Abstract
Background:
India is a tropical country with a high burden of febrile zoonotic/infectious illnesses, scrub typhus being such a cause with multiple epidemics reported from different regions of the country.
Objective:
This study was plotted to document the clinical and diagnostic manifestations, treatment, and outcomes of scrub typhus in the sub-Himalayan region of India and to compare the results with other Indian and Asian studies.
Materials and Methods:
This was a retrospective observational study involving collection of data for 54 IgM ELISA-confirmed in-patient cases of scrub typhus at a tertiary care institute in Uttarakhand, India, from their case records.
Results:
The majority of patients were from rural background. Housewives constituted 28 (51.85%) patients. The most common symptoms were due to involvement of gastrointestinal tract in the form of abdominal pain in 39 (72.22%) and vomiting in 29 (53.7%) patients. Central nervous system involvement in the form of altered sensorium in 14 (25.9%) patients and pulmonary involvement as cough in 28 (51.85%) patients was observed. An eschar was found in 7 (12.96%) patients and upper eyelid edema in 40 (74.07%) patients. The most common laboratory abnormality documented was elevation of liver transaminases (aspartate aminotransferase > alanine aminotransferase), 40 (74.07%), and blood urea levels, 47 (87.03%). There was no difference in the clinical presentation, severity, or mortality in pregnant females when compared with nonpregnant females. One (1.45% mortality) died in our study.
Conclusion:
Scrub typhus is an important cause of acute febrile illness with variable, often nonspecific and multisystem involvement. Early recognition and antibiotic administration are the key to reduce complications and mortality, especially for a primary care physician.
Keywords: Communicable diseases, Orientia tsutsugamushi, rickettsia, scrub typhus, zoonoses
Introduction
Scrub typhus is the most common rickettsial infection in the Indian subcontinent.[1] It is a zoonotic illness caused after the bite of a trombiculid mite larva carrying Orientia tsutsugamushi, an obligate intracytosolic bacterium. The mites use rodents as hosts.[1] In India, the reported incidence of Orientia in rodent carriers is very high.[2] Epidemics of scrub typhus have been reported in many parts of India, including the sub-Himalayan region.[3,4] A few studies have also been reported from the state of Uttarakhand,[5,6] and a sudden outbreak was observed in the year 2013 after the flash floods.[5] The myriad of nonspecific features involving multiple organ systems, overlooking it as a cause of pyrexia in lieu of other infective etiologies of fever in a tropical country such as India, in addition to the lacunae in diagnostic facilities in the Indian scenario is the major contributor to the resurrection of this disease.
Despite the growing awareness and reported articles, there is paucity of compendious and succinct evidence-based data about the protean manifestations, first-line drugs, optimum duration of treatment, and long-term outcomes related to this disease. This is an attempt to contribute our experiences related to scrub typhus to enhance the database for future references. This study also incorporates comparisons between Indian and other Asian studies, as well as reports the outcomes in six pregnant females with scrub typhus who were enrolled in the study.
Materials and Methods
This was a retrospective observational study, carried out in 2015 in the Department of General Medicine of a large teaching tertiary care institution in Garhwal, Uttarakhand. Case records of 54 IgM ELISA-confirmed cases of scrub typhus admitted in the general medicine ward from January 2014 to December 2014, who met the inclusion and exclusion criteria, were collected and analyzed. These case records were designed to provide pertinent demographic and clinical information including age, gender, occupation, presenting features, examination findings, laboratory results, complications, treatment instituted, and outcomes.
The diagnostic testing was carried out at the general microbiology department of the institute as per the instructions given by the manufacturer. The blood samples were subjected to detection of specific IgM antibodies against the causative agent Orientia tsutsugamushi using a commercial ELISA kit (manufactured by InBios International Inc., USA).
Inclusion criteria
Pyrexia for more than 5 days
Age >18 years
Positive serology for scrub typhus.
Exclusion criteria
Patients with other established causes of pyrexia, such as malaria, dengue, leptospirosis, enteric fever, and viral meningitis
Negative serology for scrub typhus
Incomplete case records
Patient refusal to participate in the study.
