Abstract
We report that HCl•DMPU induces the formation of (thiomethyl)methyl carbenium ion from DMSO under mild conditions. Homoallylic amines react with this electrophile to generate 4-chloropiperidines in good yields. The method applies to both aromatic and aliphatic amines. The use of HCl•DMPU as both non-nucleophilic base and chloride source constitutes an environmentally benign alternative for piperidine formation. The reaction has a broad substrate scope, and the conditions offer good chemical yields with high functional group tolerance and scalability.
Graphical Abstract
Introduction
Functionalized piperidines are ubiquitous in natural products1 and pharmaceuticals (Figure 1).2 Despite an extensive literature on piperidine syntheses,3 there are still demands for more efficient syntheses. The common strategies for the synthesis of piperidine skeletons involve intra-4, and intermolecular5 cyclization reactions, ring expansion processes6 and reduction of pyridines.7 Cycloaddition is the more effective approach, achieved either by a nucleophilic substitution process (Scheme 1a),3c, 8 transition metal catalysis (Scheme 1b),3a, b, 9 or an electrophile-or radical-induced cyclization (Scheme 1c).10 The use of designed or protected substrates and expensive transition metals limit the application of some of these methods. Another important method for obtaining 4-substituted piperidines is through the aza-Prins cyclization method (Scheme 1e).5a, 11 This method usually requires transition metal or Lewis acid catalysis.
Figure 1.
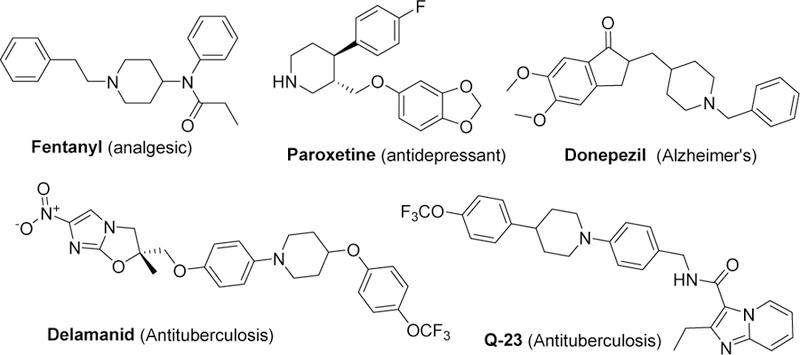
Examples of piperidine-containing natural products and drug molecules.
Scheme 1.
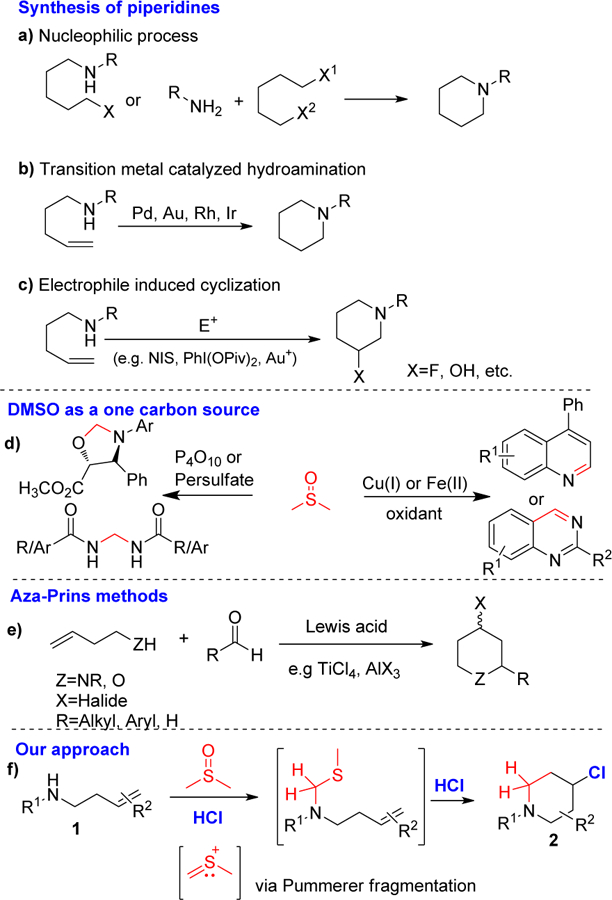
Literature background.
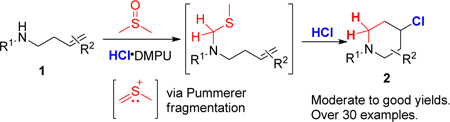
In our search for applications of the newly formulated HCl•DMPU,12 a highly concentrated, bench stable, readily prepared and easily dispensable anhydrous source of HCl, we observed the activation of DMSO. Activation of dimethyl sulfoxide by electrophiles13 has been widely reported and has led to the application of DMSO as a viable synthon,14 as noted by the increased use of DMSO as a one carbon source in the recent literature (Scheme 1d).15 We describe herein application of our HCl•DMPU-mediated DMSO activation to prepare 4-chloropiperidines.
Despite the tremendous progress made in alkene amino cyclization reactions,4, 11i, 16 there are still limitations in the substrate scope for intramolecular construction of piperidines. We wanted to avoid the use of toxic formaldehyde as a one carbon synthon. To address these limitations, we surmised one possible solution would be to exploit the formation of (thiomethyl)methyl carbenium ion from DMSO17 will be trapped by homoallylic amines (Scheme 1f).18 The thiocarbenium ion generation could arise from a Pummerer fragmentation through the interaction of the sulfoxide with an electrophile. Such activations are common in activated high-molecular weight sulfoxides.19 However, protic acid activation of DMSO is rare.20 We envisioned that reaction of the thiocarbenium ion with a homoallylic amine might initiate an intramolecular cyclization to form a piperidine ring. Specifically, tandem electrophilic capture of the DMSO-derived (thiomethyl)methyl carbenium ion by the homoallylic amine followed by intramolecular reaction of the pendant vinyl system with subsequent counterion trapping of the resulting electrophilic center could afford access to 4-substituted piperidines. Such an approach would provide a nice compliment to current halo-piperidation techniques.5a, 11a, 21
Results and Discussion
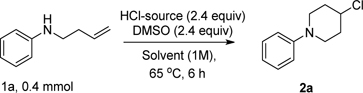
We began our investigation using the homoallylic amine 1a, HCl•DMPU (2.4 equiv) and DMSO (2.4 equiv) in DCE at 65 °C (Table 1). We were pleased to obtain the desired cyclized product 2a in decent yield. A solvent screening indicated a better conversion in ethyl acetate (Table 1, entries 1–5). Reaction concentration played an essential role in improving conversion and limiting side product of methylthiolation. (Table 1, entries 6 and 7).
Table 1. Reaction optimization.a.
 | ||||
|---|---|---|---|---|
| Entry | Solvent | HCl source | Conc. | Conv./%a |
| 1 | DCE | HCl•DMPU | 1.0 | 64 |
| 2 | CH3CN | HCl•DMPU | 1.0 | 22 |
| 3 | CH3NO3 | HCl•DMPU | 1.0 | 30 |
| 4 | EtOAc | HCl•DMPU | 1.0 | 90 |
| 5 | DMSO | HCl•DMPU | 1.0 | 18 |
| 6 | EtOAc | HCl•DMPU | 0.5 | 89 |
| 7 | EtOAc | HCl•DMPU | 0.2 | 99 |
| 8 | EtOAc | HCl, Et2O | 0.2 | 32 (98)b |
| 9 | EtOAc | HCl, 2-propanol | 0.2 | 99c |
| 10 | EtOAc | HCl, dioxane | 0.2 | 65 (94)b |
| 11 | EtOAc | HCl, AcOH | 0.2 | 99c |
| 12 | EtOAc | HCl, H2O | 0.2 | 29 (65)b |
| 13 | EtOAc | CH3COCl/EtOH | 0.2 | 99 |
| 14 | EtOAc | TMSCl/MeOH | 0.2 | 95 |
Determined by GC-MS with dodecane as the internal standard.
24 h
combined with thiolated side product.
We also investigated other HCl sources. As expected, the lower concentration sources were sluggish (Table 1, entries 8, and 10) while the more concentrated sources gave appreciable conversions with lower desired product ratios thwarted by thiolated side products (Table 1, entries 9 and 11). Use of aqueous HCl gave a dismal outcome; however, the conversions with in situ generated HCl Table 1, entries 13 and 14) proceeded with comparable selectivity to the ready-made HCl•DMPU reagent. Encouraged by these results, we examined the substrate scope of this new cyclization reaction with the optimum condition in hand (Table 2).
Table 2. Substrate scope for the synthesis of 4-chloropiperidines.a.
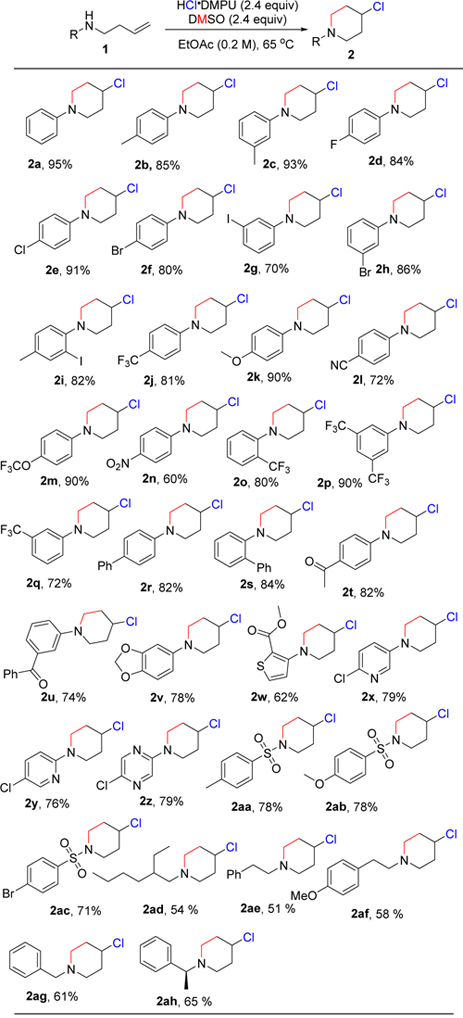 |
1 (0.2 mmol), HCl•DMPU (2.4 equiv), DMSO (2.4 equiv), 65 °C, 9–24 h. All yields are isolated yields.
We first examined the scope of homoallylic anilines. The study revealed that there was broad tolerance of substituents with various electronic properties on all positions on the aromatic ring giving good to excellent yields. An array of para-substituted anilines containing groups such as methyl (2b), halo (2d, 2e, 2f), trifluoromethyl (2j), methoxy (2k), cyano (2l), trifluoromethoxy (2m), nitro (2n), phenyl (2r) and acetyl (2t) all proceeded in excellent yields. Single crystal X-ray structure of the 4-nitrophenyl derivative (2n), was obtained showing the chlorine atom locked in the axial position (Figure 3). The inclusion of similar substituents at the ortho (2i, 2o, 2s) and meta (2c, 2g, 2h, 2q) positions did not affect the yields. The method displayed good functional group tolerance to groups like nitrile (2l), ester (2w), ethers (2k, 2m, 2v) and ketones (2t, 2u). Interestingly, homoallylic sulfonamides (2aa, 2ab, 2ac) transformed excellent yields. Heteroaromatic amines that contain benzodioxole (2v), thiophene (2w), pyridine (2x, 2y) and pyrazine (2z) moieties also gave desired cyclization products in good yields. While meta-polysubstitution (2p) was highly selective resulting in a yield of 90%, the trisubstituted substrate (2ai) gave an inseparable 3:2 mixture of piperidine and pyrrolidine respectively. Unfortunately, the method was unsuccessful with the hydrazide (2aj), hydroxylamine (2ak) and indole (2al) substrates likely due to substrate intolerance and product instability (Figure 2). We also examined aliphatic amines too. Though strongly basic, we were delighted to observe satisfactory product yields of 51-64%. The reaction conditions tolerated benzyl (2ag, 2ah) and longer aliphatic chain (2ad, 2ae, 2af) substrates.
Figure 3.
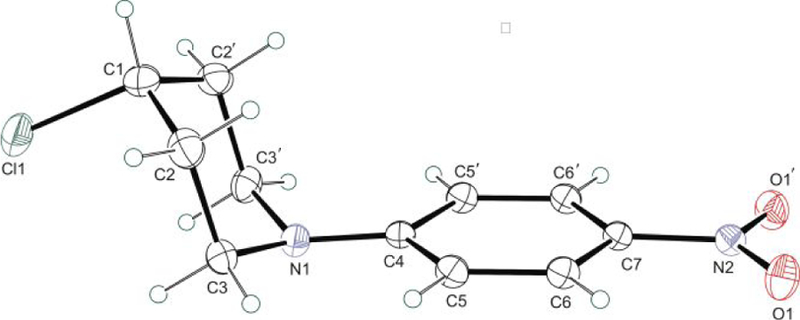
ORTEP representation of (2n) with thermal ellipsoids shown at the 50% probability level.
