Abstract
Background
Low skeletal muscle mass is associated with increased postoperative morbidity and worse survival following resection for perihilar cholangiocarcinoma (PHC). We investigated the predictive value of skeletal muscle mass and density for overall survival (OS) of all patients with suspected PHC, regardless of treatment.
Methods
Baseline characteristics and parameters regarding disease and treatment were collected from all patients with PHC from 2002 to 2014. Skeletal muscle mass and density were measured at the level of the third lumbar vertebra on CT. The association between skeletal muscle mass and density with OS was investigated using the Kaplan-Meier method and Cox survival.
Results
Median OS in 233 included patients did not differ between those with and without low skeletal muscle mass (p = 0.203), whereas a significantly different median OS (months) was observed between patients with low (HR 7.0, 95% CI 4.7–9.3) and high (HR 12.1, 95% CI 8.1–16.1) skeletal muscle density (p = 0.004). Low skeletal muscle density was independently associated with decreased OS (HR 1.78, 95% CI 1.03–3.07, p = 0.040) within the first 6 months but not after 6 months (HR 0.68, 95% CI 0.44–1.07, p = 0.093), after adjusting for age, tumour size and suspected peritoneal or other distant metastases on imaging.
Conclusion
A time-dependent effect of skeletal muscle density on OS was found in patients with PHC, regardless of subsequent treatment. Low skeletal muscle density may identify patients at risk for early death.
Keywords: Perihilar cholangiocarcinoma, Skeletal muscle density, Skeletal muscle mass, Sarcopenia, Computed tomography, Prognosis
Introduction
The prognosis of patients with perihilar cholangiocarcinoma (PHC) is poor. After curative-intent resection, the median survival is 19–39 months with a 5-year survival rate of 10–40% [1, 2, 3]. However, only about 1 in 4 patients with suspected PHC undergoes surgical resection. The majority of patients receive palliative treatment or best supportive care and have a median survival of only 6 months [4, 5, 6, 7].
Multiple staging systems are available to predict prognosis in patients with (suspected) PHC [4, 8, 9, 10]. However, the majority of staging systems, such as the frequently used American Joint Committee on Cancer (AJCC) staging system, are applicable only to a minority of patients who undergo resection [8]. Prognostic factors and models for all PHC patients regardless of treatment are rare [10].
Recently, low skeletal muscle mass (i.e., sarcopenia), as part of the cancer cachexia syndrome [11, 12], has been introduced as a biomarker to predict treatment complications and worsened survival in gastrointestinal and hepatopancreatobiliary cancer patients [13, 14]. It may detect malnutrition that is not visible otherwise [15]. Three studies found that preoperative low skeletal muscle mass was also associated with worse outcome in patients undergoing surgical resection of extrahepatic biliary cancer [16] and PHC [17, 18]. Moreover, low skeletal muscle density, as a measure of intramuscular adipose tissue infiltration, has been identified as a prognostic parameter that might be even stronger than skeletal muscle mass [19]. However, the association between sarcopenia and outcome in all PHC patients, regardless of treatment, and the prognostic value of skeletal muscle density remain unknown.
Methods
Patients and Data Collection
All patients aged 18 years and older with suspected PHC who presented between 2002 and 2014 were identified. Demographics and clinical, drainage, laboratory, and treatment parameters were collected from medical records. Body mass index (BMI) was categorized according to the World Health Organization classification [20]. PHC was defined as a mass at or near the biliary confluence, arising between the origin of the cystic duct and the segmental bile ducts [21]. In the absence of histopathological evidence, the diagnosis of suspected PHC was based on the opinion of the multidisciplinary hepatopancreatobiliary team based on clinical, radiological, endoscopic, and laboratory observations. Patients were excluded if benign disease was considered more likely during follow-up. Patients who visited our centre for drainage only once, or who already underwent treatment in the referral centre, were also excluded.
Radiological examinations (contrast-enhanced CT and/or MRI with or without cholangiopancreatography [MRCP]) were re-assessed by an experienced abdominal radiologist. Parameters assessed on imaging were tumour visibility, tumour size, Bismuth-Corlette classification [22], vascular involvement [9], lobar atrophy, lymph node status, and the presence of distant metastases. Based on these findings, the AJCC stage (7th edition) was assessed [21]. Stages I and II were analysed together for the clinical AJCC stage, because T1 (stage I) and T2 (stage II) cannot be distinguished on imaging alone. Vascular involvement was defined as tumour contact of at least 180 degrees around the portal vein and/or hepatic artery and its side branches. Involvement of lymph nodes along the cystic duct, common bile duct, hepatic artery and portal vein was classified as N1 and lymph node involvement around the aorta, caval vein, superior mesenteric artery and celiac artery as N2 [21].
