Abstract
Virus-like particles (VLPs) derived from the bacteriophage P22 offer an interesting and malleable platform for encapsulation and multivalent presentation of cargo molecules. The packaging of cargo in P22 VLP is typically achieved through genetically enabled directed in vivo encapsulation. However, this approach does not allow control over the packing density and composition of the encapsulated cargos. Here, we have adopted an in vitro assembly approach to gain control over cargo packaging in P22. The packaging was controlled by closely regulating the stoichiometric ratio of cargo-fused-scaffold protein and wild-type scaffold protein during the in vitro assembly. In a “one-pot assembly reaction” coat protein subunits were incubated with varied ratios of wild-type scaffold protein and cargo-fused-scaffold protein, which resulted in the encapsulation of both components in a co-assembled capsid. These experiments demonstrate that an input stoichiometry can be used to achieve controlled packaging of multiple cargos within the VLP. The porous nature of P22 allows the escape and re-entry of wild-type scaffold protein from the assembled capsid but scaffold protein fused to a protein-cargo cannot traverse the capsid shell due to the size of the cargo. This has allowed us to control and alter the packing density by selectively releasing wild-type scaffold protein from the co-assembled capsids. We have demonstrated these concepts in the P22 system using an encapsulated streptavidin protein and have shown its highly selective interaction with biotin or biotin derivatives. Additionally, this system can be used to encapsulate small molecules coupled to biotin, or display large proteins, that cannot enter the capsid and thus remain available for the multivalent display on the exterior of the capsid when attached to a flexible biotinylated linker. Thus, we have developed a P22 system with controlled protein cargo composition and packing density, to which both small and large molecules can be attached at high copy number on the interior or exterior of the capsid.
Introduction
Nature has inspired us with the intriguing and sophisticated design of functional micro- and nano-containers formed from either lipids or proteins to accomplish specialized tasks, including catalysis and genome housing.1–6 Amongst naturally occurring containers, protein based containers are very interesting because they spontaneously self-assemble into highly symmetric and homogeneous architectures from a defined set of identical subunits in a non-native host cell.7 Many protein cages possess structural and functional plasticity towards synthetic (genetic and chemical) modifications, allowing the integration of new functionalities without altering cage architecture.8 In addition, accessibility to the three-dimensional structural information at the atomic level, enables precise modification and insertion of amino acid residues within protein cage. Because of these desirable and well-defined features, protein cages, including ferritin, encapsulin, lumazine synthase, and a range of virus-like particles (VLPs) have been shown to be versatile platforms for the encapsulation of wide range of synthetic and gene product cargos, e.g. enzymes, fluorescent proteins, polymers, inorganic molecules, and immunogenic proteins. Encapsulation of cargo molecules have been performed both in vivo and in vitro,5, 9–34 and a variety of approaches have been adopted to direct encapsulation in protein cages, including physical entrapment,5 non-covalent affinity tags, e.g. coiled-coil motifs15, charged peptide tags and nucleic acid based linkers18,22,35,36, SpyTag/SpyCatcher37, and genetic fusions38,39.
We have previously shown encapsulation of a wide variety of proteins within VLPs derived from the P22 bacteriophage. The P22 VLP assembles from 420 copies of a 47 kDa coat protein (CP) to form a T=7, 58 nm nanoparticle known as the procapsid (PC).40 The VLP assembly requires 100–300 copies of a scaffolding protein (SP), which templates assembly of the CP into the P22 PC through a helix-turn-helix domain at its C-terminus.41 In the process of P22 assembly the SP is encapsulated on the interior of the resulting P22 PC.40 Mild treatment of the PC with a chaotrope disrupts the non-covalent interactions between the CP and SP, and the SP can freely diffuse out of the PC form to generate an empty shell (ES).42 The ES can be ‘restuffed’ by incubation with free SP, which can enter the capsid and rebind to the CP to re-form the PC.42 The SP can be extensively truncated to a minimal assembly domain and be modified at the N- or C-terminus without affecting its ability to template capsid assembly.32,38,43 This truncated scaffold, fused to a protein cargo, has been used to direct the assembly and encapsulation of a number of different proteins in an in vivo directed-encapsulation approach. Unlike the SP, the SP-cargo fusion cannot leave the PC when the cargo is larger than the pores (2.5 nm diameter44) in the capsid shell.38,45 Although, this approach has been very effective in protein encapsulation it suffers from some limitations. It does not allow control over packaging stoichiometry of multiple cargos, which can only be achieved by catenating proteins to each other and the SP.27 Reduced catalytic efficiency of encapsulated enzymes in comparison to free enzyme has been observed in a number of cases, which could be due to factors such as molecular crowding, inhibited substrate-product diffusion, and spatial constraints on enzyme conformation during catalysis,14,18,22,28,45,46 as this approach leads to dense packing of the cargo inside the capsid.27,38,45 But there is also the possibility that encapsulation of some inactive, perhaps misfolded, proteins can occur during in vivo assembly of P22 with no clear way to establish what fraction of the encapsulated proteins are inactive.
To overcome some of these limitations and challenges an in vitro assembly approach was adopted to control packaging and packing density of cargo in P22 in the present work. Significant work has been done to establish and study the in vitro assembly of P22 from individually purified CP and SP subunits.47,48 When these components are mixed together in the presence of low concentration of chaotrope and subsequently dialyzed back into buffer, procapsid-like P22 particles are formed.47 Here we have used this approach to control the packaging amount and packing density of a cargo by co-templating the P22 assembly using in vitro assembly conditions with both wild-type scaffold (wtSP) and cargo-fused scaffold protein together, and later by removing wtSP from the resulting co-assembled capsids.
We have demonstrated the concepts of controlled cargo stoichiometry and packing density system through the selective encapsulation of the tetrameric streptavidin (StAv) protein on the interior of the P22 capsid (P22-StAv). The encapsulation of StAv generates sites for the non-covalent binding of biotin tagged molecules on the interior and exterior of the capsid surface. Through these experiments we demonstrate the in vitro assembly of a streptavidin-fused-scaffold protein (StAv-SP) together with wild-type SP and CP of P22 provides a modular platform that allows: 1) packaging of functionally active cargo, 2) control over the packaging stoichiometry of cargos, 3) control over packing density of the cargo, and 4) exterior modification of the capsid through binding of a flexible biotinylated linker, tethered to big cargo molecule, to StAv on the interior of P22.
