Abstract
Studies show maternal obesity is a risk factor for metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) in offspring. Here we evaluated potential mechanisms underlying these phenotypes. Female C57Bl6 mice were fed chow or an obesogenic high-fat/high-sucrose (HF/HS) diet with subsequent mating of F1 and F2 female offspring to lean males to develop F2 and F3 generations, respectively. Offspring were fed chow or fibrogenic (high transfat, cholesterol, fructose) diets, and histopathological, metabolic changes, and bile acid (BA) homeostasis was evaluated. Chow-fed F1 offspring from maternal HF/HS lineages (HF/HS) developed periportal fibrosis and inflammation with aging, without differences in hepatic steatosis but increased BA pool size and shifts in BA composition. F1, but not F2 or F3, offspring from HF/HS showed increased steatosis on a fibrogenic diet, yet inflammation and fibrosis were paradoxically decreased in F1 offspring, a trend continued in F2 and F3 offspring. HF/HS feeding leads to increased periportal fibrosis and inflammation in chow-fed offspring without increased hepatic steatosis. By contrast, fibrogenic diet-fed F1 offspring from HF/HS dams exhibited worse hepatic steatosis but decreased inflammation and fibrosis. These findings highlight complex adaptations in NAFLD phenotypes with maternal diet.
Keywords: bile acid metabolism, fatty liver, liver fibrosis, maternal high-fat/high-sucrose diet
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in adults and children, with a prevalence estimated to affect ~64 million individuals in the United States alone (43). About 21% of adults with NAFLD progress to nonalcoholic steatohepatitis (NASH), and in the pediatric population 25% progress to NASH and 15% develop fibrosis (34, 43). However, it is unclear why some patients with NAFLD develop progressive disease, which has limited the ability to develop effective targeted therapies.
Among the potential pathways of NAFLD progression, the developmental origins of health and disease hypothesis posits that in utero or early life exposures influence susceptibility to later develop chronic diseases (14). Whereas the initial studies examined the impact of undernutrition, there is now a focus on the impact of maternal obesity, since 55% of women are overweight or obese at the time of conception (15). Obesity in women is associated with development of metabolic syndrome in their offspring (2, 14, 27, 28) while rodent or nonhuman primate models have shown that exposure to a maternal high-fat diet (HFD) in utero and postnatally (by nursing) increases the propensity for development of NAFLD (3, 6, 23, 39). The mechanisms behind the developmental programming of NAFLD are poorly understood, although recent studies are beginning to address this question by evaluating DNA methylation and microbiome alterations (35, 41). Although these studies have focused on developmental programming of hepatic metabolism, they have yet to evaluate mechanisms for NAFLD progression to fibrosis. This is a critical question because fibrosis is the strongest predictor of outcome in patients with NAFLD, including liver-related complications, liver transplantation, and death (1).
We have shown in mice that exposure to maternal HFD leads to hepatic stellate cell activation, increased expression of profibrotic genes, and liver fibrosis in the offspring weaned to a low-fat diet (38). This finding was confirmed by a subsequent study showing increased hepatic fibrogenesis in maternal HFD offspring placed on a methionine- and choline-deficient (MCD) diet (41). However, there is no information concerning the development of fibrosis in progressive NAFLD models that induce obesity, and the mechanisms underlying potential disease progression have not been defined. It is also unclear if this phenotype is passed beyond the first generation of offspring.
In the current study we evaluated the impact of maternal diet on NAFLD progression using a transgenerational maternal high-fat/ high-sugar (HF/HS) diet model in which offspring were fed an obesogenic fibrogenic diet. We also explored changes in bile acid (BA) homeostasis given the presence of ductular reaction in our prior study (38). We hypothesized that offspring exposed, in utero, to an obesogenic maternal HF/HS diet might develop more aggressive liver disease when subsequently fed a fibrogenic diet.
EXPERIMENTAL PROCEDURES
Mouse breeding scheme, feeding paradigm, and metabolic analysis.
All procedures in this study were approved by the Animal Studies Committee at Washington University School of Medicine and conformed to National Institutes of Health guidelines. Female C57Bl/6J mice (4 wk old) were fed either a HF/HS diet [Test Diet 58R3; 59% fat, 26% carbohydrates (17% sucrose), and 15% protein] or standard chow (CON) [Pico Laboratory Rodent diet 20; 13% fat, 62% carbohydrates (3.2% sucrose), and 25% protein] for 6 wk as previously reported (32). CON- and HF/HS-fed F0 female mice were mated with chow-fed male mice to produce the first generation (F1) (Fig. 1). All offspring [F1, second generation (F2), and third generation (F3)] were weaned onto chow diets. F1 and F2 females were mated with chow-fed males to yield the subsequent generation. All offspring were kept with the same dam until time of weaning. Serum and relevant tissues were collected in nonmated F1, F2, and F3 8-wk-old mice. Tissue was also collected from 40-wk-old mice in the F1 generation. A subset of offspring from each generation was fed a high-transfat, cholesterol, fructose (HTFC) diet to induce progressive NASH [catalog no. D09100301; 40% of kcal from fat, 40% of kcal from carbohydrate (20% kcal from fructose), 20% protein, 2% cholesterol; Research Diets] (22). For these studies, between 6 and 10 litters were represented in each group. Body and liver weights were measured for all offspring at the time of tissue collection and weekly during HTFC diet feeding.
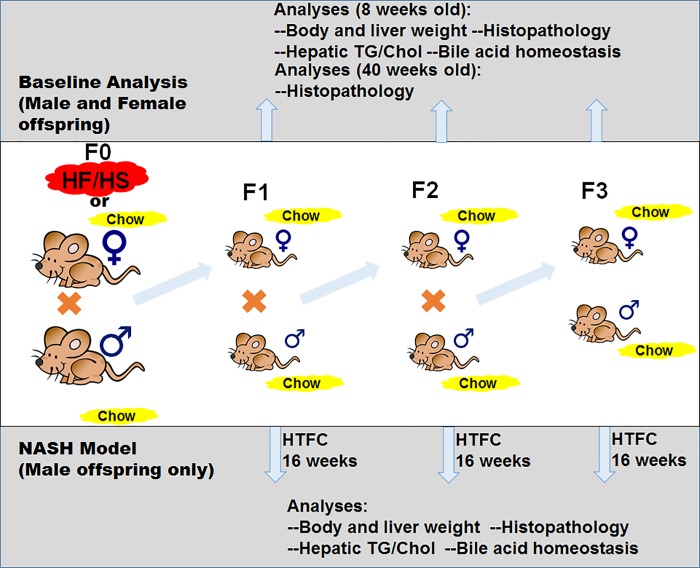
Fig. 1.
Breeding scheme and experimental design. Top, F0 female mice were fed high-fat/high-sucrose (HF/HS) diet or chow and bred with F0 male mice fed chow. All mice from the F1 through F3 generations were fed chow. For baseline analysis (bottom), offspring were evaluated at 8 and 40 wk old (data presented in Figs. 2–5). Bottom, nonalcoholic steatohepatitis (NASH) model of high-transfat, cholesterol, fructose (HTFC) diet feeding in male offspring (data presented in Figs. 6–9). Diets: Chow [kcal: 13% fat, 62% carbohydrates (3.2% sucrose), and 25% protein], HF/HS [kcal: 59% fat, 26% carbohydrates (17% sucrose), and 15% protein], and HTFC [kcal: 40% fat, 40% carbohydrate (20% fructose), 20% protein, with 2% cholesterol content]. TG, triglyceride; Chol, cholesterol.
Histology, special stains, and immunohistochemistry.