The municipal records database was checked for survival status on May 9, 2016. A waiver was granted for this study from the Institutional Review Board.
Diagnostic Work-Up and Treatment Algorithm
The diagnostic work-up included, but was not limited to, imaging with contrast-enhanced CT, and MRI with or without MRCP. Typically, patients were only considered for exploratory laparotomy in the absence of metastatic disease and with involvement of <180 degrees of the hepatic artery. A resection was performed only when a complete resection (R0) was anticipated with an adequate functional liver remnant. Patients with metastatic or locally advanced disease were offered palliative chemotherapy. All other patients received best supportive care and palliative drainage.
Skeletal Muscle Mass and Density
Skeletal muscle mass was measured on abdominal CT, using an in-house developed software package as previously described [23, 24]. In short, the cross-sectional skeletal muscle area (CSMA, in cm2) was measured at the level of the third lumbar vertebra (L3) using a Hounsfield unit range of −30 to 150. The CSMA was adjusted for patients' height squared, as is conventional for body composition measurements, resulting in the skeletal muscle index (cm2/m2) that is strongly correlated with total body skeletal muscle mass [25, 26]. Low skeletal muscle index was defined according to previously defined cut-off values in patients with PHC undergoing surgery [17]. The mean Hounsfield unit value of the CSMA, as a measure of skeletal muscle density that is closely related to muscle function [19, 27], was also recorded. Low skeletal muscle density was defined as a value below the sex-specific median [28].
The first abdominal CT during the diagnostic work-up of PHC was used. If no CT was available or not all skeletal muscles at the level of L3 were depicted on, patients were excluded.
Statistical Analyses
Continuous data are reported as median with interquartile range or mean ± SD, depending on the normality of the distribution. Normality of the distribution was tested using the Shapiro-Wilk test. Categorical variables are reported as counts with percentages. Fischer's exact or chi-square tests were used for the comparison of proportions, while continuous parameters were compared using Students t tests.
Overall survival (OS) was measured from the date of first presentation in the tertiary referral centre. Survival estimates were compared using the Kaplan-Meier method and the log-rank test. Logistic regression analysis was used to compare the 3-month, 6-month, 1-, 3-, and 5-year survival rates. The association between skeletal muscle mass and density and survival was investigated using a multivariable Cox proportional hazard regression model, adjusting for known risk factors [10] and additional factors that were associated with impaired survival in univariable analysis. Hazard ratios (HRs) with 95% CI were reported. Due to the large number of missing values, CA19-9 was not included in the final model. A subgroup analysis was performed only in unresectable patients. The effect of skeletal muscle density on the hazard was allowed to vary before and after 6 months of follow-up. Therefore, an interaction term between time and skeletal muscle density was included in the Cox regression model [29]. Finally, a sensitivity analysis was performed using the cut-off values defined by Martin et al. [19]. Two-tailed p values below 0.05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient and Tumour Characteristics
In total, 285 patients with suspected PHC in our centre were identified. Of these 285 patients, 233 (81.8%) had a contrast-enhanced abdominal CT and formed the study cohort. Body height was missing for 23 (9.9%) patients. Consequently, these patients were excluded from analyses requiring body height (i.e., skeletal muscle mass), but included in analyses using skeletal muscle density. Due to missing body height and/or weight, BMI was unknown for 50 (21.5%) patients. The median time between the first available contrast-enhanced CT performed for the suspicion on PHC and the first presentation in the tertiary referral centre was 11 (3–25) days (Table 1).
Table 1.