Results and discussion
In vivo directed-encapsulation results in functionally inactive P22-StAv particles
Using our previously demonstrated approach for protein encapsulation within P22, the gene that encodes the tetrameric form of Streptavidin (StAv; residues 15–159) was fused to the gene encoding the truncated SP (142–303 amino acids). The gene for a flexible linker (GAAG-ENLYFQS-GAAG) was incorporated between StAv and N-terminus of the truncated SP to ensure conformational flexibility, and to minimize possible steric hindrance at the binding-site of packaged StAv. The StAv-SP and CP were expressed independently in E. coli using a two-vector system bearing different promoters. StAv-SP and CP were produced via a staggered, temporal, expression to allow time for the StAv cargo to mature and achieve native state conformation, before P22 assembly and encapsulation (Figure 1). The expression of StAv-SP was induced with L-arabinose for 30 min followed by the induction of CP expression with isopropyl β-D-1-thiogalactopyranoside (IPTG) for 2 h. The resultant assembled P22-StAv capsids were isolated and purified as mentioned in materials and method section. The characterization of P22-StAv particles by sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), size exclusion chromatography coupled with multi-angle light scattering/quasi-elastic light scattering (SEC-MALS/QELS), and transmission electron microscopy (TEM) confirmed that the StAv was encapsulated within intact P22 VLPs (Figure S1A, S1B, and S1C). However, when the functional activity of the encapsulated StAv was evaluated by titrating the biotin-binding sites with biotin-4-fluorescein (B4F)49, no binding of B4F to the P22-StAv was observed (Figure S1D and S1E). Thus, although the encapsulation of the StAv inside the P22 occurred with high efficiency, the encapsulated StAv protein was somehow inactivated during in vivo directed-encapsulation (Figure 1).
Figure 1.
Expression schematic showing directed-encapsulation of a cargo during in vivo assembly of P22. Two plasmids separately containing StAv-SP and CP, under the control of different promoters, were induced sequentially in E.coli with L-arabinose and IPTG, respectively. The resulting self-assembled P22 particles packaged the StAv (P22-StAv). This methodology, however, produced functionally inactive P22-StAv particles.
We identified three potential reasons for the inactivity of the encapsulated StAv, a) encapsulation of misfolded StAv protein, b) inaccessibility to the binding site due to molecular crowding, and c) pre-saturation of binding site with biotin from E. coli. To address the possibility of encapsulation of a misfolded recombinant StAv, StAv-SP was expressed in presence of two different chaperone systems, GroEL-GroES and DnaK-DnaJ-GrpE, prior to the induction of CP expression for P22 assembly (Figure S2). Previously, we have observed an improvement in the activity of an enzyme that was expressed in presence of these two chaperone systems before its encapsulation and assembly in P22 (data unpublished). However, despite similar number of encapsulated StAv copies, no improvement in binding activity of StAv was observed with P22 materials expressed in the presence of either of these chaperone systems. When the StAv-SP was produced independently, in the absence of CP, the majority of the overexpressed protein was found to traffic to insoluble inclusion bodies. These results suggested that P22 VLPs may be encapsulating a misfolded form of StAv and that the presence of chaperones was not effective in assisting the native protein folding.
in vitro assembled P22-StAv particles are active and bind Biotin-4-fluorescein (B4F)
Overexpressed StAv-SP, isolated as insoluble protein, was refolded to its native conformation and subsequently used for in vitro assembly and encapsulation within P22 (Figure 2). The StAv-SP, with a poly-histidine (6xhis) tag at N-terminus, was expressed independently in E. coli. The overexpressed protein was found mainly in inclusion bodies and collected as a pellet in the cell debris after centrifugation of lysed cells. The protein was recovered from inclusion bodies after treatment with 6 M guanidine hydrochloride (GuHCl) and refolded on a Ni-affinity column (Figure S3A). The refolded StAv-SP protein was characterized by SDS-PAGE and mass spectrometry (Figure S3B, S3C), and when the biotin binding activity was assessed it showed a 1:1 binding stoichiometry to B4F (Figure S3D), indicating that all binding sites of StAv were fully functional.
Figure 2.
Schematic of packaging of an active StAv in P22 using in vitro assembly. Expression of StAv-SP in E.coli, yielded an insoluble protein, which was solubilized and refolded into native conformation from 6 M guanidine hydrochloride. CP subunits were obtained from disassembly of empty shells in 3 M guanidine hydrochloride. These two components were mixed together in presence of low concentration of chaotrope (1.5 M GuHCl), and subsequently dialyzed back to buffer, resulting in formation of P22-StAv particles.
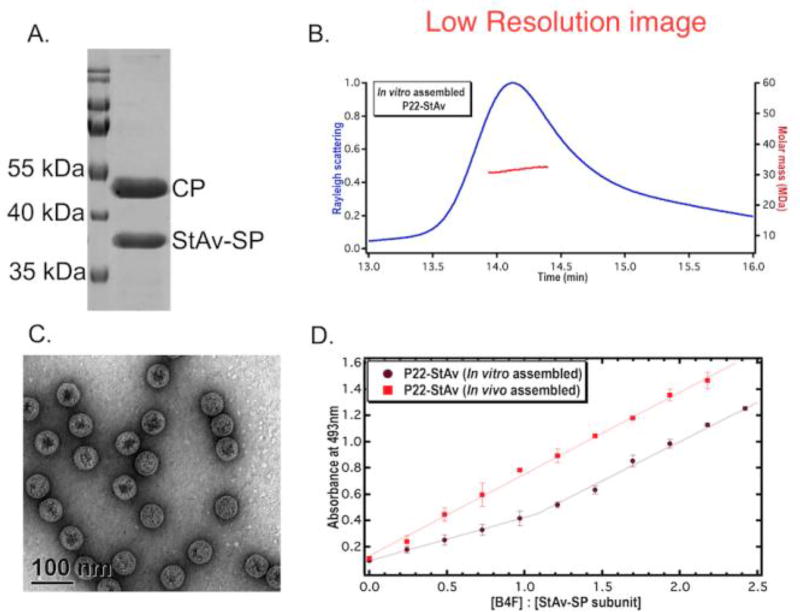
The StAv-SP protein was dialyzed into an assembly buffer (50mM Tris-HCl, 25 mM NaCl, 2mM EDTA, 3mM β-mercaptoethanol, and 1% glycerol) for in vitro assembly. P22 CP subunits were prepared by dissociating the P22 empty shells (ES) in 3M GuHCl for 30 min at room temperature. CP (~2 mg/ml) was mixed with purified StAv-SP in a 1:1 molar ratio. The volume of the StAv-SP was adjusted such that the final concentration of GuHCl was 1.5 M in all assembly reaction mixtures. The mixture was dialyzed into assembly buffer for 12–18 h and resulted in assembly of P22-StAv particles, which were isolated by ultracentrifugation. SDS-PAGE analysis confirmed the encapsulation of StAv-SP in P22 VLPs (Figure 3A) and analysis by size exclusion chromatography coupled to multiangle light scattering (SEC-MALS) indicated an average molecular weight of 31.3 ± 0.2 MDa (Figure 3B). This corresponds to 328 copies of StAv subunits, which is similar to the number of copies encapsulated during in vivo assembly of P22-StAv (Figure S1). The radius of gyration (Rg) of 27.3 ± 0.1 nm and radius of hydration (Rh) of 29.1 ± 0.3 nm of the particles were also similar to the procapsid and in vivo assembled P22-StAv (Figure S1). TEM confirmed the morphology of the P22-StAv particles suggesting that the in vitro assembly recapitulates the anticipated self-assembly of the T=7 P22 VLP (Figure 3C). The average sizes of in vitro and in vivo assembled particles were measured by TEM and found to be 55.0 ± 3.1 nm, 53.0 ± 2.6 nm, respectively (Figure S4). TEM measurement, however, is not the true measure of particle size in solution, so we have reported particle size values measured by light scattering in Figure 2. The B4F binding activity of the in vitro assembled P22-StAv was tested and the encapsulated StAv was found to be fully functional. Therefore in vitro encapsulation did not alter the binding activity (Figure 3D) despite being packaged at similar density to the in vivo assembled material. This suggests that crowding is not the cause of the observed StAv inactivity within the in vivo assembled P22-StAv. The binding activity of P22-StAv was also verified with a non-cumulative titration, where P22-StAv was incubated with varied amounts of B4F, separately. The unbound B4F was removed by extensive washing of the particles. The absorbance at 493 nm showed a concentration dependent behaviour that increased with the addition of B4F but plateaued at high [B4F], suggesting saturation of the binding sites. These experiments confirm the binding activity of StAv in P22 (Figure S5) and the ability of encapsulated StAv to non-covalently functionalize the interior of P22 with small molecules, able to diffuse freely into the capsid, at high copy number. Future work will explore the use of commercially available biotin analogs50, with weaker affinity to StAv, to tune the non-covalent attachment to the encapsulated StAv for triggered release of biotin-conjugated molecules.
