Abstract
Background
High oncogenic-risk human papillomavirus (hrHPV) is necessary, although insufficient, to promote cervical cancer. Like HPV, Epstein-Barr virus (EBV) is a common pathogen with the capacity to promote epithelial neoplasms. We examined the association between cervical EBV, hrHPV, and cytology in female sex workers in Nairobi, Kenya.
Methods
Women (n=332) with known cervical cytology and hrHPV mRNA results were evaluated for cervical EBV DNA by conventional PCR. Prevalence ratios (PR) were calculated to assess the relationships between EBV, hrHPV and cervical cytology. Prospective analyses utilized risk ratios (RR) and time-to-event analyses to determine the association of EBV with hrHPV clearance and abnormal cytology outcomes.
Results
Baseline prevalence of hrHPV and EBV was 29% and 19%, respectively. Higher EBV prevalence was found among women with older age, HIV, hrHPV, abnormal cytology, Mycoplasma genitalium infection, smoking habits, younger age at sexual debut and less frequent condom use. At baseline, women with EBV had a higher prevalence of hrHPV infection than EBV-negative women (52% versus 24%; HIV-adjusted PR [95% CI]: 1.8 [1.3, 2.6]). EBV-positive women had a higher prevalence than EBV-negative women of high-grade precancer (15% versus 2%), and abnormal cytology (37% versus 15%), although HIV- and hrHPV-adjusted associations were not significant (high-grade precancer - PR: 2.0 [0.7, 5.9]; abnormal cytology - PR: 1.4 [0.9, 2.2]). In prospective analyses, a marginal association was observed between baseline EBV detection and delayed hrHPV clearance.
Conclusions
Our data support a possible role for EBV as a high-risk marker or co-factor for HPV-mediated cervical cancer development.
Keywords: Human Papillomavirus, Epstein-Barr virus, cervical cancer, cervical cytology, sexually transmitted infections
INTRODUCTION
Despite a global reduction in incidence, cervical cancer remains the leading cause of cancer-related mortality in women in Sub-Saharan Africa (1). Kenya ranks 16th in the world in cervical cancer incidence, with >40 annual cases per 100,000 women (1). Cervical cancer develops as a result of persistent infection with oncogenic high-risk human papillomavirus (hrHPV). In contrast to the observation that hrHPV infection is found in nearly 100% of cervical cancer cases, the likelihood of developing cancer is quite low among women exposed to hrHPV infection. Therefore, hrHPV infection is an essential etiological cause of cervical cancer, yet hrHPV infection alone is not considered sufficient to cause cancer. These observations suggest that other factors interact with hrHPV to promote cancer development.
Similar to HPV, infection with the human herpesvirus Epstein-Barr virus (EBV) is extremely common and typically asymptomatic, but occasionally leads to epithelial neoplasms, for example nasopharyngeal carcinoma (2) and gastric carcinoma (3). EBV establishes a lifelong infection in its human host, resides in a quiescent (latent) state with periodic lytic reactivation, and has a predilection for mucosal surfaces (4–6). Sixbey et al. revealed that the female genital tract is a site of EBV shedding by demonstrating the recovery of live, infectious EBV from cervical secretions (6). Little is known about the biology and natural history of genital EBV infection, or the risk factors for EBV detection from the female genital tract.
The presence of EBV in the female genital tract provides an opportunity for interactions between co-existing hrHPV and EBV infections. Given the ability of EBV to promote epithelial tumor formation, there is a possibility that EBV might act in conjunction with hrHPV infection to facilitate the oncogenic potential of hrHPV and the progression of low-grade cervical dysplasia to high-grade cervical dysplasia or cancer. In this study, we utilized stored samples from a high-risk cohort of Kenyan women who had previously undergone cervical HPV testing to examine the association between detection of EBV and hrHPV at baseline, and the diagnosis of abnormal cervical cytology at baseline and over study follow-up.
MATERIALS AND METHODS
Study population
This study utilized archived specimens collected as part of a research study investigating approaches to cervical cancer screening in a high-risk cohort of African women. Participants in the study were female sex workers (FSWs) attending the Korogocho health clinic in Nairobi, Kenya, from 2009-2011, as previously described (7). Baseline specimens (n = 332) were used for this study. Sociodemographic and behavioral characteristics were collected at baseline. Participants were followed prospectively at 6-month intervals for 2-year study follow-up, and results of HPV testing and cytology were included in time-to-event analyses.
Enrollees provided informed consent. The primary study protocol was approved by local Institutional Review Boards (IRBs) and the IRB of the University of North Carolina and was conducted in accordance with all applicable ethical standards for research on human subjects. Further approval for the analysis presented herein was obtained from the IRB of the LSU Health Sciences Center, New Orleans.
