Ternary oxides, especially BaTiO3 and SrTiO3, have attracted long-standing interest in nanostructure syntheses because of their extraordinary ferroelectric, dielectric, pyroelectric, piezoelectric, and electro-optic properties. As such, they can be widely used in dynamic random access memories, capacitors, electromechanics, and nonlinear optics.[1–6] Miniaturization of ferroelectric BaTiO3 crystals to the nanometer scale is desirable for their application in the next generation of electronics;[7–10] however, the main challenge lies in the growth of barium titanate nanocrystals at room temperature in a tetragonal crystalline structure, which induces the ferroelectric property. In the past, BaTiO3 nanoparticles have been grown in the cubic structure, the most stable form at room temperature, and annealing of the nascent BaTiO3 nanoparticles at high temperature has been necessary to transform their structure from the cubic to the tetragonal form.[3,5,11–14] Here, a novel method that uses ring-shaped peptide assemblies as templates for the one-step biomimetic synthesis of ferroelectric BaTiO3 nanoparticles in a tetragonal structure at room temperature is reported. Biological and biomimetic systems produce remarkable structures of crystals controlled by the chemical structure, the morphology, and the shape of the biological templates.[15–26] For example, sea urchin larvae grow single-crystalline calcite in a curved compartment,[23] and our approach mimics those systems by growing the unusual structures of the BaTiO3 crystals on curved and confined peptide assemblies. To the best of the authors’ knowledge, this is the first report on the growth of ferroelectric BaTiO3 nanoparticles at room temperature.
Peptide templates are used to hydrolyze BaTi(O2CC7-H15)[OCH(CH3)2]5 inside the cavities in order to produce the tetragonal BaTiO3 nanoparticles at room temperature. Previously, it has been found that ring-shaped peptide assemblies could template Au-nanoparticle formation inside the peptide rings.[27] While the size of the Au nanoparticles can be controlled by the cavity size, they grow with or without the peptide nanorings. However, the new discovery reported here is that the peptide nanorings have the biomimetic function of crystallizing nanometer-scale crystals that have never been grown under ambient conditions. In other words, tetragonal BaTiO3 nanoparticles can only grow in the peptide nanorings at room temperature. When the peptide monomer solutions are added to a precursor, BaTi(O2CC7H15)[OCH(CH3)2]5,[1] ring-shaped self-assemblies appear after 1–4 days in the dark. During this process, the peptide nanoring templates are self-assembled simultaneously as the precursors are hydrolyzed in the cavities (Fig. 1a). The peptide nanoring structure has previously been investigated.[27] Figure 1b shows the phase image of BaTiO3 nanoparticles inside the peptide nanorings in a pH 4.5 solution determined by atomic force microscopy (AFM). In this AFM phase image, the harder barium titanate nanoparticles appear in a brighter contrast at the centers of the peptide nanorings, which appear as softer assemblies in a darker contrast. The peptide nanorings are monodisperse with a size of 49 ± 11 nm, as determined by AFM and transmission electron microscopy (TEM). This phase contrast is consistent with that of Au nanocrystals grown inside the peptide nanorings imaged previously.[27] The magnified TEM image of the barium titanate nanoparticle in the peptide nanoring, Figure 1c, also clearly shows a darker BaTiO3 particle grown inside the peptide template. The Raman spectra of the ring/BaTiO3 complexes shown in Figure 2 reveal the presence of Ti–carboxylate ligation. For example, peaks at 1582, 1362, and 1060 cm−1, marked in Figure 2, correspond to the v(C–O) carbonate bands in the oxalate-bridged metal complex, COO–Ti–OOC.[28] The peak at 767 cm−1 represents the Ti–O–Ti stretching mode. This chelate structure is consistent with the one between the barium titanate precursors and the hydroxylated block-copolymer surfaces.[29]
Figure 1.
