Abstract
Neuromodulation of spinal networks can improve motor control after spinal cord injury (SCI). The objectives of this study were to (1) determine whether individuals with chronic paralysis can stand with the aid of non-invasive electrical spinal stimulation with their knees and hips extended without trainer assistance, and (2) investigate whether postural control can be further improved following repeated sessions of stand training. Using a double-blind, balanced, within-subject cross-over, and sham-controlled study design, 15 individuals with SCI of various severity received transcutaneous electrical spinal stimulation to regain self-assisted standing. The primary outcomes included qualitative comparison of need of external assistance for knee and hip extension provided by trainers during standing without and in the presence of stimulation in the same participants, as well as quantitative measures, such as the level of knee assistance and amount of time spent standing without trainer assistance. None of the participants could stand unassisted without stimulation or in the presence of sham stimulation. With stimulation all participants could maintain upright standing with minimum and some (n = 7) without external assistance applied to the knees or hips, using their hands for upper body balance as needed. Quality of balance control was practice-dependent, and improved with subsequent training. During self-initiated body-weight displacements in standing enabled by spinal stimulation, high levels of leg muscle activity emerged, and depended on the amount of muscle loading. Our findings indicate that the lumbosacral spinal networks can be modulated transcutaneously using electrical spinal stimulation to facilitate self-assisted standing after chronic motor and sensory complete paralysis.
Keywords: balance control, neuromodulation, neuroplasticity, paralysis, transcutaneous electrical spinal cord stimulation
Introduction
Spinal cord injury (SCI) is a severe disorder resulting in paralysis and dysfunction of multiple physiological systems. Recovery of the ability to control full weight-bearing standing posture independently of weight-supporting devices or manual trainer assistance is one of the most desired goals of individuals with paralysis.1 Regaining this function provides a greater level of physical independence and mobility and can facilitate general health maintenance,2,3 including an enhancement of autonomical control of blood pressure, as well as bladder, bowel, and sexual functions.4–7 Finally, the same point of importance applies in the recovery of balance during standing as the foundation for regaining the ability to walk,8–11 including stepping assisted by robotic devices.12,13
Having observed the recovery of standing, stepping, and voluntary control of lower limb movements with epidural stimulation,14–21 one can predict that similar results could be achieved with a non-invasive and more readily adaptable approach. For example, it is feasible to neuromodulate excitability at multiple levels of the spinal neuraxis, ranging from the cervical to the coccygeal cord levels, to facilitate motor function using transcutaneous electrical spinal cord stimulation (tSCS).22–28 Most recently, we demonstrated that, after more than 2 years following the onset of complete or partial paralysis, stimulation over the lumbosacral enlargement improved postural control of upright sitting.29 At the same time, there have been no focused efforts to test the effectiveness of this strategy in the recovery of full weight-bearing standing, and to examine the physiological effects and mechanisms that underlie such functional outcome in individuals with chronic motor and sensory complete paraplegia.
We hypothesized that tonic tSCS can modulate spinal circuitry in humans into a physiological state that enables sensory inputs during weight-bearing to serve as a primary source of neural control to maintain externally unassisted upright posture and balance after SCI. Further, we sought to identify the progression of neuroplasticity of postural networks to repeated bouts of stand training comprising both no stimulation and spinal stimulation conditions. Our primary outcomes included: (1) qualitative comparison of need of assistance for knee and hip extension provided by trainers in standing without and during optimized or intentionally ineffective, sham stimulation in the same participants, and (2) quantitative measures of the level of manual assistance provided to each knee and amount of time spent standing independently from trainer support, during each condition. Here we demonstrate that non-invasive spinal stimulation exhibits the neuromodulatory potential as reported for epidural stimulation, including independent or minimally assisted knee and hip extension, resulting in self-assisted standing. The observed tSCS-induced enabling effects on standing occurred in every participant and within the first experimental session. Further studies will be necessary to determine whether the changes observed will result in improved self-care and quality of life in a broad SCI population.
Methods
Participants
The major inclusion criteria were non-progressive SCI, American Spinal Injury Association Impairment Scale (AIS) A-C, of at least 1-year post-injury, inability to stand without external assistance (i.e., mechanical constrain of the joints or trainer support) prior to the intervention, and stable medical condition without active ongoing treatment. Twenty-one volunteers with SCI were screened for this study. Seven candidates were not recruited because they did not fit into inclusion criteria (e.g., neurological level of injury was too high [above C5] or too low [below T12; n = 4], presence of stem cells implant in the injury site [n = 1], pressure sores [n = 1], or contractures [n = 1]), and the remaining two decided to not participate due to scheduling conflicts. One participant later withdrew from the study due to an adverse event independent of the intervention, and the data were excluded from the analysis. Fifteen individuals with SCI participated in this study (Table 1). Demographic information of each participant, as well as the AIS and Modified Ashworth Scale (MAS) assessments were performed during examination before and after the study by an independent clinician. Participants provided written informed consent to the experimental procedures, which were approved by the University of California, Los Angeles (UCLA) Institutional Review Board. During the experiment, the subjects did not participate in any professional activity-based program prior to or during the intervention.
Table 1.
Demographic Characteristics of Participants
| Participant | Sex | Age | Post-SCI (years) | NLI | AIS | Sham stimulation | tSCS-induced effects (first session) | Post-training effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tSCS | Outcomes | Duration (min) | tSCS | Outcomes | Duration (min) | |||||||
| P1 | F | 27 | 2.5 | T12 | A | Biphasic stimulation over T11, up to 110 mA. Sessions 1–12 | L1 50 mA |
Minimally assisted standing (one knee support, knee and hips are independent) | 3 | L1 30 mA |
Minimally assisted standing (no knee and hip support) | 7 |
| P2 | M | 26 | 2 | T9 | A | Biphasic stimulation over T11, up to 200 mA. Sessions 3, 5 | L1 80 mA |
Self-assisted standing (no knee and hip support) | 11 | L1 40 mA |
Minimally assisted standing (one hand self-assistance) | 30 |
| P3 | M | 26 | 8 | T2 | A | Monophasic stimulation over T7-T8, 80 mA. | L1 80 mA |
Minimally assisted standing (no knee support) | 1 | L1 60 mA |
Self-assisted standing (no knee and hip support) | 16 |
| P4 | M | 25 | 7 | T4 | A | Biphasic stimulation over T11, up to 200 mA. Sessions 1–12 | L1 120 mA |
Minimally assisted standing (one knee and hip support, other knee is independent) | 3 | L1 90 mA |
Minimally assisted standing (no knee support) | 14 |
| P5 | M | 23 | 4 | T2 | A | Biphasic stimulation over T11, up to 200 mA. Sessions 1, 9 | L1 120 mA |
Minimally assisted standing (one knee and hip support, other knee is independent) | 0.5 | L1 90 mA |
Minimally assisted standing (no knee support) | 6 |
| P6 | M | 30 | 10 | T3 | A | Biphasic stimulation over T11, up to 200 mA. Session 11 | L1 120 mA |
Minimally assisted standing (one knee and hip support, other knee is independent) | 0.5 | L1 80 mA |
Minimally assisted standing (no knee support) | 12 |
| P7 | M | 28 | 2 | T4 | C | Biphasic stimulation over T11, up to 150 mA. | L1 70 mA |
Self-assisted standing (no knee and hip support) | 8 | No training | ||
| P8 | M | 47 | 6 | T3 | A | None | L1 120 mA |
Self-assisted standing (no knee and hip support) | 3 | No training | ||
| P9 | M | 40 | 6 | T9 | A | Biphasic stimulation over T11, up to 200 mA. | L1 100 mA |
Self-assisted standing (no knee and hip support) | 2 | No training | ||
| P10 | M | 53 | 5 | T8 | A | None | L1 150 mA |
Self-assisted standing (no knee and hip support) | 6 | No training | ||
| P11 | M | 26 | 9 | C5 | B | Monophasic stimulation over T7-T8, 100 mA. | L1 100 mA |
Self-assisted standing (no knee and hip support) | 4 | No training | ||
| P12 | F | 32 | 7 | C6 | A | Monophasic stimulation over T7-T8, 80 mA. | L1 80 mA |
Minimally assisted standing (no knee, only hip support) | 2 | No training | ||
| P13 | M | 26 | 7 | C4 | C | None | L1 120 mA |
Minimally assisted standing (one knee and hip support, other knee is independent) | 4 | No training | ||
| P14 | M | 27 | 2 | C5 | A | Monophasic stimulation over T7-T8, 70 mA. | L1 70 mA |
Minimally assisted standing (one knee and hip support, other knee is independent) | 1.5 | No training | ||
| P15 | F | 32 | 13 | C5 | C | None | L1 50 mA |
Self-assisted standing (no knee and hip support) | 11 | No training | ||
AIS, American Spinal Injury Association Scale (AIS) classification; Duration, duration of self-assisted standing, without or with minimum support provided to the knees or hips in the presence of tSCS (without tSCS no participants were able to maintain their standing posture without trainer assistance); L1, L1–L2 intervertebral space; NLI, neurological level of injury; tSCS, transcutaneous electrical spinal cord stimulation location and intensity of delivery.
Transcutaneous electrical spinal cord stimulation (tSCS)
A custom-built constant current stimulator was used to deliver tSCS via self-adhesive electrodes with a diameter of 3.2 cm placed on the skin between the spinous processes of the T11–T12 or L1–L2 vertebrae (hereafter referred to as T11 and L1, respectively) as cathodes, and two 7.5-cm × 13-cm self-adhesive electrodes (ValuTrode, Axelgaard Ltd., US) located over the iliac crests as anodes.30,31 The stimulation waveform consisted of monophasic 1-msec pulses, at a frequency ranging between 0.2 and 30 Hz, with each pulse filled by a carrier frequency of 10 kHz, and stimulation intensity up to 150 mA. Reference signals were recorded from the stimulator to monitor stimulation artifacts.
