Abstract
Aging is a key contributor for subclinical progression of late-onset lung diseases. Basal, club, and type II alveolar epithelial cells (AECs) are lung epithelial progenitors whose capacities of differentiation are extensively studied. The timely transition of these cells in response to environmental changes helps maintain the intricate organization of lung structure. However, it remains unclear how aging affects their behavior. This paper demonstrates that the protein expression profiles of a type II AEC marker, prosurfactant protein C (pro-SPC), and a basal cell marker, p63, are altered in the lungs of 14-mo-old versus 7- to 9-wk-old mice. Expression of NH2-terminal-truncated forms of p63 (ΔNp63), a basal cell marker, and claudin-10, a club cell marker, in cytoplasmic extracts of lungs of 14-mo-old mice was upregulated. In contrast, nuclear expression of full-length forms of p63 (TAp63) decreases with age. These alterations in protein expression profiles coincide with dramatic changes in lung functions including compliance. Whole tissue lysates of middle-aged versus aged rhesus monkey lungs display similar age-associated alterations in pro-SPC expression. An age-associated decrease of TAp63 in nuclear lysates was observed in aged monkey group. Moreover, the lungs of 14-mo-old versus 7- to 9-wk-old mice display a wider spreading of ΔNp63-positive CCSP-positive bronchiolar epithelial cells. This expansion did not involve upregulation of Ki67, a representative proliferation marker. Collectively, it is postulated that 1) this expansion is secondary to a transition of progenitor cells committed to club cells from ΔNp63-negative to ΔNp63-positive status, and 2) high levels of cytoplasmic ΔNp63 expression trigger club cell migration.
Keywords: aging, basal cells, club cells, p63/CKAP4, prosurfactant protein C (pro-SPC)
INTRODUCTION
Aging is a complex biological process in which organs gradually require greater levels of repair and regeneration. It is thought that aging plays an important role in various lung diseases, e.g., chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) (9). In particular, the senescence of lung epithelial cells is thought to trigger the development of human lung diseases for which the incidence increases with age (17, 24, 27, 35). However, the precise mechanism underlying this process is still unclear. Therefore, a need exists to determine how aging contributes to the pathogenesis of age-associated human lung diseases with special focus on lung epithelial cells.
The lung epithelium protects lungs against toxic and infectious agents. Club cells are a lung epithelial cell type that has multiple functions including 1) xenobiotic metabolism, 2) immunomodulation, and 3) regeneration (19). A specific subpopulation of club cells are apoptosis resistant and reside in specific areas in bronchioles, close to alveoli, where lung injury and lung regeneration occur more frequently than other sites of the lung (33). However, how aging affects the overall behavior and functional integrity of club cells remains unknown. Another cell type that closely cooperates with club cells is basal cells (BCs). BCs are progenitor cells that reside in the basal layer of stratified epithelia of many organs including the skin, prostate, esophagus, and lung. They help maintain the homeostasis of the lung epithelium and facilitate its orderly regeneration when damaged (26). A marked difference in the distribution and arrangement of BCs along the proximal-distal axis of the lung exists between rodents and primates (26, 31). The BC-containing pseudostratified epithelium extends distally to the terminal bronchioles in humans and monkeys while in mice it is largely restricted to the trachea (26, 31).
One or more lineage markers generally characterize each cell type in lung epithelium even though there exist specific populations that express multiple lineage markers of different cell types. p63, a transcription factor encoded by the TP63 gene, is a cell lineage marker for BCs, extensively studied in multiple organs and helps maintain both the proliferative and differentiation capacity of tissue-specific progenitor cells (30). It also plays a critical role in the determination of cell fate; its loss affects cell-type switching and epithelial stratification (16). Studies also show that p63-positive progenitors in the distal lung proliferate and repopulate damaged alveolar epithelium (3, 32, 36). However, how aging affects their numbers and behavior as progenitor cells remains unknown.
To date, aging of lungs has been widely studied by varied approaches in vitro as well as in vivo. While lung samples of rodents have yielded a lot of data regarding aging lungs, the information obtained by human lung samples is very limited. This is probably due to the difficulty of procuring numerous undiseased lung tissues from different age groups. Huge variations of genetic backgrounds as well as environmental factors between the sample donors would reasonably make it a challenge for researchers to elicit consistent results out of using human lung samples. In the current study, monkey lung samples are used as an alternative for human lung samples and representative of primate lungs. This study demonstrates for the first time that p63, as well as other lineage markers, are altered in an age-dependent manner in lung samples from mice and monkeys.
MATERIALS AND METHODS
Constructs.
The expression plasmid pBI-MCS-EGFP-ΔNp63α was a gift from Dr. Kurt Engeland (University of Leipzig, Leipzig, Germany), and the plasmids pcDNA3.1/HisC-ΔNp63α, ΔNp63β and ΔNp63γ were a gift from Dr. Francesca Bernassola (University of Rome, Rome, Italy).
Cell culture and transfection.
Human embryonic kidney 293 (HEK293) cells were seeded on 100-mm culture dishes and grown in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. When cell density reached 70–80% confluence, the complete medium was replaced with serum-free Opti-MEM I medium (cat. no. 51985034; Thermo Fisher Scientific, Waltham, MA). Subsequently, for control group, the cells were either untreated (no siRNAs and no transfection reagent; 1st control) or transfected with nontargeting siRNAs with a final concentration of 30 nM using Lipofectamine 2000 (cat. no. 11668019; Invitrogen, Life Technologies, Carlsbad, CA; 2nd control). For p63 knockdown group, the cells were transfected with either of four different p63 siRNAs (cat. no. MQ-012755-01-0002; Dharmacon, Lafayette, CO) with a final concentration of 30 nM using Lipofectamine 2000. Six hours after transfection, the medium was replaced with complete medium. Forty-eight to seventy-two hours after transfection, cells were harvested and whole cell lysates were prepared. The sequences of nontargeting siRNAs were as follows: sense: 5′-GACUUCCGUCGACAUUAUUUU-3′, antisense 5′-AAUAAUGUCGACGGAAGUCUU-3′.
H441 human lung epithelial cells were seeded on collagen-coated six-well plates in RPMI1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin. When cell density reached 90–100% confluence, the complete medium was replaced with serum-free Opti-MEM I medium. Subsequently, for control group, the cells were either untreated or transfected with empty plasmid. For overexpression group, either pBI-MCS-EGFP-ΔNp63α, pcDNA3.1/HisC-ΔNp63α, ΔNp63β, or ΔNp63γ was transfected using Lipofectamine 2000. For each well, 2.5 μg of plasmid DNA were added. Three hours after transfection, the medium was replaced with complete medium. Twenty-four hours after transfection, cells were harvested and whole cell lysates were prepared.
Mice and monkeys.
All procedures were approved by the Animal Care and Use Committee of University of South Florida. Each animal facility at University of South Florida, including the one we used, is a distinct and secure area that is not accessible to the public and isolated from research, teaching, and clinical areas. C57BL/6J mice (Harlan laboratories, Indianapolis, IN) at 6–7 wk of age were purchased and housed in individually ventilated cage/rack systems until they reached the ages designated for each experiment (7–9 wk, 7 mo, 8–9 mo, 1 yr, 13–14 mo, and 20–21 mo old). They were euthanized immediately before sample collection. Frozen lung tissue samples of rhesus monkeys with different ages were provided by Dr. B. Hansen. The monkeys had been colony born in the United States and maintained consistently under laboratory conditions throughout life. The rhesus monkeys’ lung samples were collected after death by natural causes. In the current study, the expression of different proteins using the lungs of middle-aged (15 to 20 yr) and aged (>28 yr) monkeys was examined. Further details about the monkeys used in the current study are presented in Table 1.
Table 1.
Information about the monkeys whose samples were used in the current study
| Age at Death and Sample Collection, yr | Age Group | |
|---|---|---|
| Monkey no. 1 | 19.7 | Middle-aged |
| Monkey no. 2 | 18.7 | Middle-aged |
| Monkey no. 3 | 19.4 | Middle-aged |
| Monkey no. 4 | 18.9 | Middle-aged |
| Monkey no. 5 | 16.9 | Middle-aged |
| Monkey no. 6 | 28.0 | Aged |
| Monkey no. 7 | 29.0 | Aged |
| Monkey no. 8 | 30.4 | Aged |
| Monkey no. 9 | 28.6 | Aged |
| Monkey no. 10 | 30.6 | Aged |
| Monkey no. 11 | 31.9 | Aged |
Collection of bronchoalveolar lavage fluid.
Bronchoalveolar lavage (BAL) fluid was collected as described previously (12). Briefly, after the mice were euthanized, the trachea was exposed. A catheter was inserted inside the trachea and whole lung lavage was performed using ice-cold sterile PBS. BAL fluid (2–2.5 ml) was centrifuged at 400 g for 10 min at 4°C. The supernatants were removed and stored at −80°C until use. In Western blot analysis, 15 μl of BAL fluid supernatants from each sample boiled in Laemmli 4× SDS sample buffer (Boston BioProducts, Worcester, MA) were applied to each lane in polyacrylamide gel.
Blood collection and analysis.
Blood samples from mice were collected by cardiac puncture. Complete blood count was performed using an automated blood counter.
Lung mechanics analysis.
Mice were sedated by intraperitoneal administration of dexmedetomidine 10 min before the treatment with anesthetic agents ketamine and xylazine. The trachea was cannulated using 18-G catheter. Note that for each mouse a new catheter was prepared and calibrated before use. Immediately after tracheal cannulation, the catheter was attached to a FlexiVent ventilator (SCIREQ, Montreal, QC, Canada). Mice were then anesthetized using isoflurane (3–4% induction, 1–2% maintenance/inhalation) delivered with oxygen using a calibrated vaporizer. Initially a “Deep Inflation” perturbation was run to verify that the cannula had been properly inserted without obstruction or misplacement. Then, a sequence of baseline measurement (SnapShot-150, Quick Prime-3 and pressure-driven perturbation) was run in triplicate. This protocol was repeated twice. Data were obtained from five mice (7–9 wk of age) and four mice (13–14 mo of age). The following parameters were shown as results: static compliance (Cst), respiratory system elastance (Ers), tissue elastance (H), tissue damping (G), Newtonian resistance (Rn), and respiratory system resistance (Rrs).
Western blot analysis.