Figure 2.
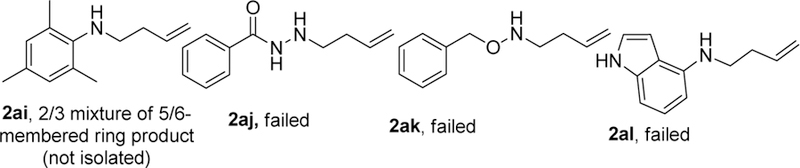
Failed substrates.
Next, we investigated the scope of the alkene chain. Both terminal and internal substituted alkenes afforded desired products (Table 3). The disubstituted alkenes (3a, 3b, 3c, 3d) furnished the desired cyclized products (4a, 4b, 4c, 4d) in good yields of 81%, 84%, 53% and 65% respectively. The major diastereomer, 4d, exhibited an anti-stereochemistry. The sterically hindered homoallylic amine derivative of nopol (3c) underwent the cyclization in a modest 53% yield. Due to steric demands, the 1,1,2-trisubstituted alkene substrate (3e) failed to achieve the desired outcomes. Rather it formed the kinetically favored pyrrolidine product in 64% yield. The mass obtained by GCMS was consistent with that of the pyrrolidine product. Also, the cyclic alkene substrate (3f) was unsuccessful probably due to ring strain barrier associated with its formation.
Table 3. Scope for the synthesis of 4-chloropiperidines.a.
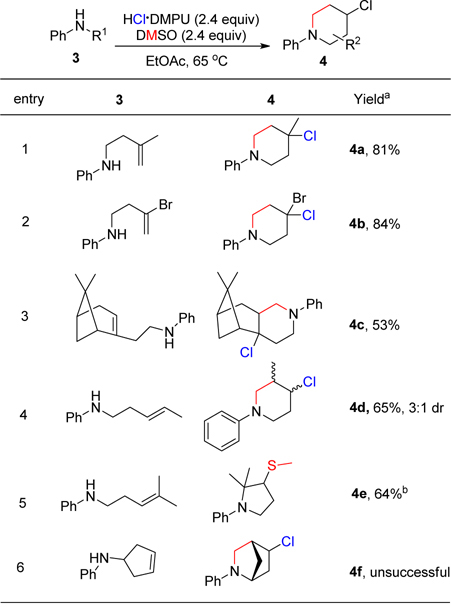 |
3 (0.2 mmol), HCl DMPU (2.4 equiv), DMSO (2.4 equiv), 65 °C, 9–18 h, isolated yields, b 1H NMR with CH2Br2 as internal standard.
To demonstrate the practicality of the method, we conducted a ten mmol reaction (Scheme 2, eq 1) of 1a without any further modifications and obtained the desired product 2a in high yield. To probe the mechanism of the reaction, we carried a deuterium labeling experiment using deuterated DMSO. We observed high deuterium incorporation of over 99% for the resulting piperidine (Scheme 2, eq 2). This result indicates that the extra carbon arises from DMSO. During the preparation of this manuscript, Zhong and co-workers22 reported that DMSO could serve as a formaldehyde surrogate. Using paraformaldehyde in place of DMSO under similar reaction conditions as ours also yielded the 4-chloropiperidine product (Scheme 2, eq 4). It is, however, inconclusive if that is the only operating mechanism because as earlier referenced, DMSO can equally serve as a one-carbon source. Given the abundance of chloride ion during the reaction, the in-situ generation of chloromethyl methyl sulfide (IIb) as an intermediate is possible. Indeed, the reaction of the starting amine with commercially available chloromethyl methyl sulfide led to the formation of 2a in 64% yield (Scheme 2, eq 3). Finally, to seek insight as to the intermediary of an iminium ion before cyclization, we performed the reaction with tertiary amine 7 and observed no cyclization to give 2a. Instead, the reaction gave the chlorothiolated product 823 was formed in 67% (Scheme 2, eq 4).
Scheme 2.
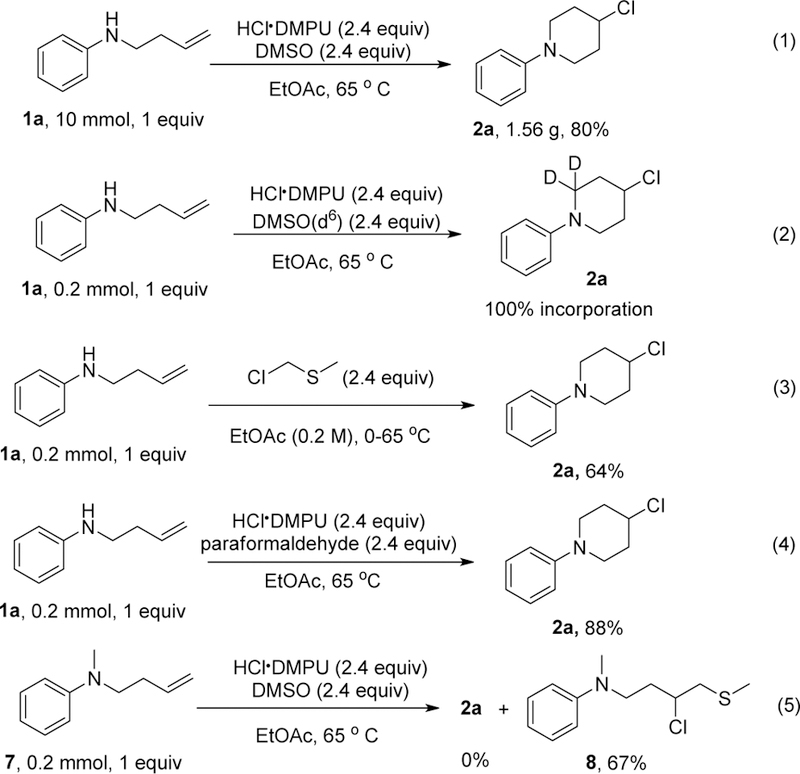
Gram scale reaction and mechanistic study.
Based on the above results, a plausible mechanism is proposed (Scheme 3). Electrophilic activation of DMSO by HCl generates sulfonium salt I, which undergoes base-assisted elimination of water to produce (thiomethyl)methyl carbenium ion IIa. Interchangeable formation of chloromethyl methyl sulfide (IIb) may also be operative. IIa/b reacts with the starting amine to ultimately generate iminium ion V from ammonium salt IV via proton transfer (P.T.) and elimination of methyl mercaptan. A 6-endo-trig cyclization24 followed by nucleophilic addition of chloride ion gives the desired product.
Scheme 3.
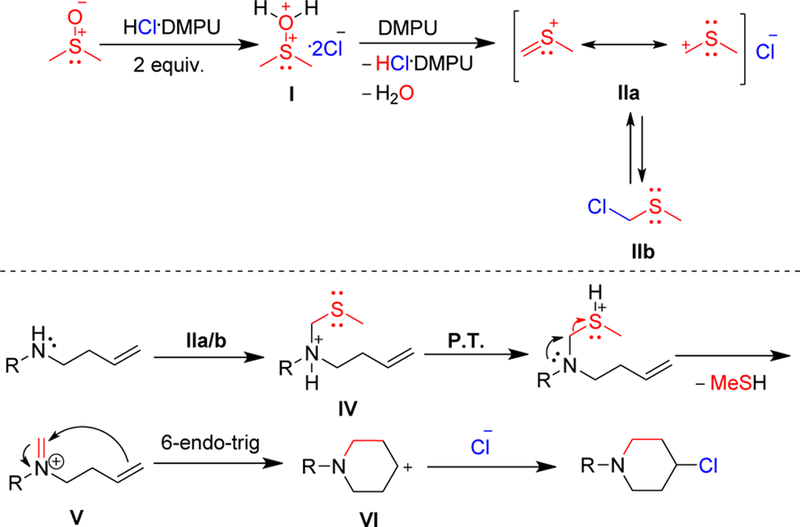
Proposed mechanism.
Conclusion
In summary, we have developed a convenient protic acid-catalyzed formation of (thiomethyl)methyl carbenium ion from DMSO under mild conditions. In the presence of homoallylic amines, the in situ-generated species reacts in aza-Pummerer fashion to generate an iminium ion intermediate that cyclizes to form 4-chloropiperidines in good yield. The method applies to both aromatic and aliphatic amines. The use of HCl•DMPU as protic acid, non-nucleophilic base and chloride source provides an environmentally benign process for piperidine formation. The reaction has a broad substrate scope and is scalable.
Experimental Section
1. General
1H and 13C (1H) decoupled NMR spectra were recorded either at 400 MHz or 500 MHz, and 101 MHz using CDCl3 or CD2Cl2 as a solvent. The chemical shifts are reported in δ (ppm) values (1H and 13C NMR relative to CHCl3, δ 7.26 ppm for 1H NMR and δ 77.0 ppm for 13C NMR, multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), p (pentet), h (hextet), m (multiplet) and br (broad). Coupling constants (J), are reported in Hertz (Hz). The HRMS data was obtained from an Agilent Technologies QTOF spectrometer. All reagents and solvents were employed without further purification. The products were purified using a commercial flash chromatography system. TLC was developed on silica gel 60 F254 aluminum sheets.
2. General procedures
2.1. Procedure for generation of HCl/DMPU
The reagent HCl•DMPU was prepared as reported in the literature.12a, b
2.2. General procedure for the preparation of homoallylic amines, 1 and 325.
To a round-bottomed flask equipped with a stirring bar was charged with aryl or alkylamine 1 or 3 (1.2 mmol, 1.2 equiv), K2CO3 (2 mmol, 2 equiv) and dry DMF (3 mL). Homoallyl bromide (1 mmol, 1 equiv) was slowly added to the mixture and heated to 110 °C. We monitored the progress of the reaction by GC-MS or TLC. Upon completion, the reaction mixture was cooled to room temperature and water (10 mL) and extracted with ethyl acetate (3 X 10 mL). The combined organic layers were then dried over anhydrous Na2SO4, filtered, concentrated and eventually purified silica gel column chromatography with hexanes/ethyl acetate (typically 70/30) or petroleum ether/ethyl acetate (80/20 for 1v, 1x, 1y, 1af, 1ag, and 3c) as eluent.
N-(but-3-en-1-yl)aniline (1a) Light yellow oil, 89.8 mg, 61% yield. 1H NMR (400 MHz, CDCl3) δ 7.19 (t, J = 7.9 Hz, 2H), 6.72 (t, J = 7.3 Hz, 1H), 6.63 (d, J = 7.8 Hz, 2H), 5.85– 5.79 (m, 1H), 5.21 – 5.07 (m, 2H), 3.63 (s, 1H), 3.20 (t, J = 6.7 Hz, 2H), 2.40 (q, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 148.4, 136.0, 129.4, 117.6, 117.3, 113.1, 43.0, 33.8.
N-(but-3-en-1-yl)-4-methylaniline (1b) Colorless oil, 103.2 mg, 64% yield. 1H NMR (400 MHz, CDCl3) δ 7.00 (d, J = 8.0 Hz, 2H), 6.56 (d, J = 8.0 Hz, 2H), 5.87– 5.78 (m, 1H), 5.17 – 5.10 (m, 2H), 3.52 (s, 1H), 3.17 (t, J = 6.7 Hz, 2H), 2.38 (q, J = 6.7 Hz, 2H), 2.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ = 146.1, 136.0, 129.8, 126.7, 117.1, 113.2, 43.3, 33.8, 20.5.
N-(but-3-en-1-yl)-3-methylaniline (1c) Colorless oil, 109.8 mg, 68% yield. 1H NMR (400 MHz, CDCl3) δ 7.07 (t, J = 7.9 Hz, 1H), 6.53 (d, J = 7.6 Hz, 1H), 6.43 (d, J = 7.7 Hz, 2H), 5.86 – 5.78 (m, 1H), 5.17 – 5.10 (m, 2H), 3.60 (s, 1H), 3.18 (t, J = 6.7 Hz, 2H), 2.42 – 2.32 (m, 2H), 2.28 (s, 3H). 13C NMR (100 MHz, CDCl3 ) δ 148.2, 138.9, 135.7, 129.0, 118.2, 116.9, 113.6, 109.9, 42.7, 33.6, 21.5.
N-(but-3-en-1-yl)-4-fluoroaniline (1d) Colorless oil, 94.1 mg, 57% yield. 1H NMR (400 MHz, CDCl3) δ 6.88 (t, J = 8.8 Hz, 2H), 6.54 (dd, J = 9.0, 4.4 Hz, 2H), 5.85 – 5.76 (m, 1H), 5.17 – 5.10 (m, 2H), 3.54 (s, 1H), 3.14 (t, J = 6.7 Hz, 2H), 2.37 (q, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 156.8, 144.6, 135.7, 117.2, 115.7, 115.6, 113.7, 43.5, 33.6. 19F NMR (376 MHz, CDCl3) δ −128.35.
