Abstract
Heparin induced thrombocytopenia (HIT) is a serious adverse drug reaction caused by transient antibodies against platelet factor 4 (PF4)/heparin complexes, resulting in platelet activation and potentially fatal arterial and/or venous thrombosis. Most cases of HIT respond to cessation of heparin and administration of an alternative non-heparin anticoagulant, but there are cases of persisting HIT, defined as thrombocytopenia due to platelet activation/consumption for greater than seven days despite standard therapy. These patients remain at high risk for thrombotic events, which may result in limb-loss and mortality. Intravenous immunoglobulin (IVIg) has been proposed as an adjunct therapy for these refractory cases based on its ability to saturate FcγRIIa receptors on platelets, thus preventing HIT antibody binding and platelet activation. We describe 2 cases of persisting HIT (strongly positive antigen and functional assays, and persisting thrombocytopenia >7 days) with rapid clinical response to IVIg. We performed in-vitro experiments to support IVIg response. Healthy donor platelets (1 × 10e6) were treated with PF4 (3.75 µg/mL) for 20 min followed by 1-hour incubation with patients’ sera. Platelet activation with and without addition of IVIg (levels equivalent to those reached in a patient after treatment with 2gm/Kg) was evaluated in the PF4-dependent P-selectin expression assay (PEA). A significantly decreased platelet activation was demonstrated after the addition of IVIg to both patient samples, which correlated well with the rapid clinical response that each patient experienced. Thus, our study supports the use of IVIg as an adjunct therapy for persisting HIT.
Keywords: Heparin-induced thrombocytopenia (HIT), intravenous immunoglobulin (IVIg), persisting HIT
Introduction:
Heparin-induced thrombocytopenia (HIT) is a potentially lethal transient autoimmune hypercoagulable condition associated with both venous and arterial thrombosis due to the production of platelet-activating IgG antibodies against PF4/heparin complexes[1]. The circulating immune complexes bind to platelet and monocyte Fcγ receptors to promote cellular activation resulting in procoagulant microparticle release and thrombin generation[2]. Alternatively, the antibodies may activate platelets directly by recognizing PF4 bound to the platelet membrane[3].
Diagnosis requires clinical and laboratory evaluation, and treatment is based on the use of alternative anticoagulation with parental direct thrombin inhibitors, fondaparinux (off-label), or direct oral anticoagulants (off-label). Duration of therapy is in part guided by the response in platelet count and presence of thrombosis[4]. Recent studies suggest that despite current therapies, the HIT morbidity/mortality burden remains high[5, 6]. Patients may have persisting HIT as defined by persistence of thrombocytopenia beyond 7 days despite cessation of heparin and treatment with alternative anticoagulation[7], for which there are no well-defined therapeutic options.
While some studies have shown rapid reduction in antibody titers by plasma exchange[2] mainly peri-operatively, others have proposed the use of intravenous immunoglobulin (IVIg) as a potential adjunct treatment based on studies that support IVIg-mediated inhibition of HIT antibody-induced platelet activation[8].
We describe two cases of persisting HIT with protracted thrombocytopenia on standard therapy who experienced rapid platelet recovery with IVIg treatment.
Cases:
Patient 1:
An 82-year-old man was admitted for persistent gross hematuria and subsequently found to have a large bladder mass. He underwent surgical resection of the tumor and the post-operative course was complicated by heart failure exacerbation. Echocardiogram revealed a thrombus in the right ventricle and the patient was initiated on a continuous unfractionated heparin (UFH) infusion. On day five of heparin therapy, there was an abrupt decrease in the platelet count from 144×109/L to 62×109/L, which slowly reached a nadir of 19×109/L on day 13, prompting discontinuation of all heparin products and a consult to the hematology service. Doppler ultrasound revealed a new upper extremity deep venous thrombosis. The 4Ts score was calculated to be 6, indicating intermediate probability of HIT. A PF4/heparin antigen enzyme linked immunosorbent assay (ELISA, PF4 IgG assay, Immucor GTI Diagnostics, Waukesha, WI), showed an optical density (OD) of 3.559 and 31% inhibition with high concentration (100 U/mL) of heparin. The serotonin release assay (SRA, BloodCenter of Wisconsin, Milwaukee, WI) confirmed the diagnosis of HIT, showing 100% release with low concentration of heparin and 100% inhibition with high concentration of heparin.
