Abstract
Purpose:
Wide field swept-source optical coherence tomography angiography (SS-OCTA) was compared with ultra wide field (UWF) fluorescein angiography (FA) for evaluating neovascularization (NV) before and after panretinal photocoagulation (PRP) in eyes with treatment-naive proliferative diabetic retinopathy (PDR).
Design:
Prospective, observational, consecutive case series
Participants:
Patients with treatment-naive PDR
Methods:
Patients were imaged using the SS-OCTA 12×12mm field of view (PLEX® Elite 9000; Carl Zeiss Meditec, Inc, Dublin, CA) at baseline and at 1 week, 1 month, and 3 months after PRP. Select eyes were imaged with five SS-OCTA 12×12mm scans to create posterior pole montages. UWF fundus photography and UWF FA were obtained at baseline and 3 months after PRP.
Main Outcome Measures:
NV visualized using wide field SS-OCTA and UWF FA
Results:
From January through May 2018, wide field SS-OCTA was performed on 20 eyes with treatment-naive PDR from 15 patients. The en face SS-OCTA 12×12mm vitreoretinal interface (VRI) slab images showed NV at baseline in 18 of 20 eyes (90%). Of the remaining 2 eyes, the posterior pole montage captured peripheral NV in one eye, and in the other eye, no evidence of NV was detected with either UWF FA or SS-OCTA. After PRP, both SS-OCTA and FA demonstrated similar progression or regression of NV, but SS-OCTA provided more detailed visualization of the vascular changes.
Conclusions:
NV in PDR can be identified at baseline and imaged serially after PRP using wide field SS-OCTA. In patients with a high clinical suspicion for PDR, wide field SS-OCTA will likely be the only imaging modality needed for diagnosis and longitudinal evaluation of NV.
INTRODUCTION
Diabetic retinopathy (DR) is the most common cause of blindness among working-age adults in developed countries.1 DR features retinal vascular changes that include microaneurysms (MAs), intraretinal microvascular abnormalities (IRMA), macular ischemia, and diabetic macular edema (DME).2 The sine qua non of proliferative diabetic retinopathy (PDR) is preretinal neovascularization (NV), which can lead to vitreous hemorrhage, tractional retinal detachment, and severe vision loss.2
Since its development in the 1960s,3,4 fluorescein angiography (FA) has remained an important imaging method for the diagnosis and monitoring of DR, particularly PDR. In PDR, FA enables the direct visualization of NV by the leakage of fluorescein from areas of NV.5,6 However, FA is time-consuming, requires intravenous access, and exposes patients to potential systemic adverse effects.7 Furthermore, FA does not differentiate whether leakage is from preretinal NV or from other sources. Finally, FA provides limited detailed characterization of NV and the surrounding retinal microvasculature because the area becomes obscured rapidly by the leakage of fluorescein, and only one eye can be chosen as the early transit eye for characterization.
In contrast to FA, swept-source optical coherence tomography angiography (SS-OCTA) is fast, non-invasive, safe, and easily repeatable.8–10 Moreover, boundary-specific segmentation strategies can be used to visualize the imaging data collected with OCTA by using en face angiograms of specific anatomical regions, such as the preretinal, superficial retinal, deep retinal, choriocapillaris, and deep choroidal vasculature. Simultaneously, OCTA provides cross-sectional B-scans that are useful for detecting DME and other signs of DR such as inner retinal disorganization and choroidopathy.11,12 OCTA has been used in DR to demonstrate the enlargement and irregularity of the foveal avascular zone (FAZ), macular capillary abnormalities, capillary ischemia, choriocapillaris flow impairment, and preretinal NV.13–29 Developing evidence suggests that OCTA detection of diabetic macular ischemia is correlated with visual acuity, and that OCTA may potentially facilitate staging of DR 13,21 or even detection of early DR changes prior to clinically detectable DR.15 Thus, OCTA shows promise in supplementing or potentially replacing FA for the diagnosis and monitoring of DR.
To date, only two studies have utilized OCTA to demonstrate longitudinal changes in PDR after a clinical intervention. Ishibazawa et al.20 used spectral domain (SD) OCTA to visualize regression of NV in eyes with PDR after PRP. In their study, patients were imaged at baseline and then only at a single time-point two months after the initiation of staged PRP. Their sample size was small and heterogenous, few eyes were imaged using FA, and they used a very small 3×3mm field of view that restricted visualization to a single area of NV per image. Zhang et al.30 also used SD-OCTA to serially image NV of the disc (NVD) after intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors and supplemental PRP, but their sample size was small with few comparisons with FA and their OCTA field of view was also small with limited visualization of NVD.
In this current report, we describe a prospective, observational study in which wide field SS-OCTA imaging was compared with UWF FA for evaluating NV before and after PRP in eyes with treatment-naive PDR.
METHODS
This study was performed in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996, and was approved by the Institutional Review Board of the University of Miami Miller School of Medicine. Informed consent was obtained from all patients. Patients were prospectively imaged during the course of routine clinical care in the resident physician clinics of J.F.R., J.W.H., N.L.S., and K.C.F. at the Bascom Palmer Eye Institute, Miami, FL. The principal inclusion criterion was treatment-naive PDR. PDR was diagnosed by detection of presumed preretinal NV on dilated ophthalmologic examination. Exclusion criteria included a history of retinal laser, history of intravitreal injection, glaucoma, dense vitreous hemorrhage, and cataract that limited the ability to obtain an SS-OCTA scan with a signal strength that was seven or greater as defined by the manufacturer. DME was not an exclusion criterion.
Patient information recorded at baseline included age, sex, ethnicity, type of diabetes, years since diabetes was diagnosed, use of oral antiglycemics, use of insulin, medical and ocular comorbidities, character and duration of ocular symptoms, and history of prior retinal laser or intravitreal injection. In patients who could not report a recent hemoglobin A1C value, an A1C was ordered. At baseline, blood pressure (BP) and body mass index (BMI) were measured. Ophthalmological examinations including Snellen best corrected visual acuity (BCVA) were performed at baseline and at 1 week, 1 month, and 3 months after PRP. All Snellen BCVA measurements were converted to approximate equivalent Early Treatment of Diabetic Retinopathy (eETDRS) visual acuity letter scores for the purposes of statistical manipulation and then converted back to Snellen BCVA.31
Ultra wide-field (UWF) FA (Optos, Inc., Marlborough, MA), UWF fundus photography (Optos, Inc.), macular SD-OCT (Spectralis, Heidelberg Engineering Inc., Franklin, MA), and SS-OCTA were performed at baseline. SS-OCTA was repeated at each follow-up visit. UWF FA, UWF fundus photography, and SD-OCT were repeated at the 3 month follow-up.