N-(but-3-en-1-yl)-4-chloroaniline (1e) Colorless oil, 116.2 mg, 63% yield. 1H NMR (500 MHz, CDCl3) δ 7.11(d, J = 7.8, 2H), 6.53 (d, J = 7.9, 2H), 5.81 – 5.77 (m, 1H), 5.15 – 5.10 (m, 2H), 3.66 (s, 1H), 3.14 (t, J = 6.4 Hz, 2H), 2.37 (q, J = 6.7, 2H). 13C NMR (126 MHz, CDCl3) δ 146.8, 135.5, 129.0, 121.9, 117.3, 113.9, 42.9, 33.5.
4-bromo-N-(but-3-en-1-yl)aniline (1f) Colorless oil, 146.9 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 8.8 Hz, 2H), 6.60 (d, J = 8.8 Hz, 2H), 5.97 – 5.88 (m, 1H), 5.29 – 5.23 (m, 2H), 3.80 (s, 1H), 3.27 (t, J = 6.7 Hz, 2H), 2.50 (q, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 147.3, 135.6, 132.0, 117.4, 114.5, 108.9, 42.9, 33.5.
N-(but-3-en-1-yl)-3-iodoaniline (1g) Colorless oil, 128.2 mg, 47% yield. 1H NMR (500 MHz, CDCl3) δ 7.02 (d, J = 7.7 Hz, 1H), 6.99 – 6.92 (m, 1H), 6.88 (t, J = 8.0 Hz, 1H), 6.56 (dd, J = 8.2, 2.2 Hz, 1H), 5.82 (ddt, J = 17.0, 10.1, 6.8 Hz, 1H), 5.29 – 4.97 (m, 2H), 3.69 (s, 1H), 3.16 (dd, J = 11.9, 6.4 Hz, 2H), 2.39 (dt, J = 7.9, 6.1 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 149.4, 135.4, 130.6, 126.1, 121.3, 117.4, 112.2, 95.3, 42.5, 33.4.
3-bromo-N-(but-3-en-1-yl)aniline (1h) Colorless oil, 115.3 mg, 51% yield. 1H NMR (500 MHz, CDCl3) δ 7.02 (t, J = 8.0 Hz, 1H), 6.81 (d, J = 7.1 Hz, 1H), 6.75 (d, J = 1.7 Hz, 1H), 6.52 (dd, J = 8.2, 2.1 Hz, 1H), 5.75–5.63 (m, 1H), 5.15 (t, J = 13.2 Hz, 2H), 3.74 (s, 1H), 3.17 (dd, J = 12.2, 6.4 Hz, 2H), 2.39 (q, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 149.5, 135.4, 130.5, 123.3, 120.0, 117.4, 115.3, 111.6, 42.6, 33.4.
N-(but-3-en-1-yl)-2-iodo-4-methylaniline (1i) Colorless oil, 206.7 mg, 72 % yield. 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 1.7 Hz, 1H), 7.02 (dd, J = 8.2, 1.7 Hz, 1H), 6.48 (d, J = 8.2 Hz, 1H), 5.85 (ddt, J = 17.1, 10.2, 6.9 Hz, 1H), 5.23 – 5.10 (m, 2H), 4.05 (s, 1H), 3.20 (t, J = 6.7 Hz, 2H), 2.43 (q, J = 6.8 Hz, 2H), 2.21 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 144.9, 139.1, 135.3, 129.8, 127.8, 117.3, 110.5, 85.3, 43.3, 33.3, 19.6.
N-(but-3-en-1-yl)-4-(trifluoromethyl)aniline (1j) Colorless oil, 124.7 mg, 58% yield. 1H NMR (400 MHz, CDCl3) δ 7.45 - 7.30 (m, 2H), 6.68 (d, J = 8.3 Hz, 2H), 6.59 (d, J = 8.5 Hz, 2H), 5.81 (dd, J = 17.1, 10.2 Hz, 2H), 5.14 (dd, J = 12.7, 11.0 Hz, 3H), 3.97 (s, 3H), 3.21 (t, J = 6.7 Hz, 3H), 2.39 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 150.4, 135.2, 126.6, 126.5, 117.4, 112.0, 42.4, 33.3. 19F NMR (376 MHz, CDCl3) δ −61.02.
N-(but-3-en-1-yl)-4-methoxyaniline (1k) Colorless oil, 108.1 mg, 61% yield. 1H NMR (400 MHz, CDCl3) δ 6.80 (d, J = 8.9 Hz, 2H), 6.60 (d, J = 8.9 Hz, 2H), 5.87 – 5.80 (m, 1H), 5.19 – 5.07 (m, 2H), 3.75 (s, 3H), 3.32 (s, 1H), 3.15 (t, J = 6.7 Hz, 2H), 2.38 (q, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 152.2, 142.6, 136.0, 117.1, 115.0, 114.3, 55.8, 43.9, 33.8.
4-(but-3-en-1-ylamino)benzonitrile (1l) Colorless oil, 86 mg, 50% yield. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 8.8 Hz, 2H), 6.55 (d, J = 8.8 Hz, 2H), 5.80 (ddt, J = 17.1, 10.2, 6.8 Hz, 1H), 5.20 – 5.08 (m, 2H), 4.24 (s, 1H), 3.22 (t, J = 6.7 Hz, 2H), 2.39 (q, J = 6.7, 2H). 13C NMR (100 MHz, CDCl3) δ 151.2, 135.0, 133.7, 120.5, 117.7, 112.2, 98.6, 42.0, 33.2.
N-(but-3-en-1-yl)-4-(trifluoromethoxy)aniline (1m) Colorless oil, 111 mg, 48% yield. 1H NMR (400 MHz, CDCl3) δ 7.03 (d, J = 8.9 Hz, 2H), 6.60 (d, J = 9.0 Hz, 2H), 5.87 – 5.78 (m, 1H), 5.25 – 5.14 (m, 2H), 3.78 (s, 1H), 3.21 (t, J = 6.7 Hz, 2H), 2.43 (q, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 146.9, 140.3, 135.4, 124.5, 122.3, 121.9, 120.7 (q, J = 257 Hz), 119.4, 117.2, 116.8, 112.9, 42.8, 33.4. 19F NMR (376 MHz, CDCl3) δ −58.31.
N-(but-3-en-1-yl)-4-nitroaniline (1n) Yellow solid, 38.4 mg, 20% yield. 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 9.2 Hz, 2H), 6.53 (d, J = 9.2 Hz, 2H), 5.87– 5.77 (m, 1H), 5.26 –5.11 (m, 2H), 4.50 (s, 1H), 3.29 (dd, J = 12.1, 6.6 Hz, 2H), 2.43 (q, J = 6.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 153.3, 138.2, 134.9, 126.6, 118.2, 111.2, 42.3, 33.3.
N-(but-3-en-1-yl)-2-(trifluoromethyl)aniline (1o) Colorless oil, 131.3 mg, 61% yield. 1H NMR (400 MHz, CDCl3) δ 7.79 - 7.19 (m, 2H), 6.95 – 6.62 (m, 2H), 5.92–5.80 (m, 1H), 5.69 – 5.02 (m, 2H), 4.55 (s, 1H), 3.39 (t, J = 6.4 Hz, 2H), 2.59 (ddd, J = 6.7, 6.2, 1.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 145.8, 135.2, 133.2, 126.8, 126.7, 124.0, 117.7, 115.9, 111.9, 42.5, 33.4. 19F NMR (376 MHz, CDCl3) δ −62.44.
N-(but-3-en-1-yl)-3,5-bis(trifluoromethyl)aniline (1p) Colorless oil, 113.3 mg, 40% yield. 1H NMR (400 MHz, CDCl3) δ 7.04 (s, 1H), 6.83 (s, 2H), 5.72 (td, J = 16.8, 6.6 Hz, 1H), 5.16 - 5.03 (m, 2H), 4.01 (s, 1H), 3.14 (dd, J = 12.1, 6.3 Hz, 2H), 2.33 (q, J = 6.7 Hz, 2H), 1.45 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 148.3, 134.5, 124.5, 121.8, 117.5, 111.4, 109.7, 41.9, 32.8. 19F NMR (376 MHz, CDCl3) δ −63.54.
N-(but-3-en-1-yl)-3-(trifluoromethyl)aniline (1q) Colorless oil, 116.2 mg, 54% yield. 1H NMR (400 MHz, CDCl3) δ 7.30 (dd, J = 9.2, 6.6 Hz, 1H), 6.97 (d, J = 7.6 Hz, 1H), 6.84 (s, 1H), 6.78 (d, J = 8.2 Hz, 1H), 5.87 (ddt, J = 17.0, 10.1, 6.8 Hz, 1H), 5.44 – 4.88 (m, 2H), 3.91 (s, 1H), 3.26 (s, 2H), 2.57 – 2.29 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 148.4, 135.4, 131.7, 131.4, 129.6, 125.7, 123.0, 117.5, 115.8, 113.7, 113.7, 108.9, 108.9, 42.5, 33.4. 19F NMR (376 MHz, CDCl3) δ −62.90.
N-(but-3-en-1-yl)-[1,1’-biphenyl]-4-amine (1r) Colorless oil, 160.8 mg, 72% yield. 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 7.8 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.27 (t, J = 7.4 Hz, 2H), 7.20 – 7.10 (m, 1H), 6.56 (d, J = 8.4 Hz, 2H), 5.86 – 5.61 (m, 1H), 5.03 (t, J = 14.5 Hz, 2H), 3.63 (s, 1H), 3.11 (t, J = 6.7 Hz, 2H), 2.29 (q, J = 6.6 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 147.5, 141.1, 135.6, 130.1, 128.5, 127.8, 126.1, 125.9, 117.0, 113.0, 42.7, 33.5.
N-(but-3-en-1-yl)-[1,1’-biphenyl]-2-amine (1s) Colorless oil , 133.9 mg, 60% yield. 1H NMR (500 MHz, CDCl3) δ 7.48 – 7.31 (m, 5H), 7.28 – 7.21 (m, 1H), 7.10 (d, J = 7.4 Hz, 1H), 6.77 (t, J = 7.4 Hz, 1H), 6.73 – 6.68 (m, 1H), 5.94 – 5.50 (m, 1H), 5.00 (dd, J = 8.6, 6.5 Hz, 2H), 3.99 (s, 1H), 3.18 (dd, J = 11.8, 6.4 Hz, 2H), 2.32 (q, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 145.0, 139.4, 135.6, 130.1, 129.4, 128.8, 128.7, 127.7, 127.1, 117.0, 116.8, 110.4, 42.8, 33.5.
1-(4-(but-3-en-1-ylamino)phenyl)ethanone (1t) Colourless oil, 125.1 mg, 66% yield. 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 8.9 Hz, 2H), 6.52 (d, J = 8.8 Hz, 2H), 5.78 (ddt, J = 17.1, 10.2, 6.8 Hz, 1H), 5.20 – 5.02 (m, 2H), 4.19 (s, 1H), 3.22 (t, J = 6.7 Hz, 2H), 2.46 (s, 3H), 2.37 (dtd, J = 6.8, 5.5, 1.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 196.1, 151.8, 134.9, 130.6, 126.5, 117.4, 111.2, 41.9, 33.1, 25.8.
(3-(but-3-en-1-ylamino)phenyl)(phenyl)methanone (1u) Light yellow solid, 158.3 mg, 63% yield. 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 7.7 Hz, 2H), 7.57 (t, J = 7.4 Hz, 1H), 7.47 (t, J = 7.5 Hz, 2H), 7.29 – 7.24 (m, 1H), 7.06 (d, J = 6.5 Hz, 2H), 6.82 (d, J = 8.3 Hz, 1H), 5.77–5.66 (m, 1H), 5.14 (t, J = 13.2 Hz, 2H), 3.81 (s, 1H), 3.22 (t, J = 6.7 Hz, 2H), 2.46 - 2.31 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 197.2, 148.2, 138.6, 137.9, 135.5, 132.2, 130.0, 128.9, 128.1, 119.6, 117.4, 116.9, 113.5, 42.7, 33.5.