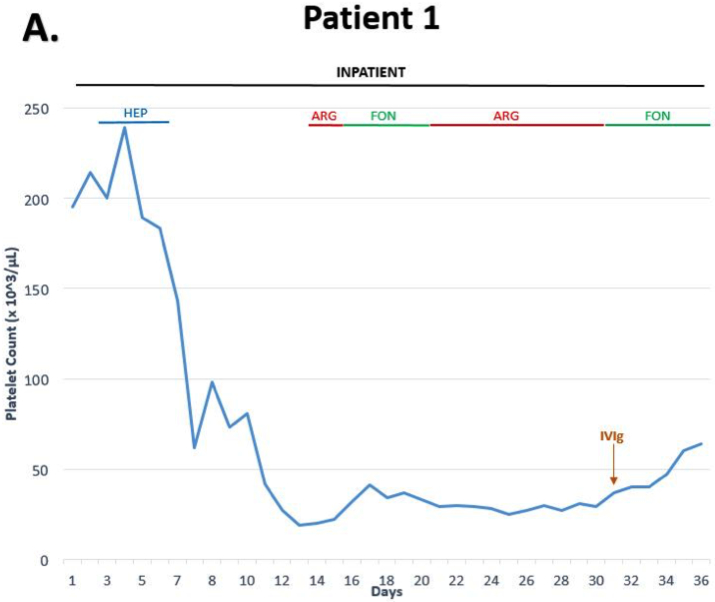
He was anticoagulated with argatroban for 2 days, titrated to therapeutic aPTT, and then switched to therapeutic dose fondaparinux 7.5mg daily. During the following six days, there was no appreciable effect on the platelet count (Figure 1A), and as a result, was switched back to argatroban along with a trial of steroids, prednisone 1mg/Kg/day, to treat for a possible component of immune thrombocytopenia. By day 31, the thrombocytopenia persisted at 37×109/L; therefore, he was administered 1 g/Kg of IVIg as a single dose. After IVIg administration, the platelet count increased over the next five days to 67×109/L (Figure 1A). Addition of IVIg to the patient’s serum in-vitro (mimicking levels attained after a 1 g/Kg dose) resulted in significant inhibition of platelet activation induced by HIT antibodies (Figure 2).
Figure 1.
Platelet recovery in response to IVIg in severe refractory HIT. ARG = argatroban; FON = fondaparinux; HEP = heparin
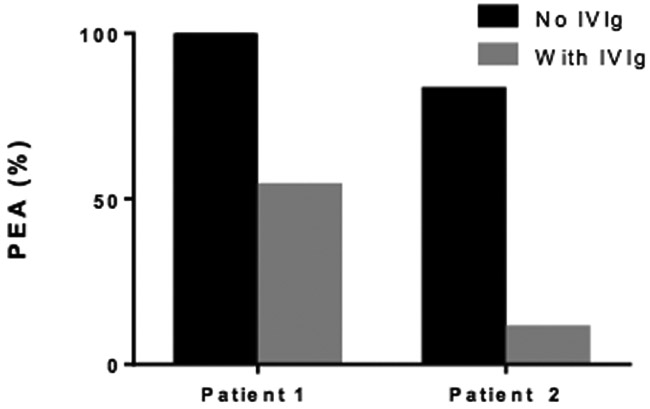
Figure 2.
PF4-dependent p-selectin expression assay (PEA). High dose IVIg inhibits HIT antibody-mediated platelet activation. The ordinate represents PEA (%) while samples used are indicated on the abscissa.
He was discharged to a subacute rehabilitation facility on therapeutic dose of fondaparinux, but was unfortunately lost to follow-up for platelet monitoring.