Baseline and treatment characteristics of the total population and for patients with low and normal/high skeletal muscle mass and skeletal muscle density respectively
| All (n = 233) |
Skeletal muscle mass |
Skeletal muscle density |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| low (n = 103) | high (n = 107) | p value | low(n = 131) | high (n = 102) | p value | ||||
| Patient characteristic | |||||||||
| Age, years, median (IQR) | 66 (57–74) | 69 (58–74) | 64 (53–72) | 0.040 | 72 (64–76) | 59 (47–67) | <0.001 | ||
| Gender,n (%) | |||||||||
| Males | 140 (60.1) | 56 (54.4) | 71 (66.4) | 81 (61.8) | 43 (42.2) | ||||
| Females | 93 (39.9) | 47 (45.6) | 36 (33.6) | 0.076 | 50 (38.2) | 59 (57.8) | 0.537 | ||
| BMI, kg/m2* | 24.7 (22.5–26.8) | 23.7 (21.3–25.7) | 25.7 (23.9–27.9) | <0.001 | 25.2 (23.4–27.6) | 24.4 (21.9–26.3) | 0.032 | ||
| ECOG (WHO) performance | |||||||||
| status+ | |||||||||
| 1–2 | 215 (95.1) | 94 (94.0) | 99 (95.2) | 118 (92.9) | 97 (98.0) | ||||
| 3–4 | 11 (4.9) | 6 (6.0) | 5 (4.8) | 0.706 | 9 (7.1) | 2 (2.0) | 0.079 | ||
| Weight loss, kg, yes | 118 (52.4) | 50 (50.5) | 60 (57.7) | 0.160 | 68 (53.5) | 50 (51.0) | 0.089 | ||
| Jaundice at presentation, yes Cholangitis at/before | 182 (80.9) | 85 (85.0) | 79 (76.7) | 0.133 | 105 (82.7) | 77 (78.6) | 0.437 | ||
| presentation or preoperative | 129 (56.8) | 8 (8.4) | 5 (4.9) | 0.320 | 69 (54.3) | 60 (60.0) | 0.392 | ||
| CA19-9, kU/L# | 220 (57–1,297) | 254 (129–1,304) | 162 (41–848) | 0.039 | 232 (67–1,351) | 206 (44–877) | 0.534 | ||
| Albumin, g/L | 38 (33–43) | 38 (31–44) | 39 (25–42) | 0.750 | 37 (31–43) | 38 (34–42) | 0.669 | ||
| Total bilirubin prior to | |||||||||
| drainage, µmol/L§ | 138 (61–225) | 146 (77–230) | 120 (53–199) | 0.185 | 155 (86–234) | 122 (57–208) | 0.134 | ||
| C-reactive protein, mg/L¥ | 13 (7–29) | 19 (9–37) | 9 (5–20) | 0.002 | 17 (9–30) | 9 (5–21) | 0.023 | ||
| Thrombocytes, ×109/L | 284 (220–338) | 287 (228–354) | 281 (206–332) | 0.266 | 259 (222–323) | 307 (208–366) | 0.174 | ||
| Disease characteristic | |||||||||
| Suspected peritoneal or other | |||||||||
| distant organ metastases | 26 (11.2) | 16 (15.5) | 5 (4.7) | 0.009 | 18 (13.7) | 8 (7.9) | 0.164 | ||
| Lymph node status on imaging† | |||||||||
| N0 | 122 (53.3) | 54 (53.5) | 60 (57.1) | 70 (54.3) | 52 (52.0) | ||||
| N1 | 67 (29.3) | 30 (29.7) | 28 (26.7) | 33 (25.6) | 34 (34.0) | ||||
| N2 | 40 (17.5) | 17 (16.8) | 17 (16.2) | 0.858 | 26 (20.2) | 14 (14.0) | 0.267 | ||
| Vascular involvement on | |||||||||
| imaging‡ | 148 (64.9) | 63 (61.2) | 67 (65.0) | 0.564 | 86 (68.3) | 62 (60.8) | 0.240 | ||
| Tumour size on imaging, mm | 22 (20–35) | 25 (19–32) | 27 (21–35) | 0.386 | 26 (21–36) | 26 (20–34) | 0.292 | ||
| Lobar atrophy on imaging AJCC stage (radiological) | 61 (26.5) | 32 (31.1) | 28 (26.7) | 0.484 | 40 (31.2) | 21 (20.6) | 0.069 | ||
| I/II | 28 (12.7) | 12 (12.4) | 14 (13.6) | 14 (11.4) | 14 (14.3) | ||||
| III | 50 (22.6) | 23 (23.7) | 24 (23.3) | 28 (22.8) | 22 (22.4) | ||||
| IV | 143 (64.7) | 62 (63.9) | 65 (63.1) | 0.968 | 81 (65.9) | 62 (63.3) | 0.810 | ||
| Blumgart classification [4, 42] | |||||||||
| Stage 1 | 60 (26.9) | 28 (28.3) | 27 (26.5) | 34 (27.6) | 26 (26.0) | ||||
| Stage 2 | 56 (25.1) | 31 (31.3) | 22 (21.6) | 33 (26.8) | 23 (23.0) | ||||
| Stage 3 | 107 (48.0) | 40 (40.4) | 53 (52.0) | 0.190 | 56 (45.5) | 51 (51.0) | 0.697 | ||
| Treatment | |||||||||
| Treatment groups | |||||||||
| Laparotomy with resection Laparotomy without | 41 (17.6) | 18 (17.5) | 23 (21.5) | 17 (13.0) | 24 (23.5) | ||||
| resection | 72 (30.9) | 29 (28.2) | 43 (40.2) | 0.062 | 24 (18.3) | 48 (47.1) | <0.001 | ||
| No laparotomy, initially | |||||||||
| unresectable | 120 (51.5) | 56 (54.4) | 41 (38.3) | 90 (68.7) | 30 (29.4) | ||||
| Chemotherapy | 31 (14.3) | 14 (14.1) | 17 (17.5) | 0.56 | 11 (8.8) | 20 (21.7) | 0.007 | ||
Categorical parameters are presented as counts (percentages) and continuous parameters as median (interquartile range). BMI, body mass index (* missing for 50 patients); ECOG, Eastern Cooperative Oncology Group (+ missing for 6 patients); CA19-9, carbohydrate antigen 19-9 (# missing for 77 patients); AJCC, American Joint Committee on Cancer.
