotemporal scan statistics to analyze each histological type of lung cancer as if it were a separate disease. Each of the 4 Poisson-based statistics relied on the same underlying population data file from the 2010 US Census. We created maps to display counties included in significant (P < .05) clusters using QGIS 3.14 and tabulated additional results using Stata 15.1 (StataCorp, College Station, Texas). We also created a map displaying high obesity and high smoking ADDs and layered with high poverty census tracts and rural–urban continuum codes (RUCC) from the US Department of Agriculture, for comparison with the map of specific lung cancer histology clusters.
Results
There were 83 946 lung cancer cases among black and white Kentuckians during the study period 1995 to 2014. During this time in Kentucky, squamous cell accounted for 20 754 (24.7%) cases, small cell 14 253 (17.0%) cases, adenocarcinoma 21 916 (26.1%) cases, and all others 27 023 (32.1%) cases (Table 1). We observed 1 cluster in the multinomial spatiotemporal statistic, and 1 or more clusters in each of the Poisson-based statistics.
Table 1.
Distribution of lung cancer cases by histological type in SEER 13, Kentucky, Appalachian Kentucky (1995-2014), and multinomial cluster (2009-2014).
| %Adenocarcinoma | %Small Cell | %Squamous Cell | %Other Types | |
|---|---|---|---|---|
| SEER 13 | 36.6 | 12.3 | 18.6 | 32.5 |
| Kentucky | 26.1 | 17.0 | 24.7 | 32.1 |
| Appalachian Kentucky | 22.9 | 17.5 | 24.6 | 35.1 |
| Multinomial cluster | 34.8 | 15.7 | 25.3 | 24.3 |
Multinomial Spatiotemporal Scan Statistic
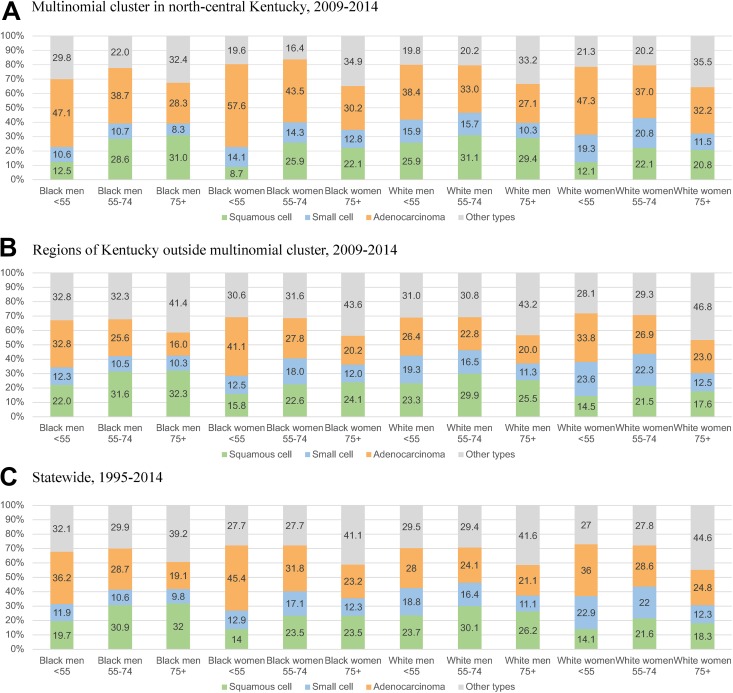
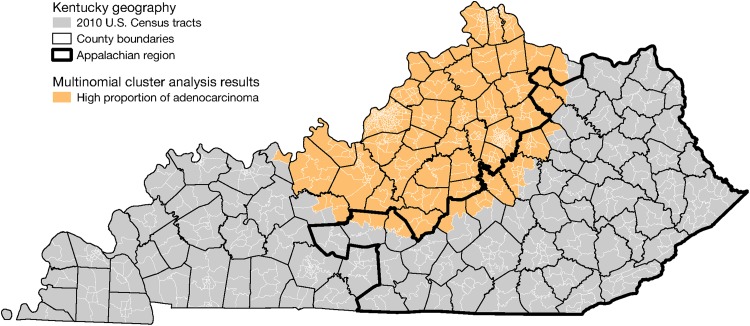
The multinomial spatiotemporal scan statistic identified one region with significantly different proportions of lung cancer histological types from 2009 to 2014 when compared to the rest of Kentucky. This cluster comprised 13 517 cases. Most notably, we observed a higher adenocarcinoma proportion (34.8%) in this region when compared to all of Kentucky (26.1%). Figure 1 displays, by population subgroup (race/age/gender), the distribution of histological types among cases in (a) the multinomial cluster from 2009 to 2014, as well as (b) regions outside the multinomial cluster from 2009 to 2014, and (c) statewide throughout the entire study period. Figure 2 shows the cluster’s location in the north-central region. Compared to the rest of Kentucky during the same time period, and to statewide during the entire study period, the adenocarcinoma proportion in the multinomial cluster was substantially higher across all 