Abstract
Introduction: Renal papillary pits are commonly encountered during ureteroscopy. The mechanism by which such pits arise is unclear. One hypothesis is that pits represent sites where stones overgrowing Randall's plaque (RP) were dislodged. We sought to examine this theory by using digital ureteroscopy and stone μCT.
Materials and Methods: Patients undergoing endoscopic stone removal had procedures recorded and stones analyzed by using μCT. Stones with evidence of Randall's plaque anchors (RPAs) were identified in a blinded fashion. Surgical videos were reviewed independently by two urologists.
Results: Twenty-eight patients had μCT-confirmed stones with RPA. Among them, 93% were recurrent stone formers and 75% had had prior stone procedures. Metabolic abnormalities were present in 87%, with 79% classified as idiopathic calcium oxalate stone formers. A mean of 7.6 stones with RPA were identified per procedure. In each case, papillary pits were visualized before any stone manipulation and in several cases the active dislodgement of an attached stone led to immediate identification of an underlying pit. Such stones routinely demonstrated an RPA on μCT. The average depth of RPA was 302 ± 172 μm, consistent with the corresponding shallow pits visualized on the papillary surface.
Conclusions: Stones overgrowing RP are capable of pulling away a piece of papilla when dislodged, resulting in a visible papillary pit. This process manifests as an RPA on the undersurface of the stone and a papillary pit on the corresponding area of attachment. Identification of pits may help identify patients who form stones primarily by the RP mechanism.
Keywords: nephrolithiasis, ureteroscopy, calculus
Introduction
Papillary pathology is increasingly recognized as a potential source of information in studying kidney stone pathogenesis. The renal papillae of stone formers differ from non-stone formers and have unique appearances depending on underlying pathophysiologies that are responsible for stone formation.1,2 Mechanisms by which changes in the papilla occur are poorly understood, though their presence has been hypothesized to have clinical relevance in classifying patients more precisely for the purposes of research and clinical care.
Recently, two endoscopic papillary classification systems have been described to help standardize the description of papillary abnormalities.3,4 One feature common to each is recognition of papillary pits (surface erosion) whereby the normally smooth urothelial surface of the papilla is disrupted. The presence of papillary pits has been reported by Traxer and colleagues as the most common abnormality identified after Randall's plaque (RP).4 Recent investigation of papillary classification among patients undergoing ureteroscopy demonstrated a strong association between RP and papillary pitting and hypothesized that pitting occurred as a result of stone overgrowth on RP with subsequent tearing off of a piece of superficial epithelium with the stone when dislodgement occurred.5 However, concrete mechanistic evidence for this process is lacking.
Our research team has recently described the ability of using μCT to reliably identify RP attachments to stones that we term Randall's plaque anchors (RPA).6,7 Patients with such stones represent an ideal cohort to test the hypothesis that papillary pits occur secondary to RP stone dislodgement as the presence of RPA on the stone should presumably correspond to papillary pits if this process does, in fact, occur. Confirmation of this mechanism would enhance our understanding of one feature of abnormal papillary pathology commonly observed during renal endoscopy for stone treatment.
Methods
Patients were enrolled as part of an ongoing IRB-approved study (IRB No. 1010002261) to investigate mechanisms of stone pathogenesis using endoscopic surgical observations and metabolic testing. Our research methodology has been previously described.6 Attempts are made to remove all stones intact. The endoscopic video is recorded and saved in high definition.
Stones were examined individually, first grossly and then with μCT by an expert in this area (J.C.W.). μCT is non-destructive and allows identification of mineral subtypes and microstructure.6–8 Mineral composition was confirmed in each case by using infrared spectroscopy. Reports from all stone μCTs were generated and recorded in a manner blinded to surgical observations and patient data.
Surgical videos from the corresponding procedures were reviewed independently by two urologists specializing in endourology and stone disease to determine whether any papillae showed evidence of pits. The identification of pits was based on previously published descriptions of this process whereby a superficial ulceration is visualized on the normally smooth and intact papillary surface.3,4
Results of corresponding serum and urine metabolic testing performed while off preventive pharmacologic agents were reviewed. Patients were categorized based on stone mineral composition and metabolic pathophysiology as previously described in prior publications.1,9,10 Descriptive statistics were performed by using JMP 12.2 (SAS, Cary, NC).