Figure 3.
Analysis of purified in vitro assembled P22-StAv particles. (A) SDS-PAGE analysis showing two bands corresponding to CP (~47 kDa) and StAv-SP (~35 kDa) confirms the encapsulation of StAv-SP. (B) SEC-MALS shows the monodisperse population of P22-StAv (blue trace) and distribution of particle weight (MW) across the peak (red trace). (C) TEM image shows that morphology and size of the P22 is maintained during in vitro assembly. (D) Binding activity of an in vitro and in vivo assembled P22-StAv particles, evaluated by titrating biotin binding sites with biotin-4-fluorescein (B4F). Binding of B4F to StAv results in reduction of the B4F absorbance at 493 nm until all sites are saturated, after which slope changes and absorbance follows Beer’s law. With in vitro assembled P22-StAv particles, the change in slope indicates the functionally active state of particles and the break point at which slope changed provides 1:1 binding stoichiometry of StAv-SP subunit to B4F. In case of in vivo assembled particles, the slope remains unchanged throughout the titration and reflects the lack of functional activity. The data were fit with a user-defined piecewise linear regression function. Error bars shows standard deviation of the mean.
To investigate the possibility that the binding sites in the in vivo assembled P22-StAv could be presaturated with biotin from the E.coli, the refolded StAv-SP was saturated with B4F or biotin and subsequently used for in vitro assembly. The StAv-SP was treated with two equivalents of B4F, excess B4F was removed by dialysis, and then mixed with CP in 1:1 molar ratio in the assembly assay. The assembled particles were found to have similar morphology and size by TEM (Figure S6A). The SDS-PAGE analysis indicated encapsulation of StAv (Figure S6B, L2 and L4), however, the StAv-SP band was extremely faint, and instead a cluster of high molecular weight bands were observed (Figure S6B, L2 and L4), which were absent in the SDS-PAGE of in vivo assembled P22-StAv (Figure S6B, L1). These high molecular weight bands are likely aggregates or oligomers of streptavidin tetramers formed in presence of biotin as reported previously in the literature.51 These bands are thus diagnostic for the biotin bound StAv and were not observed in the in vivo assembled P22-StAv materials suggesting that available binding sites were not pre-saturated with biotin.
In vitro assembly allows control over composition of StAv in P22-StAv
Building on the demonstrated success of in vitro assembly to form active P22-StAv particles, we designed a novel approach to control both the packaging stoichiometry and packing density of StAv inside P22 using in vitro assembly from defined stoichiometric mixtures of wtSP and StAv-SP together with CP (Figure 4A). The wtSP and StAv-SP were mixed at molar ratios of 1:0, 0.85:0.15, 0.75:0.25, 0.5:0.5, 0.25:0.75, 0.15:0.85, 0:1, and added to one equivalent of CP under our in vitro assembly conditions. After co-assembly, the capsids (P22-[StAv-SP][wtSP]) were isolated, purified, and analyzed by SDS-PAGE (Figure 4B). Despite the similar molecular weights of wtSP and StAv-SP slightly different electrophoretic mobilities between the two were observed, which could be due to difference in their theoretical pI values (5.58 and 9.0 respectively), a behavior that has been described in the literature52 The average amount of encapsulated wtSP and StAv-SP was quantified from the SDS-PAGE gel after a line-scan densitometry profile was plotted for each lane (Figure S7). Line scan profiles were analyzed relative to known standards of each component and the area under each peak was determined for CP, wtSP and StAv-SP (Figure S8). A calibration curve from the standards was used to calculate the mole ratios of wtSP, and StAv-SP (relative to CP) incorporated into the isolated co-assembled capsids (Table S1). The encapsulation of each component was plotted (Figure 4C) showing the input moles used for assembly and output mole ratios encapsulated in the co-assembled capsids. The data indicate that preferential packaging of StAv-SP over wtSP occurred but also that the packing stoichiometry could be controlled by the input ratio of wtSP and StAv-SP.
Figure 4.
Controlled packaging of cargo using in vitro co-assembly of P22 CP with cargo-fused SP and wtSP. (A) Schematic showing controlled packaging of StAv in P22. For controlled packaging, a constant amount of CP was added to variable amounts of wtSP and StAv-SP in separate reaction mixtures. After in vitro assembly, the resultant co-assembled particles were isolated and analyzed. (B) SDS-PAGE analysis of standards (L1, L2, and L3) that contained mixture of CP (top band), wtSP (middle band) and StAv-SP (lower band), and isolated co-assembled capsids (L4, L5, L6, L7, L8, L9, L10). (C) Plots of the encapsulation of StAv-SP and wtSP over a range of input stoichiometry ratios of each component, showing preferential encapsulation of StAv-SP over wtSP.
This preferential packaging is likely due to the tetrameric structure of StAv that presents four SP, two on each face, allowing the StAv-SP to engage in multivalent interactions with the CP during assembly. Previous work on P22 assembly has demonstrated that mutant disulfide-linked SP dimers accelerate the rate of P22 capsid assembly as compared to wtSP.53 Thus, we anticipate that multivalent StAv-SP may enhance the rate of capsid assembly and because StAv-SP is a tetramer the SP has a higher probability for rebinding to the capsid surface compared to wtSP during assembly. There seems to be a useful relationship between input and output ratios of StAv-SP and wtSP that allows us to control the packaging of cargo within the P22.
