Abstract
Even with the benefit of assisted reproductive technologies (ART), many women are unable to conceive and deliver healthy offspring. One common cause of infertility is the inability to produce eggs capable of contributing to live birth. This can occur despite standard-of-care treatment to maximize the recovery of eggs from growing ovarian follicles. Dormant primordial follicles in the human ovary are a ‘reserve’ that can be exploited clinically to overcome this problem. We discuss how controlling primordial follicle growth activation (PFGA) can produce increased numbers of high-quality eggs available for fertility treatment(s). We consider the state-of-the-art in interventions used to control PFGA, and consider genetic and epigenetic strategies on the horizon that might improve compromised oocyte quality to increase live births.
Keywords: cryopreservation, follicle, infertility, *in vitro* fertilization (IVF), oncofertility, ovary
Assisted Reproduction and the Supply of High Quality Eggs
Extraordinary advances have been made in the study and treatment of infertility in the last 40 years [1–4]. The first in vitro fertilization (IVF) birth in 1978 revolutionized the field of fertility treatment and since that time, overall IVF success rates have been improving steadily year after year [5,6]. Initially, IVF treatments resulted in a low successful pregnancy rate of 9%, and a delivery rate of 7% per egg retrieval procedure (see Glossary) [7]. Recent data from the United States of America report an approximate 38% live birth rate per retrieval; remarkably, this exceeds the fecundability of natural conception cycles in the general population on a per cycle basis [8] Access to Assisted Reproductive Technology (ART) services has also steadily improved, with treatments now available within most academic centers and an even greater number of private centers worldwide.
The abovementioned clinical progress has been accompanied by steady progress in our understanding of ovarian physiology and the endocrinology of female fertility. For example, optimization of the amount of injectable gonadotropin medications used during the so-called “controlled ovarian stimulation” prior to a given egg retrieval has been studied exhaustively [9–11]. The number of eggs retrieved is a key parameter within IVF cycles, both as a practical necessity for treatment and a measure of “ovarian response.” Historical studies and more recently, regulatory requirements for clinical reporting to large databases, e.g. the Society for Assisted Reproductive Technology Clinical Outcome Reporting System (www.sart.org) [12] in the US have resulted in a more precise accounting of IVF statistics. These data suggest that stimulation is approaching its limits in terms of maximizing the chances of conception and pregnancy within an IVF cycle [9–11]. An additional practical limit for gonadotrophic stimulation for some women is the risk of the adverse reaction termed ovarian hyper-stimulation. Patients that respond to stimulation in this fashion can exhibit major fluid/electrolyte imbalances, blood clots or other severe outcomes that might place the patient and her chance of pregnancy at risk [13].
It is commonly accepted that the greater the number of eggs retrieved, the greater the chances of generating one or more embryos that meet the criteria for transfer back into a patient’s uterus. However, the critical factor is that at least one egg needs to be of sufficient quality to give rise to a successful pregnancy. Thus, increasing the supply of high quality eggs for use in assisted reproduction attempts is a desirable goal for all patients, and particularly, for those faced with contraindications that limit this supply. After discussing the profiles of particularly difficult-to-treat IVF patients, we discuss how very recent advances might eventually be applied to enable overcoming infertility based on increasing the number of high quality eggs.
Two large patient sub-populations known to have reduced chances of pregnancy and live birth are those referred to as “advanced maternal age” and “diminished ovarian reserve (DOR)” [14,15], each of whom usually produce very few eggs during treatment. These patient sub-populations are discussed as follows. It is well-known that fertility decreases in women with age. The likelihood of conception per unit time is relatively stable between the onset of puberty and a woman’s early thirties. “Advanced maternal age” is typically assumed to start around age 35, and soon after, oocyte quantity and quality can be seen to decline precipitously [16]. At about age 37, several parameters of egg developmental competence have been shown to change significantly [17,18], including markers of cytoplasmic and chromosomal competence that begin to favor aberrant development and chromosomal segregation after fertilization [19,20]. This can manifest in eggs that are developmentally incompetent even if successfully fertilized (for individual or an entire retrieved cohort of eggs). Because many women might seek to delay pregnancy and childbirth to fit lifestyle choices and needs, often until their late thirties [21], the population faced with overcoming poor egg quality will likely increase.
Women with DOR are also characterized by low indirect markers of the “reserve” of ovarian primordial follicles [14]. These markers include serum levels of Antimüllerian hormone (AMH; commonly accepted threshold level < 1ng/ml) or, elevated serum levels of Follicle Stimulating Hormone (FSH; thresholds differ but > 10 IU/ml is commonly accepted) [22], measured on day 2 or 3 of the menstrual cycle. DOR diagnosis is not by definition, age- specific and can be seen in young women attempting to conceive; commonly interpreted as evidence of accelerated ovarian aging characterized by an accelerated loss of primordial follicles. In addition, DOR patients often respond poorly to gonadotropin ovarian stimulation during IVF cycles [14,15]. Critically, exposure to certain chemicals, including chemotherapeutic agents, or ovarian surgery, can result in significant depletion of the ovarian reserve, leading, again, to accelerated ovarian aging [13,24],. General terms for capturing optimal ovarian health at a given time include “fertility preservation” and “oncofertility” [23]. All of the above features of DOR patients correspond to a reduced chance of conception and live birth [25,26], both spontaneously and with assisted conception. Women of advanced age, or, those diagnosed with DOR that have failed assisted reproduction treatments, are often counseled to consider egg donation programs, precluding their ability to give birth to their own genetic offspring.
A central question for the field is therefore determining a means to increase the number of available high-quality eggs for all women undergoing IVF, but especially those with advanced chronological age, DOR, or for women with low yield of oocytes during a given cycle of treatment. An increasingly plausible strategy may be to access dormant primordial follicles within the ovarian cortex (Figure 1). Specifically, during normal ovarian function within the menstrual cycle, a small number of primordial follicles begin to grow daily, supplying the ovary with its population of growing follicles [27,28]. The vast majority of follicles will die within the ovary during normal reproductive cycling in a process referred to as atresia [29,30], usually leaving only a single dominant follicle [31] that survives to ovulate a mature, fertilization-competent egg. The above-mentioned hormonal stimulation strategies are largely relevant to this growing proportion of follicles. The remaining dormant population of ovarian follicles [32]-- including the primordial pool of follicles -- is currently being probed for their potential clinical utility. Of relevance, primordial follicles of many species, including humans and mice, remain growth-arrested, even if isolated and placed into “permissive” tissue culture conditions [33]. Thus, overcoming the signaling cues that enforce growth-arrest of primordial follicles may be a critical step to clinically exploit this pool of ovarian follicles.
