Abstract
Background and Purpose:
Cerebral venous thrombosis (CVT) is characterized by its clinical pleomorphism and pathogenetic variability. We studied 87 patients with CVT associated with pregnancy, and puerperium to find clinical profile, neuroradiological presentation and prognosis.
Methods:
A retrospective analysis was conducted based on clinical records at our institute between June 2008 and June 2018 without any personally identifying information. The diagnosis of CVT was confirmed by magnetic resonance imaging, angiography, or neuropathological study.
Results:
Among 87 patients, there were 82 of CVT associated with puerperium and 5 during pregnancy. Fifty-five women were multiparous. Cesarean delivery, hypertension, and anemia were strongly and significantly associated with CVT. Final outcome was considered good with anticoagulation; mortality rate was 10.34%.
Conclusion:
CVT associated with pregnancy, and puerperium is not an uncommon entity and has acute onset and a better prognosis. Pregnancy-related hypertension and Cesarean delivery and anemia are important risk factors for CVT. Further studies are needed to evaluate the need for therapeutic strategies for patients with CVT during pregnancy and puerperium.
Keywords: Pregnancy, prognosis, puerperium, venous thrombosis
Introduction
In 1825, Ribes described the clinical history of a 45-year old who died after a 6-month history of severe headache, epilepsy, and delirium. Postmortem examination showed thrombosis of superior sagittal sinus thrombosis (SSS), left lateral sinus (LS), and a cortical vein in parietal cortex. This was probably the first detailed description of cerebral venous thrombosis (CVT). The first ever description of superior SSS occurring in puerperium was by Abercrombie in 1828.[1]
CVT may result from multiple factors, including hypercoagulable states, as found in pregnancy and the puerperium, oral contraceptive pills (OCPs), infection, malignancy, and direct injury to the venous system in the head or neck. Pregnancy or the use of OCPs in patients with an underlying genetic susceptibility, such as factor V Leiden or factor II G20210Aa mutations or hyperhomocysteinemia, compounds the risk of CVT.[2,3,4]
In general population and during pregnancy, CVT is a rare diagnosis, but it is one of the most common cerebrovascular complications in pregnancy, affecting 11.6 per 100,000 deliveries.[5] Studies have demonstrated a wide range of incidence of CVT-attributable pregnancy-related stroke, from 6% to 64%.[6] we report clinical profile of 87 patients presenting to our institute who were diagnosed to have CVT over a period of 10 years. The aim of this paper was to analyze these 87 cases, to disclose some clinical, paraclinical, or prognostic features that may be distinctive for patients with CVT associated with pregnancy and puerperium.
Methods
Patient with a diagnosis of CVT admitted to our hospital were included during pregnancy, delivery, and puerperium (within 6 weeks after delivery). We identified 87 women aged 18–40 years at the time of index event admitted to our center from June 2008 through June 2018. CVT was suspected from the history or clinical examination and proven after magnetic resonance imaging and magnetic resonance venography study of the brain. Exclusion criteria were (1). Women over 40 years of age, (2). Clinical and radiological records were incomplete, (3) radiological studies were inconclusive, (4) Cavernous sinus thrombosis.
The demographic characteristics of patients were collected. History and pertinent examination findings were recorded including obstetric systemic complications and maternal prognoses. Imaging studies of patients were collected. The study was approved by institutional ethics board, and the requirement for informed consent was waived because of the retrospective nature of the study and deidentification of data. Clinical evaluation of patients was assessed using the modified Rankin scale on the day of discharge.
Results
The mean age of patients was 26 years (range 18–40 years). Regarding puerperal patients, 29 cases of CVT (33.3%) occurred during the first week after delivery and 53 (60.91%) during weeks second and third postpartum. Pregnant patients were affected in any trimester (one in first, two in the second, and two in the third trimester. There were 55 multiparous (63.22%), 54 women (62%) from low or rural stratum, and 37 (42.5%) whose delivery was conducted at home by midwives and who did not have proper prenatal care. Two patients had a history of venous thrombosis outside central nervous system (CNS) during previous pregnancies (one patient had swelling of limbs and was found to have nephritic syndrome).