For histological analysis, tissues fixed in 10% formalin were embedded in paraffin, and 5-μm sections were cut and used for hematoxylin and eosin and picrosirius red (PSR) staining as previously reported (38). For immunohistochemistry, sections were rehydrated by passing through xylene, graded alcohol, and distilled water followed by staining using select primary antibodies (CD45, CD11b, F4/80) and the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) protocol. Antigen retrieval was performed by microwaving for 8 min in citrate buffer. After antigen retrieval, endogenous peroxide inactivation, and blocking, sections were incubated with primary antibody for ~20 h at 4°C. Sections were then washed and incubated with appropriate biotin-conjugated secondary antibody for 30 min. Sections were washed, incubated with avidin-biotin complex reagent, washed, and incubated with 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin and coverslipped using Cytoseal XYL (Richard Allen Scientific, Kalamazoo, MI). Representative sections were evaluated by a pathologist (Chatterjee), blinded to group identity, for NAFLD Activity Score (NAS) and fibrosis scoring (18). A subjective analysis was used to provide an estimation of large versus small droplet steatosis (presented as percent of tissue area) and degree of pericellular fibrosis (presented as none, mild, moderate, or severe), which are not addressed by standard NAS and fibrosis scoring. PSR staining was quantified as previously described (38).
Triglyceride and cholesterol measurement.
Liver triglyceride and cholesterol levels were measured after 4 h of fasting. The levels were determined by enzymatic assay performed in the Diabetes Models Phenotyping Core in the Diabetes Research Center at Washington University.
Bile acid analysis.
BA pool size was measured in offspring as previously reported (42). Briefly, after 4 h of fasting, gallbladder, liver, and intestines including luminal contents were collected, homogenized, and incubated in ethanol to extract BAs. Total BA content was determined enzymatically (Genway Biotech, San Diego, CA) and normalized to body weight. Individual intrahepatic BA species were measured using high-performance liquid chromatography and tandem mass spectrometry as previously described (42). Mass spectrometry was performed in the Metabolomics Facility at Washington University (P30 DK-020579). Individual BA levels were normalized to tissue weight and converted to molar concentration. The levels of each individual BA type are presented as a percent of the total intrahepatic BA pool.
To evaluate cyp7a1 activity, we measured serum levels of 7α-hydroxy-4-cholesten-3-one (C-4). A protein precipitation procedure was used to extract C-4 from 50 μl of mouse serum in the presence of deuterated internal standards. The C-4 level was measured on a SecurityGuard Gemini C18 (4 × 3 mm) and ACE C8 column (3 μm, 100 × 4.6 mm). C-4 was detected with positive multiple-reaction monitoring on an Applied Biosystems Sciex 4000QTRAP tandem mass spectrometer.
Quantitative PCR analysis.
Hepatic and ileal total RNA was extracted and cDNA prepared using an ABI high-capacity cDNA reverse transcription kit with 1 µg of total RNA. Real-time quantitative PCR used cDNAs from six animals per group and was performed in duplicate on an ABI Prism7000 sequence detection system using SYBR Green PCR Master Mix (Applied Biosystems) and primer pairs (provided on request) designed by Primer Express software (Applied Biosystems). Relative mRNA abundance is expressed as fold change to maternal chow-offspring chow group after normalization to GAPDH.
Serum analysis.
Blood was collected when the animals were euthanized, and serum was isolated. Aspartate aminotransferase and alanine aminotransferase were measured using colorimetric kits (TECO diagnostics). Serum BA levels were also measured using colorimetric assay kits (Genway Biotech). Serum fibroblast growth factor 15 (FGF15) was measured by sandwich ELISA (Lifespan Biosciences).
Statistical analysis.
Unpaired Student’s t-test and ANOVA were used when appropriate using GraphPad Prism software. Data are presented as means ± SD, with P < 0.05 representing significance.
RESULTS
Histological and metabolic analysis in 8- to 10-wk-old offspring after exposure to maternal HF/HS diet.
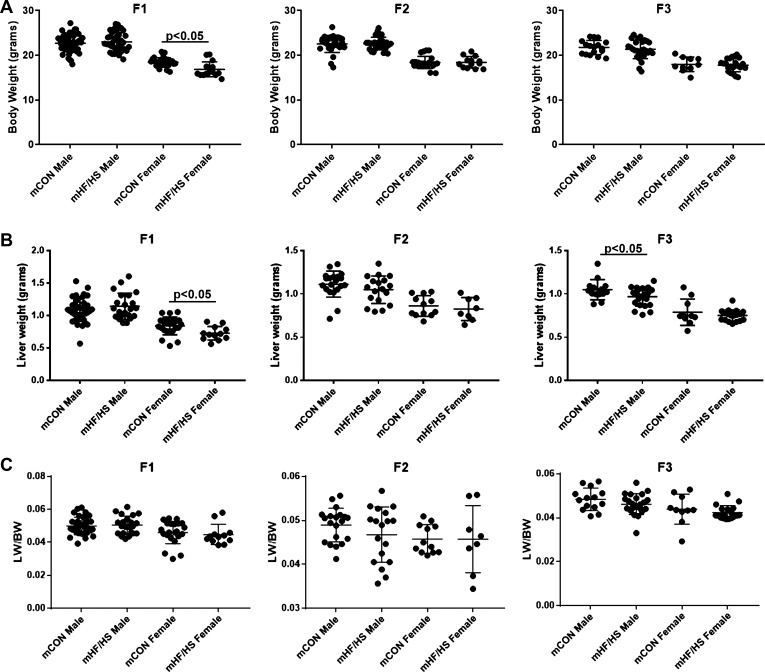
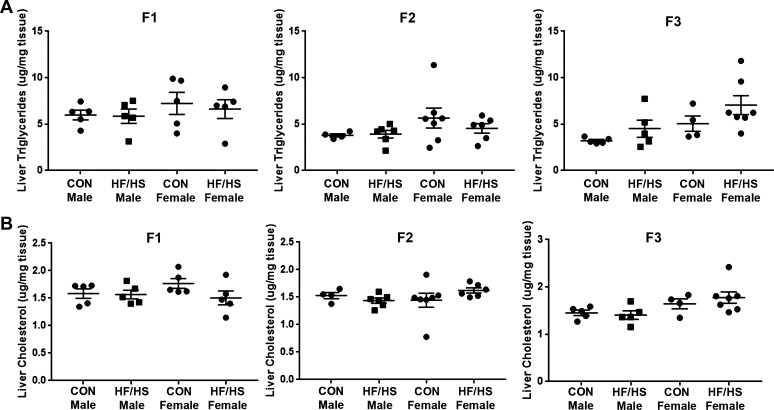
To evaluate the transgenerational impact of maternal obesogenic diet on offspring liver, F0 female mice were placed on control chow or HF/HS diet for 6 wk and mated as previously reported to generate three generations of offspring (32). Offspring body weights were similar between groups across all three generations at 8–10 wk of age, except for a small decrease in F1 females from the HF/HS lineage (Fig. 2A). Liver weight was similar between groups across all three generations, except for a minor decrease in F1 female and F3 male offspring from the maternal HF/HS lineage (Fig. 2B). No differences in liver weight-to-body weight ratio were observed in male or female offspring from either group in any of three generations (Fig. 2C). Hepatic triglyceride and cholesterol levels were also similar across all generations in both the maternal chow and maternal HF/HS diet lineage (Fig. 3, A and B).
Fig. 2.
Body and liver weights in F1–F3 offspring from maternal high-fat/high-sucrose (HF/HS) diet lineage. A: body weight of male and female offspring from maternal control (CON) and HF/HS lineage from F1, F2, and F3 generations. B: liver weight of male and female offspring from maternal CON and HF/HS lineage from F1, F2, and F3 generations. C: liver weight-to-body weight (LW/BW) ratio of male and female offspring from maternal CON and HF/HS lineage from F1, F2, and F3 generations. Quantitative data are presented as means ± SD with n ≥ 10 in each group and ≥5 separate litters represented in each group. P values are as indicated.