Specimens, clinical evaluation, and laboratory testing
Specimens collected included a physician-collected cervical brush and a conventional Pap smear, as previously described (7). Cytological smears were evaluated by two independent cytopathologists and were classified according to the 2001 Bethesda System. Low-grade cervical abnormalities were defined as atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL), and high-grade abnormalities included high-grade squamous intraepithelial lesions (HSIL) and cancer. Cervical abnormalities overall were defined as ≥ ASCUS. Cervical brush specimens were tested for hrHPV using the APTIMA HPV test (Hologic Inc., San Diego, Calif.), which indiscriminately detects the presence of E6/E7 mRNA transcripts of hrHPV genotypes 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, 66, and 68. Women were also tested for sexually transmitted infections (STIs) including Chlamydia trachomatis (CT) and Neisseria gonorrhea (NG) by APTIMA Combo-2 assay, Trichomonas vaginalis (TV) by APTIMA TV assay, and Mycoplasma genitalium (MG) by APTIMA MG assay (research use only). Testing for HIV serum antibodies was performed by ELISA.
To prepare the samples for EBV molecular testing, genomic DNA (including viral and host DNA) was isolated from a 1.5-mL aliquot of cervical brush specimen in PreservCyt transport media (Cytyc Corp., Marlborough, Mass.) using the QIAamp DNA Mini-kit (Qiagen N.V., Hilden, Germany) according to the manufacturer’s protocol. Samples that tested positive for β-globin DNA via PCR amplification (n = 330, 99.4%) were tested for EBV by PCR amplification of the BamH1-W repeats, a multi-copy region of the EBV genome (8). Presence of a 192-bp product on 2% agarose gel indicated the presence of EBV. Results were interpreted by two independent and blinded observers.
Statistical analysis
The prevalence of HIV, hrHPV mRNA, and EBV DNA were estimated for the 330 women with baseline EBV test results. Since HIV infection is likely to promote HPV persistence and might influence frequency and duration of EBV detection, we conducted analyses that adjusted for or stratified by HIV status. Cervical cytology was performed on all women at baseline and during follow-up, and therefore we chose cervical cytology as the primary clinical outcome for our analysis. In this cohort, cervical biopsy/histopathology was only performed on women with a cytology diagnosis of HSIL or LSIL/ASCUS/AGUS diagnosis at two consecutive visits. The prevalence of high-grade cervical cytology at baseline stratified by hrHPV and EBV co-infection status was estimated, overall and by HIV status. Prevalence ratios (PR, [95% confidence interval]) were calculated using Log-binomial regression to determine risk factors associated with EBV detection. Log-binomial regression was also used to estimate the association between EBV and hrHPV, cervical abnormalities, and high-grade lesions overall and adjusted for HIV status.
For longitudinal analyses, the observation period comprised 5 clinic visits at 6-month intervals over 24 months. Crude and adjusted risk ratios (RRs) compared the incidence of cervical abnormalities, and worsening of cervical cytology (defined as progression from normal to abnormal cytology or from low-grade or indeterminate to high-grade cytology) by EBV status. Women with prevalent high-grade abnormalities at baseline were referred for treatment and were excluded from the study. Women with incident high-grade abnormalities during observation were also referred for immediate treatment but were included in the analysis as a study endpoint of progression.
We conducted time-to-event analysis in which risk of hrHPV persistence was indicated by a ratio of the time to hrHPV clearance, comparing women with baseline EBV infections to women without baseline EBV infections. A time ratio (TR) greater than the null value (i.e. TR > 1.0) indicates that the time to clearance of a hrHPV infection was longer in the EBV-infected group compared to the EBV-uninfected group. Crude and adjusted TRs estimated the 1) relative time to clearance of hrHPV infection, and 2) relative time to regression of cervical lesions (defined as change from any abnormal cytology to normal cytology at a subsequent visit) comparing EBV-positive to EBV-negative participants.
The risk period for clearance began when hrHPV was first detected. The calculation was based on the number of intervals from the first positive hrHPV test until at least one subsequent hrHPV test was negative, or until the participant was lost to follow-up. Relative time-to-clearance of hrHPV was estimated using a minimum time to clearance – beginning at enrollment and ending just after the last positive hrHPV result – and a maximum time to clearance –the minimum time plus the additional unobserved time between the dates of the last positive hrHPV result and the first negative hrHPV result. Time-to-regression of cervical lesions was computed using the same strategy. Further details of the statistical considerations have been previously published (9).
Missing cytological results were imputed when the results in the visits before and after the missing visit were identical. For time-to-clearance of hrHPV, missing data were handled by right-censoring observations that had missed two or more consecutives study visits following a positive hrHPV diagnosis (lost to follow-up), and by imputing data for single missing visits, carrying over the last observed value for hrHPV status. We conducted sensitivity analyses excluding severely immunosuppressed participants (CD4+ T cell counts <200; n=6). Adjustment variables for all analyses were determined based on a review of the literature and analysis of causal diagrams. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics
The median age of the 330 participants in this study was 28.5 years (range: 25-34; Table 1). In this high-risk cohort of women, the majority had only a primary education or less (76%), and while 44% reported being single, 96% were mothers (68% multiparous; data not shown). The median duration of sex work was 5 years (Interquartile Range [IQR]: 3-7), with a median of 10 clients per week (IQR: 7-15). At the baseline visit, a quarter of women (N=81; 25%) tested positive for HIV, 97 women (29%) had hrHPV E6/E7 mRNA, 62 women (19%) had cervical EBV DNA, and 78 women (24%) tested positive for at least one other sexually transmitted infection (STI): CT, NG, TV, and/or MG. Sixty-four women (19%) had abnormal cervical cytology at baseline.
