Abstract
Selection of a proper spinal cord injury (SCI) rat model to study therapeutic effects of cell transplantation is imperative for research in cardiovascular functional recovery, due to the local harsh milieu inhibiting cell growth. We recently found that a crushed spinal cord lesion can minimize fibrotic scarring and grafted cell death compared with open-dura injuries. To determine if this SCI model is applicable for studying cardiovascular recovery, we examined hemodynamic consequences following crushed SCI and tested cardiovascular responses to serotonin (5-HT) or dopamine (DA) receptor agonists. Using a radio-telemetric system, multiple cardiovascular parameters were recorded prior to, 2, and 4 weeks after SCI, including resting mean arterial pressure (MAP) and heart rate (HR), as well as spontaneous or colorectal distension (CRD)-induced autonomic dysreflexia (AD). The results showed that this injury caused tachycardia at rest as well as the occurrence of spontaneous or artificially induced dysreflexic events. Four weeks post-injury, specific activation of 5-HT2A receptors by subcutaneous (s.c.) or intrathecal (i.t.) delivery of Dimethoxy-4-iodoamphetamine (DOI) remarkably increased resting MAP levels in a dose-dependent fashion. During CRD-induced autonomic dysreflexia, systemic administration of DOI alleviated the severity of bradycardia responsive to episodic hypertension. In contrast, selective stimulation of 5-HT1A receptors with 8-OH-DPAT or non-selective activation of DA receptors with apomorphine did not affect cardiovascular performance. Thus, crush injuries induce cardiovascular abnormalities in rats that are sensitive to 5-HT2A receptor stimulation, indicating a reliable SCI model to study how cell-based approaches impact the severity of autonomic dysreflexia and identify a possible target for pharmacological interventions.
Keywords: autonomic dysreflexia, blood pressure, heart rate, serotonin receptors, spinal cord injury
Introduction
Spinal cord injury (SCI) affects multiple organ systems. When supraspinal vasomotor pathways are interrupted in a high-level injury, spinal sympathetic activity loses cerebral regulation, which results in disordered hemodynamics at rest and the development of autonomic dysreflexia (AD). AD manifests as an incidence of hypertension accompanied by bradycardia in response to visceral or somatic stimuli below the level of injury, such as a full bladder or bowel.1 It usually occurs after severe SCI above the sixth thoracic (T6) level. The high blood pressure (BP) causes some severe symptoms, including sweating, flushing, headaches, and nausea, which decrease the patient's quality of life.2 Cardiovascular dysfunction has been shown to be one of leading causes of death in patients with SCI.3,4 Currently, there is no definitive clinical intervention that can effectively rectify unbalanced hemodynamics and prevent the occurrence of AD.
SCI-induced hemodynamic disorders are mainly attributed to the loss of supraspinal autonomic control. The descending axonal transportation of neurotransmitters to the lower spinal cord is interrupted following injury. Due to refractory regenerative capabilities, cell transplantation holds a promising prospect that can not only reconnect the damaged neuronal circuits, but also provide neurotransmitters to the caudal spinal cord. Indeed, many studies have demonstrated that transplanting early-stage neurons into the injured spinal cord reconstitutes impaired neuronal pathways by means of signal relay mechanisms, leading to improved somatic and autonomic functions.5–7 Previously, we transplanted embryonic brainstem-derived neural stem cells/progenitors into the rat injured spinal cord and found that grafted cells survived and integrated to the host tissue. Numerous graft-derived axonal extensions topographically projected to the caudal autonomic regions and synapsed onto sympathetic pre-ganglionic neurons (SPNs). As a result, basal BP recovered to normal levels and the severity of AD was remarkably mitigated.5 Despite the accomplishment of partial cardiovascular restoration, however, this approach is still hindered by some negative factors, especially severe fibrotic scarring and cavitation in the lesion site, which limit the extent of graft-host integration in the local milieu.8 To study SCI-induced cardiovascular dysfunction, animal models with mild SCIs are not suitable due to sparing of supraspinal vasomotor pathways that enable the maintenance of BP and heart rate (HR).9 Thus, establishing an SCI model that is severe enough to cause cardiovascular dysfunction and lacks heavy scarring and cavitation is pivotal to enhance graft survival and integration in current cell-based therapeutic strategies.
We recently compared several rat models with completely injured spinal cords, produced with different surgical techniques, that is, transection, aspiration, and crush. The complete crush injury generates minimum fibrotic scarring and cyst formation, and is more hospitable for grafted cell growth and integration when transplanting embryonic neural stem cells.8 Accordingly, this injury model is ideal for studying the effect of transplanted cells. To identify if the complete spinal cord crush gives rise to disordered cardiovascular consequences, such as hypotension, tachycardia, and development of AD, we examined hemodynamic parameters in this rat model with SCI.
Under normal conditions, serotonin (5-hydroxytryptamine, 5-HT) contributes to sympathetic tone via their receptors to maintain a certain level of BP. 5-HT1A and 5-HT2A receptors are distributed to the spinal cord and involved in cardiovascular performance.10–12 In parallel, previous studies reported the presence of dopamine (DA) receptors in the spinal cord that participate in the regulation of multiple body functions.13 Recent investigations revealed that specific interneurons are capable of synthesizing DA or have the potential to produce serotonin after an SCI interrupts descending neurotransmitter transport.14,15 Albeit their spinal receptors may undergo plasticity to up/downregulate activity, it is evidenced that these neurotransmitter receptors remain present and functional in the spinal cord after injury regardless of the lack of supraspinal inputs.10 Therefore, these spinal receptors could be a potential target for drug treatment to repair cardiovascular function. The purpose of this study was first to characterize cardiovascular consequences of a crushed rat SCI model, and then to define the effects of stimulating spinal serotonin or DA receptors on hemodynamic performance.
Methods
Animals
Adult female Fischer 344 rats (150–175 g, n = 12) were used for experiments. All rats were cared for and treated according to Institutional Animal Care and Use Committee and Society for Neuroscience guidelines throughout all experiments. The animals were housed three per micro-isolator cage. All cages were maintained in a temperature and light-cycle controlled environment.