The SS-OCTA instrument (PLEX® Elite 9000, Carl Zeiss Meditec, Inc., Dublin, CA) used a laser with a central wavelength of ~1060nm (1000-1100nm full width) and a scanning rate of 100,000 A-scans per second. The full width at half maximum axial resolution was ~5μm in tissue, and the lateral resolution at the retinal surface was estimated at ~14μm. This instrument has an A-scan depth of 3.0 mm in tissue (1536 pixels). The 12×12mm scans used 500 A-scans per B-scan at 500 B-scan positions, resulting in an A-scan and B-scan separation of 24 μm. Two sequential B-scans were performed at each fixed position before proceeding to the next transverse location on the retina. The raster scans were centered on the fovea. For nine eyes, four additional 12×12mm scans were performed to create posterior pole montages. These included scans positioned on the superotemporal, superonasal, inferotemporal, or inferonasal macula. The montage field of view spanned ~6 disc diameters peripheral to the optic disc and macular arcades.
The en face SS-OCTA images visualized the flow that was detectable within a volume defined by selected boundaries. Each of these segmented volumes is referred to as a slab. The total retinal slab was defined as the volume between the internal limiting membrane (ILM) and the retinal pigment epithelium (RPE). Automatic segmentation algorithms of the ILM typically encompass the inner surface of NV, so much of the NV was visible on total retinal slabs. The vitreoretinal interface (VRI) slab was defined with an inner boundary 200μm above the ILM and an outer boundary 30μm below the ILM. To be sure that the entire neovascular complex was contained within the slab, especially in cases of extensive NV, some VRI slabs required manual corrections.
All eyes were treated with a single-session of PRP at the baseline visit. One drop of topical 1% proparacaine was applied for anesthesia. PRP was performed using an argon indirect ophthalmoscopy laser or slit-lamp based PAttern SCAnning Laser (PASCAL, Topcon Medical Systems, Oakland, NJ) using 400μm spots. Burns were placed with ~1 spot size between burns and distributed in all 4 quadrants extending from ~1 disc diameter from the macular arcades and optic disc to at least the equator while sparing the long posterior ciliary nerves. In the macula, the temporal demarcation border of the PRP was defined as ~1.5 times the disc-to-fovea distance positioned temporal to the fovea. Power and duration of laser spots were titrated in order to achieve moderately white burns.
No additional PRP was performed over the course of the study. Intravitreal injections of VEGF inhibitors, either aflibercept or bevacizumab as dictated by insurance approvals rather than study protocol, were performed at the discretion of the treating physician. In keeping with routine clinical care in this largely indigent and uninsured patient population, intravitreal injections were reserved for development of visually significant vitreous hemorrhage in the absence of tractional retinal detachment or for clinically significant DME, which was interpreted as foveal DME as determined by OCT imaging. However, because of difficulties with insurance and/or payment, some patients who met these criteria were unable to receive intravitreal injections during the study period.
Responses to PRP were assessed by J.F.R. and P.J.R. in an un-masked fashion. In assessing response to PRP, we defined progression of NV as obvious enlargement in the size of the areas of leakage on FA or enlargement in the size of the areas of NV based on detectable flow using SS-OCTA (see Results). Conversely, regression of NV was defined as an obvious decrease or absence of leakage on FA, or a decrease or absence of detectable flow in the NV using SS-OCTA (see Results). The caliber of NV vessels was not quantified, and larger-caliber versus smaller-caliber NV vessels were defined by their relative rather than absolute size.
RESULTS
Patient Demographics and Treatment
A total of 20 eyes with treatment-naive PDR from 15 patients were enrolled and treated (Table 1). Two additional patients dropped out after the informed consent was signed because they were unable to undergo SS-OCTA scanning: one patient was unable to position in the SS-OCTA machine due to body habitus and another patient was unable to maintain fixation. Twelve of the 20 eyes that were enrolled and treated met criteria for high-risk PDR.32 Seventeen of the 20 eyes underwent UWF FA, UWF fundus photography, and macular SD-OCT imaging at baseline. In 3 eyes of 2 patients, FA was not performed because of renal failure or fluorescein allergy. Hemoglobin A1C measurements were not obtained on 5 of 15 patients because they could not afford the test.
Table 1.
Clinical Characteristics of Study Patients
| Treatment-naïve eyes diagnosed with PDR | 20 |
| High-risk PDR | 12 |
| Age (SD) | 48.9 (11.5) |
| Gender | 53% female |
| Ethnicity | 80% Hispanic, 20% black |
| Type of DM | 87% type 2, 13% type 1 |
| Years since DM diagnosis (SD) | 11.3 (9.2) |
| HbA1C (SD) | 9.6 (2.4) |
| Comorbidities | 73% HTN, 27% HLD, 20% CAD |
| Oral DM medication use | 80% |
| Insulin use | 73% |
| BP systolic (SD) | 147 (27) |
| BP diastolic (SD) | 83 (17) |
| BMI (SD) | 29.6 (7.1) |
Values are reported as mean (standard deviation).
Abbreviations: PDR, proliferative diabetic retinopathy; DM, diabetes mellitus; HbA1C, hemoglobin A1C; HTN, hypertension; HLD, hyperlipidemia; CAD, coronary artery disease; BP, blood pressure; BMI, body mass index; SD, standard deviation.
The average patient age was 48.9 (standard deviation (SD), 11.5) years old. Type 2 diabetes predominated (87%). For those patients able to report an A1C, the average was 9.6 (SD, 2.4). Additional patient characteristics are listed in Table 1. Mean baseline BCVA was 20/50-2 (range, 20/20 to E at 1 foot; eETDRS: 63 letters, range 85 to 0 letters). At baseline, DME was present in 18 of 20 eyes. Foveal or juxtafoveal DME judged to be visually significant was present in 5 of 20 eyes at baseline. Mean BCVA at 1 week, 1 month, and 3 months were 20/50, 20/50, and 20/50-2 (range, 20/20 to CF; eETDRS: 65, 65, and 63 letters (range 85 to 0 letters)), respectively. There were no statistically significant changes in mean BCVA over the study; however, one eye experienced a large macular subhyaloid hemorrhage and BCVA declined from 20/20 at baseline to CF (eETDRS: 85 to 0 letters) at 3 months, another eye improved from E at 1 foot to 20/100 (eETDRS: 0 to 50 letters), and 2 eyes improved from 20/200 at baseline to 20/60+1 (eETDRS: 35 to 61 letters) at 1 week, but then they were lost to follow-up. The remainder of the eyes had BCVA measurements at 3 months that were within 3 lines (eETDRS: 15 letters) of baseline BCVA.
PRP was performed in a single session using argon laser in 16 of 20 eyes (80%) and PASCAL laser in 4 of 20 eyes (20%). Thirteen eyes were treated with argon laser by a single physician (J.F.R.). The remaining 7 eyes were treated by J.W.H., N.L.S., or K.C.F. The average number of burns was 2069 (SD: 398) in the argon group and 1274 (SD: 296) in the PASCAL group. Four eyes received a single intravitreal anti-VEGF injection at baseline and 2 eyes received injections at both 2 weeks and 2 months of follow-up. There were no adverse effects noted from either PRP or injections.