Table 1.
Socio-demographic characteristics of the populationa and association with detection of Epstein-Barr virus (EBV) in cervical specimens.
| Number overall (N=330) |
EBV-positive (N=62) |
Age-adjusted PR (95%CI)b |
|
|---|---|---|---|
| Age in years (median; IQR) | 28.5 (25-34) | 30 (26-36) | 1.04 (1.01, 1.07) |
| Age at sexual debut in years (median; IQR) | 16 (15-18) | 15 (14-17) | 0.88 (0.80, 0.97) |
| Percent condom use last week (median; IQR) | 100 (70-100) | 83 (57-100) | 0.42 (0.21, 0.83) |
| Regular smokerc | |||
| Never | 204 | 14% | 1.0 (ref) |
| Former | 44 | 23% | 1.56 (0.83, 2.03) |
| Current | 82 | 28% | 2.00 (1.24, 3.23) |
| Years as sex worker (median; IQR) | 5 (3-7) | 5 (3-7) | 1.00 (0.95, 1.06) |
| Clients per week (median; IQR) | 10 (7-15) | 10 (7-15) | 1.01 (0.97, 1.04) |
| HIV serum status | |||
| Negative | 247 | 10% | 1.0 (ref) |
| Positive | 81 | 44% | 4.33 (2.72, 6.91) |
| Peripheral CD4+ T cell count (median; IQR) | 898 (618-1182) | 728 (353-997) | 0.90 (0.84, 0.96)d |
| High-oncogenic risk HPV E6/E7 mRNA (hrHPV) | |||
| Negative | 233 | 13% | 1.0 (ref) |
| Positive | 97 | 33% | 2.62 (1.70, 4.02) |
| Other sexually transmitted infection (CT, NG, TV or MG) |
|||
| Negative for all | 252 | 16% | 1.0 (ref) |
| Positive for any | 78 | 28% | 1.83 (1.17, 2.85) |
| Chlamydia trachomatis (CT) | |||
| Negative | 316 | 18% | 1.0 (ref) |
| Positive | 13 | 31% | 2.27 (0.95, 5.50) |
| Neisseria gonorrhea (NG) | |||
| Negative | 322 | 19% | 1.0 (ref) |
| Positive | 8 | 25% | 1.59 (0.47, 5.38) |
| Trichomonas vaginalis (TV) | |||
| Negative | 306 | 18% | 1.0 (ref) |
| Positive | 24 | 25% | 1.25 (0.61, 2.58) |
| Mycoplasma genitalium (MG) | |||
| Negative | 288 | 17% | 1.0 (ref) |
| Positive | 42 | 29% | 1.73 (1.03, 2.92) |
| Cervical cytologye | |||
| Normal | 266 | 15% | 1.0 (ref) |
| Low-grade | 50 | 28% | 1.89 (1.11, 3.20) |
| High-grade | 14 | 64% | 3.81 (2.31, 6.28) |
Abbreviations: EBV=Epstein-Barr virus; hrHPV=high-risk human papillomavirus; HIV=human immunodeficiency virus; IQR=interquartile range; PR=prevalence ratio; ASCUS=atypical squamous cells of undetermined significance; AGUS; atypical glandular cells of undetermined significance; LSIL=low-grade squamous intraepithelial lesions; HSIL=high-grade squamous intraepithelial lesions.
Missing data: age at sexual debut (1); HIV serology (2); Chlamydia (1).
Age-adjusted PR (prevalence ratio) is the prevalence of Epstein-Barr virus (EBV) by each potential risk factor. PRs shown in bold face are statistically significant (p<0.05).
Regular smoker was defined as ≥1 cigarette/day for 6 months.
Prevalence ratio for CD4 count is per 100 cell count change.
Low grade includes ASCUS (12), AGUS (2), and LSIL (36); High-grade includes HSIL (12) and squamous cell carcinoma (SCC) (2).
Risk factors associated with a positive cervical test for EBV
Increasing age was associated with cervical EBV detection (PR: 1.04 [1.01, 1.07], Table 1). In age-adjusted analyses, women were more likely to test positive for cervical EBV if they were infected with MG (PR: 1.73 [1.03, 2.92]), hrHPV (PR: 2.62 [1.70, 4.02]), or HIV (PR: 4.33 [2.72, 6.91]). Further, women with abnormal cytology (low-grade PR: 1.89 [1.11, 3.20]; high-grade PR: 3.81 [2.31, 6.28]) and those who reported current smoking (PR: 2.00 [1.24, 3.23]) were also more likely to test positive for cervical EBV. EBV prevalence decreased as age at sexual debut increased (PR: 0.88 [0.80, 0.97]) and with more frequent condom use (PR: 0.42 [0.21, 0.83]).