Following were the predisposing factors for CVT: nine patients were using OCP, thrombocytosis in four patients, secondary polycythemia in three cases, circulating lupus anticoagulant in three (patients had history of recurrent abortions and found to have antiphospholipid antibody syndrome), and protein S deficiency in three patients. In addition, an arteriovenous malformation was present in two patients and one patient was found to have adenocarcinoma of the stomach and metastatic carcinoma in two patients [Figure 1]. Risks of CVT increased with increasing maternal age, Cesarean delivery, in the presence of several comorbid conditions: hypertension; infection; excessive vomiting in pregnancy; and fluid, electrolyte, and acid-base disorders.
Figure 1.
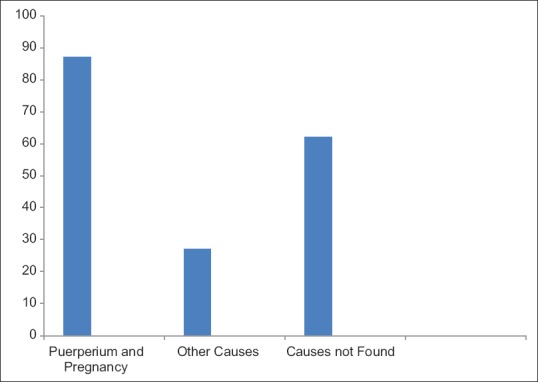
Risk factors for cerebral venous thrombosis
Most of patients presented with history of headache and vomiting. Headache was the complaint in 64 (73.5%) and vomiting occurred in 53 (60.6%) patients. Seven (8%) of the patients presented with focal seizures and two (2.29%) with generalized seizures. Six (6.89%) patients presented with altered sensorium [Table 1].
Table 1.
Presenting symptoms in 87 patients with cerebral venous thrombosis
| (n=87) | ||
|---|---|---|
| n | % | |
| Headache seizures | 64 | 73.8 |
| Generalized | 2 | 2.29 |
| Focal | 7 | 8 |
| Disorder of consciousness | 6 | 6.8 |
| Vomiting | 53 | 60.6 |
The most common clinical finding was the disc edema which was seen in 51 (58.16%) patients. Hemiparesis was seen in 24 (27.58) and aphasia had 6 (6.8%). Seventeen (19.5%) had bilateral lateral rectus palsy and multiple extraocular cranial nerve involvement was seen in three (3.44%) patients.
Anemia was more often present and was usually related to inadequate supply of iron during pregnancy with or without blood loss during delivery and puerperium (hypo chromic 61%; normochromic 29%). About 43.4% had leukocytosis with neutrophilic predominance.
Cesarean delivery was a strong and highly significant predictor (50.5%). Hypertension was also a strong and highly significant predictor. Among hypertensive CVT, seven had essential hypertension, one had renovascular hypertension, four had gestational hypertension, five had preeclampsia, two had eclampsia, three had pre-eclampsia or eclampsia superimposed on pre-existing hypertension, and three had unspecified hypertension.
The diagnosis was confirmed only after magnetic resonance imaging, magnetic resonance venography, or if needed digital section angiography (DSA) of brain study. The most commonly involved sinus was SSS. Thrombosis of SSS was seen in 50 patients (57.4%). Fifteen (17.24%) patients had involvement of right transverse sinus. Nine (10.34%) patients had involvement of left transverse sinus. Seven (8.04%) patients had involvement of right sigmoid sinus. Five (5.74%) patients had involvement of left sigmoid sinus. One (1.14%) patient had internal cerebral vein thrombosis [Figure 2]. Parenchymal involvement (venous infarct and intracerebral hemorrhage) was seen in 48 patients (55.17%) with frontal lobe involvement in 26 patients (29.8%), left parietal lobe involvement in 9 patients (10.34%), and bilateral thalamic involvement in 2 (2.29%). Twenty-six (29.8%) had bilateral hemorrhagic infarction in frontal lobe. Eight (10.34%) had bilateral frontal lobe hemorrhage. Twelve (13.79%) had infarction in frontal lobe and nine (10.34%) in parietal lobe [Figure 3]. Out of 87 patients studied, 9 patients (10.34%) were expired. Three patients had to keep on ventilator for 3 weeks and one improved later on. Most of patients received anticoagulation therapy, because it is well known that anticoagulation therapy in the early stages of CVT modifies its natural history and had good outcome. Our series shows that prognosis of patients treated with anticoagulation therapy is good.
Figure 2.