The definitions used to describe complications in the study are as follows:
Acute kidney injury (AKI): as per the latest Acute Kidney Injury Network (AKIN) classification guidelines[7]
Acute hepatitis: elevation of serum transaminases [aspartate aminotransferase (AST)/alanine aminotransferase (ALT)] more than four times the upper limit of normal
Acute respiratory distress syndrome (ARDS): acute-onset noncardiogenic pulmonary edema which manifests with bilateral alveolar or interstitial infiltrates on a chest radiograph, with a PaO2/FiO2≤200 mmHg on arterial blood gas analysis
Pneumonia: acute-onset fever and chills with cough/breathlessness, with or without crackles/rhonchi, with radiographic evidence of consolidation or interstitial infiltrates
Myocarditis: chest complaints such as pain, breathlessness, or palpitations with electrocardiographic evidence of either diffuse ST elevation or T wave inversion, with elevated markers of myocardial damage: creatine kinase-MB/troponin
Meningitis: fever with altered mental status, with or without nausea/vomiting, having signs of meningeal irritation and an abnormal cerebrospinal fluid (CSF) analysis
Hepatic encephalopathy: acute hepatitis with an altered level of consciousness with overt signs of liver failure.
This study was conducted after clearance from the institutional ethics committee.
Results
Demographic characteristics
The mean age of the patients was 30.80 ± 12.32 years [Table 1]. The female-to-male ratio was 2.8:1. The maximum number of cases, that is, 35 (64.82%), belonged to the 20–40 years age group. Most of the afflicted patients belonged to a rural background with a history of working in open fields. Housewives and students constituted the two major occupational subgroups. Most cases were seen in the months of monsoon and the postmonsoon period, that is, July, August, and September.
Table 1.
A table summarising the demographic variables of the study subjects
| Variable | No. of cases | Percentage |
|---|---|---|
| Gender | ||
| Male | 14 | 25.92 |
| Female | 40 | 74.08 |
| Age (years) | ||
| <20 | 7 | 12.96 |
| 20-40 | 35 | 64.82 |
| >40 | 12 | 22.22 |
| Occupation | ||
| Housewives | 28 | 51.85 |
| Farmers | 6 | 11.11 |
| Students | 15 | 27.77 |
| Others* | 5 | 9.25 |
*Businessman, army, teacher, guard, laborer
Clinical profile
All the patients presented with pyrexia, with an average duration of illness being 9.8 ± 4 days [Graphs 1 and 2]. The most common symptoms in our study were gastrointestinal (GI) and/or respiratory in origin. Fourteen (25.9%) patients presented with altered sensorium, of which 4 (22.22%) developed seizures during their hospital stay. In our study, six (11.11%) patients were pregnant when they acquired scrub typhus (two in the first trimester, one in the second trimester, and three in the third trimester). There were a few unusual presentations documented: two (3.70%) patients presented with hepatic encephalopathy in the emergency department, one (1.85%) patient had signs and symptoms suggestive of pneumonia, while two patients (3.70%) had complaints of dysuria.
Graph 1.
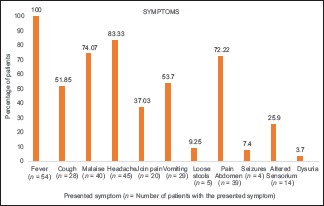
A graph summarising the symptoms at presentation observed in the study subjects
Graph 2.
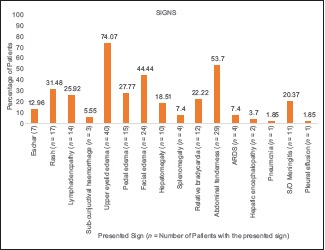
A graph summarising the signs observed in the study subjects
The most common examination finding observed in our study was upper eyelid edema (n = 40, 74.07%). The findings considered more suggestive of a rickettsial infection, that is, eschar and skin rash, were found only in (n = 7) 12.96% and (n = 17) 31.48% of cases, respectively. The most common site of eschar in our study was the abdomen (n = 4, 57.14%) at the site where the saree is tied [Figure 1]. Other sites where the eschar was seen were perineum [Figure 2], leg, and arm. There was no difference among clinical presentation and severity of illness between pregnant and nonpregnant female patients.
Figure 1.