Relationship between hrHPV, EBV, and cervical cytology
We examined the relationships between viral infections (hrHPV, EBV, and HIV) and cervical cytology. High-risk HPV E6/E7 mRNA and EBV DNA were concurrently detected in the cervical specimens of 32 women (9.7%, Table 2). Of the co-infected women, 65.6% were also infected with HIV. Women were over twice as likely to test positive for cervical hrHPV if they were also EBV DNA positive (PR: 2.1 [1.5, 2.9]). This association was attenuated when adjusting for HIV infection status (adjusted PR: 1.8 [1.3, 2.6]) but remained significant.
Table 2.
Prevalence of high-risk human papillomavirus and cervical abnormalities stratified by Epstein-Barr virus and HIV infection
| EBV | N | hrHPV n (%) | PR (95%CI) | HIV-adjusted PR (95%CI) |
|
|---|---|---|---|---|---|
| Overall | − | 268 | 65 (24%) | 1.0 (ref) | |
| + | 62 | 32 (52%) | 2.1 (1.5, 2.9) | 1.8 (1.3, 2.6) | |
| HIV-positive only | − | 45 | 14 (31%) | 1.0 (ref) | |
| + | 36 | 21 (58%) | 1.9 (1.1, 3.1) | ||
| HIV-negative only | − | 222 | 51 (23%) | 1.0 (ref) | |
| + | 25 | 10 (40%) | 1.7 (1.0, 3.0) | ||
|
| |||||
| EBV | N | High-grade cervical abnormalities n (%) |
PR (95%CI) | HIV and hrHPV adjusted PR (95%CI) |
|
|
| |||||
| Overall | − | 268 | 5 (2%) | 1.0 (ref) | |
| + | 62 | 9 (15%) | 7.8 (2.7, 22.4) | 2.0 (0.7, 5.9) | |
| HIV-positive only | − | 45 | 3 (7%) | ||
| + | 36 | 8 (22%) | |||
| HIV-negative only | − | 222 | 2 (1%) | ||
| + | 25 | 1 (4%) | |||
|
| |||||
| EBV | N | Any cervical abnormalitiesb n (%) |
PR (95%CI) | HIV and hrHPV adjusted PR (95%CI) |
|
|
| |||||
| Overall | − | 268 | 41 (15%) | 1.0 (ref) | |
| + | 62 | 23 (37%) | 2.4 (1.6, 3.7) | 1.4 (0.9, 2.2) | |
| HIV-positive only | − | 45 | 11 (25%) | ||
| + | 36 | 19 (53%) | |||
| HIV-negative only | − | 222 | 30 (14%) | ||
| + | 25 | 3 (12%) | |||
Abbreviations: hrHPV= high-risk human papillomavirus; EBV= Epstein-Barr virus; HIV= human immunodeficiency virus; PR= prevalence ratio; CI= confidence interval. PRs shown in bold face are statistically significant (p<0.05).
N=2 missing observations for HIV status.
Cervical abnormalities are defined as ≥ASCUS.
EBV-positive women had a higher prevalence than EBV-negative women of high-grade precancer (15% versus 2%) and abnormal cytology (37% versus 15%) (Figure 1). However, when adjusted for hrHPV and HIV status, EBV was not independently associated with high-grade cervical cytology (adjusted PR: 2.0 [0.7, 5.9] or abnormal cytology (adjusted PR: 1.4 [0.9, 2.2]) (Table 2). Further adjusting for smoking and age did not appreciably change the magnitude of these estimates or alter the conclusions. However, among women who were hrHPV positive, those who tested positive for EBV DNA were significantly more likely to have abnormal cervical cytology than women with hrHPV alone (53% versus 20%, p=0.01) (Figure 1). This observation was more pronounced in women who were HIV-positive than in women who were HIV-negative (Figure 1, panels B and C respectively). One-quarter of women (25%) with cervical hrHPV-EBV co-infection had concurrent high-grade cytology. By comparison, 7.6% of women with hrHPV alone had concurrent high-grade cytology (Fisher’s exact test, p= 0.027).
FIGURE 1.