Ineffective (sham) stimulation
In seven participants, P1–P2, P4–P7, and P9 (Table 1), intentionally ineffective for standing sham stimulation was administered at one of the locations as effective tSCS, between the T11 and T12 spinous processes, at a frequency of 30 Hz, and intensities up to 200 mA, but utilizing biphasic pulses instead of monophasic, with each 1-msec pulse filled by a carrier frequency of 10 kHz. Such an approach mimicked the experience and visceral sensation of the effective tSCS, from the perspective of the participant, by creating strong tonic contraction of the paraspinal muscles. Even with a higher stimulation intensity, however, biphasic pulses filled by a 10 kHz carrier frequency did not induce detectible activity of motor pools projecting to the leg muscles, and therefore, were not producing motor output in the lower limbs. In four participants P3, P11–P12, and P14 (Table 1), stimulation was delivered remotely from the lumbosacral enlargement, between the spinous processes of T7–T8, with the pulse configuration, frequency, and intensities of stimulation being the same as the effective tSCS. Similarly, with such an approach, the participants stated perceiving the contraction of the paraspinal muscles; however, the motor pools projecting to the leg muscles were not activated. During all sham and effective tSCS conditions, the procedures of placement of the stimulating and recording electrodes, order of tests, and instructions remained constant from the perspective of the participants and trainers (Fig. 1A–B).
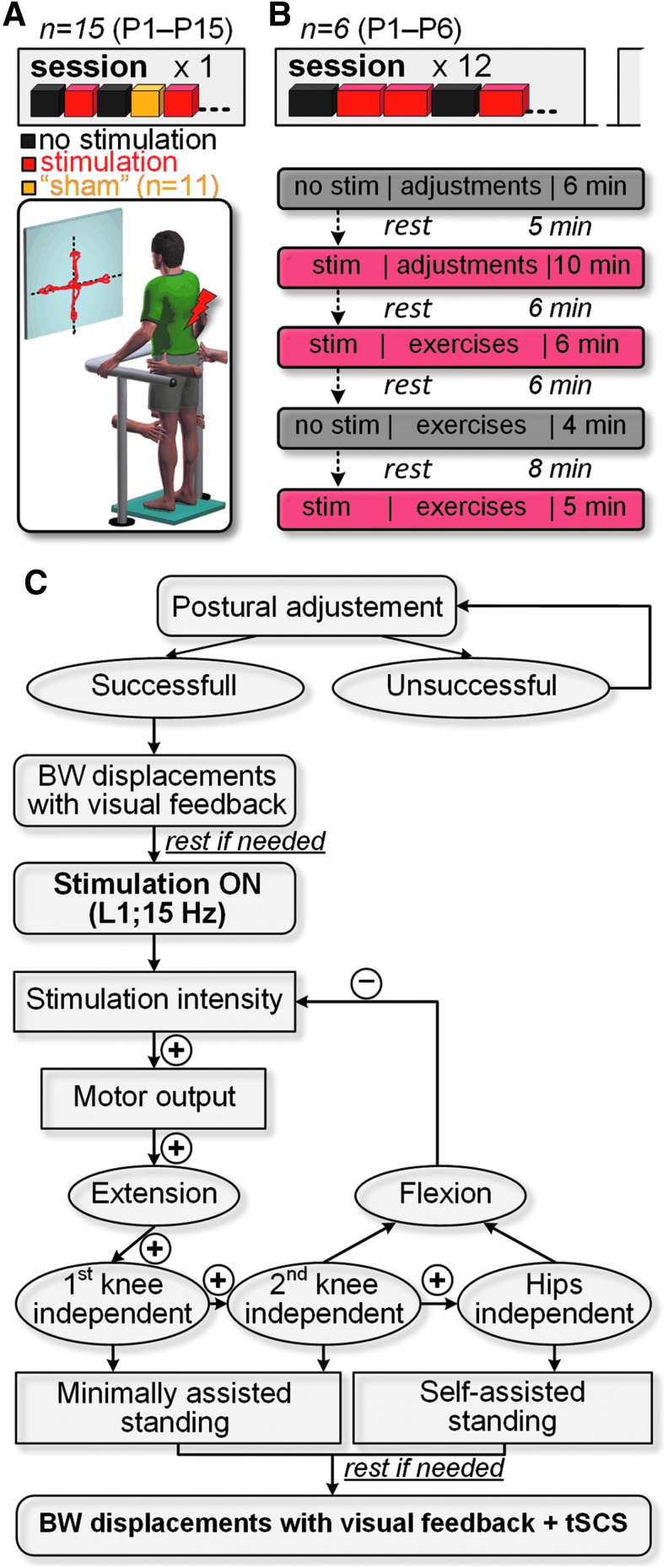
FIG. 1.
Schematics presenting the experimental design and interventions. (A) Fifteen individuals were tested in a single experimental session without and in the presence of tSCS. Eleven participants received sham stimulation by using intentionally ineffective for standing pulse configuration or location of delivery. (B) Six participants underwent 12 training sessions, where trials without and with spinal stimulation were randomly assigned within each session. Shown is an example of protocol during a single training session. (C) Decision tree exemplifying the adjustment of the stimulation parameters during “ostural adjustments” in each experimental session (see description in the text).”+” and “−” indicate an increase or decrease of spinal stimulation intensity, respectively. tSCS, transcutaneous electrical spinal cord stimulation.
EMG recording
Surface electromyogram (EMG) signals were recorded bilaterally using bipolar surface electrodes (Noraxon, US) placed over the soleus (SOL), tibialis anterior (TA), vastus lateralis (VL), and medial hamstring (MH) muscles with fixed inter-electrode distance of 20 mm. The EMG signals were amplified using PowerLab system (ADInstruments, Australia) with a band-pass filter of 10 Hz to 2 kHz, and digitized at a sampling rate of 4 kHz.
During tSCS, evoked motor potentials were induced in the leg muscles, and their peak-to-peak amplitude was calculated within a −5 to 45 msec time window in relation to the reference signals from the stimulation channel. Because of the relatively low stimulation frequency and remoteness of stimulation electrodes from the EMG, there was little interference between the stimulation artifacts and recorded signals, allowing for derivation of the induced responses in the time windows free of artifacts. Typically, six spinally evoked motor potentials from a given muscle were analyzed and compared between different tSCS characteristics, including stimulation intensity, frequency, location, and motor task.
To compare the muscle output without stimulation and with tSCS or sham stimulation conditions, the digitized EMG time series were full-wave rectified and processed as described previously.29 Briefly, the reference signal from the stimulation channel was used to determine the onset and offset of stimulation artifacts; to set the portion of the artifact corresponding to the stimuli to zero, a linear adaptive filter was utilized to reduce the magnitude of the noise signal and remove any significant outliers while retaining prominent muscle activation features, for example, muscle activation peaks. Muscle activity was quantified by calculating the mean EMG signal amplitude within a 1-sec measurement window, immediately before and during leaning in the mediolateral or anteroposterior direction as described below.
Apparatus for standing
A custom-designed stand frame was used to provide balance assistance during upright standing, and did not passively constrain the participant's torso or legs. This frame included height adjustable handrails that participants could use for weight-bearing and/or balance if required. External assistance was provided as needed by trainers to the participants' knees and/or hips. The amount of applied assistance was titrated to be as minimal as possible to promote knee and hip joint extension and maintain upright posture; the trainers were instructed to remove their hands from the participant's knees or hips if independent extension was achieved and the assistance was not required. Two 4-cm × 4-cm force-sensing resistor (FSR) sensors (Interlink Electronics, Inc., US),were placed above the participant's knees, and they were used to measure the interactive force between the surface of the participant's leg and the trainer's hands, to quantify the level of assistance provided by the trainer to facilitate each knee's extension. The FSR sensors were powered from a regulated 5-V supply, and calibrated using a set of fixed weights. The trainer placed their palms on the FSR sensors and the amount of force during the manual assistance was recorded. The system also incorporated a force plate system Stabilan-01 (Rhythm Ltd., Russia), which measured the participants' center of pressure (COP) excursions. The data from the FSR sensors and force plate were digitized at a sampling rate of 4000 and 50 Hz, respectively.
Experimental protocol
The experimental sessions typically involved at least three team members: two trainers who assisted in standing, and one researcher controlling the stimulation parameters. Neither participants nor trainers knew whether and at which intensity spinal stimulation was initiated by the researcher, thereby blinding the study.
Prior to the sessions, the participants had been requested to empty the bladder. All participants were examined initially during sitting to characterize the level of recruitment of motor pools innervating leg muscles, using stimulation over a range of intensities at the T11 and L1 vertebral sites. The participants were then transferred to the standing position, and the tests were performed without and in the presence of tSCS. During all tests in standing, participants were instructed to minimize the use of their arms for weight-bearing and to attempt to extend their knees and hips. We have defined “self-assisted” standing as the one during which the participants were able to maintain their standing posture with knees and hips extended without any additional support via mechanical constrain of the joints or trainer assistance. “Minimally assisted standing” was characterized as the one when some trainer assistance was applied to the knee or/and hip joint. In both conditions, the participants could use their hands for upper body balance.
Each 120-min session routinely included “postural adjustments,” during which trainers positioned the participant on the force plate and, using a mirror on a side, prompted them to align the body and limbs to achieve an upright position that was biomechanically close to natural standing. Specifically, the effort was made to ensure full knee and hip extension bilaterally, and to maintain the body's center of mass toward the forward limit of stability32 (Supplementary Movie S1; see online supplementary material at http://www.liebertpub.com). Once the standing posture was optimized, the participants were instructed to view the monitor placed in front of the stand frame, where real-time biofeedback of their COP position was displayed. The participants were asked to lean in the mediolateral or anteroposterior direction and maintain this position for 1 sec, with the goal to maximize the COP limits of stability during these self-initiated body-weight displacements.33 Subsequently, tSCS was administered by the researcher. Stimulation intensity was set to induce independent extension in at least one knee with the ultimate goal of no assistance provided to the knees and hips. If stimulation induced flexion in the leg antigravity muscles, the intensity was reduced. Once the researcher verified the stimulation parameters corresponding to the level minimizing the external support during standing, the self-initiated body-weight displacements were performed as described above in the presence of tSCS (Fig. 1C).
In six participants (P1–P5, P8) the effects of tSCS delivered during standing over the T11 and L1 at frequencies of 5, 15, and 25 Hz, and at intensities up to 100 mA, were investigated during the first experimental session to induce self-assisted or minimally assisted standing. In the remaining participants, the L1 and a frequency of 15 Hz were used consistently, with only stimulation intensity adjusted to the level sufficient to maximally facilitate standing.