Results
μCT stone reports were available for 166 patients undergoing stone removal procedures from 2010 to 2016. Twenty-eight of these patients (17%) had at least one stone with evidence of an RPA and are the subject of this study (Fig. 1). RPA were identified on high-resolution (generally 3 μm voxel size) μCT by the following criteria7: The stone possessed a region of apatite at its surface that was distinct from the mineral making up the stone; the apatite did not have a layered appearance (common to apatite growing in the urine)11 but instead showed a moderate level of X-ray attenuation that diminished in the edges of the apatite region away from the stone. Most often, these apatite regions contained empty cylindrical spaces that twisted through the mineral, typically with a diameter of 20 to 30 μm, consistent with these being the remaining lumens of tubules and vessels that ran through the mineralized region. Finally, not uncommonly, a profile at the edge of the apatite away from the stone was seen that appeared to be a tubule with a calcified wall, consistent with what has been proposed to be the earliest stage of the formation of RP, namely the deposition of mineral into the basement membrane of a loop of Henle.10
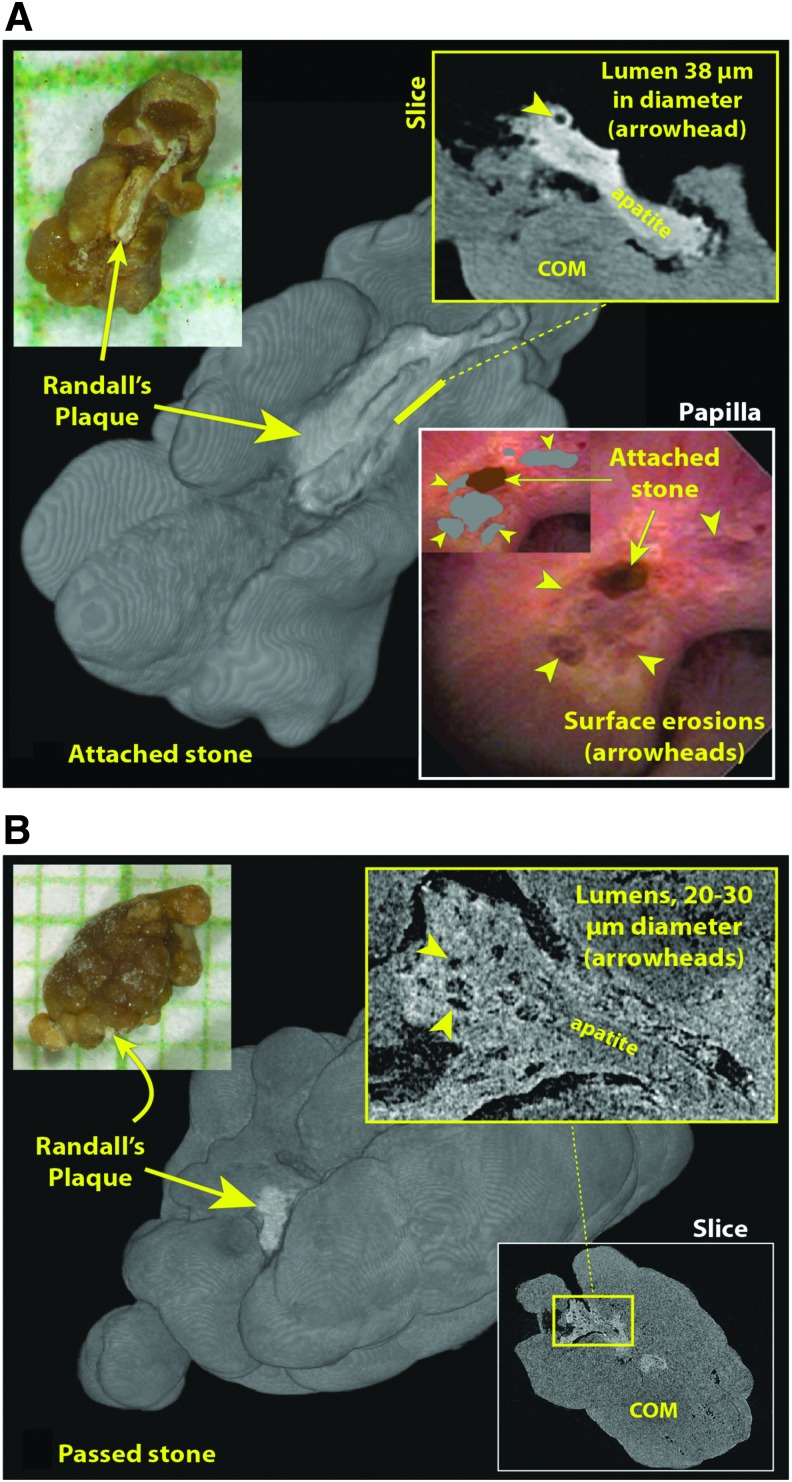
FIG. 1.
