Abstract
Human and animal studies have confirmed that inhalation of nanoparticles from ambient air or occupational settings not only causes pathophysiological changes in the respiratory system, but in the other system as well. Accumulated evidence from animal studies has led to heightened concerns about the potential short- and long-term deleterious effects of inhalation of engineered nanoparticles on the cardiovascular system. This review highlights the new observations from animal studies, which document the adverse effects of pulmonary exposure to engineered nanoparticles on the cardiovascular system and elucidate the potential mechanisms involved in regulation of cardiovascular function, in particular how the neuronal system plays a role and reacts to pulmonary nanoparticle exposure based on our in vivo and in vitro studies. In addition, this review also discussed the possible influence of altered autonomic nervous activity on pre-existing cardiovascular conditions. Whether engineered nanoparticle exposure serves as one of the risk factors in the development of cardiovascular diseases warrants further investigation.
Keywords: Engineered nanoparticles, Occupation exposure, autonomic activity, cardiovascular diseases
Incidental nanoparticles, existing on a nanoscale (with diameters of < 100 nm), can agglomerate to form naturally occurring fine particles (with diameters of 0.1–1.0 μm) or remain as ultrafine particles (with diameters of < 100 nm) in ambient air. In addition, engineered nanomaterials, which are used in various manufacturing and medical applications, are increasing rapidly in production. The adverse health effects linking ambient nanoparticle exposure to cardiovascular diseases have been proposed. In the past decades, epidemiological studies indicate that there is a strong correlation between an increased level of nano-sized particles, mainly as ultrafine particles and agglomerated fine particles, in ambient air and increased incidences of cardiovascular diseases such as angina, arrhythmia, ischemic heart failure, and sudden death (Schwartz and Dockery 1992, Pope, Burnett et al. 2004). In vivo and in vitro animal studies also confirmed that either systemic administration or perfusion of the isolated rat heart with nanoparticles prepared from ambient air or diesel engine exhaust could alter cardiac contractility, increase left-ventricular end-diastolic pressure (LVEDP), significantly decrease left-ventricular systolic pressure (LVSP), and trigger ventricular premature beats depending on the type of nanoparticle (Wold, Simkhovich et al. 2006).
Recently, the potential adverse cardiovascular effects of exposure to engineered nanoparticles have been receiving a great deal of attention from investigators. This is because engineered nanoparticles have various applications in the fields of electronics, mechanical design, environmental remediation, and biomedicine (Mazzola 2003, Bunger 2007, Bar, Yacoby et al. 2008, Campbell 2013, Yan, Lien et al. 2013, Samadishadlou, Farshbaf et al. 2017), which has resulted in dramatic increases in their production. Due to this increasing production, occupational exposures are more likely. Additionally, with a rapidly growing manufacturing industry, release of such materials into the air, water, and soil will also be expected to increase in the coming years (Marquis, Love et al. 2009, Simonet and Valcarcel 2009).
Engineered nanoparticles are very similar in size to incidental nanoparticles in ambient air, but unlike incidental ambient nanoparticles, they are made of certain types of metals and other materials that are not found as commonly in incidental nanoparticles and possess varied physical and chemical properties depending on the source and the nature of the application (Mazzola 2003, Paull, Wolfe et al. 2003, Weiss 2008). Compared to inhaled ambient nanoparticles, the complicated properties of engineered nanoparticles may be more likely to cause significant adverse health impacts on the cardiovascular system. Evidence accumulated from animal studies have already indicated that exposure to engineered nanoparticles can cause pathophysiological changes not only in the respiratory system but also in the cardiovascular system (Li, Georas et al. 2016, Stone, Miller et al. 2016). For example, Li and colleagues discovered that pharyngeal aspiration of single-walled carbon nanotubes (SWCNTs) stimulated the progression of atherosclerosis in ApoE–/– transgenic mice (Li, Hulderman et al. 2007). Furthermore, Tong and colleagues documented that pharyngeal aspiration of SWCNTs led to lower cardiac functional recovery, greater infarct size, higher coronary flow rate, and signs of focal cardiac myofiber degeneration (Tong, McGee et al. 2009). Other in vitro studies have also shown that direct exposure of the cardiovascular system to engineered nanoparticles can result in tissue damage or functional change. For examples, direct exposure of the human aortic vessel to cerium oxide or metal oxide nanoparticles not only causes an endothelial cell-based inflammatory response at low concentrations but also induces cell injury and even death of these endothelial cells at higher concentrations (Gojova, Guo et al. 2007, Gojova, Lee et al. 2009). Helfenstein and colleagues reported that direct exposure of neonatal rat ventricular cardiomyocytes (NRVCMs) to ultrafine titanium dioxide (UFTiO2) or SWCNTs in culture could induce reactive oxygen species (ROS) production, change cardiomyocyte function, and deteriorate myofibrillar structure in a dose-dependent manner (Helfenstein, Miragoli et al. 2008). A separate study conducted by Totlandsdal and colleagues also demonstrated that exposure of NRVCM’s to ultrafine carbon black elicited a notable cytokine response (Totlandsdal, Skomedal et al. 2008). Similarly, Jawad and colleagues showed that direct exposure of adult ventricular cardiomyocytes to ultrafine TiO2 reduced the contraction amplitude and led to an increased cell death in fibroblasts, which can affect cardiovascular function (Jawad, Boccaccini et al. 2011). These studies provided compelling evidence to propose the deleterious effects of engineered nanoparticles on the cardiovascular system.
