Abstract
Introduction:
Classic dengue fever presentation has expanded its horizon by involving various organ systems and is named as expanded dengue syndrome. This changing presentation and rising burden across the globe may lead to delayed diagnosis and under reporting of this syndrome.
Aim of Study:
To analyze clinicolaboratory profile of patients with expanded dengue syndrome.
Materials and Methods:
About 520 cases of expanded dengue syndrome as per World Health Organization definition criteria 2012 were studied with their informed consent. Detailed history, thorough clinical examination, and relevant investigations were done. Their clinical and laboratory parameters were analyzed. Standard treatment guidelines were followed in all cases.
Observation:
About 301 patients were male and 219 were female with male-to-female ratio of 3:2. Their age varied from 12 to 76 years with the average age of 47.5 years. About 92% of cases presented with various gastro hepatic manifestations. The commonest gastrohepatic manifestation was transaminitis (57.5%) that is asymptomatic elevation of liver enzymes followed by acalculous cholecystitis (21%) and acute pancreatitis (13.9%). Twenty-nine patients presented with various neurological manifestations. Three patients presented with acute kidney injury and eight patients had coinfection with malaria. Fever with nausea and vomiting was the most common presentation. About 15% of patients presented with bleeding manifestations. About 40.6% of patients presenting as abdominal manifestations had platelet count <20,000/mm3 and needed platelet transfusion versus 9.8% with other system involvement (central nervous system, cardiovascular system (CVS), renal). Hepatomegaly was the most common ultrasonography (USG) finding being present in 57.5% of patients followed by acalculous cholecystitis in 21.3%. Total mortality was 1.9% in our series. We lost eight patients presenting with neurological manifestations and two patients with coinfection with malaria.
Conclusion:
Atypical presentations should prompt us to investigate for dengue especially during ongoing epidemics so that expanded dengue syndrome can be diagnosed and treated early.
Keywords: Acalculous cholecystitis, expanded dengue syndrome, transaminitis
Introduction
Dengue fever is a mosquito-borne viral disease, which has reached alarming proportions in the past few years. It is endemic in over 100 countries.[1] About 40% of people all over the world live in countries where dengue is endemic. Annually 390 million infections and 5,00,000 dengue hemorrhagic fever cases have been identified. Dengue cases in India are also on a sharp rise over past few years to the tune of 28,292 cases in 2010 to 1,11880 cases in 2016.[2] posing not only a challenge to clinicians but also a threat to the community at large. Dengue virus is an arthropod borne virus of genus flavivirus belonging to family Flaviviridae. It is a single-stranded RNA virus. There are four genetically related but antigenically distinct DEN serotypes (DENV-1, DENV-2, DENV-3, DENV-4) all of which are prevalent in India.
The classic presentation of dengue fever has expanded its horizon by involving various organ systems. These system specific presentations pose a diagnostic dilemma. Therefore, World Health Organization (WHO), in 2012, incorporated a new terminology known as expanded dengue syndrome. In expanded dengue syndrome, patient usually presents with atypical manifestations with various systemic involvement. Recognition of features of expanded dengue syndrome (EDS) is very important for targeting treatment option.
Aim of the study
We have undertaken this study with the aim to analyze the clinicolaboratory profile of patients with expanded dengue syndrome.
Materials and Methods
This is a hospital-based prospective observational study. All adults with clinical and laboratory parameters of serologically confirmed cases of expanded dengue syndrome as per WHO definition criteria were taken in to the study design with their informed consent and was conducted over a period of one year from April 2017 to March 2018. Cases with classical dengue fever, dengue hemorrhagic fever, and dengue shock syndrome were excluded from the study. Detailed history and thorough clinical examination was done in all cases. Complete blood picture, fasting blood sugar, serum creatinine, liver function tests, serum protein A/G ratio, viral markers (HIV, HBSAG, HCV), blood for malaria parasite, chest radiograph, and ultrasonography of abdomen and pelvis were done in all cases. Other investigations, such as computed tomography (CT) brain, electroencephalogram (EEG), magnetic resonance imaging (MRI) brain and spine, lumbar puncture, and cerebrospinal fluid (CSF) examination were done as and when indicated. Their age and sex distribution, signs and symptoms, hematological parameters, clinical course of the illness, and outcome were analyzed in detail. They were treated with intravenous fluids, antibiotics, blood and blood component therapy, and other supportive care as indicated. Statistical analysis was performed using the SPSS software, Version 34. Total numbers and percentages were calculated for different categorical variables, such as clinical features and biochemical parameters.
