Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) affects individuals with 55–200 CGG repeats (premutation) in the 5’-untranslated region of the fragile X mental retardation 1 (FMR1) gene. FXTAS is a progressive neurodegenerative disorder associated with an action tremor, cerebellar ataxia memory and executive function deficits, autonomic dysfunction and neuropathy. Females with the fragile X premutation are often affected by fragile X-associated primary ovarian insufficiency (FXPOI), and may have other medical conditions such as fibromyalgia, depression, anxiety, and immune-mediated disorders like hypothyroidism. Here we present a case of a 54-year-old woman with tremor, ataxia, average memory skills, and executive function deficits who meets criteria for FXTAS. She also has anxiety, Major Depressive Disorder, fibromyalgia, chronic pain and was treated chronically with opioids and she overdosed on fentanyl leading to significant CNS dysfunction.
Keywords: Fentanyl, FMR1 premutation, fragile X syndrome, fragile X–associated tremor/ataxia syndrome, FXTAS
Introduction:
Patients with the premutation in FMR1 (55–200 CGG repeats) have elevated FMR1 mRNA expression levels, which have been associated with neurotoxicity, potentially causing neurodevelopmental problems or neurological problems associated with aging in both males and females.1,2 Women with the premutation face many physical and emotional challenges in their life especially when raising a child with fragile X syndrome (FXS).3 Women with the premutation are at risk for early menopause before age 40 (fragile X-associated primary ovarian insufficiency (FXPOI)), fibromyalgia, hypothyroidism, migraines, restless legs syndrome, depression, and anxiety.2,4 The estimated carrier prevalence of the premutation in women in the USA is approximately 1:178.5,6 Premutation carriers may also develop fragile X–associated tremor/ataxia syndrome (FXTAS), a neurodegenerative disorder with increased prevalence with age.7 FXTAS clinical features include progressive cerebellar ataxia and intention tremor in addition to autonomic dysfunction, peripheral neuropathy, and cognitive impairment.2,8 Many individuals experience chronic pain and often opioids are prescribed to relieve pain.9 However, anecdotal evidence suggests that those on long term opioid treatment may experience an increase in the white matter brain changes observed in those with FXTAS.10 Here we report a case of a woman with FXTAS and autonomic dysfunction who experienced an overdose from fentanyl.
Materials and methods:
The patient in this study was evaluated at the Fragile X Research and Treatment Center located at the UC Davis MIND Institute. The patient signed an IRB approved consent form for this research when she was seen. Data were acquired from the medical history obtained during study visits.
Clinical Report:
The patient is a 54-year-old Caucasian women with a normal allele of 30 CGG repeats and a premutation allele of 93 CGG repeats, with an activation ratio (AR) of 0.15 meaning that only 15% of her cells have the normal X chromosome as the active X. FMR1 mRNA level was 2.71 ± 0.15 times normal. She has a long history of anxiety beginning in childhood and intermittent depression in her adult life. She has a son with FXS who is relatively high functioning. She experienced onset of an intermittent intention tremor and postural tremor at age 49 bilaterally, but more pronounced in her right arm. Progressive balance problems began at age 48, which caused her to fall; her gait difficulties gradually worsened over the next few years.
Her memory problems began at age 47. Her stamina has decreased profoundly over the last few years. She had neuropathy with tingling and numbness in her legs beginning at age 39. Her medical history includes acid reflux, migraines with visual aura, chronic vertigo and chronic pain secondary to fibromyalgia. She started to smoke marijuana daily at age 34 to help her chronic pain. Her history also includes restless legs syndrome, ovarian cysts (which were treated by oophorectomy at age 27), orthostatic hypotension, insomnia (for many years), recurrent urinary tract infections, recurrent nausea and vomiting (treated for the past 10 years with ondansetron). She has a long psychiatric history including severe anxiety, major depression, mood swings, bulimia, and post-traumatic stress disorder (PTSD) (Table 1).