Although the previous studies indicated the adverse effects of engineered nanoparticles on the cardiovascular system, the underlying mechanisms which directly result in cardiovascular dysfunction by engineered nanoparticles, particularly through pulmonary exposure, are not yet clear. Based on current evidence from animal and epidemiological studies, three general mechanisms by which pulmonary exposure to engineered nanoparticles may cause detrimental effects on the cardiovascular system have been proposed: (1) engineered nanoparticles may translocate from lung to circulatory system and directly induce pathological changes in cardiovascular tissue, (2) engineered nanoparticles may trigger a lung-mediated systemic oxidative stress/inflammatory response, which alters cardiovascular function, or (3) engineered nanoparticles may alter the cardiovascular function through a neurogenic pathway. However, because of their complex nature, engineered nanoparticles may not just utilize only one of the proposed mechanisms but instead act via some combination of the three to bring about changes in the cardiovascular system.
It seems very understandable that due to their nano-meter size, inhaled engineered nanoparticles may easily pass through the alveolar air-blood barrier of lung. The alveolar air-blood barrier is less than 0.5 micrometers thick. Additionally, its permeability could be significantly changed and increased under certain pathological conditions, such as by lung inflammation which often occurs as a result of occupational and environmental exposure to airborne toxins or nanoparticles (Koskela, Mutanen et al. 2005, Byrne and Baugh 2008). Epithelial and endothelial injury as a result of inflammation can affect the permeability of the alveolar air-blood barrier (Planes, Valeyre et al. 1994, Tillie-Leblond, Guery et al. 2002) in ways that would allow nanoparticles to more easily translocate from the lung to blood compartments. Much support for the translocation of inhaled engineered nanoparticles comes from studies that detected inhaled nanoparticles in several organs besides that of the lungs (Kreyling, Semmler et al. 2002, Kreyling, Semmler-Behnke et al. 2009, Mercer, Scabilloni et al. 2013). Furthermore in vitro studies have indicated that direct exposure of cardiac muscle or vasculature to engineered nanoparticles can reduce contractile function and induce endothelial inflammatory responses or atherosclerotic alterations (Helfenstein, Miragoli et al. 2008, Duan, Yu et al. 2013, Yan, Wang et al. 2017). Combined, these two facets of support seem to provide evidence that nanoparticle translocation may be one of the pathophysiological mechanisms by which inhaled engineered nanoparticles regulate cardiovascular function. However, it is important to note that the fraction of inhaled nanoparticles, which are found in the cardiovascular system such as the heart and vascular tissue, is very low compared to the concentrations of nanoparticles applied during in vitro experiments. Due to dilution by the systematic blood volume, high blood concentrations of translocated nanoparticles may never be achieved following pulmonary exposure. In addition, although in vivo animal studies indicate that inhaled engineered nanoparticles can be transported to other parts of the body, such as the cardiovascular system, and accumulate with time following exposure, these findings do not explain why inhaled engineered nanoparticles cause transient cardiovascular alterations.
The second proposed mechanism is that inhaled engineered nanoparticles may trigger a lung-mediated systemic oxidative stress/inflammatory response, which may alter platelet function, coagulation or other cardiovascular endpoints (Ilinskaya and Dobrovolskaia 2013) and harm the cardiovascular system. This hypothetical mechanism was based on two major findings. First, inhaled engineered nanoparticles are able to reach the deepest part of the lung, the alveoli. From there, depending on their specific properties, the nanoparticles may stimulate either alveolar or endothelial cells. In this manner, engineered nanoparticles can interact with macrophages, epithelial cells, endothelial cells and blood leukocytes to evoke the production of reactive oxygen species (ROS) or pro-inflammatic cytokines, which could affect the cardiovascular system (Kumar, Kumari et al. 2012, Bengalli, Gualtieri et al. 2017, Wu and Tang 2017). A close correlation between oxidative stress, systemic inflammation, and increased risk of a cardiovascular incidents has been well-documented (Nijm, Wikby et al. 2005, Chrysohoou, Pitsavos et al. 2009, Dhingra, Sharma et al. 2009, Zanotti-Cavazzoni and Hollenberg 2009). Furthermore, animal studies have confirmed that engineered nanoparticles can induce lung-mediated systemic oxidative stress and inflammation. For example, pulmonary exposure to CeO2 NPs induced pulmonary and systemic inflammation and oxidative stress, which can later promote thrombosis in vivo (Nemmar, Al- Salam et al. 2017). Acute pulmonary and thrombotic effects of cerium oxide nanoparticles were noted after intratracheal instillation in mice. Similar results were also observed in mice exposed to single walled carbon nanotubes (SWCNTs) by pharyngeal aspiration (Li, Hulderman et al. 2007). Both studies suggested that systemic oxidative stress following pulmonary exposure to engineered nanoparticles was associated with the observed atherosclerotic plaque formation, a pathological process that can increase the severity of coronary artery disease (Li, Hulderman et al. 2007, Araujo, Barajas et al. 2008, Zakynthinos and Pappa 2009). Of interest, some animal studies reported that acute pulmonary exposure to engineered nanoparticles can result in a rapid and transient alterations in the cardiovascular system at lung burden too low to cause detectable pulmonary or systemic oxidative stress or inflammation.