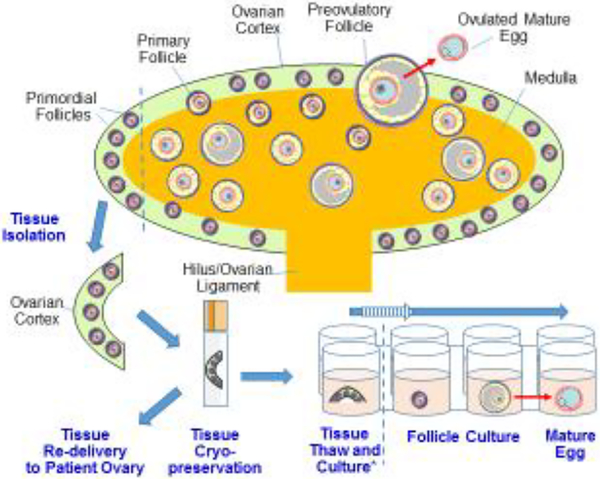
Figure 1. Human Ovarian anatomy and tissue handling for fertility support and preservation.
A cartoon representation of the human ovary, its organization, and its constituent follicles is shown. The exterior cortex (green) is the location of most if not all primordial follicles, while growing follicles are found more centrally within the medulla (orange). Once per cycle, a follicle survives to ovulate (top, red arrow). Clinical access to the primordial reserve of follicles can be gained by surgical removal of “strips” of ovarian cortex (left, TISSUE ISOLATION). Cortical strips can be cryopreserved, and optionally thawed and re-grafted to a patient’s ovary, whereupon follicle growth can resume and conception might follow [33–36]. Alternatively, tissue can be thawed and placed into a tissue culture scheme (bottom right). In a ‘one-step’ system, follicles are immediately isolated and cultured singly. In a ‘two-step’ system (*), cortex strips are first cultured until a growing follicle is visible, after which the growing follicle is mechanically isolated for further culture. Ideally, optimization of follicle culture will result in the production of a mature, offspring competent egg (bottom right, red arrow). Note that for each stage of tissue handling and culture in vitro, follicle-enclosed eggs are accessible for treatment, including the possible injection of reagents designed to improve egg quality.
When considering possible fertility treatments, the isolation of primordial follicles from the ovarian cortex (in addition to non-growing, transitional and primary follicles [32]) might offer several advantages in terms of numbers and accessibility. For instance, the number of primordial follicles exceeds the number of growing follicles until very late in reproductive life [32]. The human ovary is organized into distinct cortex and medullary layers (Figure 1) [34]. The cortex is a thin layer of fibrous, dense tissue analogous to the peel of an orange, and this is where most primordial follicles are found [34]. The medullary region, akin to the fruit within the orange peel, mostly consists of stromal tissue surrounding blood vessels, lymphatic ducts, and connective tissue, contiguous with the ovarian ligament/hilus. Because the primordial follicles are superficial within the organ, it is relatively trivial for an experienced practitioner to collect cortical tissue via a minimally invasive, laparoscopic approach, and a simple scalpel “shaving” of the surface. Also, human ovarian cortex is highly compatible with cryopreservation and particularly, vitrification, so biopsied tissue can be stored for long periods of time prior to thawing and use [35–37]. Depending on the overall primordial follicle content in an ovary, anywhere from a few to tens of primordial follicles can be present in a single cortical piece approximately 10 mm x 10 mm x 1 mm deep [33,38]. Such collection and preservation of cortical tissue is now being routinely performed for cancer patients, envisioning that mature eggs might be one day produced for fertilization purposes [23,24]. We expand upon existing workflows and additional experimental strategies below.
Clinical Strategies and Workflows
In vivo: Cortex removal, preservation, and replacement
One clinical strategy that has been used successfully is the collection, cryopreservation, thawing, and surgical re-delivery of ovarian cortex tissue to the surface of a patient’s ovary [39]. Patients newly diagnosed with cancer may not be able to take advantage of an IVF cycle in order to cryopreserve oocytes or embryos for fertility preservation prior to undertaking gonadotoxic therapies, due to either the time-sensitive need for treatment, or the clinical need to avoid gonadotrophic stimulation. However, in these situations, removal and cryopreservation of ovarian cortex pieces might be undertaken. Re-delivery of pieces of ovarian cortex to the surface of the ovary after patient recovery (process described as orthotopic transplantation; [40]) has resulted in the resumption of menstrual cycling for predictable lengths of time, and has led to a growing number of live births, both due to natural conception and IVF [41]. The length of time that menstrual cycling returns is generally thought to be directly related to the number of primordial follicles delivered in the graft(s). This first strategy can be executed while also using pharmacological agents shown to induce the growth activation of primordial follicles (see below). A second, less mature strategy for the use of patient ovarian cortex avoids the need for surgical re-delivery of tissue and instead, relies on in vitro tissue and follicle culture.
In vitro: Cortex removal followed by tissue and/or follicle culture
Individual primordial and other small growing follicles can be isolated from fresh or frozen- and-thawed pieces of human ovarian cortex. A recent study reported what appears to be the highly efficient recovery of small follicles from cortical pieces 60 – 300 mm3 in volume. Treatment with liberase and DNase I enzymes in combination with mechanical filtration of the disrupted cortex was undertaken [42]. Compared to an earlier method reported by the same group [43], the newer improved method [42] increased both the number of isolated intact follicles (e.g., follicles with central, non-extruding oocytes), and the viability of isolated follicles (96%). Following isolation, some follicles were embedded within fibrin clots -- a biological matrix the authors had previously shown to be compatible with follicle survival and growth [44] In this case, clots were grafted under the ovarian bursa of immunocompromised mice and examples of surviving human follicles were noted 7 days after transplantation. Primordial follicles were shown to maintain growth arrest after survival in the grafts, showing that the technique can maintain the follicles’ physiological state of either growth or arrest, even 7 days after isolation, handling, and grafting into the host animals [42]. This means that primordial follicles can withstand impressive isolation and re-location while maintaining their growth arrest and potential to support ovarian function over extended time periods (see below).