N-(but-3-en-1-yl)benzo[d][1,3]dioxol-5-amine (1v) Colorless oil, 86.0 mg, 45% yield. 1H NMR (500 MHz, CDCl3) δ 6.67 (d, J = 8.3 Hz, 1H), 6.26 (s, 1H), 6.07 (d, J = 8.3 Hz, 1H), 5.97 – 5.74 (m, 3H), 5.10–5.01 (m, 2H), 3.45 (s, 1H), 3.13 (td, J = 6.7, 1.4 Hz, 2H), 2.44 - 2.31 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 148.3, 144.1, 139.6, 135.7, 117.1, 108.6, 104.5, 100.5, 96.1, 43.8, 33.6.
methyl 3-(but-3-en-1-ylamino)thiophene-2-carboxylate (1w) colorless liquid, 109.8 mg, 52% yield. 1H NMR (500 MHz, CDCl3) δ 7.33 (d, J = 5.5 Hz, 1H), 6.76 (s, 1H), 6.63 (d, J = 5.5 Hz, 1H), 5.86 –5.79 (m, 1H), 5.18 – 5.11 (m, 2H), 3.81 (s, 3H), 3.34 (d, J = 2.7 Hz, 2H), 2.50 – 2.28 (m, 2H).. 13C NMR (100 MHz, CDCl3) δ 165.4, 156.1, 135.1, 132.2, 117.4, 116.2, 98.5, 51.1, 44.4, 34.3.
N-(but-3-en-1-yl)-6-chloropyridin-3-amine (1x) Colorless oil, 98.6 mg, 44% yield. 1H NMR (500 MHz, CDCl3) δ 7.77 (s, 1H), 7.09 (d, J = 8.6 Hz, 1H), 6.87 (d, J = 8.6, 1H), 5.76–5.64 (m, 1H), 5.16 (dd, J = 13.3, 6.3 Hz, 2H), 3.74 (s, 1H), 3.18 (dd, J = 12.5, 6.4 Hz, 2H), 2.40 (q, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 143.2, 139.0, 135.0, 134.5, 124.0, 122.2, 117.7, 42.5, 33.3.
N-(but-3-en-1-yl)-4-chloropyridin-2-amine (1y) Colorless oil, 122.2 mg, 67% yield. 1H NMR (500 MHz, CDCl3) δ 8.03 (s, 1H), 7.36 (d, J = 8.8, 1H), 6.33 (d, J = 8.9 Hz, 1H), 5.93 – 5.69 (m, 1H), 5.34 – 4.99 (m, 2H), 4.53 (s, 1H), 3.41 – 3.27 (m, 2H), 2.38 (dt, J = 6.6, 5.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 143.4, 138.8, 135.1, 134.5, 124.1, 122.3, 117.7, 42.6, 33.4.
N-(but-3-en-1-yl)-5-chloropyrazin-2-amine (1z) Colorless oil, 117.4 mg, 64% yield. 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 1.2 Hz, 1H), 7.64 (d, J = 1.2 Hz, 1H), 5.76–5.65 (m, 1H), 5.32 – 4.98 (m, 2H), 4.68 (s, 1H), 3.40 (dd, J = 12.4, 6.6 Hz, 2H), 2.40 (q, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 153.3, 141.2, 136.0, 135.0, 129.9, 117.7, 40.7, 33.4.
N-(but-3-en-1-yl)-4-methylbenzenesulfonamide (1aa) white solid, 168.8 mg, 75% yield. 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H), 5.62 (ddt, J = 17.1, 10.3, 6.8 Hz, 1H), 5.04 (t, J = 12.6 Hz, 2H), 4.61 (bs, 1H), 3.00 (q, J = 6.5 Hz, 2H), 2.42 (s, 3H), 2.19 (q, J = 6.8 Hz, 2H).13C NMR (126 MHz, CDCl3) δ 143.2, 136.7, 134.0, 129.5, 126.9, 117.9, 77.1, 76.8, 76.5, 41.9, 33.4, 21.3.
N-(but-3-en-1-yl)-4-methoxybenzenesulfonamide (1ab) white solid, 173.6 mg, 72% yield. 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.9 Hz, 2H), 6.91 (d, J = 8.9 Hz, 2H), 5.72 - 5.39 (m, 1H), 5.13 – 4.78 (m, 2H), 4.65 (d, J = 6.0 Hz, 1H), 3.80 (d, J = 0.6 Hz, 3H), 3.07 – 2.71 (m, 2H), 2.13 (q, J = 6.8 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 162.8, 134.1, 131.4, 129.1, 117.9, 114.2, 55.6, 42.0, 33.5.
4-bromo-N-(but-3-en-1-yl)benzenesulfonamide (1ac) white solid , 220.4 mg, 76% yield. 1H NMR (500 MHz, CDCl3) δ 7.77 – 7.69 (m, 2H), 7.68 – 7.62 (m, 2H), 5.58–5.46 (m, 1H), 5.16 - 4.95 (m, 2H), 4.90 (s, 1H), 3.03 (dd, J = 12.9, 6.7 Hz, 2H), 2.22 (qt, J = 6.8, 1.3 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 139.0, 133.9, 132.3, 128.6, 127.5, 118.2, 42.1, 33.6.
N-(but-3-en-1-yl)-2-ethylhexan-1-amine (1ad) light yellow oil, 69.6 mg, 38% yield. 1H NMR (400 MHz, CDCl3) δ 5.78 (m, J = 13.7, 10.2, 5.1 Hz, 1H), 5.18 - 4.93 (m, 2H), 2.66 (t, J = 6.9 Hz, 2H), 2.49 (d, J = 6.2 Hz, 2H), 2.32 - 2.13 (m, 2H), 1.52 – 1.36 (m, 1H), 1.35 - 1.20 (m, 7H), 0.88 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 136.6, 116.1, 53.1, 49.2, 39.4, 34.3, 31.4, 29.0, 24.5, 23.1, 14.1, 10.8.
N-phenethylbut-3-en-1-amine (1ae) light yellow oil, 105.2 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.56 – 7.33 (m, 5H), 5.99 – 5.90 (m, 1H), 5.30 – 5.15 (m, 2H), 3.13 – 3.05 (m, 2H), 3.03 – 2.97 (m, 2H), 2.90 (t, J = 6.9 Hz, 2H), 2.44 (q, J = 6.9 Hz, 2H), 1.31 (s, 1H).. 13C NMR (100 MHz, CDCl3) δ 140.2, 136.5, 128.8, 128.5, 126.2, 116.4, 51.2, 48.9, 36.5, 34.4.
N-(4-methoxyphenethyl)but-3-en-1-amine (1af) light yellow oil, 127.2 mg, 62% yield. 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 8.5 Hz, 2H), 6.92 (d, J = 8.6 Hz, 2H), 5.79 – 5.67 (m, 1H), 5.25 – 4.97 (m, 2H), 3.87 (s, 2H), 2.98 – 2.90 (m, 2H), 2.86 – 2.74 (m, 2H), 2.32 (q, J = 6.9 Hz, 2H), 1.83 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 158.1, 136.4, 132.1, 129.7, 116.4, 113.9, 55.3, 51.3, 48.8, 35.5, 34.3.
N-benzylbut-3-en-1-amine (1ag) light yellow oil, 107.9 mg, 67% yield. 1H NMR (400 MHz, CDCl3) δ = 7.39 – 7.19 (m, 5H), 5.84 – 5.74 (m, 1H), 5.16 – 4.98 (m, 2H), 3.79 (s, 2H), 2.70 (t, J=6.8, 2H), 2.28 (dt, J=6.9, 6.2, 2H), 1.29 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 140.4, 136.5, 128.4, 128.1, 126.9, 116.3, 53.9, 48.3, 34.3.
(R)-N-(1-phenylethyl)but-3-en-1-amine (1ah) light yellow oil, 129.6 mg, 74% yield. 1H NMR (400 MHz, CDCl3) δ 8.15 – 6.33 (m, 5H), 5.69 – 5.58 (m, 1H), 5.31 – 4.69 (m, 2H), 3.76 (q, J = 6.6 Hz, 1H), 2.72 – 2.36 (m, 2H), 2.29 – 2.07 (m, 2H), 1.34 (d, J = 6.6 Hz, 3H), 1.27 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 145.7, 136.5, 128.4, 126.8, 126.5, 116.2, 58.2, 46.5, 34.3, 24.3.
N-(3-methylbut-3-en-1-yl)aniline (3a) light yellow oil, 106.4 mg, 66% yield. 1H NMR (400 MHz, CDCl3) δ 7.19 (t, J = 7.6 Hz, 2H), 6.71 (t, J = 7.2 Hz, 1H), 6.62 (d, J = 8.2 Hz, 2H), 4.87 (s, 1H), 4.81 (s, 1H), 3.65 (s, 1H), 3.23 (t, J = 6.7 Hz, 2H), 2.36 (t, J = 6.6 Hz, 2H), 1.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 148.3, 142.9, 129.2, 117.3, 112.8, 112.3, 41.3, 37.4, 21.9.
N-(3-bromobut-3-en-1-yl)aniline (3b) light yellow oil, 144.6 mg, 64% yield. 1H NMR (500 MHz, CDCl3) δ 7.26 – 7.17 (m, 2H), 6.75 (td, J = 7.4, 1.0 Hz, 1H), 6.71 - 6.58 (m, 2H), 5.75 – 5.63 (m, 1H), 5.55 (d, J = 1.6 Hz, 1H), 3.77 (s, 1H), 3.41 (t, J = 6.5 Hz, 2H), 2.74 (t, J = 6.5 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 147.6, 131.3, 129.3, 118.9, 117.7, 113.0, 41.6, 40.9.
N-(2-(6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)aniline (3c) colorless liquid, 139.9 mg, 58% yield. 1H NMR (400 MHz, CDCl3) δ 7.25 – 7.11 (m, 2H), 6.72 (tt, J = 7.4, 1.0 Hz, 1H), 6.65 – 6.55 (m, 2H), 5.37 (dt, J = 4.2, 1.3 Hz, 1H), 3.66 (s, 1H), 3.27 – 2.93 (m, 2H), 2.41 (dt, J = 8.6, 5.6 Hz, 1H), 2.36 – 2.24 (m, 4H), 2.17 – 1.99 (m, 2H), 1.41 – 1.22 (m, 5H), 0.93 – 0.81 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 148.4, 145.6, 129.2, 118.7, 117.2, 112.9, 45.4, 41.2, 40.8, 38.0, 36.5, 31.8, 31.4, 26.3, 22.7, 21.2, 14.2.
(E)-N-(pent-3-en-1-yl)aniline (3d) light yellow liquid, 111.2 mg, 69% yield. 1H NMR (400 MHz, CDCl3) δ 7.18 (t, J = 7.9 Hz, 2H), 6.70 (t, J = 7.3 Hz, 1H), 6.61 (d, J = 8.1 Hz, 2H), 5.54–5.44 (m, 1H), 5.50 – 5.37 (m, 1H), 3.65 (s, 1H), 3.13 (t, J = 6.7 Hz, 2H), 2.31 (q, J = 6.7 Hz, 2H), 1.70 (d, J = 6.0 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 148.4, 129.2, 128.2, 127.7, 117.2, 112.8, 43.3, 32.4, 18.0.
N-(4-methylpent-3-en-1-yl)aniline (3e) light yellow oil, 114 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 7.30 (t, J = 7.7 Hz, 2H), 6.82 (t, J = 7.3 Hz, 1H), 6.74 (d, J = 8.0 Hz, 2H), 5.28 (t, J = 7.2 Hz, 1H), 3.76 (s, 1H), 3.24 (t, J = 6.9 Hz, 2H), 2.44 (d, J = 7.0 Hz, 2H), 1.86 (s, 3H), 1.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 148.5, 134.4, 129.3, 121.4, 117.3, 113.0, 43.9, 28.2, 25.9, 18.0.
2.3. Procedure for the synthesis of 4-chloropiperidines, 2 and 4.
A glass vial equipped with a screw cap and a stirring bar was charged with alkene 1 (0.5 mmol). Then ethyl acetate was added (2.5 mL) followed by DMSO (2.4 mmol) and HCl/DMPU (102 μL, 2.4 mmol). We stirred the reaction mixture at 65 °C and monitored the progress of the reaction by GC-MS or TLC. Upon completion, the reaction mixture was quenched with water and extracted with DCM. The combined organic layers were then dried over anhydrous Na2SO4, filtered, and the solvent evaporated. We purified the crude product by silica gel column chromatography (hexanes/ethyl acetate typically 97/3).
4-chloro-1-phenylpiperidine (2a) Colorless oil, 92.6 mg, 95% yield. 1H NMR (400 MHz, CDCl3) δ 7.23 (t, J = 8.0 Hz, 2H), 6.92 (d, J = 8.0 Hz, 2H), 6.82 (t, J = 7.3 Hz, 1H), 4.25 – 4.14 (m, 1H), 3.54 – 3.45 (m, 2H), 3.07 – 2.97 (m, 2H), 2.25 – 2.15 (m, 2H), 2.03 – 1.92 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 151.1, 129.2, 119.8, 116.6, 57.2, 47.5, 35.1. HRMS (EI+) calcd. for [C11H14NCl] (MH+) 196.1044; found 196.1041.