One patient (2 study eyes) was lost to follow-up after the 1 week visit. All other eyes underwent repeat SS-OCTA imaging 1 week, 1 month, and 3 months after PRP. Five follow-up SS-OCTA scans (2 scans for one eye, and 3 individual scans for 3 other eyes) were partially obscured by new vitreous hemorrhage. At the 3 month visit, 17 of 20 eyes again underwent UWF FA, UWF fundus photography, and macular SD-OCT.
SS-OCTA Characteristics of PDR at Baseline
All areas of multifocal NV seen on UWF fundus photography (Figure 1A) and on UWF FA (Figure 1B) within the macula or in close proximity to the arcades were captured on the SS-OCTA 12×12mm scans. On the SS-OCTA scans, these areas of multifocal NV were visualized on the total retinal slab en face images (Figure 1C). They appeared as irregular, convoluted masses of larger-caliber and smaller-caliber vessels. IRMAs were also apparent on the total retinal slabs (Figure 1C) and were readily classified as IRMAs rather than NV because of their absence on the corresponding VRI slabs (Figure 1D). For most eyes, the majority or all neovascular foci identified via UWF FA were present within the macula or close to the arcades and therefore were also present on the 12×12mm VRI slab (Figure 1B, D). The VRI slabs excluded the underlying retinal vasculature and facilitated identification and characterization of areas of NV (Figure 1D). VRI slabs were particularly helpful for differentiating small buds of NV from intraretinal MAs and IRMA from NVE, as well as for delineating the margins of large neovascular fronds. The VRI slabs alone were adequate for visualization of NV, but corresponding B-scans were always used to confirm that areas of NV seen on the VRI slabs were indeed superficial to the ILM and contained vascular flow (Figure 1F).
Figure 1. Wide field swept source OCT angiography (SS-OCTA) of a representative eye with treatment-naive proliferative diabetic retinopathy (PDR) at baseline.

(A) Ultra wide field (UWF) fundus photograph showing multifocal neovascularization (NV), mild vitreous hemorrhage, and featureless peripheral retina.
(B) UWF fluorescein angiography (FA) confirmed NVD (white arrowhead), multiple foci of NVE (yellow arrowheads) and peripheral ischemia.
(C) SS-OCTA 12×12mm en face total retinal slab showed multifocal NV. Intraretinal microvascular abnormalities (IRMAs) (blue asterisks) were also visualized but were difficult to distinguish from preretinal NV.
(D) SS-OCTA 12×12mm en face vitreoretinal interface (VRI) slab facilitated the identification of multifocal NV and the margins of individual areas of NV. NVD is highlighted with a white arrowhead, and select areas of identifiable NVE are highlighted with yellow arrowheads, as on the corresponding FA (B). Blue asterisks mark locations where IRMAs were seen on the total retinal slab (C) but not on the VRI slab (D). The dashed yellow lines in (C) and (D) depict the location of the cross-sectional B-scans shown in (E) and (F).
(E) B-scan corresponding to the yellow line in (C) showing flow (red represents flow within retinal vessels and green represents flow within choroidal vessels) and segmentation boundaries for the total retinal slab. The slab extends from the internal limiting membrane (ILM) to the retinal pigment epithelium (boundaries shown by dotted yellow lines). NV is included within the total retinal slab because automatic segmentation algorithms do not differentiate ILM from the inner surface of NV.
(F) B-scan corresponding to the yellow line in (D) showing flow and the segmentation boundaries for the VRI slab. The slab extends from 30μm deep to the ILM to 200μm superficial to the ILM. The B-scan in (F) shows disc NV (white arrowhead) defined as preretinal vessels with detectable flow, located within the VRI slab and shown by a white arrowhead in the en face image in (D).
The 12×12mm VRI slabs centered on the fovea were adequate for the diagnosis of both NV of the disc (NVD) and NV elsewhere (NVE) (Figures 2, 3). Multiple B-scans demonstrated NV growing along the posterior hyaloid (Figure 3G, 3H, 3N), which were in some cases associated with focal areas of retinal traction (Figure 3N). In one eye, fibrovascular proliferation (FVP) off the disc contained NV with flow, but there were also large areas without detectable flow on the VRI slab (Figure 2E) or corresponding B-scans (Figure 2H). In multiple eyes, UWF FA showed leakage along the course of a major retinal vessel that could have been consistent with NV or retinal vasculitis, but these areas were readily identified with SS-OCTA as NV using the corresponding VRI slabs and B-scans (Figure 3A, 3B). In total, 18 of 20 eyes (90%) at baseline had identifiable NV on the foveal-centered 12×12mm VRI slab. Of the remaining 2 eyes, a posterior pole montage of five 12×12mm scans in one eye readily captured multiple areas of peripheral NV seen on UWF FA (Figure 4). In the final eye, there was no NV detected with UWF FA or SS-OCTA, calling into question the diagnosis of PDR which had been made based on the presence on examination of multiple IRMAs mistakenly identified as preretinal NV and vitreous hemorrhage in the absence of a detectable retinal break. Altogether, wide field SS-OCTA identified NV at baseline in 100% of eyes in which NV was detectable using UWF FA.
Figure 2. Wide field swept source OCT angiography (SS-OCTA) from 3 representative eyes with neovascularization of the disc (NVD) at baseline.
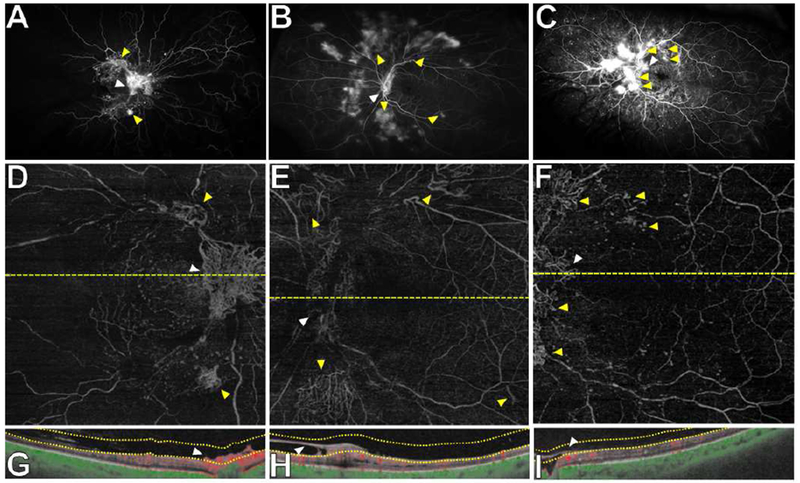
(A-C) Ultra wide field fluorescein angiography (FA) demonstrating NVD (white arrowheads) and neovascularization elsewhere (NVE) (yellow arrowheads).
(D-F) SS-OCTA 12×12mm vitreoretinal interface (VRI) slabs showing NVD (white arrowheads) and several areas of NVE (yellow arrowheads) as in the corresponding FA imaging (A-C). (G-H) Corresponding cross-sectional B-scans confirming that areas of NVD (white arrowheads) were located above the ILM as shown by the VRI slab boundaries.