Missing for 92 patients. § Missing for 49 patients.
Involvement of lymph nodes was assessed according to the AJCC (7th edition) [21].
Vascular involvement on imaging was defined as tumour contact of at least 180 degrees.
Treatment Characteristics
Forty-one (17.6%) patients underwent surgical resection including 2 liver transplantations, and 72 (30.9%) patients underwent a laparotomy without resection. In these 72 patients, the intraoperative finding of metastases and locally advanced disease were the most common reasons for renouncing resection. The remaining 120 (51.5%) patients were considered unresectable at initial presentation, of whom 13 (11.3%) received palliative chemotherapy.
Low Skeletal Muscle Mass
In total, 103 of the 210 (49.0%) of patients were considered to have low skeletal muscle mass (Table 1). Patients with low skeletal muscle mass were significantly older compared with patients with high skeletal muscle mass (69 vs. 64 years, p = 0.040) and had significantly higher C-reactive protein and CA19-9 levels. Median BMI was significantly lower in patients with low versus high skeletal muscle mass (23.7 vs. 25.7, p < 0.001). The rate of metastatic disease at initial presentation was significantly higher in patients with low skeletal muscle mass (15.5 vs. 4.7%, p = 0.009) and non-significant differences were observed in treatment groups.
Low Skeletal Muscle Density
Low skeletal muscle density was observed in 131 (56.2%) patients (Table 1). BMI was significantly higher in patients with low skeletal muscle density compared with high skeletal muscle density (25.2 vs. 24.4, p = 0.032). Furthermore, patients with low skeletal muscle density had a higher CRP level (17 vs. 9, p = 0.023), more often had unresectable disease (87.0 vs. 78.0%, p < 0.001) and were less frequently treated with chemotherapy (8.8 vs. 21.7%, p = 0.007).
Overall Survival
In total, 221 (94.8%) patients died during the study period. Median follow-up of the included patients who were alive at last follow-up was 25.3 (18.3–85.5) months. The 3-month, 6-month, 1-, 3-, and 5-year OS rates in the entire cohort were 79.0, 60.9, 42.1, 7.7, and 3.0% respectively. Median OS for the entire cohort was 9.6 (4.1–20.5) months. Median OS for patients who underwent resection was 29.1 months compared with 7.9 months in patients who did not undergo resection (p < 0.001).