Observations
Overall, 1,493 cases of dengue fever were diagnosed as per WHO criteria during the last academic year mentioned. About 520 cases were diagnosed to have expanded dengue syndrome and were taken into study design. About 301 patients were male and 219 were female with male-to-female ratio of 3:2. Their age varied from 12 to 76 years with the average age of 47.5 years. About 69% of patients belong to the age group of 31–60 years indicating that mostly young and active people are getting the infection [Figure 1].
Figure 1.
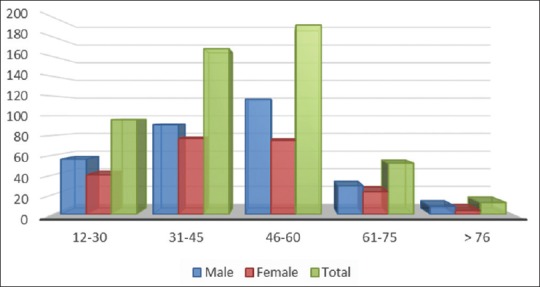
Age and Sex variation
Analysis of clinical manifestations revealed that most commonly the gastrohepatic system was affected followed by CNS involvement. In our series, we had 92% of cases of EDS who presented with different gastrohepatic manifestation. About 5.5% cases presented with neurological symptoms. Three cases had acute kidney injury who needed renal replacement therapy in the form of haemodialysis. Eight patients had coinfection with malaria and one patient had cardiovascular involvement in the form of sinus bradycardia [Figure 2].
Figure 2.
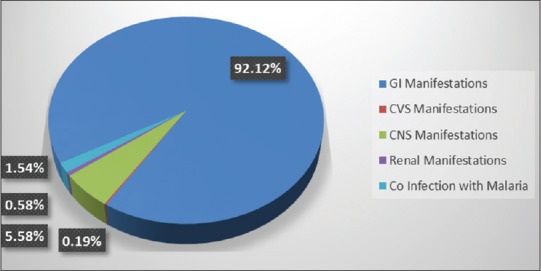
Clinical manifestations
Further system wise analysis was done. Out of 92% of cases presenting with various gastrointestinal and hepatic manifestations, 57.5% of the cases had features of asymptomatic elevation of liver enzymes followed by 21.35% of cases who had features of acalculous cholecystitis. Acute pancreatitis was detected in 12.88% of cases. Two patients had features of subacute intestinal obstruction. They presented as acute abdomen. Initially, they were admitted in surgical ward and later transferred to medical ward after physicians’ review. All of them improved with supportive care. Cerebral infarct and transverse myelitis were rare findings both contributing 0.19% each. Fever with nausea and vomiting was the most common presentation followed by malaise and pain abdomen. About 9% of patients presented with rashes; 15% of patients (n = 72) presented with bleeding manifestations; 36% of patients with abdominal manifestations had petechiae followed by features of upper gastrointestinal bleeding in 23% of cases [Figure 3].
Figure 3.