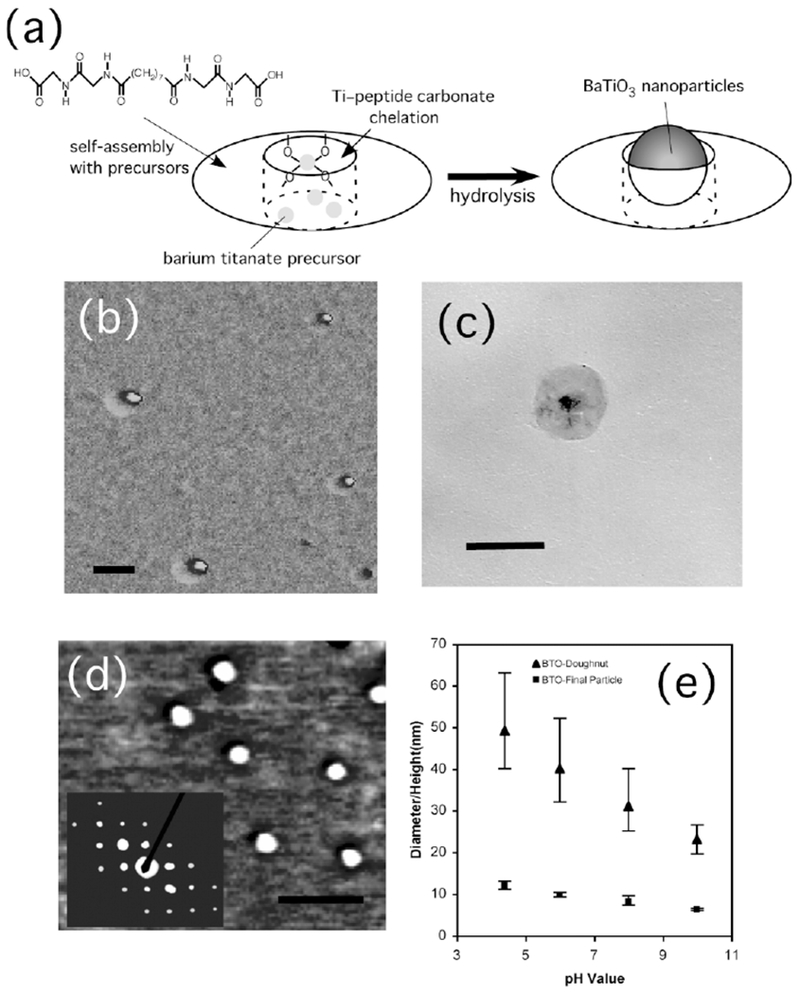
a) Illustration of the nanoring structure. Objects in this figure are not to scale and not all of the precursors that self-assemble with the peptide to form the nanoring are shown. b) Atomic force microscopy (AFM) phase image of barium titanate nanoparticles inside peptide nanoring templates. Scale bar = 50 nm. c) TEM image of a barium titanate nanoparticle inside the peptide nanoring template. Scale bar = 60 nm. d) AFM phase image of barium titanate nanoparticles after removal of peptide nanoring templates. Scale bar = 60 nm. Inset shows an electron diffraction pattern of the barium titanate nanoparticles with the (110), (111), (210), and (211) faces. e) Size distributions of peptide nanorings (▴) and barium titanate nanoparticles (■) as a function of pH of the growth solution.
Figure 2.