In vitro assembly allows control over packing density of co-assembled P22-StAv particles
We have developed an approach to alter the packing density of cargo within the capsids based on capsid assembly. We have reproduced the release of SP in vitro using 0.5 M GuHCl42 allowing us to achieve alteration of density of cargo within P22. The approach exploits the stoichiometric control demonstrated above by varying the input ratios of StAv-SP and wtSP in the assembly and taking advantage of the fact that wtSP, but not bulky SP-protein cargo, can escape across the capsid shell upon treatment with 0.5 M GuHCl. Thus, the wtSP was selectively removed from co-assembled capsids (P22-[StAv-SP][wtSP]) by dialyzing against buffer containing 0.5 M GuHCl (Figure 5A). Samples of co-assembled capsids with different ratios of StAv-SP and wtSP were treated in this way and the resulting materials were analyzed by SDS-PAGE to determine the degree of wtSP release. Line scan analysis of SDS-PAGE showed complete removal of wtSP in the co-assembled capsid samples (Figure 5B, L1, before treatment and L2, after GuHCl extraction) but no loss of the StAv-SP cargo. We were able to get rid of most (but in some cases, not all) of the wtSP from co-assembled capsids. Additional data can be found in supplemental information (Figure S9).
Figure 5.
Exterior functionalization of P22-StAv particle. (A) A cartoon showing StAv on the interior of P22 able to present GFP on the exterior surface via SP. (B) SDS-PAGE analysis of Ni-NTA purified Cys-6xhis-SP-GFP (SP-GFP) fusion protein shows the band at expected molecular weight (~62 kDa). (C) & (D) Mass-spectroscopic analysis of SP-GFP and biotinylated Cys-6xhis-SP-GFP (B-SP-GFP), respectively. Mass difference of 453 Da confirms the conjugation of one biotin molecule per Cys-6xhis-SP-GFP. (E) Purified P22-StAv particles after treatment with B-SP-GFP or iodoacetic acid protected SP-GFP (I-SP-GFP). SDS-PAGE analysis of P22-StAv (L1), P22-StAv treated with B-SP-GFP (L2), and P22-StAv treated with I-SP-GFP (L3) shows that encapsulated StAv binds B-SP-GFP but not I-SP-GFP. (F) UV-Vis analysis, shows the GFP signature peak at 495 nm in purified P22-StAv particles treated with B-SP-GFP but not with I-SP-GFP, confirming the selective binding of encapsulated StAv to B-SP-GFP. (G)SEC-MALS analysis shows an increased molecular weight of P22-StAv-B-SP-GFP over P22-StAv. (H) TEM image shows that the morphology of surface decorated particles (P22-StAv-B-SP-GFP) is maintained.
Selective functionalization of the exterior P22-StAv capsid surface
The SP can diffuse into preformed empty P22 VLP capsids and “re-stuff” them42 and we have used the ability of SP to traverse the capsid shell as a means to functionalize the exterior surface of the P22-StAv construct (Figure 6A). The SP is largely unstructured and here we used it as a flexible linker to which GFP was attached at the C-terminus while the N-terminus was biotinylated allowing it to bind to the encapsulated StAv on the interior of the P22. We hypothesized that GFP would be too large to enter the capsid through the pores and would therefore be displayed on the exterior of the capsid, tethered to the biotinylated SP which was in turn bound to the StAv on the interior of the capsid. The fusion protein Cys-6xhis-SP-GFP was expressed in E. coli and purified using Ni-affinity chromatography as described in the materials and method section, and SDS-PAGE analysis revealed a band at the expected molecular weight (Figure 6B). Biotinylated Cys-6xhis-SP-GFP (B-SP-GFP) was obtained by treating with 10 equivalents of biotin-maleimide. Mass spectrometry confirmed an increase in molecular weight of 453 Da, corresponding to addition of biotin-maleimide, indicating conjugation of one biotin per Cys-6xhis-SP-GFP (Figure 6C and 6D).
Figure 6.
Selective removal of wtSP from co-assembled capsids. (A) Schematic showing wtSP release upon treatment with 0.5 M guanidine hydrochloride. (B) SDS-PAGE analysis and densitometric profiling of co-assembled capsids, before (L1) and after (L2) guanidine hydrochloride treatment, shows complete removal of wtSP (middle band) while retaining StAv-SP in P22.
P22-StAv (in vitro assembled) was incubated with an excess of B-SP-GFP and the resultant capsids were isolated by ultracentrifugation after 2 h and extensively washed to remove unbound B-SP-GFP. As a negative control, P22-StAv was incubated with the SP-GFP fusion protein whose cysteine was blocked by treatment with iodoacetic acid (I-SP-GFP). Samples were analyzed by SDS-PAGE and clearly showed the biotinylated B-SP-GFP bound and co-purified with the capsid while the non-biotinylated I-SP-GFP did not bind (Figure 6E). This indicates that the SP can in fact diffuse across the cargo encapsulated capsid and when biotinylated can bind effectively to the interior StAv. By UV-Vis analysis, the presence of GFP is clear from its signature peak at 495 nm in purified P22-StAv-B-S-GFP, whereas this peak is absent in negative control, which further reinforces the selective binding of B-SP-GFP to P22-StAv (Figure 6F). SEC-MALS analysis of P22-StAv treated with B-SP-GFP showed an increase in molecular weight of ~2.2 MDa when compared with P22-StAv and the negative control sample. This increase in molecular weight corresponds to 36 ± 5 copies of B-SP-GFP bound to each capsid. (Figure 6G). We anticipated the attachment of up to 60 copies of B-SP-GFP to P22-StAv, because SP likely escapes through the large central pores in hexamers, and there are 60 hexamers in P22 capsid.44 However, the difference observed in molecular weights of P22-StAv-B-SP-GFP and P22-StAv in SEC-MALS analysis falls under the error range of this technique and may not be accurate, so we resorted to the densitometric analysis of SDS-PAGE to calculate bound copies of B-SP-GFP. Varied concentrations of B-SP-GFP and CP standards were run on the SDS-PAGE along with the samples (Figure S10). Densitometric analysis was performed on the standards and samples, as mentioned before. From the calibration plot of standards, the ratio of CP to bound B-SP-GFP was calculated, which translated into ~56 copies of B-SP-GFP bound to P22, considering 420 copies of CP in a capsid. This result was in accordance with the concentration of GFP calculated from absorbance at 495 nm in UV-Vis spectroscopy when a molar extinction coefficient of 55, 000 M−1cm−1 was used to determine the concentration GFP.54 SEC-MALS analysis revealed minimal increase in the radius of gyration (Rg) of 24.6 ± 0.1 nm for P22-StAv-B-SP-GFP, as compared to 24.1 ± 0.1 nm for P22-StAv. Dynamic light scattering revealed an average particle diameter of 62.3 nm (PDI: 0.06) for P22-StAv-B-SP-GFP, as compared to 60.3 nm (PDI: 0.03) for P22-StAv, suggesting a slight increase in diameter due to the bound B-SP-GFP (Figure S11). To test our hypothesis that the GFP remained exposed on the exterior capsid surface, we performed an immunodot-blot using antibodies against GFP as described in the materials and methods section. The immunodot-blot of P22-StAv-B-SP-GFP was compared to a sample of P22 in which the GFP was encapsulated on the interior of the capsid (P22-GFP)38, and thus inaccessible to the Ab binding. B-SP-GFP and P22 with varied concentrations were evaluated as controls to assess non-specific binding and qualitatively indicate the dynamic range of detection sensitivity (Figure S12). The amount of P22-StAv-B-SP-GFP and P22-GFP applied on nitrocellulose membrane for immunodot-blot was normalized to GFP concentration. This experiment clearly shows that the GFP is accessible to the Ab in the P22-StAv-B-SP-GFP sample but not in the P22-GFP where the GFP is encapsulated, thus supporting our assertion that the GFP is exposed on the exterior surface. Analysis by TEM (Figure 6H) shows that the morphology of surface decorated particles (P22-StAv-B-SP-GFP) is maintained. However, we have not been able to visualize GFP on the surface of P22, perhaps because the GFP occupies the valley at the centre of the hexamers on the surface of P22.