Fig. 3.
Liver triglyceride and cholesterol levels in F1–F3 offspring after maternal high-fat/high-sucrose (HF/HS) diet exposure. A: liver triglyceride levels from male and female offspring from maternal control (CON) and HF/HS lineage from F1, F2, and F3 generations. Triglyceride levels normalized to tissue weight. B: total liver cholesterol levels in male and female offspring (F1–F3) from maternal CON and maternal HF/HS lineages. Cholesterol levels normalized to tissue weight. Quantitative data are presented as means ± SD with n ≥ 4 in each group and ≥4 separate litters represented in each group.
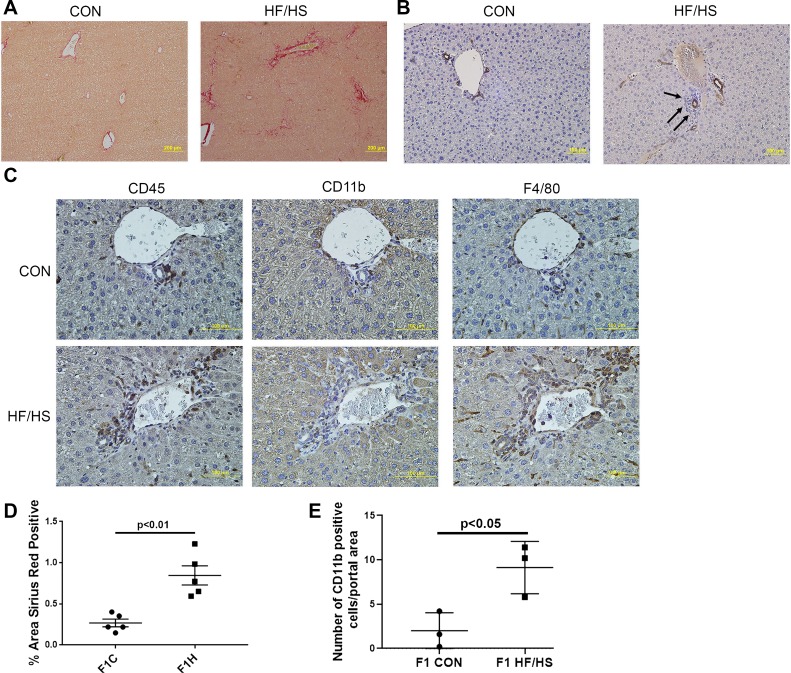
Increased periportal fibrosis and inflammation in 40-wk-old F1 offspring exposed to maternal HF/HS diet.
Because we previously found increased hepatic fibrosis in offspring exposed to maternal HFD in the low-fat-diet-fed F1 generation (38), we evaluated fibrosis in older mice (~40 wk) and found that offspring exposed to maternal HF/HS diet exhibited increased periportal and pericellular fibrosis (Fig. 4, A and D). Cytokeratin 19 staining as a surrogate for ductular proliferation revealed no change in offspring exposed to maternal HF/HS feeding, but the cells surrounding those cytokeratin 19-positive bile ducts (Fig. 4B) revealed evidence of monocyte (CD45 and CD11b) and macrophage (F4/80) infiltration (Fig. 4C). CD11b-positive cells were also increased in the periportal area in offspring from the maternal HF/HS lineage (Fig. 4, C and E). These findings suggest the presence of periportal inflammation in the F1 offspring of the maternal HF/HS lineage.
Fig. 4.
Periportal fibrosis and inflammation in offspring exposed to maternal high-fat/high-sucrose (HF/HS) diet. A: representative photomicrographs of picrosirius red (PSR) staining. B: representative photomicrographs of immunohistochemistry (IHC) for cytokeratin 19 (CK19). Arrows point to infiltrate around CK19-positive bile duct. C: representative photomicrographs of IHC for CD45, CD11b, and F4/80 in consecutive sections from maternal control (CON) and HF/HS offspring liver. D: quantitative analysis of PSR staining. E: no. of CD11b-positive cells per portal area (5 portal areas counted in each of 3 offspring/group). Quantitative data are presented as means ± SD with n = 5 (from 5 separate litters) in each group for PSR quantification and n = 3 (from 3 separate litters) in each group for CD11b cell counts. P values are as indicated.
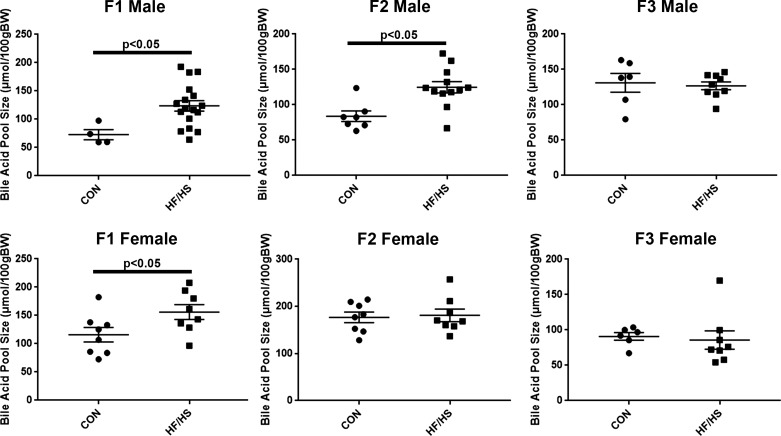
Increased BA pool size and altered intrahepatic BA composition in F1 and F2 offspring exposed to maternal HF/HS diet.
We next evaluated BA homeostasis in offspring from the various groups, beginning with measuring the total BA pool size in male and female offspring in each group across all three generations. In the F1 generation, both male and female offspring exposed to maternal HF/HS diet exhibited a significant increase in the total BA pool size (Fig. 5). In the F2 generation, only male offspring from the maternal HF/HS lineage continued to show an increase in the BA pool size. No differences in BA pool size were observed in the F3 generation.
Fig. 5.
Altered bile acid (BA) homeostasis in offspring exposed to maternal high-fat/high-sucrose (HF/HS) diet. BA pool size in male and female offspring (F1–F3) exposed to maternal control (CON) or HF/HS diet. Quantitative data are presented as means ± SD with n ≥ 4 in each group. P values are as indicated.
Maternal HF/HS diet impacts NAFLD progression and BA homeostasis in F1 offspring fed a fibrogenic diet.