Preparation of whole tissue lysates was performed as described previously (12). Briefly, lung tissue samples that had been stored in liquid nitrogen dewars were thawed and mechanically homogenized in lysis buffer supplemented with protease and phosphatase inhibitors. After two freeze-thaw cycles, the lung homogenates were centrifuged at 15,000 g for 10 min at 4°C. Supernatants were collected and stored at −80°C until use. Protein concentrations were determined by BCA assay (cat. no. 23227; Pierce, Rockford, IL). Supernatants from lung homogenates were boiled in Laemmli 4× SDS sample buffer, and equal amounts of protein were subjected to SDS-PAGE. Proteins were then transferred onto polyvinylidene difluoride or nitrocellulose membranes. The primary antibodies used in this study were rabbit anti-prosurfactant protein C (pro-SPC) antibody (cat. no. AB3786; EMD Millipore, Temecula, CA), goat anti-CCSP antibody (cat. no. sc-9772; Santa Cruz Biotechnology, Dallas, TX), rabbit anti-p63/CKAP4 antibody (cat. no. ab84712; Abcam, Cambridge, MA), rabbit anti-p21 antibody (cat. no. ab109199; Abcam), rabbit anti-Cdc42 antibody (cat. no. ab64533; Abcam), rabbit anti-ΔNp63 antibody (cat. no. ABS552; MilliporeSigma, St. Louis, MO), rabbit anti-Ki67 antibody (cat. no. AB9260; MilliporeSigma), rabbit anti-claudin-10 antibody (cat. no. 38–8400; Life Technologies, Carlsbad, CA), rabbit anti-α-tubulin antibody (cat. no. 11224-1-AP; ProteinTech, Chicago, IL), rabbit anti-TBP antibody (cat. no. 22006-1-AP, ProteinTech), rabbit anti-alpha-1-anti-trypsin antibody (cat. no. sc-69752; Santa Cruz Biotechnology), horseradish peroxidase (HRP)-conjugated rabbit anti-β-actin antibody (cat. no. 5125S; Cell Signaling, Danvers, MA), HRP-conjugated rabbit anti-GAPDH antibody (cat. no. 3683; Cell Signaling), and rabbit anti-histone H3 antibody (cat. no. 4620S; Cell Signaling). The secondary antibodies used in this study are HRP-conjugated donkey anti-rabbit IgG antibody, HRP-conjugated donkey anti-mouse IgG antibody, HRP-conjugated donkey anti-goat IgG antibody (Thermo Fisher Scientific), and HRP-conjugated goat anti-rabbit IgG antibody (Jackson Laboratories, Bar Harbor, ME). The proteins were visualized using Pierce ECL Western blotting substrate, SuperSignal West Femto maximum sensitivity substrate (Thermo Fisher Scientific) or KwikQuant Ultra Digital-ECLTM Substrate Solution (Kindle Biosciences). Note that p63 antibody (ab84712), one of the two p63 antibodies used in the current study, targets the central DNA-binding domain of p63 shared among all p63 isoforms, while the other p63 antibody (ABS552) targets NH2-terminal transcription activation domain that is solely seen in NH2-terminal-truncated (ΔNp63) forms.
Preparation of frozen lung sections.
The left lobes of mouse lungs collected from 7- to 9-wk-old and 14-mo-old mice were fixed in 4% paraformaldehyde in PBS and then processed in sucrose gradients (10%, 20% and 30%). The tissues were snap-frozen by immersion in a plastic cup filled with 2-methylbutane (Thermo Fisher Scientific) prechilled with liquid nitrogen and stored in an ultra-low temperature (−80°C) freezer until sectioning. Cryosections, 7- to 8-μm thickness, were prepared using cryostat, mounted onto positively charged slides (Superfrost Plus microscope slides, Thermo Fisher Scientific), and stored at an ultra-low temperature (−80°C) freezer until staining.
Immunohistochemical staining.
Cryosections that had been stored at −80°C were thawed at room temperature in PBS buffer. Sections were subjected to heat-induced antigen retrieval (HIAR) in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, and 0.05% Tween 20, pH 9.0) at 60°C for 5 min. Then, endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in PBS for 10 min. After blocking with PBS containing 5% bovine serum albumin plus 0.25% Tween 20, sections were incubated at 4°C overnight with rabbit ΔNp63 antibody (cat. no. ABS552; MilliporeSigma). The sections were then incubated for 60 min at room temperature with HRP-conjugated donkey anti-rabbit IgG antibody (cat. no. A16305; Thermo Fisher Scientific). For single-color staining, the signals for ΔNp63 were imaged at this point by using PolyDetector HRP Green Kit (Bio-SB, Santa Barbara, CA). Colocalization of ΔNp63 and CCSP proteins in the same frozen sections were examined using two different methods: simultaneous and sequential double labeling. In the former method, following the HIAR, quenching, and blocking steps as described above, sections were incubated with primary and secondary antibodies for each of ΔNp63 and CCSP in the order as follows: 1) rabbit anti-ΔNp63 antibody (cat. no. ABS552, MilliporeSigma); 2) HRP-conjugated donkey anti-rabbit IgG antibody; 3) goat anti-CCSP antibody (cat. no. sc-9772; Santa Cruz Biotechnology); and 4) ALP-conjugated donkey anti-goat IgG antibody (Thermo Fisher Scientific). ΔNp63 signals were first visualized using PolyDetector HRP Green Kit. With ΔNp63 signals left on the lung sections, CCSP signals were superimposed using ImmPACT Vector Red Alkaline Phosphatase Substrate (Vector Laboratories, Burlingame, CA). In the latter method, following HIAR, quenching, and blocking steps as described above, sections were incubated with primary and secondary antibodies for ΔNp63, and signals were developed using PolyDetector HRP Green Kit (1st round labeling). After imaging, the signals were removed by extensive washing. The sections were sequentially incubated with primary and secondary antibodies for CCSP. Signals for CCSP were developed using ImmPACT Vector Red Alkaline Phosphatase Substrate (Vector Laboratories; 2nd round labeling). The same fields as imaged in the 1st round staining were examined under microscope so that each marker could be colocalized. Digital images were taken using a microscope (Olympus BX43; Olympus, Tokyo, Japan) connected to an Olympus DP21 digital camera.
Preparation of nuclear and cytoplasmic protein extracts.
Nuclear and cytoplasmic extracts from mouse lung tissues were prepared using nuclear extraction kit (cat. no. 40010; Active Motif, Carlsbad, CA) and Biomasher II tissue grinder (Kimble Chase, Vineland, NJ). Briefly, lung tissues were minced into small pieces using disposable stainless steel blades and gently homogenized in hypotonic buffer. Effective nuclear separation in hypotonic buffer was confirmed by the observation of the homogenized samples under a phase contrast microscope at ×400 magnification. Samples were then centrifuged at 14,000 g for 30 s at 4°C. Supernatants were removed as Cyto-I. The remaining nuclear pellets were gently resuspended in an additional hypotonic buffer, centrifuged at 14,000 g for 30 s at 4°C. The supernatants were removed as Cyto-II. Cyto-I and Cyto-II were mixed together and centrifuged at 14,000 g for 10 min at 4°C. Then, supernatants were removed as cytoplasmic fractions. Nuclear pellets were mixed with nuclear lysis buffer and vortexed at 4°C. Once effective nuclear lysis was confirmed via microscopy, the nuclear lysates were centrifuged at 14,000 g for 10 min at 4°C. Supernatants were taken as nuclear fractions. Both cytoplasmic and nuclear fractions were stored at −80°C until use. Protein concentrations in both fractions were determined by BCA assay (cat. no. 23227; Pierce).
Total RNA extraction.
Total RNA from lungs of mice aged 7–9 wk and 14 mo was extracted as described previously (29). Briefly, lung samples were homogenized in TRIzol (Thermo Fisher Scientific), which was followed by the extraction of aqueous phase using chloroform and precipitation of RNA using isopropanol. The precipitated RNA was dissolved in RNase-free water and subjected to purification using RNeasy kit (Qiagen, Valencia, CA). The quality of RNA was assessed by Nanodrop.
Quantitative real-time RT-PCR analysis.
Quantitative real-time RT-PCR (qRT-PCR) analysis was performed as described previously (29). Briefly, 1 μg of total RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). qRT-PCR was run with the SsoFast EvaGreen Supermix kit (Bio-Rad) and gene-specific primers using the Bio-Rad CFX96 Real-time system (C1000 Thermal Cycler). A relative fold change in gene transcript level was determined using the Bio-Rad CFX Manager software, applying the comparative CT method (ΔCT) and expressed as 2−ΔΔCT and using 18S as an internal control. The primers used for qRT-PCR were as follows: CCSP forward: 5′-ACATCACCCCACATCTACAGACACCAA-3′; CCSP reverse: 5′-TGAGGAGGGCCTCAAGGACTTGAA-3′; 18S forward: 5′-ACCTGGTTGATCCTGCCAGTAG-3′; and 18S reverse: 5′-TTAATGAGCCATTCGCAGTTTC-3′.
Statistical analysis.
Statistical analysis was performed using Graph-Pad Prism 5 (GraphPad Software, San Diego, CA). Comparison of variables between two groups was performed using Student’s t-test. All tests were two-tailed, and values of P < 0.05 were considered significant.
RESULTS
Aging accompanies emergence of hypercellular regions in the bronchovascular interstitium in mouse lungs.
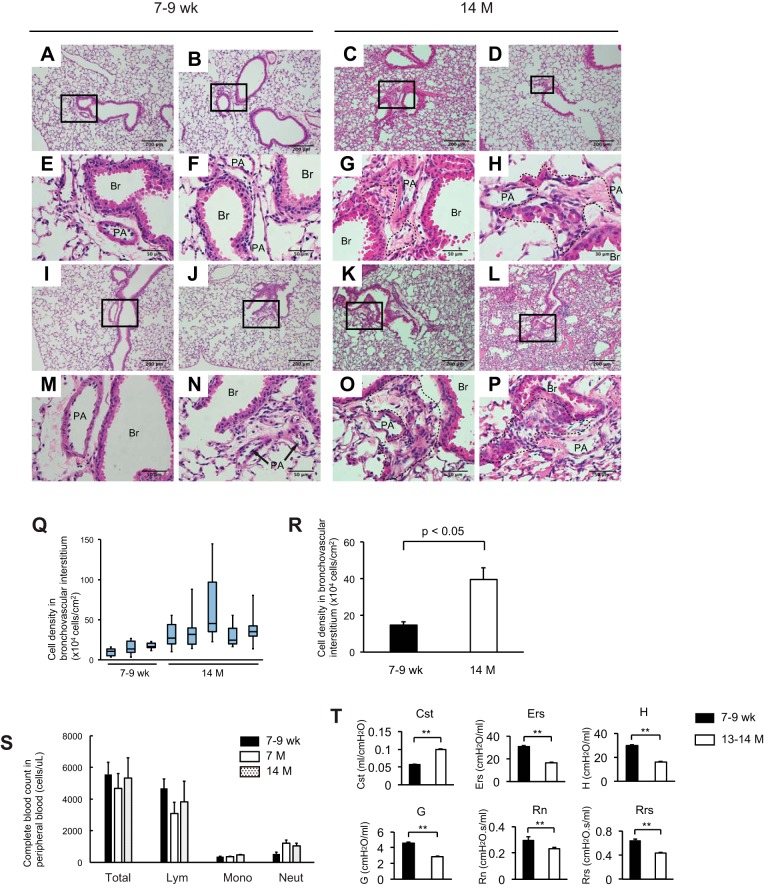
Studies reveal that a hierarchy of tissue-specific stem cell populations exists in each organ and that some reside in specific areas, e.g., perivascular regions. We first sought to determine if lung aging accompanies any histological alterations in the perivascular areas of mouse lungs by studying hematoxylin and eosin (H&E)-stained lung sections from 7 to 9-wk-old and 14-mo-old mice. Lungs of 14-mo-old mice exhibited hypercellular regions in the bronchovascular interstitium (areas circled by dashed lines in Fig. 1, G, H, O, and P). These hypercellular regions are nonuniform and frequently contain cells with different morphologies: elongated cells reminiscent of fibroblasts and cuboidal cells morphologically similar to nearby bronchiolar epithelial cells (Fig. 1O). These observations suggest that both mesenchymal and epithelial cells are involved in the development of such age-related hypercellular regions. The cell density of the bronchovascular interstitium in 14-mo-old group is significantly higher when compared with 7- to 9-wk-old group (Fig. 1, Q and R). A complete blood count (CBC) was performed using peripheral blood samples of 7- to 9-wk-old and 7-mo- and 14-mo-old mice to determine if systemic inflammation accompanies this histological alteration. The results show that there is no significant difference in the number of each immune cell type between the three groups (Fig. 1S). This suggests that systemic inflammation is not related to the observed age-associated histological alteration. In contrast, lung functions of 13- to 14-mo-old mice were totally different from 7- to 9-wk-old group (Fig. 1T). It is hypothesized that nonuniform and heterogeneous hypercellular regions are manifestations of an age-associated event in the lung involving, but not limited to, the expansion of lung-specific progenitor cells and altered lung functions.