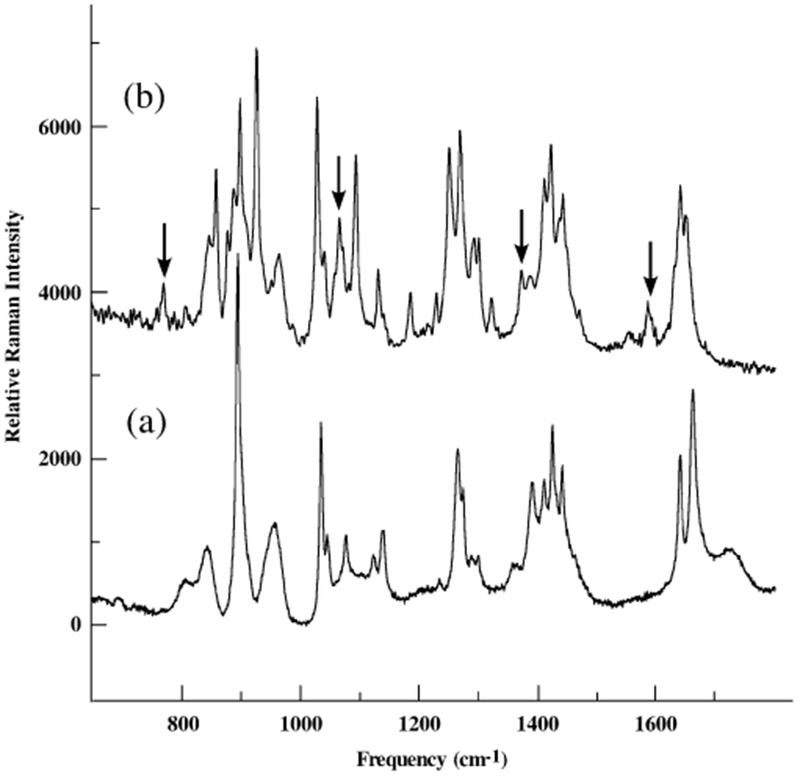
Raman spectra of a) nanotubes self-assembled from the peptide monomers without barium titanate salts, and b) nanorings self-assembled from the peptide monomers in the presence of barium titanate salts.
When the peptide nanorings with the BaTiO3 nanoparticles are irradiated by UV light (355 nm) for 10 h in solution, the peptide template shells are removed, as shown in Figure 1d. Previously, Au nanoparticles have also been grown in peptide nanorings self-assembled from peptide monomers and Au ions, and long UV irradiation destroyed the ring templates due to reduction of the Au ions that chelated the carboxylates of the peptide monomers to form the nanoring structure.[27] It is likely that a similar reduction mechanism destroys the nanoring templates of the BaTiO3 nanoparticles because the UV irradiation renders the Ti ions ineffective so they can no longer function as a glue to sustain the ring structure via the Ti-ion/peptide chelation. The AFM image in Figure 1d shows monodisperse BaTiO3 nanoparticles with a diameter of 12 ± 1 nm after UV irradiation of the ring/particle complexes for 10 h. The contrast of the nanoparticles in the AFM phase image in Figure 1d is consistent with that in the centers of the nanorings in Figure 1b, which suggests that the nanoparticles are released from the nanorings. The electron diffraction pattern of the resulting BaTiO3 nanoparticles in the inset of Figure 1d indicates that they are highly crystalline. The outer and inner diameters of the peptide nanorings are observed to change as a function of pH of the growth solution, as shown in Figure 1e. This plot shows that the size of the nanoring decreases from 49 to 23 nm as the pH is increased from 4.5 to 10. Figure 1e also shows that the decrease of the peptide nanoring cavity size directly influences the size of the BaTiO3 nanoparticles grown inside the cavities. Under all growth conditions, their sizes are observed to be very monodisperse, and their sizes can be changed from 12 to 6 nm, as shown in Figure 1e.