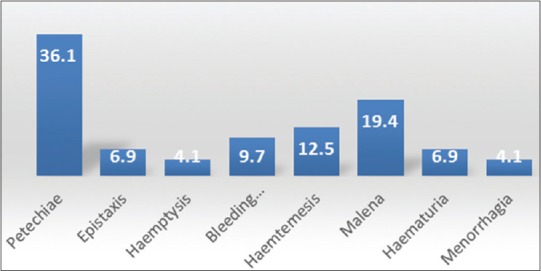
% Wise Bleeding Manifestation
About 40.6% of patients presenting as abdominal manifestations had platelet count <20,000/mm3 in comparison to 9.8% in patients who presented with other system involvement (central nervous system [CNS], CVS, renal), which is statistically significant (P-value <0.0001). About 50.84% had their platelet count between 21,000 and 49,000/mm3. Only 8.56% of cases had platelet count >50,000/mm3 [Figure 4]. About 57% of patients who presented with abdominal symptoms along with features of bleeding and low platelet count needed platelet transfusion. Gastrointestinal involvement was correlated with platelet count and Pearson correlation was found to be −0.3823 which is significant with a P value of <0.0001 and the 95% confidence interval for r was −0.4534 to −0.3064.
Figure 4.
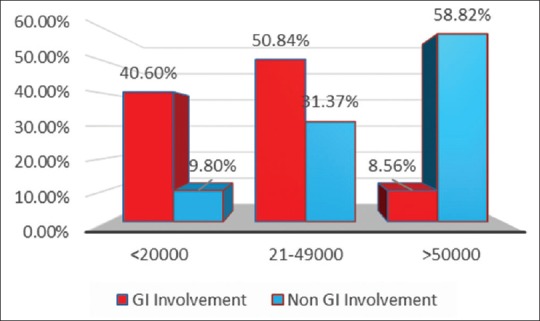
Platelet count
About 57.5% of cases of EDS showed elevation of transaminases. aspartate aminotransferase (AST) peaked at day 4 of illness, whereas alanine aminotransferase (ALT) peaked at day 6. After peaking at days 4 and 6, the AST and ALT levels gradually declined. The AST levels were much higher than ALT level. The mean AST levels on day 4 (mean 283.44, SD ± 20.1 IU) were more than twofold when compared with the mean AST values (mean = 123.4, SD ± 41.8 IU). Hepatomegaly was the most common ultrasound finding in patients with EDS being present in 299 (57.5%) patients followed by acalculous cholecystitis, which was detected in 111 patients (21.3%). Serositis in the form of ascites and pleural effusion was also found in 25.3% and 23.2% of cases, respectively. About 12.8% had features of pancreatitis. Twenty-nine cases presented with various neurological manifestations. Fifteen patients had features of acute viral encephalitis. All of them presented with complains of fever and altered sensorium. CT brain was done in all cases. Analysis of CSF was suggestive of viral encephalitis. They were treated aggressively. Ten patients recovered and five patients deteriorated and developed multiorgan dysfunction, needed mechanical ventilation, and other supportive care. Despite all measures they succumbed to the illness. Intracranial bleed occurred in five patients. Three of them had massive intracranial bleed who deteriorated rapidly and expired. Though bleeding complications are well known in dengue fever, one patient was diagnosed to have cerebral infarct after neuroimaging studies. Five patients had meningitis all of whom improved with conservative management. We had two patients who had features of hypokalemic palsy. Their nerve conduction velocity and electromyography were normal. They were treated with intravenous potassium chloride infusion resulting in rapid improvement of their motor power. Though spinal cord involvement in the form of acute transverse myelitis is an uncommon manifestation we had one patient in our series. His MRI spine showed segmental T2 hyperintensity of the spinal cord from D4 to D6 level [Figures 5 and 6] involving predominantly the central component. He was treated with injection methylprednisolone followed by adjuvant IVIG 2 g/kg (total 120 g) over 5 days and other supportive care along with physiotherapy. He improved substantially after 4 months, and after 7 months, he recovered completely.
Figure 5.