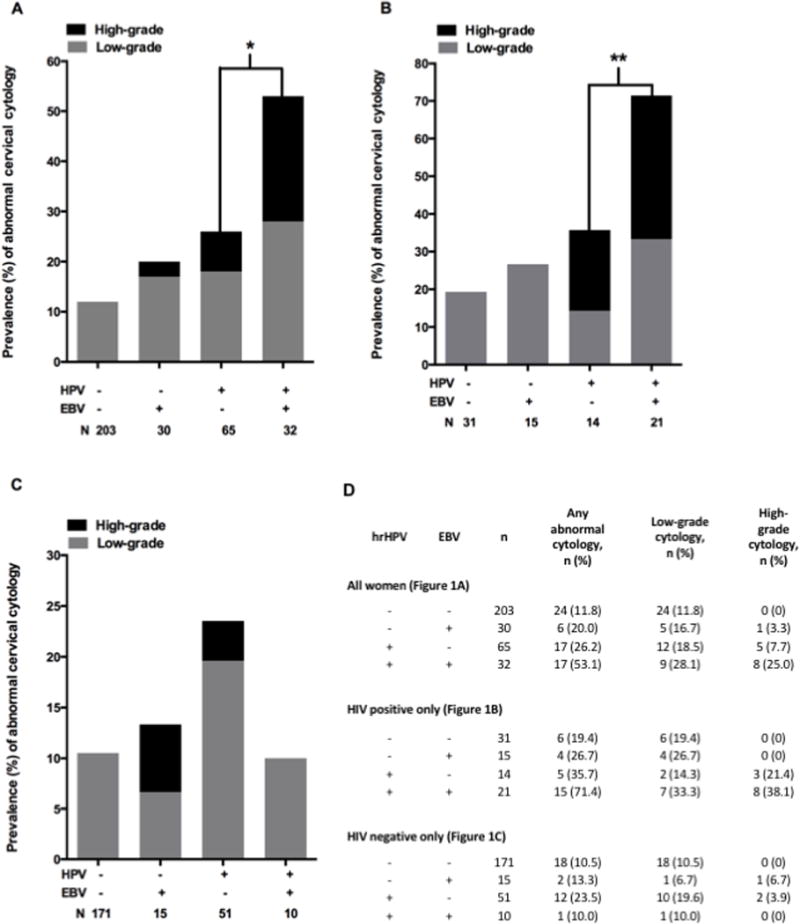
Cytology results stratified by cervical HPV and EBV detection. Low-grade abnormalities include atypical squamous cells of undetermined significance (ASCUS, 12), atypical glandular cells of undetermined significance (AGUS, 2), and low-grade squamous intraepithelial lesions (LSIL, 36). High-grade abnormalities include high-grade squamous intraepithelial lesions (HSIL, 12) and squamous cell carcinoma (SCC, 2). The number of women (N) comprising each viral infection category is shown (e.g., in panel A, the left bar represents 203 women who tested negative for both hrHPV and EBV). A) Overall population; B) HIV-positive women only; C) HIV-negative women only. B and C exclude two women with unknown HIV status. *Fisher’s exact test, p = 0.013 for abnormal cytology and p = 0.017 for high-grade cytology; **Fisher’s exact test, p = 0.02 for abnormal cytology.
Relationship between cervical EBV positivity at baseline and natural history of hrHPV infection or cervical abnormalities
A marginal and non-significant association was observed between baseline EBV positivity and risk of acquiring hrHPV infection during the 24-month observation period, when adjusting for HIV status and age (data not shown). Similarly, baseline EBV positivity was not a significant positive predictor of incident cervical abnormalities, even in women testing positive for hrHPV at baseline (Table S1). Baseline EBV positivity was associated with worsening cervical health status during observation (age and HIV status-adjusted RR: 2.21 [1.00, 4.90]), but the association was not significant when further adjusting for hrHPV status, or when restricting the analysis to women who were hrHPV positive at baseline (Table S1).
In a sensitivity analysis excluding women with CD4+ T cell counts below 200 cells/ml (n=6), associations between baseline EBV positivity and 1) abnormal cytology (PR: 2.0 [1.4, 2.8]); and 2) high-grade cytology (PR: 1.4 [0.9, 2.3]) were similar to the original estimates. Further, we observed no changes to the interpretations of our findings on the risk of 1) incident cervical abnormalities (FSW with normal cytology – RR: 1.61 [0.72, 3.60]; FSW with normal cytology and hrHPV positive – RR: 1.42 [0.50, 4.01]); or 2) progression of cervical abnormalities (FSW with normal or low-grade cytology – RR: 1.72 [0.78, 3.80]; FSW with normal or low-grade cytology and hrHPV positive – RR: 1.66 [0.61, 4.51]) after excluding women with CD4+ T cell counts below 200 cells/ml (data not shown).
The median follow-up time was 26.2 months (IQR: 18.8-27.5 months), and the median time to clearance of hrHPV infection was 9.4 months (IQR: 5.9-19.5 months). Women with baseline EBV infections had a median time to hrHPV clearance of 22.6 months, and women without baseline EBV infections had a median time to hrHPV clearance of 9.4 months. After adjusting for baseline HIV status and age, baseline EBV positivity was marginally associated with longer time to clearance of hrHPV infection among women who were co-infected with hrHPV at baseline (adjusted TR: 1.60 [1.00, 2.55]) (Table 3). In a sensitivity analysis excluding women with low CD4+ T cell counts (<200 cells/ml), we observed a slight attenuation of the association between EBV positivity and time to clearance of hrHPV infection (adjusted TR: 1.50 [0.92, 2.44]). Similar analysis estimated the TR of resolution of baseline abnormal cytology, stratified by baseline EBV status. No association was observed between baseline EBV positivity and time to resolution of abnormal cervical lesions (adjusted TR [95%CI]: 0.83 [0.27, 2.56]) (Table 3).
Table 3.