μCT appearance of stones with morphological evidence of RPAs. Both stones here were collected from the same patient. (A) One of seven attached stones was removed during the procedure, all of which showed RPAs. Upper left, photo of stone after removal, on mm grid paper. Background is 3D surface rendering of μCT image stack. Upper right is portion of μCT image slice showing lumen of tubule captured in cross-section. Lower right shows ureteroscopic view of papilla before stone was removed; inset shows stone and eroded regions colorized. (B) Spontaneously passed stone collected by patient before procedure. RPA is hidden in photo, upper left, but clearly seen in surface rendering, background. Magnified view of RPA shows evidence of lumens (inset), with appearance somewhat obscured, likely because of mineral deposited on the RPA from the urine while the stone was being passed. 3D, three-dimensional; RPAs, Randall's plaque anchors.
Demographics of these RP stone formers are described in Table 1. The majority were classified as idiopathic calcium oxalate stone formers. Complete metabolic assessment was accomplished by 22/28 patients (78.5%), a majority of whom (86.4%) were found to have at least one metabolic risk factor for stone formation. The most commonly observed abnormality was hypercalciuria (59.0%), followed by hypocitraturia (54.5%) and hyperoxaluria (36.3%) (Table 1).
Table 1.
Demographics of Patients with Randall's Plaque Anchors on Stones
| Variable | N (range) |
|---|---|
| Stone phenotype | |
| Idiopathic calcium oxalate | 22 |
| Idiopathic hydroxyapatite | 3 |
| Brushite | 1 |
| Enteric hyperoxaluria | 1 |
| Primary hyperparathyroid | 1 |
| Procedure | |
| Ureteroscopy | 24 |
| Percutaneous nephrolithotomy | 4 |
| Age at surgery | 41.6 (9–72) |
| Female (%) | 43 |
| BMI (kg/m2) | 28.8 (17.5–47.4) |
| Prior stone history, % | 92.80 |
| Prior stone procedure, % | 75 |
| Number of prior stone procedures | 2.77 (0–19) |
| Family history, % | 43 |
| Serum calcium (mg/dL) | 9.46 (8.7–10.4) |
| Urine volume (L) | 1.96 (1.2–3.3) |
| Urine sodium (mg/day) | 171.5 (86–269.5) |
| Urine calcium (mg/day) | 227.1 (45.5–420) |
| Urine oxalate (mg/day) | 39.9 (24.5–95) |
| Urine citrate (mg/day) | 560.2 (19–1484.5) |
| Urine pH | 6.16 (5.3–6.9) |
| Urine supersaturation calcium oxalate | 7.48 (2.3–12.6) |
| Urine supersaturation calcium phosphate | 1.49 (0.1–2.3) |
| Urine Ca/kg | 2.74 (0.6–4.8) |
| Urine Ca/Cr | 147.0 (46.5–294.9) |
| Urine Cr/kg | 19.4 (10.1–26) |
BMI = body mass index.
A total of 213 stones with RPA (out of 524 total stones) were identified from the 28 patients in this cohort (mean 7.6 RPA stones/patient procedure [range 1–40]). The mineral composition of the RPA itself was identified as apatite in all cases. The majority of RPA (86%) showed evidence of luminal spaces, consistent with tubules or vessels trapped in the plaque (Fig. 1). Mean dimensions of the RPA were 340 ± 224 μm wide (measured along the stone-plaque interface) and 302 ± 172 μm deep (measured from the stone-plaque interface to the furthest extension of plaque). Analysis of the overgrowing stones revealed the majority to be pure calcium oxalate (62%), with the remainder showing a mixed composition of calcium oxalate and apatite. Overall, the maximum dimension of the combined RPA and overgrowing stone was 2.0 ± 1.2 mm.