12 population subgroups defined by race/ethnicity, gender, and age, as shown in Figure 1. Furthermore, the distribution of histological types in regions outside the cluster from 2009 to 20014 was similar to statewide during the entire period. It is notable, however, that the overall adenocarcinoma proportion in the multinomial cluster was similar to that from SEER 13 (36.6%). Within the cluster, a further 25.3% of cases were squamous cell, and 15.7% were small cell. These proportions were similar for all of Kentucky (24.7% and 17.0%, respectively). But 24.3% of cases in the multinomial cluster were other types, which is a much lower proportion than for all of Kentucky (32.1%) and SEER 13 (32.5%).
Figure 1.
Lung cancer histology by population subgroups defined by age, gender, and race/ethnicity in Kentucky.
Figure 2.
Cluster of adenocarcinoma identified by multinomial spatiotemporal scan statistic.
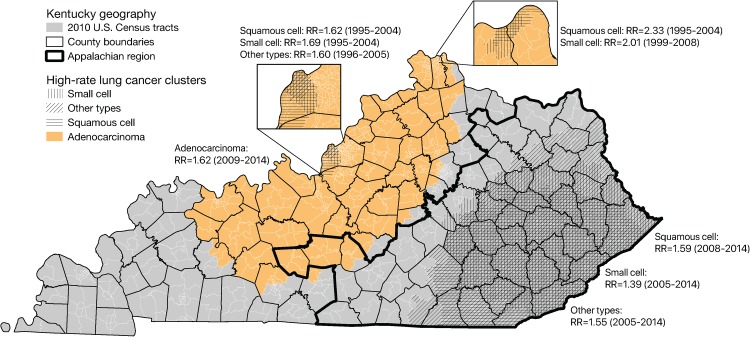
Figure 3 shows significant clusters of all histological types identified by the Poisson-based spatiotemporal scan statistics. There was an adenocarcinoma cluster (relative risk = 1.62) detected during the final 6 years of the study period (2009-2014) that is somewhat similar in geographic extent to the one detected by the multinomial scan. Furthermore, the Poisson-based analyses identified large clusters of other histological types in southeastern Kentucky, the Appalachian part of the state, during approximately the same period—2008 to 2014. We also observed a few other small clusters of squamous cell, small cell, and other types in urban areas in the north-central part of the state, but these were confined to years toward the middle of the study period, unlike the large adenocarcinoma clusters in north-central Kentucky and the larger clusters in southeastern Kentucky. Additionally, the cluster of “other types” in southeastern Kentucky was limited to 2002 to 2011. Notably, the small urban clusters of squamous cell and small cell disease were largely composed of high poverty (ie, in the top tertile statewide for percentage of families in poverty) census tracts. Within the small urban clusters of squamous cell in the Louisville–Jefferson County and Cincinnati–Northern Kentucky areas, 51.6% and 50.0% of census tracts, respectively, were high poverty census tracts (ie, in the top tertile for percentage of families in poverty—17.7% or higher). Similarly, 59.0% and 64.7% of census tracts in the small cell clusters in Louisville–Jefferson County and Cincinnati–Northern Kentucky areas, respectively, had high poverty rates.