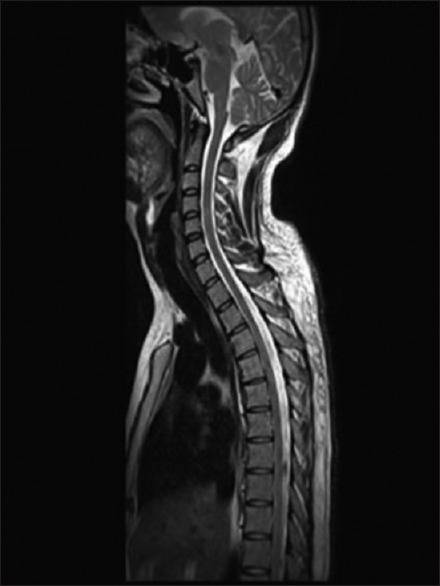
MRI Spine T2 Image showing Hyperintensity from D4-D6 level
Figure 6.
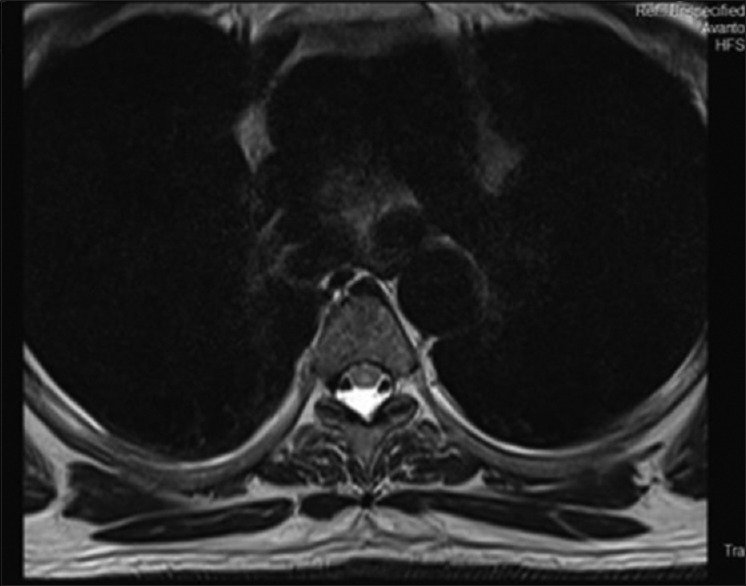
Transverse Section MRI Spine D4 level Showing Hyperintensity
Three cases of dengue presented with features of acute kidney injury (AKI) were treated with renal replacement therapy and improved. Eight cases had coinfection with malaria out of which five cases developed multiorgan dysfunction. Four patients survived and one with plasmodium falciparum expired after a long stay of 42 days in critical care unit despite aggressive management with ventilatory support, hemodialysis, blood and blood component therapy, and other supportive care. Another patient with plasmodium vivax malaria and dengue also succumbed the illness. He had G6PD deficiency too. Total mortality was 1.9% in our series. We lost eight patients presenting with neurological manifestations and two patients with coinfection with malaria.
Discussion
Expanded dengue syndrome was coined by WHO in the year 2012 to describe cases which do not fall into either dengue shock syndrome or dengue hemorrhagic fever. The atypical manifestations noted in expanded dengue are multisystemic and multifaceted with organ involvement, such as liver, brain, heart, kidney, and CNS.[3] Patients with involvement of gastrointestinal and hepatic system may present with features of asymptomatic elevation of liver enzymes, fulminant hepatic failure, acute pancreatitis, acalculous cholecystitis, peritonitis, sub acute intestinal obstruction (SAIO), and rupture of spleen. Lee et al. observed transaminitis in 30% of patients,[4] whereas we observed it in 57.5% of cases. Acalculous cholecystitis has been documented in many case reports. A study by Bhatty et al. in 2009 reported 27.5% of cases as acalculous cholecystitis.[5] In our series, we had 21.3% cases of cholecystitis. Some studies also showed as high as 38% of cholecystitis in EDS.[6] These patients had an increased levels of alkaline phosphatase, thickened gallbladder wall, and pericholecystic fluid collection. The pathogenesis of acute acalculous cholecystitis is still unclear. Many factors are thought to contribute to liver dysfunction. They are hypoxic injury due to decreased perfusion, direct damage by the virus, and immune-mediated injury. Shaprio et al. in his studies showed that cholestasis, increased bile viscosity, and infection are the probable causes.[7] However, the main cause which induces thickening of gall bladder wall is increased vascular permeability, which causes plasma leakage and serous effusion with high protein content mostly albumin. All patients with acalculous cholecystitis recovered fully with conservative management. None of them required any surgical intervention similar to other studies. Acute pancreatitis is an atypical and rare presentation.[8,9,10,11] We had 67 cases of expanded dengue presenting with features of acute pancreatitis, which was evident by raised serum amylase and lipase and ultrasound findings. The exact pathogenesis of pancreatic involvement in dengue is not known. But it can be due to result of direct invasion by the virus itself causing inflammation and destruction of pancreatic acinar cells, an autoimmune response to pancreatic islet cells, and development of edema of the ampulla of Vater with obstruction to the outflow of pancreatic fluid.[12,13] Fortunately all our patients survived. We had two patients who presented with features of subacute intestinal obstruction. They improved with supportive care. Only one case is documented so far in literature.[14] The exact pathophysiology of subacute intestinal obstruction is not known. Probably, it occurs due to edema of intestinal wall. These patients presenting as intestinal obstruction usually get admitted in surgical wards. Same thing happened to our cases. They were initially admitted in surgical ward and later transferred to medical side. Sometimes unnecessary surgery even takes place under confusion as EDS may present in form of acute abdomen.[15] Even cases are reported where three uncommon presentations occurred concomitantly (acute acalculous cholecystitis, acute pancreatitis, and pancytopenia) most likely due to hemophagocytic syndrome.[16]