Fig. 1.
Aging accompanies emergence of hypercellular regions in the bronchovascular interstitium of mouse lungs. A–P: paraffin-embedded lung tissue sections from 7- to 9-wk-old (A, B, E, F, I, J, M, and N) and 14-mo-old (C, D, G, H, K, L, O, and P) wild-type mice were subjected to hematoxylin and eosin (H&E) staining. E, F, G, H, M, N, O, and P are magnified views of the boxed regions in A, B, C, D, I, J, K, and L, respectively. Original magnification: ×100 (A–D and I–L) and ×400 (E–H and M–P). Br, bronchiolar lumen; PA, pulmonary artery. Q: cell density in each of randomly chosen high-power fields (8 or more fields from each H&E-stained slide, magnification: ×400) was calculated and the distribution of the calculated values for each mouse was shown as a box-whisker plot. Each box shows the upper and lower quartile with the central bar representing the median and the whiskers showing the minimum and maximum. R: the cell density in bronchovascular interstitium for each age group, 7- to 9-wk-old and 14-mo-old, is expressed as mean ± SE. S: peripheral blood samples from 7- to 9-wk-old and 7-mo- and 14-mo-old WT mice (n = 3–4 per each age group) were subjected to complete blood cell count. Lym, lymphocytes; Mono, monocytes; Neut, neutrophils. Results are expressed as means ± SE. T: lung functions were measured using live mice following sedation and tracheal cannulation. 7- to 9-wk-old (n = 5) and 13- to 14-mo-old (n = 4) mice were subjected to analysis. Cst, static compliance; Ers, respiratory system elastance; H, tissue elastance; G, tissue damping; Rn, Newtonian resistance; Rrs, respiratory system resistance. Results are expressed as means ± SE. Data presented are representative of one experiment. **P < 0.01.
Aging leads to altered protein expression profiles of p63 isoforms in lungs of mice.
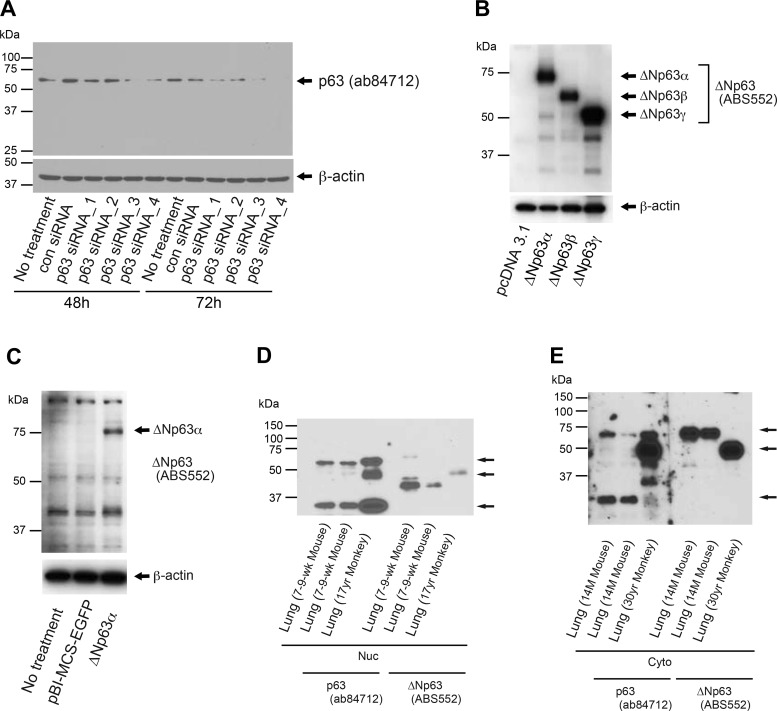
The p63 protein has multiple isoforms. Therefore, two different p63-targeting antibodies were evaluated and characterized in an attempt to eliminate the nonspecific binding effects of p63 antibodies. The human embryonic kidney 293 (HEK293) cell line coupled with siRNAs-based knockdown system was used to verify the specificity of the p63 antibody (cat. no. ab84712; Abcam) because the cell line expresses p63 and the target amino acid sequence of this antibody, located within the DNA-binding domain, is highly conserved among different species including, but not limited to, the human and mouse. The results show that this antibody detected a single band around the molecular mass of 65 kDa (Fig. 2A). The intensities of the bands are decreased in the cells treated with p63-targeting siRNAs compared with those not treated or those treated with nontargeting siRNAs indicating that p63 antibody (ab84712) detects a specific isoform of the p63 protein. The H441 human lung epithelial cell line coupled with plasmid-based overexpression system was used to verify the specificity of the ΔNp63 antibody (cat. no. ABS552; MilliporeSigma). The results show that this antibody clearly detects ΔNp63α, ΔNp63β, and ΔNp63γ isoforms even though there were several nonspecific bands detected as well (Fig. 2, B and C). Western blotting for nuclear fractions from mouse and monkey lung tissues, using the above two antibodies, was conducted to further characterize these antibodies. The results show two major bands detected by p63 antibody (ab84712) that are readily detectable both in mouse and monkey samples: one at ~65 kDa and the other 37 kDa (Fig. 2D, top and bottom arrows, respectively). It is possible that two distinct isoforms of transcriptionally active p63 (TAp63) are expressed in lung basal cells since ΔNp63 (ABS552) antibody did not show clear bands at the same molecular mass. Both of the antibodies detected a 50-kDa band in monkey samples (Fig. 2D, middle band). It is possible that this represent a ΔNp63 isoform; however, in mouse samples this isoform was not clearly detected. Likewise, it is postulated that there are nonspecific bands between 37 and 50 kDa, detected by ΔNp63 antibody (ABS552), in nuclear fractions of mouse lungs. We further examined the above two antibodies by Western blotting for cytoplasmic fractions from mouse and monkey lung tissues. The results show multiple clear bands at the same molecular mass between the two antibodies between 50 and 75 kDa (Fig. 2E, top and middle arrows). These are considered to represent ΔNp63 isoforms. The bands seen at ~75 kDa (Fig. 2E, top band) are considered to represent ΔNp63α in reference to the molecular mass of the ΔNp63α proteins overexpressed in H441 cells (Fig. 2, B and C). Next, cytoplasmic and nuclear protein extracts from different organs of mice were Western blotted using the same p63 antibodies to see if such differential expression pattern of p63 isoforms in nucleus and cytoplasm as observed in lungs of mice is shared among different organs. The results show that the expression patterns of p63 isoforms from different organs of mice are similar to each other (Supplemental Fig. S1, A and B; Supplemental Material for this article is available online at https://doi.org/10.6084/m9.figshare.7545161). Collectively, we concluded that both the p63 antibody (ab84712) and ΔNp63 antibody (ABS552) have good specificity against p63 proteins even though nonspecific binding is still encountered.
Fig. 2.
Specificity evaluation for the two p63 antibodies and their profiles when used in Western blotting. A: specificity of p63 antibody (ab84712) was evaluated by Western blot analysis of p63 protein after transfection in HEK293 cells of p63-targeting or control siRNAs. Whole cell lysates were collected 48 and 72 h after siRNA transfection. Equal amounts of protein (15 μg) were loaded per lane. B and C: specificity of ΔNp63 antibody (ABS552) was evaluated by Western blot analysis. Twenty-four hours after transfecting overexpression plasmid or empty vector into H441 cells, whole protein lysates were prepared. Equal amounts of protein (1.5 μg/lane in B; 4.5 μg/lane in C) were loaded. D and E: Western blot analysis of p63 using cytoplasmic (Cyto) and nuclear (Nuc) protein extracts from lungs of mice and monkeys. A specific set of samples was loaded in duplicate for each of D and E. Each piece of the membrane containing a set of sample was probed separately. At development, the membranes were put back together into original shape. Equal amounts of protein (6.0 μg/lane) were loaded. The ages of the animals that the protein samples were collected from are 7–9 wk old for “Lung (7-9-wk Mouse)” and 17 yr old for “Lung (17yr Monkey)” in D; 14 mo old for “Lung (14M Mouse)” and 30 yr old for “Lung (30yr Monkey)” in E. Data presented are representative of two independent experiments in A–C and three independent experiments in D and E, respectively.