Because current evidence suggests problems with direct cardiovascular effects of translocated nanoparticles or the systemic inflammation mechanisms, a neurogenic mechanism has been proposed. Indeed, epidemiological studies have shown that particulate air pollution exposure is associated with indicators of impacted autonomic input to the heart, resulting in increased heart rate, decreased heart rate variability, and increased cardiac arrhythmias (Timonen, Vanninen et al. 2006). Furthermore, epidemiological studies also demonstrate that short-term exposure to fine particulate pollutants can lead to an autonomic imbalance, which results in vasoconstriction and high blood pressure. The growing body of evidence obtained from epidemiological studies strongly suggests that a neuron-regulated pathway may play a crucial role in regulating cardiovascular function after pulmonary exposure to engineered nanoparticles.
Recently, a few animal studies, including ours, have explored the possible contribution of a neuronal pathway in engineered nanoparticle-induced cardiovascular events. Results from these in vivo studies strongly support that the nervous system can modulate cardiovascular function after pulmonary exposure to engineered nanoparticles. For instance, Legramante and colleagues studied the arterial baroreflex function (BRF), which is closely regulated by sympathetic and parasympathetic branches of the autonomic nervous system, in response to single-walled carbon nanotube exposure in an animal model. The study demonstrated that intratracheal instillation of single-walled carbon nanotubes (SWCNTs) induced a blunted BRS response (Legramante, Valentini et al. 2009), which was likely due to input from the autonomic nervous system, and supported a neurogenic pathway in the regulation of cardiovascular function after pulmonary exposure to SWCNTs. An in vivo study conducted by Nurkiewicz and colleagues demonstrated that pulmonary ultrafine titanium dioxide exposure induced systemic microvascular dysfunction in the spinotrapezius muscle, which was partially reversed with the fast sodium (Na+) channel antagonist tetrodotoxin (TTX) (Nurkiewicz, Porter et al. 2011). Another in vivo study conducted by the same authors discovered that enhanced adrenergic receptor sensitivity influenced vasoreactivity after inhalation of UFTiO2 (Knuckles, Yi et al. 2012). Both studies support a possible neurogenic mechanism.
In our studies using in vitro and in vivo experimental models, we have also provided convincing evidence to support the involvement of neural systems in engineered nanoparticle-induced cardiovascular alterations. In an in vitro study, direct incubation of cardiac myocytes isolated from the heart of a naïve adult rat with UFTiO2 did not induce detectable biological changes, such as phosphorylation of cardiac troponin I (cTnI) (Figure 1). However, the phosphorylation level of cTnI was significantly increased in the cardiac tissue obtained from the rats following pulmonary exposure to UFTiO2 (Figure 1) (Kan, Wu et al. 2012). The results indicated that UFTiO2-induced biological changes in the heart following pulmonary exposure were not likely due to the translocation of nanoparticles from the respiratory system to the circulatory system and their direct interactions with the heart, since coronary levels of UFTiO2 were below levels of detection.
Figure 1.
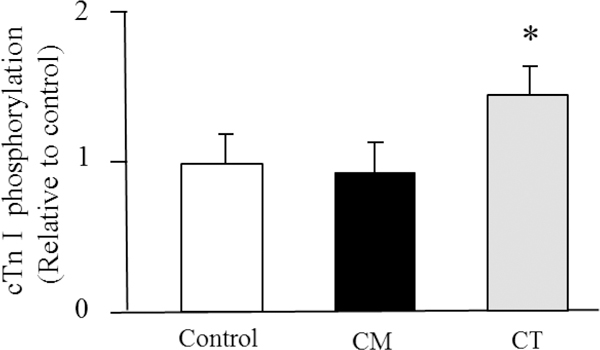
Densitometry values of phosphorylated cardiac troponin I (cTnI) from cardiac myocytes (CM) directly exposed to UFTiO2 and cardiac tissue (CT) obtained from the rat following pulmonary exposure to UFTiO2.