These experiments have demonstrated the accessibility of StAv binding site from exterior of the capsid by a biotinylated flexible protein linker attached to a well folded protein too large to traverse the capsid shell leaving it tethered on the exterior of P22. The specificity of streptavidin-biotin binding interaction resulted in the selective attachment of B-SP-GFP at high copy number on the exterior of P22-StAv capsid surface. Thus, encapsulation of StAv provides a means to decorate the exterior surface of P22 with large molecules.
Conclusions
We have successfully encapsulated tetrameric streptavidin in P22 by utilizing an in vitro assembly, which allowed refolding of a protein before packaging in P22. Using in vitro assembly, we have introduced the new methodology to control cargo stoichiometry in P22, which might open doors to package multiple enzymes with known and controlled concentration. This may also make the engineering of complex synthetic metabolons with enhanced efficiency and turnover rate of cascaded pathways in P22 significantly less challenging. Building up on this, we demonstrated the alteration in packing density of cargo by selectively releasing wtSP. Besides promoting P22 assembly, wtSP acts as a space holder in the interior of P22. Therefore, once the cargo packaging is controlled by assembling P22 with wtSP and cargo-fused-SP, the selective removal of wtSP from co-assembled capsids is expected to release some volume constraints and provide more spatial and dynamic flexibility to the cargo in confined VLP environment. These experiments will allow us to investigate the encapsulation and confinement effects. We have demonstrated that the streptavidin in P22 has the ability to attach molecules at both the interior and exterior interfaces in high copy number that makes this (P22-StAv) a viable nanoscale platform with potential applications in biomedicine.
Materials and methods
Materials
Reagents for buffer preparation were purchased from Fisher Scientific, unless otherwise specified. DNA primers were purchased from Eurofins MWG Operon. Chemically competent E.coli cells BL21(λDE3) were purchased from Lucigen or Novagen. QuikChange Lightning Site-Directed Mutagenesis kit was purchased from Agilent Technologies. Biotin-4-fluorescein was purchased from Biotium. Biotin-maleimide and streptavidin was purchased from Sigma Aldrich. Protease and phosphatase inhibitor mini tablets were purchased from Roche. For gene assembly, NEB builder kit was purchased from New England Biolabs. For Immunoblot, Opti-4CN detection kit was purchased from Biorad.
Molecular Biology. Gene assembly of 6xhis-StAv15–159-GAAG-ENLYFQS-GAAG-SP142–303 (StAv-SP) in a pBAD vector
The streptavidin (StAv, amino acids 15–159) gene was provided by Dr. Paolo Arosio in a pET11b plasmid. The pBAD expression vector with StAv-SP gene insert was constructed from a previously cloned pBAD vector that contained GFP-GAAG-ENLYFQS-GAAG-SP142–303 between NcoI/Sacl restriction sites, and pET11b that contained 6xhisStAv. The 6xhisStAv15–159 was amplified with overhangs overlapping with pBAD vector sequence on one end using FP1 and with GAAG-ENLYFQS-GAAG-SP142–303 on another end using RP1 FP1:5’GAATTAACCATGGGTCATCATCATCATCATCATGCCGGCATCACC GGC-3’, and RP1: 5’-GAAATACAGGTTTTCACCTGCTGCACCCTGCTGAACGGCGTCG AGC-3’
The GAAG-ENLYFQS-GAAG-SP142–303 pBAD vector backbone was amplified with overhangs that overlap with linker region (GAAG-ENLYF) on 5’ end and with 6xhis-StAv on 3’ end using primers 5’-GCAGGTGAAAACCTGTATTTCCAGAGCGGTGCGG-3’ and 3’-ATGATGACC CATGGTTAATTCCTCCTGTTAGCCCAAAAAACGG-3’.
The two PCR products with complementary overhangs were assembled into circular DNA using NEB builder kit (New England Biolabs) as per instruction manual.
Cysteine mutant of 6xhis-SP142–303-GGGGG-ENLYFQS-GGGGG-GFP (Cys-6xhisSP-GFP)
Cysteine residue was inserted before his-tag in previously generated 6xhis-SP142–303-GGGGG-ENLYFQS-GGGGG-GFP fusion construct. QuikChange Lightning Site-Directed Mutagenesis kit was used to introduce cysteine residue. The residue was inserted in the plasmid by PCR and a pair of following mutagenic primers 5’GGAATTAACCATGGGCTGCCATCATCACCACCATC-3’ and 5’GATGGTGGTGATGATGGCAGCCCATGGTTAATTCC-3’. The amino acid sequence for both the fusion proteins was confirmed by DNA sequencing and is provided in the supplementary information.
Expression, Lysis and Purification
All constructs were transformed into E. coli BL21 (λDE3) bacterial cells. E. coli strain harboring expression vectors were grown on LB medium at 37 °C in presence of ampicillin (50 mg ml−1) and/or kanamycin (30 mg ml−1) to maintain selection for recombinant plasmids.
Expression of P22 procapsids
The expression was induced by adding Isopropyl β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM once the cell density reached early log phase (OD600 = 0.6 – 0.8). Cultures were grown for 4 h after the addition of IPTG, then the cells were harvested by centrifugation at 3700 ×g for 20 min. The cell pellets were suspended in PBS (50 mM sodium phosphate, 100 mM sodium chloride, pH 7.0) and stored at −80 °C until further use.
Expression of P22-StAv
Two vector system was used for expression of P22-StAv. StAv-SP in pBAD/HisB vector was expressed first by addition of L-arabinose to a final concentration of 13mM at OD600 = 0.6. Cultures were grown for 0.5 h post induction and then CP expression in pRSF-Duet was induced by Isopropyl β-D-thiogalactopyranoside (IPTG, 0.5mM final concentration) and grown for additional 2 h. The cells were harvested by centrifugation, resuspended in PBS, and stored at −80 °C.