Figure 3.
Clusters of lung cancer histological types identified by Poisson-based spatiotemporal scan statistics.
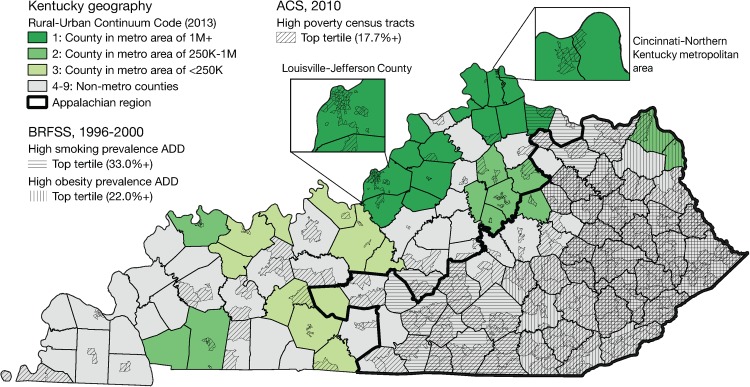
Figure 4 shows that high smoking and high obesity regions in Kentucky are mostly limited to the Appalachian region of Kentucky and appear to overlap substantially in the general region of the high rate clusters of squamous cell and small cell lung cancer identified by the Poisson-based scan statistics. It also shows the north-central region includes the largest metropolitan areas in the state, anchored by Louisville–Jefferson County and Lexington–Fayette County, as well as the Kentucky portion of the Cincinnati–Northern Kentucky metropolitan area. It is apparent from the inset maps in this figure that the smaller clusters of squamous cell, small cell, and other types appeared in large metropolitan counties in, or near, high poverty census tracts.
Figure 4.
Kentucky geography and distribution of risk factors relevant to lung cancer.
Discussion
This study confirmed that the north-central region of Kentucky, where smoking and obesity rates tend to be lower and more people reside in urban areas, has a higher proportion of lung cancer cases classified as adenocarcinomas. It is important to note, however, that this was not due to comparatively lower rates of squamous cell and small cell types, as expected. Instead, the risk of adenocarcinoma appears to be significantly higher, and perhaps even rising, in this region, despite substantially lower rates of smoking. Consistently higher proportions of adenocarcinoma across all age, gender, and racial/ethnic groups might suggest an environmental etiology. There is some research to suggest that environmental exposures associated with urban residence could be related to this high rate cluster of adenocarcinoma. Since the north-central Kentucky region includes the largest metropolitan areas in the state, it generates far more transportation-related fine particulates and other air pollution, which have been shown to contribute to lung cancer risk.34 The adenocarcinoma proportion in the multinomial cluster was similar to SEER 13, however, which might suggest this region reflects national patterns in ways that the rest of Kentucky does not.