Figure 3. Wide field swept source OCT angiography (SS-OCTA) from 8 representative eyes with neovascularization elsewhere (NVE) at baseline.

(A-D) SS-OCTA 12×12mm vitreoretinal interface (VRI) slabs showing areas of NVE (white arrowheads) confirmed on corresponding cross-sectional B-scans to be located above the ILM and containing detectable flow (white arrowheads in E-H). Four additional representative VRI slabs (I-L) and cross-sectional B-scans (M-P) are shown. Yellow arrowheads highlight additional areas of NV identified within the VRI slabs. Neovascularization of the disc (NVD) was seen in (C), (I), and (L) (yellow arrowheads). In all cases, NVE and NVD identified on the 12×12mm VRI slab corresponds with areas of leakage on ultra wide field fluorescein angiography (see Figures 2, 4, 5, 7).
Figure 4. Wide field posterior pole montage of swept source OCT angiography (SS-OCTA) scans for the identification of peripheral neovascularization (NV) at baseline in a representative eye.
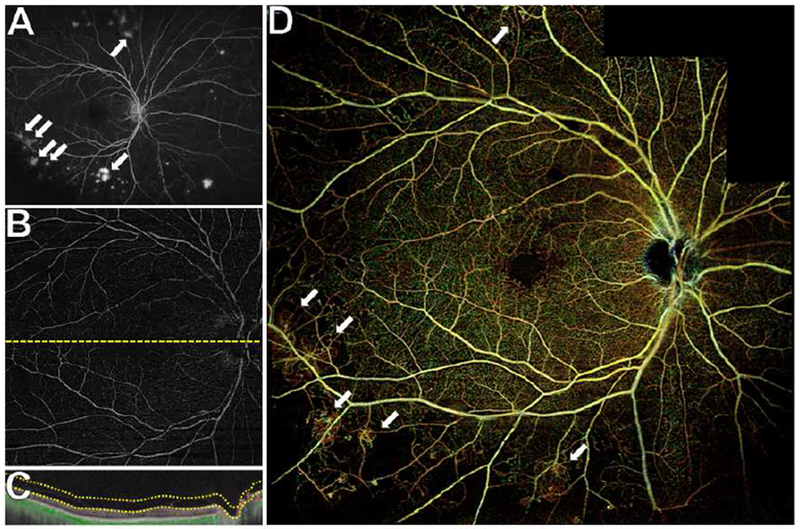
(A) Ultra wide field fluorescein angiography (UWF FA) shows multifocal peripheral NV (white arrows).
(B) The SS-OCTA 12×12mm vitreoretinal interface (VRI) does not capture the areas of NV seen on UWF FA. The corresponding cross-sectional B-scans (C) confirms proper segmentation of the VRI slab, and a careful search within the SS-OCTA 12×12mm scan centered on the fovea revealed no preretinal NV vessels with detectable flow.
(D) Posterior pole montage of 12×12mm SS-OCTA total retinal scans of the posterior pole shows most areas of peripheral NV that were seen on UWF FA but not on the single 12×12mm VRI slab (white arrows).
Longitudinal SS-OCTA of NV after PRP
After baseline imaging and PRP, eyes were serially imaged at 1 week, 1 month, and 3 months with SS-OCTA, and repeat UWF FA was performed at 3 months. Posterior pole 12×12mm montages enabled visualization of essentially all areas of NV detected on UWF FA. SS-OCTA imaging was able to monitor NV located ~6 disc diameters in all directions from the arcades and disc (Figure 5).
Figure 5. Wide field posterior pole montage of swept source OCT angiography (SS-OCTA) scans for monitoring progression of neovascularization (NV) after panretinal photocoagulation (PRP) in a representative eye.
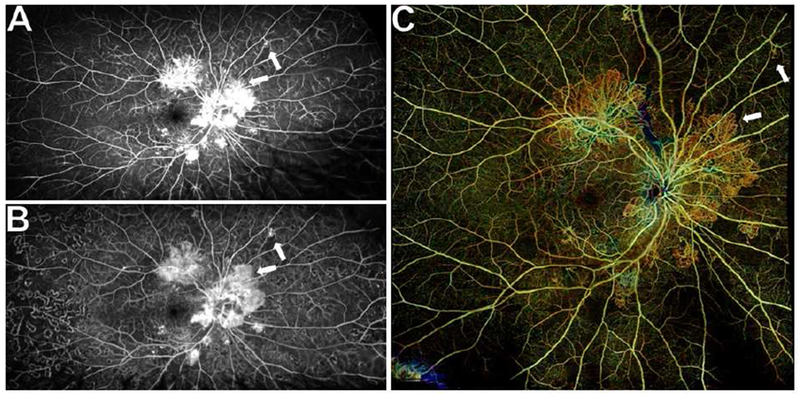
(A) Ultra wide field fluorescein angiography (UWF FA) showing multifocal NV at baseline.
(B) UWF FA 3 months after PRP demonstrating PRP scars and less intense leakage from areas of NV, but some areas of NV appear larger relative to baseline (white arrows).
(C) Wide field posterior pole total retinal slab montage of 12×12mm SS-OCTA scans 3 months after PRP showing all the areas of peripheral NV that were seen on UWF FA. Areas of NV up to ~6 disc diameters from the arcades and disc in all directions were captured. Areas of NV that changed after PRP on UWF FA demonstrated similar changes on the posterior pole montage. A pre-treatment montage of scans was not obtained, but corresponding 12×12mm SS-OCTA scans from before PRP and three time-points after PRP are shown in Figure 6.
Of the 17 eyes with both baseline and 3 month UWF FA, based on the interpretation of FA alone, 8 eyes (47%) were judged to have progressed and 9 eyes (53%) to have regressed. Upon parallel, unmasked interpretation of corresponding SS-OCTA images, all eyes judged to have progressed on UWF FA were also seen to progress on SS-OCTA, and vice versa.
In those eyes that progressed at 3 months despite PRP, the VRI slabs at 1 week after treatment did show a decrease in the density of smaller-caliber vessels with detectable flow within NV fronds (Figure 6A, 6B), while showing no significant changes in the larger-caliber vessels that constituted the perimeter and the internal major branches of the NV (Figure 6A, 6B). At 1 month, the density of smaller-caliber vessels with detectable flow had returned to the density seen at baseline, and there was again no change in the larger-caliber vessels within the NV (Figure 6A, 6C). However, at 3 months, new smaller-caliber neovascular vessels in some NV fronds were observed to sprout at the margins of the NV (Figure 6D). In smaller foci of NV, which consisted predominantly of larger-caliber rather than smaller-caliber vessels at baseline, the larger-caliber vessels were seen to remodel or to grow in length as the NV expanded in size (Figure 6D). In some large fronds of NV, this same phenomenon of larger-caliber vessel remodeling with linear expansion was observed at the margins of NV as well (Figure 6D). In cases of neovascular expansion, the corresponding B-scans demonstrated enlargement and growth of NV along the posterior hyaloid (Figure 6E-H). This progression of NV seen on SS-OCTA corresponded to neovascular progression on UWF FA (Figure 5A, 5B), although in some cases, the FA at 3 months showed less intense leakage (Figure 5B), which might have been interpreted as an adequate response to PRP in the absence of the corresponding SS-OCTA images that showed progression of NV (Figure 6D). Therefore, leakage and growth of NV are not synonymous for disease progression in PDR, and SS-OCTA VRI slab imaging allowed for precise characterization of the vascular changes associated with neovascular progression that may not be possible using the corresponding FA images (Figure 5B, 6D).