Telemeter implantation
The naïve rats underwent anesthesia through inhalation of 4% isoflurane for initial surgery preparation, and later were given 2% isoflurane for sustainment during surgery. A skin incision was made in the midline of the ventral abdomen and the muscle layer was opened to expose the abdominal cavity. The organs were pushed aside, and the descending abdominal aorta was separated from the vein. Subsequently, the catheter of a radio telemetric pressure transducer (model HD-S10, DSI) was tipped into the abdominal descending aorta for 3–4 cm. A drop of tissue glue was used to seal the lesion in the artery. The transducer body was anchored on the abdominal wall. After the organs were reoriented to their original regions, the muscles and skin were then sutured, respectively. This implanted device allows for the measurement of hemodynamics such as the mean arterial pressure (MAP) and HR. Rats were given subcutaneous (s.c.) injections of buprenex (0.035 mg/kg) and cefazolin (10 mg/kg) for post-surgical care.
T4 spinal cord crush
Rats were anesthetized with isoflurane for spinal cord crush injury at the fourth thoracic (T4) level. An isoflurane administration procedure was performed as previously described. An incision of 2–3 cm was created in the skin of the back. Connective tissues and muscles were separated with a tissue separator until the T3 spinal process was exposed. After T3 dorsal laminectomy, the T4 spinal cord was completely crushed with fine forceps. During the process, the forceps were placed around the spinal cord and touching the ventral side of the vertebrae, then the forceps were pressed tightly together for 10 sec. Visual verification was performed to ensure the crush injury was complete, the dura remained intact, and no spared tissue remained on the lateral or ventral edges. Once active bleeding was controlled, the muscles were sutured, and the skin was stapled. Rats were then given injections of buprenex and cefazolin for post-surgical care. Animals had their bladder manually expressed 3 times daily for the first 2 weeks after surgery and then 2 times daily until sacrificed.
Hemodynamic recordings
The Dataquest Acquisition & Analysis System ART (Data Sciences International) was used for telemetric monitoring of hemodynamic parameters. Using this program, pulse arterial pressure was transmitted as a radiofrequency signal to a receiver, and data from receivers were collected and analyzed. MAP and HR values were derived from the pulse arterial pressure with a computerized acquisition system. Basal cardiovascular parameters and events of spontaneous AD were recorded prior to injury (1 week after telemeter implantation) as well as 2 and 4 weeks post-injury. Colorectal distention (CRD)-induced AD was measured at 2 and 4 weeks post-SCI. Prior to any hemodynamic recordings, the bladder was manually expressed to eliminate any possible confounds due to distention.
Resting MAP and HR. For resting cardiovascular parameters, singly housed rats (n = 7) were placed on the top of individual receiver pads associated with their specific telemeters. As each receiver was associated with the Dataquest system, it allowed for specific monitoring of hemodynamic parameters for each rat. Once their telemeters were activated, the rats were then undisturbed for 10–15 min to allow for acclimation and stabilization of cardiovascular parameters. The basal MAP and BP parameters were then recorded for a total of 30 min with a sampling rate of 10 sec. The environment was kept in a quiescent state throughout the period. The resting MAP and BP recordings were collected prior to the injury, 2, and 4 weeks post-injury. Afterwards, the values of MAP and BP were averaged per animal for statistical analysis.
Events of spontaneous autonomic dysreflexia (AD). To measure events of spontaneous AD over a span of 24 h, Dataquest acquisition, as previously described, was implemented along with a specific algorithm.16 This algorithm used the waveforms of MAP and HR acquired by the Dataquest software to effectively assess the approximate number of instances a steep increase in BP occurred concurrently with a decrease in HR. The rats (n = 6) were prepared in the same manner as for the basal hemodynamic recordings. The caged rats were placed on their individual receivers and allowed an acclimation period of 10–15 min. Cardiovascular parameters were recorded with data sampling rate of 2 sec for 24 h. Each rat was housed with adequate food and water during this task in a quiet environment. Spontaneous AD recordings were collected prior to injury as well as 2 and 4 weeks post-injury. Due to the strain-related variation, the parameter settings for detecting an AD period were modified from previous reports, including MAP increase threshold over baseline 15 mm Hg, MAP increase threshold peak-to-peak 20 mm Hg, HR decrease threshold peak-to-peak 15 bpm, and MAP rise duration 35 sec. The principle was to set the threshold of AD incidence close to 0 in naïve rats.
Colorectal distention (CRD)-induced AD. To induce AD, a clean balloon catheter coated with a petroleum-based lubricant was placed 2 cm into the rectum of each rat (n = 8). A syringe was attached at the end of each catheter to inflate the balloon. The catheter was then anchored to the tail for stabilization using athletic tape. Once the catheter was secured, the rats were then placed in a restraining tube on the receiver and covered with a cloth towel to create a calming environment. Although rats might have stress right after being put into the restrain tube, they were then allowed an acclimation period of 10–15 min in a quiet environment until the BP and HR were stable at the normal level. To initiate a distended colorectum, the balloon was inflated with 1.4 mL of air and held in an inflated state for 1 min. MAP and HR were recorded 1 min before, during, and after the distension with data sampling rate of 3 sec. Each rat was subjected to two trials with at least 15 min between recordings. Once completed, the catheter was removed, and the rat was injected s.c. with buprenex. The difference between the basal and CRD-induced MAP and HR for each animal was calculated and then averaged across the two trials. The experiment was performed at both 2 and 4 weeks post-SCI.
Pharmacological interventions of cardiovascular dysfunction
To minimize the interference of freely moving, conscious rats, we administered the drugs s.c. during cardiovascular recordings. We chose to give three different doses of drugs to a same group of rats but not separated ones in order to more accurately compare the cardiovascular responses with different drug concentrations without individual variations. For each drug, the interval between different doses was chosen based on previous reports but modified according to the specific slow entry delivery. In fact, a pilot experiment confirmed the effective response of this diagram.
Subcutaneous drug delivery. To examine the possible effects of spinal receptors for serotonin and dopamine on cardiovascular performance in SCI rats, we employed several pharmacological reagents to manipulate receptor activation during basal hemodynamic recordings at 4 to 5 weeks post-injury, including a 5-HT1A agonist, 8-OH-DPAT, a 5-HT2A agonist, Dimethoxy-4-iodoamphetamine (DOI),17,18 and a non-selective dopamine receptor agonist, apomorphine (APO).19 To record basal BP and HR, a total of 100 μL of drug was administered s.c. in a cumulative manner of low, middle, and high concentrations after an equilibrium period of 10–15 min. 8-OH-DPAT (n = 4) and DOI (n = 9) were given in dosages of 5, 20, and 100 μg/kg, whereas APO (n = 7) was given at 10, 100, and 300 μg/kg. Approximately 5 min after each injection, the cardiovascular measurements were recorded for 10 min. An additional period of 10–15 min was allotted for parameters to return to baseline prior to the injection of the following concentration. Between two different drugs, a time-period of 30 min was allowed to elapse to wash out the effect. At the completion of all trials, the average MAP and HR of each concentration for each animal was calculated for analysis. After the DOI trial, ketanserin (100 μg/kg), a 5-HT2A antagonist, was given at a dose of 100 μL to counteract the effects of DOI.