We observed high rate clusters of squamous and small cell lung cancer in both urban and rural areas with high rates of poverty. As the analysis presented here did not adjust for tobacco use, these clusters likely reflect higher rates of smoking, and perhaps obesity, among those with low income.35 As shown in Figure 4, this is apparent in the Appalachian region in eastern Kentucky; smoking and obesity rates are available for the multicounty ADD regions and tend to overlap with high poverty census tracts in the map. It is somewhat less apparent in the Louisville–Jefferson County and Cincinnati–Northern Kentucky metropolitan areas because smoking and obesity prevalence estimates are not available at the scale of census tracts. That higher rates of smoking, obesity, and poverty coincide with high rate clusters of squamous, and small cell lung cancer was not wholly unexpected, but does serve to illustrate another interesting feature of the adenocarcinoma clusters—unlike all other histological types of lung cancer, they do not appear to be associated with high poverty rates. Overall, the region of high adenocarcinoma lung cancer risk is generally more affluent than the rest of Kentucky and is home to signature industries such as horse breeding and bourbon distilling. Furthermore, the only 5 counties in Kentucky with comprehensive smoking bans in all workplaces and enclosed public places (all implemented between 2007 and 2014) are in this region; several additional cities and towns also have similarly strong smoke-free ordinances. Such laws are less common, or, if they exist, are weaker, in other parts of Kentucky.36
Unlike the large adenocarcinoma cluster in north-central Kentucky, or the large squamous cell, small cell, and other clusters in the Appalachian region, the small urban clusters were limited to years toward the middle of the study period. Although we have limited information to interpret this phenomenon, it is possible that the introduction of smoking bans (by governments or, increasingly, individual establishments) might have helped to decrease smoking prevalence in these areas. It is also possible that implementation of the Affordable Care Act improved access to health-care resources, such as medications or nicotine replacement therapy, and could have moderated risk toward the end of the study period in some areas. Urban areas could have benefitted more in this scenario, due to better existing spatial accessibility of such resources compared to rural Appalachia.
Hosgood and colleagues recently completed a similar spatial analysis of lung cancer histology in Maine, another rural state with high lung cancer rates.29 Their analysis suggested a link between rurality and large cell lung cancer. Our spatial analysis did not distinguish large cell cancers from others in the “other types” category, but it is notable that the multinomial cluster region had the lowest proportion of “other types” when compared to statewide. After our primary analysis, we briefly examined the percentage of large cell (histology codes 8012-8014) cases within the multinomial cluster and outside of it, by metropolitan/nonmetropolitan status, and over the entire study period. This demonstrated that large cell cases decreased from 7.0% to 1.0% of all lung cancer cases inside the multinomial cluster during the study period and from 9.4% to 1.7% outside it. Over the entire study period, large cell decreased from 7.7% to 1.3% of lung cancers in metropolitan counties (RUCC 1-3) and from 8.7% to 1.4% in nonmetropolitan (RUCC 4-9) counties.
The squamous and small cell proportions were similar for most demographic groups in the multinomial adenocarcinoma cluster and statewide. It appears that most of the difference in adenocarcinoma proportions was offset by lower proportions of the “other types.” It is possible that this pattern—higher adenocarcinoma proportion in a region with a lower proportion of “other types” during the latter years of the study period—could merely indicate variation in reporting or coding. This seems unlikely, however, because both the multinomial cluster and adenocarcinoma cluster span the service areas of Kentucky’s 2 largest university-affiliated hospitals (in Louisville–Jefferson County and Lexington–Fayette County) where most lung cancer cases are treated, and all data have been systematically collected by the same SEER cancer registry. Still, we briefly examined the percentage of lung cancers coded to broad, nonspecific categories such as “Neoplasm, malignant,” “Tumor cells, malignant,” “Carcinoma, NOS,” or “Carcinoma, undifferentiated, NOS” (histology codes 8000, 8001, 8010, 8020, respectively). This analysis showed these cases decreased from 18.5% to 11.0% of all lung cancer cases inside the multinomial cluster during the study period and from 24.6% to 14.9% outside it. Given the more rapid decline outside the multinomial cluster, it seems unlikely that the significantly higher percentage of adenocarcinomas diagnosed inside the cluster is due to fewer of these “NOS”-type diagnoses.