Patient 2:
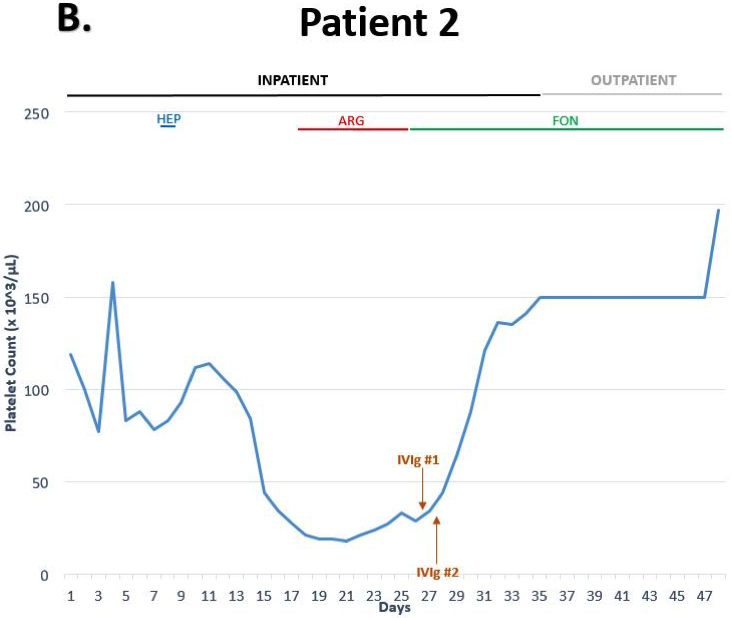
A 38-year-old man with glioblastoma who was admitted for pneumonia and subsequently developed heparin-induced thrombocytopenia (HIT) after receiving unfractionated heparin for thrombosis prophylaxis. There was a progressive decline in platelet count from a baseline of 80–100×109/L to 40×109/L within 12 days of admission, with a nadir of 18×109/L (Figure 1B). Doppler ultrasound revealed a thrombus of the right common femoral vein and his 4Ts score of 6 indicated a high clinical probability of HIT. Heparin was discontinued and he was initiated on argatroban. IgG-specific PF4/heparin ELISA was strongly positive [optical density of 2.77 and 98% inhibition with high concentration of heparin (100U/mL)] and serotonin release assay confirmed the diagnosis showing 93% release with low and 100% inhibition with high concentration of heparin.
Thrombocytopenia persisted despite one week of consistently therapeutic argatroban; therefore, he was given 2g/Kg of IVIg over two days. The platelet count had a rapid response with >two-fold increase within 48 hours and normalization of the platelet count >150×109/L seven days after administration. The patient was discharged on fondaparinux 7.5mg daily to complete a three-month course. IVIg was added to the patient’s serum in-vitro (mimicking levels attained after a 2 g/Kg dose) and resulted in significant inhibition of platelet activation induced by HIT antibodies (Figure 2), and correlated well with the rapid and sustained response in platelet count seen in our patient.
Five months later he maintained a normal platelet count (142×109/L) with no evidence of recurrent thromboses.
Materials and Methods:
The PF4-dependent p-selectin expression assay (PEA) was performed as previously described[8]. Briefly, washed O blood group normal platelets from three donors were pooled and 1 × 10e6 platelets treated with PF4 (3.75 µg/mL) for 20 min followed by patient serum for 1 h. After addition of fluorochrome-labeled anti-P-selectin (424.2 hybridoma, BloodCenter of Wisconsin) and anti-GPIIb (290.5 hybridoma, BloodCenter of Wisconsin) antibodies, platelet events were gated by GPIIb positivity, and P-selectin expression (median fluorescence intensity [MFI]) was recorded. Maximum P-selectin expression (100%) was measured by treating platelets with thrombin receptor-activating peptide (TRAP; 25 µg/mL). Results were expressed as the percentage of maximum P-selectin expression corrected for background signal obtained with normal serum as follows:
An incremental recovery rate of 2.3 mg/dL for each mg/kg body weight of IVIg (GAMMAGARD, Shire) was used to mimic IgG levels attained in vivo. Thus, a dose of 2 g/Kg IVIg administered to patients corresponds to an IgG concentration of 46 mg/mL attained in patient plasma. Studies were approved by the institutional review board of the Medical College of Wisconsin (Protocol PRO00023318).
Results and Discussion:
In both patients IVIG had rapid clinical impact, with platelet counts responding within the first two days. The proposed mechanism of action is saturation of FcγRIIa receptors on platelets that inhibits platelet activation by antibodies with specificity to glycosaminoglycan/PF4 immune complexes, although additional mechanisms cannot be ruled out. This hypothesis is supported by in-vitro studies demonstrating that the addition of IVIg to patients’ serum samples resulted in decreased platelet activation induced by HIT antibodies (Figure 2). The inhibition was higher in patient 2, which corresponds with the more robust in vivo platelet response as compared to patient 1. FcγIIa genotyping could not be performed; it is unknown if the R vs. H polymorphism at amino acid position 131 in FcγRIIa may have modulated the IVIg response[8].