Intrathecal administration of DOI. To verify specific central effects of DOI, we delivered the drug i.t. during hemodynamic recordings at 4 to 5 weeks following SCI. Prior to the recording and injection, rats were lightly anesthetized by s.c. injection of urethane (0.8 mg/kg). Afterwards, rats were placed on the receiver to record resting hemodynamics for 5 min. The i.t. injection was achieved using a revised transcutaneous lumbar puncture method.20 Briefly, the rat was placed in the prone position and a needle (30 gauge × ½ inch) attached to an insulin syringe was inserted transcutaneously into the L5–6 intervertebral space. DOI (20μL) was slowly injected into the intrathecal space.21 The MAP and BP measurements were then recorded for 5 min. Similarly, the drug was administered in three different concentrations (1, 4, and 20 μg/kg). Subsequently, a total of 20 μL of ketanserin (20 μg/kg) was also administered to counteract the effect of the 5-HT2A agonist. The values of MAP and HR for each concentration were then averaged for analysis.
Injection of DOI for CRD-induced AD. Four weeks after SCI, rats (n = 8) and equipment were prepared, and telemetric parameters were set for CRD, as previously described. Once the equilibration period had elapsed, the rats received a single s.c. injection of 100 μL of DOI (20μg/kg). After 5 min, CRD was induced and cardiovascular parameters were recorded. The difference between basal and CRD-induced MAP and HR measurements were then analyzed for each animal. Once the experiment was terminated, the catheter was removed, and each rat received an s.c. injection of buprenex.
Fluorogold injection
One week before sacrifice, selected animals (n = 4) received an intraperitoneal injection of Fluorogold (FG; 0.4 mL of 0.5% in distilled water; Fluorochrome) to label SPNs in the intermediolateral cell column (IML).22
Histological analysis
Rats were overdosed with an intraperitoneal injection of Euthasol and subsequently transcardially perfused with 4% paraformaldehyde. The spinal cord was then dissected out and placed in the same solution for post-fixation overnight. Afterwards, the segments were transferred to 30% sucrose for at least 24 h. One 3-cm spinal segment containing the lesion site was embedded in Tragacanth medium for cryosectioning. Spinal cords were serially cryosectioned in the longitudinal, horizontal plane at 35 μm in six series of free-floating sections.9 Each section was submerged in Tris-buffered saline (TBS). To further verify lesion completeness, we performed immunostaining for platelet-derived growth factor receptor (PDGFR)-β, 5-HT, and glial fibrillary acidic protein (GFAP). Floating spinal sections were blocked in TBS containing 0.5% Triton X and 5% normal donkey serum for 1 h, and then incubated in primary antibody of rabbit anti-PDGFR-β (1:1000, Abcam), rabbit anti-5-HT (1:1000, Immunostar), and mouse anti-GFAP (1:1000, Abcam) at 4°C overnight. Following three washes in TBS, secondary antibodies such as goat anti-rabbit conjugated to Alexa 488 or anti-mouse conjugated to Alexa 594 (1:300, Invitrogen) were added for 3 h at room temperature. The sections were mounted on slides at room temperature to dry. The slides were then reviewed and imaged using a Leica DM5500B fluorescent microscope.
Statistical analysis
The basal MAP and BP before or following pharmaceutical interventions were assessed using one-way repeated measures analysis of variance (ANOVA) followed by a post hoc analysis with a Bonferroni adjustment. Events of spontaneous AD were analyzed using the non-parametric Friedman's test. CRD-induced AD data were analyzed using the paired t test. For all statistical analyses, p < 0.05 was considered a value of significance and all data are expressed as mean ± standard error of the mean (SEM).
Results
Resting hemodynamics are disordered following SCI
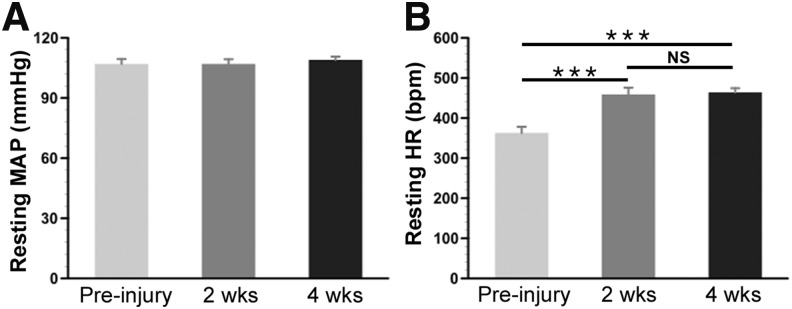
Abnormal basal hemodynamics such as hypotension and tachycardia are hallmark characteristics after high SCI in rats.23,24 To examine possible hemodynamic changes in this crush model, basal MAP and HR were measured prior to, 2, and 4 weeks post-SCI. Statistical analysis did not indicate any significant change in resting MAP between the time-points (repeated measures ANOVA, p > 0.05; pre-injury, 107.4 ± 2.4 mm Hg; 2-week SCI, 106.8 ± 2.3 mm Hg; 4-week SCI, 108.8 ± 1.6 mm Hg). Conversely, resting HR significantly increased after SCI (repeated measures ANOVA, p < 0.001) (Fig. 1). Post hoc analysis revealed significant differences between the pre-injury and 2- or 4-week post-injury time-points (pre-injury, 338 ± 11 bpm; 2-week SCI, 467 ± 2 bpm; 4-week SCI, 454 ± 15 bpm; Fisher's PLSD, both p < 0.001). Additionally, there was no meaningful change in resting HR between 2 and 4 weeks post-injury.
FIG. 1.
Resting hemodynamic changes before and after complete spinal cord crush at the T4 level. Basal mean arterial pressure (MAP) and heart rate (HR) at rest are measured prior to injury, 2, or 4 weeks after SCI. Statistical analysis shows that (A) there is no significant difference in the MAP, whereas (B) the HR increases 2 and 4 weeks following injury compared with the intact (repeated measures analysis of variance [ANOVA], p < 0.001; Fisher's PLSD, ***p < 0.001). The high resting HR is sustained between the two time-points of measurement. NS, non-significant.