Purification of P22 and P22-StAv
Cell pellets were resuspended in PBS with lysozyme, DNase and RNase added and incubated at room temperature for 30 min. The cell suspension was lysed by sonication. Cellular components were removed by centrifugation at 12000 ×g for 45 min at 4 °C. Samples were purified from the cell lysate by ultracentrifugation through a 5 mL 35% (w/v) sucrose cushion. The resulting viral pellet was resuspended in PBS and spun at 16000 ×g for 20 min in a benchtop micro-centrifuge to pellet lipids in the sample. Capsid sample was then purified over an S-500 Sephadex (GE Healthcare Life Sciences) size exclusion column using a Biorad Biologic Duoflow FPLC. Fractions containing P22 were concentrated by ultracentrifugation and the resulting viral pellet was resuspended in an adequate volume of PBS. In the final purification step, the capsids were incubated with excess amount of C-terminal cysteine mutant of Dec (DecS134C) for approx. 1 – 2 h at room temperature. It was demonstrated that DecS134C binds to aberrant particles and causes their aggregation.55 The aggregates were removed by pelleting in bench-top micro-centrifuge and free DecS134C was removed by ultracentrifugation and pellet was washed with buffer until supernatant is free from residual DecS134C. The concentration of streptavidin in P22-StAv was determined using method given in supplementary information.
Expression and purification of free StAv-SP
The expression of StAv-SP was induced by addition of L-arabinose to a final concentration of 13mM at OD600 = 0.6. The cultures were grown for 4 h after induction. The cells were harvested and resuspended in PBS with lysozyme, DNase, RNase and protease mini tablets for 30 min. The cell suspension was lysed by sonication and the protein was purified from inclusion body pelleted in cell debris. The cell debris was dissolved in 10 ml 6 M guanidine hydrochloride in a PBS background. The cell debris was centrifuged at 12000 ×g for 30 min at 4 °C and filtered through 0.45-micron filter. The solubilized and denatured protein was refolded onto cOmplete His-tag purification column. Protein was loaded on the column at 1 mL min−1 and pre-equilibrated in denaturing buffer (50mM Phosphate, 100mM NaCl, pH 7.0, 6 M guanidine hydrochloride) for 10 min at 2 ml min−1. For renaturation of protein, a linear reverse gradient of denaturing buffer (6 – 0 M guanidine hydrochloride) was run for 80 min at 0.25 ml min−1. The on-column refolded protein was washed with 20 mL of low concentration imidazole buffer (50 mM phosphate, 100 mM sodium chloride, 20 mM imidazole pH 7.8) at 2 ml min−1 and eluted with a gradient of increasing imidazole concentration (20 – 500 mM imidazole) at 2 ml min−1.
Expression and purification of free wtSP and Cys-6xhis-SP-GFP
The expression was carried out similar to StAv-SP expression, however proteins were purified from cell lysate. Cellular components were removed by centrifugation at 12000 ×g for 45 min at 4 °C. The lysate was filtered through 0.45-micron filter and loaded onto His-tag column and washed with 40 mL of low imidazole buffer (50 mM phosphate, 100 mM sodium chloride, 20 mM imidazole pH 7.8). Samples were eluted using gradient of increasing imidazole concentration (20 – 500 mM). Fractions were collected and pooled after analyzing by SDS PAGE. Samples were dialyzed in PBS to remove imidazole and concentrated by amicon® ultracentrifugal spin filters.
Preparation of coat protein subunits for in vitro assembly
CP subunits were obtained by dissociating the ES in 3 M GuHCl (final concentration). The ES was prepared by washing off SP from P22 PC, successively. The PC were incubated with 0.5 M GuHCl for 1–1.5 h at 4 °C. Capsids were pelleted in ultracentrifuge at 45,000 rpm for 50 min followed by resuspension and incubation in 0.5 M GuHCl for 1 h. This extraction process was repeated until the supernatant was free from SP. The ES were then purified over an S-500 Sephadex size exclusion column.
In vitro assembly
CP subunits (~2 mg/ml), as prepared above in 3M GuHCl, were mixed with purified StAv-SP subunits in a 1:1 molar ratio. The volume of the StAv-SP was adjusted such that the final concentration of GuHCl was 1.5 M in all assembly reaction mixtures. The mixture was dialyzed into assembly buffer (50mM Tris-HCl, 25 mM NaCl, 2mM EDTA, 3mM β-mercaptoethanol, and 1% glycerol) for 12–18 h. The resulting assembled P22-StAv particles were isolated by ultracentrifugation.
Binding activity of commercial StAv, StAv-SP and P22-StAv
Binding activity was assayed by cumulative titration of StAv with biotin-4-fluorescein (B4F). The binding of B4F to StAv, accompanies with a significant reduction in molar extinction coefficient at 493 nm, which results in a decreased absorbance at 493 nm.49 We first tested commercial StAv free in solution as a positive control. The stock solution of StAv was prepared in PBS (50 mM Phosphate, 100 mM NaCl and pH 7.0). B4F stock solution was made in DMSO and stored at −80°C in small aliquots until further use. The working standard was prepared in PBS and concentration was calculated from the absorbance at λmax and molar extinction coefficient of 68,000 M−1 cm−1.56 For titration, typically, a 100 µl aliquot of 10 µM StAv was taken into a quartz cuvette and titrated with 400 µM of B4F in 0.5 µl increments at constant time intervals of 30 seconds to allow reaction to reach equilibrium. Absorbance was measured at each titration point. For a negative control, PBS was titrated with B4F alongside. With PBS, the absorbance was increased linearly with a constant slope throughout the successive additions of B4F, whereas in StAv, reduction in absorbance was observed until all binding sites became saturated with B4F. Further addition of B4F resulted in an increase in absorbance with a slope similar to the control experiment, indicating absence of non-specific binding. The data was plotted between absorbance and molar ratio of [B4F]/[ StAv monomer] and fitted with a user-defined piecewise linear regression function in Igor pro 6.37. The breakpoint at molar ratio of 1 indicated the binding stoichiometry of 1:1, and that all sites are functional, and in agreement with the specifications provided by vendor. Binding activity of StAv-SP was tested in similar manner. The concentration of StAv-SP was calculated from absorbance at 280 nm and extinction coefficient that was obtained from ExPASy-ProtParam tool. To measure binding activity of the StAv in P22-StAv, the concentration of encapsulated StAv was calculated from an absorbance at 280 nm and molar extinction coefficient of 215,000 M−1 cm−1. Details on the determination of molar extinction coefficient is provided in supplementary information. The results are an average of three independent triplicates. The concentrations were corrected for dilutions.
Titration curve fitting
Data was fitted using a user-defined piecewise linear regression model in Igor pro 6.37. The model that fits two lines to different section of a data is given below
where m1 and m2 are the slope values of two linear functions; xb and yb are the intersection coordinates where the linear function changes over different ranges of x. Data was fit systematically to ensure reasonable initial guesses as input parameters.
