Abstract
BACKGROUND. Topical calcipotriol plus 5-fluorouracil (5-FU) combination is an effective immunotherapy against actinic keratosis (AK), which is a precursor to squamous cell carcinoma (SCC). However, the long-term effectiveness of calcipotriol plus 5-FU treatment for SCC prevention is unknown.
METHODS. We performed a blinded prospective cohort study on participants of a randomized double-blind clinical trial in which a 4-day course of topical calcipotriol plus 5-FU combination was compared to Vaseline plus 5-FU (control) for AK treatment. SCC and basal cell carcinoma (BCC) incidences were assessed at 1, 2, and 3 years after trial. Tissues were analyzed for calcipotriol plus 5-FU–induced T cell immunity in the skin.
RESULTS. Calcipotriol plus 5-FU–induced tissue-resident memory T (Trm) cell formation in face and scalp skin associated with significantly higher erythema scores compared with control (P < 0.01). Importantly, more participants in the test cohort remained SCC-free over the more than 1,500-day follow-up period (P = 0.0765), and significantly fewer developed SCC on the treated face and scalp within 3 years (2 of 30 [7%] versus 11 of 40 [28%] in control group, hazard ratio 0.215 [95% CI: 0.048–0.972], P = 0.032). Accordingly, significantly more epidermal Trm cells persisted in the calcipotriol plus 5-FU–treated face and scalp skin compared with control (P = 0.0028). There was no significant difference in BCC incidence between the treatment groups.
CONCLUSION. A short course of calcipotriol plus 5-FU treatment on the face and scalp is associated with induction of robust T cell immunity and Trm formation against AKs and significantly lowers the risk of SCC development within 3 years of treatment.
FUNDING. This research was supported by internal academic funds and by grants from the Burroughs Wellcome Fund, Sidney Kimmel Foundation, Cancer Research Institute, and NIH.
Keywords: Dermatology, Oncology
Keywords: Adaptive immunity, Cancer immunotherapy, Skin cancer
Successful treatment of actinic keratoses with a short course of calcipotriol plus 5-FU immunotherapy results in a long-term reduced risk of squamous cell carcinoma.
Introduction
Cutaneous squamous cell carcinoma (SCC) is the second most common type of cancer (1). Despite recognition of ultraviolet exposure as a preventable risk factor for SCC, there has been a 100% increase in keratinocyte carcinomas (KCs), including SCC and basal cell carcinoma (BCC), and an increase in the SCC to BCC ratio from 1992 to 2012 in the United States population (2). SCC is associated with significant morbidity including ulceration and disfigurement, and a mortality rate similar to that of melanoma, particularly in solid organ transplant recipients (3–5). Treatments for SCC include surgery and radiation for localized disease, and chemotherapy, targeted therapy, and immunotherapy for unresectable and metastatic cancer (5–8). In addition to their side effects, the treatments for SCC and BCC represent a rising public health challenge, with more than $1 billion in total annual cost in the United States (9). Therefore, skin cancer prevention is an urgent unmet need.
Actinic keratosis (AK) is a common precursor to SCC that develops in sun-damaged skin (10). Treatment for AKs is recommended due to their premalignant nature (11). While an individual AK is estimated to have a less than 1% chance of progressing to SCC within 1 year, the majority of SCCs arise from existing AKs, highlighting their shared origin (12–14). Without intervention, patients with numerous AKs have a relatively high cumulative risk of developing skin cancer (11). Treatment options for AKs include therapies directed against individual lesions, such as cryotherapy, and field treatments including photodynamic therapy (PDT) and topical 5-fluorouracil (5-FU), imiquimod, diclofenac, and ingenol mebutate (15–17). Although they are effective in eliminating AKs, the ability of these cytotoxic and innate-immune-activating agents to prevent SCC in the long term is unknown. The only agent that has been shown to reduce the risk of SCC after treatment cessation is 5-FU, and this benefit is no longer apparent 2 years after treatment (18).