Skeletal Muscle Mass and Density and OS
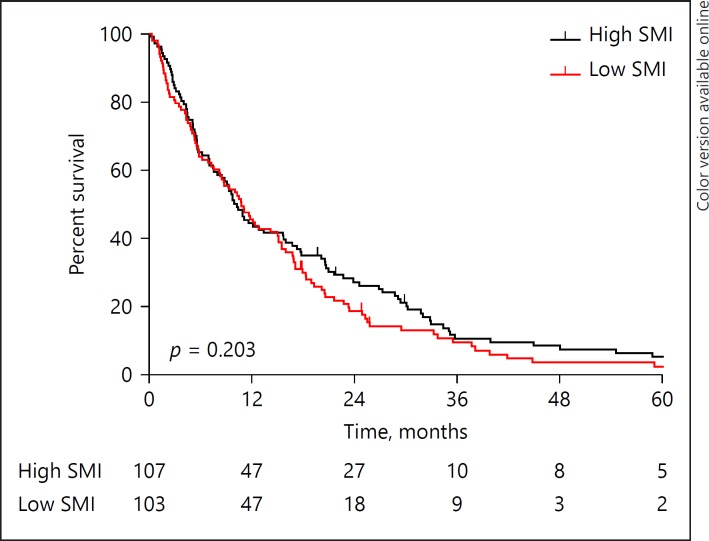
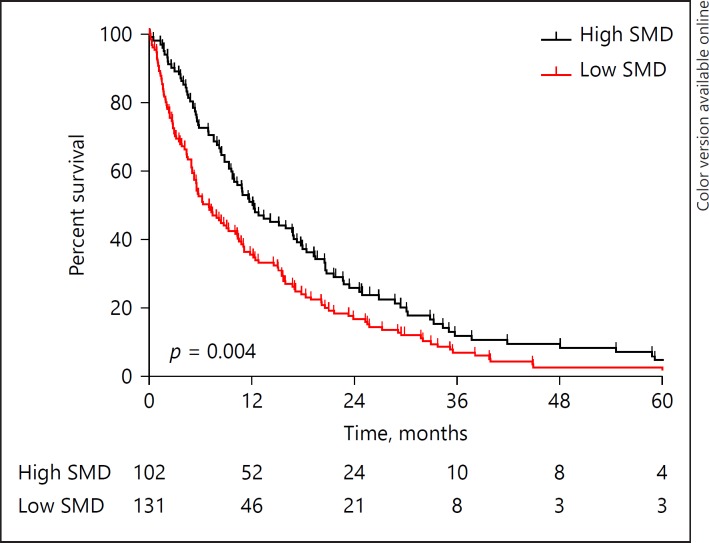
The median OS did not differ between patients with low and high skeletal muscle mass (10.8 [7.7–13.8] vs. 10.3 [8.2–12.3] months, p = 0.203; Fig. 1), whereas a significantly lower median survival was observed in patients with low skeletal muscle density compared with patients with high skeletal muscle density (7.0 [4.7–9.3] vs. 12.1 [8.1–16.1] months, p = 0.004; Fig. 2). Kaplan-Meier survival curves for patients with high/low skeletal muscle mass/density stratified for treatment group (i.e., resection, laparotomy without resection, initially unresectable) are provided in online supplementary Figures 1–3 (for all online suppl. material, see www.karger.com/doi/10.1159/000486867). A sensitivity analysis using the cut-off defined by Martin et al. [19] showed comparable results (online suppl. Fig. 4, 5).
Fig. 1.
Kaplan-Meier overall survival curves for patients with high and low skeletal muscle mass. SMI, skeletal muscle index.
Fig. 2.
Kaplan-Meier overall survival curves for patients with high and low skeletal muscle density. SMD, skeletal muscle density.
Lower OS rates were observed in patients with low skeletal muscle density compared with patients with high skeletal muscle density at 3 months (71.0 vs. 89.2%, p = 0.001; OR 3.38 [1.63–7.02], p = 0.001), 6 months (51.9 vs. 72.5%, p = 0.003; OR 2.45 [1.11–4.26], p = 0.002) and 1 year (35.1 vs. 51.0%, p = 0.015; OR 1.92, [1.13–3.26], p = 0.015), but not at 3 and 5 years (6.1 vs. 9.8%, p = 0.294, and 2.3 vs. 3.9%, p = 0.086, respectively). After adjusting for age, tumour size, and suspected peritoneal or other distant metastases on imaging, low skeletal muscle density was independently associated with decreased OS (HR 1.78 [1.03–3.07], p = 0.004) ≤6 months, but not >6 months (HR 0.68 [0.44–1.07], p = 0.093; Table 2). Similar results were observed when the sex factor was added to the analyses and in a subgroup analysis in unresectable patients only. An incremental skeletal muscle density (as a continuous measure) was also independently associated with decreased OS ≤6 months (HR 0.96 [0.93–0.99], p = 0.002) but not >6 months.
Table 2.