Table 1:
FXTAS clinical and molecular findings.
| Medical History/Clinical Findings | Age at Onset of Symptoms (years) |
|---|---|
| Generalized Anxiety | 10 |
| Bulimia | 14 |
| Migraines | 16 |
| FXPOI | 27 |
| Major Depressive Disorder | 30 |
| Dizziness and vertigo | 34 |
| Hypotdyroidism | 34 |
| Fibromyalgia | 34 |
| Chronic Pain | 34 |
| Tingling and numbness | 39 |
| Memory problems | 47 |
| Handwriting problems | 47 |
| Balance problem | 47 |
| Swallowing problem | 47 |
| Tremor | 48 |
| Hearing loss | 53 |
| Neurological Exam | Severity of Symptoms |
| Right upper extremity | Intention tremor (+++) |
| Postural tremor (++) | |
| Intention tremor(+) | |
| Left upper extremity | Postural tremor (+) |
| Molecular Tests | Results |
| Fragile X DNA test (CGG repeats) | 93 |
| FMR1 mRNA level (times normal) | 3.71 |
| Diagnosis | |
| FXTAS diagnosis | Probable |
| FXTAS stage | 4 |
Due to chronic pain from the fibromyalgia, she started using hydrocodone which made her sick and sleepy. Therefore she discontinued the use of hydrocodone and started fentanyl patches at age 51, with a 75 mcg/hour patch two to three times per day. She was found passed out at home after using a fentanyl patch and she was taken to the emergency room and then the intensive care unit for four days. She was found to have a 95% blockage of her right carotid artery and she suffered from a serious episode of hypoxia from the fentanyl overdose. After this hospitalization she felt much weaker with worsening of the tremor and ataxia, and she could not walk without crutches. She subsequently underwent surgery to alleviate the carotid blockage and a stent was placed in her carotid artery at age 55.
Her current medications include fentanyl patches (25 mcg/hour patch) for pain control, hydrocodone (10–25 mg), diazepam, pitavastatin (4 mg) for pain control, ondansetron (4 mg) for nausea, topiramate for migraine, meclizine for dizziness, paroxetine hydrochloride (40 mg), clopidogrel (75 mg) after stent replacement, probiotics, levothyroxine sodium (0.125mg per day), and cyanocobalamin (1000 mcg/ml).
Her family history includes her father who died of FXTAS, and she has a son with FXS who is 35 years old.
At age 54 before her stent was placed but after her overdose her examination demonstrated: occipital frontal circumference 56.5 cm, height 165.3 cm, weight 66.1 kg, blood pressure was 131/80, and heart rate was 59 bpm. The patient’s neurologic examination included a severe intention tremor with right hand worse than the left hand and dyskinesia in her movements, a positive snout reflex, and a positive palmomental reflex. She had allodynia (pain to touch), and was ataxic with gait. She could not tandem walk.
Her deep-tendon reflexes were 2+ in the upper extremities, 4+ at the knees, and 2+ at the ankles but she often jerked her whole upper body with the tap of the reflex hammer. Her gag reflex was exaggerated and she had bilateral skin nodules around her proximal metacarpal joints. The patient underwent numerous neuropsychological and neuropsychiatric assessments, including the Wechsler Adult Intelligence Scales, 4th edition(WAIS-IV),11 the Wechsler Memory Scales, 4th edition (WMS-IV),12 and the Mini-Mental Status Exam (MMSE)13 to test cognitive status, and the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Research Version, Non-patient Edition(SCID-I/NP)14 to diagnose psychiatric disorders. Self-reported psychological problems and symptoms of psychopathology were assessed through the Symptom Checklist-90-R (SCL-90-R).15 The SCL-90-R scores are reported in T-scores, which have an average range between 40 and 59. For the assessment of executive function, the Behavioral Dyscontrol Scale 2 (BDS-2)16 was administered. Her scores on the above mentioned assessments are seen in Table 2.