Several other biomaterials have also been shown to be compatible with follicle survival in vitro. Specifically, three-dimensional collagen [45–47], hyaluronan [48], or alginate [49,50] matrices have each been used to house follicles as they grow in vitro; these matrices can improve morphological features of follicle growth relative to those grown directly in culture dishes. For instance, alginate has been used to grow rhesus monkey follicles successfully [51,52]. Beyond providing scaffolding for follicles to grow within, these biomaterials can be modified in terms of their concentration (and thus permeability), as well as mechanical rigidity to improve follicle survival and proper development [51]. Further, different extracellular matrix or other macromolecular components might be mixed within these materials in ways that might enhance follicle development and improve the chances of producing mature eggs [53], an area warranting further investigation. Overall, however, the field has advanced to a point where attempts are being made at generating “artificial ovary” tissues [42,52] where many isolated human follicles can be aggregated within a biomaterial scaffold. With the intent of increasing the number of follicles available to support ovarian function, and possibly, fertility, such aggregates might be re-introduced in vivo to the surface of a dormant ovary lacking significant immature follicles (unlike cortex removal, preservation, and replacement; see above).
A second technique for the in vitro culture of follicles is referred to as a ‘two-step’ approach. [33,38]. First, intact pieces of ovarian cortex are cultured until a single growing follicle of sufficient size is noted. Subsequently, the growing follicle can be isolated from the surrounding cortical tissue for further culture, possibly within one of the biomaterial matrices mentioned above. One study showed that such a two-step procedure could result in the generation of large antral follicles [33,38]. Specifically, this technique has the benefit of allowing follicles to initially develop in their native ovarian tissue, in some ways mimicking in vivo conditions. However, a large number of immature follicles are lost to atresia during the first stage of cortical strip culture [33]. It remains to be seen whether the relative advantages and disadvantages of culturing follicles singly or using the two-step approach ultimately impact the quality of mature eggs produced. Regardless of the culture method used, inducing the growth and development of primordial follicles seems likely to open an ‘untapped reserve’ of high quality mature eggs for patients, and this is an area that merits further investigation.
Mechanistic Control of Primordial Follicle Growth Activation
Primordial follicle growth activation (PFGA) is the process by which individual primordial follicles leave their dormant phase and enter a growth phase that can culminate in the development of a mature peri-ovulatory follicle containing a nearly mature egg [28]. As mentioned, clear mechanistic information is available on how primordial follicles stay in this growth-arrested state for as long as 35 years within the ovary, and even remaining growth-arrested when placed into in vitro cultures. A “summary” model of the signal transduction and gene expression pathways regulating PFGA, in conjunction with a table of additional factors shown to impact PFGA, are illustrated in Figure 2.
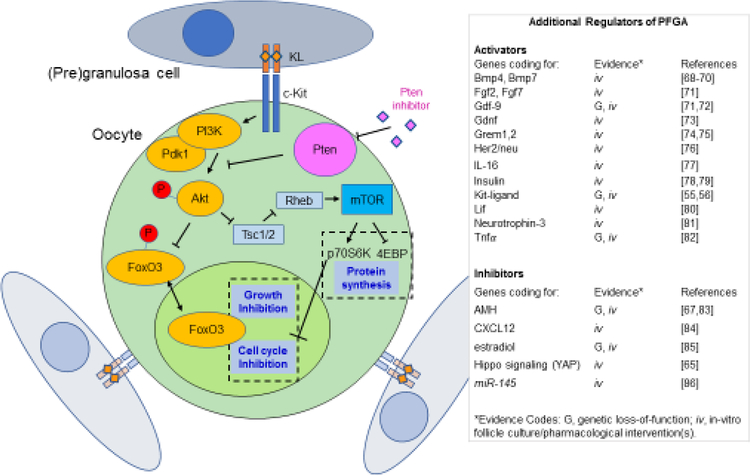
Figure 2. Molecular regulation of PFGA in Mice.
The cartoon on the left depicts primordial follicles consisting of a primordial oocyte and a few pregranulosa cells stay dormant due to the negative regulation of cell growth and the cell cycle by transcription factor FoxO3, and negative regulation of the mTOR signaling pathway, both controlled by the upstream action of Pten [69,93,105]. Accordingly, pharmacological inhibition of Pten using molecules such as bpV(HOpic) can result in enhanced PFGA. FoxO3 has been shown to inhibit the cell cycle via regulation of Cyclin dependent kinase inhibitor 1b (Cdkn1b/p27). Oocytes with low mechanistic Target of Rapamycin (mTOR) activity are likely to be held in a state of low protein synthesis due to a lack of phosphorylation of the mTOR target Eukaryotic translation initiation factor 4E-binding protein (4-EBP), remaining out of an active cell cycle due to activity of P70S6-kinase (P70S6K), itself a positive regulator of cyclin D1, cyclin dependent kinase 4 (CDK4), and Retinoblastoma (Rb) proteins [106]. The table on the right presents an updated list of genes and factors shown to impact PFGA, with experimental evidence categorized by in vitro and/or in vivo genetic evidence. Cartoon adapted from [105].
c-Kit/Kit Ligand (KL) signaling was one of the first pathways identified as a PFGA regulatory mechanism. C-Kit is a receptor tyrosine kinase, activated by its ligand KL [54]. Disruption of this pathway using the Steel panda mutant mouse that expresses low levels of Kit ligand [55], or, via use of a c-Kit blocking antibody [56], each were found to result in enhanced arrest of primordial follicles and a reduction in the number of small growing follicles [55,56]. A second key regulatory molecule was discovered some time later, where forkhead transcription factor FoxO3 knockout mice were shown to exhibit the opposite effect: a total commitment of primordial follicles to growth (depletion of the entire primordial follicle population) by 2–3 months of age was detected in histomorphometric studies, while in contrast, wild-type controls maintained ovarian function for the normal duration [57]. This suggested that FoxO3 was responsible --in large part -- for keeping primordial follicles growth-arrested [57].
Other central regulators of PFGA include the lipid phosphatase Phosphatase and tensin homolog deleted on chromosome ten (Pten), and its downstream proximal substrate, protein kinase Akt [28,60]. Pten and Akt are well-known for their role(s) in controlling cell growth and survival during normal cell and tissue function and as dysregulated in cancers [58]. Their action relies upon downstream regulation of mechanistic Target of Rapamycin (mTOR) activity (Figure 2, blue boxes), and also upon the regulation of nucleo-cytoplasmic FoxO3 transport. High Pten activity downregulates Akt due to action upon Pi3 kinase (Pi3k), resulting in low downstream mTOR activity. This culminates in blocked cell cycle progression (via p70s6 Kinase [p70s6k]) and limits upon protein translation (via Eukaryotic translation initiation factor 4E-binding protein [4E-BP]). High Pten activity also corresponds to hypo-phosphorylation and nuclear localization of FoxO3, where it can activate the transcription of genes consistent with cell cycle arrest, including Cyclin dependent kinase inhibitor 1b (Cdkn1b/p27Kip1) [59]. Conversely, low Pten activity results in upregulated mTOR and hyper-phosphorylation and nuclear export of FoxO3, relieving post-translational (mTOR) and transcriptional (FoxO3) enforcement of growth inhibition and allowing PFGA. [59] Accordingly, blocking PTEN activity and/or activating AKT have been shown to increase growth activation of primordial follicles both experimentally and in the clinic (see below).