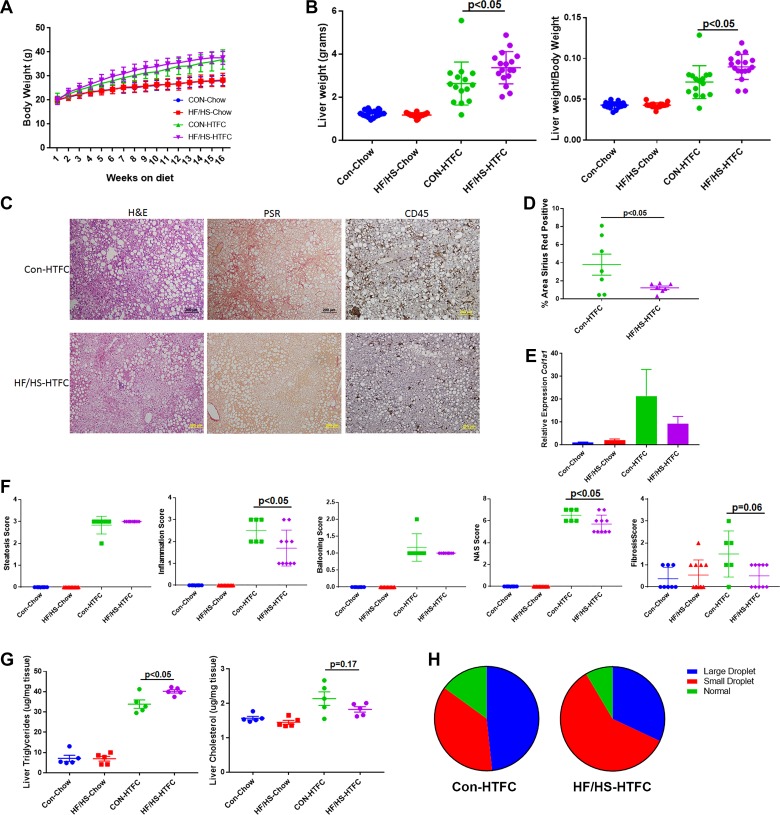
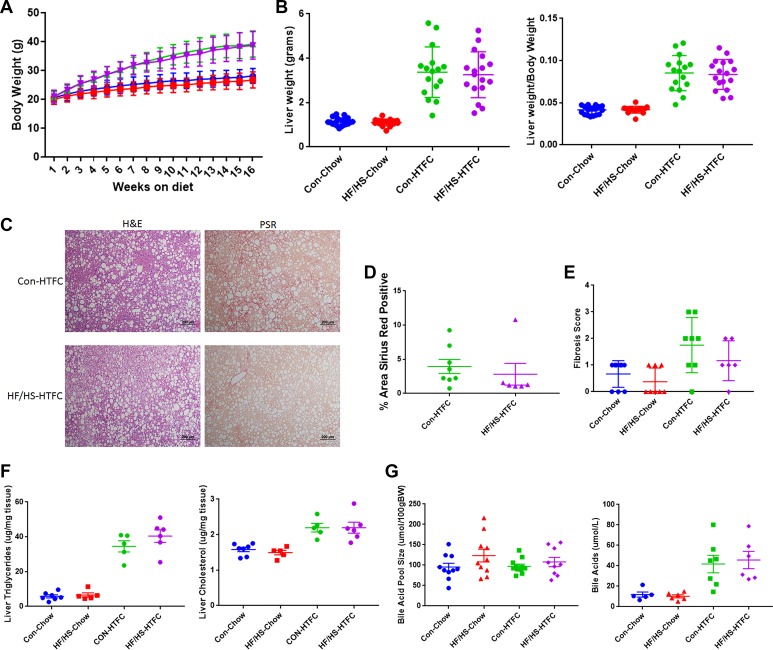
Male offspring from the F1 generation of the maternal chow and maternal HF/HS lineage were fed HTFC diet for 16 wk to induce progressive NAFLD, including inflammation and fibrosis, as previously reported (22). Body weights measured weekly revealed significant weight gain in both groups fed HTFC diet; however, no difference in weight was observed between F1 offspring from the maternal CON and maternal HF/HS lineages (Fig. 6A). After 16 wk of HTFC diet feeding, offspring from the maternal HF/HS lineage exhibited increased liver weight and liver weight-to-body weight ratio compared with maternal CON lineage (Fig. 6B).
Fig. 6.
Histopathological progression of nonalcoholic fatty liver disease (NAFLD) in F1 offspring from maternal control (CON) and high-fat/high-sucrose (HF/HS) diet-fed lineage. A: weekly body weights during 16 wk of chow or high-transfat, cholesterol, fructose (HTFC) diet feeding. B: liver weight and liver weight-to-body weight ratio after 16 wk of chow or HTFC diet feeding C: representative photomicrographs of hematoxylin and eosin (H&E), picrosirius red (PSR), and CD45 staining of liver from maternal CON and maternal HF/HS lineage offspring fed HTFC diet. D: quantification of %area positive for PSR in maternal CON and maternal HF/HS offspring fed HTFC diet. E: quantitative PCR for Col1a1 in liver from maternal CON and HF/HS lineage offspring fed chow or HTFC diet. Levels are presented as fold change to maternal CON chow-fed group as control. F: NAFLD Activity Score (NAS) (steatosis, inflammation, ballooning, and total) and fibrosis scoring on liver from maternal CON and HF/HS offspring fed chow or HTFC diet. G: hepatic triglyceride and cholesterol levels from offspring fed chow or HTFC diet. H: quantification of %area with large or small lipid droplets in liver from offspring fed HTFC diet. Five separate litters represented in each group with triglyceride and cholesterol levels; ≥6 separate litters represented for all other quantitative data. P values are as noted on each graph. All data are from male offspring.
Histopathological analysis was then performed to evaluate steatosis, inflammation, and fibrosis in F1 offspring fed HTFC diet. Offspring from both maternal CON and maternal HF/HS lineages show widespread hepatic steatosis (Fig. 6C). Blinded pathological NAS scoring revealed both groups of offspring exhibited a similar steatosis score (Fig. 6, C–F), although formal determination of hepatic triglyceride content showed a small but significant increase in the maternal HF/HS lineage offspring (Fig. 6G). Despite a similar histological steatosis score, offspring from the maternal CON lineage exhibited predominantly macrovesicular steatosis, whereas the maternal HF/HS lineage offspring exhibited predominately microvesicular steatosis (Fig. 6H). Conversely, measurement of total hepatic cholesterol showed a trend toward higher levels in maternal CON offspring (Fig. 6G). Immunohistochemical evaluation revealed decreased CD45-positive cells in maternal HF/HS lineage offspring liver compared with maternal CON lineage offspring (Fig. 6C) and also revealed a lower inflammation score in offspring from the maternal HF/HS lineage (Fig. 6F).
Because we observed mild fibrosis at baseline in offspring exposed to maternal HF/HS diet, we reasoned that these offspring would develop more rapid progression of fibrosis during fibrogenic diet feeding. However, to our surprise, F1 offspring from the maternal HF/HS lineage exhibited reduced PSR staining compared with maternal CON lineage offspring (Fig. 6C and quantified in Fig. 6D). Fibrosis scoring further confirmed less fibrosis in offspring from the maternal HF/HS lineage compared with maternal CON lineage (Fig. 6F). These findings were confirmed with blinded pathological evaluations of offspring from the maternal HF/HS lineage, which exhibited no more than mild fibrosis. Gene expression analysis by quantitative PCR showed variable, but not significantly different, Col1a1 expression in F1 maternal CON and HF/HS lineage offspring liver (Fig. 6E).
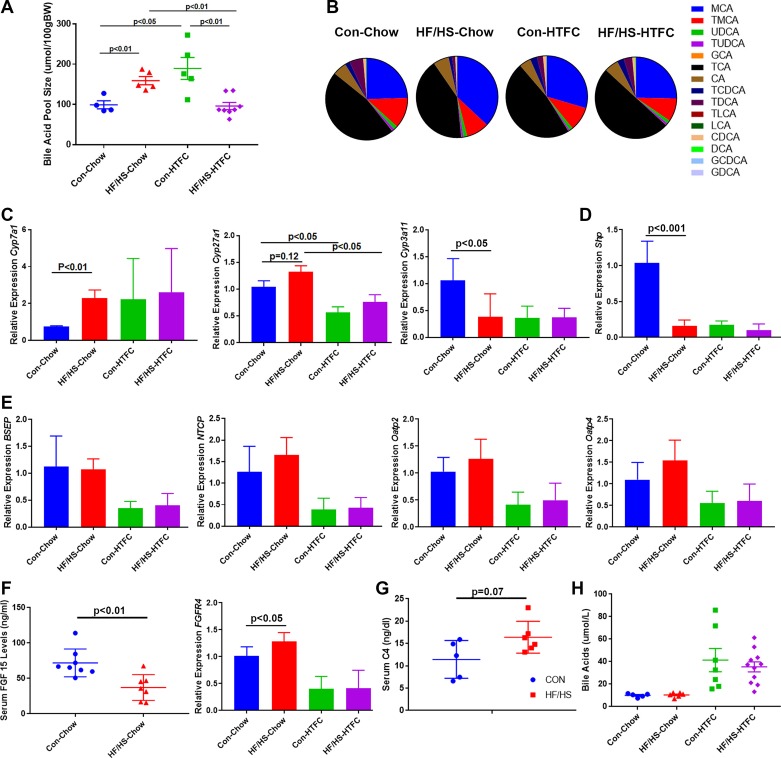
Maternal HF/HS diet impacts BA homeostasis in F1 offspring during the development of NAFLD.