Stand training
Six participants (P1–P6) underwent 12 sessions of stand training, performed 3 days per week (Fig. 1B). Two types of exercises were utilized: “Circle,” where the participants were instructed to shift their body weight in a circular pattern and follow a trajectory displayed on the monitor, and “Basketball,” where three targets of different colors appeared on the top of the screen, and the participants were instructed to direct the target into the “basket” of the matching color by shifting their COP. The duration of each exercise varied from 1 to 2 min. To motivate participants to improve their performance, they were instructed to maximize their score during each exercise. The game-based exercises were practiced both without and in the presence of optimized tSCS. Stimulated and non-stimulated conditions were interspersed in a pseudo-random sequence at the individual level to achieve balanced protocol, and each participant received an equal number of each condition within a given experimental session, which lasted as long as the participant's endurance allowed on a given day.
Multi-site spinal cord stimulation
Effects of multi-site stimulation34 were tested in the participants P1–P2 (during 12th session), and P15. In P1 and P2, during standing enabled by stimulation over L1 at 15 Hz, T11 stimulation was periodically delivered at 30 Hz, at the intensity corresponding to motor threshold responses in the leg muscles. P15 (AIS C) was instructed to lift the right foot off the ground during standing, and to maintain body weight on the left leg. These unilateral stepping-like movements were performed without stimulation, during stimulation over T11 delivered at 30 Hz, and during combined stimulation over T11 at 30 Hz and L1 at 15 Hz. The right hip and knee joint angles were measured using electrogoniometers.
Training logs and questionnaires
During training, the participants were requested to document any changes in their body, including fatigue and endurance, sleep, appetite, perspiration, muscle tone, etc., daily. During each visit, they were additionally requested to evaluate their (a) general health condition, (b) mood, (c) spasticity, and (d) bladder and bowel functions since the prior session, by grading each as: below, usual, or above the “normal” level. Blood pressure, heart rate, and skin condition were monitored by the team throughout each session.
Statistical analysis
The study comprised three conditions: standing without (n = 15), in the presence of effective tSCS (n = 15), and during sham stimulation (n = 11). Statistical analyses were performed by an independent statistician using a fully within-subject statistical design implemented through general linear model, linear mixed model, or generalized estimating equations as appropriate based on tests of statistical assumptions of primary end-points. The co-primary end-points were assistance-free standing (binary outcome), quantitative measurement of force needed for knee assistance, and amount of time spent standing. The impact of stimulation was assessed in a completely within-subject design with stimulation, no stimulation, and sham stimulation in the same participants (participants served as their own control). The statistical design consisted of three within-subject independent variables (stimulation, session, muscle/joint/activity), and the interactions of those. The within-subject study design enabled high power (η2 = 0.67–0.91) for assessing training efficacy in six participants. During training, stimulation intensity required to achieve minimally assisted standing, as well as the time in upright position, were monitored and compared. Finally, the performance was monitored and expressed as a score for each exercise. The level of significance was set at α = 0.05 for all analyses.
Results
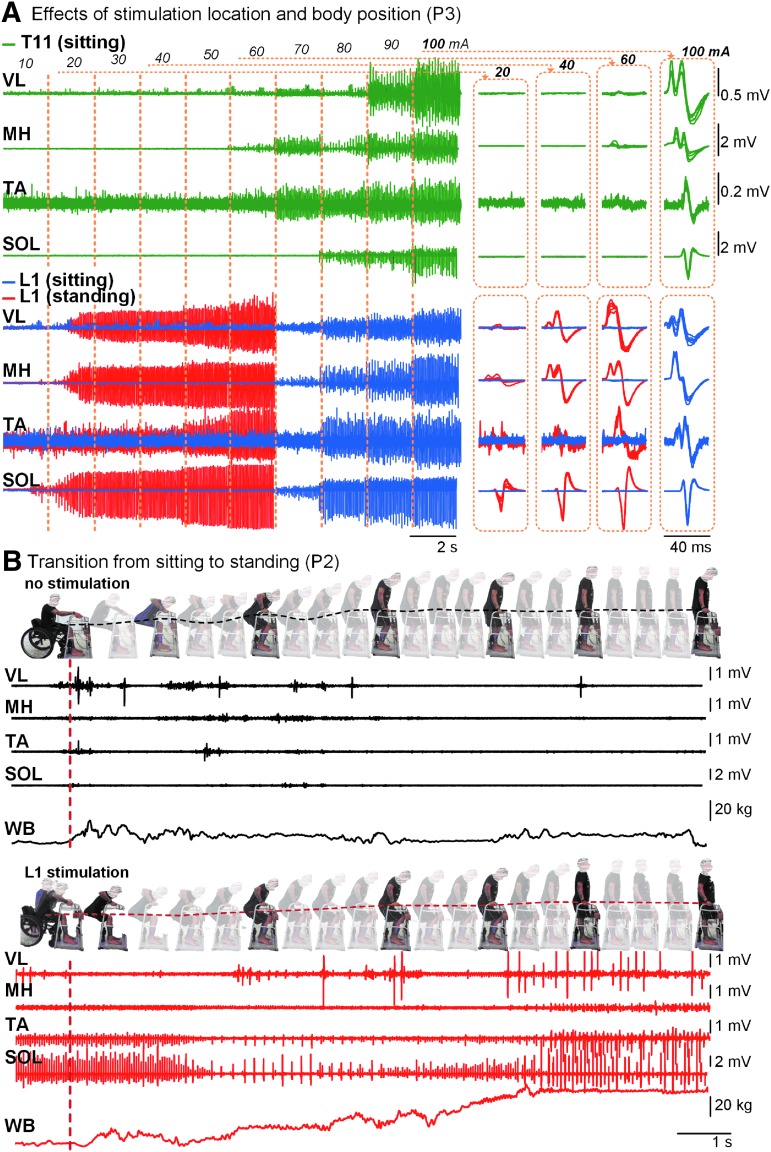
Individual recordings are presented in Figure 2. The level of leg muscles' activation depended on the rostro-caudal location of stimulation and the participant's body position. During stimulation at T11, the magnitude of response of proximal VL was higher than distal TA and SOL; whereas, during stimulation at L1, this relationship was reversed. After transition from sitting to standing, a decreased muscle activation threshold, and increased level of EMG activity, as compared with sitting, were revealed (Fig. 2A). Without tSCS, transition into standing occurred with minimal activity in leg muscles, and with little weight-bearing through the lower body: The participants supported themselves mainly by their arms. During tSCS, the leg muscles' activity emerged, and depended on the amount of weight-bearing by the lower body (Fig. 2B).
FIG. 2.
Individual reactions during tSCS. (A) EMG activity of the left leg muscles during tSCS delivered with a frequency of 15 Hz at incremental intensities over T11 and L1 during sitting and standing, recorded in a representative participant (P3). Right panels indicate spinally evoked motor potentials recorded during the indicated stimulation intensities. (B) Transition from sitting to standing, recorded in a representative participant (P2). Note minimum EMG activity and low amount of body-weight bearing on the force plate during the upright position without spinal stimulation, and modulated EMG activity and a high amount of the body-weight on the support surface during stimulation. Also note “oscillating” versus “smooth” patterns of the hip displacement during the transition without and with spinal stimulation, respectively (shown by dotted traces). EMG, electromyogram; tSCS, transcutaneous electrical spinal cord stimulation.
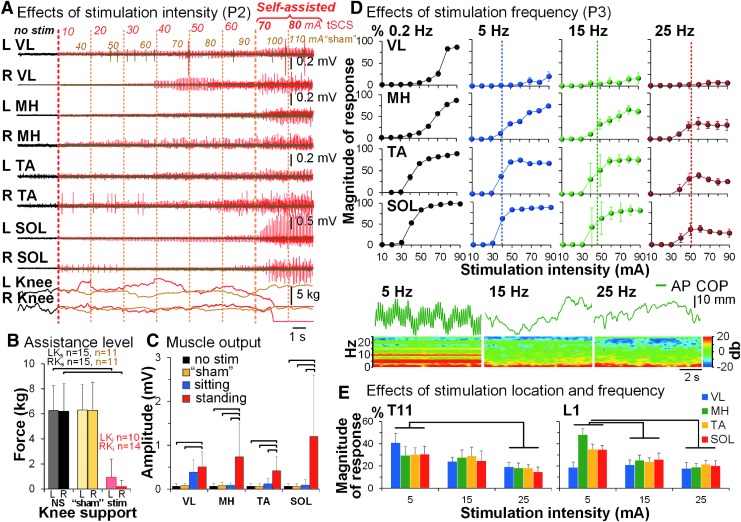
In the upright standing position without stimulation, none of the participants were able to maintain their posture without external assistance (Fig. 3A, Supplemental Movie S1). Intentionally ineffective for standing, sham, spinal stimulation was administered in 11 participants using similar parameters as effective tSCS, except with the stimulating pulse configuration (n = 7) or location of delivery (n = 4) (Table 1, Supplementary Movie S2. See online supplementary material at http://www.liebertpub.com). Although sham stimulation caused similar sensations as effective tSCS (i.e., perceived increased tone in the back muscles), there were no enabling effects on standing, and no changes in the level of assistance provided to the lower body (Fig. 3A–C). With a gradual increment of tSCS intensity delivered over the L1, leg muscle activity increased, whereas the amount of external assistance needed to sustain extended knees decreased as compared with no stimulation condition (Fig. 3B) (F1,15 = 158.23, p = 1.16e–10). The magnitude of spinally evoked motor potentials was larger during standing as compared with sitting in MH, TA, and SOL (p < 0.05) (Fig. 3C). The participants were able to bear their weight with one or both legs fully extended, and hips unsupported or minimally supported (Table 1, Supplementary Movies S1, S2. See online supplementary material at http://www.liebertpub.com). In six participants (P2, P8–P12), within the first experimental session, tSCS over the L1 with frequency 15 Hz induced robust self-assisted standing, wherein the duration varied from 0.5 (P5, P6) to 11 (P2) min (Table 1). Standing of the remaining participants required some assistance (Supplementary Movie S3; see online supplementary material at http://www.liebertpub.com).
FIG. 3.