Pharmacological inhibition of the Pten enzyme using the Pten-specific inhibitor (and indirect Akt activator) bisperoxovanadium bpV(HOpic) (bpV(pic)) during in vitro mouse primordial follicle and ovary cultures [60], and separately, oocyte-specific genetic loss-of-function Pten deficient mice[61–63], each have been shown to result in significantly increased PFGA, producing more growing follicles than (and reduced numbers of primordial follicles compared to) corresponding controls. Translating these findings to human primordial follicles, including an attempt at the treatment of infertility followed soon thereafter.
As seen in the mouse models, it was possible to induce growth activation of primordial follicles in human frozen-and-thawed ovarian cortical tissue [64,65]. In this experimental treatment paradigm, PTEN inhibition/AKT activation using bpV(HOpic) was combined with an ovarian cortex fragmentation approach to offset the growth-inhibitory Hippo signaling pathway [65]. A cohort of 27 amenorrheic patients was selected that had a mean duration of amenorrhea of nearly 7 years. Isolated cryopreserved-and-thawed ovarian cortex from these patients was cut into small cubes and treated with AKT activators and 740YP in vitro. Cubes of tissue were then surgically auto-transplanted back to the Fallopian tube serosa of patients, and the graft sites were monitored for follicle growth via ultrasound. Eggs were successfully retrieved from five patients, and following fertilization by intracytoplasmic sperm injection (ICSI), one live birth was achieved [65].
This remarkable finding suggests that indeed, inducing PFGA in primordial follicles that would otherwise remain dormant in women with POI may be a plausible strategy to increase the chances of a successful pregnancy and live birth. Despite this outcome, a cautionary set of data was published soon afterwards that showed that inhibition of PTEN by bpV(HOpic) can result in poorer survival of immature follicles in human ovarian cortex cultured in vitro [66]. The live birth outcome for even one of these previously amenorrheic patients, however, seems to justify a larger clinical trial of this strategy on pregnancy outcomes.
It is also worthwhile to consider additional secreted and other extracellular factors that have been shown to impact PFGA. These might be added to culture systems or measured in patients to determine how their concentration(s) might impact the accessibility of primordial follicles. A recent hypothesis article that evaluated and interpreted the action of AMH [67], a list of activators [55,56,68–82] and repressors [67, 83–86] of PFGA was provided. Figure 2 extends this informative list, organizing effectors based on available experimental evidence for their action on PFGA— either genetic (usually loss-of-function) models, in vitro culture experiments, or both.
The Physiological Clock that Controls PFGA
As described, much is now known of key signals that enforce the physiological growth-arrest of primordial follicles. Loss-of-function of negative regulators such as Pten [60–62], FoxO3A [57], and the mTOR protein effector Tuberous sclerosis 2 Tsc2 [87] in mouse models results in vastly accelerated PFGA and exhaustion of the primordial pool of follicles early in life. However, identifying these negative regulators has only provided clues about how the steady rate of PFGA is achieved during the normal physiological lifespan. To us, this is the major remaining question in the field. How can seemingly equivalent primordial follicles behave so differently from one another in humans, where growth-activation in some follicles can occur as far as 35 years apart (see Outstanding Questions)? In a thought experiment, might isolated human primordial follicles placed into tissue culture last years without growth-activation, finally undergoing PFGA according to an internal “clock?”
Outstanding Questions.
What regulates the “clock” that controls regular PFGA during the human/mammalian reproductive lifespan? Despite an improved understanding of the mechanisms that can either activate or repress PFGA, little is known about the regulation of PFGA rate time during reproductive maturity. Experimental models that extend the ovarian lifespan are beginning to provide clues in this area.
Is poor egg quality established as early as the primordial follicle, or can it be corrected? Because some women produce only poor-quality eggs that may be incompatible with offspring production, it is currently unclear whether any intervention can improve their situation. With the assumption that it may be possible to gain control of the continuum of follicle development, enabling the growth and maturation of primordial follicles, we may potentially overcome the problem of poor egg quality, provided that oocytes are not already compromised beyond “correction.”
As discussed, external physiological factors can impact the likelihood of growth activation of primordial factors. One such molecule is AMH, the same molecule used as a serum measurement to indirectly estimate the ovarian reserve [67,83]. Amh knockout mice [83] show an acceleration of PFGA, characterized by a decline in primordial follicle number and an increase in growing follicles at different postnatal time points. While these data point towards a definite impact of Amh upon mouse PFGA, AMH’s mechanism of action upon primordial follicles is not entirely clear at this time, as while the AMH receptor AMHR2 can be detected in human primordial follicles [88], the orthologous receptor has been shown to be lacking in rat primordial follicles [89]. This suggests that AMH might partially suppress PFGA indirectly in some species but not others.
Four additional models where the duration of ovarian function is significantly extended have been established in the mouse. Dietary supplementation with omega-3 fatty acids [90] or coenzyme Q10 [91], or alternatively, systemic (intraperitoneal) delivery of the mTOR inhibitor Rapamycin [92,93] have all been shown to extend ovarian lifespan in mice. In a transgenic mouse model (Fshb−/− HSFHBMut) where FSH is genetically “rerouted” in such a way that its expression is pulsatile due to fusion with a carboxyterminal Luteinizing Hormone motif, ovarian lifespan also appears to be significantly extended relative to controls [94]. In all four distinct models, the extension of ovarian function appears to result from a slower rate of PFGA and not a reduction in follicle atresia. Whether or not these models involve regulation of a molecular “clock” that can control the rate of PFGA over time, these are valuable lessons when considering how to regulate the process in patients in the future.
Concluding Remarks
Reproducible, near-term strategies for increasing the number of healthy offspring-competent eggs in model systems have been discussed. While some of these strategies have been successfully translated to human interventions, there is much room for improvement. When considering future perspectives for the treatment of human patients, initial attempts should be made to improve the quality of eggs produced during current stimulation-and-retrieval protocols. Because only one (or possibly, a few) high quality eggs are necessary per IVF cycle to produce an offspring, improving egg quality so that those few high-quality eggs are produced instead of none is the first priority.