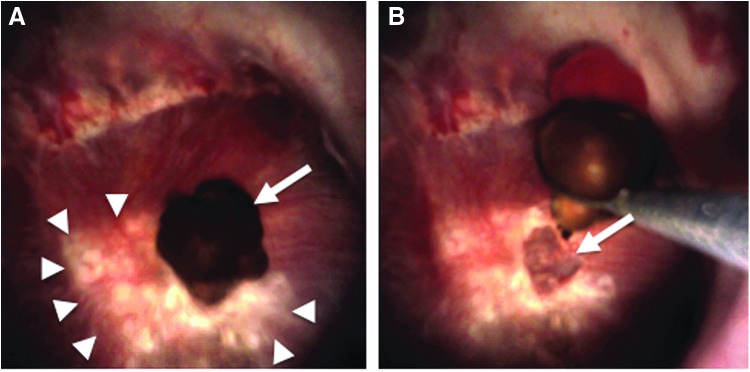
On review of the endoscopic surgical procedures, all patients demonstrated evidence of attached stones to at least one papilla. Papillary pits were identified on at least one papilla in all patients as well. Occasionally, we had the opportunity to observe a papillary pit immediately on stone removal (Fig. 2) though this was not possible in all cases owing to stone location relative to the papilla and the ureteroscope. Notably, pits were identified before any direct stone manipulation in seven patients with no history of prior stone procedures. In each case, at least one papillary pit was able to be observed immediately on entry to the kidney before any stone manipulation, suggesting that the visualized pits may have formed from spontaneous, rather than iatrogenic, dislodgement of a stone. This is supported by the stone analyses in Figure 1, whereby a patient had brought in several spontaneously passed stones before undergoing planned ureteroscopy. RPA were visualized on the spontaneously passed and mechanically removed stones alike.
FIG. 2.
Demonstration of pit creation after stone dislodgement. (A) Papilla with large amount of RP designated by arrowheads. There is an attached stone overlying the papilla (arrow). (B) Stone is mechanically dislodged from papillary tip with stone basket, revealing underlying surface pit in the papilla. RP = Randall's plaque.
We also had the opportunity to examine the papillae of one patient in two different bilateral procedures that occurred 330 days apart. A total of 32 papillae from the 2 kidneys were able to be matched between the videos of the 2 procedures, and the papillary appearances were nearly identical in every case. Figure 3 shows representative papillae from this case whereby the gross appearance of the identified pits was stable over nearly 1 year, suggesting that pitting is a relatively stable phenomenon in this type of patient and not a transient process that is only visible shortly on dislodgement of a stone.
FIG. 3.
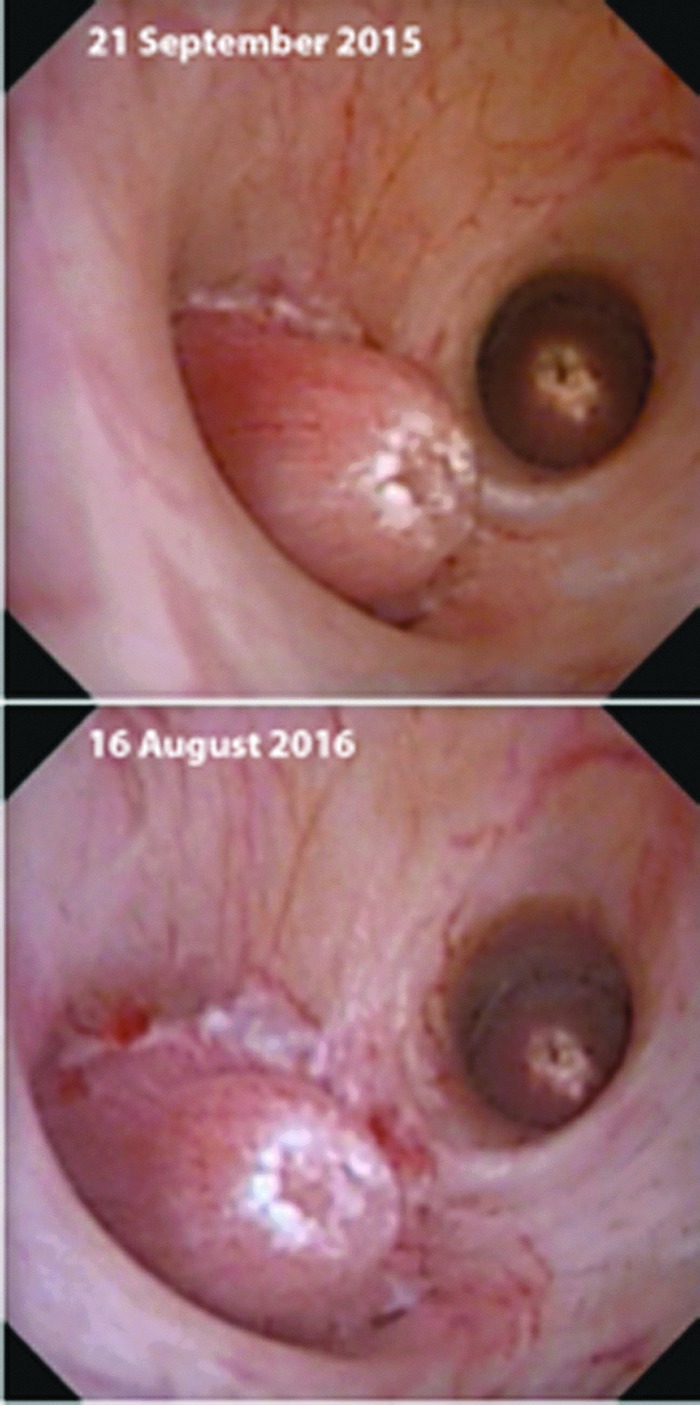
Stability of papillary pitting. These images show the same papillae from ureteroscopic procedures that took place almost 11 months apart. These papillae are quite representative of 32 such papillae that were matched between the videos of the 2 procedures. Note that the RP regions (white) of the papilla in the foreground surround a region of pitting that is apparently unchanged between 2015 and 2016.
Discussion