Cox proportional hazard regression analysis for factors associated with impaired survival
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, years, median (IQR) | 1.02 (1.01–1.03) | 0.001 | 1.02 (1.01–1.04) | 0.003 |
| Gender | ||||
| Female | 1 (ref.) | |||
| Male | 1.01 (0.77–1.32) | 0.945 | ||
| BMI ≥25 kg/m2 | 1.04 (0.77–1.40) | 0.803 | ||
| ECOG (WHO) performance status | ||||
| 1–2 | 1 (ref.) | 1 (ref.) | ||
| 3–4 | 1.31 (0.69–2.48) | 0.403 | 1.63 (0.72–3.69) | 0.243 |
| Bilirubin >200 µmol/L | 1.48 (1.00–2.19) | 0.051 | 1.04 (0.67–1.60) | 0.866 |
| CA19-9 >1,000 kU/L | 1.87 (1.29–2.70) | 0.001 | ||
| Albumin, g/dL | 0.99 (0.96–1.02) | 0.429 | ||
| C-reactive protein≥100, mg/L | 2.10 (1.05–4.18) | 0.036 | ||
| Cholangitis before or at presentation | 1.48 (0.84–2.60) | 0.180 | ||
| Tumour size >3 cm | 2.31 (1.72–3.09) | <0.001 | 2.24 (1.60–3.15) | <0.001 |
| Suspicious lymph nodes on imaging* | ||||
| N0 | 1 (ref.) | 1 (ref.) | ||
| N1 | 1.37 (1.01–1.87) | 0.046 | 1.57 (1.08–2.28) | 0.018 |
| N2 | 1.48 (1.03–2.13) | 0.033 | 1.37 (0.91–2.06) | 0.134 |
| Suspected distant metastases on imaging | 1.46 (0.97–2.20) | 0.072 | 3.74 (1.93–7.26) | <0.001 |
| Lobar atrophy on imaging | 1.04 (0.77–1.41) | 0.793 | ||
| Vascular involvement on imaging§ | 1.44 (1.09–1.91) | 0.011 | 1.30 (0.91–1.85) | 0.150 |
| Low skeletal muscle mass | 1.99 (0.91–1.59) | 0.204 | ||
| Low skeletal muscle density (<6 months)# | 2.09 (1.34–3.27) | 0.001 | 1.78 (1.03–3.07) | 0.040 |
| Low skeletal muscle density (≥6 months)# | 1.20 (0.85–1.69) | 0.306 | 0.68 (0.44–1.07) | 0.093 |
HR, hazard ratio; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization.
Involvement of lymph nodes was assessed according to the AJCC (7th edition) [21].
The effect of skeletal muscle density on the hazard varied with time. Hence, an interaction term between skeletal muscle density and time was used to calculate the time-dependent effect of skeletal muscle density on the hazard.
Vascular involvement on imaging was defined as tumour contact of at least 180 degrees around the portal vein and/or hepatic artery and its side branches.
Discussion
This is the first study showing an association between low skeletal muscle density and worse outcome in all patients with PHC in a unique Western series of patients with both resectable and unresectable PHC. In other tumours, such as follicular lymphoma, melanoma, and metastatic renal cell and gastric carcinoma, no association between skeletal muscle mass and survival was shown, whereas skeletal muscle density was an independent prognostic factor [28, 30, 31, 32, 33]. The similarity between these studies and the current study is the aggressive course of the disease, which may have led to the inability to accurately predict outcome.
Subgroup analyses based on treatment groups (i.e., resection, laparotomy without resection, initially unresectable), which should be interpreted with caution due to small sample sizes, showed non-significant differences in OS favouring patients with high skeletal muscle mass and density.
An intriguing hypothesis described by Hayashi et al. [32] is that a decrease in skeletal muscle density is detected earlier on CT than a decrease in skeletal muscle mass. Recent studies show that skeletal muscle density is mainly correlated with intramuscular adipose tissue content [27], while low skeletal muscle mass results from limited muscle growth and increased muscle wasting [34]. The mechanisms leading to and effects of these 2 processes are probably different and further research on their pathophysiology is warranted. Tumour biology may play an important role, since the effects of skeletal muscle mass and density on outcome vary per tumour sort and within tumour sorts and altered body composition is associated with an elevated inflammatory response [35, 36].
The independent association between skeletal muscle mass and density has frequently been found in survival analyses of previous studies [13, 37]. Nevertheless, this is the first study to describe a time-dependent effect, independently of previously described risk factors for impaired survival in patients with PHC [10]. Time-dependency of covariates is often not assessed, leading to bias in survival analyses in a great part of literature [29]. Low skeletal muscle density influenced OS in the 3–6 months after initial diagnosis. However, this effect faded hereafter. This suggests that patients with the poorest survival are those with the lowest skeletal muscle density and that skeletal muscle mass may identify patients at increased risk for early death. Another reason why no effect was found after 3 and 5 years could have been the low median survival time (i.e., 7.9 months in unresectable and 29.1 months in resected patients), resulting in low patient numbers. Although we did not correct for treatment in multivariable analysis, we strongly believe that the model accurately reflects daily practice. After all, the parameters assessed at first presentation greatly determine treatment and consequently (indirectly) correlate with survival. Our results should therefore be interpreted as valid in an “all-comers” patient population.