4-chloro-1-(p-tolyl)piperidine (2b) Colorless oil, 89.1 mg, 85% yield. 1H NMR (400 MHz, CDCl3) δ = 7.05 (d, J=7.7, 2H), 6.83 (d, J=7.5, 2H), 4.16 (bs, 1H), 3.42 – 3.44 (m, 2H), 2.99 – 2.95 (m, 2H), 2.29 – 2.12 (m, 5H), 2.00 – 1.98 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 149.0, 129.6, 129.3, 116.9, 57.1, 48.1, 35.2, 20.4. HRMS (EI+) calcd. for [C H NCl] (MH+) 210.1044; found 210.1042.
4-chloro-1-(m-tolyl)piperidine (2c) Colorless oil, 97.5 mg, 93% yield. 1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 7.7 Hz, 1H), 6.76 (d, J = 8.4 Hz, 2H), 6.70 (d, J = 7.4 Hz, 1H), 4.23 – 4.17 (m, 1H), 3.55 – 3.47 (m, 2H), 3.09 – 2.99 (m, 2H), 2.32 (s, 3H), 2.26 – 2.17 (m, 2H), 2.06 – 1.95 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 151.1, 138.9, 129.0, 120.8, 117.5, 113.7, 57.2, 47.7, 35.1, 21.8. HRMS (EI+) calcd. for [C12H17NCl] (MH+) 210.1044; found 210.1042.
4-chloro-1-(4-fluorophenyl)piperidine (2d) Colorless oil, 89.5 mg, 84% yield. 1H NMR (400 MHz, CDCl3) δ 7.01 – 6.84 (m, 4H), 4.19 (tt, J = 8.0, 3.9 Hz, 1H), 3.45 – 3.31 (m, 2H), 3.02 – 2.93 (m, 2H), 2.22 (dtd, J = 10.4, 7.0, 3.6 Hz, 2H), 2.09 – 1.95 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 158.3, 155.9, 147.7, 118.4, 115.5, 115.3, 56.7, 48.3, 35.0. 19F NMR (376 MHz, CDCl3) δ −124.31 (s, 1H). HRMS (EI+) calcd. for [C11H13ClFN] (M+) 213.0719; found 213.0714.
4-chloro-1-(4-chlorophenyl)piperidine (2e) Colorless oil, 104.7 mg, 91 % yield. 1H NMR (400 MHz, CDCl3) δ 7.20 (d, J = 9.0 Hz, 2H), 6.85 (d, J = 8.7 Hz, 2H), 4.21 (tt, J = 7.6, 3.7 Hz, 1H), 3.52 – 3.35 (m, 2H), 3.13 – 2.97 (m, 2H), 2.27 – 2.11 (m, 2H), 2.06 – 1.92 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 149.6, 129.0, 124.6, 117.7, 56.8, 47.4, 34.8. HRMS (EI+) calcd. for [C11H14NCl2] (MH+) 230.0498; found 230.0496.
1-(4-bromophenyl)-4-chloropiperidine (2f) Colorless oil, 109.9 mg, 80% yield. 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 9.0 Hz, 2H), 6.80 (d, J = 9.0 Hz, 2H), 4.21 (tt, J = 7.8, 3.9 Hz, 1H), 3.51 – 3.39 (m, 2H), 3.12 – 2.94 (m, 2H), 2.20 (dtd, J = 10.5, 7.0, 3.6 Hz, 2H), 2.06 – 1.94 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 150.0, 131.9, 129.1, 118.1, 56.8, 47.2, 34.7. HRMS (EI+) calcd. for [C11H14NBrCl] (MH+) 273.9993; found 273.9989.
4-chloro-1-(3-iodophenyl)piperidine (2g) Colorless oil, 112.3 mg, 70% yield. 1H NMR (400 MHz, CDCl3) δ 7.25 – 7.23 (m, 1H), 7.18 – 7.13 (m, 1H), 6.98 – 6.93 (m, 1H), 6.87 (ddd, J = 8.4, 2.4, 0.8 Hz, 1H), 4.21 (tt, J = 7.8, 3.8 Hz, 1H), 3.53 – 3.44 (m, 2H), 3.12 – 3.01 (m, 2H), 2.18 (ddd, J = 13.1, 6.8, 3.3 Hz, 2H), 2.03 – 1.92 (m, 2H). 13C NMR (100 MHz, CDCl3 δ 152.1, 130.5, 128.4, 125.2, 115.6, 95.2, 56.8, 46.9, 34.7. HRMS (EI+) calcd. for [C11H13ClIN] (M+) 320.9781; found 320.9775.
1-(3-bromophenyl)-4-chloropiperidine (2h) Colorless oil, 137 mg, 86% yield. 1H NMR (400 MHz, CDCl3) δ 7.11 (t, J=8.1, 1H), 7.05 (t, J=2.1, 1H), 6.96 (ddd, J=7.8, 1.8, 0.8, 1H), 6.87 – 6.81 (m, 1H), 4.22 (tt, J=7.8, 3.8, 1H), 3.57 – 3.45 (m, 2H), 3.15 – 3.04 (m, 2H), 2.25 – 2.15 (m, 2H), 2.05 – 1.93 (m,2H). 13C NMR (100 MHz CDCl3) δ 152.2, 130.4, 123.2, 122.2, 119.1, 114.8, 56.8, 46.8, 34.6. HRMS (EI+) calcd. For [C8H14NBrClF2] (MH+) 275.9970; found 275.9969.
4-chloro-1-(2-iodo-4-methylphenyl)piperidine (2i) Colorless oil, 106.5 mg, 81% yield. 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.28 – 4.28 (m, 1H), 3.64 – 3.53 (m, 2H), 3.25 – 3.15 (m, 2H), 2.23 – 2.17 (m, 2H), 2.04 – 1.91 (m, 2H). 13C NMR (100 MHz, CDCl3) δ = 152.7, 129.0, 126.4, 125.9, 125.6, 123.2, 120.5, 114.8, 58.6, 45.9, 34.3. 19F NMR (376 MHz, CDCl3) δ −61.35. HRMS (EI+) calcd. for [C H ClF N] (M+) 263.0691; found 263.0686.
4-chloro-1-(4-(trifluoromethyl)phenyl)piperidine (2j) Colorless oil, 106.5 mg, 81% yield. 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.28 – 4.28 (m, 1H), 3.64 – 3.53 (m, 2H), 3.25 – 3.15 (m, 2H), 2.23 – 2.17 (m, 2H), 2.04 – 1.91 (m, 2H). 13C NMR (100 MHz, CDCl3) δ = 152.7, 129.0, 126.4, 125.9, 125.6, 123.2, 120.5, 114.8, 58.6, 45.9, 34.3. 19F NMR (376 MHz, CDCl3) δ −61.35. HRMS (EI+) calcd. for [C12H13ClF3N] (M+) 263.0691; found 263.0686.
4-chloro-1-(4-methoxyphenyl)piperidine (2k) Colorless oil, 101.5 mg, 90% yield. 1H NMR (400 MHz, CDCl3) δ = 6.93 (d, J=9.0, 2H), 6.85 (d, J=8.9, 2H), 4.24 – 4.12 (m, 1H), 3.79 (s, 3H), 3.46 – 3.34 (m, 2H), 3.00 – 2.90 (m, 2H), 2.22 (s, 2H), 2.12 – 2.00 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 153.9, 145.6, 118.8, 114.4, 57.1, 55.5, 49.1, 35.4. HRMS (EI+) calcd. for [C12H17ONCl] (MH+) 226.0993; found 226.0990.
4-(4-chloropiperidin-1-yl)benzonitrile (2l) Colorless oil, 79.4 mg, 72% yield. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 9.0 Hz, 2H), 6.87 (d, J = 9.0 Hz, 2H), 4.31 – 4.26 (m, 1H), 3.69 – 3.57 (m, 2H), 3.32 – 3.26 (m, 2H), 2.21 – 2.14 (m, 2H), 2.00 – 1.92 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 152.7, 133.4, 119.8, 114.3, 100.0, 56.3, 44.9, 34.1. HRMS (EI+) calcd. for [C12H14N2Cl] (MH+) 221.0840; found 221.0837.
4-chloro-1-(4-(trifluoromethoxy)phenyl)piperidine (2m) Colorless oil, 126 mg, 90 % yield. 1H NMR (400 MHz, CDCl3) δ = 7.11 (d, J=8.7, 2H), 6.90 (d, J=8.9, 2H), 4.22 (dt, J=11.7, 3.8, 1H), 3.53 – 3.44 (m, 2H), 3.13 – 3.03 (m, 2H), 2.21 (dd, J=14.6, 11.6, 2H), 2.06 – 1.96 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 149.7, 141.9, 124.2, 121.8, 121.6, 119.2, 117.1, 116.8, 114.4, 56.6, 47.3, 34.7. 19F NMR (376 MHz, CDCl3) δ = −58.32. HRMS (EI+) calcd. for [C12H14ONClF3] (MH+) 280.0711; found 280.0708.
4-chloro-1-(4-nitrophenyl)piperidine (2n) Yellow solid, 72.0 mg, 60% yield. 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 9.4 Hz, 2H), 6.82 (d, J = 9.4 Hz, 2H), 4.32 (dq, J = 10.8, 3.7 Hz, 1H), 3.78 – 3.65 (m, 2H), 3.40 (ddd, J = 11.5, 7.3, 3.6 Hz, 2H), 2.26 – 2.11 (m, 2H), 2.01 – 1.93 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 154.4, 138.4, 126.1, 112.9, 56.3, 44.9, 34.3. HRMS (EI+) calcd. for [C11H13ClN2 O2] (M+) 240.0669; found 240.0664.
4-chloro-1-(2-(trifluoromethyl)phenyl)piperidine (2o) Colorless oil, 105.4 mg, 80% yield. 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 7.9 Hz, 1H), 7.51 (t, J = 7.7 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 4.23 (bs, 1H), 3.21 – 3.02 (m, 2H), 2.81 (ddd, J = 11.2, 7.7, 3.2 Hz, 2H), 2.21 (ddd, J = 13.5, 6.9, 3.4 Hz, 2H), 2.09 – 1.97 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 152.8, 132.9, 128.2, 127.3, 125.5, 125.0, 124.2, 122.8, 120.1, 57.4, 51.4, 35.9. 19F NMR (376 MHz, CDCl3) δ −60.5, −62.21 (side product). HRMS (EI+) calcd. for [C12H14NClF3] (MH+) 264.0761; found 264.0758.
1-(3,5-bis(trifluoromethyl)phenyl)-4-chloropiperidine (2p) Colorless oil, 149.2 mg, 90% yield. 1H NMR (400 MHz, CDCl3) δ 7.32 –7.21 (m, 3H), 4.28 (tt, J=7.4, 3.7, 1H), 3.64 – 3.55 (m, 2H), 3.29 – 3.19 (m, 2H), 2.22 (ddt, J=14.3, 7.4, 3.6, 2H), 2.06 – 1.96 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 151.3, 132.8, 132.5, 132.2, 131.8, 127.5, 124.8, 122.1, 119.4, 115.0, 112.0, 56.0, 46.0, 34.3. 19F NMR (376 MHz, CDCl3) δ = - 63.10. HRMS (EI+) calcd. for [C13H13NClF6] (MH+) 332.0635; found 332.0632.
4-chloro-1-(3-(trifluoromethyl)phenyl)piperidine (2q) Colorless oil, 95 mg, 72% yield. 1H NMR (400 MHz, CDCl3) δ 7.35 (t, J = 8.0 Hz, 1H), 7.12 (s, 1H), 7.08 (d, J = 8.2 Hz, 2H), 4.24 (tt, J = 7.8, 3.8 Hz, 1H), 3.60 – 3.48 (m, 2H), 3.20 – 3.08 (m, 2H), 2.29 – 2.15 (m, 2H), 2.07 – 1.94 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 151.1, 131.9, 131.6, 131.3, 131.0, 130.0, 125.6, 122.9, 119.2, 115.8, 112.6, 56.6, 46.8, 34.7. 19F NMR (376 MHz, CDCl3) δ −62.83. HRMS (EI+) calcd. for [C12H14NClF3] (MH+) 264.0761; found 264.0760.
1-([1,1’-biphenyl]-4-yl)-4-chloropiperidine (2r) White solid, 111.4 mg, 82% yield. 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.6 Hz, 2H), 7.43 (d, J = 8.6 Hz, 2H), 7.32 (t, J = 7.6 Hz, 2H), 7.19 (dd, J = 14.7, 7.2 Hz, 1H), 6.91 (d, J = 8.6 Hz, 2H), 4.16 – 4.10 (m, 1H), 3.51 – 3.43 (m, 2H), 3.08 – 2.97 (m, 2H), 2.19 – 2.08 (m, 2H), 1.99 – 1.87 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 150.1, 140.7, 132.2, 128.6, 127.7, 126.4, 126.4, 116.5, 57.0, 47.3, 34.8. HRMS (EI+) calcd. for [C17H19NCl] (MH+) 272.1201; found 272.1200.