Figure 6. Longitudinal wide field swept source OCT angiography (SS-OCTA) showing the progression of neovascularization (NV) after panretinal photocoagulation (PRP) in a representative eye.

(A) Baseline SS-OCTA 12×12mm vitreoretinal interface (VRI) slab showing multifocal NV also seen on the corresponding ultra wide field fluorescein angiography (UWF FA) (Figure 5A). Large NV fronds consisted of convoluted masses of smaller-caliber vessels interspersed between larger-caliber vessels that define the perimeter and internal branches. Smaller foci of NV consisted predominantly of larger-caliber vessels.
(B) SS-OCTA 12×12mm VRI slab 1 week after PRP. Note that within large NV fronds there appears to be a decrease in the density of smaller-caliber vessels with detectable flow. The larger-caliber vessels appear unchanged.
(C) SS-OCTA 12×12mm VRI slab 1 month after PRP. The density of smaller-caliber vessels with detectable flow returned to baseline levels. Again, the larger-caliber vessels were unchanged.
(D) SS-OCTA 12×12mm VRI slab 3 months after PRP. In some areas, smaller-caliber neovascular vessels appear to sprout at the margins of NV (white arrowhead). In other areas, the larger-caliber neovascular vessels remodeled or grew in length such that the neovascular lesion expanded in size (yellow arrowheads). These changes in the NV seen on SS-OCTA corresponded to changes seen on UWF FA (Figure 5B), but SS-OCTA allowed for more precise characterization of the microvascular changes associated with progression.
(E-H) Corresponding cross-sectional B-scans demonstrating enlargement and growth of NV along the posterior hyaloid (white arrowheads). The B-scan was positioned to highlight sprouting of small-caliber neovascular vessels along the margin of the NV frond (white arrowhead). The B-scan positions appear to vary slightly because of decentration of the en face images, but they are in fact aligned to the retinal vessels to ensure the exact position is depicted for all four scans.
In those eyes that demonstrated regression of NV after treatment, posterior pole 12×12mm montages were adequate for visualizing longitudinal neovascular regression in virtually all NV seen on UWF FA (Figure 7). While some serial VRI slabs showed decreased density of smaller-caliber vessels with detectable flow at 1 week (Figure 8A, 8B), by 1 month, there was a marked decrease in smaller-caliber vessels with detectable flow (Figure 8C). In some cases, especially in the large fronds of NV, larger-caliber vessels continued to have detectable flow (Figure 8C, 8D), but there was no leakage on FA (Figure 7B). As NV regressed, B-scans often showed focal release of the posterior hyaloid (Figure 8E-H).
Figure 7. Wide field posterior pole montage of swept source OCT angiography (SS-OCTA) scans showing regression of neovascularization (NV) after panretinal photocoagulation (PRP) in a representative eye.
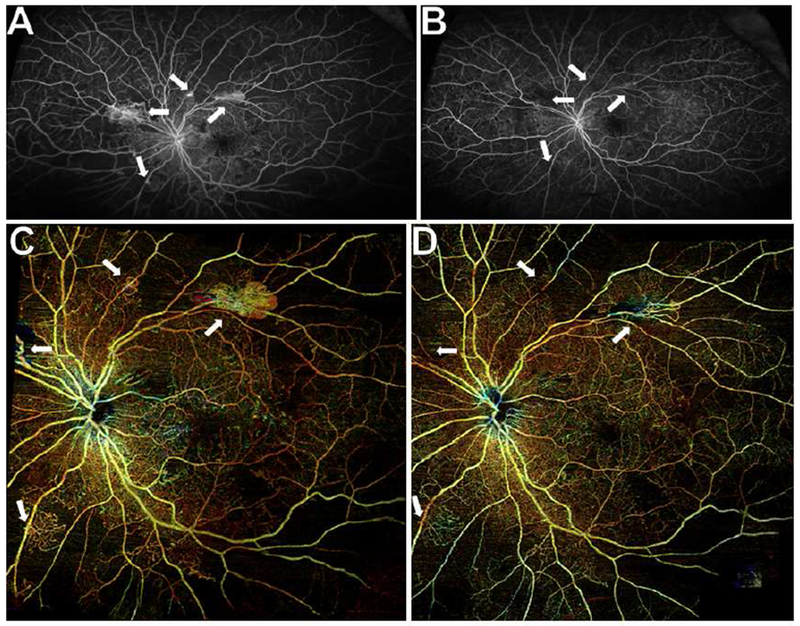
(A) Ultra wide field fluorescein angiography (UWF FA) showing multifocal NV at baseline (white arrows).
(B) UWF FA 3 months after PRP demonstrating PRP scars and complete disappearance of leakage (white arrows). This eye also received supplemental intravitreal aflibercept for subfoveal macular edema 2 weeks and 2 months after PRP.
(C) Baseline wide field total retinal posterior pole montage of SS-OCTA scans showing all areas of peripheral NV that were seen on UWF FA (white arrows).
(D) At 3 months after PRP, wide field posterior pole montage of SS-OCTA scans showing regression of NV that mirrored those changes seen on UWF FA (white arrows).
Corresponding 12×12mm SS-OCTA scans from before PRP and three time-points after PRP are shown in Figure 8.
Figure 8. Longitudinal wide field swept source OCT angiography (SS-OCTA) showing regression of neovascularization (NV) after panretinal photocoagulation (PRP) in a representative eye.

(A) The baseline SS-OCTA 12×12mm vitreoretinal interface (VRI) slab showing multifocal NV also seen on the corresponding ultra wide field fluorescein angiography (UWF FA) (Figure 7A).
(B) SS-OCTA 12×12mm VRI slab 1 week after PRP. Note that within the large NV frond the density of smaller-caliber vessels with detectable flow is decreased. The larger-caliber vessels were unchanged.
(C) SS-OCTA 12×12mm VRI slab 1 month after PRP. The smaller-caliber vessels with detectable flow have largely disappeared. Some larger-caliber vessels with detectable flow remain.
(D) SS-OCTA 12×12mm VRI slab 3 months after PRP, demonstrating similar findings to (C). There was no recurrence of smaller-caliber vessels and some larger-caliber vessels continue to have detectable flow, though there is no leakage on FA (Figure 7B).
(E-H) Cross-sectional B-scans corresponding to the dashed blue lines in (A-D) showing disappearance of preretinal NV vessels with detectable flow (white arrowheads). Regression of NV was associated with focal release of the posterior hyaloid (left arrowhead in E-H).