Digital ureteroscopes have provided an unprecedented ability to inspect the renal papillae during ureteroscopic stone treatment, leading to the realization that renal papillary abnormalities are very common in patients who form stones.6 One of the most common abnormalities encountered, papillary pits, are believed, in some instances, to occur after an adherent stone is dislodged but there is scant evidence supporting this hypothesis. Herein, we describe one such mechanism whereby a piece of the papilla is lost when the stone is shed and can be seen microscopically still adherent to the stone in the form of an RPA. Further, in patients whose stones feature RPA, papillary pits are present on at least one papilla in 100% of cases.
This process has been previously alluded to. In 1973, Elliot and colleagues analyzed 150 spontaneously passed ureteral stones with low power magnification and identified depressions in nearly half. In many, a small, cream-colored focus was seen in the depression, which, when analyzed, was found to nearly always be apatite, consistent with RP.12 In 1975, Prien hypothesized that this lesion originated as papillary plaque, sloughed from the papilla during stone passage. In some instances, he even described bits of papillary tissue still adherent to the stone.13 In 1985, through scanning electron microscopy of spontaneously passed stones, Cifuentes Delatte and colleagues later verified that luminal spaces in the RP nucleus were consistent with renal tubules, blood, and lymph capillaries running toward the surface of the papilla.14 We, too, have previously recognized that a high percentage of stones removed from kidneys of idiopathic calcium oxalate stone formers show morphologic evidence of having originated on RP with papillary depressions and apatite nuclei underlying calcium oxalate overgrowths.15
Our identification of 218 unique stones exhibiting RPA is, by far, the largest ever reported. We are also the first to report the corresponding papillary appearance from such patients. Our findings of papillary pits in 100% of such patients provide a plausible explanation as to one way in which papillary pits may form and they are further strengthened by the direct observation of papillary pits identified immediately on dislodgement of attached stones. Papillary pits are likely capable of forming spontaneously and not just via iatrogenic processes. As mentioned, RPA are known to occur on spontaneously passed stones. Moreover, 7 out of 7 patients undergoing their initial stone treatment in this series demonstrated evidence of pitting before stone extraction. Notably, all but one of these patients reported a prior history of spontaneous stone passage, making it strongly suggestive that pits seen at the time of endoscopy may have formed at the time of prior spontaneous stone dislodgement. We are also confident that pits are not just a transient process as evidenced by the re-demonstration of nearly identical papillary appearances over an 11-month period in one of the patients included in this study.