Though sinus bradycardia is more common, patient may present with features of myocarditis, pericarditis, acute myocardial infarction, cardiomyopathy, sinoatrial (SA) node, atrio-ventricular (AV) nodal block, and atrial fibrillation. Dengue myocarditis incidence is low because it is asymptomatic and diagnosis is easily missed. Almost all cases of dengue myocarditis are self-limiting and severe myocarditis leading to dilated cardiomyopathy is extremely rare.[17,18] Bradycardia followed by complete heart block is also documented.[19] We have encountered one patient who had bradycardia in expanded dengue. The pathophysiology of cardiac involvement in dengue is variable. The favoured theory is that the virus cause inflammation leading to cytokine storm causing loss of both structural and functional integrity. Localized insult due to minute bleeding involving the SA node, AV node, or within its close vicinity can also cause transient conduction abnormalities.[20,21] Neurological manifestations are more commonly observed and reported involving both central and peripheral nervous system.[22] Patient can present with features of encephalitis, meningitis, stroke (both haemorrhagic and ischemic), hypokalemic paralysis, encephalopathy, seizures, mono-neuropathy, polyneuropathy, and Guillain-Barre or Miller-Fisher syndromes. Dengue virus infection involving spinal cord is extremely rare. Only few cases of transverse myelitis in patients with dengue fever have been reported so far. We had 29 patients (5.5%) who had various neurological manifestations. Though bleeding complications are more common, we had one patient who had cerebral infarct. We had five patients with meningitis and 15 with encephalitis. In these patients, there was CSF pleocytosis along with positive enzyme linked immunosorbent assay (ELISA) for dengue. We had one patient who was diagnosed as acute transverse myelitis following dengue infection. His MRI spine showed segmental T2 hyperintensity of the spinal cord from D4 to D6 levels. There was no evidence of cord expansion or contrast enhancement or involvement of the other segments of the spinal cord. He was treated with injection methylprednisolone followed by adjuvant IVIG 2 g/kg (total 120 g) over 5 days and other supportive care and improved. There are case reports documenting transverse myelitis in dengue who were treated with steroid and immunoglobulins as we did.[23,24] The exact pathogenesis of neurological manifestations of dengue viral infection is not known. However, there are several postulations. Most important is either the neurotropic effect of the virus or the immune-mediated injury or both. When the neurological symptoms develop in peri-infectious period, it is attributed to direct viral invasion of the nervous tissue. Delayed appearance of neurological disorders usually in postinfectious phase are considered to be due to immunologically mediated neural injury.[25,26,27,28] Acute hypokalemic quadriparesis is an uncommon presentation of dengue fever, not yet widely recognized. Only few cases are reported from various institutes worldwide. The mechanism of hypokalemia could be either due to redistribution of potassium in cells or transient renal tubular abnormalities leading to increased urinary potassium wasting. We had two patients with hypokalemic quadriparesis who improved after potassium supplementation similar to other case reports.[28] Patients with neurological manifestations especially encephalitis have high mortality as shown in recent studies which is similar to our observation too.[29]