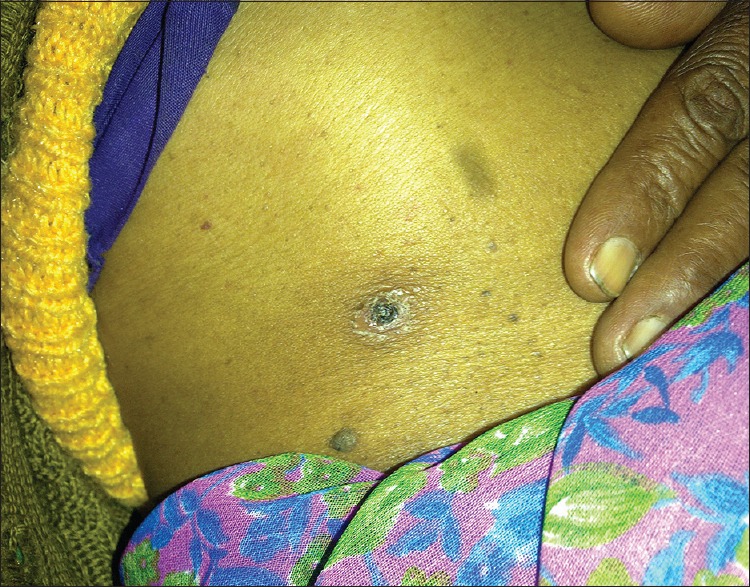
A figure demonstrating an eschar at abdomen
Figure 2.
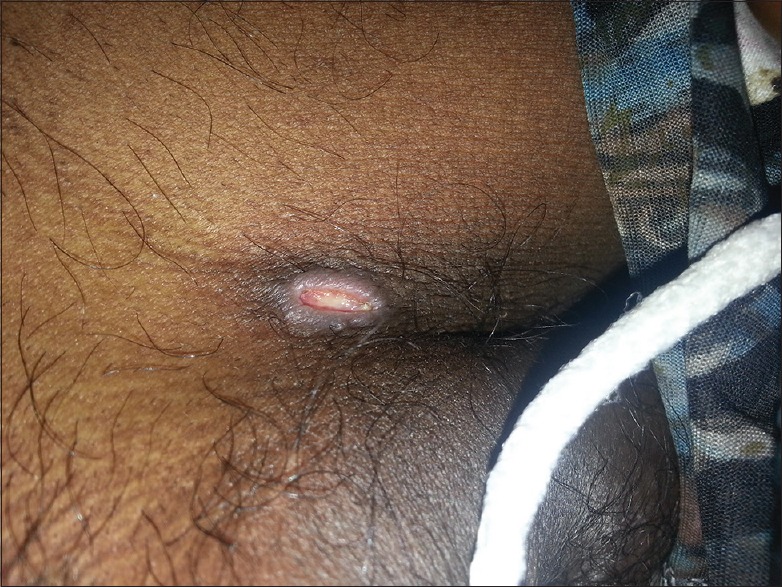
A figure demonstrating an eschar at perineum
Laboratory results
Four patients (7.40%) developed acute hepatitis [Graph 3]. Lumbar puncture was performed in 14 suspected cases of CNS involvement, of which 6 cases (42.8%) had elevated protein and lymphocytic preponderance on CSF examination. Contrast-enhanced computed tomography was performed in 10 cases, which suggested the nonspecific finding of effacement of the sulci in 2 cases. Abdominal sonography showed hepatomegaly in eight cases (14.8%) and splenomegaly in one case (1.85%). None of the patients showed any alteration of hepatic echotexture or obstructive features in ultrasonography. Urine routine/microscopic examination was abnormal in two cases (3.70%) showing elevated pus cells, with no other abnormality. Chest radiographs showed pleural effusion in 1.85% (n = 1) of case, opacities in 18.5% (n = 10), ARDS in 7.40% (n = 4), and peribronchial thickening in 9.2% (n = 5) of cases. Electrocardiography was performed for 10 patients but revealed no abnormalities in any of them.
Graph 3.
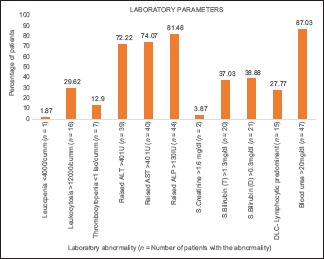
A graph summarising the laboratory abnormalities observed in the study subjects
Treatment and outcomes
A total of 48 patients were treated with supportive care and a medical regimen consisting of twice-daily oral doxycycline (100 mg). Of them, 12 patients (22.22%) developed nausea/vomiting and were switched over to once-daily oral azithromycin (500 mg). Six pregnant patients in our study were started on oral azithromycin 500 mg OD. The treatment was administered for 5 days in uncomplicated cases and for 10 days in patients with complications. Two patients developed Stage 2 AKI (as per AKIN classification),[7] and four developed ARDS during their hospital stay. Of a total of 54 patients, 53 recovered, while 1 patient died of ARDS. Comparison with other studies is summarized in Tables 2 and 3.
Table 2.