1-([1,1’-biphenyl]-2-yl)-4-chloropiperidine (2s) Colorless oil, 114.1 mg, 84% yield. 1H NMR (400 MHz, CDCl3) δ 7.65 – 7.60 (m, 2H), 7.42 (dd, J=10.5, 4.7, 2H), 7.34 – 7.24 (m, 3H), 7.08 (ddd, J=11.9, 9.2, 4.6, 2H), 4.13 – 3.97 (m, 1H), 3.18 – 3.07 (m, 2H), 2.70 (ddd, J=11.8, 8.8, 2.9, 2H), 2.01 – 1.93 (m, 2H), 1.86 – 1.71 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 150.2, 141.0, 135.1, 131.3, 128.6, 128.1, 126.7, 122.7, 118.5, 57.3, 49.4, 35.6. HRMS (EI+) calcd. for [C17H19NCl] (MH+) 272.1201; found 272.1199.
1-(4-(4-chloropiperidin-1-yl)phenyl)ethanone (2t) Colorless oil, 97.4 mg, 82% yield. 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J=9.0, 2H), 6.87 (d, J=9.0, 2H), 4.28 (tt, J=7.6, 3.8, 1H), 3.68 (ddd, J=12.8, 7.3, 3.6, 2H), 3.29 (ddd, J=13.2, 7.8, 3.5, 2H), 2.52 (s, 3H), 2.05-1.95 (m, 2H), 1.96 (dtd, J=11.3, 7.7, 3.6, 2H). 13C NMR (100 MHz, CDCl3) δ 196.4, 153.6, 130.4, 127.5, 113.6, 56.7, 45.3, 34.4, 26.1. HRMS (EI+) calcd. For [C13H17ONCl] (MH+) 238.0993; found 238.0991.
(3-(4-chloropiperidin-1-yl)phenyl)(phenyl)methanone (2u) Colorless liquid, 111 mg, 74 % yield. 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.5 Hz, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.48 (t, J = 7.6 Hz, 2H), 7.39 (s, 1H), 7.34 (t, J = 7.9 Hz, 1H), 7.18 (dd, J = 14.1, 7.7 Hz, 2H), 4.23 (tt, J = 7.8, 3.8 Hz, 1H), 3.61 – 3.51 (m, 2H), 3.19 – 3.09 (m, 2H), 2.21 (bs, 2H), 2.07 – 1.96 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 196.9, 150.8, 138.4, 137.6, 132.3, 130.0, 128.8, 128.1, 121.7, 120.4, 117.1, 56.7, 47.0, 34.7. HRMS (EI+) calcd. for [C18H19ONCl] (MH+) 300.1150; found 300.1150.
1-(benzo[d][1,3]dioxol-5-yl)-4-chloropiperidine (2v) Yellow liquid, 81.3 mg, 68 % yield. 1H NMR (400 MHz, CDCl3) δ 6.71 (d, J = 8.4 Hz, 1H), 6.56 (d, J = 2.3 Hz, 1H), 6.37 (dd, J = 8.4, 2.4 Hz, 1H), 5.90 (s, 2H), 4.17 (td, J = 8.0, 4.0 Hz, 1H), 3.40 – 3.27 (m, 2H), 2.92 (ddd, J = 12.0, 8.4, 3.4 Hz, 2H), 2.20 (dd, J = 16.4, 3.5 Hz, 2H), 2.08 – 1.94 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 110.0, 109.7, 108.1, 100.9, 100.5, 84.4, 57.0, 49.2, 35.3. HRMS (EI+) calcd. for [C12H14O2NCl] (MH+) 239.0713; found 239.0707.
methyl 3-(4-chloropiperidin-1-yl)thiophene-2-carboxylate (2w) Pale yellow oil, 80.3 mg, 62 % yield. 1H NMR (500 MHz, CDCl3) δ 7.38 (d, J = 5.4 Hz, 1H), 6.84 (d, J = 5.4 Hz, 1H), 4.37 – 4.19 (m, 1H), 3.84 (s, 3H), 3.57 – 3.45 (m, 2H), 3.23 – 3.11 (m, 2H), 2.28 (ddd, J = 13.9, 7.4, 3.9 Hz, 2H), 2.12 – 1.98 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 162.1, 157.6, 156.7, 131.1, 121.0, 56.9, 51.6, 49.8, 35.2. HRMS (EI+) calcd. for [C11H14O2NClS] (M+) 259.0434; found 259.0430.
2-chloro-5-(4-chloropiperidin-1-yl)pyridine (2x) Yellow liquid, 91.3 mg, 79 % yield. 1H NMR (400 MHz, CDCl3) δ 8.15 – 8.03 (m, 1H), 7.41 (dd, J = 9.0, 2.7 Hz, 1H), 6.61 (d, J = 9.0 Hz, 1H), 4.27 (tt, J = 7.8, 3.8 Hz, 1H), 3.87 (ddd, J = 13.0, 7.1, 3.7 Hz, 2H), 3.41 (ddd, J = 13.3, 8.1, 3.5 Hz, 2H), 2.20 – 2.08 (m, 2H), 1.96 – 1.82 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 157.3, 146.3, 137.2, 120.0, 107.8, 57.2, 43.2, 34.4. HRMS (EI+) calcd. for [C10H13N2Cl2] (MH+) 231.0450; found 231.0447.
4-chloro-2-(4-chloropiperidin-1-yl)pyridine (2y) Colorless oil, 87.4 mg, 76% yield. 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J=1.5, 1H), 7.31 (s, 1H), 7.25 – 7.19 (m, 1H), 4.29 (tt, J=7.5, 3.8, 1H), 3.58 – 3.47 (m, 2H), 3.23 – 3.12 (m, 2H), 2.26 (ddt, J=14.2, 7.3, 3.6, 2H), 2.11 – 2.00 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 146.0, 141.3, 137.9, 126.2, 124.0, 56.2, 46.5, 34.4. HRMS (EI+) calcd. for [C10 H12Cl2N2] (M+) 230.0382; found 230.0378.
2-chloro-5-(4-chloropiperidin-1-yl)pyrazine (2z) Brown oil, 91.7 mg, 79% yield. 1H NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 7.88 (s, 1H), 4.34 – 4.28 (m, 1H), 3.89 – 3.81 (m, 2H), 3.55 – 3.47 (m, 2H), 2.18 – 2.11 (m, 2H), 1.97 – 1.89 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 153.4, 141.0, 136.0, 129.2, 56.6, 42.4, 34.2. HRMS (EI+) calcd. for [C9H12N3Cl2] (MH+) 232.0403; found 232.0400.
4-chloro-1-tosylpiperidine (2aa) White solid. 89.1 mg, 85% yield. 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 4.16 – 4.09 (m, 1H), 3.21 – 3.07 (m, 4H), 2.44 (s, 3H), 2.16 – 2.09 (m, 2H), 1.98 – 1.89 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 143.5, 132.8, 129.6, 127.4, 55.2, 42.7, 33.8, 21.4. HRMS (EI+) calcd. for [C12H17O2NClS] (MH+) 274.0663; found 274.0660.
4-chloro-1-((4-methoxyphenyl)sulfonyl)piperidine (2ab) White solid, 113 mg, 78 % yield. 1H NMR (400 MHz, (CDCl3) δ 7.60 (d, J = 9.0 Hz, 2H), 6.91 (d, J = 8.9 Hz, 2H), 4.07 – 3.97 (m, 1H), 3.78 (s, 3H), 3.11 – 3.05 (m, 2H), 3.03 – 2.95 (m, 2H), 2.10 – 1.97 (m, 2H), 1.88 – 1.80 (m, 2H). 13C NMR (100 MHz, (CDCl3) δ 162.9, 129.5, 127.6, 114.1, 55.5, 55.2, 42.7, 33.9. HRMS (EI+) calcd. for [C12H17O3NClS] (MH+) 290.0612; found 290.0610.
1-((4-bromophenyl)sulfonyl)-4-chloropiperidine (2ac) White solid, 120.2mg, 71 % yield. 1H NMR (400 MHz, CDCl3) 7.68 (d, J = 8.6, 2H), 7.62 (d, J = 8.6, 2H), 4.17 – 4.13 (m, 1H), 3.15 (t, J = 5.4 Hz, 4H), 2.17 – 2.10 (m, 2H), 1.97 – 1.91 (m, 2H).13C NMR (100 MHz, CDCl3) δ 135.4, 132.6, 129.2, 128.2, 55.2, 42.9, 34.1. HRMS (EI+) calcd. for [C11H13O2NBrClNaS] (M+Na) 359.9431; found 359.9429.
4-chloro-1-(2-ethylhexyl)piperidine (2ad) Colorless oil, 64.4 mg, 54% yield. 1H NMR (500 MHz, CDCl3) δ 4.01 (bs, 1H), 2.71 (bs, 2H), 2.13 (dd, J = 6.6, 4.4 Hz, 5H), 1.98 – 1.81 (m, 3H), 1.44 (bs, 2H), 1.29 – 1.23 (m, 7H), 0.88 (dt, J = 14.9, 7.1 Hz, 7H). 13C NMR (126 MHz, CDCl3) δ 62.7, 58.0, 52.2, 36.5, 35.8, 31.5, 28.9, 24.7, 23.1, 14.1, 10.8. HRMS (EI+) calcd. for [C13H26ClN] (M+) 231.1756; found 231.1751.
4-chloro-1-(4-methoxyphenethyl)piperidine (2ae) Colorless oil, 56.9 mg, 51% yield. 1H NMR (400 MHz, CDCl3) δ 7.32 – 7.10 (m, 5H), 4.01 (s, 1H), 2.78 – 2.72 (m, 4H), 2.54 (dd, J=9.7, 6.5, 2H), 2.27 (bs, 2H), 2.07 (d, J=12.4, 2H), 1.94 – 1.80 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 140.2, 128.6, 128.3, 125.9, 60.3, 57.3, 51.2, 35.5, 33.7. HRMS (EI+) calcd. for [C13H18ClN] (M+) 223.1232; found 223.1228.
4-chloro-1-(4-methoxyphenethyl)piperidine (2af) Colorless oil, 73.4 mg, 58% yield. 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 8.4 Hz, 2H), 6.83 (d, J = 8.5 Hz, 2H), 4.12 – 4.00 (m, 1H), 3.79 (s, 3H), 2.88 – 2.79 (m, 2H), 2.78 – 2.67 (m, 3H), 2.61 – 2.52 (m, 2H), 2.38 – 2.25 (m, 2H), 2.18 – 2.08 (m, 2H), 2.00 – 1.86 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 158.0, 132.3, 129.6, 113.8, 60.6, 57.4, 55.3, 51.3, 35.6, 32.9. HRMS (EI+) calcd. for [C14H20ClNO] (M+) 253.1232; found 253.1228.
1-benzyl-4-chloropiperidine (2ag) Colorless oil, 64 mg, 61% yield. 1H NMR (400 MHz, CDCl3) δ 7.43 – 7.12 (m, 5H), 4.03 (bs, 1H), 3.49 (bs, 2H), 2.80 – 2.67 (m, 2H), 2.23 (s, 2H), 2.13 – 2.02 (m, 2H), 1.96 – 1.84 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 138.3, 129.0, 128.2, 127.0, 62.8, 57.5, 51.3, 35.6. HRMS (EI+) calcd. for [C12H16ClN] (M+) 209.0973; found 209.0967.
4-chloro-1-(1-phenylethyl)piperidine (2ah) Colorless liquid, 72.7 mg, 65% yield. 1H NMR (400 MHz, CDCl3) δ 7.38 – 7.21 (m, 5H), 3.98 (bs, 1H), 3.44 (q, J=6.7, 1H), 2.86 (bs, 1H), 2.78 – 2.66 (m, 1H), 2.29 – 2.12 (m, 2H), 2.14 – 1.99 (m, 2H), 1.97 – 1.80 (m, 2H), 1.37 (d, J=6.7, 3H). 13C NMR (100 MHz, CDCl3) δ 143.8, 128.2, 127.5, 126.9, 64.4, 57.8, 48.6, 35.9, 19.4. HRMS (EI+) calcd. for [C13 H19NCl] (MH+) 224.1201; found 224.1197.
4-chloro-4-methyl-1-phenylpiperidine (4a) Colorless liquid, 84.9 mg, 81% yield.1H NMR (400 MHz, CDCl3) δ 7.32 – 7.22 (m, 2H), 6.96 (d, J = 8.6 Hz, 2H), 6.85 (t, J = 7.0 Hz, 1H), 3.51 (d, J = 12.8 Hz, 2H), 3.25 – 3.12 (m, 2H), 1.95 (ddd, J = 22.6, 18.0, 8.3 Hz, 4H), 1.68 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 151.1, 129.1, 119.5, 116.4, 69.5, 46.0, 40.3, 33.2. HRMS (EI+) calcd. for [C12H17NCl] (MH+) 210.1044; found 210.1040.