BA pool size was increased in 8-wk-old offspring from the maternal HF/HS lineage. Similarly, at 22 wk of age, male maternal HF/HS lineage offspring from the F1 generation maintained a significant increase in BA pool size. After HTFC feeding for 16 wk, maternal CON lineage offspring exhibit increased BA pool size compared with baseline (Fig. 7A). By contrast, F1 offspring from the maternal HF/HS lineage exhibit a decline in BA pool size. Mass spectrometric analysis of individual intrahepatic BAs (Fig. 7B) revealed baseline shifts in maternal HF/HS lineage offspring with an increase in muricholic acid (MCA) and decreases in deoxycholic acid (DCA) and taurodeoxycholic acid (Table 1). However, after feeding with HTFC diet, no differences in levels of specific BA species were observed between the two lineages. To evaluate expression of enzymes involved in BA metabolism, quantitative PCR was performed, which showed a significant increase in mRNA abundance of Cyp7a1 mRNA and decreased Cyp3a11 and small heterodimer protein (Shp) mRNA in maternal HF/HS lineage offspring (Fig. 7, C and D). We also measured several BA transporter mRNAs (Bsep, Ntcp, Oatp2, and Oatp4), none of which varied in between maternal CON and HF/HS lineage offspring at baseline and after HTFC diet feeding (Fig. 7E). We also evaluated ileal FGF15 production, which signals via fibroblast growth factor receptor 4 (FGFR4) to downregulate Cyp7a1 expression (16). Serum levels of FGF15 were decreased while expression of FGFR4 was slightly increased in liver of maternal HF/HS offspring (Fig. 7F). We also evaluated Cyp7a1 activity by determining serum levels of (C-4), which showed a trend (P = 0.07) toward an increase in HF/HS offspring (Fig. 7G). Last, serum BA concentrations were increased after HTFC feeding in both groups, but no differences were found between the maternal CON and HF/HS lineages (Fig. 7H).
Fig. 7.
Bile acid (BA) homeostasis in F1 offspring during Western diet feeding. A: BA pool size in offspring fed chow or high-transfat, cholesterol, fructose (HTFC) diet. B: intrahepatic BA composition (%total) with key to individual species on right. C: quantitative PCR for Cyp7a1, Cyp27a1, and Cyp3a11 from liver of offspring fed chow or HTFC diet. Levels are presented as fold change to maternal control (CON) chow-fed group as control. D: quantitative PCR (qPCR) for small heterodimer protein (Shp) from liver of offspring fed chow or HTFC diets. Levels are presented as fold change to maternal CON chow-fed group as control. E: qPCR for several BA transporters (BSEP, NTCP, Oatp2, and Oatp4) from liver of offspring fed chow or HTFC diet. Levels are presented as fold change to maternal CON chow-fed group as control. F: serum fibroblast growth factor 15 (FGF15) levels (left) and qPCR for liver fibroblast growth factor receptor 4 (FGFR4, right). G: serum levels of 7α-hydroxy-4-cholesten-3-one (C-4) from offspring of maternal CON or fed high-fat/high-sucrose (HF/HS) diet lineage-fed chow diet. H: circulating BAs in offspring fed chow or HTFC diet. Quantitative data are presented as means ± SD with n ≥ 4 in each group. For BA pool size, ≥4 separate litters are represented in each group. For all other data, ≥6 separate litters are represented in each group. P values are as noted on each graph. All data are from male offspring.
Table 1.
BA profile for individual species
| Average Intrahepatic BA Pool, % (n ≥ 8) |
P Values for Direct Comparisons (t-test) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bile Acid Species | CC | HC | CH | HH | CC versus HC | CH versus HH | CC versus CH | HC versus HH |
| MCA | 24.5 | 29.5 | 37 | 25.5 | 0.02* | 0.18 | 0.19 | 0.01* |
| TMCA | 12 | 9.5 | 9 | 9.5 | 0.57 | 0.95 | 0.64 | 0.96 |
| UDCA | 1.5 | 1.5 | 1.5 | 1.5 | 0.99 | 0.80 | 0.53 | 0.57 |
| TUDCA | 1 | 0.5 | 1 | 1 | 0.56 | 0.39 | 0.19 | 0.93 |
| GCA | <0.5 | <0.5 | <0.5 | <0.5 | 0.18 | 0.74 | 0.68 | 0.79 |
| TCA | 46.5 | 47.5 | 42 | 50 | 0.12 | 0.54 | 0.77 | 0.02* |
| CA | 5.5 | 4.5 | 6 | 5.5 | 0.52 | 0.49 | 0.45 | 0.67 |
| TCDCA | 2 | 3 | 1.5 | 2.5 | 0.07 | 0.85 | 0.02* | <0.01* |
| TDCA | 5.5 | 2.5 | 1 | 3.5 | <0.01* | 0.18 | <0.01* | 0.01* |
| TLCA | <0.5 | <0.5 | <0.5 | <0.5 | 0.01* | 0.76 | 0.97 | <0.01* |
| LCA | <0.5 | <0.5 | <0.5 | <0.5 | 0.20 | 0.63 | 0.32 | 0.03* |
| CDCA | 1.09 | 1.5 | 0.5 | 1 | 0.07 | 0.67 | 0.49 | 0.02* |
| DCA | 0.25 | <0.5 | <0.5 | 0.5 | 0.01* | 0.14 | 0.09 | 0.03* |
| GCDCA | <0.5 | <0.5 | <0.5 | <0.5 | 0.60 | 0.28 | 0.53 | 0.95 |
| GDCA | <0.5 | <0.5 | <0.5 | <0.5 | 0.02* | 0.70 | 0.02* | 0.44 |
Data show average percent of total intrahepatic bile acid (BA) pool for each individual species and P values for t-test comparing individual BA percentages between groups with comparison noted. CC, maternal control (CON)-offspring chow; HC, maternal high-fat/high-sucrose (HF/HS)-offspring chow; CH, maternal CON-offspring high-transfat, cholesterol, fructose (HTFC); HH, maternal HF/HS-offspring HTFC. n ≥ 8 in each group.
Comparisons reaching statistical significance (P < 0.05).
Transgenerational effect of maternal HF/HS diet on development and progression on NAFLD.