Size Exclusion Chromatography with Multiangle Light Scattering and Refractive Index Detection
Samples were separated over a WTC-0200S (Wyatt Technologies) size exclusion column utilizing an Agilent 1200 HPLC system at a flow rate 0.7 ml/min of MALS buffer (50 mM phosphate, 100 mM NaCl and 200 ppm NaN3, pH 7.2). Sample volume of 25 µL per injection was loaded onto the column, and the column was run for 35 min. Eluted peaks were detected using a UV-Vis detector (Agilent), a Wyatt HELEOS multi-angle laser light scattering (MALS) detector, and an Optilab rEX differential refractometer (Wyatt Technology Corporation). The number-average particle molecular weight, Mn, was measured across FWHM of each peak with Astra 5.3.14 software (Wyatt Technology Corporation) using a previously calculated dn/dc value of 0.185 mL/g.
SDS-PAGE
Protein samples were mixed with 4x loading buffer containing 100 mM DTT, heated in a boiling water bath for 10 min, removed, and spun down on a benchtop centrifuge. Samples were separated on a 15% acrylamide gel at a constant current of 35 mA for approximately 1 h. Gels were stained with a Instant Blue protein stain, rinsed with water, and imaged. Images were recorded on a UVP MultDoc-IT Digital Imaging System. A 10–180 kDa PageRuler prestained ladder was used for reference.
Transmission Electron Microscopy
Samples (4 µL, 0.3 mg/mL protein) were applied to carbon-coated grids and incubated for 45 s. Excess liquid was wicked away with filter paper. Grids were then washed with 5 µL of distilled water. Grids were stained with 4 µL 2% Uranyl acetate for 10 s and excess stain was wicked away with filter paper. Images were taken on a JEOL 1010 transmission electron microscope at accelerating voltage of 100 kV.
Dynamic light scattering
Protein samples were spun in a benchtop centrifuge at 17000 rpm for 10 minutes to remove any aggregates. For size measurement, samples (100 µL of ~1 mg ml−1) were taken into a quartz cuvette (Hellma Analytics, ZEN2112) and analyzed by a Malvern Zetasizer Nano-S for size determination.
Immunodot blot
For blot, 5 µL aliquots of standards and samples were applied on nitrocellulose membrane and left to air dry for 5–10 min. The membrane was blocked with 5% milk in TBS buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) for 30 min at room temperature. The membrane was incubated with polyclonal anti-GFP rabbit antibody (1:10,000 dilution in 5% milk) for 2 h at room temperature on rocker, followed by washing two times with TBS, 5 min each time. The membrane was incubated with goat anti-rabbit HRP-conjugated secondary antibody (1:3000 dilution in 5% milk) for 2 h at room temperature, followed by washing two times with TBS, 5 min each time and developed with opti-4CN substrate as per instructions provided in kit for colorimetric method.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Science Foundation (BMAT DMR-1507282) and the National Institutes of Health (R01 AI104905). We thank Dr. Paolo Arosio for providing us Streptavidin gene. Polyclonal anti-GFP antibody was a generous gift from Dr. David Rudner (Harvard Medical School). We thank Dr. Barry Stein of Indiana University Electron Microscopy Center for his help in acquiring TEM images. We thank Biological mass-spectrometry lab at IU for analyzing our samples and Dr. Benjamin Schwarz and Dr. Soumyananda Chakraborty for useful suggestions in this project.
Footnotes
Electronic Supplementary Information (ESI) available.
Conflict of Interest: The authors declare no competing financial interest.
References
- 1.Douglas T, Young M. Science. 2006;312:873–875. doi: 10.1126/science.1123223. [DOI] [PubMed] [Google Scholar]
- 2.Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, Meier W. Adv. Funct Mater. 2011;21:1241–1259. [Google Scholar]
- 3.Tanner P, Balasubramanian V, Palivan CG. Nano Lett. 2013;13:2875–2883. doi: 10.1021/nl401215n. [DOI] [PubMed] [Google Scholar]
- 4.Elani Y, Law RV, Ces O. Nature Communications. 2014;5 doi: 10.1038/ncomms6305. [DOI] [PubMed] [Google Scholar]
- 5.Comellas-Aragones M, Engelkamp H, Claessen VI, Sommerdijk NA, Rowan AE, Christianen PC, Maan JC, Verduin BJ, Cornelissen JJ, Nolte RJ. Nat Nanotechnol. 2007;2:635–639. doi: 10.1038/nnano.2007.299. [DOI] [PubMed] [Google Scholar]
- 6.Rother M, Nussbaumer MG, Renggli K, Bruns N. Chem. Soc. Rev. 2016;45:6213–6249. doi: 10.1039/c6cs00177g. [DOI] [PubMed] [Google Scholar]
- 7.Kang S, Douglas T. Science. 2010;327:42–43. doi: 10.1126/science.1184318. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Ardejani MS, Orner BP. Chem. Asian J. 2016;11:2814–2828. doi: 10.1002/asia.201600769. [DOI] [PubMed] [Google Scholar]
- 9.Frey R, Mantri S, Rocca M, Hilvert D. J. Am. Chem. Soc. 2016;138:10072–10075. doi: 10.1021/jacs.6b04744. [DOI] [PubMed] [Google Scholar]
- 10.Frey R, Hayashi T, Hilvert D. Chem. Commun. (Camb.) 2016;52:10423–10426. doi: 10.1039/c6cc05301g. [DOI] [PubMed] [Google Scholar]
- 11.Azuma Y, Zschoche R, Tinzl M, Hilvert D. Angew. Chem. Int. Ed. Engl. 2016;55:1531–1534. doi: 10.1002/anie.201508414. [DOI] [PubMed] [Google Scholar]
- 12.Zschoche R, Hilvert D. J. Am. Chem. Soc. 2015;137:16121–16132. doi: 10.1021/jacs.5b10588. [DOI] [PubMed] [Google Scholar]
- 13.Brasch M, Putri RM, de Ruiter MV, Luque D, Koay MST, Caston JR, Comelissen JJLM. JACS. 2017;139:1512–1519. doi: 10.1021/jacs.6b10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minten IJ, Claessen VI, Blank K, Rowan AE, Nolte RJM, Cornelissen J. Chemical Science. 2011;2:358–362. [Google Scholar]
- 15.Minten IJ, Hendriks LJA, Nolte RJM, Cornelissen J. JACS. 2009;131:17771–17773. doi: 10.1021/ja907843s. [DOI] [PubMed] [Google Scholar]
- 16.Rurup WF, Verbij F, Koay MS, Blum C, Subramaniam V, Cornelissen JJ. Biomacromolecules. 2014;15:558–563. doi: 10.1021/bm4015792. [DOI] [PubMed] [Google Scholar]