The structure of the BaTiO3 nanoparticles has been studied by X-ray diffraction as shown in Figure 3. In this figure the (100), (110), (111), (200), (210), and (211) faces of BaTiO3 are observed. The magnified X-ray diffraction spectrum in the inset of Figure 3 resolves the characteristic (002) and (200) faces of the tetragonal BaTiO3 crystals.[30] To test the nanometer-scale ferroelectric property of the tetragonal BaTiO3 nanoparticles, electrostatic force microscopy (EFM) was used to image and manipulate the ferroelectric polarization of these nanoparticles. This procedure is summarized in Figure 4a. In the first step, the electric polarization of the BaTiO3 nanoparticles is manipulated by applying a voltage, Vwrite, to the conductive AFM tip that gently contacts the nanoparticles.[3] After the local electric polarization is written onto the nanoparticles, the resulting polarization is probed using EFM with a lower voltage, Vprobe, by measuring the shift in the resonance frequency of the AFM tip.[8] As shown in Figure 4a, during the probing process the AFM tip is raised at a constant height above the nanoparticles in order to avoid interference between the manipulated polarization and Vprobe.[31] The raised distance of 40 nm enables one to image only the contribution from the surface charges associated with the local electric polarization of the BaTiO3 nanoparticles. After a Vwrite of +12 V was applied to BaTiO3 nanoparticles with an average diameter of 12 nm (Fig. 4b), the EFM image of those nanoparticles to which a Vprobe of +2 V was applied, shown in Figure 4c, appears in a brighter contrast compared with the background due to the repulsive electrostatic interaction between the tip and the nanoparticles.[8] After a Vwrite of −12 V was applied to the same BaTiO3 nanoparticles, the EFM image of those nanoparticles to which a Vprobe of 2 V was applied, shown in Figure 4d, appears in a darker contrast compared with the background due to the attractive electrostatic interaction. It should be noted that control experiments that involved scanning the manipulated nanoparticles with Vprobe = −2 V resulted in reverse EFM images, which confirms that the probe voltage did not interfere significantly with the written polarization. These EFM images indicate that the BaTiO3 nanoparticles synthesized in the peptide nanorings at room temperature possess a ferroelectric property by reorienting the spontaneous electric polarization with an external electric field.
Figure 3.
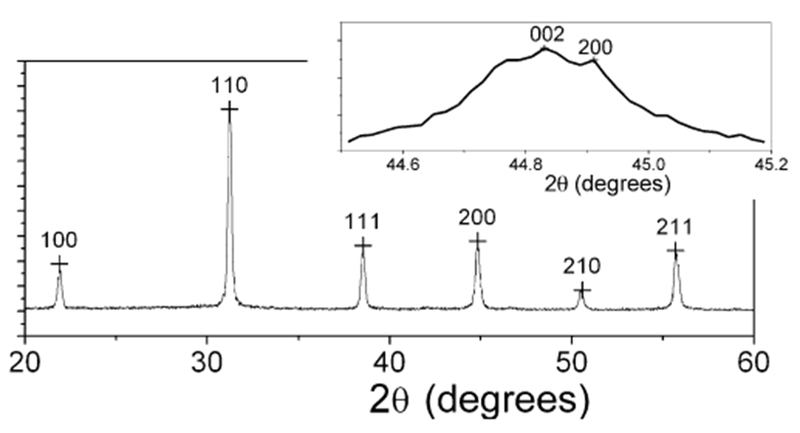
X-ray diffraction pattern of barium titanate nanoparticles synthesized in peptide nanorings at room temperature. The inset shows the magnified spectrum
Figure 4.