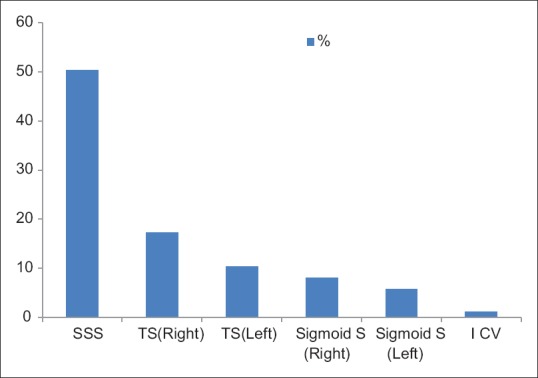
Type of cerebral sinus involvement in patients
Figure 3.
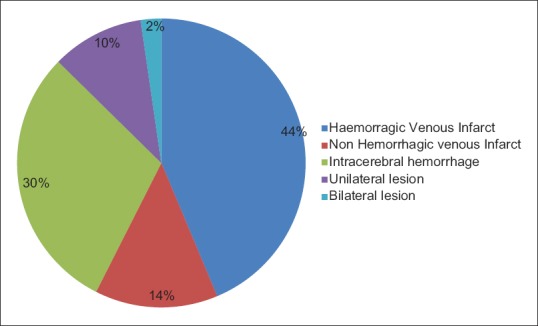
Neuroradiological findings: Parenchymal involvement in patients
Discussion
A wide variety of well-known conditions may cause or predispose to CVT, and their relative importance may defer in different areas of the world.[7] This complication is common in our country, with a prevalence of 4.5/1000 obstetric admissions. Pregnancy and puerperium are common causes of transient prothrombotic states. Approximately 2% of pregnancy-associated strokes are attributable to CVT.[8] In accordance with an obstetric CVT series, symptoms of CVT appeared in first 3 weeks after delivery in the majority of cases, and as in this study, women who had home deliveries and poor prenatal care were more often affected. CVT should be suspected in any female who develops neurological symptoms in immediate postpartum period, since nearly 33% of our cases occurred in first week after childbirth. CVT occurred 13 times more often during puerperium than during pregnancy. This study shows that multiparas are more often affected than primiparas, in a proportion of 2:1; this substantiates previous study.[7,9]
CVT presents with a remarkably wide spectrum of signs and mode of onset, thus mimicking numerous other disorders.[7,10] The most common symptoms and signs are headache, seizures, focal neurological deficits, altered consciousness, and papilledema, which can present in isolation or in association with other symptoms.[11] About 73.8% of patients in our study had history of headache. In this study, we found 55.17% of parenchymal lesions (venous infarct and intracerebral hemorrhage).[7] This neuropathological findings also explain the high mortality rate in our whole series.(10.34%). In our study, we featured a disease with sudden or acute onset with a progressive course that tends to become stable or begin to subside in a week. This suggests that the pathological process leading to venous occlusion develops faster but is usually self-limited and resolves in a shorter time; however, it should be born in mind that puerperium represents an important clue for suspecting CVT.[11,12]
Another important observation was the high frequency of anemia in our series, probably reflecting a poor diet during pregnancy and/or blood loss during delivery. Other series has also recognized high prevalence of anemia and this deserves further large-scale study to clarify its role in pathogenesis.[7]
Traditionally, the most frequently postulated mechanisms involved in CVT is hypercoagulable states associated with dehydration and anemia.[8] However, other factors may play a role, such as protein S deficiency, which is common during pregnancy and puerperium. Other studies have reported similar observations.[5,13]
The associations of CVT with Cesarean delivery may in part reflect a higher likelihood of Cesarean delivery among women who had a CVT during pregnancy, particularly in the presence of hypertension or if the event was an hemorrhagic infarction.[6] Early authors particularly advocated cesarean delivery after CVT during pregnancy to avoid the presumed increased risk of bleeding associated with the hemodynamic changes of labor and vaginal delivery. In contrast, the associations of CVT with cesarean delivery suggest that cesarean delivery may increase the risk of these events. Surgery has long been associated with and apparently precipitates venous thrombosis. Levels of protein C decrease after surgery, presumably because surgically induced tissue damage induces the activation of blood clotting with increased thrombin generation, which, in turn, both activates protein C and accelerates its clearance from plasma.[14] Postsurgical decrease in protein C may compound a hereditary or pregnancy-associated resistance to activated protein C and thereby contribute to an increased incidence of thrombosis in postsurgical patients.[5] Another less likely possibility is that underlying conditions that lead to Cesarean delivery also precipitates postpartum CVT. The very strong association of pregnancy-related hypertension with CVT is consistent with previous findings and with the known path physiology of cerebrovascular complications of hypertension.[5] A number of studies have suggested that infection may trigger CVT, particularly in young patients, although plausible underlying pathogenetic mechanisms remain to be determined.[6,14]