A new strategy consists of the inclusion of inhibin-blocking antisera in ovarian stimulation entry (during stimulation cycles). In mice, this strategy vastly increases offspring-competent oocyte yield due to the prevention of ovary-derived inhibin and its negative feedback on pituitary FSH production [95,96]. Separately, the same mouse model of pulsatile FSH expression shown to extend ovarian lifespan (above, [94]) also exhibited significantly improved oocyte yield after gonadotropin stimulation. It is not yet known whether either blocking inhibin or delivering FSH in a pulsatile fashion can similarly result in increased oocyte yield in human patients with a normal ovarian reserve, let alone a limited reserve of follicles. Thus, we return to consider the primordial follicles found in human ovarian cortex and how this reserve might be exploited.
Given increased mastery of ovarian cortex cryopreservation and handling, it should be possible to more precisely control PFGA, follicle survival, and mature egg production from frozen-and-thawed tissue. Technical hurdles remain, the first of which is the attrition of immature follicles after only a few days in culture in two-step culture protocols [33,97]. How such attrition might be overcome is presently unclear. Isolating viable immature follicles from ovarian cortical tissue is still technically challenging, but improving methods [42,97] might be bringing this closer to routine clinical performance. Some combination of cortical tissue culture followed by dissociation, isolation of individual follicles, and subsequent single follicle culture might eventually contribute to increasing the number of available mature eggs. Basic mechanistic information gained from these studies should also shed light on how the rate of PFGA is regulated over the adult reproductive lifespan of humans.
Beyond the technical challenges of tissue and follicle handling, a critical question to address is that of intrinsic egg quality (See “Outstanding Questions”, box 1, and also [30]). Can oocyte quality be compromised even as far back as the primordial follicle? In the short term, it may be that no intervention designed to isolate mature eggs can overcome what is a fixed poor quality of eggs within certain, and potentially all, follicles [29]. Because some women are never able to produce healthy offspring, and other women lose their ability to do so with age, overcoming this problem may require early intervention, perhaps by manipulating the eggs within immature follicles.
Box 1. Clinician’s Corner:
During the normal menstrual cycle, small cohorts of “resting” primordial follicles are recruited into a growth cycle; most are destined for atresia while typically only a single dominant follicle survives to ovulation
Oocyte quality and quantity declines precipitously with age; women of advancing age are at risk of “diminished ovarian reserve” (DOR), with decreasing chances of conception and live birth over time
Certain cancer treatments and other treatments for non-malignant conditions (often medical or radiological) can damage the reserve of primordial follicles within the ovary, accelerating ovarian demise. Fertility preservation/oncofertility options are becoming more widespread for patients faced with these treatments so that attempts at conception can occur after patient recovery. Eggs, embryos, and/or ovarian cortex can be cryopreserved for fertility preservation purposes.
Actionable information about the physiological control of primordial follicle growth activation, or, “recruitment” is available from studies of model systems and human ovarian cortex. Translational studies seeking to support fertility by activating dormant primordial follicles are being performed
The population of dormant follicles may represent an important target for experimental clinical strategies designed to increase the quantity of oocytes available for use in assisted reproduction treatments
The time that follicles are placed within in vitro culture can be exploited for intervention using powerful new techniques that modify the genetic and epigenetic state(s) of eggs. Injection of the egg within the growing mouse follicle has been successfully performed in multiple laboratories [98,99], showing that macromolecules can be delivered in a way that is compatible with egg and follicle survival. It is a short leap to the delivery of CRISPR/Cas9 and guide RNAs [100], or, modified enzymes that target DNA methylation enzymatic activity to the genome at precise sequence-directed location(s) [101–103]. Proof-of-concept delivery of CRISPR/Cas9 reagents into human one-cell embryos (post-fertilization), or, mature eggs (injected with the sperm cell during ICSI) has been demonstrated with detailed information available regarding embryo viability, targeting efficiency, and embryonic mosaicism where only a subset of embryonic cells harbor the genomic alteration or epigenetic mark [100]. One might anticipate delivery of these reagents to eggs inside follicles grown in vitro with the aim of improving the likelihood of survival and production of an offspring-competent egg, perhaps being able to correct specific genetic abnormalities found to compromise egg development (Figure 1).
Follicles cultured in vitro to produce mature eggs starting with the primordial stage could therefore offer a broad window of access and control, improving fertility and also reducing disease in offspring. In this way, the limitation of the number of eggs available for conception should be possible to overcome for all but those patients with the fewest remaining primordial follicles. For even those patients with zero available primordial follicles, options may lie on the more distant horizon. For example, the production of mature, fertilization-competent eggs entirely in vitro using embryonic stem cells or induced pluripotent cells (iPSC) cells, has been achieved in mouse models [104]. A careful stepwise process of iPSC generation from somatic cells, followed by in vitro stem cell differentiation, in vitro growth (of cells specified as oocytes), and in vitro oocyte maturation, resulted in the production of normal metaphase II eggs capable of giving rise to offspring [104]. Generation of patient-matched, high quality mature eggs for human infertility treatment using iPSC is daunting, but should be plausible given time and attention from the field.
While all of the possible future directions mentioned here have varying degrees of difficulty, most are likely to be reached in the next several years given the demand-driven, rapid pace of progress in reproductive and molecular genetic techniques. The rate-limiting step(s) appear to reside at the level of tissue handling and possible, inherent biological limitations of the primordial oocytes themselves, rather than the availability of molecular tools and creative clinical/translational researchers working in this exciting area.
Highlights.
Women that are currently considered infertile due to poor egg quality or are at risk of ovarian failure may have new options. Ovarian cortex can be isolated, cryopreserved and re-delivered to the ovary whereupon ovarian function might resume.
The ovarian cortex tissue is also currently being used in attempts to produce mature fertilizable eggs in vitro.
Mechanisms that control primordial follicle growth activation (PFGA) have been identified and used to induce follicle growth in mouse models (genetic and in vitro follicle cultures) and also in experimental human clinical interventions.
In vitro follicle culture offers control over follicle development (and access to the follicles themselves from early developmental stages) so that modern genetic/epigenetic tools might be used to improve egg quality. For example, genome editing (e.g via CRISPR/Cas9) reagents might be injected into growing follicles to correct defects compromising egg quality. This type of technology also aims to correct disease states in offspring; however, these newer approaches in reproductive medicine, although promising, are still in their infancy.
Acknowledgments
A.K. is supported by the Reproductive Scientist Development Program (NIH-NICHD Project #2K12 HD000849–26), the Bennack-Polan Foundation Grant, the American Society for Reproductive Medicine, and the NIH Loan Repayment Program. A.P. is supported by NICHD R01 HD081162, and J.J. is supported by University of Colorado Department of Obstetrics and Gynecology Research Funds.