(I-L) Cross-sectional B-scans corresponding to the dashed yellow lines in (A-D) showing that larger-caliber neovascular vessels (yellow arrowheads) continue to be located in the preretinal space and have detectable flow. The B-scan positions appear to vary slightly because of decentration of the en face images, but they are in fact aligned to the retinal vessels to ensure the exact positions are depicted for all four time-points.
A total of 6 eyes received supplemental intravitreal anti-VEGF injections, but 2 of these eyes were lost to follow-up after 1 week. Of the remaining 4 eyes that were injected, 2 demonstrated complete regression of NV on UWF FA at 3 months. These 2 eyes also demonstrated near-complete regression of NV on SS-OCTA, although some larger-caliber vessels in large neovascular fronds were still present at 3 months. Of the 14 eyes that received PRP alone, 2 had complete regression of NV on UWF FA, which was also visualized using SS-OCTA. These differences were not statistically significant, and there were no apparent qualitative differences in neovascular progression or regression in the PRP alone versus the combined PRP/anti-VEGF groups.
DISCUSSION
Wide field SS-OCTA imaging at baseline and 3 months after PRP appeared comparable to UWF FA in its ability to identify and monitor NV in eyes with treatment-naïve PDR. At baseline, wide field SS-OCTA was able to distinguish between IRMA and NV, and to identify the neovascular foci seen on UWF FA within the same field of view. The only neovascular foci that could not be identified using wide-field SS-OCTA were located in the far periphery, but evidence of NV was identifiable within the SS-OCTA field of view in every case. Furthermore, SS-OCTA was able to monitor eyes longitudinally for the progression or regression of multifocal NV in response to treatment over 3 months, and the treatment results documented by SS-OCTA were the same as those documented by UWF FA. In addition, SS-OCTA was able to identify microvascular changes within NV associated with progression or regression during the 3-month follow-up period, and identify microvascular changes previously unidentified due to the limitations of FA. While FA can identify leakage and overall growth of NV, SS-OCTA imaging can identify the subtle alterations in flow within a lesion that can serve as harbingers of disease progression. SS-OCTA has the added advantage of being able to be used more frequently and more safely than FA, and wide field SS-OCTA provides the equivalent of early fluorescein angiographic transit time images for both eyes. Our findings demonstrate the correspondence and critical benefits of wide field SS-OCTA over UWF FA in the clinical management of PDR.
In our study, all areas that leaked on FA and were within the field of view of the corresponding SS-OCTA scans were identified as preretinal NV with flow. While it remains an open question whether the sensitivity of SS-OCTA for detecting flow in NV matches the sensitivity of FA for detecting flow-associated leakage, we did find that all areas of NV that leaked on FA were associated with flow on SS-OCTA and all areas of regressed NV that no longer leaked on FA were either associated with no flow on SS-OCTA or associated with flow in some larger-caliber vessels. Of course, a common limitation of both FA and SS-OCTA is that the absence of detectable flow does not necessarily mean an absence of any flow, so low levels of blood flow may still be present, but not detectable using either technology.
SS-OCTA was able to detect at least one area of NV in 90% of PDR eyes with a single 12×12mm scan centered on the fovea. The SS-OCTA 12×12mm montages of the posterior pole were able to capture almost all areas of peripheral NV because the montages extended ~6 disc diameters from the macula and few NV foci were beyond this field of view. This is consistent with prior studies that have shown the overwhelming majority of NV in PDR were located in the posterior pole.33–35 The ability to capture most areas of NV with a single 12×12mm scan or by using the wider 12×12mm montaged field of view highlights the advantages of SS-OCTA over SD-OCTA, which has a smaller field of view and requires targeted scans of areas with NV.15,20,30
The ability of FA to detect leakage from NV has made FA an important imaging method for diagnosing PDR for over 50 years.5,6 NV as identified by FA has been traditionally defined as areas of preretinal NV that leak fluorescein, but interpretation of images can be challenging because FA cannot differentiate the preretinal and intraretinal vasculature. In contrast, OCTA localizes vessels as preretinal or intraretinal. Though current OCTA techniques do not provide information on vascular leakage, OCTA does detect subtle flow within vessels. Moreover, PDR leakage as visualized with FA is only a surrogate marker for presumed NV, whereas OCTA documents the NV itself.
Longitudinal SS-OCTA scans were able to readily identify gross changes in NV after PRP, such as the enlargement or disappearance of areas of NV, that were seen on FA. In addition, the repeatability and resolution of SS-OCTA enabled us to make novel observations about the response of NV to PRP by carrying out what is, to our knowledge, the first study to perform any kind of angiography one week after PRP in a cohort of eyes with PDR. One week after PRP, we observed a decrease in the density of smaller-caliber vessels with detectable flow regardless of whether those neovascular foci eventually progressed or regressed at 3 months. However, by 1 month after PRP, the density of smaller-caliber vessels with detectable flow had returned to baseline levels in the foci of NV that ultimately progressed. In contrast, in NV that regressed at 3 months, the smaller-caliber vessels with detectable flow had partially or completely regressed by 1 month.
On OCTA, the response of larger-caliber NV vessels after treatment varied and early changes seemed to correlate with eventual regression or progression. Interestingly, in the NV that progressed, larger-caliber vessels were essentially unchanged at 1 week and 1 month, but had begun to remodel and expand at 3 months. In NV that regressed, larger-caliber vessels were unchanged at 1 week, but some had no detectable flow at 1 month. In those larger-caliber vessels with detectable flow at 1 month, there was still flow at 3 months, though there was no associated leakage on FA.
Since larger-caliber vessels can continue to have detectable flow on OCTA despite a complete absence of leakage on FA (Figures 7, 8), their presence after treatment may not be an adequate rationale for re-treatment. On the other hand, when larger-caliber vessels are observed to remodel and enlarge, it likely represents progression of the NV (Figure 5D). Similarly, the growth of smaller-caliber vessels at the margin of NV suggested progression (Figure 5D). Altogether, our findings are consistent with those of Ishibazawa et al.20 in that both smaller-caliber and larger-caliber preretinal vessels are associated with leakage on FA and should be considered NV, but that smaller-caliber vessels have more intense leakage on FA at baseline. Possibly, larger-caliber NV vessels are less responsive to changes in VEGF levels, although the differential responses of larger-caliber and smaller-caliber vessels to PRP could also be related to shunting of vascular flow, or an alternative mechanism. In any case, these patterns of microvascular changes in NV after PRP could not have been previously characterized using FA because it is not practical to perform FA frequently and fluorescein leakage obscures detailed visualization of the NV.