- 17.Brasch M, de la Escosura A, Ma YJ, Uetrecht C, Heck AJR, Torres T, Cornelissen JJLM. JACS. 2011;133:6878–6881. doi: 10.1021/ja110752u. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler JD, Brown SD, Lau JL, Finn MG. Angewandte Chemie-International Edition. 2010;49:9648–9651. doi: 10.1002/anie.201005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovlid ML, Lau JL, Breitenkamp K, Higginson CJ, Laufer B, Manchester M, Finn MG. Acs Nano. 2014;8:8003–8014. doi: 10.1021/nn502043d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pokorski JK, Breitenkamp K, Liepold LO, Qazi S, Finn MG. JACS. 2011;133:9242–9245. doi: 10.1021/ja203286n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee JK, Hovlid M, Fiedler JD, Brown SD, Manzenrieder F, Kitagishi H, Nycholat C, Paulson JC, Finn MG. Biomacromolecules. 2011;12:3977–3981. doi: 10.1021/bm200983k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow JE, Capehart SL, Francis MB, Tullman-Ercek D. Acs Nano. 2012;6:8658–8664. doi: 10.1021/nn302183h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida M, Morris DS, Kang S, Jolley CC, Lucon J, Liepold LO, LaFrance B, Prevelige PE, Jr, Douglas T. Langmuir. 2012;28:1998–2006. doi: 10.1021/la203866c. [DOI] [PubMed] [Google Scholar]
- 24.Uchida M, Qazi S, Edwards E, Douglas T. Methods Mol. Biol. 2015;1252:17–25. doi: 10.1007/978-1-4939-2131-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan PC, Patterson DP, Saboda KN, Edwards EJ, Miettinen HM, Basu G, Thielges MC, Douglas T. Nat. Chem. 2016;8:179–185. doi: 10.1038/nchem.2416. [DOI] [PubMed] [Google Scholar]
- 26.Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, Douglas T. ACS Nano. 2013;7:3036–3044. doi: 10.1021/nn4006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson DP, Schwarz B, Waters RS, Gedeon T, Douglas T. ACS Chem. Biol. 2014;9:359–365. doi: 10.1021/cb4006529. [DOI] [PubMed] [Google Scholar]
- 28.O’Neil A, Prevelige PE, Basu G, Douglas T. Biomacromolecules. 2012;13:3902–3907. doi: 10.1021/bm301347x. [DOI] [PubMed] [Google Scholar]
- 29.Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, Varpness Z, Liepold LO, Young M, Douglas T. Adv. Mater. 2007;19:1025–1042. [Google Scholar]
- 30.Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman CL, Brinker CJ, Caldeira JD, Chackerian B, Wharton W, Peabody DS. Acs Nano. 2011;5:5729–5745. doi: 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruckman MA, VanMeter A, Steinmetz NF. Acs Biomaterials Science & Engineering. 2015;1:13–18. doi: 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qazi S, Miettinen HM, Wilkinson RA, McCoy K, Douglas T, Wiedenheft B. Mol. Pharm. 2016;13:1191–1196. doi: 10.1021/acs.molpharmaceut.5b00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giessen TW, Silver PA. Curr. Opin. Biotechnol. 2017;46:42–50. doi: 10.1016/j.copbio.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Rother M, Nussbaumer MG, Renggli K, Bruns N. Chem. Soc. Rev. 2016;45:6213–6249. doi: 10.1039/c6cs00177g. [DOI] [PubMed] [Google Scholar]
- 35.Douglas T, Young M. Nature. 1998;393:152–155. [Google Scholar]
- 36.Worsdorfer B, Woycechowsky KJ, Hilvert D. Science. 2011;331:589–592. doi: 10.1126/science.1199081. [DOI] [PubMed] [Google Scholar]
- 37.Giessen TW, Silver PA. Chembiochem. 2016;17:1931–1935. doi: 10.1002/cbic.201600431. [DOI] [PubMed] [Google Scholar]
- 38.O’Neil A, Reichhardt C, Johnson B, Prevelige PE, Douglas T. Angewandte Chemie-International Edition. 2011;50:7425–7428. doi: 10.1002/anie.201102036. [DOI] [PubMed] [Google Scholar]
- 39.Inoue T, Kawano MA, Takahashi RU, Tsukarnoto H, Enornoto T, Imai T, Kataoka K, Handa H. J. Biotechnol. 2008;134:181–192. doi: 10.1016/j.jbiotec.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Botstein D, Waddell CH, King J. Journal of molecular biology. 1973;80:669IN13679–13678IN18695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- 41.Padilla-Meier GP, Gilcrease EB, Weigele PR, Cortines JR, Siegel M, Leavitt JC, Teschke CM, Casjens SR. J. Biol. Chem. 2012;287:33766–33780. doi: 10.1074/jbc.M112.393132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene B, King J. Virology. 1994;205:188–197. doi: 10.1006/viro.1994.1634. [DOI] [PubMed] [Google Scholar]
- 43.Parker MH, Casjens S, Prevelige PE., Jr J. Mol. Biol. 1998;281:69–79. doi: 10.1006/jmbi.1998.1917. [DOI] [PubMed] [Google Scholar]
- 44.Chen DH, Baker ML, Hryc CF, DiMaio F, Jakana J, Wu W, Dougherty M, Haase-Pettingell C, Schmid MF, Jiang W, Baker D, King JA, Chiu W. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1355–1360. doi: 10.1073/pnas.1015739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson DP, Prevelige PE, Douglas T. Acs Nano. 2012;6:5000–5009. doi: 10.1021/nn300545z. [DOI] [PubMed] [Google Scholar]
- 46.Patterson DP, Schwarz B, El-Boubbou K, van der Oost J, Prevelige PE, Douglas T. Soft Matter. 2012;8:10158–10166. [Google Scholar]
- 47.Fuller MT, King J. Biophys. J. 1980;32:381–401. doi: 10.1016/S0006-3495(80)84963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prevelige PE, Thomas D, King J. J. Mol. Biol. 1988;202:743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- 49.Waner MJ, Mascotti DP. J. Biochem. Biophys. Methods. 2008;70:873–877. doi: 10.1016/j.jbbm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Aoki K, Sugiyama A, Doi H, Kodama T, Shimizu Y, Kanai M. Chem. Asian J. 2015;10:1071–1078. doi: 10.1002/asia.201500120. [DOI] [PubMed] [Google Scholar]
- 51.Humbert N, Zocchi A, Ward TR. Electrophoresis. 2005;26:47–52. doi: 10.1002/elps.200406148. [DOI] [PubMed] [Google Scholar]
- 52.Guan YH, Zhu QF, Huang DL, Zhao SY, Lo LJ, Peng JR. Sci. Rep. 2015;5 doi: 10.1038/srep13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker MH, Stafford WF, 3rd, Prevelige PE., Jr J. Mol. Biol. 1997;268:655–665. doi: 10.1006/jmbi.1997.0995. [DOI] [PubMed] [Google Scholar]
- 54.McRae SR, Brown CL, Bushell GR. Protein Expr. Purif. 2005;41:121–127. doi: 10.1016/j.pep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz B, Madden P, Avera J, Gordon B, Larson K, Miettinen HM, Uchida M, LaFrance B, Basu G, Rynda-Apple A, Douglas T. ACS Nano. 2015;9:9134–9147. doi: 10.1021/acsnano.5b03360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haugland RP, P R. Haugland, Handbook of fluorescent probes and research products. 9. Molecular Probes, Inc.; Eugene, Or: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.