Sinuses most frequently involved were SSS and LS. Our series shows that the most commonly involved sinuses were SSS and LS, which were involved in 57.4% and 27.5% cases, respectively. Possibly CVT in obstetric subjects is a benign entity because of limited and transient occlusion, with rapid sinovenous recanalization by spontaneous thrombolysis or development of collaterals. Prognosis and treatment are the most controversial issues of CVT. Prognosis is quite variable.[15] The outcome can range from total recovery to death. Since the introduction of magnetic resonance imaging, an increasing number of patients with CVT have been diagnosed. This may explain the well-established benefits of early anticoagulation in our study as most of patients received anticoagulation. This justify the risk of anticoagulation for severe and wherever needed for complete recovery. Our series shows that prognosis of patients treated with anticoagulation therapy is good. Based on the experience acquired at our institution, we recommend anticoagulation in patients with the factors associated with a bad prognosis. Any young female during pregnancy and in the puerperal period who present with headache and focal neurological deficit in the puerperal period may be suffering from CVT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Liang CC, Chang SD, Lai SL, Hsieh CC, Chueh HY, Lee TH. Stroke complicating pregnancy and the puerperium. Eur J Neurol. 2006;13:1256–60. doi: 10.1111/j.1468-1331.2006.01490.x. [DOI] [PubMed] [Google Scholar]
- 2.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–82. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 3.Feske SK, Singhal AB. Cerebrovascular disorders complicating pregnancy. Continuum (Minneap Minn) 2014;20:80–99. doi: 10.1212/01.CON.0000443838.95260.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Complan L. Uncommon Causes of Stroke. 2nd ed. United Kingdom: Cambridge University Press; 2008. p. 560. [Google Scholar]
- 5.Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. American Heart Association Stroke Council on Epidemiology and Prevention.Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–92. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 6.Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F ISCVT Investigators. Prognosis of Cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thromobosis (ISCVT) Stroke. 2004;35:664–70. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 7.Aberombei J. Pathological and Practical Researches of the Brain and Spinal Cord Publ. Edinburgh: John Carfare and Sons; 1828. Superior sagittal thrombosis in puerperium. [Google Scholar]
- 8.Breteau G, Mournier Vehier F, Godefroy O, Gauvrit JY, Mackowiak-Cordoliani MA, Girot M, et al. Cerebral venous thrombosis: Three year clinical outcome in 55 consecutive patients. J Neurol. 2003;250:29–35. doi: 10.1007/s00415-003-0932-4. [DOI] [PubMed] [Google Scholar]
- 9.Vladutiu DJ, Meyer ML, Malek AM, Stuebe AM, Mosher A, Aggarwal S, et al. Racial Differences in the association between parity and incident stroke: Results from the reasons for geographic and racial differences in stroke study. J Stroke Cerebrovasc Dis. 2017;26:749–55. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Takahashi JC, Takenobu Y, Suzuki N, Ogawa A, Miyamoto S. Strokes associated with pregnancy and puerperium: A nationwide study by the Japan stroke society. Stroke. 2017;48:276–82. doi: 10.1161/STROKEAHA.116.014406. [DOI] [PubMed] [Google Scholar]
- 11.Cantu C, Barinagarrementia F. Cerebral Venous thrombosis associated with pregnancy and puerperium: Review of 67 cases. Stroke. 1993;24:1880–4. doi: 10.1161/01.str.24.12.1880. [DOI] [PubMed] [Google Scholar]
- 12.Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, et al. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke. 2009;40:1162–8. doi: 10.1161/STROKEAHA.108.540880. [DOI] [PubMed] [Google Scholar]
- 13.James AH, Bushnell CD, Jaminson MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–16. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 14.Pabinger I, Grafenhofer H, Kyrle PA, Quehenberger P, Mannhalter C, Lechner K, et al. Temporary increase in the risk for recurrence during pregnancy in women with history of venous thromboembolism. Blood. 2002;100:1060–2. doi: 10.1182/blood-2002-01-0149. [DOI] [PubMed] [Google Scholar]
- 15.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. American Heart Association Stroke Council.2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke. Regarding Endovascular Treatment: A Guideline for Healthcare stroke Association. Stroke. 2015;46:3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]