Glossary
- Amenorrheic (amenorrhea)
The absence of menstruation for one or more months, indicative of the loss of reproductive cycling.
- Antimüllerian hormone (AMH)
(or Müllerian Inhibitory Substance (MIS)) is a TGFβ family peptide hormone produced by small growing mammalian ovarian follicles to repress primordial follicle growth activation. Serum AMH concentration is used clinically as an indirect measure of the ovarian reserve, where < 1 ng/ml is viewed as indicative of Diminished Ovarian Reserve.
- Antral follicles
Ovarian follicle whose growth and development has resulted in the acquisition of a fluid-filled cavity termed the antrum. Antral follicles contain multiple layers of cuboidal, proliferative granulosa cells and a growing, maturing oocyte.
- Atresia
death and involution of the ovarian follicle structure in a process that includes hallmarks of granulosa cell and oocyte apoptosis. The vast majority of ovarian follicles will die via atresia, while only a small fraction survive such that a single egg is ovulated each menstrual cycle.
- Egg Retrieval
The clinical procedure whereby human eggs (within cumulus granulosa cells) are collected. Egg retrievals usually occur as outpatient procedures, using local anaesthetic/analgesic treatment to manage the ultrasound-guided transvaginal puncture of follicles and suction at the surface of the ovary.
- Embryonic Mosaicism
Often referring to the blastocyst stage, refers to an embryo composed of cells of differing genotypes. The unique genotypes often consist of cells whose chromosomes have been segregated correctly to daughter cells after cell division (euploid cells) and cells whose chromosomes have been segregated inappropriately/unequally (aneuploid cells). In the case of genomic editing of embryos, embryonic mosaicism can occur when only a subset of cells carry the desired alteration.
- Diminished Ovarian Reserve (DOR)
A clinical diagnosis characterized by evidence of advanced ovarian aging independent of chronological age, including elevated FSH and reduced AMH relative to same-age fertile women. Women with DOR often produce low numbers of eggs during IVF cycles.
- Fertility Preservation/Oncofertility
Egg and embryo freezing, as well as ovarian cortex freezing are all core strategies for female fertility preservation. After thawing, each can be used in later attempts at conception.
- Gonadotoxic Therapies
Therapeutic treatments with an inordinate impact on gonadal function, often due to germ cell toxicity. Both female and male germ cells are known to be sensitive to radiotherapy and specific chemotherapeutic agents. If the standard-of-care or preferred treatment (for example, for a life-threatening disease) for a condition is known to place a patient at risk for the loss of ovarian function, Fertility Preservation/Oncofertility strategies can be considered.
- Growing Follicle
an ovarian follicle that has committed to a growth phase consisting of an increase in oocyte volume and an increase in the surrounding population of granulosa cells due to proliferation. Growing ovarian follicles eventually acquire a fluid-filled cavity termed the antrum as they approach ovulatory size.
- Ovarian hyperstimulation (Syndrome - OHSS)
A condition that occurs almost exclusively after fertility treatments (most often after IVF) when the ovaries get markedly enlarged and produce fluid shifts from the intravascular space to the abdominal cavity (so called “third space”).
- Ovarian Stimulation
the standard-of-care for in vitro fertilization cycles, where gonadotropin hormones (Follicle Stimulating Hormone [FSH] and Luteinizing Hormone [LH]or analogues in various formulations) support the growth and survival, then recovery of a cohort of growing ovarian follicles. Inclusion of Inhibin blocking antisera in mouse stimulation protocols (“superovulation”) abrogates endogenous suppression of FSH by ovarian inhibin, resulting in significantly increased oocyte yield.
- Primordial Follicle
a dormant ovarian follicle consisting of an oocyte surrounded by a few non-proliferative pre-granulosa cells. Compare to “Growing Follicle.”
- Primordial Follicle Growth Activation (PFGA)
The primordial follicle consists of a few squamous (“flat”) pregranulosa cells surrunding a primordial oocyte. Upon PFGA, the pregranulosa cells change to a cuboidal appearance and begin to proliferate, and the oocyte begins to grow and mature.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No conflicts of interest are noted or occurred during the preparation of this manuscript.
References
- 1.Pereira N et al. (2017) The safety of intracytoplasmic sperm injection and long-term outcomes. Reproduction 154, F61–F70 [DOI] [PubMed] [Google Scholar]
- 2.Toner JP et al. (2016) Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil. Steril 106, 541–546 [DOI] [PubMed] [Google Scholar]
- 3.Potdar N et al. (2014) Oocyte vitrification in the 21st century and post-warming fertility outcomes: a systematic review and meta-analysis. Reprod. Biomed. Online 29, 159–176 [DOI] [PubMed] [Google Scholar]
- 4.Biggers JD (2012) IVF and embryo transfer: historical origin and development. Reprod. Biomed. Online 25, 118–127 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson J et al. (2017) Developments in IVF warrant the adoption of new performance indicators for ART clinics, but do not justify the abandonment of patient-centred measures. Hum. Reprod 32, 1155–1159 [DOI] [PubMed] [Google Scholar]
- 6.Cohen J et al. (2012) Past performance of assisted reproduction technologies as a model to predict future progress: a proposed addendum to Moore’s law. Reprod. Biomed. Online 25, 585–590 [DOI] [PubMed] [Google Scholar]
- 7.Inge GB, et al. (2005). Oocyte number per live birth in IVF: were Steptoe and Edwards less wasteful?. Hum. Reprod, 20, 3, 588–92 [DOI] [PubMed] [Google Scholar]
- 8.Sunderam S, et al. (2017). Assisted Reproductive Technology Surveillance - United States, 2014. MMWR Surveill. Summ, 66, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alper MM and Fauser BC (2017) Ovarian stimulation protocols for IVF: is more better than less? Reprod. Biomed. Online 34, 345–353 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt DW et al. (2004) Reducing the dose of human chorionic gonadotropin in high responders does not affect the outcomes of in vitro fertilization. Fertil. Steril 82, 841–846 [DOI] [PubMed] [Google Scholar]
- 11.Stouffer RL and Zelinski-Wooten MB (2004) Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod. Biol. Endocrinol 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SART (1996) Assisted reproductive technology in the United States and Canada: 1994 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil. Steril 66, 697–705 [DOI] [PubMed] [Google Scholar]
- 13.Mascarenhas M, Balen AH (2017). The high responder: a review of pathophysiology and outcomes during IVF treatment. Hum Fertil (Camb), 20, 3:155–167. [DOI] [PubMed] [Google Scholar]
- 14.Rasool S and Shah D (2017) Fertility with early reduction of ovarian reserve: the last straw that breaks the Camel’s back. Fertil Res Pract 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene AD et al. (2014) Genetic associations with diminished ovarian reserve: a systematic review of the literature. J. Assist. Reprod. Genet 31, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tietze C (1947) Human fertility in Puerto Rico. Am J Sociol 53, 34–40 [DOI] [PubMed] [Google Scholar]