4-bromo-4-chloro-1-phenylpiperidine (4b) Colorless liquid, 114.7 mg, 84% yield. 1H NMR (400 MHz, CDCl3) δ 7.28 (d, J = 7.9 Hz, 2H), 6.93 (d, J = 7.9 Hz, 2H), 6.88 (t, J = 7.3 Hz, 1H), 3.45 – 3.28 (m, 4H), 2.68 (ddd, J = 13.7, 7.1, 3.7 Hz, 2H), 2.55 (ddd, J = 13.7, 7.0, 3.7 Hz, 2H). 13C NMR (100 MHz CDCl3) δ 150.2, 129.1, 120.0, 116.4, 78.9, 47.5, 46.4. HRMS (EI+) calcd. for [C11H13BrClN] (M+) 272.9917; found 272.9912.
4a-chloro-6,6-dimethyl-2-phenyldecahydro-5,7-methanoisoquinoline (4c) Colorless oil, 76.62 mg, 53% yield. 1H NMR (500 MHz, CDCl3) δ 7.23 (d, J = 8.1 Hz, 2H), 6.91 (d, J = 8.1 Hz, 2H), 6.79 (t, J = 7.2 Hz, 1H), 3.70 – 3.60 (m, 3H), 2.79 (td, J = 12.6, 3.0 Hz, 1H), 2.45 – 2.38 (m, 1H), 2.23 (td, J = 12.1, 6.4 Hz, 1H), 2.10 (td, J = 12.8, 4.5 Hz, 1H), 1.92 – 1.84 (m, 2H), 1.79 (d, J = 10.7 Hz, 1H), 1.61 – 1.54 (m, 1H), 1.35 (d, J = 11.2 Hz, 1H), 1.08 (s, 3H), 1.01 (s, 3H), 0.92 (dt, J = 13.0, 4.4 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 151.1, 129.0, 118.9, 116.3, 77.7, 55.2, 51.8, 47.5, 47.3, 39.4, 34.8, 30.5, 30.4, 29.9, 27.7, 23.2. HRMS (EI+) calcd. for [C18H24ClN] (M+) 289.1595; found 289.1590.
4-chloro-3-methyl-1-phenylpiperidine (4d) Colorless oil, 68.1 mg, 65% yield. 1H NMR (400 MHz, CDCl3) Major (anti-) diastereomer: δ 7.31 – 7.22 (m, 2H), 6.96 – 6.90 (m, 2H), 6.86 (t, J = 7.3 Hz, 1H), 3.73 – 3.57 (m, 3H), 2.80 (td, J = 12.5, 2.7 Hz, 1H), 2.50 (dd, J = 12.9, 10.6 Hz, 1H), 2.31 – 2.21 (m, 1H), 2.14 – 1.95 (m, 2H), 1.14 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 150.7, 129.1, 119.7, 116.5, 64.7, 56.4, 49.4, 39.5, 35.8, 16.9. HRMS (EI+) calcd. for [C12H17NCl] (MH+) 210.1044; found 210.1042.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health for financial support (R01GM121660). Bo Xu is thankful to the National Science Foundation of China for financial support (NSFC-21472018). We also thank Dr. Neal Stolowich for his help in some of the NMR experiments and Angela M. Hansen for her help with HRMS analyses performed at Indiana University Bloomington Mass Spectrometry Facility (NSF Grant CHE1726633).
Footnotes
ASSOCIATED CONTENT
This material is available free of charge via the internet at http://pubs.acs.org.
Copies of 1H and 13C NMR spectra
Notes
The authors declare no competing financial interests.
REFERENCES
- 1.(a) François ‐Xavier F; Jacques L Recent Advances in the Total Synthesis of Piperidine and Pyrrolidine Natural Alkaloids with Ring ‐ Closing Metathesis as a Key Step. Eur. J. Org. Chem 2003, 2003, 3693–3712; [Google Scholar]; (b) Carmen E; Mercedes A; Joan B Chiral Oxazolopiperidone Lactams: Versatile Intermediates for the Enantioselective Synthesis of Piperidine ‐ Containing Natural Products. Chem. Eur. J 2006, 12, 8198–8207. [DOI] [PubMed] [Google Scholar]
- 2.(a) Baumann M; Baxendale IR An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem 2013, 9, 2265–2319; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859; [DOI] [PubMed] [Google Scholar]; (c) Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274; [DOI] [PubMed] [Google Scholar]; (d) Zhang TY, Chapter One - The Evolving Landscape of Heterocycles in Drugs and Drug Candidates. In Adv. Heterocycl. Chem, Scriven EFV; Ramsden CA, Eds. Academic Press: 2017; Vol. 121, pp 1–12; [Google Scholar]; (e) Ye Z; Adhikari S; Xia Y; Dai M Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes. Nat. Commun 2018, 9, 721; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Pethe K; Bifani P; Jang J; Kang S; Park S; Ahn S; Jiricek J; Jung J; Jeon HK; Cechetto J; Christophe T; Lee H; Kempf M; Jackson M; Lenaerts AJ; Pham H; Jones V; Seo MJ; Kim YM; Seo M; Seo JJ; Park D; Ko Y; Choi I; Kim R; Kim SY; Lim S; Yim S-A; Nam J; Kang H; Kwon H; Oh C-T; Cho Y; Jang Y; Kim J; Chua A; Tan BH; Nanjundappa MB; Rao SPS; Barnes WS; Wintjens R; Walker JR; Alonso S; Lee S; Kim J; Oh S; Oh T; Nehrbass U; Han S-J; No Z; Lee J; Brodin P; Cho S-N; Nam K; Kim J Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med 2013, 19, 1157–1160. [DOI] [PubMed] [Google Scholar]
- 3.(a) Julian LD; Hartwig JF Intramolecular Hydroamination of Unbiased and Functionalized Primary Aminoalkenes Catalyzed by a Rhodium Aminophosphine Complex. J. Am. Chem. Soc 2010, 132, 13813–13822; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Takemiya A; Hartwig JF Rhodium-Catalyzed Intramolecular, Anti-Markovnikov Hydroamination. Synthesis of 3-Arylpiperidines. J. Am. Chem. Soc 2006, 128, 6042–6043; [DOI] [PubMed] [Google Scholar]; (c) Fujita K.-i.; Fujii T; Yamaguchi R Cp*Ir Complex-Catalyzed N-Heterocyclization of Primary Amines with Diols: A New Catalytic System for Environmentally Benign Synthesis of Cyclic Amines. Org. Lett 2004, 6, 3525–3528. [DOI] [PubMed] [Google Scholar]
- 4.(a) Qi X; Chen C; Hou C; Fu L; Chen P; Liu G Enantioselective Pd(II)-Catalyzed Intramolecular Oxidative 6-endo Aminoacetoxylation of Unactivated Alkenes. J. Am. Chem. Soc 2018, 140, 7415–7419; [DOI] [PubMed] [Google Scholar]; (b) Ortiz GX; Kang B; Wang Q One-Pot Synthesis of 3-Azido- and 3-Aminopiperidines by Intramolecular Cyclization of Unsaturated Amines. J. Org. Chem 2014, 79, 571–581. [DOI] [PubMed] [Google Scholar]
- 5.(a) Liu G-Q; Cui B; Xu R; Li Y-M Preparation of trans-2-Substituted-4-halopiperidines and cis-2-Substituted-4-halotetrahydropyrans via AlCl3-Catalyzed Prins Reaction. J. Org. Chem 2016, 81, 5144–5161; [DOI] [PubMed] [Google Scholar]; (b) Nebe MM; Opatz T, Chapter Five - Synthesis of Piperidines and Dehydropiperidines: Construction of the Six-Membered Ring. In Adv. Heterocycl. Chem, Scriven EFV; Ramsden CA, Eds. Academic Press: 2017; Vol. 122, pp 191–244. [Google Scholar]
- 6.(a) Coldham I; Collis AJ; Mould RJ; Rathmell RE Synthesis of 4-phenylpiperidines by tandem Wittig olefination– aza-Wittig rearrangement of 2-benzoylaziridines. J. Chem. Soc., Perkin Trans 1 1995, 2739–2745; [Google Scholar]; (b) Coldham I; Collis AJ; Mould RJ; Rathmell RE Ring expansion of aziridines to piperidines using the aza-Wittig rearrangement. Tetrahedron Lett 1995, 36, 3557–3560; [Google Scholar]; (c) Ohno H Synthesis and applications of vinyl aziridines and ethynyl aziridines. Chem. Rev 2014, 114, 7784–814. [DOI] [PubMed] [Google Scholar]
- 7.(a) Rueping M; Antonchick AP Organocatalytic Enantioselective Reduction of Pyridines. Angew. Chem. Int. Ed 2007, 46, 4562–4565; [DOI] [PubMed] [Google Scholar]; (b) Mahdi T; del Castillo JN; Stephan DW Metal-Free Hydrogenation of N-Based Heterocycles. Organometallics 2013, 32, 1971–1978; [Google Scholar]; (c) Liu Y; Du H Metal-Free Borane-Catalyzed Highly Stereoselective Hydrogenation of Pyridines. J. Am. Chem. Soc 2013, 135, 12968–12971; [DOI] [PubMed] [Google Scholar]; (d) Gandhamsetty N; Park S; Chang S Selective Silylative Reduction of Pyridines Leading to Structurally Diverse Azacyclic Compounds with the Formation of sp3 C–Si Bonds. J. Am. Chem. Soc 2015, 137, 15176–15184; [DOI] [PubMed] [Google Scholar]; (e) Zhou Q; Zhang L; Meng W; Feng X; Yang J; Du H Borane-Catalyzed Transfer Hydrogenations of Pyridines with Ammonia Borane. Org. Lett 2016, 18, 5189–5191; [DOI] [PubMed] [Google Scholar]; (f) Liu G-Q; Opatz T, Chapter Two - Recent Advances in the Synthesis of Piperidines: Functionalization of Preexisting Ring Systems. In Adv. Heterocycl. Chem, Scriven EFV; Ramsden CA, Eds. Academic Press: 2018; Vol. 125, pp 107–234. [Google Scholar]
- 8.(a) Ju Y; Varma RS Aqueous N-Heterocyclization of Primary Amines and Hydrazines with Dihalides: Microwave-Assisted Syntheses of N-Azacycloalkanes, Isoindole, Pyrazole, Pyrazolidine, and Phthalazine Derivatives. J. Org. Chem 2006, 71, 135–141; [DOI] [PubMed] [Google Scholar]; (b) Shao Z; Chen J; Tu Y; Li L; Zhang H A facile aminocyclization for the synthesis of pyrrolidine and piperidine derivatives. Chem. Commun 2003, 1918–1919. [PubMed]
- 9.(a) Trost BM; Maulide N; Livingston RC A Ruthenium-Catalyzed, Atom-Economical Synthesis of Nitrogen Heterocycles. J. Am. Chem. Soc 2008, 130, 16502–16503; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Z; Hartwig JF Mild, Rhodium-Catalyzed Intramolecular Hydroamination of Unactivated Terminal and Internal Alkenes with Primary and Secondary Amines. J. Am. Chem. Soc 2008, 130, 1570–1571; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lu Z; Stahl SS Intramolecular Pd(II)-Catalyzed Aerobic Oxidative Amination of Alkenes: Synthesis of Six-Membered N-Heterocycles. Org. Lett 2012, 14, 1234–1237; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Han X; Widenhoefer RA Gold(I)-catalyzed intramolecular hydroamination of alkenyl carbamates. Angew. Chem. Int. Ed, 2006, 45, 1747–9. [DOI] [PubMed] [Google Scholar]
- 10.(a) Huang HT; Lacy TC; Blachut B; Ortiz GX Jr.; Wang Q An efficient synthesis of fluorinated azaheterocycles by aminocyclization of alkenes. Org Lett 2013, 15, 1818–1821; [DOI] [PubMed] [Google Scholar]; (b) Wang Q; Zhong W; Wei X; Ning M; Meng X; Li Z Metal-free intramolecular aminofluorination of alkenes mediated by PhI(OPiv)2/hydrogen fluoride-pyridine system. Org. Biomol. Chem 2012, 10, 8566–8569; [DOI] [PubMed] [Google Scholar]; (c) Kong W; Feige P; de Haro T; Nevado C Regio- and enantioselective aminofluorination of alkenes. Angew Chem Int Ed Engl 2013, 52, 2469–73; [DOI] [PubMed] [Google Scholar]; (d) Teresa d. H.; Cristina N Flexible Gold-Catalyzed Regioselective Oxidative Difunctionalization of Unactivated Alkenes. Angew. Chem. Int. Ed 2011, 50, 906–910. [DOI] [PubMed] [Google Scholar]