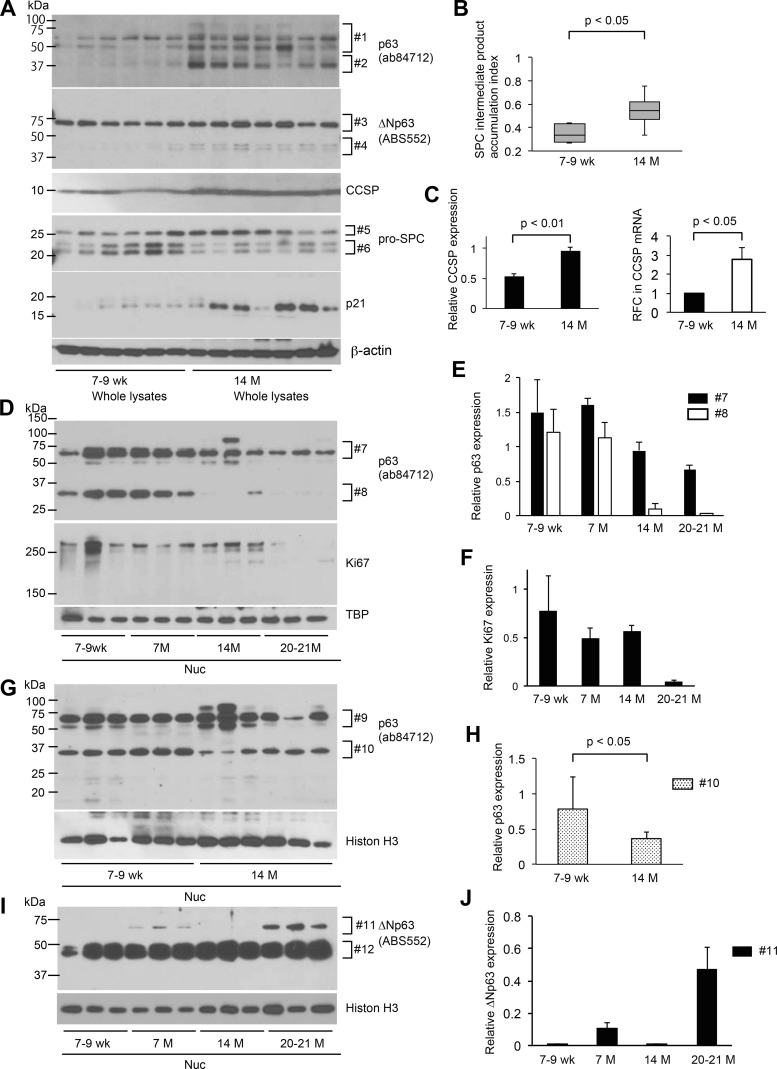
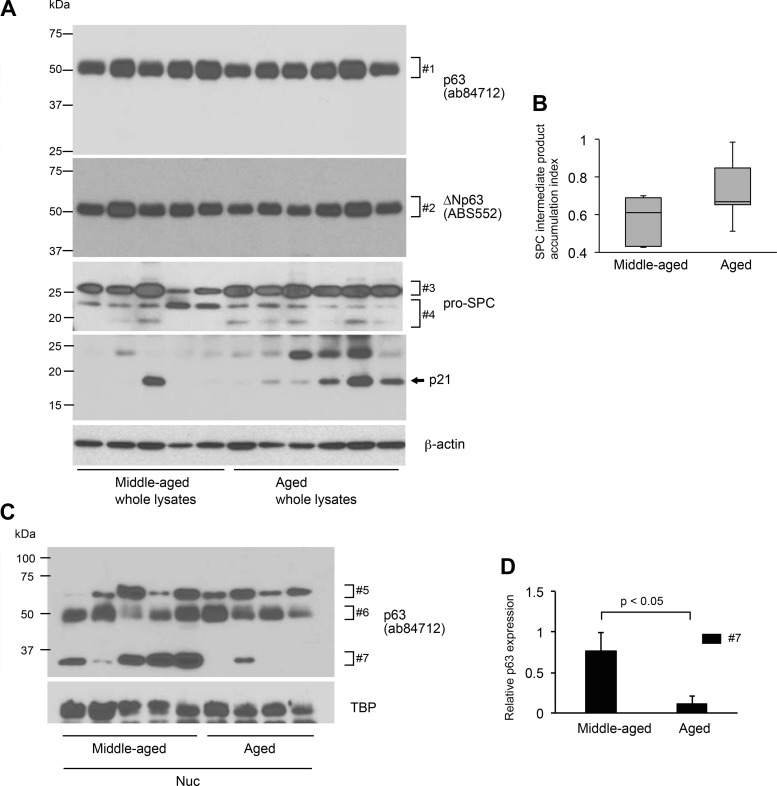
C57BL/6J mice spontaneously develop nonuniform hypercellular regions in the bronchovascular interstitium at 14 mo of age, as already demonstrated by the semiquantitative use of H&E-stained lung sections of 7- to 9-wk-old and 14-mo-old mice (Fig. 1). Likewise, Western blotting was performed using nonfractionated whole lysates of lung tissues from 7 to 9-wk-old and 14-mo-old mice to determine if total expression and/or expression patterns of lineage markers for lung-specific progenitor cells are altered in an age-dependent manner. Representative lineage markers used were as follows: p63 (basal cells), CCSP (club cells), and pro-SPC [type II alveolar epithelial cells (AECs)]. There are three major bands detected with the use of pro-SPC antibodies (Fig. 3A). Considering the previously demonstrated processing mechanism of pro-SPC, it is postulated that the middle and bottom bands (Fig. 3A, #6) represent 21-kDa precursors and the top band (Fig. 3A, #5) a 24-kDa intermediate product (referred to as SPC-IP hereinafter) in the conversion process from 21-kDa precursor to a further mature form of surfactant protein C (28). The SPC-IP index was defined to evaluate the relative accumulation of SPC-IP. Details are described in the Fig. 3 legend. The SPC-IP index of 14-mo-old versus 7- to 9-wk-old group is significantly higher (Fig. 3B). In addition, CCSP expression is significantly increased in 14-mo-old versus 7- to 9-wk-old mice (Fig. 3, A and C). These results suggest that the posttranslational processing of pro-SPC in type II AECs and the expression of CCSP proteins in club cells alter in an age-dependent manner and that these alterations begin before the mouse reaches 14 mo of age. Western blotting using p63 antibody (ab84712) reveals a clear difference between 7- and 9-wk-old versus 14-mo-old mice. The latter shows several bands with higher intensities than the former, which span a broad molecular mass range, ~37 to 75 kDa (Fig. 3A, #1 and #2). The results of Western blotting with the use of ΔNp63 antibody (ABS552) show a slight difference between the two age groups with minor bands, between 37 and 50 kDa, being higher in density in 14-mo-old mice (Fig. 3A, #3 and #4). The expression of p21, a representative senescence marker, was also evaluated to determine if 14-mo-old mice are suitable to study aging lungs. The results show that p21 expression is remarkably high in four versus the other three mice in the aged group and mice in the 7- to 9-wk-old group (Fig. 3A). These results suggest that aging of the lung begins as early as 14 mo old in mice and that there is a considerable difference in the time at which lungs age among individual mice. Collectively, it is proposed that protein expression profiles of lineage markers for lung progenitor cells alter as mice age.
Fig. 3.
Aging leads to decreased nuclear expression of p63 isoforms in lungs of mice. A: Western blot analysis using whole tissue lysates of lungs from wild-type mice at 7- to 9-wk-old and 14-mo-old WT mice. Equal amounts of protein (15 μg) were loaded per lane. B: surfactant protein C intermediate product accumulation index (SPC-IP index) defined as follows was calculated for each age group and results are shown: SPC-IP index equals band intensity of the top band (#5) divided by sum of the band intensities of all the 3 bands (#5 plus #6) detected by anti-pro-SPC antibody. Results are shown as box-whisker plots. Each box shows the upper and lower quartile with the central bar representing the median and the whiskers showing the minimum and maximum. C, left: expression of CCSP in A was normalized to β-actin and presented in arbitrary units. Results shown are the means ± SE. C, right: mRNA levels in the whole lysates of the lungs were measured by quantitative RT-PCR. Three mice per each group were tested. The error bar represents standard deviation. D, G, and I: Western blot analysis using nuclear extracts prepared from lungs of WT mice at different ages. Each lane represents a sample from an individual mouse. Equal amounts of protein (6 μg) were loaded per lane. E: expression of two major isoforms of p63 as detected by anti-p63 antibody (ab84712; D, #7 and #8) was normalized respectively to TATA-binding protein (TBP) and is presented in arbitrary units. Results shown are the means ± SE. F: expression of Ki67 shown in D was normalized to TBP and presented in arbitrary units. Results shown are the means ± SE. H: expression of p63 isoform #10 was normalized to histone H3 and presented in arbitrary units. Results shown are the means ± SE. J: expression of p63 isoform #11 was normalized to histone H3 and presented in arbitrary units. Results shown are the means ± SE. Data presented are representative of two independent experiments.
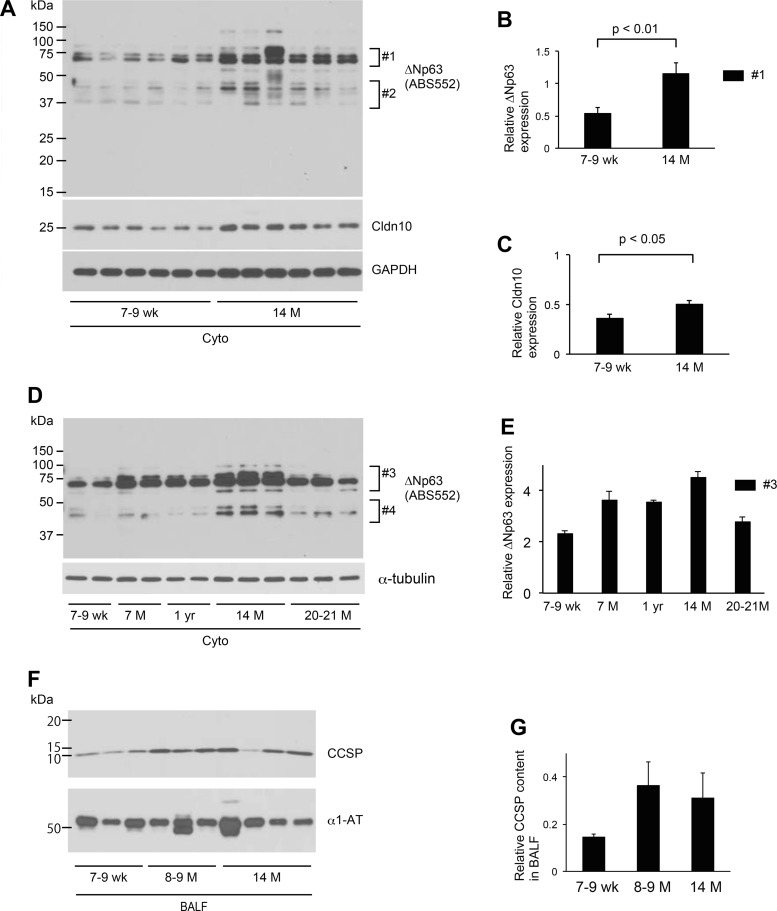
Nuclear fractions from lungs of 7- to 9-wk-old and 7-mo, 14-mo, and 20- to 21-mo-old mice were collected, and a Western blot assay using p63 antibody (ab84712) done to investigate if alterations in band patterns of p63 in whole lysates of lungs reflect age-dependent appearance and/or accumulation of specific p63 isoforms in the nucleus. Two easily recognized bands show a dramatic decrease in an age-dependent manner (Fig. 3D, #7 and #8; Fig. 3E). The intensity of the band with the smaller molecular mass decreased significantly at 14 mo versus 7–9 wk (Fig. 3G, #10; Fig. 3H). They were considered to be isoforms of TAp63 since these p63 isoforms were not as well defined by use of the ΔNp63 antibody (ABS552) (Fig. 3I). Nuclear fractions, when probed with ΔNp63 antibody (ABS552), showed one sharp band and one band clusters (Fig. 3I). The former increased markedly after age 14 mo (Fig. 3I, #11; Fig. 3J) in contrast to the decrease in this isoform in cytoplasmic fractions of mouse lungs after age 14 mo (Fig. 4D, #3). Cytoplasmic fractions, when probed with ΔNp63 antibody (ABS552), showed two band clusters, one spanning from 37 to 50 kDa and the other, 50 to 75 kDa (Fig. 4, A and D). They show 1) an age-dependent increase up to age 14 mo; and 2) a decrease after age 14 mo (Fig. 4, A and B, #1; Fig. 4D, #3; Fig. 4E). These are compatible with the result showing that whole lysates of lung tissues from 14-mo-old mice show several bands with higher intensities than 7- to 9-wk-old mice (Fig. 3A). Considering the fact that the complex protein expression profile of p63 varies with age, isoform, and location, we postulated that ΔNp63 isoforms is regulated differently from TAp63 isoforms and that aging has pleiotropic effects on the expression of each p63 isoform in the lung.
Fig. 4.
Aging leads to increased cytoplasmic expression of Δp63 isoforms in lungs of mice. A and D: Western blot analysis using cytoplasmic extracts prepared from lungs of WT mice at different ages. Each lane represents a sample from an individual mouse. Equal amounts of protein (6 μg) were loaded per lane. B: expression of p63 isoforms detected by anti-ΔNp63 antibody (ABS552; #1) was normalized to GAPDH and presented in arbitrary units. Results shown are the means ± SE. C: expression of claudin-10 (cldn10) shown in A was normalized to GAPDH and presented in arbitrary units. Results shown are the means ± SE. E: expression of p63 isoforms detected by anti-ΔNp63 antibody (ABS552) (#3 in D) was normalized to α-tubulin and presented in arbitrary units. Results shown are the means ± SE. F: the concentrations of CCSP and α1-antitrypsin (α1-AT) in bronchoalveolar lavage (BAL) fluid (n = 3–4 per each age group) were estimated by Western blotting. Equivalent volume of BAL fluid was loaded per lane. G: relative CCSP content in BAL fluid was normalized to α1-AT and presented in arbitrary units. Results shown are the means ± SE. 7 M: 7 mo; 8–9 M: 8–9 mo; 14 M: 14 mo; 20–21 M: 20–21 mo. Data presented are representative of two independent experiments.