In one of our in vivo studies, we also found that pulmonary exposure to UFTiO2 did not increase peripheral blood levels of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1)(Kan, Wu et al. 2012), both of which are known to have negative ionotropic effects on cardiac function (Ahmad, Otaal et al. 2009, Dhingra, Sharma et al. 2009, Zanotti-Cavazzoni and Hollenberg 2009). In the same study, we did not detect an increase of neutrophils in the peripheral blood or of ROS in the cardiac tissue (Kan, Wu et al. 2012). These results suggested that pulmonary exposure to UFTiO2 did not lead to systemic inflammation or oxidative stress even though it may cause inflammation at the site of lung deposition. However, our study found that pulmonary exposure to UFTiO2 greatly increased substance P, a neuronal transmitter, synthesis in the nodose ganglia, which is associated with activation of sensory receptors in airways (Figure 2). Interestingly, blocking sensory receptor activation with the transient receptor potential (TRP) channel blocker ruthenium red not only inhibited substance P synthesis in nodose ganglia, but also prevented the UFTiO2-induced alteration in cardiac diastolic function and increase of blood pressure (Figure 2, 3, 4) (Kan, Wu et al. 2014).
Figure 2.
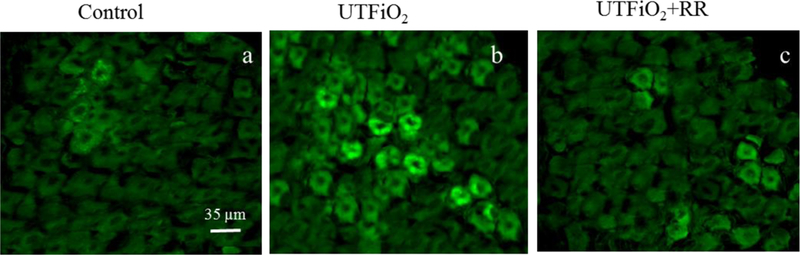
Fluorescence photomicrographs of substance P immunoreactivity in nodose ganglia.
Figure 3.
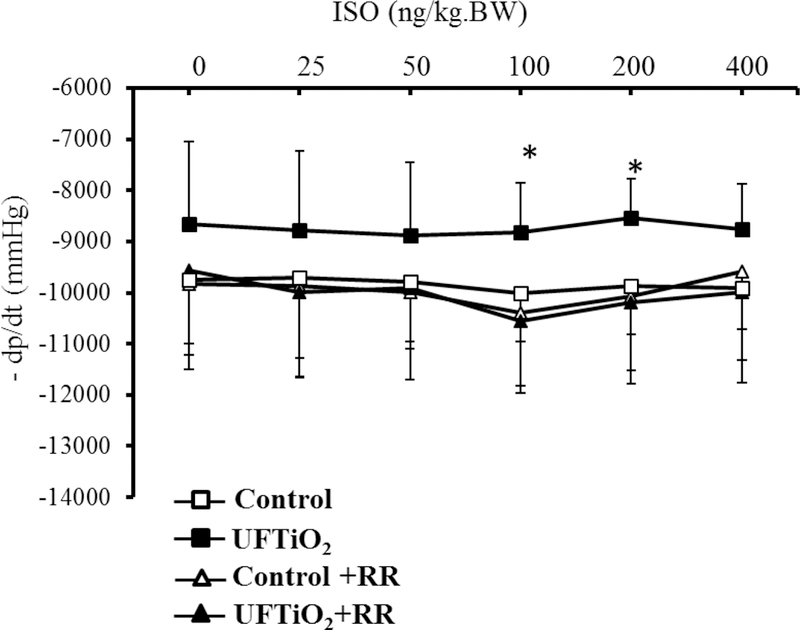
The dose–response curve of mean blood pressure (MBP) in response to norepinephrine (NE) in the presence and absence of ruthenium red (RR) after exposure to UFTiO2.
Figure 4.
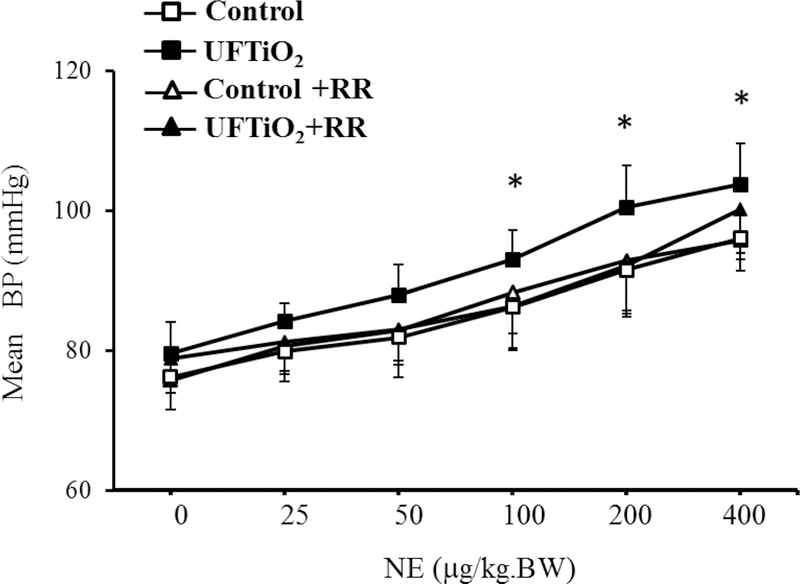
The effect of ruthenium red (RR) on diastolic function of the heart after exposure to UFTiO2
The nodose ganglia has been known to be involved in the integration and control of lung and heart function by receiving primary sensory nerve fibers from the lungs and transmitting that information to the brainstem’s medullary respiratory and cardiovascular regulatory centers (Spyer 1982). It was also reported that nodose ganglia’s efferent neurons project to different chambers of the rat heart and exhibit a variety of neurochemical phenotypes (Guic, Kosta et al. 2010, Kosta, Guic et al. 2010). Our observations revealed that pulmonary exposure to engineered nanoparticles may cause adverse effects on the cardiovascular system by altering autonomic neuronal function through the nodose ganglia. The results from our study also suggest that a neurogenic mechanism could be responsible to alterations in cardiovascular function following pulmonary exposure to engineered nanoparticles.