Spontaneous autonomic dysreflexia occurs after T4 crush
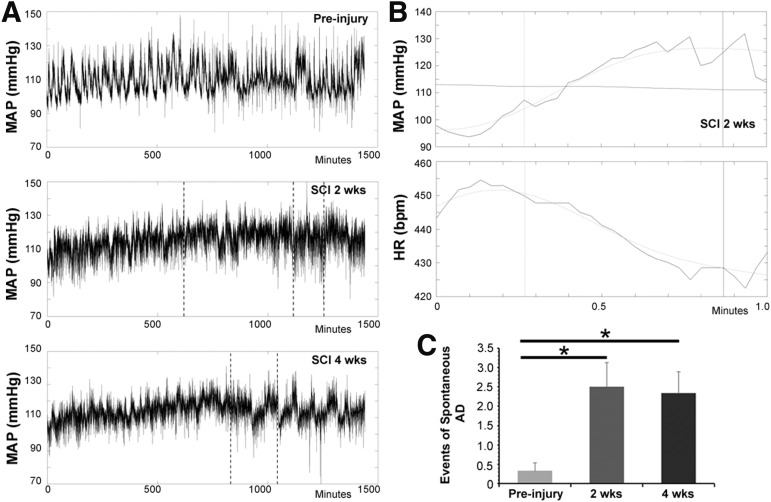
It has previously been shown that spontaneous AD occurs after spinal cord complete transection at high levels in rats.23 To determine whether this crush injury model was sufficient to induce spontaneous AD, rats underwent resting hemodynamic recordings for 24 h at three time-points: pre-injury, 2, and 4 weeks post-injury. The recording and following measurements disclosed the occurrence of spontaneous AD at both 2 and 4 weeks post-SCI, in which the MAP increases coincided with a sharp decrease in the HR. Compared with the uninjured animal, more events of spontaneous AD were detected after spinal cord crush (pre-injury, 0.33 ± 0.21 events; 2-week SCI, 2.5 ± 0.62 events; 4-week SCI, 2.33 ± 0.56 events; Friedman's test, both p < 0.05) (Fig. 2). There was no significant difference in the number of AD events between 2 and 4 weeks post-injury (p > 0.05).
FIG. 2.
Spontaneous autonomic dysreflexia occurs in the spinal cord crushed rats. (A) Representative mean arterial pressure (MAP) tracers are shown over a 24-h period. The event of spontaneous autonomic dysreflexia (AD), detected by employing the algorithm, is indicated with a vertical line. (B) A typical episode of AD is displayed in time-stretched tracers of MAP and HR, which is demonstrated as a sudden increase in MAP and a simultaneous decrease in HR. (C) Statistical analysis demonstrates a significantly higher number of spontaneous AD events at both 2- and 4-weeks post-injury when compared with the pre-injury (non-parametric data, Friedman's analysis, *p < 0.05).
CRD induces autonomic dysreflexia
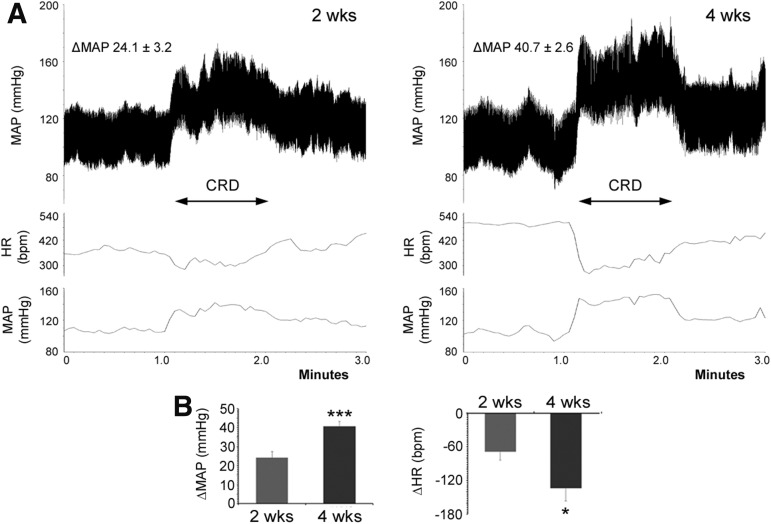
To identify if a peripheral sensory stimulation mimicking visceral pain could trigger dysreflexia, noxious CRD was performed 2 and 4 weeks after spinal cord crush during hemodynamic recordings. A typical AD episode was effectively induced at both time-points; however, the degree of change in MAP and HR differed (Fig. 3). Under CRD stimulation, the MAP increased by 24.1 ± 3.2 mm Hg at 2 weeks post-injury, whereas the change reached to 40.7 ± 2.6 mm Hg at 4 weeks (paired t test, p < 0.001). Similarly, the extent of HR decrease was smaller at 2 weeks post-injury (−69 ± 15 bpm) than the 4-week time-point (−134 ± 23 bpm, p < 0.05). Thus, the severity of CRD-induced AD is progressive during the subacute phase following spinal cord crush at the T4 level.
FIG. 3.
The noxious colorectal distention (CRD) induces autonomic dysreflexia (AD) following T4 spinal cord crush. An artificial CRD is performed with a tip-ballooned catheter to mimic visceral pain, and successfully triggers AD. The mean arterial pressure (MAP) and heart rate (HR) are recorded 1 min before, during, and after CRD. (A,B) Representative tracers of AD in MAP and averaged curves in MAP and HR are shown at 2- (A) and 4-weeks (B) post-injury, in which elevated MAP is accompanied by a decreased HR in response to CRD. (C) Statistical analysis shows a greater extent of MAP increase (paired t test, ***p < 0.001) and HR decrease (*p < 0.05) at 4 weeks compared with the 2-week time-point, demonstrating the progressive severity of AD during the subacute stage after spinal cord injury (SCI).