Table 2:
Neuropsychological/neuropsychiatric assessments
| Assessment | Index Score | Percentile | Results |
|---|---|---|---|
| WAIS-IV | |||
| Verbal comprehension | 93 | 32 | Average |
| Perceptual reasoning | 88 | 21 | Low average |
| Working memory | 86 | 18 | Low average |
| Processing speed | 76 | 5 | Borderline |
| Full scale IQ | 83 | 13 | Low average |
| WMS-IV | |||
| Auditory memory | 107 | 68 | Average |
| Visual memory | 86 | 18 | Low average |
| Visual working memory | 80 | 9 | Low average |
| Immediate memory | 94 | 34 | Average |
| Delayed memory | 98 | 45 | Average |
| MMSE | 29 | Preserved orientation and short term memory skill | |
| BDS-2 | 16 | Moderate difficulties in executive functioning skills | |
| SCID-I/NP | Current Major Depressive Disorder | ||
| SCL-90-R (T-scores) | |||
| Somatization | 81 | Clinically Significant | |
| Obsessive-compulsive symptoms | 80 | Clinically Significant | |
| Interpersonal sensitivity | 64 | Clinically Significant | |
| Depression | 69 | Clinically Significant | |
| Anxiety | 66 | Clinically Significant | |
| Hostility | 69 | Clinically Significant | |
| Phobic anxiety | 70 | Clinically Significant | |
| Psychoticism | 69 | Clinically Significant | |
| Global symptom index | 72 | Clinically Significant | |
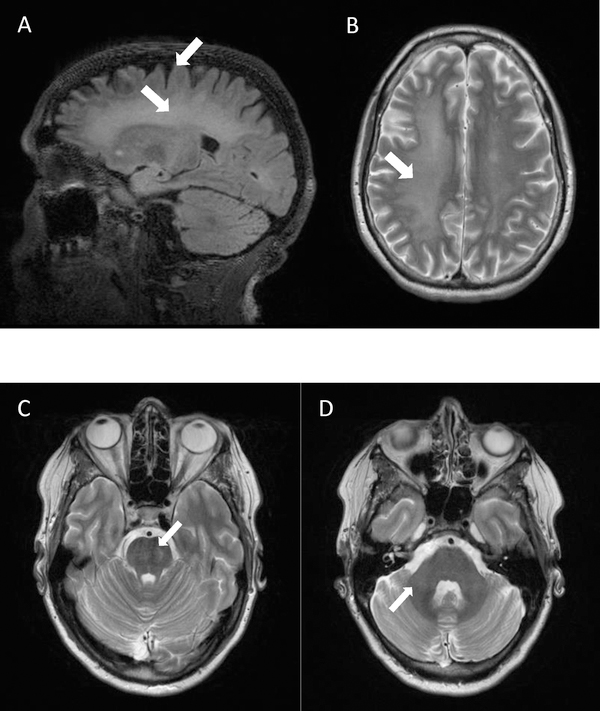
MRI of the brain at age 54 years after overdose and before vascular surgery demonstrated mild atrophy, increased T2 signal intensity in the pons, and severe diffuse increased T2 signal intensity in the deep white matter of the cerebrum. White matter damage was greater on the right side of the brain compared to the left side, suggesting hypoxic damage secondary to the carotid blockage (Figure 1).
Figure 1:
Brain MRI of a 54-year-old female fragile X premutation carrier, after fentanyl overdose and before vascular surgery. MRI demonstrated mild atrophy (A), increased T2 signal intensity in the pons (C), and severe diffuse increased T2 signal intensity in the deep white matter of the cerebrum (A, B). White matter damage was greater on the right side of the brain compared to the left side (B). Patient did not have increased T2 signal intensity in the middle cerebellar peduncles (D).
Discussion:
In this study we report on a woman who has the FMR1 premutation, FXTAS, chronic pain secondary to fibromyalgia and a fentanyl overdose that led to hypoxia because of a carotid artery blockage secondary to atherosclerosis. Opioids are commonly used for pain associated with neuropathy or fibromyalgia in individuals with FXTAS, however there is anecdotal evidence that those on opioids can have faster progression of their FXTAS symptoms.10 Premutation neurons die more easily in cell culture compared to normal neurons without the premutation so these neurons are considered to be more vulnerable to environmental toxins.1,17 This case points out another danger, specifically the high risk of an overdose from opioids such as fentanyl, which in this carrier led to hypoxic damage. Autonomic dysfunction is often seen in FXTAS and fainting or loss of consciousness from cardiac arrhythmias can also be seen in FXTAS.9 Thus, in this patient, it is possible that the overdose of the fentanyl may have exacerbated the autonomic instability, which with the combined effect of the carotid blockage, led to significant hypoxia and her FXTAS symptoms worsened after this episode.
Even though the patient’s neurocognitive functioning is mainly preserved (low average to average scores on most assessments), her scores on the BDS-2 point to impaired executive functioning, which includes poor decision-making, planning, and motor control. Her borderline low processing speed index (PSI standard score 76) is another indicator of subtle impairments that have not fully expanded to affect her general cognitive functioning (Table 2). In addition, her psychiatric history and current mental health problems indicate a particular vulnerability and could lead to further impairments in the future.
Fentanyl is a potent opioid analgesic used in the treatment of pain which is common in FXTAS and in fibromyalgia. Transdermal fentanyl patches are now widely utilized as an acceptable and efficacious method of medication delivery.18 In addition to possible exacerbation of FXTAS symptoms, long-term exposure to opioids can make individuals more sensitive to pain through neoplastic changes in the peripheral and central nervous systems, a phenomenon known as opioid-induced hyperalgesia.19 Because of these issues, we recommend avoiding opioids whenever possible in the treatment of pain in individuals with the premutation or FXTAS.