Acute kidney injury is a serious and potentially lethal complication of this disease, and the actual incidence is unknown. Several forms of renal involvement have been identified in patients with dengue, including elevation of the serum creatinine level, AKI, acute tubular necrosis, hemolytic uremic syndrome, proteinuria, glomerulopathy, and nephrotic syndrome.[30,31] Several studies reported that the AKI in expanded dengue syndrome was associated with higher mortality and longer hospital stay and sometimes required initiation of renal replacement.[32,33] We had three cases of AKI. Two patients improved with supportive care and one patient needed renal replacement therapy. The proposed mechanisms are either invasion of dengue virus in to the kidneys or immune mediated. Coinfection with malaria, Chikungunya, and Zika virus infection has been reported.[34] Malaria is the most common coinfection with dengue fever. Plasmodium falciparum is most commonly associated as per Indian studies; however, Plasmodium vivax coinfection has also been reported.[35,36] Based upon cross-sectional studies, malaria and dengue prevalence varied widely, ranging between 0.1% and 23% from south Asia, 0.01% and 9% from Africa, 0.5% and 2.5% from Southeast Asia, and 1% and 3% from South America[37] Even we had eight patients in our series with malaria coinfection. Concurrent malaria and dengue infection is a scenario that both malaria and dengue exists in a patient at the same time and the focus is on the overlapping clinical presentations and the timely diagnosis, which is crucial and may reduce the mortality and morbidity. We lost two patients of expanded dengue with malaria coinfection similar to other case reports across the globe.[38,39] In endemic areas, possibilities of concurrent infections should be thought, and despite similarities in clinical and biological characteristics of both diseases, all treating clinicians should order investigation for both. Expanded dengue syndrome in the form of organ dysfunction may require management support from various disciplines for aggressive and effective measures. Recognition of features of EDS is very important for targeting treatment option. Patients with myriads of presentations usually consult their family physicians. Therefore, it is important for them to have a high degree of suspicion for early recognition and appropriate management.
Conclusion
Expanded dengue syndrome may be unrecognized and under reported. In clinical practice, the occurrence of atypical and systemic presentations should prompt the primary or the family physician to investigate for dengue specially during epidemics. A high degree of clinical suspicion is the key for early diagnosis and treatment. There is a rising tide of EDS. Our case series may be the tip of the iceberg. Spreading increased awareness among the community at large is the need of the hour to fight this syndrome which mimic a myriad of clinical conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO Dengue and severe dengue. Fact sheet. WHO; 2017. [Google Scholar]
- 2.Pangtey GS, Prakash A, Munjal YP. Role of Carica papaya leaf extract for dengue associated thrombocytopenia. J Assoc Physicians India. 2016;64:11–3. [PubMed] [Google Scholar]
- 3.Kadam DB, Salvi S, Chandanwale A. Expanded dengue. J Assoc Physicians India. 2016;64:59–63. [PubMed] [Google Scholar]