Relative time to clearance of prevalent high-risk human papillomavirus infection and time to regression of prevalent abnormal cytology, stratified by baseline Epstein-Barr virus status
| Event | Baseline Characteristic(s) of Evaluated Cases | EBV | n (%) | Crude TR (95%CI) | Adjusted TRa (95%CI) |
|---|---|---|---|---|---|
| Relative time to clearance of hrHPV infectionb | |||||
| hrHPV-positive (n=97) | − | 65 (67.0) | 1.0 (ref) | 1.0 (ref) | |
| + | 32 (33.0) | 1.41 (0.92, 2.18) | 1.60 (1.00, 2.55) | ||
| Relative time to resolution of abnormal cytologyc | |||||
| Abnormal cytology (n=64) | − | 41 (64.0) | 1.0 (ref) | 1.0 (ref) | |
| + | 23 (35.9) | 1.53 (0.61, 3.85) | 0.83 (0.27, 2.56) | ||
| Abnormal cytology and hrHPV-positive (n=34) | − | 17 (50.0) | 1.0 (ref) | 1.0 (ref) | |
| + | 17 (50.0) | 1.27 (0.35, 4.63) | 0.43 (0.07, 2.47) |
Abbreviations: hrHPV=high-risk human papillomavirus; EBV=Epstein-Barr virus; TR=time ratio.
Adjusted for HIV status and age at baseline (continuous).
Time to clearance is calculated from the first visit at which a positive hrHPV test was obtained until the next visit at which a negative hrHPV test was obtained.
Time to resolution is calculated from baseline until the first visit at which a normal cervical cytology result was obtained.
DISCUSSION
In this cohort of FSWs in Nairobi, Kenya, we found that women testing positive for both hrHPV and EBV had a significantly higher prevalence of concurrent cervical abnormalities (overall and for high-grade precancer) compared to women infected with hrHPV alone. This difference was most pronounced in HIV-infected women. In addition, cervical EBV detection was seen more often in HIV-positive women and those with hrHPV infection. Finally, in prospective analyses, the presence of EBV was marginally associated with delayed resolution of hrHPV infection and progression to more severe cytology.
Prevalence of EBV detection at the cervix in this cohort of Kenyan FSWs was 19% among all women, with notably high detection among HIV-positive women at baseline (44%). In comparison EBV prevalence ranged widely (9-42%) in cervical samples of healthy women (10–13) while cervical EBV prevalence in women attending STD clinics exceeded 25% (8, 14). The prevalence of cervical EBV infection was reportedly 10% in an Italian cohort of women with HIV infection (15). Finally, in a large cohort of women seeking gynecological care in rural India, the prevalence of cervical EBV detection was 20% (16). The highly variable prevalence rates of cervical EBV detection is likely attributable to differences in geographic location, sociodemographic risk factors, and laboratory testing methods among published studies.
There are limited published data describing factors associated with genital tract EBV detection. In the present study and in a study from India, older age was significantly associated with a higher rate of detection of EBV at the cervix (16). In our study, women who tested positive for STIs, including HIV, were more likely to be EBV positive at the cervix. In particular, infection with MG was associated with a higher detection of EBV, as was recently reported by Dehon et al. (17). Failure to consistently use condoms was also significantly associated with higher EBV prevalence in our study, possibly due to increased exposure to STIs. The association of EBV with sexually transmitted pathogens could indicate that EBV is being deposited at the cervix during sexual intercourse, similar to the sexual transmission route of the more traditional STI pathogens. Alternatively, since the reservoir of latent EBV is memory B cells (18), EBV could be a passenger in infiltrating lymphocytes trafficking to the cervix in response to infection and/or associated cervical pathology. Our study was not designed to distinguish between these two possibilities.
In our cohort, we found that women positive for hrHPV were twice as likely to have an abnormal cytology result or high-grade precancer if they were simultaneously positive for EBV at the cervix. This relationship was even stronger in women with HIV. Despite differences in geographic location, population, sampling, and testing methods, our data corroborate the findings of other investigators. In the rural Indian population, cervical EBV detection was associated with a positive test for HPV and abnormal cytology, as in our study, while detection of the cytomegalovirus was not associated with cervical screening test outcomes (16). The authors found that a biopsy-confirmed histological diagnosis of cervical intraepithelial neoplasia was a significant positive predictor of cervical EBV detection after adjusting for other factors, including age, with an adjusted prevalence ratio of 3.92 (95%CI: [2.49-6.16]). Among HIV-seropositive Italian women with HPV infection, prevalence of abnormal cytology was higher in women testing positive for EBV and HPV (76% vs. 67% in women with HPV alone). This finding was not statistically significant, possibly owing to immune suppression (average CD4+ T cell count, 291 cells/ml) leading to unusually high rates of abnormal cytology in the HIV-HPV co-infected women (15). Results of our sensitivity analyses excluding women with CD4+ T cell counts <200 found similar associations between EBV positivity and risk of both prevalent cervical abnormalities, although the excess time to hrHPV clearance observed among participants testing positive for EBV was may have partially been driven by a small number of participants with severe HIV-associated immune suppression, as HIV-infected participants in our sample were generally not severely immunocompromised (median CD4+ T cell count [IQR]: 507 [353-814]).