- 17.La Marca A et al. (2017) Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril 108, 777–783 [DOI] [PubMed] [Google Scholar]
- 18.Lim AS and Tsakok MF (1997) Age-related decline in fertility: a link to degenerative oocytes? Fertil. Steril 68, 265–271 [DOI] [PubMed] [Google Scholar]
- 19.Velde E.R. te and Pearson PL. (2002) The variability of female reproductive ageing. Hum. Reprod. Update 8, 141–154 [DOI] [PubMed] [Google Scholar]
- 20.Levi M et al. (2013) Morphological and molecular markers are correlated with maturation-competence of human oocytes. Hum. Reprod 28, 2482–2489 [DOI] [PubMed] [Google Scholar]
- 21.Mathews TJ and Hamilton BE (2016). Mean Age of Mothers is on the Rise: United States, 2000–2014. NCHS Data Brief, 232, 1–7 [PubMed] [Google Scholar]
- 22.Shahine LK et al. (2016) Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertil. Steril 106, 1124–1128 [DOI] [PubMed] [Google Scholar]
- 23.Salama M and Woodruff TK (2017) Anticancer treatments and female fertility: clinical concerns and role of oncologists in oncofertility practice. Expert Rev Anticancer Ther 17, 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JL and Wang ET (2017) Oncofertility for women with gynecologic malignancies. Gynecol. Oncol 144, 631–636 [DOI] [PubMed] [Google Scholar]
- 25.Cohen J et al. (2017) Outcomes of first IVF/ICSI in young women with diminished ovarian reserve. Minerva Ginecol 69, 315–321 [DOI] [PubMed] [Google Scholar]
- 26.Tannus S et al. (2017) The role of intracytoplasmic sperm injection in non-male factor infertility in advanced maternal age. Hum. Reprod 32, 119–124 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H and Liu K (2015) Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 21, 779–786 [DOI] [PubMed] [Google Scholar]
- 28.Reddy P et al. (2010) Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab 21, 96–103 [DOI] [PubMed] [Google Scholar]
- 29.Tilly JL et al. (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129, 2799–2801 [DOI] [PubMed] [Google Scholar]
- 30.Thomson TC et al. (2010) Intrinsic and extrinsic mechanisms of oocyte loss. Mol. Hum. Reprod 16, 916–927 [DOI] [PubMed] [Google Scholar]
- 31.Mihm M and Evans AC (2008) Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod. Domest. Anim 43 Suppl 2, 48–56 [DOI] [PubMed] [Google Scholar]
- 32.Hansen KR et al. (2012) Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause 19, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin M et al. (2011) mTOR kinase inhibition results in oocyte loss characterized by empty follicles in human ovarian cortical strips cultured in vitro. Fertil. Steril 96, 1154–1159 [DOI] [PubMed] [Google Scholar]
- 34.Motta PM et al. (1995) Ultrastructure of human reproduction from folliculogenesis to early embryo development. A review. Ital J Anat Embryol 100, 9–72 [PubMed] [Google Scholar]
- 35.Oktay K et al. (1997) Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil. Steril 67, 481–486 [DOI] [PubMed] [Google Scholar]
- 36.Gosden RG (2000) Low temperature storage and grafting of human ovarian tissue. Mol. Cell. Endocrinol 163, 125–129 [DOI] [PubMed] [Google Scholar]
- 37.Dunlop CE et al. (2016) Re-implantation of cryopreserved ovarian cortex resulting in restoration of ovarian function, natural conception and successful pregnancy after haematopoietic stem cell transplantation for Wilms tumour. J. Assist. Reprod. Genet 33, 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin M et al. (2015) An externally validated age-related model of mean follicle density in the cortex of the human ovary. J. Assist. Reprod. Genet 32, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnez J et al. (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364, 1405–1410 [DOI] [PubMed] [Google Scholar]
- 40.Meirow D et al. (2016) Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril 106, 467–474 [DOI] [PubMed] [Google Scholar]
- 41.Donnez J et al. (2011) Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil. Steril 95, 1–4 [DOI] [PubMed] [Google Scholar]
- 42.Chiti MC et al. (2017) A modified and tailored human follicle isolation procedure improves follicle recovery and survival. J Ovarian Res 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, Amorim CA (2011). Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil. Steril, 96, 2:379–383.e3. [DOI] [PubMed] [Google Scholar]
- 44.Chiti MC, Dolmans MM, Donnez J, Amorim CA (2017). Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann Biomed Eng, 45, 7:1650–1663. [DOI] [PubMed] [Google Scholar]
- 45.Telfer E et al. (1990) Morphological study of cultured preantral ovarian follicles of mice after transplantation under the kidney capsule. J. Reprod. Fertil 89, 565–571 [DOI] [PubMed] [Google Scholar]
- 46.Torrance C et al. (1989) Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J. Reprod. Fertil 87, 367–374 [DOI] [PubMed] [Google Scholar]
- 47.Loret de Mola JR et al. (2004) Comparison of two culture systems for the in-vitro growth and maturation of mouse preantral follicles. Clin Exp Obstet Gynecol 31, 15–19 [PubMed] [Google Scholar]
- 48.Desai N et al. (2012) Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreeger PK et al. (2006) The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 27, 714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shikanov A et al. (2011) A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J et al. (2010) Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 140, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salama M and Woodruff TK (2015) New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 34, 807–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tagler D, Makanji Y, Tu T, Bernabé BP, Lee R, Zhu J, Kniazeva E, Hornick JE, Woodruff TK, Shea LD (2014). Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol. Bioeng, 111, 7:1417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H (2013). The C-kit receptor-mediated signal transduction and tumor-related diseases. Int. J. Biol. Sci, 9, 5:435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang EJ et al. (1993) The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev. Biol 157, 100–109 [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H et al. (1997) Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev. Biol 184, 122–137 [DOI] [PubMed] [Google Scholar]
- 57.Castrillon DH et al. (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301, 215–218 [DOI] [PubMed] [Google Scholar]
- 58.Xie Y et al. (2016) Power of PTEN/AKT: Molecular switch between tumor suppressors and oncogenes. Oncol Lett 12, 375–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K (2007). Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development, 134, 1:199–209. [DOI] [PubMed] [Google Scholar]
- 60.Li J et al. (2010) Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. U.S.A. 107, 10280–10284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy P et al. (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 [DOI] [PubMed] [Google Scholar]