The p63 gene expressed in stem cells of many stratified epithelia has been associated with their proliferation. Therefore, we examined the expression of Ki67, a representative nuclear protein associated with cell proliferation, to determine the relationship between these age-associated alterations in differential expression of p63 isoforms and cell proliferation. The results show that the expression of Ki67 is stable up to the age of 14 mo but dramatically decreases thereafter (Fig. 3, D and F). This age-associated decline pattern of Ki67 is similar to that of p63 isoforms in the nucleus, suggesting that p63 protein in the nucleus regulates the ability of the progenitor cell in the distal lungs of mice to proliferate. The content of CCSP in air space using bronchoalveolar lavage (BAL) fluid was determined to corroborate the age-associated CCSP increase in whole lysates of lung tissues. The results of Western blotting displayed higher CCSP content in BAL fluid in 8- to 9-mo- and 14-mo-old versus 7- to 9-wk-old mice (Fig. 4, F and G). We also determined the expression of claudin-10 (cldn10; another marker for club cells) in cytoplasmic fractions. According to the CCSP increase in whole lung lysates and air space in 14-mo-old mice, the expression of cldn10 showed significant increase in cytoplasmic extracts of lungs from 14-mo-old mice compared with 7- to 9-wk-old mice (Fig. 4, A and C). These results indicate that the number and/or function of club cells change with age and that this age-associated alteration begins before the mouse reaches 14 mo of age.
Aging leads to decreased expression of TAp63 in the nucleus in lungs of monkeys.
The distribution of basal and club cells in the lungs of primates versus rodents differs (26, 31). Therefore, the experimental result that progenitor cell profiles alter with age needs to be reproduced by an experiment using primate lung samples. Western blotting using protein extracts of the lungs from rhesus monkeys was performed to determine if the age-associated alterations in lineage marker expression observed in mouse lungs also occur in primate lungs. Whole lung lysates, when probed with the p63 antibody (ab84712), show strong bands at ~50 kDa (Fig. 5A, #1). The major bands observed at ~50 kDa were considered to be an isoform of ΔNp63, since ΔNp63 antibody (ABS552) detects a band at similar molecular mass (Fig. 5A, #2). No age-associated alterations were observed regarding the expression of this 50-kDa ΔNp63 isoform (Fig. 5A, #1 and #2). Conversely, nuclear fractions from monkey lungs, when probed with p63 antibody (ab84712), revealed sharp bands in the middle-aged group with a molecular mass slightly lower than 37 kDa (Fig. 5C, #7). The expression of this p63 isoform, which is thought to be TAp63, is significantly decreased in the aged versus middle-aged group (Fig. 5D). This suggests that an age-associated decrease in the expression of a specific TAp63 isoform as observed in monkey lungs has similar molecular mechanism as is observed in mouse lungs (Fig. 3, D, E, G, and H). This also suggests that the altered protein expression profile of p63, specifically TAp63 isoforms, is related to the senescence of p63-positive progenitor cells in the lung. The protein expression profile of pro-SPC by Western blotting using whole lung lysates of monkey lungs was also examined. The results show that the SPC-IP index of aged versus the middle-aged group is higher (Fig. 5B) similar to the result obtained with mouse lungs (Fig. 3B). This suggests that the aging of lungs elicits a shift in the maturation process of pulmonary surfactant protein C. The expression of p21, a representative senescence marker, was also evaluated. The results show that p21 expression is remarkably high in one middle-aged and three aged versus the other four middle-aged and three aged monkeys (Fig. 5A), suggesting that there is a considerable difference in the time at which lungs age among individual monkeys.
Fig. 5.
Aging leads to decreased nuclear expression of p63 isoforms and altered protein expression profile of surfactant protein C in lungs of monkeys. A: Western blot analysis using whole tissue lysates of lungs from middle-aged (n = 5) and aged monkeys (n = 6). Each lane represents a sample from an individual monkey. B: surfactant protein C intermediate product accumulation index (SPC-IP index) defined as follows was calculated for each lane and results are shown: SPC-IP index equals band intensity of the top band (#3) divided by sum of the band intensities of all the three bands (#3 plus #4) detected by anti-pro-SPC antibody. Results are shown as box-whisker plot. Each box shows the upper and lower quartile with the central bar representing the median and the whiskers showing the minimum and maximum. C: Western blot analysis using nuclear extracts prepared from lungs of middle-aged (n = 5) and aged monkeys (n = 4). Each lane represents a sample from an individual monkey. D: expression of a p63 isoform detected by anti-p63 antibody (ab84712; C, #7) was normalized to TATA-binding protein (TBP) and presented in arbitrary units. Results shown are the means ± SE. Middle aged: 17–20 yr old; aged: 28–32 yr old. Data presented are representative of two independent experiments.
Patchy expansion of ΔNp63-positive CCSP-positive cells in bronchiolar epithelium develops with age.
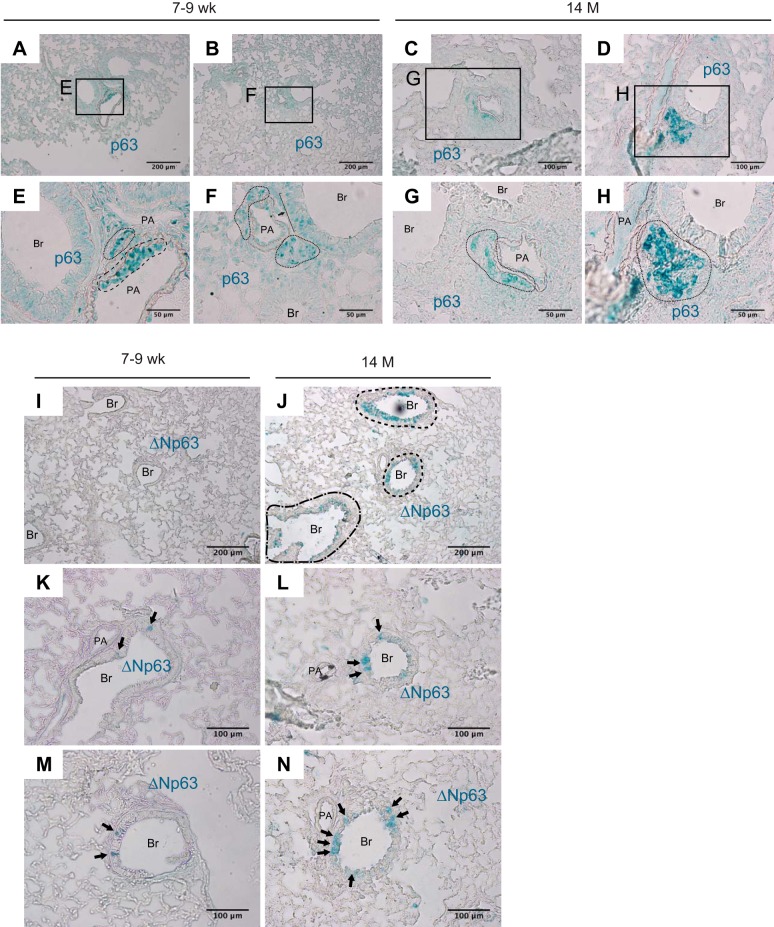
Western blot data using protein lysates from the lungs of mice at different ages demonstrate that 1) the expression of TAp63 isoforms in nuclear fraction decreases with age, and, in stark contrast, 2) ΔNp63 isoforms in cytoplasmic fraction increase until age 14 mo. However, it is unclear where TAp63 and ΔNp63 proteins localize in the distal lungs of mice. It is also not understood how the expression patterns of TAp63 and ΔNp63 change with age. Immunohistochemically labeled frozen lung sections of mice aged 7–9 wk and 14 mo, using p63 antibody (ab84712) and/or ΔNp63 antibody (ABS552Np63 antibody (ABS552), were utilized to answer these questions. Single staining of lungs from 7- to 9-wk-old mice using p63 antibody (ab84712) display patchy and apparently random signals in the bronchovascular interstitium and bronchiolar epithelium (Fig. 6, A, B, E, and F). Patchy signals were also detected in the bronchovascular interstitium and bronchiolar epithelium of mice at age 14 mo (Fig. 6, C, D, G and H). Note that the intensity of p63 signal in bronchiolar epithelium in 14-mo-old mice is relatively weak compared with 7- to 9-wk-old mice. p63 signals are also occasionally encountered in cytoplasm of the cells located by pulmonary veins in 14-mo-old mice (data not shown). Next, lung sections were labeled using ΔNp63 antibody (ABS552). Immunohistochemical labeling of lungs from 7- to 9-wk-old mice with ΔNp63 antibody display patchy signals in bronchiolar epithelial cells (Fig. 6, I, K, and M). Many of these cells display cuboidal morphology and are spotted beneath the basal membrane. In contrast, lungs from 14-mo-old versus 7- to 9-wk-old mice display a wider signal distribution (Fig. 6, J, L, and N). These results are compatible with Western blot results in which the expression of ΔNp63 isoforms in 14-mo-old versus 7- to 9-wk-old group was higher.
Fig. 6.
Patchy distribution of ΔNp63-positive bronchiolar epithelial cells expands with age in mice. Frozen lung sections prepared from lungs of 7- to 9-wk-old and 14-mo-old wild-type mice were immunohistochemically labeled using either anti-p63 (ab84712) or anti-ΔNp63 (ABS552) antibody. Representative images are shown out of two independent experiments with 4 mice per group, respectively. E–H are magnified views of the boxed regions in A–D, respectively. A, B, E, and F: in distal parts of the lungs of 7- to 9-wk-old mice, strong p63 signals are seen in the interstitium of bronchovascular bundle (areas circled by dotted lines in E and F) and on the inside walls of the pulmonary artery (area circled by dashed line). Weak signals are detected in the nucleus of the bronchiolar epithelial cells (arrow in F). C, D, G, and H: in distal parts of the lungs of 14-mo-old mice, p63 signals are observed with a scattered distribution in the interstitium of bronchovascular bundle (areas circled by dotted lines in G and H). Signals in the nucleus of bronchiolar epithelial cells are absent or lower compared with 7- to 9-wk-old mice. I, K, and M: in distal parts of the lungs of 7- to 9-wk-old mice, ΔNp63 signals are sparsely observed beneath the basement membrane in bronchiolar epithelium. Most of the ΔNp63-positive cells are observed as a solitary cell (arrows in K and M). J, L, and N: in distal parts of the lungs of 14-mo-old mice, ΔNp63-positive cells are more widely distributed in bronchiolar epithelium and display varied morphology with some cells being cuboidal and others being columnar in shape. The density of ΔNp63-positive cells in terminal and respiratory bronchioles (area circled by dot-dashed line in J) is lower than the more proximal bronchiolar epithelium (areas circled by dashed lines in J). Original magnification: ×100 (A, B, I, and J), ×200 (C, D, and K–N), and ×400 (E–H). Br: bronchiolar lumen, PA: pulmonary artery.