IVIg carries an FDA black box warning for thrombosis. The incidence of thrombosis has previously been difficult to ascertain from the literature, but a recent large meta-analysis of 31 randomized controlled trials with >4000 patients showed no increased risk of arterial or venous thrombosis[9]. There are no reports of extension of existing thrombosis or new thrombosis after IVIg administration in any of the 24 HIT cases reported in the literature (Table 1). Nevertheless, this potential risk should be discussed with patients.
Table 1:
Cases reported in the literature of IVIG for treatment HIT.
| Author and Year |
Age/Sex | Context of Heparin Use |
Platelet Nadir (×109/L) |
Optical Density |
SRA (%) with heparin 0.1 U/mL |
Thrombosis | IVIg Dose | Time to Platelet Response |
|---|---|---|---|---|---|---|---|---|
| Frame et al 1989[12] | 62/F | Deep vein thrombosis of lower extremity and pulmonary embolism | 7.5 | - | - | Yes | 0.4 g/kg/day for 3 doses | Normalization of platelet count by 50 hours |
| Grau et al 1992[13] | 76/F | Prophylaxis during admission for unstable angina and heart failure exacerbation | 35 | - | - | Yes | 0.4 g/kg/day for 5 days | 2 days |
| Prull et al 1992‡[14] | 51/F | Prophylaxis following orthopedic surgery | 33 | - | - | Yes | 5 g/day for 7 consecutive days | 20 hours |
| Winder et al 1998[15] | 46/M | Unstable angina | 3 | 0.58 | - | - | 2 g/kg for 2 days | 1 day |
| 88/F | Prophylaxis following orthopedic surgery | 13 | 1.16 | - | Yes | 1 g/kg for 2 days | 2 days | |
| 74/F | Prophylaxis following orthopedic surgery | 19 | 0.76 | - | - | 2 g/kg for 2 days | 4 days | |
| Betrosian et al 2003‡[14] | 62/F | Orthopedic surgery | 15 | - | - | Yes | 1 g/kg/day for 2 days | 7 days |
| Betrosian et al 2004‡[14] | 60/M | Aortic valve replacement | 10 | - | - | - | 1 g/kg/day for 2 days | 4 days |
| Cho and Liu 2008[16] | 24/F | Pulmonary embolism on postpartum day 3 | 18 | - | - | - | 15 g/day for 3 days | 4 days |
| Gul et al 2011[17] | 69/F | Prophylaxis following orthopedic surgery | 14 | - | - | Yes | 1 g/kg/day for 3 days | Approximately 2 days |
| Warkentin and Shepard 2014[18] | 68/M | Intraoperative heparin for cardiac surgery | 20 | 2.74 | 100 | Yes | 1 g/kg once | 9 days |
| Tvito 2015[14] | 85/F | Prophylaxis following orthopedic surgery | 3 | 2.610 | - | Yes | 1 g/kg for 2 days | Approximately 2 days |
| Azimov 2017[19] | 58/F | Pulmonary embolism | 26 | 2.668 | 93 | - | 1 g/kg followed by 0.45 g/kg once | Approximately 4 days |
| Doucette 2017[20] | 49/F | Thoracic aorta thrombus and thoracic endovascular aortic aneurysm repair | 11 | 2.79 | 95 | Yes | 1 g/kg/day for 2 days (with steroids), then 1 more dose one week later after the platelet count dropped again | Approximately 4 days after the first series of doses, then 3 days after the second administration |
| 84/F | Thoracic endovascular aortic aneurysm repair | 16 | 2.805 | 100 | Yes | 1 g/kg/day for 5 days (with steroids) | Approximately 3 days | |
| Ibrahim and Rice 2017[11] | 78/F | Procedural heparin for hemodialysis | 15 | 1.69 | 82 | Yes | 0.4 g/kg/day for 4 days | 5 days |
| 35/M | Use of heparin for heart transplantation in the setting of acute HIT with OD of 2.1 after plasma exchange | 31, with rapid response to bivalirudin | 2.50 | - | - | 0.4 g/kg/day for 2 days | No unexpected thrombocytopenia or thrombosis with use of heparin during heart transplant | |
| Lei et al 2017[21] | 47/M | Coronary artery bypass grafting and prophylaxis | 8 | 2.722 | 100 | Yes | 1 g/kg for 2 days | Approximately 2 days |
| Padmanabhan et al 2017*[8] | 73/M | Coronary artery bypass grafting and prophylaxis | 25 | 2.30 | 100 | Yes | 1 g/kg for 2 days | Approximately 1 day |