The autonomic nervous system (ANS) is crucial in maintaining proper cardiovascular function. A slight disturbance of ANS balance (parasympathetic to sympathetic input) can greatly affect cardiovascular function (Frontiers in Physiology Editorial 2015, Fukuda, Kanazawa et al. 2015, Grassi, Mark et al. 2015). Although studies indicated that ANS is likely involved in a neural pathway that regulates cardiovascular function following engineered nanoparticle exposure, how ANS activity is influenced by engineered nanoparticle exposure has only been recently explored. To determine the influence of engineered nanoparticle exposure on the autonomic system, we designed an experiment to study heart rate variability (HRV) following pulmonary exposure to multi-walled carbon nanotubes (MWCNTs), an engineered nanomaterial, in freely moving rats pre-implanted with telemetry devices. HRV, which analyzes beat-to-beat variability of the heart rate measured in an electrocardiogram (EKG), has been considered to be an indicator that reflects fluctuations in the activity of the sympathetic and parasympathetic nervous systems (Moulopoulos 2005). Therefore, changes of HRV reflects fluctuations in autonomic nervous system activity. In this study, we found that pulmonary exposure to MWCNTs significantly changed HRV indices of root mean square of successive differences (RMSSD), low frequency (LF) and high frequency (HF) (Figure 5), which were associated with increased blood pressure and reduced cardiac function (Zheng, McKinney et al. 2016). The results also indicated that pulmonary exposure to MWCNTs changed both parasympathetic and sympathetic nervous activity, which may shift the balance point of the autonomic nervous system and alter cardiovascular function.
Figure 5.
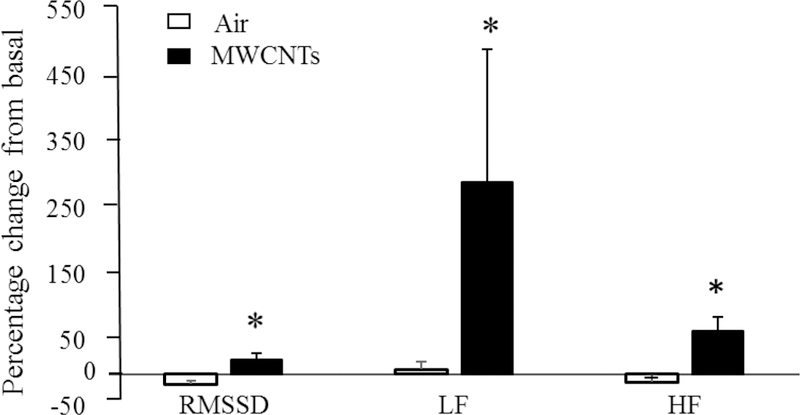
Effects of multi-walled carbon nanotubes (MWCNTs) on the heart rate variability (HRV). Bar graph depicting % change of root mean square of successive differences (RMSSD), low frequency (LF) and high frequency (HF) from the basal level before exposure.
Our observations, together with other in vivo and in vitro studies, support the role of a neuronal pathway in the regulation of cardiovascular function following pulmonary exposure to engineered nanoparticles. It appears that inhaled engineered nanoparticles can cause local irritation at the site of deposition, which activates lung sensory receptors. Then a sensory signal can be relayed to the nervous system through the nodose ganglia, which in turn influences autonomic nervous output that regulates cardiovascular function. In our study, we also noticed that neuron-regulated alterations in HRV and cardiovascular function occurred rapidly but were only transient (Zheng, McKinney et al. 2016). This time course fits well with characteristic of the sensory neurons. The mechanism by which the inhalation of MWCNTs only induces a transient or short effect is most likely a quickly adapted reaction of either the lung sensory receptor or the central nervous system to constantly detected stimulations. Such normal adaption after pulmonary exposure to nanomaterials may serve as a protective mechanism to maintain homeostasis in the cardiovascular system.