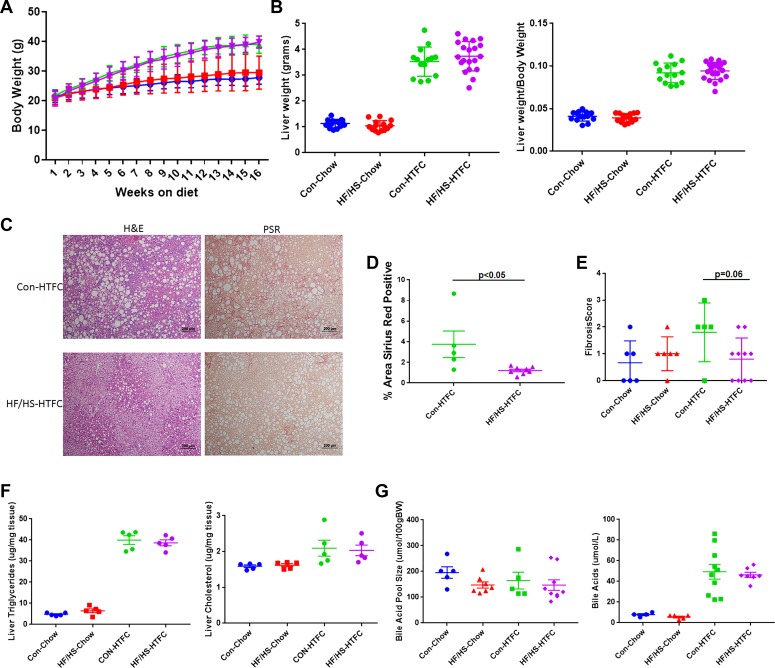
We also evaluated the effect of maternal HF/HS diet on NAFLD progression in male offspring from the F2 and F3 generations fed HTFC diet for 16 wk. In the F2 and F3 generation offspring, no differences in body weight, liver weight, and liver weight-to-body weight ratio were present between the maternal CON and HF/HS lineages (Figs. 8, A and B, and 9, A and B). Histopathological analysis showed similarly extensive steatosis in both lineages that was confirmed by measurement of both hepatic triglyceride (Figs. 8, C and F, and 9, C and F) and cholesterol (Figs. 8F and 9F) content. Despite no difference in steatosis compared with F2 from CON dams, F2 offspring in the maternal HF/HS lineage exhibited less fibrosis by both pathological scoring and measurement of PSR staining (Fig. 8, C–E). Also, histopathological analysis showed all but one F3 offspring from the maternal HF/HS lineage showed protection from fibrosis (Fig. 9, C–E).
Fig. 8.
Histopathological progression of nonalcoholic fatty liver disease (NAFLD) and bile acid (BA) homeostasis in F2 offspring from maternal control (CON) and fed high-fat/high-sucrose (HF/HS) diet lineage. A: weekly body weights during 16 wk of chow or high-transfat, cholesterol, fructose (HTFC) diet feeding. B: liver weight and liver weight-to-body weight ratio after 16 wk of chow or HTFC diet feeding C: representative photomicrographs of hematoxylin and eosin (H&E) and picrosirius red (PSR) staining of liver from maternal CON and maternal HF/HS lineage offspring fed HTFC diet. D: quantification of %area positive for PSR in maternal CON and maternal HF/HS offspring fed HTFC diet. E: fibrosis scoring on liver from offspring fed chow or HTFC diet. F: hepatic triglyceride and cholesterol levels from offspring fed chow or HTFC diet. G: measurement of BA pool size and serum BAs in offspring fed chow or HTFC diet. Quantitative data are presented as means ± SD with n ≥ 5 in each group with ≥5 separate litters represented in each group. P values are as noted on each graph. All data are from male offspring.
Fig. 9.
Histopathological progression of nonalcoholic fatty liver disease (NAFLD) and bile acid (BA) homeostasis in F3 offspring from maternal control (CON) and high-fat/high-sucrose (HF/HS) diet lineage. A: weekly body weights during 16 wk of chow or high-transfat, cholesterol, fructose (HTFC) diet feeding. B: liver weight and liver weight-to-body weight ratio after 16 wk of chow or HTFC diet feeding. C: representative photomicrographs of hematoxylin and eosin (H&E) and picrosirius red (PSR) staining of liver from maternal CON and maternal HF/HS lineage offspring fed HTFC diet. D: quantification of %area positive for PSR in maternal CON and maternal HF/HS offspring fed HTFC diet. E: fibrosis scoring on liver from offspring fed chow or HTFC diet. F: hepatic triglyceride and cholesterol levels from offspring fed chow or HTFC diet. G: measurement of BA pool size and serum BAs in offspring fed chow or HTFC diet. Quantitative data are presented as means ± SD with n ≥ 5 in each group and ≥5 separate litters represented in each group. P values are as noted on each graph. All data are from male offspring.
Transgenerational effect of maternal HF/HS diet on BA homeostasis in offspring.
To evaluate whether the observed changes in BA metabolism are passed on to subsequent generations, we measured the BA pool size and serum BA concentrations in male F2 and F3 offspring from both the maternal CON and HF/HS lineages. No significant differences in BA pool size were observed at baseline or after HTFC feeding between the two lineages in the F2 and F3 generation (Figs. 8G and 9G). There was also no difference in serum BA levels.
DISCUSSION
The transmission of NAFLD risk to offspring exposed to maternal obesity has been shown in the literature across multiple species (3, 4, 25, 39). These studies have evaluated this risk in the immediate (F1)-generation offspring but not subsequent generations (3, 4, 23, 25, 39). In the current study, we evaluated NAFLD development and progression across three generations and the effect of maternal HF/HS diet feeding on BA homeostasis in offspring. Our key findings include: 1) increased steatosis and hepatic triglyceride accumulation in maternal HF/HS lineage offspring occurs in a direct but not intergenerational (only transmitted to F2) or transgenerational (transmitted to F3) fashion, 2) BA homeostasis is altered, but only in the F1 generation offspring exposed to maternal HF/HS diet, evidenced by increased BA pool size and shifted hepatic BA composition, and 3) despite periportal inflammation and fibrosis at baseline, maternal HF/HS lineage offspring fed a high-fat fibrogenic diet were paradoxically protected from progression of NAFLD to inflammation and fibrosis. Furthermore, this protection from fibrosis occurred in a transgenerational manner. Several components of these conclusions merit expanded discussion.
Maternal HF/HS diet exposure alters offspring BA homeostasis.
A key finding in this study is the impact of maternal HF/HS diet exposure on BA homeostasis in the offspring. The presence of periportal fibrosis and inflammation, as well as our previous observation of ductular reaction in offspring exposed to maternal HFD alone (38), prompted an evaluation of BA metabolism. Of specific relevance, steatosis and inflammation in the periportal region rather than the pericentral zone is commonly reported in pediatric NAFLD compared with adult NAFLD (33). Periportal inflammation is associated with an increase in fibrosis and more advanced liver disease in both children and adults (5). Furthermore, periportal inflammatory infiltrate is a strong predictor of fibrosis severity in both adult and pediatric NAFLD (7, 8). Development of disease in the periportal region is also associated with cholangiocyte proliferation, or ductular reaction, in animal models and has been reported in biopsies from both children and adults with NASH and fibrosis (20, 24). We found that the bile pool size was increased in F1 generation male and female offspring. This would support the observation of periportal inflammation/fibrosis present in the offspring. We also observed a shift in the intrahepatic BA pool composition toward increased MCA and decreased DCA and taurodeoxycholic acid. This shift indicates a more farnesoid X receptor (FXR) antagonistic versus agonistic profile in maternal HF/HS lineage offspring. The increased expression of Cyp7a1 mRNA is indirect support for FXR antagonism in view of work showing that FXR agonists negatively regulate Cyp7a1 expression and reduce BA synthesis (10). We also observed a significant decrease in Shp1 gene expression. Shp1 is a known negative regulator of Cyp7a1 acting downstream of FXR, so a decrease in Shp1 would be consistent with a concurrent increase in Cyp7a1 levels (10). Serum levels of FGF15 were decreased, which may also contribute to an increase in Cyp7a1 expression, since FGF15 signaling through FGFR4 decreases Cyp7a1 levels (16). To evaluate Cyp7a1 activity as an indicator of function, serum levels of C-4 were measured (9). In F1 offspring exposed to maternal HF/HS diet, C-4 levels were increased in the circulation, findings that indirectly suggest increased Cyp7a1 enzyme activity in addition to the aforementioned increased Cyp7a1 mRNA abundance. Although we also observed changes in expression of other enzymes (Cyp27a1, Cyp3a11) involved in BA metabolism, no difference was noted in expression of several major BA transporters. Our current data support a change in the BA metabolism pathway as the primary driver for increased BA pool size.