- 11.(a) Durel V; Lalli C; Roisnel T; van de Weghe P Synergistic Effect of the TiCl4/p-TsOH Promoter System on the Aza-Prins Cyclization. J. Org. Chem 2016, 81, 849–59; [DOI] [PubMed] [Google Scholar]; (b) Katamura T; Shimizu T; Mutoh Y; Saito S Synthesis of Tricyclic Benzazocines by Aza-Prins Reaction. Org. Lett 2017, 19, 266–269; [DOI] [PubMed] [Google Scholar]; (c) Mahía A; Badorrey R; Gálvez JA; Díaz-de-Villegas MD Diastereoselective Construction of the 6-Oxa-2-azabicyclo[3.2.1]octane Scaffold from Chiral α -Hydroxyaldehyde Derivatives by the Aza-Prins Reaction. J. Org. Chem 2017, 82, 8048–8057; [DOI] [PubMed] [Google Scholar]; (d) Launay GG; Slawin AMZ; O’Hagan D Prins fluorination cyclisations: Preparation of 4-fluoro-pyran and - piperidine heterocycles. Beilstein J. Org. Chem 2010, 6, 41; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yadav JS; Reddy BVS; Ramesh K; Kumar GGKSN; Grée R An expeditious synthesis of 4-fluoropiperidines via aza-Prins cyclization. Tetrahedron Lett 2010, 51, 1578–1581; [Google Scholar]; (f) Reddy BVS; Borkar P; Chakravarthy PP; Yadav JS; Gree R Sc(OTf)3-catalyzed intramolecular aza-Prins cyclization for the synthesis of heterobicycles. Tetrahedron Lett 2010, 51, 3412–3416; [Google Scholar]; (g) Clarisse D; Pelotier B; Fache F Solvent-Free, Metal-Free, Aza-Prins Cyclization: Unprecedented Access to δ -Sultams. Chem. Eur. J 2013, 19, 857–860; [DOI] [PubMed] [Google Scholar]; (h) Sun Y; Chen P; Zhang D; Baunach M; Hertweck C; Li A Bioinspired Total Synthesis of Sespenine. Angew. Chem. Int. Ed 2014, 53, 9012–9016; [DOI] [PubMed] [Google Scholar]; (i) Nallasivam JL; Fernandes RA A Cascade Aza-Cope/Aza-Prins Cyclization Leading to Piperidine Derivatives. Eur. J. Org. Chem 2015, 2015, 2012–2022; [Google Scholar]; (j) Okoromoba OE; Hammond GB; Xu B Preparation of Fluorinated Tetrahydropyrans and Piperidines using a New Nucleophilic Fluorination Reagent DMPU/HF. Org Lett 2015, 17, 3975–7; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Chio FKI; Guesné SJJ; Hassall L; McGuire T; Dobbs AP Synthesis of Azabicycles via Cascade Aza-Prins Reactions: Accessing the Indolizidine and Quinolizidine Cores. J. Org. Chem 2015, 80, 9868–9880; [DOI] [PubMed] [Google Scholar]; (l) Ma D; Zhong Z; Liu Z; Zhang M; Xu S; Xu D; Song D; Xie X; She X Protecting-Group-Free Total Synthesis of (−)-Lycopodine via Phosphoric Acid Promoted Alkyne Aza-Prins Cyclization. Org. Lett 2016, 18, 4328–4331; [DOI] [PubMed] [Google Scholar]; (m) Subba Reddy BV; Nair PN; Antony A; Lalli C; Grée R The Aza-Prins Reaction in the Synthesis of Natural Products and Analogues. Eur. J. Org. Chem 2017, 2017, 1805–1819. [Google Scholar]
- 12.(a) Ebule R; Liang S; Hammond GB; Xu B Chloride-Tolerant Gold(I)-Catalyzed Regioselective Hydrochlorination of Alkynes. ACS Catal 2017, 7, 6798–6801; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liang S; Ebule R; Hammond GB; Xu B A Chlorinating Reagent Yields Vinyl Chlorides with High Regioselectivity under Heterogeneous Gold Catalysis. Org. Lett 2017, 19, 4524–4527; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zeng X; Liu S; Hammond GB; Xu B Hydrogen-Bonding-Assisted Brønsted Acid and Gold Catalysis: Access to Both (E)- and (Z)-1,2-Haloalkenes via Hydrochlorination of Haloalkynes. ACS Catal 2018, 8, 904–909; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zeng X; Lu Z; Liu S; Hammond GB; Xu B Metal-free, Regio-, and Stereo-Controlled Hydrochlorination and Hydrobromination of Ynones and Ynamides. J. Org. Chem 2017, 82, 13179–13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Varkey TE; Whitfield GF; Swern D Activation of dimethyl sulfoxide by electrophiles and use of the reactive intermediates in the preparation of iminosulfuranes. J. Org. Chem 1974, 39, 3365–3372; [Google Scholar]; (b) Mancuso AJ; Swern D ACTIVATED DIMETHYLSULFOXIDE - USEFUL REAGENTS FOR SYNTHESIS. Synthesis 1981, 165–185.
- 14.Wu X-F; Natte K The Applications of Dimethyl Sulfoxide as Reagent in Organic Synthesis. Adv. Synth. Catal 2016, 358, 336–352. [Google Scholar]
- 15.(a) Liu H; Jiang X Transfer of sulfur: from simple to diverse. Chem Asian J 2013, 8, 2546–63; [DOI] [PubMed] [Google Scholar]; (b) Sun K; Lv Y; Zhu Z; Zhang L; Wu H; Liu L; Jiang Y; Xiao B; Wang X Oxidative C–S bond cleavage reaction of DMSO for C–N and C–C bond formation: new Mannich-type reaction for β -amino ketones. RSC Adv 2015, 5, 3094–3097; [Google Scholar]; (c) Zhang R; Shi XQ; Yan QQ; Li ZJ; Wang Z; Yu HF; Wang XK; Qi J; Jiang ML Free-radical initiated cascade methylation or trideuteromethylation of isocyanides with dimethyl sulfoxides. RSC Adv 2017, 7, 38830–38833; [Google Scholar]; (d) Patel OPS; Anand D; Maurya RK; Yadav PP H2O2/DMSO-Promoted Regioselective Synthesis of 3,3’-Bisimidazopyridinylmethanes via Intermolecular Oxidative C(sp(2))-H Bond Activation of Imidazoheterocycles. J. Org. Chem 2016, 81, 7626–34; [DOI] [PubMed] [Google Scholar]; (e) Jadhav SD; Singh A Oxidative Annulations Involving DMSO and Formamide: K2S2O8 Mediated Syntheses of Quinolines and Pyrimidines. Org Lett 2017, 19, 5673–5676; [DOI] [PubMed] [Google Scholar]; (f) Lv Y; Li Y; Xiong T; Pu W; Zhang H; Sun K; Liu Q; Zhang Q Copper-catalyzed annulation of amidines for quinazoline synthesis. Chem. Commun 2013, 49, 6439–41; [DOI] [PubMed] [Google Scholar]; (g) Yuan J; Yu JT; Jiang Y; Cheng J Carbon annulation of ortho-vinylanilines with dimethyl sulfoxide to access 4-aryl quinolines. Org Biomol Chem 2017, 15, 1334–1337. [DOI] [PubMed] [Google Scholar]
- 16.Lu D-F; Liu G-S; Zhu C-L; Yuan B; Xu H Iron(II)-Catalyzed Intramolecular Olefin Aminofluorination. Org. Lett 2014, 16, 2912–2915. [DOI] [PubMed] [Google Scholar]
- 17.Wen Z-K; Liu X-H; Liu Y-F; Chao J-B Acid Promoted Direct Cross-Coupling of Methyl Ketones with Dimethyl Sulfoxide: Access to Ketoallyl Methylsulfides and -sulfones. Org. Lett 2017, 19, 5798–5801. [DOI] [PubMed] [Google Scholar]
- 18.Kappe CO, Product Class 2: Alkylidenesulfonium Salts or α -Sulfanyl Carbocations. In Category 4. Compounds with Two Carbon Heteroatom Bonds, 2005 ed.; Padwa A; Bellus D, Eds. Georg Thieme Verlag: Stuttgart, 2005; Vol. 27. [Google Scholar]
- 19.(a) Smith LH; Coote SC; Sneddon HF; Procter DJ Beyond the Pummerer reaction: recent developments in thionium ion chemistry. Angew. Chem. Int. Ed 2010, 49, 5832–44; [DOI] [PubMed] [Google Scholar]; (b) Bur SK; Padwa A The Pummerer Reaction: Methodology and Strategy for the Synthesis of Heterocyclic Compounds. Chem. Rev 2004, 104, 2401–2432; [DOI] [PubMed] [Google Scholar]; (c) Akai S; Kita Y, Recent Advances in Pummerer Reactions. In Sulfur-Mediated Rearrangements I, Schaumann E, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2007; pp 35–76. [Google Scholar]
- 20.(a) Jiang X; Wang C; Wei Y; Xue D; Liu Z; Xiao J A general method for N-methylation of amines and nitro compounds with dimethylsulfoxide. Chem. Eur. J 2014, 20, 58–63; [DOI] [PubMed] [Google Scholar]; (b) Xue L; Cheng G; Zhu R; Cui X Acid-promoted oxidative methylenation of 1,3-dicarbonyl compounds with DMSO: application to the three-component synthesis of Hantzsch-type pyridines. RSC Advances 2017, 7, 44009–44012. [Google Scholar]
- 21.(a) Wu T; Yin G; Liu G Palladium-Catalyzed Intramolecular Aminofluorination of Unactivated Alkenes. J. Am. Chem. Soc 2009, 131, 16354–16355; [DOI] [PubMed] [Google Scholar]; (b) Kong W; Feige P; de Haro T; Nevado C Regio- and Enantioselective Aminofluorination of Alkenes. Angew. Chem. Int. Ed 2013, 52, 2469–2473; [DOI] [PubMed] [Google Scholar]; (c) Liu G-Q; Li Y-M Regioselective (Diacetoxyiodo)benzene-Promoted Halocyclization of Unfunctionalized Olefins. J. Org. Chem 2014, 79, 10094–10109. [DOI] [PubMed] [Google Scholar]
- 22.Ni Y; Zuo H; Yu H; Wu Y; Zhong F Synergistic Catalysis-Enabled Thia-Aza-Prins Cyclization with DMSO and Disulfides: Entry to Sulfenylated 1,3-Oxazinanes and Oxazolidines. Org. Lett 2018, 20, 5899–5904. [DOI] [PubMed] [Google Scholar]
- 23.Ebule R; Hammond GB; Xu B Metal-Free Chlorothiolation of Alkenes Using HCl and Sulfoxides. Eur. J. Org. Chem 2018, 4705–4708. [DOI] [PMC free article] [PubMed]
- 24.(a) For intramolecular iminium cyclizations see: Memeo MG; Quadrelli P Iminium Ions as Dienophiles in Aza-Diels– Alder Reactions: A Closer Look. Chem. Eur. J 2012, 18, 12554–12582; [DOI] [PubMed] [Google Scholar]; (b) Remuson R; Gelas-Mialhe Y A Convenient Access to the Piperidine Ring by Cyclization of Allylsilyl Substituted N-acyliminium and Iminium Ions: Application to the Synthesis of Piperidine Alkaloids. Mini Rev. Org. Chem 2008, 5, 193–208; [Google Scholar]; (c) Dounay AB; Humphreys PG; Overman LE; Wrobleski AD Total Synthesis of the Strychnos Alkaloid (+)-Minfiensine: Tandem Enantioselective Intramolecular Heck−Iminium Ion Cyclization. J. Am. Chem. Soc 2008, 130, 5368–5377; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Maryanoff BE; Zhang H-C; Cohen JH; Turchi IJ; Maryanoff CA Cyclizations of N-Acyliminium Ions. Chem. Rev 2004, 104, 1431–1628; [DOI] [PubMed] [Google Scholar]; (e) Snider BB Intramolecular cycloaddition reactions of ketenes and keteniminium salts with alkenes. Chem. Rev 1988, 88, 793–811; [Google Scholar]; (f) Overman LE; Sharp MJ Nucleophile-promoted electrophilic cyclization reactions of alkynes. J. Am. Chem. Soc 1988, 110, 612–614. [Google Scholar]
- 25.Zheng J; Huang L; Huang C; Wu W; Jiang H Synthesis of Polysubstituted Pyrroles via Pd-Catalyzed Oxidative Alkene C–H Bond Arylation and Amination. J. Org. Chem 2015, 80, 1235–1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