In summary, pulmonary exposure to engineered nanoparticles could initiate two pathophysiological phases in the cardiovascular system. The first involves neuron-regulated fast-onset and short-lasting cardiovascular reactions that include alterations in heart rate, blood pressure and cardiac contractility. The second, depending on the composition and structure of engineered nanoparticles, may involve inhaled engineered nanoparticles gradually and continuously crossing the air-blood barrel into the circulatory system or inducing an inflammation and oxidative stress response systemically in the circulation. The second pathophysiological phase may promote the development of chronic cardiovascular events such as atherosclerosis (Li, Hulderman et al. 2007, Yan, Wang et al. 2017). Neurogenic cardiovascular reactions through the autonomic nervous system by engineered nanoparticles, although short-lasting and potentially producing minor adverse impacts on healthy subjects, could be a risk factor to people with pre-existing cardiovascular conditions. For instance, people with heart failure or hypertension are usually more prone to sudden alterations in autonomic nervous activity (Triposkiadis, Karayannis et al. 2009). Exposure to engineered nanoparticle-induced unbalance or activity changes in the autonomic system may trigger arrhythmias, further reduce already lowered cardiac output or cause vasculature constriction, which could further lead to coronary artery spasms and myocardial ischaemia. The reaction of cardiovascular system in response to inhaled engineered nanomaterials is complicated and could very depend on the physical, chemical or even biological properties of nanomaterials. All three mechanisms, translocation, inflammation and neuronal regulation, could contribute to alterations in cardiovascular system following a given type of nanomaterial exposure, but neuronal-regulated cardiovascular reaction appears responsible for the first notable changes. Research associating pulmonary exposure to engineered nanoparticles and their impacts on the cardiovascular system is still at its early stage. More studies are warranted to better understand the significance of neurogenic mechanism in evaluation and prevention of the adverse effects on the cardiovascular system following occupational exposure to engineered nanoparticles.
Footnotes
Disclaimer: The opinions expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Center for Disease Control and Prevention of the USA.
References
- Ahmad S, Otaal PS, Rai TS, Bahl A, Saikia UN, Manoj RK, Thungapathra M, Talwar KK and Khullar M (2009). “Circulating proinflammatory cytokines and N-terminal pro-brain natriuretic peptide significantly decrease with recovery of left ventricular function in patients with dilated cardiomyopathy.” Mol Cell Biochem 324(1–2): 139–145. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ and Nel AE (2008). “Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress.” Circ Res 102(5): 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar H, Yacoby I and Benhar I (2008). “Killing cancer cells by targeted drug-carrying phage nanomedicines.” BMC Biotechnol 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengalli R, Gualtieri M, Capasso L, Urani C and Camatini M (2017). “Impact of zinc oxide nanoparticles on an in vitro model of the human air-blood barrier.” Toxicol Lett 279: 22–32. [DOI] [PubMed] [Google Scholar]
- Bunger M (2007). “Strategies for developing and commercializing nanobio drugs, diagnostics and devices.” Nanomedicine (Lond) 2(2): 137–141. [DOI] [PubMed] [Google Scholar]
- Byrne JD and Baugh JA (2008). “The significance of nanoparticles in particle-induced pulmonary fibrosis.” Mcgill J Med 11(1): 43–50. [PMC free article] [PubMed] [Google Scholar]
- Campbell CT (2013). “The energetics of supported metal nanoparticles: relationships to sintering rates and catalytic activity.” Acc Chem Res 46(8): 1712–1719. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Pitsavos C, Barbetseas J, Kotroyiannis I, Brili S, Vasiliadou K, Papadimitriou L and Stefanadis C (2009). “Chronic systemic inflammation accompanies impaired ventricular diastolic function, detected by Doppler imaging, in patients with newly diagnosed systolic heart failure (Hellenic Heart Failure Study).” Heart Vessels 24(1): 22–26. [DOI] [PubMed] [Google Scholar]
- Dhingra S, Sharma AK, Arora RC, Slezak J and Singal PK (2009). “IL-10 attenuates TNF-alpha- induced NF kappaB pathway activation and cardiomyocyte apoptosis.” Cardiovasc Res 82(1): 59–66. [DOI] [PubMed] [Google Scholar]