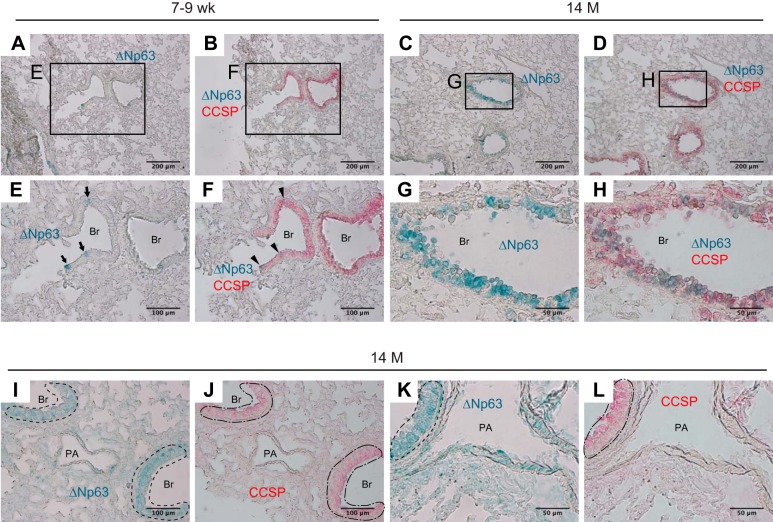
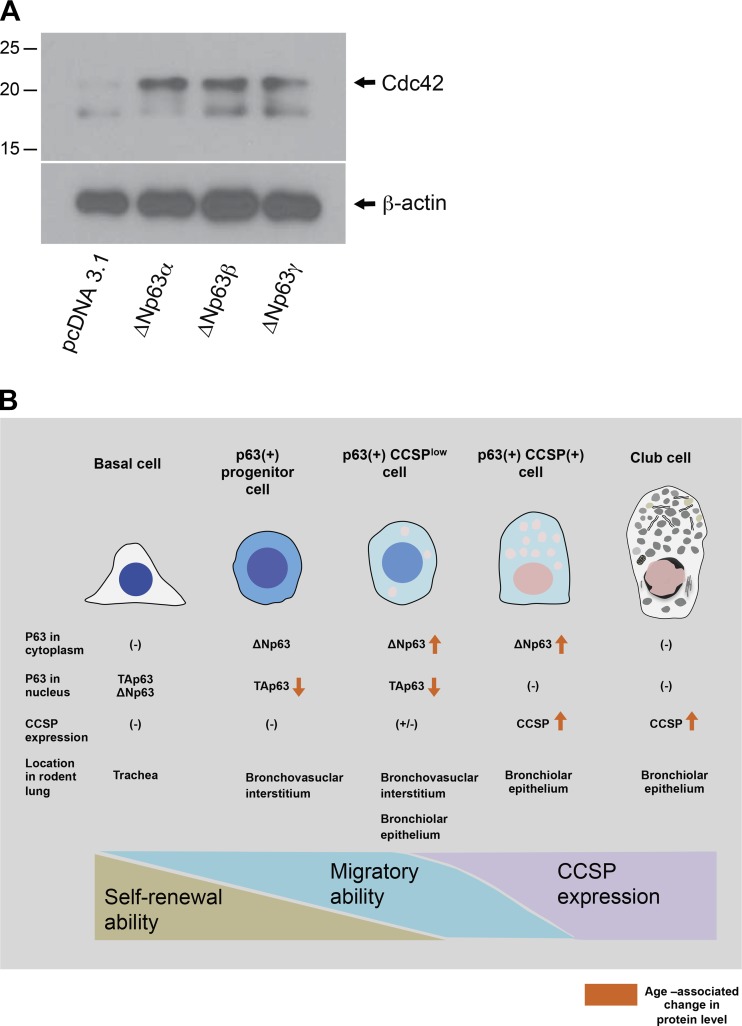
Immunohistochemical double staining for ΔNp63 and CCSP demonstrates that many of ΔNp63-positive bronchiolar epithelial cells are positive for CCSP in both 7- to 9-wk-old (Fig. 7, A, B, E, and F) and 14-mo-old mice (Fig. 7, C, D, G, and H). The bronchiolar epithelium featuring layers of ΔNp63-positive CCSP-positive cells is frequently encountered in the lungs of 14-mo-old but not 7- to 9-wk-old mice (Fig. 7H). These results suggest that aging causes expansion of ΔNp63-positive CCSP-positive epithelial cells in mouse lungs, which mainly occurs in bronchiolar epithelium. It is hypothesized that age-associated expansion of ΔNp63-positive CCSP-positive club cells is mainly due to an active shift of ΔNp63-negative club cells into a new form where they express high levels of ΔNp63 in cytoplasm since Western blot data show that active cell proliferation is not occurring in the lungs of mice at 14 mo of age (Fig. 3, D and F). Sequential double labeling for ΔNp63 and CCSP was performed to further support this hypothesis (Fig. 7, I–L). Interestingly, CCSP signals in the ΔNp63-positive cells that are located adjacent to the basement membrane were relatively low when compared with the double-positive cells that are located at the luminal side (Fig. 7, J and L, dot-dashed lines) while the signals for ΔNp63 were observed at a high intensity in bronchiolar epithelial cells that are adjacent to the basement membrane (Fig. 7, I and K, areas circled in dashed lines). These results indirectly support the hypothesis that the age-associated expansion of ΔNp63-positive CCSP-positive club cells in bronchiolar epithelium is mainly due to a shift from ΔNp63-negative into a ΔNp63-positive status. Next, to determine if upregulation of ΔNp63 isoforms gives club cells motility or not, each of ΔNp63α, β, γ was overexpressed in club cell line H441 by plasmid transfection. The results show that the expression of Cdc42 protein was increased in H441 cells in which each of the ΔNp63 isoforms was overexpressed (Fig. 8A). These results suggest that the hypercellularity of bronchovascular interstitium observed in mice at 14 mo of age is, at least in part, due to the expansion of club cells that has acquired motility through ΔNp63α.
Fig. 7.
Patchy expansion of ΔNp63-positive CCSP-positive cells in bronchiolar epithelium expands with age. Frozen lung sections from 7- to 9-wk-old and 14-mo-old wild-type mice were immunohistochemically double-stained using anti-ΔNp63 (ABS552) and anti-CCSP antibodies. Representative images are shown out of two independent experiments with 4 mice per group respectively. A–H: after sequentially incubating the sections with primary and secondary antibodies for each of ΔNp63 and CCSP proteins, ΔNp63 signals were visualized by the addition of HRP-reacting substrate (Green) and photomicrographs were taken (A, C, E, and G); ALP-reacting substrate (red) was sequentially added to visualize CCSP (B, D, F, and H). I–L: the sections were labeled for ΔNp63 (1st round staining; green signals in I and K). The same sections are extensively washed to remove signals. Sequentially the sections were probed for CCSP. The same fields as imaged in 1st round were photomicrographed so that each marker can be colocalized (2nd round staining; red signals in J and L). A, B, E, and F: in distal parts of the lungs of 7- to 9-wk-old mice, ΔNp63-positive cells are scarcely observed in bronchiolar epithelium (arrows in E). Most of these ΔNp63-positive cells were also positive for CCSP (arrowheads in F). C, D, G, and H: in distal parts of the lungs of 14-mo-old mice, ΔNp63-positive cells are more widely distributed and they are frequently spotted in clusters (C and G); colocalization of ΔNp63 and CCSP signals are observed in most of these cells (H). I–L: in bronchiolar epithelium of 14-mo-old mice, a slight basal-to-luminal decreasing gradient of ΔNp63 signals is observed (areas circled by dashed lines in I and K). In stark contrast, basal-to-luminal increasing gradient of CCSP signals is observed (J and L). Original magnification: ×100 (A–D), ×200 (E, F, I, and J), and ×400 (G, H, K, and L). Br, bronchiolar lumen; PA, pulmonary artery.
Fig. 8.
Overexpression of ΔNp63 isoforms induces Cdc42 upregulation in club cells. A: 24 h after transfecting overexpression plasmid or empty vector into H441 cells, whole cell protein lysates were prepared. Equal amounts of protein (1.5 μg/lane) were loaded. Data presented are representative of two independent experiments. B: hypothesis regarding the locations and profiles of the putative progenitor cells expressing p63 and/or CCSP in rodent lungs.
DISCUSSION
Evidence from this paper provides compelling evidence that aging is associated with elevated expression of specific ΔNp63 isoforms in bronchiolar epithelium of mouse lungs. ΔNp63 signals were mainly observed in a patchy manner in the bronchiolar epithelium of lungs of mice at 14 mo of age, where ΔNp63 frequently colocalized with CCSP. The paper also reveals that the expression of TAp63 isoforms dramatically decrease with age in the lungs of mice, corroborating similar data using monkey lung samples. These age-associated changes in different p63 isoforms are accompanied by 1) appearance of nonuniform hypercellular regions with heterogeneity in the interstitium of the bronchovascular bundle, 2) an altered protein expression profile of prosurfactant protein C (pro-SPC), and 3) altered lung functions (increased lung compliance and decreased elastance). A similar age-associated altered protein expression profile of pro-SPC was also observed in aged monkeys. Collectively, these data indicate that the property of progenitor cells in the lung alter with age.
p63 is a member of the p53, p63, and p73 transcription factor family (18). The p63 gene has two promoters, which permit it to generate two opposing classes of proteins, full-length (TAp63) and NH2-terminal-truncated (ΔNp63) forms. Each class comprises different isoforms generated by a variety of splicing transcripts. Given that the p63 protein family has numerous splice isoforms possessing different molecular mass, the specificity of the p63 antibodies used in the current study was thoroughly evaluated. Western blotting with the p63 antibody (cat. no. ab84712; Abcam) detects a single clear band at ~65 kDa in HEK293 cells (Fig. 2A). The band density markedly decreases in the cells treated with p63-targeting siRNAs. Therefore, it was proposed that this single band represents one of the TAp63 isoforms in light of a previous report demonstrating that TAp63 isoforms are much more actively transcribed than ΔNp63 isoforms in HEK293 cells (21). In addition, TAp63α and TAp63γ have been detected at ~80 and 50 kDa, respectively (2, 14, 22). Therefore, it is postulated that the 65-kDa single band detected by p63 antibody (ab84712) in HEK293 cells is TAp63β. Clear bands with similar molecular mass appeared in Western blot experiments where mouse and monkey samples were utilized (Fig. 2D, top arrow; Supplemental Fig. S1A, arrow). These bands are considered to correspond to TAp63β, given the fact that this isoform is detected by p63 antibody (ab84712) but not by ΔNp63 isoform-specific antibody (ABS552). The bands detected by both p63 (ab84712) and ΔNp63 antibody (ABS552) in cytoplasmic fractions, whose molecular mass is ~75 kDa (Fig. 2E, top arrow; Supplemental Fig. S1B, top arrow), are considered to be ΔNp63α since our data show that the molecular mass of ΔNp63α is ~75 kDa (Fig. 2, B and C). Therefore, it was speculated that the bands with approximate molecular mass of 50-kDa, detected by both p63 (ab84712) and ΔNp63 antibody (ABS552), are either ΔNp63β or ΔNp63γ (Fig. 2, D and E, middle arrows; Supplemental Fig. S1B, bottom arrows). The bands detected in cytoplasmic and nuclear fractions with an approximate molecular mass of 35 kDa (Fig. 2, D and E, bottom arrows; Fig. 3D, #8; Fig. 3G, #10; Fig. 5C, #7), are considered to be TAp63ε or unidentified TAp63 isoform based on its small molecular mass (21). However, further studies are necessary because the information about the molecular mass of small-sized p63 isoforms, as detected by Western blotting, is limited. It is postulated that the multiple bands, which were detected in the range of 37 to 50 kDa (Fig. 2 B–D; Fig. 3A, #2 and #4; Fig. 3I, #12; Fig. 4A, #2; Fig. 4D, #4) are nonspecific.