Whereas the levels of genes involved in BA metabolism may be under epigenetic regulation in our model, another potential driver for changes in BA metabolism is the offspring microbiome. We have yet to evaluate the microbiome in our studies, but two recently published manuscripts suggest a role in developmental programming of metabolic liver disease. Soderborg et al. (36) isolated stool samples from newborn offspring of obese and lean mothers and performed fecal microbiome transplantation in gnotobiotic mice. Gnotobiotic mice receiving stool from offspring of obese mothers exhibited periportal inflammation, changes in BA levels, and increased Cyp7a1 expression consistent with our observations, although, in contrast to our observations, Shp1 expression was unchanged. Another study evaluated expression of genes involved in BA metabolism with associated microbiome changes, but only in the setting of postweaning HFD exposure (40). Taken together these studies implicate alterations in the microbiome as one determinant of altered BA homeostasis in offspring exposed to maternal HFD.
Although the phenotype of increased BA pool size and composition shift was not passed on to the F3 generation, increased BA pool size was observed in 8-wk-old F2 males in the maternal HF/HS lineage. Those findings suggest intergenerational, but not transgenerational, transmission of the changes in BA homeostasis. However, BA pool size was no longer increased in 22-wk-old male offspring in the F2 maternal HF/HS lineage, suggesting that this difference is not sustained, unlike the F1 generation. Given the lack of a sustained effect on BA metabolism in the F2 generation or any difference in the F3 generation, we speculate that the phenotypes observed here could be explained by a direct effect of maternal HF/HS diet on BA metabolism in the developing fetus rather than by an epigenetic mechanism passed down to future generations. Future studies will be essential to delineate the mechanism behind changes in the pool size and intrahepatic composition on a molecular and subsequently epigenetic level.
Varied pathological response to high-fat fibrogenic diet in offspring from maternal HF/HS lineage.
Based on our findings of increased baseline hepatic fibrosis in chow-fed offspring exposed to maternal HF/HS diet, we reasoned that these offspring would develop exaggerated steatosis along with more rapid progression of fibrosis when they were fed a high-fat fibrogenic diet. Indeed, these expectations were fulfilled in regard to hepatic lipid accumulation in the F1 generation, findings consistent with prior studies (3, 23). In addition, our findings suggest that offspring from the maternal HF/HS lineage exhibit predominately microvesicular steatosis while maternal CON lineage offspring manifested predominately macrovesicular steatosis. Because prior studies in NAFLD subjects indicated that microvesicular steatosis was associated with more severe injury (37), we expected that the offspring from the maternal HF/HS lineage would manifest increased fibrosis when fed a fibrogenic diet. However, we found that offspring from the maternal HF/HS lineage exhibited less fibrosis on HTFC diet.
The phenotype of reduced fibrosis in the maternal HF/HS lineage compared with CON offspring fed a fibrogenic diet was further unexpected because we observed periportal fibrosis in maternal HF/HS offspring at baseline and increased hepatic triglyceride accumulation in the maternal HF/HS lineage offspring. These apparently paradoxical findings are, however, internally consistent with our prior work that showed that offspring exposed to maternal HFD developed more severe steatosis but no fibrosis when fed a HFD for 12 wk after weaning (38). These studies both argue that, while offspring exposed to maternal HFD develop worse steatosis, they are protected from disease progression to fibrosis.
Our findings and conclusions contrast with those from Wankhade et al. (41) who showed that maternal HFD-exposed offspring fed a MCD diet developed worse fibrosis. Among the differences between the MCD model and our high-fat cholesterol fructose dietary model is that MCD feeding induces significant weight loss rather than weight gain and induces a more inflammatory injury (21, 31). Accordingly, we suspect that differences in fibrotic response between the two studies may be explained by divergent mechanisms with these two dietary models (21). Given this discrepancy, future studies evaluating fibrosis across different models of liver injury in maternal HF/HS offspring will be essential to identify if this protection is specific to Western diet-induced fibrosis versus other forms of injury. While this work was in review, Gutierrez Sanchez and colleagues published findings in which maternal high-fat, fructose, and cholesterol diet exposure induced greater fibrosis in the offspring fed the same diet (11). The discrepancy between our findings highlights the complexity of developmental programming and suggests that the timing and specific composition of the maternal diet are key variables in evaluating the impact of maternal exposures on development of necroinflammatory steatotic injury in offspring.
A further finding from our studies was that fibrosis protection during fibrogenic diet feeding extends beyond the F1 generation. There is precedent for developmental programming of protection from hepatic fibrosis with other models, as illustrated by Zeybel et al. (44) who previously showed transgenerational protection from CCl4-induced fibrosis through the male lineage with an epigenetic link through peroxisome proliferator-activated receptor-γ. Our observation that offspring from the maternal HF/HS lineage across all three generations are protected from fibrosis during HTFC diet feeding would likewise suggest an epigenetic mechanism is involved. Future studies will be needed to confirm this finding in additional models and begin to identify the mechanisms that are driving fibrosis protection.
We also acknowledge some limitations to the current studies. First, the maternal HF/HS diet has a lower percent of kilocalories provided from protein than the CON diet. This is important because low-protein maternal diets could potentially contribute to developmentally programed metabolic phenotypes in the offspring, specifically in liver metabolism (29, 30). It will be important, therefore, to evaluate if protein restriction replicates the phenotypes we observe in the maternal HF/HS model. Second, only maternal inheritance was evaluated. Although the role of the maternal environment has been the focus of many studies related to the developmental origins of liver disease, there is emerging evidence for programming via paternal nutrition. Indeed, paternal HFD feeding leads to increased steatosis in the offspring, and there is an additive effect if both parents consume a HFD (26). Of note, paternal HFD led to worse periportal fibrosis in female offspring (19). Further studies will be essential to delineate the impact of paternal HFD on offspring BA metabolism and progression of liver disease during the development of NASH. Another factor to take into account is the role of litter size. Offspring raised in small litters gain more weight and experience alterations in metabolism through to adulthood likely because of postnatal overnutrition (12). Although litter size was not strictly controlled in this study, very small litters (<3 pups) were not used for analysis. We did not observe any major differences in weight gain (data not shown), which might be expected in the setting of litter size affecting metabolism, but controlling litter size more tightly will be essential in studies moving forward. Last, serum FGF15 was measured by ELISA, which may not be the most reliable method (17). It will be important to also measure ileal FGF15 gene expression in future studies.
In summary, we have shown that maternal HF/HS diet exposure leads to changes in BA homeostasis and increased hepatic steatosis in F1 offspring exposed to maternal HF/HS diet and fed a Western diet into adulthood. Despite those phenotypes, we find that offspring exposed to maternal HF/HS diet are protected from fibrosis during Western diet feeding, a phenotype passed across three generations. Future studies may explore the mechanisms of the complex and varied pathological responses observed in the liver after maternal HF/HS diet exposure.