Characteristics of spinal stimulation to induce self-assisted standing. (A) EMG activity in the leg muscles during standing without (black traces), in the presence of sham (olive traces and font, show intensities from 40 to 110 mA) and effective (red traces and font, show intensities from 10 to 80 mA) tSCS delivered over the L1 at 15 Hz, recorded in a representative participant (P2). “L Knee” and “R Knee,” readings from the pressure sensors placed above the participant's knees quantifying assistance provided by the trainer. “Self-assisted” label indicates both knees and hips extension independent of trainer assistance. (B) Pooled effects of the amount of assistance applied to the participants' knees during standing without stimulation (n = 15, black font), during sham stimulation (n = 11, olive font), and during standing enabled by tSCS (n = 15, red font). Without and during sham stimulation, all participants required trainers' assistance applied to the knees and hips, whereas in the presence of tSCS, the incidence and required force of knee assistance were reduced; hip assistance was not required in eight participants (also see Table 1). “LKs” and “RKs” indicate the left and right knee being supported, respectively; “LKi” and “RKi” indicate the left and right knee being independent, respectively; “NS” indicates standing with no stimulation (gray and black); “sham” indicates sham stimulation (light and dark yellow); “stim” indicates standing during tSCS (pink and red). (C) The mean muscle EMG amplitude during standing with no stimulation (n = 15), in the presence of sham stimulation (n = 11), and sitting and standing (n = 15) in the presence of tSCS of the same intensity during the first experimental session. (D) Recruitment curves of motor evoked potentials in the left leg muscles obtained at different frequencies of spinal stimulation delivered over the L1 during standing in a representative participant (P3). Vertical dashed lines indicate the stimulation intensity sufficient to generate self-assisted standing at indicated frequency. Note the decrease in the evoked potentials' magnitude at higher stimulation frequencies. Lower panels present the center of pressure (COP) oscillations in the anteroposterior (AP) direction, as well as the frequency spectrum corresponding to the AP COP. With tSCS frequency of 5 Hz, the dominant frequency of the COP oscillations corresponds to that of the stimulation (e.g., 5 Hz). During tSCS of higher frequencies, the COP oscillations are independent from the stimulation frequency. (E) Pooled effects (n = 6) of tSCS delivered over the T11 and L1 at 5, 15, and 25 Hz on EMG responses in leg muscles. The magnitude of responses was normalized to the maximum amplitude of each muscle during stimulation delivered at 0.2 Hz. EMG, electromyogram; tSCS, transcutaneous electrical spinal cord stimulation.
When comparing various stimulation frequencies (Fig. 3D–E), 5 Hz, although producing high-amplitude muscle responses, was effective in three of six tested participants and was stated as being uncomfortable due to strong pulsatile leg extensors' contractions correspondent to the stimulation frequency. Contrastingly, 25 Hz produced the lowest magnitude of muscle responses at similar intensities, yet enabled standing in five participants. The frequency 15 Hz generated the most robust effects on standing in all tested participants. A linear mixed model analysis revealed that stimulation had a specific, frequency-dependent effect on motor output, and that the L1 stimulation site was more effective in inducing differential proximal-distal muscle response than the T11 sites (F2,6 = 15.21, p < 0.0001).
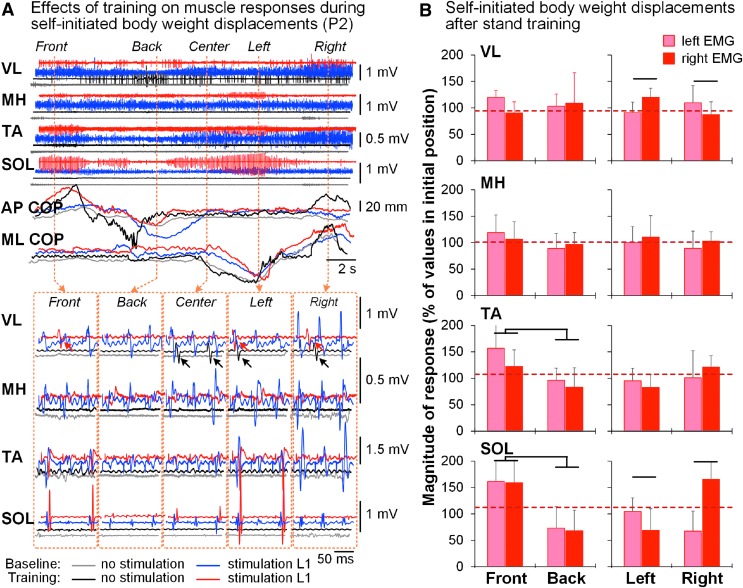
During self-initiated body-weight displacements without tSCS, minimal to no muscle activity occurred (Fig. 4A). In the presence of tSCS, more pronounced activity was observed in the VL and TA pre-training. Post-training, the EMG pattern across the muscles changed, and the most prominent activity in the presence of tSCS occurred in the SOL ipsilateral to weight-bearing. Pooled data revealed conjoint bilateral modulation of SOL and TA during anteroposterior body-weight displacements, as well as reciprocal modulation of ipsilateral SOL and VL during mediolateral displacements, depending on the loading side (p < 0.05) (Fig. 4B).
FIG. 4.
Self-initiated body-weight displacements. (A) Modulation of EMG activity in the left leg muscles and the COP signal during self-initiated body-weight displacements in the mediolateral or anteroposterior directions before (blue traces) and after (red traces) stand training in a representative participant (P2) without and in the presence of tSCS delivered over the L1 with frequency of 15 Hz. The directions of the body-weight displacements are indicated on the top. Lower panel presents individual spinally evoked motor potentials in the initial (center) position and during the body-weight displacements in a particular direction. Note VL activity (arrows) occurred independently of the stimulation during the body-weight displacements following stand training. (B) Pooled data (n = 6) demonstrating modulatory effects of the body-weight displacements in the mediolateral or anteroposterior directions on the magnitude of spinally evoked motor potentials in the left and right leg muscles during standing enabled by tSCS following training. The magnitude of spinally evoked motor potentials was normalized to the peak-to-peak amplitude of each muscle at the initial position, that is prior to movement in either direction (shown by red dashed line). COP, center of pressure; EMG, electromyogram; tSCS, transcutaneous electrical spinal cord stimulation; VL, vastus lateralis.
Training effects
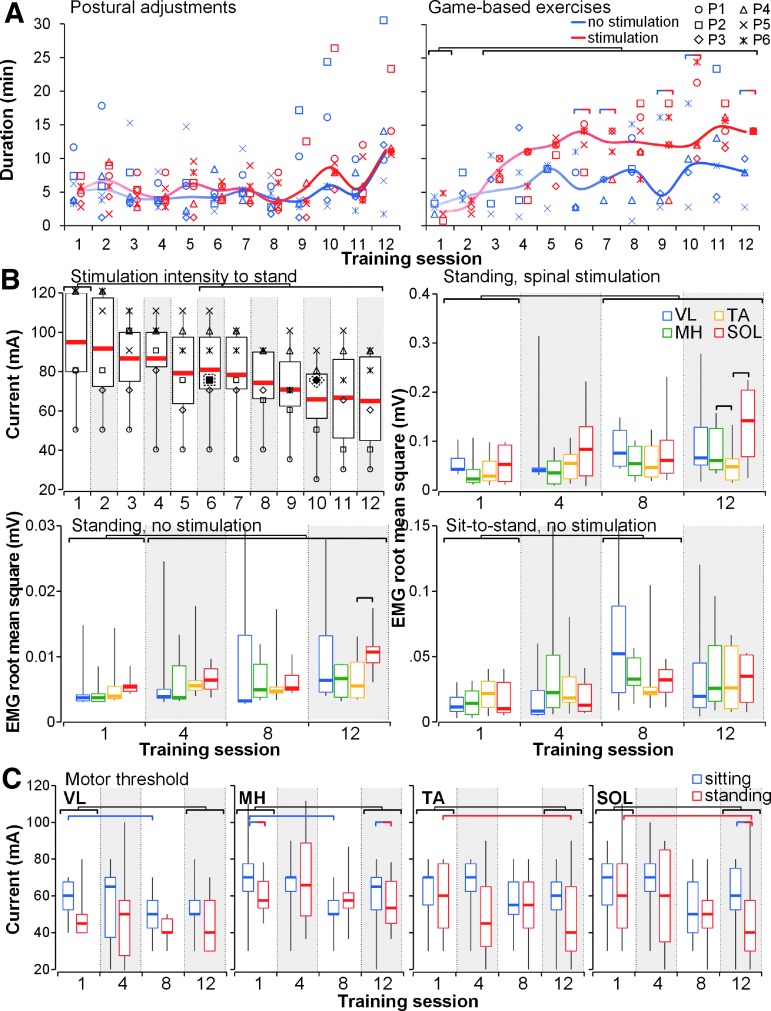
There were significant effects of stimulation (χ2 [Wald Chi-Square] = 20.43, p < 0.001), number of training session (χ2 = 2.95^12, p < 0.0001), activity (χ2 = 6.25, p < 0.05), stimulation × training session (χ2 = 4.79^12, p < 0.0001), training session × activity (χ2 = 1641.39, p < 0.0001), and stimulation × training session × activity (χ2 = 5.67^12, p < 0.0001). The duration of postural adjustment was relatively consistent throughout the training session; however, time spent in “game-based exercises” progressively increased through the training period, reflecting improvements in the participants' endurance (Fig. 5A). As the intervention progressed, stimulation intensity inducing self-assisted or minimally assisted standing decreased in all participants (χ2 = 1.15^12, p < 0.0001), whereas the leg muscle activity increased during tSCS-enabled standing (χ2 = 17.78, p < 0.0001), as well as during supported standing without stimulation (χ2 = 29.145, p < 0.0001) and sit-to-stand transitions without stimulation (χ2 = 10.97, p < 0.05) (Fig. 5B). The tSCS intensity to reach motor threshold in the leg muscles decreased during the training period both in the sitting and standing positions (Fig. 5C). There were significant effects of position (χ2 = 3.138, p < 0.05), training session (χ2 = 26.75, p < 0.0001), training session × muscle (χ2 = 10.89, p < 0.05), and position × training session × muscle (χ2 = 1.65^12, p < 0.0001).
FIG. 5.
Effects of stand training intervention on electrophysiological characteristics (n = 6). (A) Types and duration of activities practiced during the training period. “Postural adjustments” included the participant's positioning in the stand frame and on the force plate, optimization of stimulation parameters, and self-initiated body-weight displacements without and in presence of spinal stimulation; “game-based exercises” included “Circle” and “Basketball” tasks practice. (B) Stimulation intensity required to induce self-assisted or minimally assisted standing, leg muscle activity during standing without and in the presence of spinal stimulation, and during sit-to-stand transitions through the training period. Filled and outlined by dashed line symbols correspond to intensities during which the micturition reflexes occurred in participants P2 and P3 during sessions 6 and 10, respectively. (C) Stimulation intensity required to evoke motor threshold response in the leg muscles in the sitting and standing positions during the training period. Horizontal bars indicate significant differences between training sessions, stimulated versus unstimulated trials, and muscles.