Limitations of this study included insufficient follow-up time for some women to experience definitive outcomes and a low incidence of high-grade cytology In addition, the impact of false-negative tests for EBV or HPV would be that some women with abnormal cytology may have falsely tested negative for one of the two viruses. This would lead to misclassification and subsequent underestimation of the risk of abnormal cytology associated with viral co-infection. False-negative classification of a single cytology result (i.e., normal cytology when in fact an abnormality exists) is another potential source of misclassification bias. Using the gold standard histopathology diagnosis of cervical biopsy tissue would improve diagnostic certainty and might also alter the results of our analysis. We conducted a sensitivity analysis for Table 2 in which we reclassified baseline high-grade cervical abnormalities based on histopathology results (i.e. comparing CIN2+ versus CIN1, normal histology or normal cytology). We identified four additional CIN2+ outcomes, which gave more power to detect a difference in prevalence of high-grade cervical abnormalities by EBV status (HIV- and hrHPV-adjusted PR: 2.63 [1.01, 6.84]). However, as not all participants had histopathology data, we opted for cytology as our primary clinical outcome for consistency and to avoid verification bias.
Previous reports of cervical EBV detection and cervical abnormalities in the context of HPV infection have assessed HPV status by detection of viral DNA using highly sensitive PCR assays (8, 15, 19–24). Sensitive DNA-based assays would detect both productive, clinically relevant infection and unproductive, clinically benign hrHPV (for instance, recent deposition in the absence of true infection). Our study defined hrHPV infection by detection of E6/E7 mRNA transcripts, which is more indicative of a productive and clinically relevant hrHPV infection. If EBV promotes cervical abnormalities by inducing expression of hrHPV E6/E7 transcripts, then restricting the analysis to infections with detectable E6/E7 transcripts could underestimate the potential role of EBV in promoting productive hrHPV infections and corresponding abnormalities.
To our knowledge, this is the first prospective study that attempts to determine associations between EBV detection at the cervix and hrHPV persistence and cervical dysplasia outcomes. Despite the aforementioned limitations to our study, we observed trends that would suggest that EBV at the cervix may alter the natural history of hrHPV infection and cervical abnormalities. Alternatively, EBV detection at the cervix may be a correlate of other biological processes that portend the outcome of cervical dysplasia in the absence of a direct causal role in promoting disease. Larger prospective cohort studies with longer observation periods will be necessary to clarify the associations between cervical EBV detection and hrHPV persistence or progression of cervical abnormalities. Further studies are also warranted to determine whether or not EBV plays a direct role in the pathogenesis of hrHPV mediated cervical abnormalities and cancer.
Supplementary Material
SUMMARY.
Women with hrHPV and EBV had a higher prevalence of cervical abnormalities compared to women with only hrHPV, indicating a possible role for EBV as a co-factor of cervical precancer.
Acknowledgments
JSS has received unrestricted educational grants, consultancy, and research grants from Hologic Corporation, Trovagene, and BD Corporation over the past 5 years. This work was supported by Hologic; a UNC Center for AIDS Research grant (Grant No. 5-51060 awarded to JSS); and the National Cancer Institute (supplement to the Lineberger Cancer Center awarded to JSS and research grant R01 CA121979 awarded to MEH). During the completion of this work, JEC received support from the National Institute of General Medical Sciences through the Center of Biomedical Research Excellence (P20 GM103501) and the Louisiana Clinical and Translational Science Center (U54 GM104940), AFR received support from the Johns Hopkins University Center for AIDS Research (P30 AI094189), and NAV received support from the National Institute of Allergy and Infectious Diseases (T32 AI070114). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring agencies and affiliate institutions.
SOURCES OF FUNDING
This work was supported by Hologic; a UNC Center for AIDS Research grant (Grant No. 5-51060 awarded to JSS); and the National Cancer Institute (supplement to the Lineberger Cancer Center awarded to JSS and research grant R01 CA121979 awarded to MEH). During the completion of this work, JEC received support from the National Institute of General Medical Sciences through the Center of Biomedical Research Excellence (P20 GM103501) and the Louisiana Clinical and Translational Science Center (U54 GM104940), AFR received support from the Johns Hopkins University Center for AIDS Research (P30 AI094189), and NAV received support from the National Institute of Allergy and Infectious Diseases (T32 AI070114). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring agencies and affiliate institutions.
Footnotes
CONFLICTS OF INTEREST
All other authors have no conflicts to declare.
References
- 1.Ferlay JS, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11[Internet] 2013 Accessed 07/31/2016; Available from: http://globocan.iarc.fr.