Notably, the rate of patients that underwent resection or received chemotherapy was lower in the low skeletal muscle density group. Furthermore, patients undergoing resection were significantly younger. These findings suggest a preoperative selection process of patients considered fit for surgery and chemotherapy. After all, none of the parameters representing tumour load (i.e., bilirubin level, CA19-9 level, vascular involvement, tumour size) that possibly may have influenced resectability, were significantly higher in patients with low skeletal muscle density. However, it should be noted that the median time interval between first presentation in the tertiary hospital and resection was 79 days. This time window may have led to further clinical deterioration and these findings should therefore be interpreted with caution. The significantly lower BMI and higher age in patients with low skeletal muscle density are in line with previous findings, as increasing age and adiposity are known for its association with intramuscular adipose tissue content [19, 38].
PHC can be treated surgically or, if surgical resection is impossible, with non-surgical methods such as chemotherapy and palliative stenting. The majority of all prognostic models for PHC have been developed in patients undergoing surgical resection [10, 21]. However, the latter group forms the greatest number of patients with PHC, since only around a quarter of all patients undergo resection [4, 5, 6]. The value of skeletal muscle mass and density measurements to identify patients at risk for impaired outcome seems promising, particularly in hepaticopancreatobiliary cancer patients [13, 17, 18, 39]. Unfortunately, the number of patients who underwent surgical resection was too small to validate previously described findings regarding CT-assessed skeletal muscle mass and impaired outcome in patients with PHC undergoing surgery [17, 18]. Future studies should include low skeletal muscle density as a poor prognostic factor.
Because no uniform cut-off has been determined for density measurements, and optimum stratification was not possible due to sample size, we choose to use the sex-specific median to group patients into low and high skeletal muscle density [17, 19, 23, 25, 40]. Skeletal muscle density and survival were entered into the survival analysis as a continuous variable, since previous reports with large cohorts did not report sex differences in skeletal muscle attenuation [19]. Ideally, definitive cut-off points should be developed that are derived from healthy persons.
Previous studies as well as the current study show that sarcopenia is heavily correlated with cancer stage and treatment; yet across all strata of treatment and cancer stage, patients with sarcopenia perform worse [41, 42]. This indicates that, regardless of cancer stage and treatment, sarcopenia is an independent predictor of outcome. By only taking into account the preoperative sarcopenia and radiological imaging, we believe we have described the predictive ability of patient predisposition regardless of any treatment decisions. Moreover, a subgroup analysis in non-resectable patients only showed similar results. This predictive information could improve clinical decision-making.
Some limitations of the current study should be acknowledged. A drawback is the retrospective character of the study design. Although a systematic search was performed in the electronic patient records, this may have led to selection bias. Furthermore, some variables had a high number of missing values. In 77 patients, for example, CA19-9 was unknown because this tumour marker assessment has not routinely been performed before 2010. Although only contrast-enhanced CTs were used for skeletal muscle mass and density measurements, possible differences as a consequence of the use of different CT scanners and scanning protocols in various hospitals could not be precluded. Skeletal muscle mass and density were measured at one time only. Future studies could evaluate consecutive CT examinations over time to allow analysing changes over time.
In conclusion, a time-dependent association between skeletal muscle density and mortality was found in patients with PHC, regardless of subsequent treatment. Low skeletal muscle density may identify patients with PHC at risk for early death. This finding should be validated in a larger, external cohort, and future studies are needed to investigate the additional value of skeletal muscle density measurements in prognostic models.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Source
None declared.
Supplementary Material
Supplementary data
Acknowledgment
The authors would like to thank Wiro J. Niessen and Marcel Koek from the Department of Radiology and Medical Informatics, Erasmus MC University Medical Centre, Rotterdam, the Netherlands, for providing the FatSeg software program for skeletal muscle mass and density measurements, Gregorios Papageorgiou from the department of Biostatistics, Erasmus MC University Medical Centre, Rotterdam, the Netherlands, for providing statistical advice, and Leontien Heiligers, Laurens Groenendijk and Ivo Cornelissen, from the Trial Centre Radiology, Erasmus MC University Medical Centre, Rotterdam, the Netherlands for the collection of CT examinations.