A table of comparison of clinical presentations and complications observed in our study compared to studies done in other parts of India
| Vivekanandan et al.[8] | Subbalaxmi et al [9] | Sivarajan et al.[10] | Takhar et al.[11] | This study [Graphs 1–3] | |
|---|---|---|---|---|---|
| Place; sample size | Puducherry; 50 | Andhra Pradesh; 176 | Meghalaya; 90 | Rajasthan; 66 | Uttarakhand; 54 |
| Clinical features | |||||
| Fever | 50 (100%) | 176 (100%) | 75 (83.3) | 66 (100%) | 54 (100%) |
| Myalgia | 19 (38%) | NA | 56 (62.2) | 20 (30.3%) | 40 (74.07%) |
| Altered sensorium | 10 (20%) | 23 (13.1%) | 5 (19%) | 26 (39.4%) | 14 (25.92%) |
| Headache | 20 (40%) | 92 (52.3%) | 24 (26.7) | 22 (33.3%) | 45 (83.33%) |
| Cough | 20 (40%) | 94 (53.4%) | 21 (23.3) | 32 (48.5%) | 28 (51.85%) |
| Nausea/vomiting | 29 (58%) | NA | 21 (23.3) | 18 (27.3%) | 29 (53.7%) |
| Abd. pain | 10 (20%) | NA | 24 (26.7) | NA | 39 (72.22%) |
| Loose stools | 8 (16%) | 28 (15.9%) | NA | NA | 5 (9.25%) |
| Hepatosplenomegaly | 24 (48%) | 51 (28.9%) | Hepatomegaly 24 (26.7), splenomegaly 22 (24.4) | 23 (34.8%) | Hepatomegaly 10 (18%), splenomegaly 4 (7.4%) |
| Eschar | 23 (46%) | 23 (13.1%) | 10 (11.1) | 8 (12.1%) | 7 (12.96%) |
| Upper lid edema | NA | NA | NA | NA | 40 (74.07) |
| Conjunctival congestion/hemorrhage | NA | NA | NA | NA | 3 (5.55%) |
| Complications | |||||
| Leukopenia (<4000/mm3) | 1 (2%) | 42 (23.9%) | 11 (12%) | 10 (15.1%) | 1 (1.87%) |
| Leucocytosis (>11,000/mm3) | 15 (30%) | 18 (10.2%) | 23 (25%) | 20 (30.3%) | 16 (29.62%) |
| Thrombocytopenia (<100,000/mm3) | 5 (10.8%) | 53 (30.1%) | 18 (20%) | 52 (78.8%) | 7 (12.9%) |
| Raised transaminases | 47 (95.9%) | 153 (86.9%) | ALT 85 (94%), AST 90 (100%) | 32 (48.5%) | 40 (74.07%) |
| Renal failure (criteria not defined) | 6 (12%) | 49 (27.8%) | 11 (12.2%) | 34 (51.5%) | 2 (3.70%) |
| Mortality | 1 (2%) | 8 (4.5%) | 5 (5.15%) | 21.2% | 1 (1.87%) |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; NA: Data not available
Table 3.
A table of comparison of clinical presentations and complications observed in our study compared to studies done in other Asian countries
| Zhang et al.[12] | Kun Ming Wu et al.[13] | Hamaguchi et al.[14] | Brummaier et al.[15] | This study [Graphs 1–3] | |
|---|---|---|---|---|---|
| Place; sample size | Shandong; 102 | Taiwan; 136 | Hanoi, Vietnam; 237 | North-western Thailand; 422 | Uttarakhand, India; 54 |
| Clinical features | |||||
| Fever | 102 (100%) | 134 (98.5%) | 180 (76%) | 378 (89.6%) | 54 (100%) |
| Relative bradycardia | NA | NA | 92 (39.8%) | NA | 12 (22.22%) |
| Headache | 64 (62.7%) | 85 (62.5%) | 167 (70.5%) | 224 (53.1%) | 45 (83.33%) |
| Myalgia | 48 (47.1%) | NA | 161 (67.9%) | 151 (35.8%) | 40 (74.07%) |
| Altered mental status | NA | NA | 23 (9.7%) | 7 (1.7%) | 14 (25.92%) |
| Abdt tenderness | 17 (16.7%) | 42 (30.9%) | 10 (4.3%) | 98 (23.2%%) | 29 (53.7%) |
| Nausea/vomiting | 28 (27.4%) | NA | NA | 85 (20.1%)/116 (27.5%) | 29 (53.70%) |
| Cough | 14 (13.7%) | 71 (52.5%) | 104 (43.9%) | 115 (27.3%) | 28 (51.85%) |
| Conjunctival congestion/hemorrhage | 8 (7.8%) | NA | NA | NA | 3 (5.55%) |
| Eschar | 88 (86.3%) | 82 (60.3%) | 149 (62.9%) | 38 | 7 (12.96%) |
| Rash | 70 (68.6%) | 35 (25.7%) | 74 (31.2%) | 17 (31.48%) | |
| Hepatosplenomegaly | Hepatomegaly 7 (6.9%), splenomegaly 14 (13.7%) | Hepatosplenomegaly 35 (34.7%) | Hepatosplenomegaly 118 (49.8%) | NA | Hepatomegaly 10 (18.51%), splenomegaly 4 (7.40%) |
| Upper eyelid edema | NA | NA | NA | NA | 40 (74.07%) |
| Complications | |||||