The role of cytoplasmic ΔNp63α in physiological conditions remains elusive even though the literature shows that nucleo-cytoplasmic transport of ΔNp63α occurs in squamous carcinoma cells treated with cisplatin, a DNA-damaging agent (11). Higher levels of cytoplasmic p63 are associated with increased proliferative activity while cytoplasmic ΔNp63α is a known target for proteasome-mediated degradation (7, 10, 11). ΔNp63α is also associated with acquisition of cell mobility (6). ΔNp63-positive epithelial cells were found to be widely distributed in 14-mo-old mice lungs in the current study. They exhibit cytoplasmic staining of ΔNp63 (Fig. 6, L and N, arrows; Fig. 7, G and K), consistent with Western blot data (Fig. 4, A and D). In addition, semiquantitative analysis using H&E-stained lung sections reveals the appearance of nonuniform hypercellular regions with heterogeneity in the interstitium of the bronchovascular bundle in 14-mo-old mice (areas circled by dashed lines in Fig. 1, G, H, O, and P). Therefore, it was postulated that age-dependent progressive accumulation of cellular damage in progenitor cells residing at the bronchovascular interstitium causes ΔNp63α accumulation in their cytoplasm and, as a consequence, confers proliferative ability. However, since the expression of Ki67, a proliferation marker, does not increase with age even before 14 mo (Fig. 3, D and F), it makes this hypothesis untenable. These results led to an alternative hypothesis as follows: the expression of ΔNp63α in club cells, which remains low under normal conditions, gradually increases with age secondary to age-associated changes in the microenvironment. Once club cells acquire migratory ability via ΔNp63α activation, some, instead of migrating to luminal side, infiltrate into the bronchovascular interstitium. To corroborate this hypothesis, additional experiments were performed in vitro using human club cells (H441 cell line). In support of our hypothesis, ΔNp63α overexpression induced the upregulation of Rho-GTPase family member Cdc42 (a protein that plays a role in cell migration) (Fig. 8A). On the other hand, scratch wound-healing assay showed that ΔNp63α overexpression did not alter the migratory ability of H441 cells (Supplemental Fig. S1, C and D). However, actual effects of ΔNp63α on club cells in physiological conditions may be different from the results of wound healing assay because H441 cells are a cancer cell line, not primary cells. In addition, in this assay we used cell culture plate coated solely with collagen type I, which is totally different from the physiological extracellular matrix (ECM) consisting of varied components such as collagen, elastin, fibronectin, laminin, glycosaminoglycans, etc. Furthermore, the following information supports the aforementioned our hypothesis: 1) some of the hypercellular regions spotted in the bronchovascular interstitium of the lungs of 14-mo-old mice contain cuboidal cells morphologically close to nearby bronchiolar epithelial cells (Fig. 1O); 2) double-staining of lung sections for CCSP and cldn10 show that in 14-mo-old mice double-positive cells are not only observed in bronchiolar epithelium but also spotted in such areas as bronchovascular interstitium and thickened interstitium of alveoli, where no typical bronchiolar structures are observed (data not shown); and 3) vascular endothelial layer near hypercellular regions as observed in lungs of 14-mo-old mice often exhibits a disorganized cellular arrangement and varied cell morphology (Fig. 1G). A paper published by Zheng et al. (34) supports this concept. It uses a lineage-tracing approach demonstrating that club cells-derived p63-positive cells appear in alveoli following severe alveolar damage, i.e., after bleomycin treatment or influenza virus infection. Further investigation is required to establish this novel concept about club cell phenoconversion and migration in aging lungs.
As previously mentioned, the distribution of basal cells along the proximal-distal axis of the lung differs significantly between mice and primates. In stark contrast to human lungs, BCs are rarely observed in the bronchiolar epithelium of mouse lungs (26). Data demonstrate that ΔNp63α-positive CCSP-positive cells are distributed mainly in the bronchiolar epithelium in distal airways of both 7- to 9-wk-old and 14-mo-old mice. In the former, ΔNp63α-positive CCSP-positive cells are frequently observed as a solitary cell in the bronchiolar epithelium (Fig. 7, E and F). ΔNp63α-positive CCSP-positive cells are found as clusters in 14-mo-old mice (Fig. 7, G–L). Unexpectedly, no bronchiolar epithelial cells were clearly labeled by use of the p63 antibody (ab84712) that recognizes the DNA-binding domain shared by all p63 isoforms. These opposing results are reconciled by the possibility that p63 antibody (ab84712) does not efficiently recognize and/or bind to ΔNp63 isoforms in the cytoplasm. In support of this explanation, when mouse skin sections are immunohistochemically labeled using p63 antibody (ab84712), signals are barely detected in cell cytoplasm (Supplemental Fig. S1F). However, when cytoplasmic extracts from mouse skin tissues are Western blotted, this same antibody as well as ΔNp63 antibody (ABS552) detects a clear band at ~75 kDa (Supplemental Fig. S1B). It is possible that ΔNp63 isoforms undergo posttranslational modifications in the DNA-binding domain that in turn makes it difficult for the p63 antibody (ab84712) to detect them since the target amino acid sequence of this antibody (ab84712) is “SDGIHVVKDARERDFTSLENTVEERLTELTKSINDNIAIFTEVQKRSQKE” and highly conserved among different species (homology between human and mouse is 98%).
The maturation process of surfactant protein C (SPC) is extensively studied, however there is limited information about how the process gets modified or impaired when type II alveolar epithelial cells (AECs) are functionally disturbed by infection, injury or aging. SPC is initially synthesized in the endoplasmic reticulum (ER) as a 21-kDa pro-protein (referred to as pro-SPC21 hereinafter). Pro-SPC21 is then modified to a 24-kDa higher molecular mass form (referred to as pro-SPC24 hereinafter) (28). This intermediate product, pro-SPC24, matures through stepwise proteolytic cleavage during its transport from the ER to the Golgi apparatus and subsequently from the Golgi apparatus to multivesicular bodies (20). Therefore, an easily available index that simply uses the densities of Western blot bands was devised in light of a complex modification and cleavage process required for the maturation of SPC. This index, which was created to quantify the extent of accumulation of processing intermediates of SPC, increases with age: 1.6-fold statistically significant increase in 14-mo-old versus 7- to 9-wk-old mice (Fig. 3B). Fourteen months corresponds with the early stages of aging for mice since the average lifespan of laboratory mice is ~24 mo (8). Therefore, these data alone are not sufficient to justify the use of this index for more advanced aging. However, similar data using monkey lungs were obtained wherein the accumulation index for aged monkeys is higher than middle-aged monkeys: 1.3-fold increase in aged versus middle-aged monkeys (Fig. 5B). In addition, the expression of p21, a representative senescence marker, increased in mice aged 14 mo versus 7–9 wk (Fig. 3A). Therefore, the index used in the current study, the SPC-IP index, is a sensitive detector to measure the alteration of SPC maturation process. Data indicate that this index is a good marker for aging of the lung given that the expression of p21 increases concurrently with the index even though the expression of p21 in aged mice shows huge variation between individual animals (Fig. 3, A and B; Fig. 5, A and B).
Cytoplasmic and nuclear fractions of lung tissues to quantify each compartment-derived different isoforms of p63 were used in this study because whole tissue lysates show multiple overlapping bands (Fig. 3A). This approach allows bands to be detected that were not detected when whole lysates of lung tissues were examined (Fig. 3D, #8, Fig. 3G, #10; Fig. 5C, #7). It also permits the detection of differences in the expression of specific p63 isoforms in nuclear and cytoplasmic fractions of lung tissues (Fig. 3, D, G, and I; Fig. 4, A and D; Fig. 5C). However, the results obtained by running such fractions of lung tissues show band patterns that are incompatible with those obtained by using whole lysates. One possible explanation for this discrepancy between Western blot results is that the intensity of the homogenization required to obtain cytoplasmic extracts at the initial step of fractionation is not as high as that to obtain whole lysates. The tissues are manually homogenized in hypotonic buffer to release cytoplasmic proteins into the supernatants at the first step of fractionation. The tissues had to be homogenized using mild grinding strength to avoid the undesired release of nuclear proteins during this initial step. Therefore, the cytoplasmic fractions could have been derived primarily from cells that are easily dissociated from the chunks of lung tissues, e.g., alveolar and bronchiolar epithelial cells. This hypothesis is compatible with the observation that the age-associated increase in the expression of ΔNp63 isoforms was clearly observed when tested with cytoplasmic fractions but not with whole lysates (Fig. 3A vs. Fig. 4A, #1). However, a possible reason for discrepancy between results obtained using nuclear fractions versus those obtained by using whole lung lysates is probably the difference in the efficiency of nuclear extraction. The suspension of isolated intact nuclei was vortexed long enough to prepare nuclear lysates, which consequently led to completely deformed shapes of nuclei. When whole lysates of lung tissues were prepared in the current study, strong detergents were not utilized. This process may not have been adequate to release a large amount of nuclear proteins, even though repeated freeze-thaw cycles to facilitate the rupture of the membranous structures, including nuclear membranes, were utilized instead. Therefore, it is not necessarily a contradiction that signals from a specific p63 isoform are only detectable when using nuclear fractions but not when using whole lysates as demonstrated (Fig. 3A vs. Fig. 3D, #8; Fig. 5A vs. Fig. 5C).
Club cells are progenitor cells in the distal lung. Progenitor cell function deterioration is associated with aging. Unfortunately, there is limited information as to how aging alters and/or affects the functional integrity and behavior of club cells. Data in this study demonstrate that the expression of CCSP in lungs of mice aged 14 mo versus 7–9 wk is significantly higher (Fig. 3A and C) and that the contents of CCSP in air space in mice at 8–9 mo and 14-mo versus 7–9 wk of age are both higher (Fig. 4, F and G). The expression of cldn10, another marker for club cells, in cytoplasmic fractions of lungs from 14-mo-old mice was significantly increased in parallel (Fig. 4A). However, there is no clear evidence as to how this acceleration of CCSP secretion is related to increased expression of cldn10 in cytoplasmic fractions, not to mention its relation to the club cells’ status of differentiation. Further studies are needed to determine how CCSP expression and secretion, and cldn10 expression are correlated with each other. This may help understand the behavior of club cells in aged lungs.