- 4.Lee LK, Gan VC, Lee VJ, Tan AS, Leo YS, Lye DC. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. Plos Negl Trop Dis. 2012;6:e1676. doi: 10.1371/journal.pntd.0001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatty S, Shaikh NA, Fatima M, Sumbhuani AK. Acute acalculous cholecystitis in dengue fever. J Pak Assoc. 2009;59:519–21. [PubMed] [Google Scholar]
- 6.Chandey M, Kaur H, Kaur S. Acute acalculous cholecystitis in dengue fever patients. Int J Adv Med. 2017;4:375–7. [Google Scholar]
- 7.Shapiro MJ, Luchtefeld WB, Kurzweil S, Kaminski DL, Durham RM, Mazuski JE. Acute acalculous cholecystitis in the critically ill. Am Surg. 1994;60:335–9. [PubMed] [Google Scholar]
- 8.Gonzalez-Fontal GR, Henao-Martinez AF. Dengue hemorrhagic fever complicated by pancreatitis. Braz J Infect Dis. 2011;15:490–2. doi: 10.1016/s1413-8670(11)70235-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee IK, Lee K, Khor BS, Kee KM, Yang KD, Liu JW. Hyperlipasemia/pancreatitis in adults with dengue hemorrhagic fever. Pancreas. 2007;35:381–2. doi: 10.1097/01.mpa.0000297828.05678.7a. [DOI] [PubMed] [Google Scholar]
- 10.Wijekoon CN, Wijekoon PW. Dengue hemorrhagic fever presenting with acute pancreatitis. Southeast Asian J Trop Med Public Health. 2010;41:864–6. [PubMed] [Google Scholar]
- 11.Jain V, Gupta OP, Rao T, Rao S. Acute pancreatitis complicating severe dengue. J Glob Infect Dis. 2014;6:76–8. doi: 10.4103/0974-777X.132050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karoli R, Fatima J, Singh G, Maini S. Acute pancreatitis: An unusual complication of dengue fever. J Assoc Physicians India. 2012;60:64–5. [PubMed] [Google Scholar]
- 13.Gogna A, Pathak S, Dhamija K, Jharyan P, Arora BS. Changing clinical profile of dengue fever in Delhi in 2011. JIACM. 2015;16:20–6. [Google Scholar]
- 14.Arifijanto MV, Luqmana HP, Rusli M, Bramantono An expanded dengue syndrome patient with manifestation myocarditis: Case report. IOP Conf Ser Earth Environ Sci. 2018;125:012094. [Google Scholar]
- 15.Anam AM, Shumy F, Rabbani R, Polash MMI, Huq SM, Shareef A, et al. Expanded dengue syndrome: Gastrointestinal manifestations.Review article. Bangladesh Crit Care J. 2018;6:34–9. [Google Scholar]
- 16.Anam AM, Rabbani R, Shumy F. Expanded dengue syndrome: Three concomitant uncommon presentations in the same patient. Trop Doct. 2017;47:167–70. doi: 10.1177/0049475517696638. [DOI] [PubMed] [Google Scholar]
- 17.Zea D, Foley K, Carey J. Myocarditis in a traveler returning from the dominican republic: An unusual presentation of dengue fever. Am J Trop Med Hyg. 2014;91:156–8. doi: 10.4269/ajtmh.13-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter R, 3rd, Hinojosa-Laborde C, Convertino VA. Heart rate variability in patients being treated for dengue viral infection: New insights from mathematical correction of heart rate. Front Physiol. 2014;5:46. doi: 10.3389/fphys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SMS, Hoo FK, Sulaiman WAW. A case of dengue hemorrhagic fever with myocarditis and complete heart block. RMJ. 2014;39:104–6. [Google Scholar]
- 20.Kaushik JS, Gupta P, Rajpal S, Bhatt S. Spontaneous resolution of sinoatrial exit block and atrioventricular dissociation in a child with dengue fever. Singapore Med J. 2010;51:e146–8. [PubMed] [Google Scholar]
- 21.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 22.Chanthamat N, Sathirapanya P. Acute transverse myelitis associated with dengue viral infection. J Spinal Cord Med. 2010;33:425–7. doi: 10.1080/10790268.2010.11689722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty B, Mehta S, Ahmed A. Acute transverse myelitis: A rare presentation of dengue fever. Arch Gen Intern Med. 2018;2:23–6. [Google Scholar]