| 72/M | Cardiac catheterization and coronary artery bypass grafting | 16 | 2.60 | 100 | Yes | 1 g/kg for 2 days, then 0.5 g/kg once 3 days later after platelets dropped from 124 to 111 109/L | >50% increase in platelet count within 24 hours after each 1g/kg dose | |
| McKenzie et al 2018[22] | 48/M | Peripheral stem cell harvesting | 14 | 2.824 | 61 | Yes | 0.5 g/kg for 5 days | 5 days |
| Warkentin et al 2018[11] | 59/M | Use of heparin for femoral artery vascularization in the setting of acute HIT with OD of 2.66 | 34 | 2.66 | 100 | Yes | 90 g before and during surgery | No serious thrombocytopenia or thrombosis with the use of heparin during revascularization† |
| Park et al 2018[current] | 82/M | Right ventricle thrombus | 19 | 3.559 | - | Yes | 1 g/kg once | 5 days |
| 38/M | Prophylaxis following aspiration pneumonia | 18 | 2.77 | - | Yes | 2 g/kg/day for 2 total doses | 2 days |
= Padmanabhan et al 2017 included one additional case that was reported in Lei et al 2017
= data extracted from Tvito 2015; original articles either in a different language or unable to be located
= there was a mild reduction in platelet fount from 130×109/L to 112 ×109/L, which was attributed to volume resuscitation with 3 L of fluid perioperatively
“Time to platelet response” = time until greater than two-fold increase from platelet nadir
Blank spaces = not reported
g/kg = grams per kilogram; IVIg = intravenous immunoglobulin; SRA = serotonin release assay
These two cases add to the growing number of case reports that demonstrates the efficacy of IVIg in persisting HIT (Table 1). In addition, Warkentin et al[10] and Ibrahim et al[11] have reported the use of IVIg as a novel treatment approach in patients requiring urgent surgery with intra-operative heparin in the setting of residual platelet-activating antibodies. Warkentin et al recently described a patient who had developed HIT after heparin exposure for symptomatic atherothrombosis of both superficial femoral arteries and revascularization surgery had been postponed while awaiting the disappearance of HIT antibodies. However, the patient developed progressive distal lower limb ischemia and necrosis that required urgent limb salvage and revascularization surgery. The patient’s serum demonstrated residual HIT activity with strongly positive SRA. Therefore, the patient was given high dose IVIg before and during the surgery to block the functional activity of HIT antibody. This was confirmed in vitro by negative SRA after testing the patient’s pre-operative serum obtained immediately after IVIg administrations in the setting of persistently positive PF4/heparin complex antigen testing. Thereafter, the surgery was successfully performed using UFH without thrombotic complications. The authors noted a mild reduction in the platelet count following surgery from 130×109/L to 112×109/L; however, this was attributed to significant perioperative volume resuscitation with 3 liters of fluid and no additional decrease was noted.
A recent survey of more than 400 patients with HIT demonstrates that morbidity and mortality of HIT is high even in patients who are treated optimally with alternative anticoagulants[6]. The rapid and sustained responses of patients with persisting HIT treated with IVIg described in this and other recent reports suggest that IVIg could provide an adjunctive therapy for patients with both “persisting” and “conventional” HIT requiring use of UFH during surgery.
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Addendum: A. Padmanabhan designed and performed the in-vitro experiments. All authors contributed toward writing and final review of the manuscript.