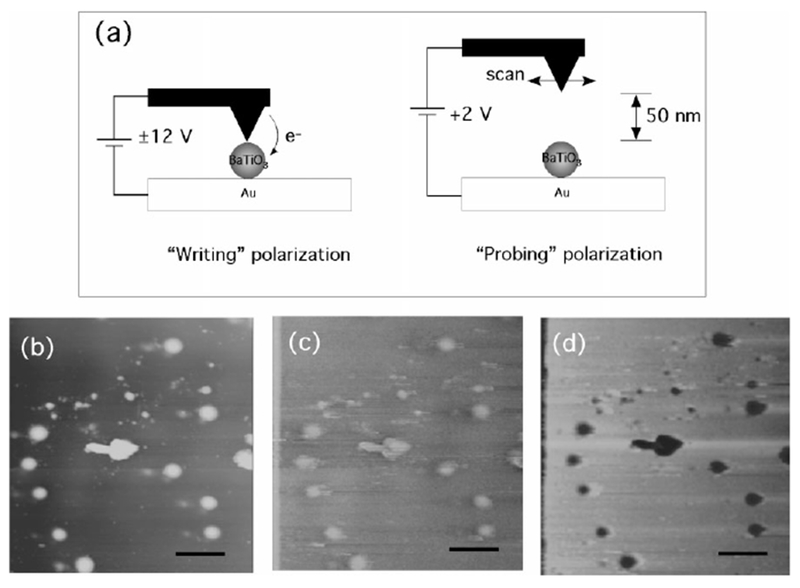
a) Schematic representation of manipulating and probing the electric polarization of BaTiO3 nanoparticles with EFM. b) Topological AFM image of barium titanate nanoparticles; scale bar = 30 nm. c) EFM image of barium titanate nanoparticles with Vprobe = +2 V after Vwrite = +12 V was applied on the nanoparticles across a conductive AFM tip and a gold substrate; scale bar = 30 nm. d) EFM image of barium titanate nanoparticles with Vprobe = +2 V after Vwrite = −12 V was applied on the nanoparticles across a conductive AFM tip and a gold substrate; scale bar = 30 nm.
Previously, when mineralization of calcite has occurred in micrometer-scale pores of membranes, the resulting crystals in the pores have been observed to have unusual crystalline forms and structures due to unusual surface tensions and reaction kinetics of the crystal growth in such small confined regions.[23] The shapes of crystallizing templates in organisms are also observed to have a significant impact on the resulting crystalline structures grown on biological templates.[24,25] The nanometer-scale peptide rings used here as the confining templates for the crystal growth of BaTiO3 took this strategy of the confinement-controlled mineralization one step further to the nanometer scale. As observed by the crystal growth in the membranes, the confinement effect of the peptide nanorings induced the unusual crystallization of tetragonal nanoparticles of BaTiO3. Since the arrangement and the distribution of self- assembled monolayers (SAMs) of nucleating functional groups have previously been observed to influence the resulting crystalline structures,[32] the chemical structure, such as the location of carboxylic acid groups that chelate Ti ions on the curved surfaces of the peptide nanorings, should also be a critical factor in growing tetragonal BaTiO3 nanoparticles without annealing. The exact chemical structure of the peptide assemblies is still under investigation.
In conclusion, ferroelectric BaTiO3 nanoparticles are hydrolyzed inside peptide-ring templates at room temperature and under ambient pressure, and these nanoparticles have a tetragonal crystalline structure. Because the cavity sizes of the nanorings change as a function of pH, the diameters of the monodisperse BaTiO3 nanoparticles could be controlled between 6 and 12 nm. The resulting BaTiO3 nanoparticles are also demonstrated to possess a switching behavior by using external electric fields to reorient their spontaneous electric polarization as the direction of the electric fields is switched. This unusual crystallization of tetragonal BaTiO3 nanoparticles at room temperature is likely to be induced by the surface chemical structure of the peptide templates and the high surface tension in the nanometer-scale peptide cavities. In general, various important electric materials are synthesized at high temperature; however, if these syntheses can be conducted under milder experimental conditions, such as room temperature, it can reduce the production cost, and the facilities (such as cooling systems) and the manpower needed, which will have a huge impact on manufacturing. High-temperature processing could also lead to nanoparticle aggregation and defects due to local thermal stresses. Ring-shaped peptides enable the synthesis of crystals at low temperatures, as seen here for the case of tetragonal BaTiO3 nanoparticles, and this approach will provide a new route for novel material syntheses.