Recognition that pits can occur in response to RP stone dislodgement is useful as continued efforts are being made to improve the way in which patients with nephrolithiasis are classified. Current classification mechanisms are insufficient and lack insight into the stone formation mechanism. For example, stone mineral analysis varies widely between labs16 and results of 24-hour urine testing have been found to be inadequate in nearly 50% of cases when performed in non-research settings.17 Recently, Letavernier and colleagues suggested that the mechanism of stone formation be considered when classifying stone formers. RP formation, in particular, has been suggested to have clinical relevance as patients who form stones this way have been found to have earlier onset of stone disease and high likelihoods of metabolic abnormalities.18
Two endoscopic papillary grading systems have been recently introduced to serve as an adjunct to established mechanisms to classify stone formers.3,4 Each of these systems is based predominantly on the presence of papillary abnormalities; however, the corresponding meaning and implications of the abnormalities have yet to be fully appreciated. Herein, we provide a previously undescribed correlation between papillary abnormality and stone formation that might help advance the field.
The clinical and research implications of identifying this mechanism for papillary pitting are significant. First, papillary pits are a very common endoscopic finding. They are a central component of both endoscopic papillary classification systems. In the classification system described by Almeras and coworkers, pitting was the most commonly observed abnormality besides RP itself.4 Further, application of the system described by Borofsky and coworkers3 found a strong correlation between pitting and RP.5 We are now well poised to study the clinical relevance of this finding. For example, might papillary pits be useful as a surrogate marker of stone activity? Data from this cohort would suggest this to be the case as the vast majority of patients with pits were recurrent stone formers (93%). Application of this concept might be useful in combating known underutilization of 24-hour urine testing by urologists by helping identify patients with the highest probability of having the most meaningful abnormalities.19 Further, the identification of papillary pits opens numerous opportunities for research. For example, it remains unclear as to whether these pits are associated with alterations in renal physiology. If so, perhaps this process may explain why patients with recurrent calcium oxalate stones occasionally convert to forming calcium phosphate stones.20 Alternatively, it remains unclear as to whether this apparent injury to the papilla has any corresponding impact on renal function, suggested to occur in association with repeated stone events.21 Finally, we do not know whether RP is able to reform at all once dislodged. If not, perhaps the superficial shedding of the RP might explain why the incidence of stone disease declines with age.22
Our findings must be interpreted in the context of several limitations. For one, the concept of papillary pitting is relatively new and has only recently gained appreciation. An alternative papillary pathology with the potential to look similar to a pit is a widely dilated Bellini duct. Dilated Bellini ducts are found commonly in the papillae of stone formers with high degrees of tubular plugging by crystals such as occur in cystine, brushite, and calcium phosphate stone disease. Classically, dilated Bellini ducts have a different appearance from pits and can be distinguished by a deep rather than superficial extension into the papilla. Further, dilated ducts classically have discrete edges whereas pits tend to have more ragged edges, frequently with adjacent RP. We acknowledge that in certain disease states pits and dilated ducts may coexist and we are hopeful to formally assess this as our experience with endoscopic papillary assessment grows. An additional limitation is that we are unable to definitively match each RP stone to a corresponding pit. In several instances, however, a papillary pit was able to be appreciated immediately on dislodgement of an attached stone (Fig. 2). In many cases, we presume that pits formed at previous time points. Another limitation is that the patients included in this study were identified retrospectively on the basis of stone μCT, making it possible that other mechanisms may also lead to papillary pits. Finally, our study did not have a control group. Given our hypothesis that pits form after stone dislodgement, we would not anticipate seeing any in the papilla of non-stone formers and plan to formally assess this in future research efforts.
Our study has several strengths as well. Most notably, the μCT data presented is the largest in the published literature. Further, the inclusion of seven patients undergoing their first stone treatment, each of whom demonstrated pitting, suggests that this is not merely a surgical induced process resulting from direct stone removal.