References:
- 1.Lee GM, Arepally GM. Heparin-induced thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2013;2013:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salter BS, Weiner MM, Trinh MA, Heller J, Evans AS, Adams DH, et al. Heparin-Induced Thrombocytopenia: A Comprehensive Clinical Review. J Am Coll Cardiol. 2016;67(21):2519–32. [DOI] [PubMed] [Google Scholar]
- 3.Padmanabhan A, Jones CG, Bougie DW, Curtis BR, McFarland JG, Wang D, et al. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood. 2015;125(1):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Gates C, Neave L. How we manage patients with heparin induced thrombocytopenia. Br J Haematol. 2016;174(1):9–15. [DOI] [PubMed] [Google Scholar]
- 5.Dhakal B, Kreuziger LB, Rein L, Kleman A, Fraser R, Aster RH, et al. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: a population-based study. Lancet Haematol. 2018;5(5):e220–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuter DJ, Konkle BA, Hamza TH, Uhl L, Assmann SF, Kiss JE, et al. Clinical outcomes in a cohort of patients with heparin-induced thrombocytopenia. Am J Hematol. 2017;92(8):730–8. [DOI] [PubMed] [Google Scholar]
- 7.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–114. [DOI] [PubMed] [Google Scholar]
- 8.Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, et al. IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest. 2017;152(3):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammann EM, Haskins CB, Fillman KM, Ritter RL, Gu X, Winiecki SK, et al. Intravenous immune globulin and thromboembolic adverse events: A systematic review and meta-analysis of RCTs. Am J Hematol. 2016;91(6):594–605. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE, Climans TH, Morin PA. Intravenous Immune Globulin to Prevent Heparin-Induced Thrombocytopenia. N Engl J Med. 2018;378(19):1845–8. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim IF, Rice L. Intravenous Immunoglobulin for Heparin-Induced Thrombocytopenia. Chest. 2017;152(4):906–7. [DOI] [PubMed] [Google Scholar]
- 12.Frame JN, Mulvey KP, Phares JC, Anderson MJ. Correction of severe heparin-associated thrombocytopenia with intravenous immunoglobulin. Ann Intern Med. 1989;111(11):946–7. [DOI] [PubMed] [Google Scholar]
- 13.Grau E, Linares M, Olaso MA, Ruvira J, Sanchis J. Heparin-induced thrombocytopenia--response to intravenous immunoglobulin in vivo and in vitro. Am J Hematol. 1992;39(4):312–3. [DOI] [PubMed] [Google Scholar]
- 14.Tvito A, Bakchoul T, Rowe JM, Greinacher A, Ganzel C. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol. 2015;90(7):675–8. [DOI] [PubMed] [Google Scholar]
- 15.Winder A, Shoenfeld Y, Hochman R, Keren G, Levy Y, Eldor A. High-dose intravenous gamma-globulins for heparin-induced thrombocytopenia: a prompt response. J Clin Immunol. 1998;18(5):330–4. [DOI] [PubMed] [Google Scholar]
- 16.Cho FN, Liu CB. Potential role of intravenous immunoglobulin in the management of peripartum maternal thrombocytopenia due to various causes. J Chin Med Assoc. 2008;71(5):267–9. [DOI] [PubMed] [Google Scholar]
- 17.Gul EE, Abdulhalikov T, Aslan R, Aydogdu I. A rare and undesirable complication of heparin-induced thrombocytopenia: acute massive pulmonary embolism. Clin Appl Thromb Hemost. 2011;17(5):546–8. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin TE, Sheppard JA. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are reexposed to heparin. Blood. 2014;123(16):2485–93. [DOI] [PubMed] [Google Scholar]
- 19.Azimov MB, Slater ED. Persistent Heparin-Induced Thrombocytopenia Treated With IVIg. Chest. 2017;152(3):679–80. [DOI] [PubMed] [Google Scholar]
- 20.Doucette K DC, Jain NA, Cruz AL, Malkovska V, Fitzpatrick K. Treatment of refractory delayed onset heparin-induced thrombocytopenia after thoracic endovascular aortic repair with intravenous immunoglobulin (IVIG). Res Pract Thromb Haemost. 2017;1:134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei BZ, Shatzel JJ, Sendowski M. Rapid and durable response to intravenous immunoglobulin in delayed heparin-induced thrombocytopenia: a case report. Transfusion. 2017;57(4):919–23. [DOI] [PubMed] [Google Scholar]
- 22.McKenzie DS, Anuforo J, Morgan J, Neculiseanu E. Successful Use of Intravenous Immunoglobulin G to Treat Refractory Heparin-Induced Thrombocytopenia With Thrombosis Complicating Peripheral Blood Stem Cell Harvest. J Investig Med High Impact Case Rep. 2018;6:2324709618755414. [DOI] [PMC free article] [PubMed] [Google Scholar]