References
- 1.Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbeck's Arch Surg. 2014;399:693–705. doi: 10.1007/s00423-014-1210-x. [DOI] [PubMed] [Google Scholar]
- 2.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, Blumgart LH, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, van Gulik TM, Jarnagin WR. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2016;27:753. doi: 10.1093/annonc/mdw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Youssef BM, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, Coelen RJ, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, Jarnagin WR, van Gulik TM. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221:1041–1049. doi: 10.1016/j.jamcollsurg.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg. 2012;36:2179–2186. doi: 10.1007/s00268-012-1638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelen RJS, Gaspersz MP, Labeur TA, van Vugt JLA, van Dieren S, Willemssen F, Nio CY, IJzermans JNM, Klumpen HJ, Groot Koerkamp B, van Gulik TM. Validation of the mayo clinic staging system in determining prognoses of patients with perihilar cholangiocarcinoma. Clin Gastroenterol Hepatol. 2017;15:1930–1939. doi: 10.1016/j.cgh.2017.04.044. e1933. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 10.Chaiteerakij R, Harmsen WS, Marrero CR, Aboelsoud MM, Ndzengue A, Kaiya J, Therneau TM, Sanchez W, Gores GJ, Roberts LR. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881–1890. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006;42:31–41. doi: 10.1016/j.ejca.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 13.Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–1458. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 14.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, JN IJ. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 15.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 16.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Kobayashi A, Iida T, Yagi S, Taura K, Hatano E, Uemoto S. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159:821–833. doi: 10.1016/j.surg.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Coelen RJ, Wiggers JK, Nio CY, Besselink MG, Busch OR, Gouma DJ, van Gulik TM. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB (Oxford) 2015;17:520–528. doi: 10.1111/hpb.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39:1494–1500. doi: 10.1007/s00268-015-2988-6. [DOI] [PubMed] [Google Scholar]
- 19.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. ed 5. New York: Springer; 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 22.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 23.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 24.van Vugt JL, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, Willemsen SP, de Bruin RW, IJzermans JN. A comparative study of software programs for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12158. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 28.Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, Fizazi K, di Palma M, Baracos VE, Escudier B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119:3377–3384. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 29.van Walraven C, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57:672–682. doi: 10.1016/j.jclinepi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Chu MP, Lieffers J, Ghosh S, Belch AR, Chua NS, Fontaine A, Sangha R, Turner AR, Baracos VE, Sawyer MB. Skeletal muscle radio-density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PLoS One. 2015;10:e0127589. doi: 10.1371/journal.pone.0127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18:3579–3585. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi N, Ando Y, Gyawali B, Shimokata T, Maeda O, Fukaya M, Goto H, Nagino M, Kodera Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep. 2016;35:1727–1731. doi: 10.3892/or.2015.4475. [DOI] [PubMed] [Google Scholar]
- 33.Van Rijssen LB, van Huijgevoort NC, Coelen RJ, Tol JA, Haverkort EB, Nio CY, Busch OR, Besselink MG. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol. 2017;24:272–280. doi: 10.1245/s10434-016-5495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by actriib antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Reisinger KW, Derikx JP, van Vugt JL, Von Meyenfeldt MF, Hulsewe KW, Olde Damink SW, Stoot JH, Poeze M. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35:924–927. doi: 10.1016/j.clnu.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Richards CH, Roxburgh CS, MacMillan MT, Isswiasi S, Robertson EG, Guthrie GK, Horgan PG, McMillan DC. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7:e41883. doi: 10.1371/journal.pone.0041883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang DD, Chen XX, Chen XY, Wang SL, Shen X, Chen XL, Yu Z, Zhuang CL. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol. 2016;142:2347–2356. doi: 10.1007/s00432-016-2230-4. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DE, D'Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci. 2013;68:317–323. doi: 10.1093/gerona/gls168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levolger S, van Vledder MG, Muslem R, Koek M, Niessen WJ, de Man RA, de Bruin RW, Ijzermans JN. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J Surg Oncol. 2015;112:208–213. doi: 10.1002/jso.23976. [DOI] [PubMed] [Google Scholar]
- 40.Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxford) 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, Young PM, Bakkum-Gamez JN, Langstraat CL, Dowdy SC, Jatoi A, Mariani A. Muscle composition measured by ct scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142:311–316. doi: 10.1016/j.ygyno.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Murton AJ, Maddocks M, Stephens FB, Marimuthu K, England R, Wilcock A. Consequences of late-stage non-small-cell lung cancer cachexia on muscle metabolic processes. Clin Lung Cancer. 2016;18:e1–e11. doi: 10.1016/j.cllc.2016.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data