Spinal cord crush interrupts supraspinal serotonergic pathways
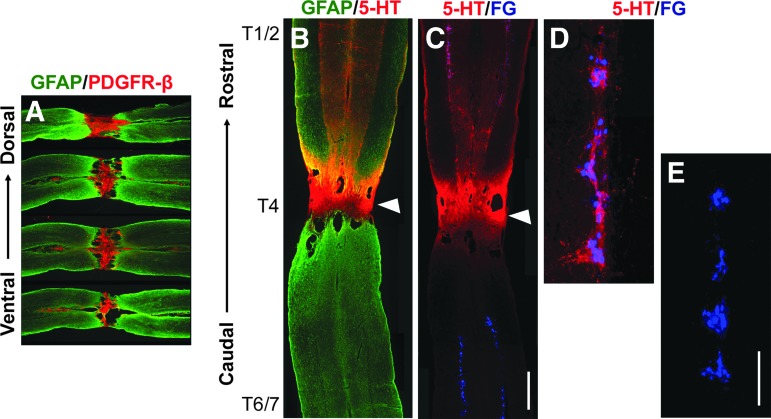
The completeness of supraspinal vasomotor pathways was examined in histological analysis. Serotonergic neurons in the caudal raphe nuclei project to autonomic regions in the spinal cord regulating spinal sympathetic function. The loss of serotonergic innervation after SCI has been reported to be one major reason for disordered hemodynamics.25,26 Accordingly, the descending neuronal pathways can be a representative to study supraspinal vasomotor circuits. Four to 5 weeks after spinal cord crush, immunostaining showed intense PDGFR-β labeling in the lesion of the spinal cord crush site, indicating the occurrence of fibrotic scarring.27 Above the lesion, numerous 5-HT+ axons projected to the IML and innervated FG-labeled SPNs. In contrast, no serotonergic fibers were detected in the autonomic region below the spinal cord lesion (Fig. 4), suggesting the crush injury adequately interrupted the descending serotonergic pathways.
FIG. 4.
A spinal cord crush injury at T4 level adequately severs descending supraspinal serotonergic fibers. (A) Four weeks post-injury, immunolabeling shows the injury site in the cord filled with platelet-derived growth factor receptor (PDGFR)-β+ fibroblasts. The spinal cord tissue is identified by glial fibrillary acidic protein (GFAP; green) in a serial of longitudinal sections from dorsal to ventral. (B) As a representative, 5-HT immunolabeling (red) detects serotonergic axons above the lesion but not below, suggesting a complete interruption of supraspinal pathways. (C) 5-HT immunostained serotonergic fibers (red) innervate the sympathetic preganglionic neurons (SPNs) retrogradely labeled by Fluorogold (FG, blue) in the rostral segment of the intermediolateral column (IML). White triangles in B and C indicate the injury site at T4 level. Higher magnification rostral (D) or caudal (E) to the lesion. Scale bars: A, 0.5 mm; C, 0.35 mm; E, 200 μm. Color image is available online.
Stimulating spinal 5-HT2A receptors improves hemodynamic performance
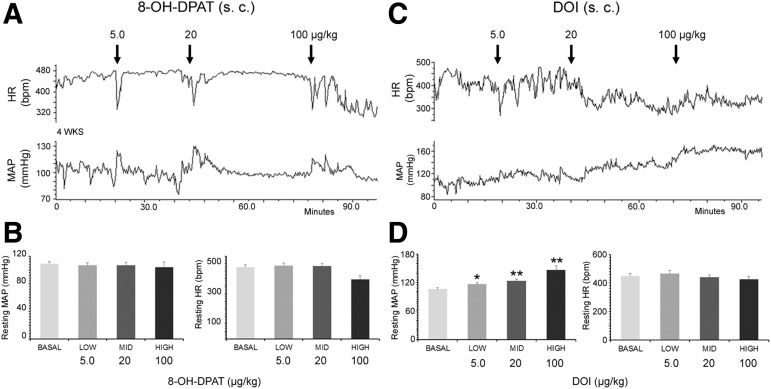
Once the descending hemodynamic regulation had been interrupted, we tested cardiovascular responses to pharmacological interventions of 5-HT and DA receptors. Four to 5 weeks after SCI, s.c. administration of 8-OH-DPAT to stimulate 5-HT1A receptors did not cause notable changes in resting MAP or HR (repeated measures ANOVA, both p > 0.05), compared with basal parameters in the SCI rats (Fig. 5A). However, stimulating 5-HT2A receptors by s.c. delivering DOI elicited a significant increase in resting MAP in a dose-dependent manner (repeated measures ANOVA, p < 0.001; Fisher's PLSD, p < 0.05 in low dose, p < 0.01 in both middle and high doses). Although resting HR was decreased after the delivery, the change did not reach to a significant level (p > 0.05) (Fig. 5B). In parallel, administration of APO, a non-selective dopamine receptor agonist, did not result in a significant change in resting MAP and HR (all p > 0.05, Table 1).
FIG. 5.
Systemic stimulation of 5-HT2A but not 5-HT1A receptors increases resting mean arterial pressure (MAP) in spinal cord crushed rats. (A,B) Four weeks after SCI, a serial dose of 8-OH-DPAT (5.0, 20, 100 μg/kg), an agonist of 5-HT1A receptors, is subcutaneously (s.c.) delivered during basal hemodynamic recordings. This does not induce meaningful changes in resting MAP and heart rate (HR). (C,D) In contrast, administration of Dimethoxy-4-iodoamphetamine (DOI; 5.0, 20, 100 μg/kg), an agonist of 5-HT2A receptors, increases resting MAP in a dose-dependent manner. Statistical analysis revealed that pharmacological activation of 5-HT2A receptors with DOI elevates resting MAP (repeated measures analysis of variance [ANOVA]; p < 0.001; Fisher's PLSD, *p < 0.05, **p < 0.01), whereas resting HR does not change in rats with spinal cord crush injury.
Table 1.
Effects of Serotonin and Dopamine Receptor Agonists on Resting Hemodynamic Parameters
| Drugs | Resting parameters | |||||
|---|---|---|---|---|---|---|
| Name | Type | Delivery | Con. (μg/kg) | Animals (n) | MAP | HR |
| 8-OH-DPAT | 5-HT1A agonist | s.c. | Basal | 4 | 109.2 ± 2.8 | 476 ± 17 |
| Low (5.0) | 106.7 ± 4.0 | 487 ± 15 | ||||
| Mid (20) | 107.0 ± 3.8 | 484 ± 16 | ||||
| High (100) | 104.1 ± 7.6 | 396 ± 23 | ||||
| DOI | 5-HT2A agonist | Basal | 9 | 106.3 ± 3.2 | 448 ± 19 | |
| Low (5.0) | 116.4 ± 3.6** | 467 ± 21 | ||||
| Mid (20) | 132.5 ± 3.9** | 440 ± 16 | ||||
| High (100) | 146.5 ± 8.8** | 425 ± 18 | ||||
| DOI | 5-HT2A agonist | i.t. | Basal | 4 | 92.2 ± 2.7 | 339 ± 18 |
| Low (1.0) | 97.1 ± 1.7 | 341 ± 17 | ||||
| Mid (4.0) | 104.1 ± 1.1* | 342 ± 11 | ||||
| High (20) | 111.1 ± 3.6* | 313 ± 12 | ||||
| Apomorphine | Non-selective DA receptor agonist | s.c. | Basal | 7 | 102.8 ± 3.8 | 445 ± 24 |
| Low (10) | 104.9 ± 3.2 | 453 ± 21 | ||||
| Mid (100) | 106.0 ± 3.3 | 432 ± 17 | ||||
| High (300) | 101.7 ± 3.2 | 431 ± 11* | ||||
*p < 0.05, **p < 0.01, repeated measures ANOVA followed by Fisher's PLSD.