| Hepatitis | 4 (3.9%) | NA | NA | 1 (0.3%) | 4 (7.40%) |
| Bronchopneumonia | 22 (21.6%) | 13 (20.6%) | 83 (35%) | 30 (9.9%) | 1 (1.87%) |
| Pleural effusion | 1 (1%) | 27 (42.9%) | NA | NA | 1 (1.87%) |
| Renal failure | 0 | 9 (6.8%) | 19 (11.5%) | 8 (2.6%) | 2 (3.70%) |
| ARDS | NA | 4 (6.3%) | 1 (0.4%) | NA | 4 (7.4%) |
| Meningitis | 0 | NA | NA | 4 (1.3%) | 11 (20.3%) |
| Leucocytosis | 13.5% | 39 (29.8%) | 96 (40.7%) | NA | 16 (29.6%) |
| Leukopenia | 4.1% | NA | NA | NA | 1 (1.87%) |
| Thrombocytopenia | 25.4% | NA | 104 (45%) | NA | 7 (12.96%) |
| Elevated AST | 75% | NA | NA | 74 (24.4%) | 40 (74.07%) |
| Elevated ALT | 80.3% | 109/127 (85.8%) | 194 (97.5%) | 39 (72.22%) | |
| Elevated BUN | 5.7% | NA | 82 (36.4%) | 47 (87.03%) | |
| Mortality | NA | 2 (1.47%) | 1 (0.4%) | 1 (1.87%) |
NA: Data not available; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ARDS: Acute respiratory distress syndrome; BUN: Blood urea nitrogen
Discussion
Orientia tsutsugamushi, the causative agent, has >20 antigenically distinct serotypes due to a wide variation in the antigenic protein –56 kDa TSA which is the most abundant protein of Orientia genus.[1,16] This serotypal variability contributes to the wide variability in disease severity and disease presentation of scrub typhus.
Young adults (20–40 years) were the most affected group [Table 1], which is due to the increased time spent outdoors. Female preponderance was seen [Table 1], which was in concordance with other risk factor assessment studies.[17] This is because in hilly areas, it is usually the women who search for and bring firewood from the forests, along with contribution in domestic farming activities. The maximum burden of cases was seen during the monsoon months of July, August, and September, which correlates with other studies as well.[5] This is attributable to the abundant vegetation growth, increased occupational exposure due to the concurrent harvesting season, leading to more people in the fields and for longer periods of the day in the rainy season. Therefore, preventive measures such as personal protective equipment can have a noticeable impact in decreasing the transmission, especially in these areas.
Eschar, which is described as the characteristic lesion, was noted only in seven of our patients [Graph 2]. A similar frequency of its occurrence was reported in the other Indian studies [Table 2]. Cases from other Asian countries usually have a higher incidence of eschar [Table 3]. This may be due to variability in the infecting serotype in different regions, high skin color in the Indian subcontinent, and high rates of underdetection due to the painless and nonitchy characteristics of the eschar.
Another frequently observed finding documented in our series was edema, in particular upper eye lid edema, facial edema, and pedal edema [Graph 2]. This is much more when compared with the prevalence of facial and pedal edema reported in north-east Himalayan studies.[18] The edema develops due to multiplication of Orientia in the endothelial cells of small blood vessels resulting in breaks in the vascular barrier (perivasculitis) and subsequent accumulation of fluid in the interstitial spaces.[19] A study done on 451 patients depicting ocular changes in scrub typhus described optic nerve and retinal edema but not upper eyelid edema.[20] In our study, three (5.55%) patients had subconjunctival hemorrhage [Figure 3] which is in concordance with the studies by Zhang et al.[12] and Scheie[20] but has not been described in other Indian studies [Table 2].