GRANTS
This work was supported by Child Health Research Center at Washington University School of Medicine NIH Grant K12-HD-076224 (M. D. Thompson), Digestive Diseases Research Cores Center Grant DK-52574 (M. D. Thompson, N. O. Davidson), a Washington University Institute for Clinical and Translational Science Just in Time Award (M. D. Thompson), and NIH Grants DK-56260 (N. O. Davidson), HL-38180 (N. O. Davidson), HL-140848 (J. L. A. Ferey), and HD-83895 (K. H. Moley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.T., K.H.M., and N.O.D. conceived and designed research; M.T., A.D., J.L.F., M.R., Y.X., M.C., D.C., C.N., N.H., and F.G. performed experiments; M.T., A.D., and D.C. analyzed data; M.T. and N.O.D. interpreted results of experiments; M.T. and M.C. prepared figures; M.T. drafted manuscript; M.T., J.L.F., K.H.M., and N.O.D. edited and revised manuscript; M.T., J.L.F., N.H., F.G., K.H.M., and N.O.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the assistance of David Scherrer and Xuntian Jiang in the Metabolomics Core for performance of bile acid composition analysis and Sangeeta Adak for completing triglyceride and cholesterol measurements.
REFERENCES
- 1.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149: 389–97.e10, 2015. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 3.Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50: 1796–1808, 2009. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 4.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, Reynolds R, Alston M, Hoffman C, Pan Z, Friedman JE, Barbour LA. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr 162: 930–6.e1, 2013. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA; NASH Clinical Research Network . Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 49: 809–820, 2009. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgueño AL, Cabrerizo R, Gonzales Mansilla N, Sookoian S, Pirola CJ. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J Nutr Biochem 24: 6–13, 2013. doi: 10.1016/j.jnutbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Carpino G, Nobili V, Renzi A, De Stefanis C, Stronati L, Franchitto A, Alisi A, Onori P, De Vito R, Alpini G, Gaudio E. Macrophage activation in pediatric nonalcoholic fatty liver disease (NAFLD) correlates with hepatic progenitor cell response via Wnt3a pathway. PLoS One 11: e0157246, 2016. doi: 10.1371/journal.pone.0157246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59: 1393–1405, 2014. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 9.Gälman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res 44: 859–866, 2003. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517–526, 2000. doi: 10.1016/S1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez Sanchez LH, Tomita K, Guo Q, Furuta K, Alhuwaish H, Hirsova P, Baheti S, Alver B, Hlady R, Robertson KD, Ibrahim SH. Perinatal nutritional reprogramming of the epigenome promotes subsequent development of nonalcoholic steatohepatitis. Hepatol Commun 2: 1493–1512, 2018. doi: 10.1002/hep4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J Nutr 143: 553–562, 2013. doi: 10.3945/jn.112.172825. [DOI] [PubMed] [Google Scholar]
- 14.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94: 1027–1076, 2014. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinkle SN, Sharma AJ, Kim SY, Park S, Dalenius K, Brindley PL, Grummer-Strawn LM. Prepregnancy obesity trends among low-income women, United States, 1999-2008. Matern Child Health J 16: 1339–1348, 2012. doi: 10.1007/s10995-011-0898-2. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Katafuchi T, Esterházy D, Lemoff A, Ding X, Sondhi V, Kliewer SA, Mirzaei H, Mangelsdorf DJ. Detection of FGF15 in plasma by stable isotope standards and capture by anti-peptide antibodies and targeted mass spectrometry. Cell Metab 21: 898–904, 2015. doi: 10.1016/j.cmet.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Lu YP, Tsuprykov O, Hasan AA, Reichetzeder C, Tian M, Zhang XL, Zhang Q, Sun GY, Guo J, Gaballa MMS, Peng XN, Lin G, Hocher B. Folate treatment of pregnant rat dams abolishes metabolic effects in female offspring induced by a paternal pre-conception unhealthy diet. Diabetologia 61: 1862–1876, 2018. doi: 10.1007/s00125-018-4635-x. [DOI] [PubMed] [Google Scholar]
- 20.Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, Diehl AM. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J Hepatol 63: 962–970, 2015. doi: 10.1016/j.jhep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One 10: e0127991, 2015. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCommis KS, Hodges WT, Brunt EM, Nalbantoglu I, McDonald WG, Holley C, Fujiwara H, Schaffer JE, Colca JR, Finck BN. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology 65: 1543–1556, 2017. doi: 10.1002/hep.29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, Butt A, Saraswati R, Novelli M, Fusai G, Poston L, Taylor PD, Oben JA. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 58: 128–138, 2013. doi: 10.1002/hep.26248. [DOI] [PubMed] [Google Scholar]
- 24.Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 56: 2142–2153, 2012. doi: 10.1002/hep.25742. [DOI] [PubMed] [Google Scholar]
- 25.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, Taylor PD. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 52: 913–920, 2010. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Ornellas F, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Programming of obesity and comorbidities in the progeny: lessons from a model of diet-induced obese parents. PLoS One 10: e0124737, 2015. doi: 10.1371/journal.pone.0124737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia 40: 1094–1100, 1997. doi: 10.1007/s001250050792. [DOI] [PubMed] [Google Scholar]
- 28.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord 21: 451–456, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Qasem RJ, Cherala G, D’mello AP. Maternal protein restriction during pregnancy and lactation in rats imprints long-term reduction in hepatic lipid content selectively in the male offspring. Nutr Res 30: 410–417, 2010. doi: 10.1016/j.nutres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Qasem RJ, Li J, Tang HM, Pontiggia L, D’mello AP. Maternal protein restriction during pregnancy and lactation alters central leptin signalling, increases food intake, and decreases bone mass in 1 year old rat offspring. Clin Exp Pharmacol Physiol 43: 494–502, 2016. doi: 10.1111/1440-1681.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res 47: 2280–2290, 2006. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep 16: 1–8, 2016. doi: 10.1016/j.celrep.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 42: 641–649, 2005. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 34.Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, Vanderwall K, Fontanesi J. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther 38: 1267–1277, 2013. doi: 10.1111/apt.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seki Y, Suzuki M, Guo X, Glenn AS, Vuguin PM, Fiallo A, Du Q, Ko YA, Yu Y, Susztak K, Zheng D, Greally JM, Katz EB, Charron MJ. In utero exposure to a high-fat diet programs hepatic hypermethylation and gene dysregulation and development of metabolic syndrome in male mice. Endocrinology 158: 2860–2872, 2017. doi: 10.1210/en.2017-00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D’Alessandro A, Barbour LA, El Kasmi KC, Friedman JE. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 9: 4462, 2018. doi: 10.1038/s41467-018-06929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Ünalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol 55: 654–659, 2011. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson MD, Cismowski MJ, Trask AJ, Lallier SW, Graf AE, Rogers LK, Lucchesi PA, Brigstock DR. Enhanced steatosis and fibrosis in liver of adult offspring exposed to maternal high-fat diet. Gene Expr 17: 47–59, 2016. doi: 10.3727/105221616X692135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 63: 2702–2713, 2014. doi: 10.2337/db14-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Piccolo BD, Mercer KE, Andres A, Thakali KM, Shankar K. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci Rep 8: 16502, 2018. doi: 10.1038/s41598-018-34453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, Shankar K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One 12: e0175675, 2017. doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y, Cifarelli V, Pietka T, Newberry EP, Kennedy SM, Khalifeh-Soltani A, Clugston R, Atabai K, Abumrad NA, Davidson NO. Cd36 knockout mice are protected against lithogenic diet-induced gallstones. J Lipid Res 58: 1692–1701, 2017. doi: 10.1194/jlr.M077479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73–84, 2016. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 44.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, Burt AD, Wilson CL, Anstee QM, Barter MJ, Masson S, Elsharkawy AM, Mann DA, Mann J. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med 18: 1369–1377, 2012. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]