Conclusions
RP is able to be reliably identified by using μCT. Papillary pits were identifiable on at least one papilla from such patients in 100% of cases. Increasing awareness of this common finding and elucidation of the papillary findings associated with it may allow greater precision in accurately classifying stone formers not only by the type of mineral that their stones are composed of but also by the underlying formation mechanism itself. Further work is needed to understand the implications of such findings, in particular how the presence and relative degree of papillary pits may be used to classify and risk stratify stone formers.
Abbreviations Used
- μCT
micro-computed tomography
- RP
Randall's plaque
- RPA
Randall's plaque anchors
Author Disclosure Statement
M.S.B.: Boston Scientific Corporation; Auris. C.A.D.: Boston Scientific Corporation. J.E.L.: Boston Scientific Corporation. All other authors have no competing financial interests.
References
- 1. Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res 2010;38:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evan AP, Worcester EM, Coe FL, Williams J, Jr., Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis 2015;43(Suppl 1):19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borofsky MS, Paonessa JE, Evan AP, et al. A proposed grading system to standardize the description of renal papillary appearance at the time of endoscopy in patients with nephrolithiasis. J Endourol 2016;30:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almeras C, Daudon M, Ploussard G, Gautier JR, Traxer O, Meria P. Endoscopic description of renal papillary abnormalities in stone disease by flexible ureteroscopy: A proposed classification of severity and type. World J Urol 2016;34:1575–1582 [DOI] [PubMed] [Google Scholar]
- 5. Cohen A, Borofsky M, Anderson BB, et al. Endoscopic evidence that Randall's plaque is associated with surface erosion of the renal papilla. J Endourol 2017;31:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borofsky MS, Dauw CA, Cohen A, Williams JC, Jr, Evan AP, Lingeman JE. Integration and utilization of modern technologies in nephrolithiasis research. Nat Rev Urol 2016;13:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zarse CA, McAteer JA, Sommer AJ, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urol 2004;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams JC, Jr, McAteer JA, Evan AP, Lingeman JE. Micro-computed tomography for analysis of urinary calculi. Urol Res 2010;38:477–484 [DOI] [PubMed] [Google Scholar]
- 9. Evan AP, Lingeman JE, Worcester EM, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec 2014;297:731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 2005;67:576–591 [DOI] [PubMed] [Google Scholar]
- 11. Pramanik R, Asplin JR, Jackson ME, Williams JC., Jr Protein content of human apatite and brushite kidney stones: Significant correlation with morphologic measures. Urol Res 2008;36:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elliot JS. Structure and composition of urinary calculi. J Urol 1973;109:82. [DOI] [PubMed] [Google Scholar]
- 13. Prien EL. The riddle of Randall's plaques. J Urol 1975;114:500–507 [DOI] [PubMed] [Google Scholar]
- 14. Cifuentes Delatte L, Minon-Cifuentes JL, Medina JA. Papillary stones: Calcified renal tubules in Randall's plaques. J Urol 1985;133:490–494 [DOI] [PubMed] [Google Scholar]
- 15. Miller NL, Williams JC, Jr, Evan AP, et al. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall's plaque. BJU Int 2010;105:242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krambeck AE, Khan NF, Jackson ME, Lingeman JE, McAteer JA, Williams JC., Jr Inaccurate reporting of mineral composition by commercial stone analysis laboratories: Implications for infection and metabolic stones. J Urol 2010;184:1543–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGuire BB, Bhanji Y, Sharma V, et al. Predicting patients with inadequate 24- or 48-hour urine collections at time of metabolic stone evaluation. J Endourol 2015;29:730–735 [DOI] [PubMed] [Google Scholar]
- 18. Letavernier E, Vandermeersch S, Traxer O, et al. Demographics and characterization of 10,282 Randall plaque-related kidney stones: A new epidemic? Medicine (Baltimore) 2015;94:e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dauw CA, Alruwaily AF, Bierlein MJ, et al. Provider variation in the quality of metabolic stone management. J Urol 2015;193:885–890 [DOI] [PubMed] [Google Scholar]
- 20. Mandel N, Mandel I, Fryjoff K, Rejniak T, Mandel G. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol 2003;169:2026–2029 [DOI] [PubMed] [Google Scholar]
- 21. Zisman AL, Evan AP, Coe FL, Worcester EM. Do kidney stone formers have a kidney disease? Kidney Int 2015;88:1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curhan GC. Epidemiology of stone disease. Urol Clin North Am 2007;34:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]