ANOVA, analysis of variance; DOI, Dimethoxy-4-iodoamphetamine; i.t., intrathecal; HR, heart rate; MAP, mean arterial pressure; s.c., subcutaneous.
To determine the drug effect over the central nervous system (CNS), serial doses of DOI were i.t. administered in urethane-anesthetized rats. Basal hemodynamic recordings revealed that resting MAP increased as a cumulative response compared with baseline (repeated measures ANOVA, p < 0.01). Post hoc analysis revealed a significant increase when the middle and high concentrations of DOI were delivered (Fisher's PLSD, both p < 0.05) (Fig. 6A, B). Similarly, the delivery resulted in a decrease in resting HR (repeated measures ANOVA, p < 0.05); however, the significant change was displayed only between the low and high concentrations (p < 0.05). There was no difference between the basal measurements and the varying concentrations (p > 0.05). The results indicate the cardiovascular effect of peripheral delivery of DOI is also via the central mechanism at spinal levels.
FIG. 6.
Intrathecal (i.t.) stimulation of 5-HT2A receptors increases resting MAP. (A) A tracer shows resting MAP and heart rate (HR) recordings during serial dosages (1, 4, 20 μg/kg) of Dimethoxy-4-iodoamphetamine (DOI) administration (i.t.). (B) Statistical analysis confirms that resting mean arterial pressure (MAP) significantly increases in a dose-dependent manner (repeated measures analysis of variance [ANOVA], p < 0.05) in response to DOI delivery, while the resting HR remains unchanged (repeated measures ANOVA, p > 0.05). Subsequently, delivery of ketanserin (20 μg/kg, i.t.), a specific 5-HT2A receptor antagonist, dilutes the effect of DOI. (C) Systemic administration of DOI (20 μg/kg) attenuates CRD-induced bradycardia following spinal cord crush. Representative tracers of autonomic dysreflexia in MAP and averaged curves in MAP and HR are shown before and after subcutaneous (s.c.) administration of DOI in the spinal cord injured (SCI) rats. (D) Compared with pre-intervention CRD parameters, the degree of HR decrease is significantly smaller (paired t test, *p < 0.05) after stimulating 5-HT2A receptor with DOI, whereas MAP change has no difference (p > 0.05).
DOI delivery alleviates CRD-induced AD
To observe the effect of 5-HT2A activation on dysreflexia, CRD-induced AD was performed before and after DOI delivery 4 to 5 weeks post-SCI. Following s.c. injection of DOI, CRD-induced AD exhibited smaller changes in MAP and HR compared with parameters before drug administration. Specifically, there was a significant decrease in the change of HR (control, −134 ± 23 bpm; DOI, −63 ± 21 bpm; paired t test, p < 0.05), whereas an insignificant reduction in the change of MAP (control, 40.7 ± 2.6 mm Hg; DOI, 33.9 ± 4.4 mm Hg, p > 0.05) (Fig.6C, D). This illustrates that pharmacological activation of spinal 5-HT2A receptors alleviates bradycardia during an AD episode following SCI.
Discussion
The present study demonstrates that cardiovascular dysfunction occurs in a completely crushed SCI rat model. The hemodynamic disorders manifest as dramatic tachycardia at rest and the development of spontaneous or artificially induced AD. Importantly, cardiovascular parameters in this model are responsive to pharmacological stimulation of spinal 5-HT2A receptors. This upper thoracic SCI subject can be used to investigate AD and employ serotonin-related drugs for hemodynamic improvement.
Although previous studies often reported a low resting BP in SCI rats, we here observed an elevated HR but no meaningful change in MAP in the spinal cord crush model. The normal level of MAP could be a result of compensatory mechanisms via the baroreceptor reflex regarding the high degree of persistent tachycardia.28 Another explanation is the possible presence of very few non-serotonergic spared tissues in the ventral part of the lesion that transmit some supraspinal signals to maintain MAP. Moreover, the crush injury leaves the dura unopened, which may have some influence on the regulation of bodily functions with regard to the effect of epidural stimulation.29 Indeed, various hemodynamic symptoms have been recognized in different SCI models, which are usually associated with the severity and level of injury in the spinal cord.30
In the present study, the occurrence of spontaneous AD in the spinal cord crushed rats is much less than that previously reported in the spinal transected models.16,31 This discrepancy is also likely due to different severity of injury, distinct animal strains, or the extent of bladder function recovery. Additionally, noxious CRD stimulation successfully induced AD episodes at both 2 and 4 weeks following injury, and the condition was exacerbated over time. Following SCI, intraspinal plasticity develops in the form of aberrant pelvic primary afferent sprouting and propriospinal circuit reorganization modulating SPNs.32,33 That is to say, what we perceived here is the developing process of AD based on the reestablishment of spinal reflex circuits. In spite of varied hemodynamic responses in SCI models, the fundamental reason for disorders is the interruption of supraspinal vasomotor pathways regulating sympathetic activity. Thus, the variation of hemodynamic responses does not prevent the application of this crushed SCI model to study the reconnection of supraspinal neuronal regulation for cardiovascular recovery.