- 24.Leao RN, OiKawa T, Rosa ES, Yamaki JT, Rodrigues SG, Vasconcelos HB, et al. Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis) Rev Soc Bras Med Trop. 2002;35:401–4. doi: 10.1590/s0037-86822002000400018. [DOI] [PubMed] [Google Scholar]
- 25.Kunishige M, Mitsui T, Tan BH, Leong HN, Takasaki T, Kurane I, et al. Preferential gray mater involvement indengue myelitis. Neurology. 2004;63:1980–1. doi: 10.1212/01.wnl.0000144194.29643.d0. [DOI] [PubMed] [Google Scholar]
- 26.Carod-Artal FJ, Wichmann O, Farrar J, Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906–19. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 27.Sundaram C, Uppin SG, Dakshinamurthy KV, Borgahain R. Acute disseminated encephalomyelitis following dengue hemorrhagic fever. Neurol India. 2010;58:599–601. doi: 10.4103/0028-3886.68666. [DOI] [PubMed] [Google Scholar]
- 28.Gutch M, Agarwal A, Amar A. Hypokalemic quadriparesis: An unusual manifestation of dengue fever. J Nat Sci Biol Med. 2012;3:81–3. doi: 10.4103/0976-9668.95976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajapakse S, Wattegama M, Weeratunga P, Sigera PC, Fernando SD. Beyond thrombocytopaenia, haemorrhage and shock: The expanded dengue syndrome. Pathog Global Health. 2018;1-11 doi: 10.1080/20477724.2018.1552645. doi: 10.1080/20477724.2018.1552645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardi R, Yu L, Younes-Ibrahim M, Schor N, Burdmann EA. Epidemiology of acute kidney injury in Latin America. Semin Nephrol. 2008;28:320–9. doi: 10.1016/j.semnephrol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Lizarraga KJ, Nayer A. Dengue-associated kidney disease. J Nephropathol. 2014;3:57–62. doi: 10.12860/jnp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repizo LP, Malheiros DM, Yu L, Barros RT, Burdmann EA. Biopsy proven acute tubular necrosis due to rhabdomyolysis in a dengue fever patient: A case report and review of literature. Rev Inst Med Trop São Paulo. 2014;56:85–8. doi: 10.1590/S0036-46652014000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehra N, Patel A, Abraham G, Reddy YN, Reddy YN. Acute kidney injury in dengue fever using Acute Kidney Injury Network criteria: Incidence and risk factors. Trop Doct. 2012;42:160–2. doi: 10.1258/td.2012.120023. [DOI] [PubMed] [Google Scholar]
- 34.Khalil MA, Sarwar S, Chaudry MA, Maqbool B, Khalil Z, Tan J, et al. Acute kidney injury in dengue virus infection. Clin Kidney J. 2012;5:390–4. doi: 10.1093/ckj/sfs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villamil-Gómez WE, González-Camargo O, Rodriguez-Ayubi J, Zapata-Serpa D, Rodriguez-Morales AJ. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016;9:684–6. doi: 10.1016/j.jiph.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Magalhaes BM, Alexandre MA, Siqueira AM, Melo GC, Gimaque JB, Bastos MS, et al. Clinical profile of concurrent dengue fever and Plasmodium vivax malaria in the Brazilian Amazon: Case series of 11 hospitalized patients. Am J trop Med Hyg. 2012;87:1119–24. doi: 10.4269/ajtmh.2012.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohapatra MK, Patra P, Agrawala R. Manifestation and outcome of concurrent malaria and dengue infection. J Vector Borne Dis. 2012;49:262–6. [PubMed] [Google Scholar]
- 38.Salam N, Mustafa S, Hafiz A, Chaudhary AA, Deeba F, Parveen S. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: A systematic review. BMC Public Health. 2018;18:710. doi: 10.1186/s12889-018-5626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yunsheng ZH, Xia WU, Fei LI. Severe cerebral falciparum malaria with dengue coinfection: A case report. Iran J Parasitol. 2018;13:323–7. [PMC free article] [PubMed] [Google Scholar]