Conclusion:
We recommend evaluating premutation carriers with chronic pain carefully, and avoiding long-term opioid use to prevent possible exacerbation of symptoms of FXTAS. Alternatively treatments for pain include gabapentin, cannabidiol and anti-inflammatory medication.
Finally, appropriate management of depression, anxiety, chronic pain and preventing long-term use of opioids may slow the progression of white matter disease in those with FXTAS.
Acknowledgements:
This research was supported by the United States National Institute of Child Health and Human Development (grant R01 HD036071), the MIND Institute Intellectual and Developmental Disabilities Research Center (grant U54 HD079125) and the National Center for Advancing Translational Sciences and National Institutes of Health (grant UL1 TR001860).
Footnotes
Conflicts of interest: RH has received funding from Roche, Novartis, Neuren, Marinus, and Alcobra for carrying out treatment studies in patients with fragile X syndrome. She has also consulted with Roche, Novartis, Alcobra, Fulcrum and Zynerba regarding treatment studies in individuals with fragile X syndrome. FT received funds from Asuragen, Inc. The other authors declare no conflicts of interest.
References
- 1.Saldarriaga W, Lein P, Gonzalez Teshima LY, et al. Phenobarbital use and neurological problems in FMR1 premutation carriers. Neurotoxicology. 2016;53:141–147. doi: 10.1016/j.neuro.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12(8):786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyos LR, Thakur M. Fragile X premutation in women: recognizing the health challenges beyond primary ovarian insufficiency. J Assist Reprod Genet. 2017;34(3):315–323. doi: 10.1007/s10815-016-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall D, Tassone F. FXTAS, FXPOI and FMR1 Associated Disorders. Springer; 2016. [Google Scholar]
- 5.Hantash FM, Goos DM, Crossley B, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: Insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency i. Genet Med. 2011;13(1):39–45. doi: 10.1097/GIM.0b013e3181fa9fad. [DOI] [PubMed] [Google Scholar]
- 6.Tassone F, Iong KP, Tong T-H, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4(12):100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. Jama. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 8.Hall DD a, O’keefe JJ a. Fragile x-associated tremor ataxia syndrome: the expanding clinical picture, pathophysiology, epidemiology, and update on treatment. Tremor Other Hyperkinet Mov (N Y). 2012;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagerman RJ, Hagerman P. Fragile X ‑ associated tremor / ataxia syndrome — features, mechanisms and management. Nat Publ Gr. 2016;12(7):403–412. doi: 10.1038/nrneurol.2016.82. [DOI] [PubMed] [Google Scholar]
- 10.Muzar Z, Adams PE, Schneider A, Hagerman RJ, Lozano R. Addictive substances may induce a rapid neurological deterioration in fragile X-associated tremor ataxia syndrome: A report of two cases. Intractable rare Dis Res. 2014;3(4):162–165. doi: 10.5582/irdr.2014.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler D Wechsler Adult Intelligence Scale (WAIS).; 2008.
- 12.Wechsler D Wechsler Memory Scale-Fourth Edition (WMS-IV). San Antonio, TX, Pearson Assessment; 2009. doi: 10.1037/t15175-000. [DOI] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.First MB et, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV).; 1997. [Google Scholar]
- 15.Derogatis LR, Lazarus L. SCL-90—R, Brief Symptom Inventory, and matching clinical rating scales. In: The Use of Psychological Testing for Treatment Planning and Outcome Assessment. 1994:217–248. doi: 10.1037/t01210-000. [DOI] [Google Scholar]
- 16.Grigsby J, Kaye K, Kowalsky J, Kramer AM. Association of behavioral self-regulation with concurrent functional capacity among stroke rehabilitation patients. [References]. J Clin Geropsychology. 2002;8(1):25–33. doi: 10.1023/A:1013094023856. [DOI] [Google Scholar]
- 17.Polussa J, Schneider A, Hagerman R. Molecular Advances Leading to Treatment Implications for Fragile X Premutation Carriers. Brain Disord Ther. 2014;03(02):997–1003. doi: 10.4172/2168-975X.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer CKMW, Shand JAD, Reynolds TM. The Fentanyl Patch Boil-Up - A Novel Method of Opioid Abuse. Basic Clin Pharmacol Toxicol. 2015;117(5):358–359. doi: 10.1111/bcpt.12412. [DOI] [PubMed] [Google Scholar]
- 19.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]