Experimental
Bis(N-α-amido-glycylglycine)heptane-1,7-dicarboxylate and bolaamphiphile peptide were used as monomers to self-assemble nanorings. The chemical synthesis and the characterization of the peptide monomers have been reported previously [33,34]. When the peptide monomer solutions (10 mL) in pH 4.5–10 citric acid/NaOH (10 ×10−3 M) were added to 20 μL of BaTi(O2CC7H15)[OCH(CH3)2]5 (13% in isopropyl alcohol, Alfa Aesar) [1], ring-shaped self-assemblies appeared after 1–4 days in the dark. This solution containing the nanoring-BaTiO3 complexes was washed with deionized water and centrifuged at 14.5 krpm. After this process was repeated twice, the solution was then irradiated by long-wave UV light (355 nm) for 10 h to remove the peptide templates. The final product was then washed and centrifuged to purify the tetragonal BaTiO3 nanocrystals. The nanorings and the extracted BaTiO3 nanoparticles were imaged by AFM (Nanoscope III, Vecco, Inc.) on freshly cleaved mica surfaces. These samples were also dried on carbon-coated copper grids at room temperature and studied by TEM and electron diffraction (JOEL 1200 EX) at an acceleration voltage of 100 kV. X-ray diffraction was measured using a Philips PW3040. A confocal Raman microscope (LabRam, Jobin Yvon/Horiba) was used to obtain two-dimensional Raman images. The 632.8 nm line of an air-cooled He/Ne laser was injected into an integrated Olympus BX 40 microscope and focused to a spot size of approximately 0.7 μm by an 80× long-working-distance objective. EFM images of the BaTiO3 nanoparticles were recorded by the same atomic force microscope with a NanoScope Extender (Vecco, Inc) on Au substrates.
Footnotes
This work was supported by the U.S. Department of Energy (DE-FG-02-01ER45935) and the National Science Foundation CARRER Award (ECS-0103430). Hunter College infrastructure is supported by the National Institutes of Health and the RCMI program (G12-RR-03037).
Contributor Information
Nurxat Nuraje, Department of Chemistry, City University of New York, Hunter College, New York, NY 10021 (USA).
Kai Su, Department of Chemistry, City University of New York, College of Staten Island, Staten Island, NY 10314 (USA).
Amit Haboosheh, Department of Chemistry, City University of New York, Hunter College, New York, NY 10021 (USA).
Jacopo Samson, Department of Chemistry, City University of New York, Hunter College, New York, NY 10021 (USA).
Edward P. Manning, Department of Physics, City University of New York, Hunter College, New York, NY 10021 (USA)
Prof. Nan-loh Yang, Department of Chemistry, City University of New York, College of Staten Island, Staten Island, NY 10314 (USA).
Prof. Hiroshi Matsui, Department of Chemistry, City University of New York, Hunter College, New York, NY 10021 (USA).
References
- [1].O’Brien S, Brus L, Murray CB, J. Am. Chem. Soc. 2001, 123, 12085. [DOI] [PubMed] [Google Scholar]
- [2].Urban JJ, Yun WS, Gu Q, Park H, J. Am. Chem. Soc 2002,124, 1186. [DOI] [PubMed] [Google Scholar]
- [3].Yun WS, Urban JJ, Gu Q, Park H, Nano Lett. 2002,2, 447. [Google Scholar]
- [4].Ahn CH, Rabe KM, Triscone JM, Science 2004,303, 488. [DOI] [PubMed] [Google Scholar]