This study has some limitations that warrant consideration. First, we did not assess individual exposures or behaviors in this population-based study. Few details relating to such factors are available from most cancer registries, however; even lifetime prevalence of tobacco use is often not available. Regardless, the overall purpose of this study was to describe larger trends in lung cancer histology in the population of Kentucky and to assess their concordance with individual-level evidence from genetic epidemiology, rather than to firmly establish causal links. Additional limitations of this study relate to use of spatiotemporal scan statistics. For example, clusters of disease that do not manifest in a circular or elliptical manner might be more difficult to detect with this method, given its reliance on circular or elliptical scan windows. It is possible that other techniques could discern clustering in areas overlapping with the clusters we found, or in other areas altogether. Another limitation of this study lies in the use of BRFSS data to characterize smoking and obesity patterns at the county level, and for years that were not congruent to the entire study period. We combined 5 years of these data to overcome the relatively small sample sizes among counties, but we were not able to combine BRFSS data from before 2011 with later years, given important changes to the sampling and weighting protocols of the survey.37 Furthermore, BRFSS sample sizes during earlier years of the study period were smaller and thus less useful for examining geographic trends. We ultimately chose to present smoking patterns during the 2011- to 2015 period because it roughly overlapped with the statistically significant multinomial cluster and the largest significant Poisson-based clusters of adenocarcinoma, squamous cell, and small cell types.
This study also has important strengths. We used high-quality population-based lung cancer incidence data from an SEER cancer registry site, minimizing the likelihood of information bias. Also, this study is one of very few to examine risk for specific histological types of lung cancer. Furthermore, we conducted this spatial and temporal analysis of lung cancer in a high-incidence state known for high prevalence of important risk factors, smoking and obesity.
Conclusions
This study has demonstrated that significant and substantial spatial variation exists in the histological types of lung cancer diagnosed in Kentucky, a state with an especially high burden of lung cancer. Along with significantly higher risk for most types of lung cancer in high poverty areas, likely related to higher smoking rates, we observed recent increases in risk for adenocarcinoma in a region that has lower smoking and obesity rates than the rest of the state and is additionally more urban and affluent. Future research should examine data from additional cancer registries in other states and explore potential risks associated with air pollution and other environmental exposures in metropolitan areas to better understand spatial and temporal trends in lung cancer histology.
Acknowledgments
The authors would like to acknowledge the efforts of Dr Sarojini Kanotra and the Kentucky BRFSS Program, Kentucky Cabinet for Health and Family Services. Furthermore, the authors appreciate the efforts of the anonymous reviewers who contributed insightful comments that helped to improve this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Cancer Research Informatics and Biostatistics and Bioinformatics Shared Resource Facilities of the University of Kentucky Markey Cancer Center (P30CA177558).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Noone AM, Krapcho M, Miller D, et al. (eds). SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 3. de Groot P, Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am. 2012;50(5):863–876. [DOI] [PubMed] [Google Scholar]
- 4. Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31(2-3):139–148. [DOI] [PubMed] [Google Scholar]
- 7. Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89(21):1580–1586. [DOI] [PubMed] [Google Scholar]
- 8. Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–299. [DOI] [PubMed] [Google Scholar]
- 9. Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US; ); 2010. [PubMed] [Google Scholar]
- 10. Barbone F, Bovenzi M, Cavallieri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest. 1997;112(6):1474–1479. [DOI] [PubMed] [Google Scholar]
- 11. Bush ML, Christian WJ, Bianchi K, Lester C, Schoenberg N. Targeting regional pediatric congenital hearing loss using a spatial scan statistic. Ear Hear. 2015;36(2):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behavioral Risk Factor Surveillance System (BRFSS) Prevalence & Trends Data. National Center for Chronic Disease Prevention and Health Promotion DoPH, ed. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2017. [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2016). 2017. http://www.seer.cancer.gov/popdata. Accessed September 19, 2018.