Figure 3.
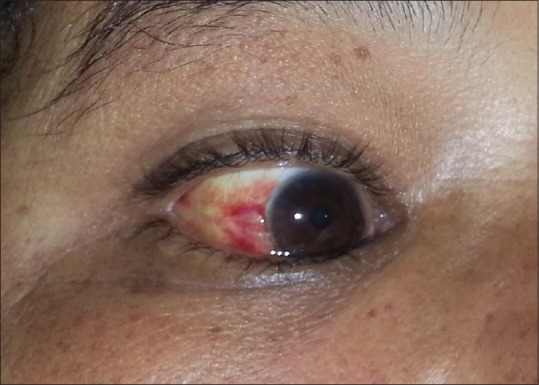
A figure demonstrating sub-conjunctival haemorrhage in a study subject
The most predominant organ system involved was GI [Graphs 1 and 2]. The findings corroborate well with the ones described in the literature [Tables 2 and 3]. The salient differentiating feature of scrub typhus from other causes of infective GI involvement is anicteric hepatitis.[21] In our study as well as in other reported studies, serum glutamic oxaloacetic transaminase (AST) is more often elevated when compared with serum glutamic pyruvic transaminase (ALT) [Tables 2 and 3], indicating that inflammation in scrub typhus is not just limited to the liver but involves other organs as well. A study done by Mokta et al. has mentioned scrub typhus to be the most common cause of febrile jaundice in the Himalayan region.[22]
The pulmonary involvement in our study is low when compared with other Indian and Asian studies [Tables 2 and 3]. Similar radiologic findings were observed in a cohort study of 348 patients.[23] Hence, in endemic areas, a good understanding and familiarity with the various radiologic findings of scrub typhus is essential in identifying pulmonary complications. The CNS findings of altered sensorium, meningitis, seizures with CSF findings of high protein levels, normal glucose, and lymphocytic preponderance were also described in another study done in 37 patients.[24] This has significant diagnostic implications, as an acute febrile illness with altered sensorium and signs of meningitis with no eschar may prompt the diagnosis of viral meningoencephalitis.
It was reported that serious complications often developed in the second week during the course of untreated illness,[25] which correlated with our findings: of 20 patients with complications, 13 developed in the second week of illness (65%), and 7 developed in the third week (35%). Nearly all complicated patients usually presented late, with complications already manifesting due to ignorance, illiteracy, and superstitious beliefs in the local population. According to our study, two patients had stage 2 AKI as per AKIN classification,[7] but none of them required dialysis. Different studies have reported different results [Tables 2 and 3]. Some studies in adults have shown AKI in 12%–22% of patients.[9] In our study, four of our patients developed ARDS while in the hospital. Among them, one died (case-specific mortality = 25%), which is equivalent to a study reported in Taiwan.[26] Two patients presented to us in the emergency department with overt hepatic failure in the setting of hepatic encephalopathy. A similar prevalence (5.2%) of hepatic encephalopathy has been described by Vikrant et al. in the Himalayan region.[27]
Leucocytosis with lymphocytic preponderance and thrombocytopenia (as in our study) have been noted in studies both from the Indian subcontinent and other Asian endemic zones [Tables 2 and 3]. In a study by Varghese et al. (n = 50), a combination of elevated transaminases, thrombocytopenia and leucocytosis displayed 80% specificity and 80% positive predictive value for scrub typhus diagnosis.[28] This could be especially useful to primary care physicians in hilly areas like ours, who may not have immediate access to confirmatory tests. However, this association needs to be confirmed by analytical epidemiological studies on larger samples. Favorable maternal and fetal outcomes were observed in our study which was comparable to another study in sub-Himalayan region by Kumar et al.[29] and in contrast to a study done by Rajan et al.[30] This may be due to a less virulent strain and/or earlier immunity due to persistent exposure to the vector in the Himalayan region.
This study was limited by small sample size. The study relied on IgM positivity through ELISA while indirect immunofluorescence assay is considered the gold standard test for scrub typhus diagnosis. (However, a correlation of 97% between IgM ELISA and SD BIOLINE tsutsugamushi rapid test was reported in India.[31]). In this study, serotyping and genotyping were not performed due a lack of resources.