In this study, p63-positive cells are encountered in distal lungs of mice (Fig. 6). In 7- to 9-wk-old mice, strong signals of p63 are mainly observed in the nuclei of the cells that are spotted in the interstitium, next to pulmonary arteries (Fig. 6, E and F). Weak nuclear signals of p63 are often encountered in the bronchiolar epithelium beneath the basement membrane near to the interstitium where strong p63 signals are observed (Fig. 6F). In contrast, in 14-mo-old mice, nuclear signals of p63 are sparsely encountered compared with 7- to 9-wk-old mice (Fig. 6, C, D, G, and H). These results are compatible with Western blot data showing that nuclear expression of p63 isoforms as detected by p63 antibody (ab84712) decreases in 14-mo-old versus 7- to 9-wk-old mice (Fig. 3 D and G). The identities of the p63-positive cells that are spotted in distal lungs of mice are not clear. However, given the fact that their location and cell shapes display huge variations, it is likely that they consist of different cell types. On the other hand, weak p63 signals are observed in the cytoplasm of cells in bronchiolar epithelium and bronchovascular interstitium (Fig. 6, E–H). These observations are apparently inconsistent with the fact that basal cells express p63 only in the nucleus. To reconcile the widely accepted profiles of basal cells and our findings, we postulate that in addition to basal cells there are other types of epithelial progenitors resident in the lung that also express p63 in the cytoplasm and/or nucleus (Fig. 8B). This hypothesis is supported by the immunohistochemical staining results using mouse skin tissue sections where cytoplasmic expression of ΔNp63 signals, as detected by ABS552, was observed in cells that are located at hair follicles and epidermis while nuclear p63 signals, as detected by ab84712, were observed in nucleus of cells located at epidermis (Supplemental Fig. S1F). Our data showing that TAp63 isoforms in the nucleus downregulate and ΔNp63 isoforms in cytoplasm upregulate at age 14 mo can be explained by our hypothesis as follows: age-associated alteration in p63 isoform expression corresponds to the status change of “p63(+) progenitor cell” (Fig. 8B), i.e., loss of its self-renewal ability and concomitant cell differentiation toward functionally active club cells. Our data also demonstrate that CCSP expression increases with age (Fig. 3C) while the expression of Ki67, a proliferation marker, in lungs decreases with age (Fig. 3, D and F). Our hypothesis reconciles these observations by an explanation that “p63(+) CCSPlow cell” and “p63(+) CCSP(+) cell” as described in Fig. 8B increase in number not by their proliferation but by differentiation of “p63(+) progenitor cells“ toward functionally active club cells. These explanations led by our observations are in harmony with the reported roles of TAp63 and ΔNp63: TAp63 suppresses the differentiation of committed progenitor cells while ΔNp63 acts to promote terminal differentiation via its competition and suppression of TAp63 actions (23). Meanwhile, we have no clues as to whether the function of basal cells in the mouse trachea is affected at 14-mo of age.
Our data demonstrate that compliance was significantly increased and elastance was significantly decreased in lungs of mice aged 14 mo versus 7–9 wk (Fig. 1T). We have no clues as to whether this dramatic alteration in lung functions is related, directly or indirectly, to other phenotypic changes such as increased CCSP and altered expression profiles of p63 isoforms. One possible scenario that can reasonably explains all these changes is that age-associated alterations in the extracellular matrix (ECM), e.g., degradation of ECM, modulate the microenvironment and affect the migratory activity and differentiation status of progenitor cells in the lung. A previous report shows that elastic fibers dramatically decrease while type III collagen increases in the lungs of elderly human subjects versus non-elderly controls (5). The mechanical signals in ECM can be transmitted to intracellular signaling through cell surface receptors, such as integrin family members (15). In addition, not only intact ECM but also fragmented ECM components released through ECM cleavage dynamically regulate and influence cell behavior (1). Given such dynamic cell-ECM interaction as well as observed ECM alterations in lungs of elderly people, it is possible that the degradation of ECM in the lung of aged mice is a direct cause of age-associated alterations in lung functions and an indirect cause of altered behaviors of various progenitor cells. Further investigations are required to elucidate the interaction between ECM and progenitor cells in aging lungs with special focus on basal and club cells.
Our study also provides additional insights as follows: 1) dysregulation of type II AECs and expansion of p63+ CCSP+ distal airway stem cells are closely linked in the aging lung, and 2) aging of the lung consists of two phases: an early age phase when club cells actively migrate and a late age phase when this club cell activation subsides. This hypothesis may explain the widely shared clinical observations that human lung cancer commonly develops during post-middle age in relatively peripheral regions of the lung. Idiopathic pulmonary fibrosis (IPF) is also a representative age-associated lung disease, in which 1) hyperplastic epithelial cells expressing ΔNp63 are observed at bronchiolo-alveolar junctions (4), 2) elevated alveolar epithelial cell (AEC) apoptosis is frequently juxtaposed with hyperplastic cells (25), and 3) immature epithelial cells with club cell markers are widely distributed in various patterns (13). Observations in the current study suggest that age-associated functional deterioration of type II AECs is temporally related to expansion of epithelial progenitors in the distal lung, consistent with the histopathological features of IPF. Collectively, 1) understanding the mechanisms of how nuclear expression of TAp63 isoforms and cytoplasmic expression of ΔNp63 isoforms are linked at molecular levels during the differentiation of lung progenitor cells and 2) revealing how the expression of each of the p63 isoform in rodent lungs is timely and spatially controlled during development and under stress conditions may offer a new avenue for creating new therapeutic options against age-associated lung diseases.
GRANTS
N. Kolliputi was funded by the American Heart Association National Scientist Development Grant 09SDG2260957, National Heart, Lung, and Blood Institute Grant R01 HL-105932, and the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology. J. Fukumoto was funded by the American Heart Association Postdoctoral Fellowship Award 14POST18200004. L. Galam was funded by the American Heart Association National Scientist Development Grant 17SDG32780002. V. R. Narala was supported by an award from the University Grants Commission, Raman Fellowship for Post Doctoral Research for Indian Scholars in the United States of America [F.No. 5-90/2016 (IC)], New Delhi, India. The primate colony of B. C. Hansen was supported by National Institute on Aging Grants N01AG31012 and HHSN2532008002C. U. K. Chaudhari was supported by National Institute for Research in Reproductive Health of the Indian Council of Medical Research (Department of Health Research/Human Resource Development/LTFF Fellowship 2016-2017) at the Department of Internal Medicine, University of South Florida.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F. and U.K.C. conceived and designed research; J.F., S.S.P., S.K., S.S., I.J., V.R.N., H.M.H.-C., and L.G. performed experiments; J.F. analyzed data; J.F. interpreted results of experiments; J.F. prepared figures; J.F. drafted manuscript; J.F., M.A., M.T.B., R.S., B.C.H., and R.F.L. edited and revised manuscript; J.F. and N.K. approved final version of manuscript.
REFERENCES
- 1.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801, 2014. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, Knight RA, Melino G. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ 13: 1037–1047, 2006. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- 3.Chernaya O, Shinin V, Liu Y, Minshall RD. Behavioral heterogeneity of adult mouse lung epithelial progenitor cells. Stem Cells Dev 23: 2744–2757, 2014. doi: 10.1089/scd.2013.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, Piccoli P, Cangi G, Semenzato G, Doglioni C. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest 82: 1335–1345, 2002. doi: 10.1097/01.LAB.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 5.D’Errico A, Scarani P, Colosimo E, Spina M, Grigioni WF, Mancini AM. Changes in the alveolar connective tissue of the ageing lung. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol 415: 137–144, 1989. doi: 10.1007/BF00784351. [DOI] [PubMed] [Google Scholar]
- 6.Dang TT, Esparza MA, Maine EA, Westcott JM, Pearson GW. ΔNp63α promotes breast cancer cell motility through the selective activation of components of the epithelial-to-mesenchymal transition program. Cancer Res 75: 3925–3935, 2015. doi: 10.1158/0008-5472.CAN-14-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon PK, Barry M, Stampfer MJ, Perner S, Fiorentino M, Fornari A, Ma J, Fleet J, Kurth T, Rubin MA, Mucci LA. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev 18: 595–600, 2009. doi: 10.1158/1055-9965.EPI-08-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 152: 244–248, 2016. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186: 306–313, 2012. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 10.Ferronika P, Triningsih FX, Ghozali A, Moeljono A, Rahmayanti S, Shadrina AN, Naim AE, Wudexi I, Arnurisa AM, Nanwani ST, Harijadi A. p63 cytoplasmic aberrance is associated with high prostate cancer stem cell expression. Asian Pac J Cancer Prev 13: 1943–1948, 2012. doi: 10.7314/APJCP.2012.13.5.1943. [DOI] [PubMed] [Google Scholar]
- 11.Fomenkov A, Zangen R, Huang YP, Osada M, Guo Z, Fomenkov T, Trink B, Sidransky D, Ratovitski EA. RACK1 and stratifin target deltaNp63α for a proteasome degradation in head and neck squamous cell carcinoma cells upon DNA damage. Cell Cycle 3: 1285–1295, 2004. doi: 10.4161/cc.3.10.1155. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol 305: C182–C189, 2013. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumoto J, Soundararajan R, Leung J, Cox R, Mahendrasah S, Muthavarapu N, Herrin T, Czachor A, Tan LC, Hosseinian N, Patel P, Gone J, Breitzig MT, Cho Y, Cooke AJ, Galam L, Narala VR, Pathak Y, Lockey RF, Kolliputi N. The role of club cell phenoconversion and migration in idiopathic pulmonary fibrosis. Aging (Albany NY) 8: 3091–3109, 2016. doi: 10.18632/aging.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res 64: 6900–6905, 2004. doi: 10.1158/0008-5472.CAN-04-1657. [DOI] [PubMed] [Google Scholar]
- 15.Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng 5: 2041731414557112, 2014. doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development 131: 4955–4964, 2004. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano K, Araya J, Hara H, Minagawa S, Takasaka N, Ito S, Kobayashi K, Nakayama K. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Respir Investig 54: 397–406, 2016. doi: 10.1016/j.resinv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 113: 1661–1670, 2000. [DOI] [PubMed] [Google Scholar]
- 19.López IP, Piñeiro-Hermida S, Pais RS, Torrens R, Hoeflich A, Pichel JG. Involvement of Igf1r in bronchiolar epithelial regeneration: role during repair kinetics after selective club cell ablation. PLoS One 11: e0166388, 2016. doi: 10.1371/journal.pone.0166388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Rodriguez E, Pérez-Gil J. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim Biophys Acta 1838: 1568–1585, 2014. doi: 10.1016/j.bbamem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Mangiulli M, Valletti A, Caratozzolo MF, Tullo A, Sbisà E, Pesole G, D’Erchia AM. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res 37: 6092–6104, 2009. doi: 10.1093/nar/gkp674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matin RN, Chikh A, Chong SL, Mesher D, Graf M, Sanza’ P, Senatore V, Scatolini M, Moretti F, Leigh IM, Proby CM, Costanzo A, Chiorino G, Cerio R, Harwood CA, Bergamaschi D. p63 is an alternative p53 repressor in melanoma that confers chemoresistance and a poor prognosis. J Exp Med 210: 581–603, 2013. doi: 10.1084/jem.20121439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKeon F. p63 and the epithelial stem cell: more than status quo? Genes Dev 18: 465–469, 2004. doi: 10.1101/gad.1190504. [DOI] [PubMed] [Google Scholar]
- 24.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, Wolters PJ. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: e86704, 2016. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 127: 266–274, 2005. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 26.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556, 2010. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivshankar P, Brampton C. Miyasato S, Kasper M, Thannickal VJ, Le Saux CJ. Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 47: 28–36, 2012. doi: 10.1165/rcmb.2011-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solarin KO, Ballard PL, Guttentag SH, Lomax CA, Beers MF. Expression and glucocorticoid regulation of surfactant protein C in human fetal lung. Pediatr Res 42: 356–364, 1997. doi: 10.1203/00006450-199709000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Soundararajan R, Stearns TM, Czachor A, Fukumoto J, Turn C, Westermann-Clark E, Breitzig M, Tan L, Lockey RF, King BL, Kolliputi N. Global gene profiling of aging lungs in Atp8b1 mutant mice. Aging (Albany NY) 8: 2232–2252, 2016. doi: 10.18632/aging.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev 20: 3185–3197, 2006. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyler NK, Plopper CG. Morphology of the distal conducting airways in rhesus monkey lungs. Anat Rec 211: 295–303, 1985. doi: 10.1002/ar.1092110310. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625, 2015. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair 7: 8, 2014. 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng D, Yin L, Chen J. Evidence for Scgb1a1+ cells in the generation of p63+ cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol 50: 595–604, 2014. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 35.Zhou F, Onizawa S. Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res 12: 78, 2011. doi: 10.1186/1465-9921-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517: 616–620, 2015. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]