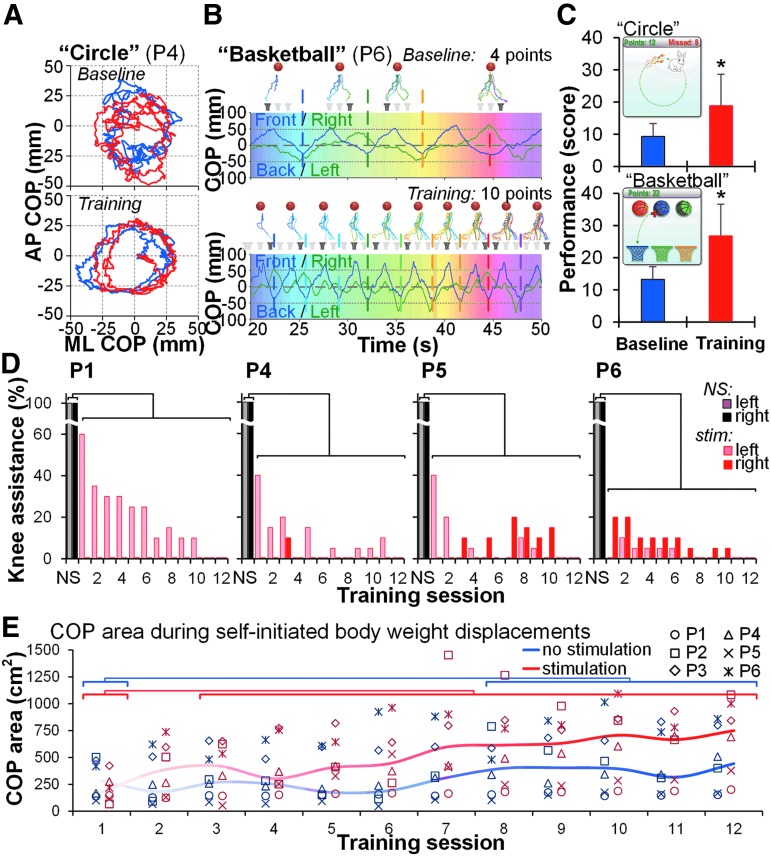
The performance during game-based training significantly improved (p < 0.05) (Fig. 6A–C). The level of assistance provided to the knees in the presence of tSCS progressively decreased (p < 0.01) (Fig. 6D, Table 1, Supplementary Movies S4, S5. See online supplementary material at http://www.liebertpub.com). The range of the COP excursions during self-initiated body-weight displacements performed both without and in the presence of tSCS increased throughout the training period (Fig. 6E): There were significant effects of stimulation (χ2 = 11.77, p < 0.001), training session (χ2 = 3.83, p < 0.0001), and stimulation × training session (χ2 = 6.25^9, p < 0.0001). The magnitude of the COP displacements with stimulation, however, was achieved in half the time as compared with that without tSCS. The participants improved their upright balance control in the presence of tSCS (Table 1). Post-training, one participant (P2) was able to stand with just one finger touch; four (P1, P4–P6) who initially required one knee support, regained the ability to stand with both knees independently extended; one (P3) who initially required hip assistance, was able to stand without any external support (Table 1).
FIG. 6.
Effects of stand training intervention on functional features. (A) The COP trajectory during the “Circle” game-based exercise without (blue traces) and in presence of spinal stimulation (red traces) in a representative participant (P4). (B) The COP trajectory during the “Basketball” exercise during spinal stimulation in a representative participant (P6). Note the increased score after the stand training. Gradient colors at the horizontal bars present time series synchronized with the cumulative COP trajectories displayed above. (C) Pooled data (n = 6) of the performance during the first and last training sessions of the stand training. The average score was calculated from three trials of each exercise. Examples of the game-based exercises are presented in the top left corner of each graph. (D) Progressive decrease of the level of assistance provided by trainer to the left and right knees during standing in the presence of tSCS (pink and red) as percent of the values during standing without stimulation (NS, gray and black) recorded using the FSR sensors within each session. Zero value indicates that the knee is independently extended (no assistance needed). Participants P2 and P3 (not displayed) did not require any knee assistance during standing in the presence of tSCS starting from training session 1. Hip assistance was not needed during standing in the presence of tSCS for participants P1 and P2 starting from training session 1, and for participant P3—starting from training session 4. Participants P4–P6 required hip assistance during standing in the presence of tSCS throughout the training period. (E) The COP area during self-initiated body-weight displacements performed without and in the presence of spinal stimulation during the training period (n = 6). Horizontal bars indicate significant differences of the COP area between training sessions. COP, center of pressure; FSR, force-sensing resistor; tSCS, transcutaneous electrical spinal cord stimulation.
Safety and tolerability of the procedure
The stimulation procedures were well tolerated by all participants with no pain or discomfort associated with tSCS being reported. The participants noted tightness in the trunk muscles during stimulation delivered at higher intensities; however, it was readily tolerated because it helped to stabilize the upper body. In general, the participants were in a positive mood at the end of each treatment session. Following the sessions, some participants noticed a modest but not disconcerting increase in spasticity or muscle tone within the first 24 h. Participants P1 and P3 indicated overall positive perception of their bladder function toward the end of the training period, and P4 reported decreased bladder function during a part of the study due to recurrent symptoms of urinary tract infection (UTI). The UTI was confirmed by the primary care physician during the 2nd and 4th weeks of the study, and the intervention was suspended for a week during antibiotic treatment. In P2, P3, and P7, there was unintentional activation of the micturition reflex and active voiding during tSCS in standing during sessions 6, 10, and 1, respectively. These events occurred spontaneously, and without apparent connection with particular activity or stimulation parameters, given that P2 and P3 tolerated similar activity and parameters without the micturition reflex during prior and following sessions (Figs. 5, 6). No changes in bowel or sexual functions were indicated. Trained participants reported in their personal logs that their overall physical endurance had been enhanced, and trunk muscle control during sitting improved. Three participants (P4, P6, P9) noticed perspiration below the lesion during the first intervention session, and one participant (P1) indicated a feeling of her calf muscles being “pumped up” after nine sessions for the first time since being paralyzed. The AIS examination did not reveal statistically significant effects of the intervention. However, P2 reported that he was able to voluntarily move his right big toe at the end of the study (Supplementary Table 1; see online supplementary material at http://www.liebertpub.com). Post-training MAS measurements revealed increased level of muscle tone in all individuals who underwent the intervention (Supplementary Table 2; see online supplementary material at http://www.liebertpub.com).
In the upright position, participants were requested to immediately initiate active body-weight displacements, to engage skeletal muscle pump of the venous vasculature, and to prevent development of orthostatic hypotension (OH).35,36 If OH symptoms occurred, they were readily mitigated by adjusting the spinal stimulation intensities.
There were no confirmed incidents of autonomical dysreflexia (AD), defined as increased systolic blood pressure greater than 20 to 30 mm Hg.37 During the first visit, participant P3 demonstrated elevation of systolic blood pressure of greater than 60 mm Hg during spinal stimulation delivered at the intensity of 10 mA in the sitting position, after which the stimulation was stopped and the participant was referred to his primary care physician. After about 2 months, during which the participant did not experience any signs of autonomical dysfunction, and confounding factors, such as UTI, pressure sores, and tight clothing were ruled out, the participant was re-enrolled in the study, with no further incidents of abruptly increased blood pressure.
Following a training session with tSCS some redness was typically observed under and around stimulating electrodes, but within minutes after the electrodes were removed, the redness was insignificant and had dissipated. There was one incident (P5) of surface skin breakage of approximately1 mm diameter during week 3, which occurred due to a defect of external conducting layer of the stimulating electrode. After a week without stimulation, the skin completely healed and the intervention was continued.
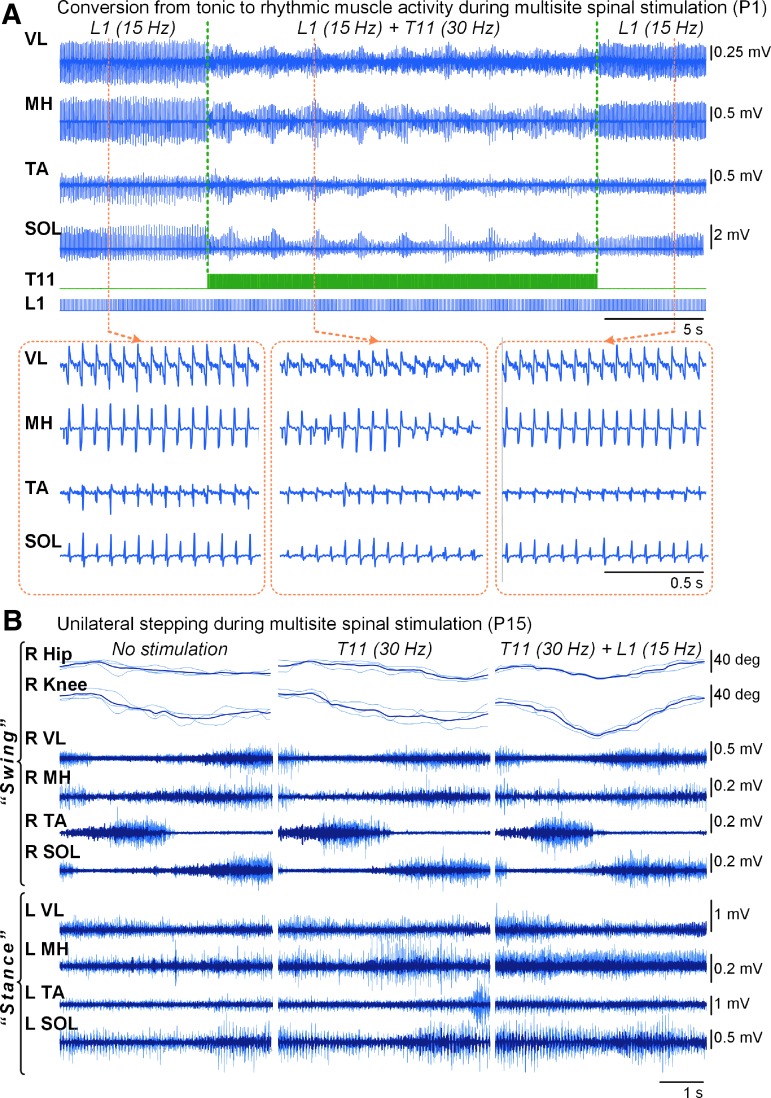
Multi-site spinal cord stimulation during standing
Multi-site stimulation delivered at different frequencies in participants P1 and P2, converted “tonic” patterns of muscle activity into a rhythmic pattern (Fig. 7A, Supplementary Movie S4). During unilateral stepping motion without tSCS, the participant P15 (AIS C) was able to produce minimal flexion in the ipsilateral hip and knee joints (Fig.7B). Weight-bearing on the contralateral leg, however, was impossible because of insufficient knee extension. Stimulation delivered over the T11 facilitated the movement, although the participant was still unable to bear body-weight on the contralateral side. Combined stimulation over the L1 and T11 considerably facilitated both the ipsilateral stepping motion and contralateral weight-bearing, and the participant was able to maintain the body-weight on one leg.