In cell transplantation research, we recently demonstrated that grafted cell survival and integration were closely related to the type and severity of SCI. A dura-opened complete spinal cord transection with ensuing microaspiration always caused excessive tissue damage and released massive injury signals for fibroblast proliferation and migration, which in turn, induced severe fibrosis and huge cysts in the lesion site. As a consequence, grafted cells poorly survived and integrated to the host tissue, limiting the extent of graft-relayed supraspinal signal transmission.8 In fact, grafted cells have better growth into a small lesion of the spinal cord due to less inhibitory components. However, a mild injury often spares some descending vasomotor projections, which sustains the normal cardiovascular function, thereby rendering the model inadequate. For example, a contusive SCI may better model the situation of injury in the clinic. However, the partial lesion easily sustains normal cardiovascular function albeit few labs are using this model.34 Further, contusion injury is not ideal to study the effect of cell transplantation for cardiovascular recovery because abundant spared tissues make the evaluation of physiological and histological results difficult. On the contrary, disordered resting hemodynamic parameters and AD are always displayed in the spinal cord crush rat model. It would be concise to assess graft-derived axon growth and innervation to the denervated SPNs because of completely disrupted descending vasomotor pathways. Hence, this crushed SCI model is applicable to study cell-based therapeutic approaches for the severity of AD.
It is well known that serotonin plays a vital role in regulating cardiovascular function under normal conditions.11 Indeed, central 5-HT1A and5-HT2A receptors are the major subtypes important in cardiovascular regulation. 5-HT1A receptors are mainly expressed on presympathetic vasomotor neurons in the rostral ventrolateral medulla (RVLM) of the brainstem, and they have very little effect on resting BP or sympathetic outflow. Conversely, 5-HT2A receptors are expressed on the midbrain and spinal cord. Their modifications of sympathetic outflow are via distinct pathways. The midbrain 5-HT2A receptors are involved in a central angiotensinergic pathway, whereas the spinal cord population directly excites SPNs to raise BP.12 The loss of supraspinal neurotransmitter transportation induces up/downregulation of receptor expression or sensitivity in the caudal spinal cord. Despite this plasticity, the presence of these receptors after injury implies that their activation may compensate dysregulated cardiovascular function.
Previous studies reported a lack of 5-HT1A expression in the IML of the human spinal cord,35 indicating that this subtype of receptors may not be involved in cardiovascular function following SCI. Consistent with that study, systemic administration of 8-OH-DPAT, a 5-HT1A agonist, did not produce effects on hemodynamics. However, s.c. application of DOI, a 5-HT2A agonist, demonstrated a dose-dependent increase in resting MAP and no significant decrease in resting HR. Because 5-HT2A can induce vasoconstriction via both peripheral and central mechanisms,11 we repeated the hemodynamic recording with i.t. delivery of DOI to differentiate the origin of response. The similar results suggest that 5-HT2A activation increases BP in SCI rats at least partially via the spinal central machinery. The lack of remarkable corresponding changes in HR could be due to the impaired baroreceptor sensitivity after SCI.36 Another possibility is that activation of 5-HT2A receptors in spinal segments T1–T4 caused sympathoexcitation of the cardiac reflex, which counteracts the compensatory decrease in HR.
To further investigate the role of 5-HT2A receptors in the cardiovascular reflexic response, we administered DOI during CRD induction. Compared with dysreflexia parameters without drug, DOI induces a dramatically decreased change in HR and an insignificant reduction of change in MAP during CRD. It therefore denotes that dysregulated spinal 5-HT2A receptors in SPNs may be attributable to the development of AD after SCI. In consideration of increased basal MAP after DOI administration, the alleviated bradycardia might also be resulted from the lower extent of MAP elevation during CRD stimulation. Nevertheless, the results indicate that stimulation of spinal 5-HT2A receptors can manipulate cardiovascular function in SCI subjects.
The neurotransmitter DA has been reported to regulate BP. Previous studies demonstrated that D1, D3, and D4 receptors interact with the renin-angiotens for cardiovascular function, whereas the D2 and D5 receptors interact with the sympathetic nervous system.37 Although this role is mainly caused by the modulation of renal proximal tubular and jejunal sodium transport, one cannot rule out the possibility of central mechanisms in light of the same types of DA receptors located in the CNS, particularly after an SCI removes cerebral controls. Recent studies have identified the presence of DA receptors within the autonomic IML of the spinal cord, which modulates sympathetic activity by means of pharmacological manipulation.19,37,38 In the present study, delivering varying concentrations of the non-selective DA receptor agonist, APO, did not yield significant changes in resting hemodynamics. It indicates that spinal DA receptors may not play a role in cardiovascular regulation after SCI.
In summary, this study validates the usage of a complete spinal cord crush model for studying AD. Pharmacological activation of spinal 5-HT2A receptors improves hemodynamic performance and alleviates CRD-induced dysreflexic bradycardia, indicating a possible target for therapeutic interventions following SCI.
Acknowledgments
We thank Tatiana M. Saltos for technical assistance, and are grateful to Dr. Alexander G. Rabchevsky and Khalid Eldahan for providing algorithm tools. Support for this work was provided by NIH NINDS R01NS099076, Craig H. Neilsen Foundation (280072), and Morton Cure Paralysis Funds (MCPF) to S.H., and NIH NINDS R01NS085426, R01NS106908, and CDMRP/DoD W81XWH-14-1-0605 to V.J.T.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Lindan R., Joiner E., Freehafer A.A., and Hazel C. (1980). Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia 18, 285–292 [DOI] [PubMed] [Google Scholar]
- 2. Hou S., and Rabchevsky A.G. (2014). Autonomic consequences of spinal cord injury. Compr. Physiol. 4, 1419–1453 [DOI] [PubMed] [Google Scholar]
- 3. Myers J., Lee M., and Kiratli J. (2007). Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am. J. Phys. Med. Rehabil. 86, 142. [DOI] [PubMed] [Google Scholar]
- 4. Karlsson A.K. (2006). Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Prog. Brain Res. 152, 1–8 [DOI] [PubMed] [Google Scholar]
- 5. Hou S., Tom V.J., Graham L., Lu P., and Blesch A. (2013). Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J. Neurosci. 33, 17138–17149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jakeman L.B., and Reier P.J. (1991). Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J. Comp. Neurol. 307, 311–334 [DOI] [PubMed] [Google Scholar]