While there is general agreement that NV regresses after PRP, there is uncertainty as to the time-frame and extent of regression.2 Studies have been limited by different PRP protocols, follow-up periods, and definitions of regression. In general, complete regression of NV after PRP, defined as total absence of leakage on FA, is uncommon.2 For example, in the Diabetic Retinopathy Study (DRS), only 20% of the eyes treated with single-session argon PRP had complete regression of NV at 1 year.36 Of the 14 eyes in our study that received PRP alone, we observed complete regression in 2 eyes (14%) at 3 months. Recent evidence suggests that supplementation of PRP with intravitreal anti-VEGF agents may hasten and increase the extent of neovascular regression.37–39 We observed complete regression of NV at 3 months in 2 of 4 eyes (50%) that received PRP plus an anti-VEGF treatment, versus 14% in the PRP-only group. Importantly, patients that regressed to no leakage on FA also regressed to no flow on OCTA, with the exception of some larger-caliber NV vessels, which continued to have flow on OCTA but did not leak on FA. However, the sample sizes in our study were too small to draw statistical conclusions or for quantitative assessment of neovascular regression on longitudinal SS-OCTA. Future, larger studies using wide field SS-OCTA should more precisely define both the time-course and extent of neovascular regression after treatment.
Currently, clinical decisions about whether to treat or re-treat PDR with PRP or intravitreal anti-VEGF therapies are typically based on the presence of NV, presence of visually significant DME, the response of NV and DME to treatment, and new onset vitreous hemorrhage. As we have shown, once the areas of NV have been identified, SS-OCTA imaging can be used to follow eyes for the progression or regression of NV in response to treatment. Our observation that smaller-caliber vessels with detectable flow decrease in density 1 week after PRP, but then may increase in density after 1 month, could serve as an early marker of disease progression. This may enable determination of success or failure of PRP sooner than was previously possible, such that further treatment can be initiated promptly in eyes at high risk for progression despite PRP. Alternatively, evidence of microvascular changes, such as smaller-caliber NV vessel sprouting or larger-caliber NV vessel remodeling or linear expansion, on the SS-OCTA at 3 months, could indicate progression of NV and impel the clinician to re-treat. Importantly, wide field SS-OCTA simultaneously provides detailed information about NV via en face angiography slabs and about DME via B-scans. This combination encompasses all of the imaging information that a clinician relies on to decide upon treatment in PDR. Therefore, our study suggests that in most cases, wide field SS-OCTA alone may be the only imaging modality that is needed for the clinical management of PDR.
OCTA has a number of additional advantages compared to FA. It is inexpensive to perform, non-invasive, easily repeatable, and safe. It may be the only practical means of angiography in patients with fluorescein allergy, renal failure, or difficult intravenous access, as it was for 3 eyes in our study. OCTA also enabled differentiation of NV from large vessel vasculitis, and distinguished areas of FVP with regressed NV from active NV (Figure 2B, E). The B-scans that corresponded to en face angiograms provided information about whether NV is subhyaloid, growing along the posterior hyaloid, or associated with retinal traction. Also, cross-sectional B-scans can be used to identify DME and other OCT signs of diabetic retinopathy. Notable disadvantages of OCTA are that patients must have adequate visual acuity to fixate on a target, whereas FA does not require fixation, and OCTA performs less well than FA in the presence of vitreous hemorrhage. Currently, the disadvantages of SS-OCTA instruments include that they are more expensive than traditional SD-OCT instruments, although comparable to the cost of UWF FA instruments, and a disadvantage of the OCTA procedure is that it is not yet reimbursed separately by Medicare or private insurances in the U.S.
Our study has a number of limitations. It involved only 20 eyes, the follow-up period was just 3 months, and our study was only prospective in the sense that eyes were serially imaged during the course of routine clinical care. There was no true standardization of treatment, although routine care of the patients was similar and the majority were treated by a single physician. Assessment of responses to PRP was subjective, and there was no masking. Other limitations of our study include the lack of FA in 3 eyes, although these 3 eyes had florid NV on fundus exam and OCTA, and the loss of 2 eyes to follow-up. Also, we did not analyze the eyes based upon the presence or progression of DME. Finally, we did not quantify the number and size of neovascular foci. Questions to be investigated in future prospective studies include the concordance in diagnosis of NV and PDR by masked clinicians using UWF FA versus wide field SS-OCTA, whether SS-OCTA imaging is adequate to monitor the response of NV to PRP or whether peripheral NV not captured by wide field SS-OCTA imaging behaves differently, whether PRP affects macular perfusion, either immediately after PRP and months later, whether macular ischemia worsens after PRP (Figure 2D), whether vascular changes are associated with the development and worsening of DME after PRP, and whether PRP affects choriocapillaris perfusion. Ideally, all clinically significant NV (CSNV) will lie within the fovea-centered 12×12mm SS-OCTA montaged field of view and these areas of NV can be used as sentinel vessels capable of reflecting disease activity without requiring the need to image any NV in the far periphery that is outside the field of view of the montaged SS-OCTA images.
In summary, longitudinal wide field SS-OCTA imaging was compared with UWF FA imaging before and after PRP of treatment-naive eyes with PDR. We found that in the same field of view, wide field SS-OCTA could identify areas of NV as well as UWF FA, and that changes in NV seen on FA could be monitored longitudinally using wide field SS-OCTA. Moreover, SS-OCTA was better than FA at providing detailed imaging of NV that could be useful for assessing the likelihood of disease progression within 1 month of PRP. Taken together, these observations suggest a novel approach to clinical management of PDR using information from SS-OCTA alone. Whether SS-OCTA imaging can serve as a stand-alone imaging modality comparable or superior to UWF FA for the detection, monitoring, and management of NV in PDR remains to be determined and will require a prospective randomized study.
Wide field swept source optical coherence tomography angiography was comparable to ultra wide field fluorescein angiography in detecting neovascularization in diabetic retinopathy while providing clinically useful information about the response of neovascularization to panretinal photocoagulation.
ACKNOWLEDGMENTS
We thank Ying Chen, MD, Jacob Duker, MD, Michelle Falcone, MD, Alexandra Levitt, MD, and Van Ann Tran, MD, for referring patients.
Financial Support: Research supported by grants from Carl Zeiss Meditec, Inc. (Dublin, CA), the Salah Foundation, an unrestricted grant from the Research to Prevent Blindness, Inc. (New York, NY), and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organizations had no role in the design or conduct of this research.
Dr. Gregori and Dr. Rosenfeld received research support from Carl Zeiss Meditec, Inc. Dr. Gregori and the University of Miami co-own a patent that is licensed to Carl Zeiss Meditec, Inc. Dr. Rosenfeld also received additional research support from Genentech; consultancy for Allergan, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, Genentech, Healios K.K., F. Hoffmann-La Roche Ltd., Isarna Pharmaceuticals, MacRegen Inc., Ocudyne, Ocunexus Therapeutics, and Unity Biotechnology; equity interests in Apellis, Digisight, and Ocudyne.
Abbreviations/Acronyms:
- SS
swept-source
- OCTA
optical coherence tomography angiography
- PRP
panretinal photocoagulation
- DR
diabetic retinopathy
- PDR
proliferative diabetic retinopathy
- NV
neovascularization
- DME
diabetic macular edema
- FA
fluorescein angiography
- UWF
ultra wide-field
- VRI
vitreoretinal interface
- IRMA
intraretinal microvascular abnormalities
- MAs
microaneurysms
- FVP
fibrovascular proliferation
- NVD
neovascularization of the disc
- NVE
neovascularization elsewhere
- VEGF
vascular endothelial growth factor
- CSNV
clinically significant neovascularization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The other authors have no disclosures.