- 62.Jagarlamudi K et al. (2009) Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS ONE 4, e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng Y et al. (2015) Promotion of ovarian follicle growth following mTOR activation: synergistic effects of AKT stimulators. PLoS ONE 10, e0117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novella-Maestre E et al. (2015) Short-Term PTEN Inhibition Improves In Vitro Activation of Primordial Follicles, Preserves Follicular Viability, and Restores AMH Levels in Cryopreserved Ovarian Tissue From Cancer Patients. PLoS ONE 10, e0127786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawamura K et al. (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. U.S.A. 110, 17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLaughlin M et al. (2014) Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol. Hum. Reprod 20, 736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pankhurst MW (2017) A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J. Endocrinol 233, R1–R13 [DOI] [PubMed] [Google Scholar]
- 68.Ding X et al. (2013) Effects of BMP4/SMAD signaling pathway on mouse primordial follicle growth and survival via up-regulation of Sohlh2 and c-kit. Mol. Reprod. Dev 80, 70–78 [DOI] [PubMed] [Google Scholar]
- 69.Tanwar PS et al. (2008) In vivo evidence of role of bone morphogenetic protein-4 in the mouse ovary. Anim. Reprod. Sci 106, 232–240 [DOI] [PubMed] [Google Scholar]
- 70.Lee WS et al. (2001) Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol. Reprod 65, 994–999 [DOI] [PubMed] [Google Scholar]
- 71.Tang K et al. (2012) GDF-9 and bFGF enhance the effect of FSH on the survival, activation, and growth of cattle primordial follicles. Anim. Reprod. Sci 131, 129–134 [DOI] [PubMed] [Google Scholar]
- 72.Kedem A et al. (2011) Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J. Clin. Endocrinol. Metab 96, E1246–1254 [DOI] [PubMed] [Google Scholar]
- 73.Dole G et al. (2008) Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction 135, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda Y et al. (2016) Effects of gremlin-2 on the transition of primordial follicles during early folliculogenesis in the human ovary. Eur. J. Obstet. Gynecol. Reprod. Biol 203, 72–77 [DOI] [PubMed] [Google Scholar]
- 75.Nilsson EE et al. (2014) Roles of Gremlin 1 and Gremlin 2 in regulating ovarian primordial to primary follicle transition. Reproduction 147, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li-Ping Z et al. (2010) Proto-oncogene c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways. Reprod. Biol. Endocrinol 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feeney A et al. (2014) Cytokine (IL16) and tyrphostin actions on ovarian primordial follicle development. Reproduction 148, 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang P et al. (2010) Murine folliculogenesis in vitro is stage-specifically regulated by insulin via the Akt signaling pathway. Histochem. Cell Biol. 134, 75–82 [DOI] [PubMed] [Google Scholar]
- 79.Kezele PR et al. (2002) Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol. Cell. Endocrinol 192, 37–43 [DOI] [PubMed] [Google Scholar]
- 80.Nilsson EE et al. (2002) Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol. Cell. Endocrinol 188, 65–73 [DOI] [PubMed] [Google Scholar]
- 81.Nilsson E et al. (2009) Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction 138, 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenfeld CR et al. (2007) Tumor necrosis factor (TNF) receptor type 2 is an important mediator of TNF alpha function in the mouse ovary. Biol. Reprod 76, 224–231 [DOI] [PubMed] [Google Scholar]
- 83.Durlinger AL et al. (1999) Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 140, 5789–5796 [DOI] [PubMed] [Google Scholar]
- 84.Holt JE et al. (2006) CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev. Biol 293, 449–460 [DOI] [PubMed] [Google Scholar]
- 85.Britt KL et al. (2004) Estrogen actions on follicle formation and early follicle development. Biol. Reprod 71, 1712–1723 [DOI] [PubMed] [Google Scholar]
- 86.Yang S et al. (2013) Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol. Reprod 89, 126. [DOI] [PubMed] [Google Scholar]
- 87.Adhikari D et al. (2009) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod 15, 765–770 [DOI] [PubMed] [Google Scholar]
- 88.Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY (2014). Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol. Hum. Reprod, 20, 4:293–308. [DOI] [PubMed] [Google Scholar]
- 89.Baarends WM et al. (1995) Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136, 4951–4962 [DOI] [PubMed] [Google Scholar]
- 90.Nehra D et al. (2012) Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 11, 1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Meir A et al. (2015) Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14, 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou L et al. (2017) Rapamycin Prevents cyclophosphamide-induced Over-activation of Primordial Follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J Ovarian Res 10, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dou X et al. (2017) Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell 16, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H et al. (2014) Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc. Natl. Acad. Sci. U.S.A. 111, 5735–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takeo T and Nakagata N (2015) Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS ONE 10, e0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakagawa Y et al. (2016) Ultra-superovulation for the CRISPR-Cas9-mediated production of gene-knockout, single-amino-acid-substituted, and floxed mice. Biol Open 5, 1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Telfer EE et al. (2008) A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod 23, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 98.Lowther KM and Mehlmann LM (2015) Embryonic Poly(A)-Binding Protein Is Required During Early Stages of Mouse Oocyte Development for Chromatin Organization, Transcriptional Silencing, and Meiotic Competence. Biol. Reprod 93, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaffe LA et al. (2009) Microinjection of follicle-enclosed mouse oocytes. Methods Mol. Biol 518, 157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang E et al. (2016) Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275 [DOI] [PubMed] [Google Scholar]
- 101.Okada M et al. (2017) Stabilization of Foxp3 expression by CRISPR-dCas9-based epigenome editing in mouse primary T cells. Epigenetics Chromatin 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minkenberg B et al. (2017) CRISPR/Cas9-Enabled Multiplex Genome Editing and Its Application. Prog Mol Biol Transl Sci 149, 111–132 [DOI] [PubMed] [Google Scholar]
- 103.Huang YH et al. (2017) DNA epigenome editing using CRISPR-Cas SunTag-directed DNMT3A. Genome Biol. 18, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hikabe O, et al. (2016). Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature, 539, 299–303 [DOI] [PubMed] [Google Scholar]
- 105.Adhikari D, Liu K (2009). Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev, 30, 5:438–64. [DOI] [PubMed] [Google Scholar]
- 106.Zacharek SJ, Xiong Y, Shumway SD (2005). Negative regulation of TSC1-TSC2 by mammalian D-type cyclins. Cancer Res, 65, 24:11354–60. [DOI] [PubMed] [Google Scholar]