Scrub typhus should be included in the list of differentials while evaluating a patient of acute undifferentiated febrile illnesses from rural background in a tropical country like India. In conclusion, scrub typhus can have a variety of clinical presentations, and indigenous patients hailing from the hilly regions of Uttarakhand regularly tend to have a less severe course of illness, often without a rash or eschar. Whether this is due to past exposure to the organism, variation in strain type, or other factors needs to be explored. Upper eyelid edema, facial edema, and pedal edema should also be considered as pointers to scrub typhus. The outcomes are better when compared with studies in other places, therefore indicating a less virulent strain and milder disease in the Himalayan regions. Primary care physicians can play a pivotal role to prevent this potentially fatal clinical entity from spiralling into a major public health issue by early recognition and treatment based on clinical presentation and simple laboratory parameters in the absence of gold standard confirmatory tests in resource-poor setting.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank the Principal of Veer Chandra Singh Garhwali Government Institute of Medical Science and Research (VCSGGIMS and R), Srinagar, Uttarakhand, India, for his help.
References
- 1.Chang WH. Current status of tsutsugamushi disease in Korea? J Korean Med Sci. 1995;10:227–38. doi: 10.3346/jkms.1995.10.4.227. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilak R, Kunwar R, Tyagi PK, Khera A, Joshi RK, Wankhade UB. Zoonotic surveillance for rickettsiae in rodents and mapping of vectors of rickettsial diseases in India: A multicentric study? Indian J Public Health. 2017;61:174–81. doi: 10.4103/ijph.IJPH_156_17. doi: 10.4103/ijph.IJPH_156_17. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Thakur S, Bhawani R, Kanga A, Ranjan A. Clinical profile and complications of scrub typhus: Hospital-based study in sub-Himalayan region. J Assoc Physicians India. 2016;64:30–4. [PubMed] [Google Scholar]
- 4.Mahajan SK, Rolain JM, Kashyap R, Bakshi D, Sharma V, Prasher BS, et al. Scrub typhus in Himalayas. Emerg Infect Dis. 2006;12:1590–2. doi: 10.3201/eid1210.051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal S, Sharma M, Kotian S, Juyal D, Singh A, Sharma N. Post disaster outbreak of scrub typhus in sub-Himalayan region of Uttarakhand. J Acad Clin Microbiol. 2016;18:95–9. [Google Scholar]
- 6.Bhargava A, Kaushik R, Kaushik RM, Sharma A, Ahmad S, Dhar M, et al. Scrub typhus in Uttarakhand & adjoining Uttar Pradesh: Seasonality, clinical presentations & predictors of mortality? Indian J Med Res. 2016;144:901–9. doi: 10.4103/ijmr.IJMR_1764_15. doi: 10.4103/ijmr.IJMR_1764_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–8. [PubMed] [Google Scholar]
- 9.Subbalaxmi MVS, Chandra N, Teja VD, Lakshmi V, Rao MN, Raju YSN. Scrub typhus – Experience from a South Indian tertiary care hospital. BMC Infect Dis. 2012;12:77. [Google Scholar]
- 10.Sivarajan S, Shivalli S, Bhuyan D, Mawlong M, Barman R. Clinical and paraclinical profile, and predictors of outcome in 90 cases of scrub typhus, Meghalaya, India? Infect Dis Poverty. 2016;5:91. doi: 10.1186/s40249-016-0186-x. doi:10.1186/s40249-016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takhar RP, Bunkar ML, Arya S, Mirdha N, Mohd A. Scrub typhus: A prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India. 2017;30:69–72. [PubMed] [Google Scholar]
- 12.Zhang M, Zhao ZT, Wang XJ, Li Z, Ding L, Ding SJ. Scrub typhus: Surveillance, clinical profile and diagnostic issues in Shangdong, China? Am J Trop Med Hyg. 2012;87:1099–104. doi: 10.4269/ajtmh.2012.12-0306. doi:10.4269/ajtmh.2012.12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu KM, Wu ZW, Peng G, Wu JL, Yilee S. Radiologic pulmonary findings, clinical manifestations and serious complications in scrub typhus: Experiences from a teaching Hospital in Eastern Taiwan. Int J Gerontol. 2009;3:223–32. [Google Scholar]