FIG. 7.
Effects of multi-site spinal stimulation. (A) Changes in the EMG activity pattern during one-site stimulation delivered over the L1 at frequency of 15 Hz, and combined stimulation over the T11 (30 Hz) and L1 (15 Hz) in participant P1. Note changes of the tonic pattern of the muscle activity during L1 stimulation to the rhythmic, “bursting” pattern, resembling “stepping” during the combined stimulation. (B) Goniograms of the right hip and knee and EMG activity of the leg muscles during unilateral stepping motion in participant P15, performed by the right foot, without (left panel), during spinal stimulation applied over the T11 at 30 Hz (middle panel), and over the T11 at 30 Hz and L1 at 15 Hz levels (right panel). Note increased angular displacement in the ipsilateral hip and knee joints, as well as larger activity in the contralateral SOL, during combined T11+L1 stimulation. The thin gray traces indicate individual responses (n = 3), whereas the bold black traces indicate the average of three responses. EMG, electromyogram; SOL, soleus.
Discussion
The present results qualitatively and quantitatively demonstrate the feasibility and effectiveness of non-invasive spinal stimulation in regaining self-assisted standing, without or with minimum assistance provided to the knees or hips, following chronic SCI. We identified specific stimulation characteristics that enabled effective standing equilibrium in paralyzed individuals. We demonstrated that spinal neuronal networks, which are relatively non-responsive after SCI, become reactive to somatosensory information in the presence of tSCS. These networks can generate and sustain independent knee and hip extension with the aid of tSCS within a single treatment session. Sham stimulation, which was utilized by altering the stimulating pulse configuration or location of delivery, did not induce enabling effects, despite being perceived by the participants as helping them to maintain their trunk extension, similar to the effective tSCS. Postural control was further improved following repeated sessions of stand training both without and in the presence of tSCS, suggesting learning effects.
Similarities of epidurally and transcutaneously mediated mechanisms
It is evident from the present findings and studies with epidural spinal stimulation18,20,38 that no participant with SCI is able to stand using self-assistance only without sufficient motor output generated by the leg muscles. To what extent are these enabling effects mediated via the polysynaptic spinal cord circuitry as opposed to mono-synaptically or post-synaptically excited motor axons projected to the leg muscles? Recent electrophysiological39–42 and computational43–45 studies demonstrated that the structures, stimulated directly and electrically by epidural or transcutaneous lumbar spinal cord stimulation, are indeed predominantly afferent fibers of the posterior roots. However, it was later supported that the volleys can activate lumbar interneuronal circuits via synaptic projections.46,47 The degree to which different components of the spinal networks are activated, depends, to a large extent, on stimulation intensity as it determines the “depth” of the current penetration (due to the induced current field), as well as on frequency, as it determines the circuitry output.25,48–50 Principally based on this concept of neuromodulating the functional state of spinal circuitry below the lesion, regaining of significant levels of clinically relevant functions, such as stepping, standing, and voluntary leg movements, after SCI has been demonstrated using both invasive19,20,38 and non-invasive22,24,25,48,51 spinal stimulation. The unique features of epidural spinal stimulation reported previously18,38,52 are strikingly similar to those identified in this work, suggesting similar electrophysiological mechanisms of invasive and non-invasive approaches. Indeed, in the present study, we consistently observed other phenomena highly akin to those reported in studies using epidural stimulation, namely: periodic modulation of repetitively evoked motor potentials,53 unique modulatory effects of different stimulation frequencies,38 and the adjustment of the muscle responses to the changes in the body position.18 The revealed adaptation of the muscle responses to the changes in the sensory environment in real time cannot be interpreted as being generated by direct activation of action potentials of motor axons. On the contrary, our data indicate that the proprioceptive inputs were processed by the spinal networks, which resulted in specific motor pools' activation depending on the task or sensory environment. The differences in the COP behavior recorded during spinal stimulation delivered at different frequencies is an example of multiple mechanisms in play: When the stimulation frequency is 5 Hz (Fig. 3D) or lower, the motor outputs seem highly synchronous among different muscles, which results in the frequency of the COP oscillation highly corresponding to that of the stimulation, suggesting relatively simple “stimulus → (Ia afferents) → α-motoneuron → muscle response” order of actions. However, spinal stimulation of higher frequencies induced motor output, which led to more robust COP behavior and facilitated self-assisted standing. As such, although facilitated standing cannot be achieved after motor complete paralysis without spinally induced leg motor output, interneuronal spinal mechanisms must be also involved, contributing to multi-segmental intraspinal interplay, which results in complex behavioral regulation below the lesion.
Earlier findings obtained in experiments with animals provided evidence that the spinal cord contains the networks generating “postural limb reflex (PLR),”54–56 and whereas in intact animals they are activated by the tonic supraspinal drive from the posture-related brain structures, the EMG pattern of PLRs can be evoked in spinal animals using epidural stimulation of the spinal cord below the lesion.56,57 In spinalized rabbits, PLRs are absent or dysregulated without neuromodulation, as observed by the reversed pattern of flexors versus extensors activation in response to the changes in the body position.55 Similarly, little or a reversed pattern of muscle activity during body-weight displacements was revealed in the present study during standing without stimulation (Figs. 2A, 4A). tSCS delivered in these participants resulted in re-appearance of the motor output and generated force responses contained both static and dynamic components. As such, our observations indicate that human spinal networks feature the critical level of posture-specific automaticity that can be exploited by utilizing non-invasive spinal neurostimulation, and can function effectively even in the lack of supraspinal excitatory drive to facilitate standing posture and some balance control.
Differences in epidurally and transcutaneously mediated effects
The results presented in the studies using epidural stimulation18,38 were obtained after research participants underwent 80 sessions of locomotor training prior to stimulator implantation, with subsequent 80 sessions of stand training and optimization in the presence of stimulation. The rapid effects occurred in the present study may be due at least in part to the ability of tSCS to neuromodulate broader components of the neural networks that are necessary to engage for successful standing, including multi-segmental projections to the trunk and lower limb musculature, due to wider current field, as compared with epidural stimulation.
Acute and cumulative effects of tSCS on spinal excitability
It is plausible that changes in the geometry of the thoracolumbar spine and the relative position of the stimulating electrodes can influence the flow of current58 and thus can contribute to the observed difference in the neuromodulation of the induced muscle responses during the sitting and standing positions. For instance, it has been shown that depending on the body position, different neural structures can be involved in the response during spinal stimulation.59 At the same time, positive augmentative interaction between spinal stimulation and weight-bearing has been demonstrated previously,16,20,26,28,60,61 and is prominent in the present study. It is critical to note that without tSCS, very low levels of the leg muscle activity were observed even during self-initiated postural adjustments, and, therefore, it was impossible for the individuals with SCI to maintain upright posture without external assistance. During tSCS, once its intensity reached a critical threshold during sitting, the transition to standing increased the excitability of the postural-specific interneuronal networks to the level adequate to generate and maintain the body weight-bearing. It appears that in the initial stages of neuromodulation, higher levels of tSCS intensity are needed to engage the networks to a physiological state necessary to process an effective level and range of somatosensory inputs required for generation of the upright posture. With subsequent sessions of neuromodulation, the level of stimulation could be reduced and still achieve a similar level of engagement of the spinal networks, as it was initially (Fig. 5B), as such the external assistance required to maintain standing proportionally reduced or was no longer required (Fig. 6D).
The force plate was reset to zero every time the participant stepped on it. Although the recordings allowed for quantification of the COP displacement, the body-weight transmitted to the plate was not monitored. The body-weight measurement, as well as more comprehensive assessment of the external versus self-assistance, including the activity of the upper body musculature, are needed for quantifying changes both below and above the injury in future trials.
Training effects
Considerable improvements occurred in the exercises practiced, as well as in the specificity of the EMG modulation during body-weight displacement (Figs. 4–6, Supplementary Movies S4 and S5). Functionally, each participant progressively improved their balance control during standing both without and with stimulation (Fig. 6E). Previous studies demonstrated that repetitive task-oriented training combined with neurostimulation is critical for augmentation or restoration of supraspinal–spinal connectivity.62,63 At the same time, individuals with chronic motor complete paralysis do not recover standing or walking without some activating neuromodulatory factors, even after intensive activity-based training.64–66 Early experiments with animals demonstrated that repetitive, long-term application of pharmacological agents or epidural spinal stimulation can cause plastic changes in the sublesional spinal postural networks, leading to restoration of postural limb reflexes and to substantial recovery of postural functions.10,55,67 Such restored intrinsic spinal automaticity and, presumably, an increase in quality and quantity of supraspinal descending motor control after clinically complete, but anatomically discomplete68 SCI in the presence of spinal stimulation can facilitate functional recovery through the regaining of movement strategies.69 Presumably, an increase in quality and quantity of supraspinal descending motor control after discomplete68 SCI in the presence of spinal stimulation70–72 can facilitate functional recovery through the regaining of movement strategies.69 Given the extensive reorganization of cortico–brainstem–spinal, corticospinal, and spinal networks after SCI,73 the level of functionality demonstrated during stand training is likely to be reflected in the level of synergism within and between the brain and spinal cord. The fact that improved motor strategies and sensorimotor responses occurred within a few sessions after years following SCI, indicates the potential of supraspinal–spinal plasticity.