REFERENCES
- 1).Zhang X, Saaddine JB, Chou CF, et al. 2010. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 304: 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Schachat AP, Wilkinson CP, Hinton DR, Sadda SR, Wiedemann P, eds. 2017. Ryan’s Retina, 6th edition Edinburgh: Elsevier. [Google Scholar]
- 3).Novotny HR, Alvis DL. 1961. A method of photographing fluorescence in circulating blood in the human retina. Circulation 24: 82–86 [DOI] [PubMed] [Google Scholar]
- 4).Gass JDM, Sever RJ, Sparks D, Goren J. 1967. A combined technique of fluorescein funduscopy and angiography of the eye. Arch Ophthalmol 78: 455–461 [DOI] [PubMed] [Google Scholar]
- 5).Norton EWD, Gutman F. 1965. Diabetic retinopathy studied by fluorescein angiography. Trans Am Ophthalmol Soc, 63: 108–128 [PMC free article] [PubMed] [Google Scholar]
- 6).Gass JDM. 1968. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. IV. Diabetic retinal angiopathy. Arch Ophthalmol 80: 583–591 [DOI] [PubMed] [Google Scholar]
- 7).Kwiterovitch KA, Maguire MG, Murphy RP, et al. 1991. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology 98: 1139–1142 [DOI] [PubMed] [Google Scholar]
- 8).Choi W, Mohler KJ, Potsaid B, et al. 2013. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One 11: e81499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Huang Y, Zhang Q, Thorell MR, et al. 2014. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina 45: 382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Schaal KB, Legarreta AD, Gregori G, et al. 2015. Widefield en face optical coherence tomography imaging of subretinal drusenoid deposits. Ophthalmic Surg Lasers Imaging Retina 46: 550–559 [DOI] [PubMed] [Google Scholar]
- 11).Sun JK, Lin MM, Lammer J, et al. 2014. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol 132: 1309–1316 [DOI] [PubMed] [Google Scholar]
- 12).Wang JC, Lains I, Providencia J, et al. 2017. Diabetic choroidopathy: choroidal vascular density and volume in diabetic retinopathy with swept-source optical coherence tomography. Am J Ophthalmol 184: 75–83 [DOI] [PubMed] [Google Scholar]
- 13).Agemy SA, Scripsema NK, Shah CM, et al. 2015. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 35: 2353–2363 [DOI] [PubMed] [Google Scholar]
- 14).Couturier A, Mane V, Bonnin S, et al. 2015. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina 35: 2384–2391 [DOI] [PubMed] [Google Scholar]
- 15).De Carlo TE, Chin AT, Bonini Filho MA, et al. 2015. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 35: 2364–2370 [DOI] [PubMed] [Google Scholar]
- 16).Takase N, Nozaki M, Kato A, et al. 2015. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 35: 2377–2383 [DOI] [PubMed] [Google Scholar]
- 17).De Carlo TE, Bonini Filho MA, Baumal CR, et al. 2016. Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 47: 115–119 [DOI] [PubMed] [Google Scholar]
- 18).Freiberg FJ, Pfau M, Wons J, et al. 2016. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 254: 1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Hwang TS, Gao SS, Liu L, et al. 2016. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmology 134: 367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ishibazawa A, Nagaoka T, Yokota H, et al. 2016. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57: 6247–6255 [DOI] [PubMed] [Google Scholar]
- 21).Salz DA, de Carlo TE, Adhi M, et al. 2016. Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol 134: 644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Stanga PE, Papayannis A, Tsamis E, et al. 2016. New findings in diabetic maculopathy and proliferative disease by swept-source optical coherence tomography angiography. Dev Ophthalmol 56: 113–121 [DOI] [PubMed] [Google Scholar]
- 23).Choi W, Waheed NK, Moult EM, et al. 2017. Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina 37: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Gill A, Cole ED, Novais EA, et al. 2017. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retina Vitreous 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Krawitz BD, Mo S, Geyman LS, et al. 2017. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res 139: 177–186 [DOI] [PubMed] [Google Scholar]
- 26).Moein HR, Novais EA, Rebhun CB, et al. 2017. Optical coherence tomography angiography to detect macular capillary ischemia in patients with inner retinal changes after resolved diabetic macular edema. Retina doi: 10.1097/IAE.0000000000001902 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 27).Pedinielli A, Bonnin S, Sanharawai ME, et al. 2017. Three different optical coherence tomography angiography measurement methods for assessing capillary density changes in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina 48: 378–384 [DOI] [PubMed] [Google Scholar]
- 28).Samara WA, Shahlaee A, Adam MK, et al. 2017. Quantification of diabetic macular ischemia using optical coherence tomography and its relationship with visual acuity. Ophthalmology 124: 235–244 [DOI] [PubMed] [Google Scholar]
- 29).Ting DSW, Tan GSW, Agrawal R, et al. 2017. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol 135: 306–312 [DOI] [PubMed] [Google Scholar]
- 30).Zhang X, Wu C, Zhou LJ, et al. 2018. Observation of optic disc neovascularization using OCT angiography in proliferative diabetic retinopathy after intravitreal conbercept injections. Sci Rep 8: 3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Gregori N, Feuer W, Rosenfeld PJ. 2010. Novel method for analyzing Snellen visual acuity measurements. Retina 30: 1046–1050 [DOI] [PubMed] [Google Scholar]
- 32).Early Treatment Diabetic Retinopathy Study Research Group. 1991. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 98: 823–833. [PubMed] [Google Scholar]
- 33).Taylor E, Dobree JH. 1970. Proliferative diabetic retinopathy. Site and size of initial lesions. Br J Ophthalmol 54: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Feman SS, Leonard-Martin TC, Semchyshyn TM. 1998. The topographic distribution of the first sites of diabetic retinal neovascularization. Am J Ophthalmol 125: 704–706 [DOI] [PubMed] [Google Scholar]
- 35).Jansson RW, Froystein T, Krohn J. 2012. Topographical distribution of retinal and optic disc neovascularization in early stages of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 53: 8246–8252 [DOI] [PubMed] [Google Scholar]
- 36).Diabetic Retinopathy Study (DRS) Research Group. 1978. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of the Diabetic Retinopathy Study findings. Ophthalmology 85: 82–106 [DOI] [PubMed] [Google Scholar]
- 37).Tonello M, Costa RA, Almeida FP, et al. 2008. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol 86: 385–389 [DOI] [PubMed] [Google Scholar]
- 38).Zhou AY, Zhou CJ, Yao J, et al. 2016. Panretinal photocoagulation versus panretinal photocoagulation plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy. Int J Ophthalmol 9: 1772–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Figueira J, Fletcher E, Massin P, et al. 2018. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology 125: 691–700 [DOI] [PubMed] [Google Scholar]