- Duan J, Yu Y, Li Y, Yu Y, Li Y, Zhou X, Huang P and Sun Z (2013). “Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint.” PLoS One 8(4): e62087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontiers in Physiology Editorial, O. (2015). “Retraction: Physiology and Pharmacology of the Cardiovascular Adrenergic System.” Front Physiol 6: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kanazawa H, Aizawa Y, Ardell JL and Shivkumar K (2015). “Cardiac innervation and sudden cardiac death.” Circ Res 116(12): 2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM and Barakat AI (2007). “Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition.” Environ Health Perspect 115(3): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Lee JT, Jung HS, Guo B, Barakat AI and Kennedy IM (2009). “Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells.” Inhal Toxicol 21 Suppl 1: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Mark A and Esler M (2015). “The sympathetic nervous system alterations in human hypertension.” Circ Res 116(6): 976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guic MM, Kosta V, Aljinovic J, Sapunar D and Grkovic I (2010). “Characterization of spinal afferent neurons projecting to different chambers of the rat heart.” Neurosci Lett 469(3): 314–318. [DOI] [PubMed] [Google Scholar]
- Helfenstein M, Miragoli M, Rohr S, Muller L, Wick P, Mohr M, Gehr P and Rothen-Rutishauser B (2008). “Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro.” Toxicology 253(1–3): 70–78. [DOI] [PubMed] [Google Scholar]
- Ilinskaya AN and Dobrovolskaia MA (2013). “Nanoparticles and the blood coagulation system. Part II: safety concerns.” Nanomedicine (Lond) 8(6): 969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad H, Boccaccini AR, Ali NN and Harding SE (2011). “Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications.” Nanotoxicology 5(3): 372–380. [DOI] [PubMed] [Google Scholar]
- Kan H, Wu Z, Lin YC, Chen TH, Cumpston JL, Kashon ML, Leonard S, Munson AE and Castranova V (2014). “The role of nodose ganglia in the regulation of cardiovascular function following pulmonary exposure to ultrafine titanium dioxide.” Nanotoxicology 8(4): 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, Wu Z, Young SH, Chen TH, Cumpston JL, Chen F, Kashon ML and Castranova V (2012). “Pulmonary exposure of rats to ultrafine titanium dioxide enhances cardiac protein phosphorylation and substance P synthesis in nodose ganglia.” Nanotoxicology 6(7): 736–745. [DOI] [PubMed] [Google Scholar]
- Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V and Nurkiewicz TR (2012). “Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways.” Nanotoxicology 6(7): 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela RS, Mutanen P, Sorsa JA and Klockars M (2005). “Respiratory disease and cardiovascular morbidity.” Occup Environ Med 62(9): 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosta V, Guic MM, Aljinovic J, Sapunar D and Grkovic I (2010). “Immunohistochemical characteristics of neurons in nodose ganglia projecting to the different chambers of the rat heart.” Auton Neurosci 155(1–2): 33–38. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S and Oberdorster G (2009). “Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs.” Inhal Toxicol 21 Suppl 1: 55–60. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdorster G and Ziesenis A (2002). “Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low.” J Toxicol Environ Health A 65(20): 1513–1530. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kumari A, Guleria P and Yadav SK (2012). “Evaluating the toxicity of selected types of nanochemicals.” Rev Environ Contam Toxicol 215: 39–121. [DOI] [PubMed] [Google Scholar]
- Legramante JM, Valentini F, Magrini A, Palleschi G, Sacco S, Iavicoli I, Pallante M, Moscone D, Galante A, Bergamaschi E, Bergamaschi A and Pietroiusti A (2009). “Cardiac autonomic regulation after lung exposure to carbon nanotubes.” Hum Exp Toxicol 28(6–7): 369–375. [DOI] [PubMed] [Google Scholar]
- Li N, Georas S, Alexis N, Fritz P, Xia T, Williams MA, Horner E and Nel A (2016). “A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects.” J Allergy Clin Immunol 138(2): 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova A, Luster MI and Simeonova PP (2007). “Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes.” Environ Health Perspect 115(3): 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis BJ, Love SA, Braun KL and Haynes CL (2009). “Analytical methods to assess nanoparticle toxicity.” Analyst 134(3): 425–439. [DOI] [PubMed] [Google Scholar]
- Mazzola L (2003). “Commercializing nanotechnology.” Nat Biotechnol 21(10): 1137–1143. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V and Porter DW (2013). “Extrapulmonary transport of MWCNT following inhalation exposure.” Part Fibre Toxicol 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulopoulos SD (2005). “Heart rate variability and the autonomic nervous system.” Eur J Intern Med 16(1): 1–2. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al-Salam S, Beegam S, Yuvaraju P and Ali BH (2017). “The acute pulmonary and thrombotic effects of cerium oxide nanoparticles after intratracheal instillation in mice.” Int J Nanomedicine 12: 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijm J, Wikby A, Tompa A, Olsson AG and Jonasson L (2005). “Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease.” Am J Cardiol 95(4): 452–456. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Moseley AM, Cumpston JL, Goodwill AG, Frisbee SJ, Perrotta PL, Brock RW, Frisbee JC, Boegehold MA, Frazer DG, Chen BT, Castranova V and Committee HEIHR (2011). “Pulmonary particulate matter and systemic microvascular dysfunction.” Res Rep Health Eff Inst(164): 3–48. [PubMed] [Google Scholar]