- [5].Fong DD, Stephenson GB, Streiffer SK, Eastman JA, Auciello O, Fuoss PH, Thompson C, Science 2004,304,1650. [DOI] [PubMed] [Google Scholar]
- [6].Choi KJ, Biegalski M, Li YL, Sharan A, Schubert J, Uecker R, Reiche P, Chen YB, Pan XQ, Gopalan V, Chen LQ, Schlom DG, Eom CB, Science 2004,306,1005. [DOI] [PubMed] [Google Scholar]
- [7].Shaw TM, Trolier-McKinstry S, McIntyre PC, Ann. Rev. Mater. Sci. 2000,30, 263. [Google Scholar]
- [8].Urban JJ, Spanier JE, Lian OY, Yun WS, Park H, Adv. Mater. 2003,15, 423. [Google Scholar]
- [9].Fu HX, Bellaiche L, Phys. Rev. Lett. 2003, 91, 257 601. [DOI] [PubMed] [Google Scholar]
- [10].Naumov II, Bellaiche L, Fu HX, Nature 2004, 432, 737. [DOI] [PubMed] [Google Scholar]
- [11].Clark IJ, Takeuchi T, Ohtori N, Sinclair DC, J. Mater. Chem 1999, 9, 83. [Google Scholar]
- [12].Niederberger M, Garnweitner G, Pinna N, Antonietti M, J. Am. Chem. Soc 2004,126, 9120. [DOI] [PubMed] [Google Scholar]
- [13].Mao YB, Banerjee S, Wong SS, J. Am. Chem. Soc 2003, 125, 15718. [DOI] [PubMed] [Google Scholar]
- [14].Luo Y, Szafraniak I, Zakharov ND, Nagarajan V, Steinhart M, Wehrspohn RB, Wendorff JH, Ramesh R, Alexe M, Appl. Phys. Lett. 2003,83, 440. [Google Scholar]
- [15].Cha NJ, Stucky GD, Morse DE, Deming TJ, Nature 2000, 403, 289. [DOI] [PubMed] [Google Scholar]
- [16].Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO, Nat. Mater 2002,1, 169. [DOI] [PubMed] [Google Scholar]
- [17].Sarikaya M, Tamerler C, Jen AKY, Schulten K, Nat. Mater. 2003,2, 577. [DOI] [PubMed] [Google Scholar]
- [18].Whitling JM, Spreitzer G, Wright DW, Adv. Mater. 2000, 12, 1377. [Google Scholar]
- [19].Reches M, Gazit E, Science 2003,300, 625. [DOI] [PubMed] [Google Scholar]
- [20].Bansal V, Sanyal A, Rautaray D, Ahmad A, Sastry M, Adv. Mater. 2005,17, 889. [DOI] [PubMed] [Google Scholar]
- [21].Walsh D, Arcelli L, Ikoma T, Tanaka J, Mann S, Nat. Mater. 2003, 2, 386. [DOI] [PubMed] [Google Scholar]
- [22].Douglas T, Young M, Adv. Mater. 1999,11, 679. [Google Scholar]
- [23].Loste E, Park RJ, Warren J, Meldrum FC, Adv. Funct. Mater 2004,14, 1211. [Google Scholar]
- [24].Aizenberg J, Muller DA, Grazul JL, Hamann DR, Science 2003, 299, 1205. [DOI] [PubMed] [Google Scholar]
- [25].Yang S, Chen G, Megens M, Ullal CK, Han YJ, Rapaport R, Thomas EL, Aizenberg J, Adv. Mater. 2005,17, 435. [Google Scholar]
- [26].Banerjee IA, Yu L, Matsui H, Proc. Natl. Acad. Sci. USA 2003, 100,14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Djalali R, Samson J, Matsui H, J. Am. Chem. Soc. 2004,126, 7935. [DOI] [PubMed] [Google Scholar]
- [28].Dutta PK, Gallagher PK, Twu J, Chem. Mater. 1993,5,1739. [Google Scholar]
- [29].Lee T, Yao N, Imai H, Aksay IA, Langmuir 2001,17, 7656. [Google Scholar]
- [30].Takeuchi T, Tabuchi M, Ado K, Honjo K, Nakamura O, Kageyama H, Suyama Y, Ohtori N, Nagasawa M, J. Mater. Sci. 1997, 32, 4053. [Google Scholar]
- [31].Heim T, Lmimouni K, Vuillaume D, Nano Lett. 2004, 4, 2145. [Google Scholar]
- [32].Aizenberg J, Adv. Mater. 2005, 16, 1295. [Google Scholar]
- [33].Matsui H, Gologan B, J. Phys. Chem. B 2000, 104, 3383. [Google Scholar]
- [34].Kogiso M, Ohnishi S, Yase K, Masuda M, Shimizu T, Langmuir 1998,14, 4978. [Google Scholar]