- 2.zur Hausen H, Schulte-Holthausen H, Klein G, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 3.Shibata D, Tokunaga M, Uemura Y, et al. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139(3):469–474. [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber P, Lucas S, Nonoyama M, et al. Oral excretion of Epstein-Barr virus by healthy subjects and patients with infectious mononucleosis. Lancet. 1972;2(7785):988–989. doi: 10.1016/s0140-6736(72)92402-6. [DOI] [PubMed] [Google Scholar]
- 5.Yao QY, Rickinson AB, Epstein MA. Oropharyngeal shedding of infectious Epstein-Barr virus in healthy virus-immune donors. A prospective study. Chin Med J (Engl) 1985;98(3):191–196. [PubMed] [Google Scholar]
- 6.Sixbey JW, Lemon SM, Pagano JS. A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet. 1986;2(8516):1122–1124. doi: 10.1016/s0140-6736(86)90531-3. [DOI] [PubMed] [Google Scholar]
- 7.Ting J, Mugo N, Kwatampora J, et al. High-risk human papillomavirus messenger RNA testing in physician- and self-collected specimens for cervical lesion detection in high-risk women, Kenya. Sex Transm Dis. 2013;40(7):584–589. doi: 10.1097/OLQ.0b013e31828e5a91. [DOI] [PubMed] [Google Scholar]
- 8.Voog E, Ricksten A, Lowhagen GB. Prevalence of Epstein-Barr virus and human papillomavirus in cervical samples from women attending an STD-clinic. Int J STD AIDS. 1995;6(3):208–210. doi: 10.1177/095646249500600313. [DOI] [PubMed] [Google Scholar]
- 9.Vielot N, Hudgens MG, Mugo N, et al. The Role of Chlamydia trachomatis in High-Risk Human Papillomavirus Persistence Among Female Sex Workers in Nairobi, Kenya. Sex Transm Dis. 2015;42(6):305–311. doi: 10.1097/OLQ.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor Y, Melvin WT, Sewell HF, et al. Prevalence of Epstein-Barr virus in the cervix. J Clin Pathol. 1994;47(1):92–93. doi: 10.1136/jcp.47.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradilone A, Vercillo R, Napolitano M, et al. Prevalence of human papillomavirus, cytomegalovirus, and Epstein-Barr virus in the cervix of healthy women. J Med Virol. 1996;50(1):1–4. doi: 10.1002/(SICI)1096-9071(199609)50:1<1::AID-JMV1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Enbom M, Strand A, Falk KI, et al. Detection of Epstein-Barr virus, but not human herpesvirus 8, DNA in cervical secretions from Swedish women by real-time polymerase chain reaction. Sex Transm Dis. 2001;28(5):300–306. doi: 10.1097/00007435-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Berntsson M, Dubicanac L, Tunback P, et al. Frequent detection of cytomegalovirus and Epstein-Barr virus in cervical secretions from healthy young women. Acta Obstet Gynecol Scand. 2013;92(6):706–710. doi: 10.1111/aogs.12134. [DOI] [PubMed] [Google Scholar]
- 14.Naher H, Gissmann L, Freese UK, et al. Subclinical Epstein-Barr virus infection of both the male and female genital tract–indication for sexual transmission. J Invest Dermatol. 1992;98(5):791–793. doi: 10.1111/1523-1747.ep12499958. [DOI] [PubMed] [Google Scholar]
- 15.Ammatuna P, Giovannelli L, Giambelluca D, et al. Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J Med Virol. 2000;62(4):410–415. doi: 10.1002/1096-9071(200012)62:4<410::aid-jmv3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Silver MI, Paul P, Sowjanya P, et al. Shedding of Epstein-Barr virus and cytomegalovirus from the genital tract of women in a periurban community in Andhra Pradesh, India. J Clin Microbiol. 2011;49(7):2435–2439. doi: 10.1128/JCM.02206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehon PM, Hagensee ME, Sutton KJ, et al. Histological Evidence of Chronic Mycoplasma genitalium-Induced Cervicitis in HIV-Infected Women: A Retrospective Cohort Study. J Infect Dis. 2016;213(11):1828–1835. doi: 10.1093/infdis/jiw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babcock GJ, Decker LL, Volk M, et al. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 19.Koffa M, Koumantakis E, Ergazaki M, et al. Association of herpesvirus infection with the development of genital cancer. Int J Cancer. 1995;63(1):58–62. doi: 10.1002/ijc.2910630112. [DOI] [PubMed] [Google Scholar]
- 20.Sasagawa T, Shimakage M, Nakamura M, et al. Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: a comparative study with human papillomavirus (HPV) infection. Hum Pathol. 2000;31(3):318–326. doi: 10.1016/s0046-8177(00)80245-2. [DOI] [PubMed] [Google Scholar]
- 21.Lattario F, Furtado YL, Fonseca R, et al. Analysis of human papillomavirus and Epstein-Barr virus infection and aberrant death-associated protein kinase methylation in high-grade squamous intraepithelial lesions. Int J Gynecol Cancer. 2008;18(4):785–789. doi: 10.1111/j.1525-1438.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 22.Szostek S, Zawilinska B, Kopec J, et al. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim Pol. 2009;56(2):337–342. [PubMed] [Google Scholar]
- 23.Kahla S, Oueslati S, Achour M, et al. Correlation between ebv co-infection and HPV16 genome integrity in Tunisian cervical cancer patients. Braz J Microbiol. 2012;43(2):744–753. doi: 10.1590/S1517-83822012000200039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khenchouche A, Sadouki N, Boudriche A, et al. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol J. 2013;10:340. doi: 10.1186/1743-422X-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