- Paull R, Wolfe J, Hebert P and Sinkula M (2003). “Investing in nanotechnology.” Nat Biotechnol 21(10): 1144–1147. [DOI] [PubMed] [Google Scholar]
- Planes C, Valeyre D, Loiseau A, Bernaudin JF and Soler P (1994). “Ultrastructural alterations of the air-blood barrier in sarcoidosis and hypersensitivity pneumonitis and their relation to lung histopathology.” Am J Respir Crit Care Med 150(4): 1067–1074. [DOI] [PubMed] [Google Scholar]
- Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D and Godleski JJ (2004). “Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease.” Circulation 109(1): 71–77. [DOI] [PubMed] [Google Scholar]
- Samadishadlou M, Farshbaf M, Annabi N, Kavetskyy T, Khalilov R, Saghfi S, Akbarzadeh A and Mousavi S (2017). “Magnetic carbon nanotubes: preparation, physical properties, and applications in biomedicine.” Artif Cells Nanomed Biotechnol: 1–17. [DOI] [PubMed] [Google Scholar]
- Schwartz J and Dockery DW (1992). “Increased mortality in Philadelphia associated with daily air pollution concentrations.” Am Rev Respir Dis 145(3): 600–604. [DOI] [PubMed] [Google Scholar]
- Simonet BM and Valcarcel M (2009). “Monitoring nanoparticles in the environment.” Anal Bioanal Chem 393(1): 17–21. [DOI] [PubMed] [Google Scholar]
- Spyer KM (1982). “Central nervous integration of cardiovascular control.” J Exp Biol 100: 109–128. [DOI] [PubMed] [Google Scholar]
- Stone V, Miller MR, Clift MJ, Elder A, Mills NL, Moller P, Schins RP, Vogel U, Kreyling WG, Jensen KA, Kuhlbusch TA, Schwarze PE, Hoet P, Pietroiusti A, De Vizcaya-Ruiz A, Baeza-Squiban A, Tran CL and Cassee FR (2016). “Nanomaterials vs Ambient Ultrafine Particles: an Opportunity to Exchange Toxicology Knowledge.” Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillie-Leblond I, Guery BP, Janin A, Leberre R, Just N, Pittet JF, Tonnel AB and Gosset P (2002). “Chronic bronchial allergic inflammation increases alveolar liquid clearance by TNF-alpha - dependent mechanism.” Am J Physiol Lung Cell Mol Physiol 283(6): L1303–1309. [DOI] [PubMed] [Google Scholar]
- Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef B, Gold DR, Heinrich J, Hoek G, Lanki T, Peters A, Tarkiainen T, Tiittanen P, Kreyling W and Pekkanen J (2006). “Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: the ULTRA study.” J Expo Sci Environ Epidemiol 16(4): 332–341. [DOI] [PubMed] [Google Scholar]
- Tong H, McGee JK, Saxena RK, Kodavanti UP, Devlin RB and Gilmour MI (2009). “Influence of acid functionalization on the cardiopulmonary toxicity of carbon nanotubes and carbon black particles in mice.” Toxicol Appl Pharmacol 239(3): 224–232. [DOI] [PubMed] [Google Scholar]
- Totlandsdal AI, Skomedal T, Lag M, Osnes JB and Refsnes M (2008). “Pro-inflammatory potential of ultrafine particles in mono- and co-cultures of primary cardiac cells.” Toxicology 247(1): 23–32. [DOI] [PubMed] [Google Scholar]
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G and Butler J (2009). “The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications.” J Am Coll Cardiol 54(19): 1747–1762. [DOI] [PubMed] [Google Scholar]
- Weiss PS (2008). “Meeting global challenges by investing in nanoscience and nanotechnology.” ACS Nano 2(11): 2193. [DOI] [PubMed] [Google Scholar]
- Wold LE, Simkhovich BZ, Kleinman MT, Nordlie MA, Dow JS, Sioutas C and Kloner RA (2006). “In vivo and in vitro models to test the hypothesis of particle-induced effects on cardiac function and arrhythmias.” Cardiovasc Toxicol 6(1): 69–78. [DOI] [PubMed] [Google Scholar]
- Wu T and Tang M (2017). “Review of the effects of manufactured nanoparticles on mammalian target organs.” J Appl Toxicol. [DOI] [PubMed] [Google Scholar]
- Yan W, Lien HL, Koel BE and Zhang WX (2013). “Iron nanoparticles for environmental cleanup: recent developments and future outlook.” Environ Sci Process Impacts 15(1): 63–77. [DOI] [PubMed] [Google Scholar]
- Yan Z, Wang W, Wu Y, Wang W, Li B, Liang N and Wu W (2017). “Zinc oxide nanoparticle- induced atherosclerotic alterations in vitro and in vivo.” Int J Nanomedicine 12: 4433–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakynthinos E and Pappa N (2009). “Inflammatory biomarkers in coronary artery disease.” J Cardiol 53(3): 317–333. [DOI] [PubMed] [Google Scholar]
- Zanotti-Cavazzoni SL and Hollenberg SM (2009). “Cardiac dysfunction in severe sepsis and septic shock.” Curr Opin Crit Care 15(5): 392–397. [DOI] [PubMed] [Google Scholar]
- Zheng W, McKinney W, Kashon M, Salmen R, Castranova V and Kan H (2016). “The influence of inhaled multi-walled carbon nanotubes on the autonomic nervous system.” Part Fibre Toxicol 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