Multi-system effects
Weight-bearing itself has a number of therapeutic and functional benefits for autonomical functions, such as bladder control4 and blood pressure homeostasis.6 Our findings include signs of changes affecting the lower urinary tract function in the form of unintentional voiding onset during standing in the presence of active tSCS, as well as the positive subjective perception of bladder function, including better awareness of its filling. Somato-to-pudendal inhibitory reflex, in which hindlimb extensor contraction can inhibit the external urethral sphincter activity and reduce urethral resistance, is well known in animal models,74 and can be attributed to activation of the micturition reflex observed in our study during leg extension promoted by spinal stimulation. Anecdotal reports from participants regarding change in perspiration also suggest some recovery of autonomical control. Such integrative effects between sensorimotor and autonomical systems concur with above reports on multi-system effects of upright standing.
Multi-site spinal stimulation facilitates postural–locomotor interaction
The spinal network is highly susceptible to stimulation location,34,75 intensity, and frequency.10 For instance, it has been demonstrated that stimulation of the rostral portion of the lumbosacral enlargement (corresponding approximately to the T11–T12 vertebral level) at a frequency of 30 Hz is more specific for facilitating rhythmic stepping movements,24,48,76,77 whereas stimulation delivered over the caudal area of the lumbosacral enlargement (corresponding approximately to the L1–L2 vertebral level) at a frequency of 15 Hz results in facilitation of tonic extensor activity specific for postural control.20,38,52 At the same time, the current data on the interaction between posture- and locomotor-specific networks are inconclusive. Specifically, it has not been previously investigated in SCI whether it is possible to initiate stepping from sustained standing, or whether one can improve balance during stepping by modulating the extensor tonic activity. Application of multi-site stimulation using variation of frequency and intensity within an epidural electrode array is currently impossible due to technical limitations. At the same time, tSCS allows for testing such modalities. We combined locomotor-specific stimulation over the T11 at 30 Hz, with the postural-specific stimulation over the L1 at 15 Hz, and observed facilitation of the mediolateral body-weight transitions allowing effective stepping motions to be performed in participant P15 (Fig. 7B). In participants P1 and P2, the EMG pattern during T11+L1 stimulation generated rhythmic muscle activity (Fig. 7A, Supplementary Movie S4), which demonstrates the interplay of various stimulation characteristics in generation of continuous and alternating weight-bearing, as occurs during stepping. Further, we implemented this stimulation paradigm during self-assisted over-ground stepping in participant P1, and observed considerably enhanced muscle activity compared with when there was no stimulation (Supplementary Movie S6; see online supplementary material at http://www.liebertpub.com). As such, multi-site tSCS delivered at different frequencies can concomitantly facilitate balance control and enhance weight-bearing during stepping tasks. These findings together indicate the functional continuum and important locomotor and postural networks' interactions, even though that may seem counterintuitive given the highly repetitive and rather predictable features of stepping compared with the more variable and subtle features of balance control during standing.78,79 The critical relationship, however, is in the tight interaction of sensory interplay with the multi-segmental spinal networks, which seems to have the remarkable ability to readily associate a specific sensory ensemble with a particular motor outcome. We suggest that multi-site spinal stimulation can effectively engage and synergistically modulate postural and locomotor networks, and concurrently enhance generation of motor patterns appropriate for both standing and stepping.
Conclusion
After years following complete paralysis, self-assisted or minimally assisted standing was recovered by the enabling effect of a non-invasive electrical neuromodulatory approach, and improved using repeated training sessions. The observed functional and electrophysiological effects were similar qualitatively and quantitatively to those seen in experiments with epidural stimulation. The similarities between the non-invasive and invasive approaches are of critical importance in further exploration of the mechanisms of functional recovery following spinal cord stimulation therapies. The physiological impact of these findings encompasses multiple functional systems that may contribute to the independence and quality of life in a broad population of individuals with SCI. The present data demonstrate that extensive neuroplastic changes of spinal and potentially supraspinal networks can occur using the non-invasive neurostimulating intervention while engaging sensory inputs associated with a motor task. These data are consistent with a growing body of evidence of the potential for functional recovery of multiple physiological systems years after the onset of severe paralysis when treated with a combination of activity-dependent and neuromodulatory interventions.
Supplementary Material
Acknowledgments
We would like to thank the research volunteers for their valuable contributions to this study. We are very grateful to the student-volunteers for their contribution to this research. We also thank Ms. Amanda Turner for invaluable administrative support and assistance for this project. This work was supported by the Paralyzed Veterans of America (PVA) Research Foundation (Grant #3068) and National Institutes of Health (NIH) SBIR grant R43EB018232. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Author Disclosure Statement
V.R.E., Y.P.G., and J.W.B., researchers on the study team, hold shareholder interest in NeuroRecovery Technologies. They hold certain inventorship rights on intellectual property licensed by the regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- 1. Snoek G.J., IJzemas M.J., Hermens H.J., Maxwell D., and Biering-Sorensen F. (2004). Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegic. Spinal Cord 42, 526–532 [DOI] [PubMed] [Google Scholar]
- 2. Anneken V., Hanssen-Doose A., Hirschfeld S., Scheuer T., and Thietje R. (2009). Influence of physical exercise on quality of life in individuals with spinal cord injury. Spinal Cord 48, 393–399 [DOI] [PubMed] [Google Scholar]
- 3. Fernhall B., Heffernan K., Jae S.Y., and Hedrick B. (2008). Health implications of physical activity in individuals with spinal cord injury: a literature review. J. Health Hum. Serv. Adm. 30, 468–502 [PubMed] [Google Scholar]
- 4. Walter J.S., Sola P.G., Sacks J., Lucero Y., Langbein E., and Weaver F. (1999). Indications for a home standing program for individuals with spinal cord injury. J. Spinal Cord Med. 22, 152–158 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal S., Triolo R.J., Kobetic R., Miller M., Bieri C., Kukke S., Rohde L., and Davis J.A., Jr (2003). Long-term user perceptions of an implanted neuroprosthesis for exercise, standing, and transfers after spinal cord injury. J. Rehabil. Res. Dev. 40, 241–252 [PubMed] [Google Scholar]
- 6. Harkema S.J., Ferreira C.K., van den Brand R.J., and Krassioukov A.V. (2008). Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J. Neurotrauma 25, 1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biering-Sorensen F., Hansen B., and Lee B.S. (2009). Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord 47, 508–518 [DOI] [PubMed] [Google Scholar]
- 8. Fong A.J., Roy R.R., Ichiyama R.M., Lavrov I., Courtine G., Gerasimenko Y., Tai Y.C., Burdick J., and Edgerton V.R. (2009). Recovery of control of posture and locomotion after a spinal cord injury: solutions staring us in the face. Prog. Brain Res. 175, 393–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deliagina T.G., Zelenin P.V., and Orlovsky G.N. (2012). Physiological and circuit mechanisms of postural control. Curr. Opin. Neurobiol. 22, 646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Courtine G., Gerasimenko Y., van den Brand R., Yew A., Musienko P., Zhong H., Song B., Ao Y., Ichiyama R.M., Lavrov I., Roy R.R., Sofroniew M.V., and Edgerton V.R. (2009). Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Earhart G.M. (2013). Dynamic control of posture across locomotor tasks. Mov. Disord. 28, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grasmucke D., Zieriacks A., Jansen O., Fisahn C., Sczesny-Kaiser M., Wessling M., Meindl R.C., Schildhauer T.A., and Aach M. (2017). Against the odds: what to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Neurosurg. Focus 42, E15. [DOI] [PubMed] [Google Scholar]
- 13. Gad P., Gerasimenko Y., Zdunowski S., Turner A., Sayenko D., Lu D.C., and Edgerton V.R. (2017). Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front. Neurosci. 11, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Illis L.S. (2012). Central nervous system regeneration does not occur. Spinal Cord 50, 259–263 [DOI] [PubMed] [Google Scholar]
- 15. Brown J.M., Deriso D.M., and Tansey K.E. (2012). From contemporary rehabilitation to restorative neurology. Clin. Neurol. Neurosurg. 114, 471–474 [DOI] [PubMed] [Google Scholar]
- 16. Minassian K., and Hofstoetter U.S. (2016). Spinal cord stimulation and augmentative control strategies for leg movement after spinal paralysis in humans. CNS Neurosci. Ther. 22, 262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edgerton V.R., and Harkema S. (2011). Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev. Neurother. 11, 1351–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angeli C.A., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grahn P.J., Lavrov I.A., Sayenko D.G., Van Straaten M.G., Gill M.L., Strommen J.A., Calvert J.S., Drubach D.I., Beck L.A., Linde M.B., Thoreson A.R., Lopez C., Mendez A.A., Gad P.N., Gerasimenko Y.P., Edgerton V.R., Zhao K.D., and Lee K.H. (2017). Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 92, 544–554 [DOI] [PubMed] [Google Scholar]
- 21. Gill M.L., Grahn P.J., Calvert J.S., Linde M.B., Lavrov I.A., Strommen J.A., Beck L.A., Sayenko D.G., Van Straaten M.G., Drubach D.I., Veith D.D., Thoreson A.R., Lopez C., Gerasimenko Y.P., Edgerton V.R., Lee K.H., and Zhao K.D. (2018). Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nature Med. doi: 10.1038/s41591-018-0248-7. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 22. Hofstoetter U.S., Hofer C., Kern H., Danner S.M., Mayr W., Dimitrijevic M.R., and Minassian K. (2013). Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. (Berl.) doi: 10.1515/bmt-2013-4014. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 23. Hofstoetter U.S., McKay W.B., Tansey K.E., Mayr W., Kern H., and Minassian K. (2014). Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 37, 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hofstoetter U.S., Krenn M., Danner S.M., Hofer C., Kern H., McKay W.B., Mayr W., and Minassian K. (2015). Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif. Organs. 39, E176–E186 [DOI] [PubMed] [Google Scholar]
- 25. Minassian K., Hofstoetter U.S., Danner S.M., Mayr W., Bruce J.A., McKay W.B., and Tansey K.E. (2016). Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil. Neural Repair 30, 233–243 [DOI] [PubMed] [Google Scholar]