- 14. Christian WJ, Hopenhayn C. Early stage lung cancer survival in Kentucky: associations with smoking history and Appalachian geography. J Ky Med Assoc. 2010;108:97–105. [Google Scholar]
- 15. Hopenhayn C, Christian WJ, Christian A, Studts J, Mullet T. Factors associated with smoking abstinence after diagnosis of early stage lung cancer. Lung Cancer. 2013;80(1):55–61. [DOI] [PubMed] [Google Scholar]
- 16. Huang B, Dignan M, Han D, Johnson O. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. J Rural Health. 2009;25(4):366–371. [DOI] [PubMed] [Google Scholar]
- 17. Schoenberg NE, Howell BM, Fields N. Community strategies to address cancer disparities in Appalachian Kentucky. Fam Community Health. 2012;35(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carreras-Torres R, Johansson M, Haycock PC, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: a Mendelian randomization study. PLoS One. 2017;12(6):e0177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petridou ET, Sergentanis TN, Antonopoulos CN, et al. Insulin resistance: an independent risk factor for lung cancer? Metabolism. 2011;60(8):1100–1106. [DOI] [PubMed] [Google Scholar]
- 20. Carreras-Torres R, Haycock PC, Relton CL, et al. The causal relevance of body mass index in different histological types of lung cancer: a Mendelian randomization study. Sci Rep. 2016;6:31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control & Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 22. Christian WJ, Huang B, Rinehart J, Hopenhayn C. Exploring geographic variation in lung cancer incidence in Kentucky using a spatial scan statistic: elevated risk in the Appalachian coal-mining region. Public Health Rep. 2011;126(6):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hendryx M, O’Donnell K, Horn K. Lung cancer mortality is elevated in coal-mining areas of Appalachia. Lung Cancer (Amsterdam, Netherlands). 2008;62(1):1–7. [DOI] [PubMed] [Google Scholar]
- 24. North American Association of Central Cancer Registries (NAACCR). Who is Certified? http://www.naaccr.org/Certification/WhoisCertified.aspx. Published 2018. Accessed April 12, 2019.
- 25. Kentucky Association For Economic Development (KAED). Kentucky Area Development Districts. https://kaedonline.org/kentucky-area-development-districts/. Published 2018. Accessed February 28, 2019.
- 26. Center for Disease Control (CDC). Best Practices User Guide: Health Equity in Tobacco Prevention and Control. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2015. [Google Scholar]
- 27. US Census Bureau. DP03: Selected Economic Characteristics, 2006–2010. 2011. American FactFinder http://factfinder.census.gov. Published April 12, 2019. [Google Scholar]
- 28. Amin R, Hendryx M, Shull M, Bohnert A. A cluster analysis of pediatric cancer incidence rates in Florida: 2000, Äì2010. Stat Public Policy. 2014;1(1):69–77. [Google Scholar]
- 29. Hosgood HD, III, Farah C, Black CC, Schwenn M, Hock JM. Spatial and temporal distributions of lung cancer histopathology in the state of Maine. Lung Cancer. 2013;82(1):55–62. [DOI] [PubMed] [Google Scholar]
- 30. DeChello LM, Sheehan TJ. Spatial analysis of colorectal cancer incidence and proportion of late-stage in Massachusetts residents: 1995–1998. Int J Health Geogr. 2007;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kulldorff M, Athas WF, Feurer EJ, Miller BA, Key CR. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am J Public Health. 1998;88(9):1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kulldorff M. A spatial scan statistic. Commun Stat Theory Meth. 1997;26(6):1481–1496. [Google Scholar]
- 33. Jung I, Kulldorff M, Richard OJ. A spatial scan statistic for multinomial data. Stat Med. 2010;29(18):1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. BMJ. 2006;333(7557):25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frazer K, McHugh J, Callinan JE, Kelleher C. Impact of institutional smoking bans on reducing harms and secondhand smoke exposure. Cochrane Database Syst Rev. 2016;(5):CD011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. BRFSS. Behavioral Risk Factor Surveillance System: Comparability of Data BRFSS 2013. Atlanta, GA: Center for Disease Control; 2014. [Google Scholar]