- 14.Hamaguchi S, Cuong NC, Tra DT, Doan YH, Shimizu K, Tuan NQ, et al. Clinical and epidemiological characteristics of scrub typhus and murine typhus among hospitalized patients with acute undifferentiated fever in Northern Vietnam. Am J Trop Med Hyg. 2015;92:972–8. doi: 10.4269/ajtmh.14-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brummaier T, Kittitrakul C, Choovichian V, Lawpoolsri S, Namaik-larp C, Wattanagoon Y. Clinical manifestations and treatment outcomes of scrub typhus in a rural health care facility on the Thailand-Myanmar border? J Infect Dev Ctries. 2017;11:407–13. doi: 10.3855/jidc.8912. doi:https://doi.org/10.3855/jidc.8912. [DOI] [PubMed] [Google Scholar]
- 16.Prakassh JA, Kavitha ML, Mathai E. Nested polymerase reaction on blood clots for gene encoding 56Kda antigen and serology for the diagnosis of scrub typhus. Indian J Med Microbiol. 2011;29:47–50. doi: 10.4103/0255-0857.76524. [DOI] [PubMed] [Google Scholar]
- 17.Trowbridge P, P D, Premkumar PS, Varghese GM. Prevalence and risk factors for scrub typhus in South India? Trop Med Health. 2017;22:576–82. doi: 10.1111/tmi.12853. doi:10.1111/tmi.12853. [DOI] [PubMed] [Google Scholar]
- 18.Gurung S, Pradhan J, Bhutia PY. Outbreak of scrub typhus in the North East Himalayan region – Sikkim: An emerging threat. Indian J Med Microbiol. 2013;31:72–4. doi: 10.4103/0255-0857.108729. [DOI] [PubMed] [Google Scholar]
- 19.Allen AC, Spitz S. A comparative study of the pathology of scrub typhus (tsutsugamushi disease) and other rickettsial diseases. Am J Pathol. 1945;21:603–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Scheie HG. Ocular changes in scrub typhus.A study of 451 patients. Trans Am Opthamol Soc. 1947;45:637–77. [PMC free article] [PubMed] [Google Scholar]
- 21.Elsom KA, Beebe GW, Sayen JJ, Scheie HG, Gammon GD, Wood FC. Scrub typhus: A follow-up study. Ann Intern Med. 1961;55:785–95. doi: 10.7326/0003-4819-55-5-784. [DOI] [PubMed] [Google Scholar]
- 22.Mokta J, Yadav R, Mokta K, Panda P, Ranjan A. Scrub typhus – The most common cause of Febrile Jaundice in a tertiary care hospital of Himalayan State. J Assoc Physicians India. 2017;65:47–50. [PubMed] [Google Scholar]
- 23.Abhilash K, Mannam PR, Rajendran K, John RA, Ramasami P. Chest radiographic manifestations of scrub typhus. J Postgrad Med. 2016;62:235–8. doi: 10.4103/0022-3859.184662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rana A, Mahajan SK, Sharma A, Sharma S, Verma BS, Sharma A. Neurological manifestations of scrub typhus in adults. Trop Doct. 2017;47:22–5. doi: 10.1177/0049475516636543. [DOI] [PubMed] [Google Scholar]
- 25.Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31:240–4. [PubMed] [Google Scholar]
- 26.Wang CC, Liu SF, Liu JW, Chung YH, Su MC, Lin MC. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–52. [PubMed] [Google Scholar]
- 27.Vikrant S, Dheer SK, Parashar A, Gupta D, Thakur S, Sharma A, et al. Scrub typhus associated acute kidney injury – A study from a tertiary care hospital from western Himalayan state of India? Ren Fail. 2013;35:1338–43. doi: 10.3109/0886022X.2013.828257. doi:10.3109/0886022X.2013.828257. [DOI] [PubMed] [Google Scholar]
- 28.Varghese G, Abraham O, Mathai D, Thomas K, Aaron R, Kanitha M, et al. Scrub typhus among hospitalised patients with febrile illness in South India: Magnitude and clinical predictors. J Infect. 2006;52:56–60. doi: 10.1016/j.jinf.2005.02.001. doi: 10.1016/j.jinf.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Thakur S, Bhawani R, Kanga A, Ranjan A. Clinical profile of scrub typhus in pregnancy in Sub-Himalayan Region. J Obstet Gynaecol India. 2016;66(Suppl 1):82–7. doi: 10.1007/s13224-015-0776-8. doi:10.1007/s13224.015.0776.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajan SJ, Sathyendra S, Mathuram AJ. Scrub typhus in pregnancy: Maternal and foetal outcomes. Obstet Med. 2016;9:164–6. doi: 10.1177/1753495X16638952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalawat U, Rani ND, Chaudhary A. Seroprevalence of scrub typhus at a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol. 2015;33:68–72. doi: 10.4103/0255-0857.148381. doi:10.4103/0255-0857.14831. [DOI] [PubMed] [Google Scholar]