- 7. Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., Brock J., Blesch A., Rosenzweig E.S., Havton L.A., Zheng B., Conner J.M., Marsala M., and Tuszynski M.H. (2012). Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell1 50, 1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou S., Saltos T.M., Iredia I.W., and Tom V.J. (2018). Surgical techniques influence local environment of injured spinal cord and cause various grafted cell survival and integration. J. Neurosci. Methods 293, 144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hou S., Lu P., and Blesch A. (2013). Characterization of supraspinal vasomotor pathways and autonomic dysreflexia after spinal cord injury in F344 rats. Auton. Neurosci. 176, 54–63 [DOI] [PubMed] [Google Scholar]
- 10. Zhang M. (2016). Normal distribution and plasticity of serotonin receptors after spinal cord injury and their impacts on motor outputs, in: Recovery of Motor Function Following Spinal Cord Injury. Fuller H., and Gates M. (eds). InTech: Rijeka, Ch: 5 [Google Scholar]
- 11. Ramage A.G., and Villalon C.M. (2008). 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol. Sci. 29, 472–481 [DOI] [PubMed] [Google Scholar]
- 12. Ramage A.G. (2001). Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res. Bull. 56, 425–439 [DOI] [PubMed] [Google Scholar]
- 13. Zhu H., Clemens S., Sawchuk M., and Hochman S. (2007). Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience 149, 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hou S., Carson D.M., Wu D., Klaw M.C., Houle J.D., and Tom V.J. (2016). Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp. Neurol. 285, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wienecke J., Ren L.Q., Hultborn H., Chen M., Moller M., Zhang Y., and Zhang M. (2014). Spinal cord injury enables aromatic L-amino acid decarboxylase cells to synthesize monoamines. J. Neurosci. 34, 11984–12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabchevsky A.G., Patel S.P., Lyttle T.S., Eldahan K.C., O'Dell C.R., Zhang Y., Popovich P.G., Kitzman P.H., and Donohue K.D. (2012). Effects of gabapentin on muscle spasticity and both induced as well as spontaneous autonomic dysreflexia after complete spinal cord injury. Front. Physiol. 3, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gradin K., Pettersson A., Hedner T., and Persson B. (1985). Acute administration of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), a selective 5-HT-receptor agonist, causes a biphasic blood pressure response and a bradycardia in the normotensive Sprague-Dawley rat and in the spontaneously hypertensive rat. J. Neural Transm. 62, 305–319 [DOI] [PubMed] [Google Scholar]
- 18. Ramage A.G., and Daly M.B. (1998). The central action of the 5-HT2 receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) on cardiac inotropy and vascular resistance in the anaesthetized cat. Br. J. Pharmacol. 125, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mugabo P., Buylaert W., and Bogaert M. (1983). Cardiovascular effects of apomorphine in the rat. Arch. Int. Pharmacodyn. Ther. 262, 324–325 [PubMed] [Google Scholar]
- 20. Mestre C., Pelissier T., Fialip J., Wilcox G., and Eschalier A. (1994). A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods 32, 197–200 [DOI] [PubMed] [Google Scholar]
- 21. Mbaki Y., and Ramage A.G. (2008). Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br. J. Pharmacol. 155, 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akhavan M., Hoang T.X., and Havton L.A. (2006). Improved detection of fluorogold-labeled neurons in long-term studies. J. Neurosci. Methods 152, 156–162 [DOI] [PubMed] [Google Scholar]
- 23. Laird A.S., Carrive P., and Waite P.M. (2006). Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J. Physiol. 577, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teasell R.W., Arnold J.M., Krassioukov A., and Delaney G.A. (2000). Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil. 81, 506–516 [DOI] [PubMed] [Google Scholar]
- 25. Cormier C.M., Mukhida K., Walker G., and Marsh D.R. (2010). Development of autonomic dysreflexia after spinal cord injury is associated with a lack of serotonergic axons in the intermediolateral cell column. J. Neurotrauma 27, 1805–1818 [DOI] [PubMed] [Google Scholar]
- 26. Llewellyn-Smith I.J., Weaver L.C., and Keast J.R. (2006). Effects of spinal cord injury on synaptic inputs to sympathetic preganglionic neurons. Prog. Brain Res. 152, 11–26 [DOI] [PubMed] [Google Scholar]
- 27. Soderblom C., Luo X., Blumenthal E., Bray E., Lyapichev K., Ramos J., Krishnan V., Lai-Hsu C., Park K.K., Tsoulfas P., and Lee J.K. (2013). Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 33, 13882–13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swenne C.A. (2013). Baroreflex sensitivity: mechanisms and measurement. Neth. Heart J. 21, 58–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayr W., Krenn M., and Dimitrijevic M.R. (2016). Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr. Opin. Neurol. 29, 721–726 [DOI] [PubMed] [Google Scholar]
- 30. Furlan J.C., and Fehlings M.G. (2008). Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg. Focus 25, E13. [DOI] [PubMed] [Google Scholar]
- 31. West C.R., Popok D., Crawford M.A., and Krassioukov A.V. (2015). Characterizing the temporal development of cardiovascular dysfunction in response to spinal cord injury. J. Neurotrauma 32, 922–930 [DOI] [PubMed] [Google Scholar]
- 32. Hou S., Duale H., Cameron A.A., Abshire S.M., Lyttle T.S., and Rabchevsky A.G. (2008). Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J. Comp. Neurol. 509, 382–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weaver L.C., Verghese P., Bruce J.C., Fehlings M.G., Krenz N.R., and Marsh D.R. (2001). Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma 18, 1107–1119 [DOI] [PubMed] [Google Scholar]
- 34. Squair J.W., West C.R., Popok D., Assinck P., Liu J., Tetzlaff W., and Krassioukov A.V. (2016). High thoracic contusion model for the investigation of cardiovascular function after spinal cord injury. J. Neurotrauma 34, 671–684 [DOI] [PubMed] [Google Scholar]
- 35. Perrin F.E., Gerber Y.N., Teigell M., Lonjon N., Boniface G., Bauchet L., Rodriguez J.J., Hugnot J.P., and Privat A.M. (2011). Anatomical study of serotonergic innervation and 5-HT(1A) receptor in the human spinal cord. Cell Death Dis. 2, e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips A.A., Krassioukov A.V., Ainslie P.N., and Warburton D.E. (2012). Baroreflex function after spinal cord injury. J. Neurotrauma 29, 2431–2445 [DOI] [PubMed] [Google Scholar]
- 37. Jose P.A., Eisner G.M., and Felder R.A. (2003). Regulation of blood pressure by dopamine receptors. Nephron. Physiol. 95, p19–p27 [DOI] [PubMed] [Google Scholar]
- 38. Gladwell S.J., Pyner S., Barnes N.M., and Coote J.H. (1999). D(1)-like dopamine receptors on retrogradely labelled sympathoadrenal neurones in the thoracic spinal cord of the rat. Exp. Brain Res. 128, 377–382 [DOI] [PubMed] [Google Scholar]