- 26. Gerasimenko Y., Gorodnichev R., Moshonkina T., Sayenko D., Gad P., and Reggie Edgerton V. (2015). Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 58, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerasimenko Y., Gorodnichev R., Puhov A., Moshonkina T., Savochin A., Selionov V., Roy R.R., Lu D.C., and Edgerton V.R. (2015). Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 113, 834–842 [DOI] [PubMed] [Google Scholar]
- 28. Gerasimenko Y., Gad P., Sayenko D., McKinney Z., Gorodnichev R., Puhov A., Moshonkina T., Savochin A., Selionov V., Shigueva T., Tomilovskaya E., Kozlovskaya I., and Edgerton V.R. (2016). Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion. J. Neurophysiol. 116, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rath M., Vette A.H., Ramasubramaniam S., Li K., Burdick J., Edgerton V.R., Gerasimenko Y.P., and Sayenko D.G. (2018). Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J, Neurotrauma 35, 2540–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dy C.J., Gerasimenko Y.P., Edgerton V.R., Dyhre-Poulsen P., Courtine G., and Harkema S.J. (2010). Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J. Neurophysiol. 103, 2808–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorodnichev R.M., Pivovarova E.A., Pukhov A., Moiseev S.A., Savokhin A.A., Moshonkina T.R., Shcherbakova N.A., Kilimnik V.A., Selionov V.A., Kozlovskaia I.B., Edgerton V.R., and Gerasimenko Iu P. (2012). [Transcutaneous electrical stimulation of the spinal cord: non-invasive tool for activation of locomotor circuitry in human]. Fiziol Cheloveka 38, 46–56 [PubMed] [Google Scholar]
- 32. Horak F.B. (2006). Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35(Suppl. 2), ii7–ii11 [DOI] [PubMed] [Google Scholar]
- 33. Sayenko D.G., Alekhina M.I., Masani K., Vette A., Obata H., Popovic M., and Nakazawa K. (2010). Positive effect of balance training with visual feedback on standing balance abilities in people with incomplete spinal cord injury. Spinal Cord 48, 886–893 [DOI] [PubMed] [Google Scholar]
- 34. Sayenko D.G., Atkinson D.A., Floyd T.C., Gorodnichev R.M., Moshonkina T.R., Harkema S.J., Edgerton V.R., and Gerasimenko Y.P. (2015). Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 609, 229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Claydon V.E., Steeves J.D., and Krassioukov A. (2006). Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44, 341–351 [DOI] [PubMed] [Google Scholar]
- 36. Laughlin M.H., and Schrage W.G. (1999). Effects of muscle contraction on skeletal muscle blood flow: when is there a muscle pump? Med. Sci. Sports Exerc. 31, 1027–1035 [DOI] [PubMed] [Google Scholar]
- 37. Krassioukov A., Warburton D.E., Teasell R., Eng J.J.; Spinal Cord Injury Rehabilitation Evidence Research Team. (2009). A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rejc E., Angeli C., and Harkema S. (2015). Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One 10, e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hunter J.P., and Ashby P. (1994). Segmental effects of epidural spinal cord stimulation in humans. J. Physiol. 474, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maertens de Noordhout A., Rothwell J.C., Thompson P.D., Day B.L., and Marsden C.D. (1988). Percutaneous electrical stimulation of lumbosacral roots in man. J. Neurol. Neurosurg. Psychiatry 51, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Minassian K., Jilge B., Rattay F., Pinter M.M., Binder H., Gerstenbrand F., and Dimitrijevic M.R. (2004). Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42, 401–416 [DOI] [PubMed] [Google Scholar]
- 42. Murg M., Binder H., and Dimitrijevic M.R. (2000). Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches - a functional method to define the site of stimulation. Spinal Cord 38, 394–402 [DOI] [PubMed] [Google Scholar]
- 43. Rattay F., Minassian K., and Dimitrijevic M.R. (2000). Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 38, 473–489 [DOI] [PubMed] [Google Scholar]
- 44. Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., and Rattay F. (2010). Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 637–645 [DOI] [PubMed] [Google Scholar]
- 45. Danner S.M., Hofstoetter U.S., Ladenbauer J., Rattay F., and Minassian K. (2011). Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif. Organs 35, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jilge B., Minassian K., Rattay F., and Dimitrijevic M.R. (2004). Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol. Cybern. 91, 359–376 [DOI] [PubMed] [Google Scholar]
- 47. Minassian K., Persy I., Rattay F., Pinter M.M., Kern H., and Dimitrijevic M.R. (2007). Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 26, 275–295 [DOI] [PubMed] [Google Scholar]
- 48. Gerasimenko Y.P., Lu D.C., Modaber M., Zdunowski S., Gad P., Sayenko D.G., Morikawa E., Haakana P., Ferguson A.R., Roy R.R., and Edgerton V.R. (2015). Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Danner S.M., Hofstoetter U.S., and Minassian K. (2013). Finite element models of transcutaneous spinal cord stimulation. In: Jaeger D., Jung R.(eds.), Encyclopedia of Computational Neuroscience, New York, NY: Springer New York, pp. 1–6 [Google Scholar]
- 50. Minassian K., McKay W.B., Binder H., and Hofstoetter U.S. (2016). Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics 13, 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Minassian K., Hofstoetter U.S., Danner S.M., Mayr W., McKay W.B., Tansey K., and Dimitrijevic M.R. (2013). Mechanisms of rhythm generation of the human lumbar spinal cord in response to tonic stimulation without and with step-related sensory feedback. Biomed. Tech. (Berl.) doi: 10.1515/bmt-2013-4013 [DOI] [PubMed] [Google Scholar]
- 52. Jilge B., Minassian K., Rattay F., Pinter M.M., Gerstenbrand F., Binder H., and Dimitrijevic M.R. (2004). Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp. Brain Res. 154, 308–326 [DOI] [PubMed] [Google Scholar]
- 53. Hofstoetter U.S., Danner S.M., Freundl B., Binder H., Mayr W., Rattay F., and Minassian K. (2015). Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J. Neurophysiol. 114, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu L.J., Zelenin P.V., Orlovsky G.N., and Deliagina T.G. (2012). Effects of galvanic vestibular stimulation on postural limb reflexes and neurons of spinal postural network. J. Neurophysiol. 108, 300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Musienko P.E., Zelenin P.V., Orlovsky G.N., and Deliagina T.G. (2010). Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J. Neurophysiol. 103, 1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deliagina T.G., Beloozerova I.N., Orlovsky G.N., and Zelenin P.V. (2014). Contribution of supraspinal systems to generation of automatic postural responses. Front. Integrat. Neurosci. 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lavrov I., Gerasimenko Y., Burdick J., Zhong H., Roy R.R., and Edgerton V.R. (2015). Integrating multiple sensory systems to modulate neural networks controlling posture. J. Neurophysiol. 114, 3306–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ranger M.R., Irwin G.J., Bunbury K.M., and Peutrell J.M. (2008). Changing body position alters the location of the spinal cord within the vertebral canal: a magnetic resonance imaging study. Br. J. Anaesth. 101, 804–809 [DOI] [PubMed] [Google Scholar]
- 59. Danner S.M., Krenn M., Hofstoetter U.S., Toth A., Mayr W., and Minassian K. (2016). Body position influences which neural structures are recruited by lumbar transcutaneous spinal cord stimulation. PLoS One 11, e0147479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herman R., He J., D'Luzansky S., Willis W., and Dilli S. (2002). Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40, 65–68 [DOI] [PubMed] [Google Scholar]
- 61. Sayenko D.G., Angeli C., Harkema S.J., Edgerton V.R., and Gerasimenko Y.P. (2014). Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 111, 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Field-Fote E.C. (2015). Exciting recovery: augmenting practice with stimulation to optimize outcomes after spinal cord injury. Prog. Brain Res. 218, 103–126 [DOI] [PubMed] [Google Scholar]
- 63. Kwakkel G. (2006). Impact of intensity of practice after stroke: issues for consideration. Disabil. Rehabil. 28, 823–830 [DOI] [PubMed] [Google Scholar]
- 64. Colombo G., Wirz M., and Dietz V. (1998). Effect of locomotor training related to clinical and electrophysiological examinations in spinal cord injured humans. Ann. N. Y. Acad. Sci. 860, 536–538 [DOI] [PubMed] [Google Scholar]
- 65. Dietz V., Colombo G., Jensen L., and Baumgartner L. (1995). Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 37, 574–582 [DOI] [PubMed] [Google Scholar]
- 66. Harkema S.J. (2008). Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 57, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lyalka V.F., Hsu L.J., Karayannidou A., Zelenin P.V., Orlovsky G.N., and Deliagina T.G. (2011). Facilitation of postural limb reflexes in spinal rabbits by serotonergic agonist administration, epidural electrical stimulation, and postural training. J. Neurophysiol. 106, 1341–1354 [DOI] [PubMed] [Google Scholar]
- 68. Sherwood A.M., Dimitrijevic M.R., and McKay W.B. (1992). Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J. Neurol. Sci. 110, 90–98 [DOI] [PubMed] [Google Scholar]
- 69. Raineteau O., and Schwab M.E. (2001). Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 [DOI] [PubMed] [Google Scholar]
- 70. Knikou M. (2014). Transpinal and transcortical stimulation alter corticospinal excitability and increase spinal output. PLoS One 9, e102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Knikou M., Dixon L., Santora D., and Ibrahim M.M. (2015). Transspinal constant-current long-lasting stimulation: a new method to induce cortical and corticospinal plasticity. J. Neurophysiol. 114, 1486–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roy F.D., Bosgra D., and Stein R.B. (2014). Interaction of transcutaneous spinal stimulation and transcranial magnetic stimulation in human leg muscles. Exp. Brain Res. 232, 1717–1728 [DOI] [PubMed] [Google Scholar]
- 73. Topka H., Cohen L.G., Cole R.A., and Hallett M. (1991). Reorganization of corticospinal pathways following spinal cord injury. Neurology 41, 1276–1283 [DOI] [PubMed] [Google Scholar]
- 74. Tai C., Shen B., Wang J., Chancellor M.B., Roppolo J.R., and de Groat W.C. (2008). Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am. J. Physiol. Renal Physiol. 294, F591–F602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shah P.K., Sureddi S., Alam M., Zhong H., Roy R.R., Edgerton V.R., and Gerasimenko Y. (2016). Unique spatiotemporal neuromodulation of the lumbosacral circuitry shapes locomotor success after spinal cord injury. J. Neurotrauma 33, 1709–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dimitrijevic M.M., Dimitrijevic M.R., Illis L.S., Nakajima K., Sharkey P.C., and Sherwood A.M. (1986). Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent. Nerv. Syst. Trauma 3, 129–144 [DOI] [PubMed] [Google Scholar]
- 77. Barolat G., Myklebust J.B., and Wenninger W. (1986). Enhancement of voluntary motor function following spinal cord stimulation–case study. Appl. Neurophysiol. 49, 307–314 [DOI] [PubMed] [Google Scholar]
- 78. De Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1998). Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 80, 83–91 [DOI] [PubMed] [Google Scholar]
- 79. de Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1998). Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 79, 1329–1340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.