Previously, we discovered calcipotriol plus 5-FU combination as a novel topical immunotherapy for AKs (19). Calcipotriol is a low-calcemic vitamin D analog that is FDA approved for the treatment of psoriasis (20). Calcipotriol induces the expression of thymic stromal lymphopoietin (TSLP) cytokine in keratinocytes (19, 21, 22). In a randomized, double-blind clinical trial with 130 participants, we demonstrated that a 4-day course of 0.005% calcipotriol ointment plus 5% 5-FU cream was superior to Vaseline plus 5-FU in eliminating AKs (19). Calcipotriol plus 5-FU treatment induced the expression of TSLP which, together with cellular stress signals from the premalignant keratinocytes, led to a robust CD4+ T cell immunity against AKs and thus their elimination (19). While our clinical trial provided evidence that calcipotriol plus 5-FU combination is an effective immunotherapy for skin cancer precursors, it remained unknown whether this reduction in AKs would translate into a decreased risk of SCC over time.
Herein, we investigated the effectiveness of topical calcipotriol plus 5-FU treatment in preventing SCC development. We analyzed the clinical data and tissue samples obtained from the participants in our randomized controlled trial followed for over 1,500 days after treatment. We determined that a short course of calcipotriol plus 5-FU was associated with the induction of a tissue-resident memory T (Trm) cell response and a decrease in risk of SCC development on the face and scalp over a 3-year period following treatment.
Results
Study population.
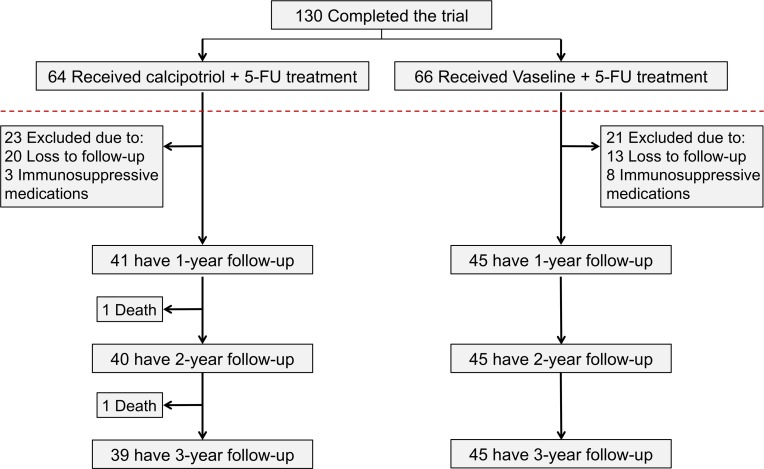
Among the 130 patients who completed the randomized controlled trial, 64 were in the calcipotriol plus 5-FU test arm and 66 were in the Vaseline plus 5-FU control arm. Of these, 33 patients (20 in the test arm and 13 in the control arm) were excluded from the present study because of loss to follow-up due to death, lack of subsequent dermatologic care, or inability to retrieve outside records. An additional 11 participants (3 in test arm and 8 in control arm) were excluded because of the use of immunosuppressive agents during the follow-up period. Two participants in the test arm had 536 and 889 days of follow-up before their death and were excluded from the 2/3–year and 3-year analyses, respectively. Thus, the final study population included 86 participants (41 test and 45 control) for 1-year outcomes, 85 participants (40 test and 45 control) for 2-year outcomes, and 84 participants (39 test and 45 control) for 3-year outcomes (Figure 1). For outcomes limited to the treated face and scalp, 72 participants (32 test and 40 control) were eligible.
Figure 1. CONSORT diagram of clinical trial followed by prospective cohort study.
Flow chart illustrates the number of clinical trial participants randomized to each treatment group who were included in analyses at 1, 2, and 3 years after treatment. Participants were excluded if they had fewer than 365 days of follow-up after the clinical trial or were immunosuppressed due to a medical condition or therapy during the follow-up period.
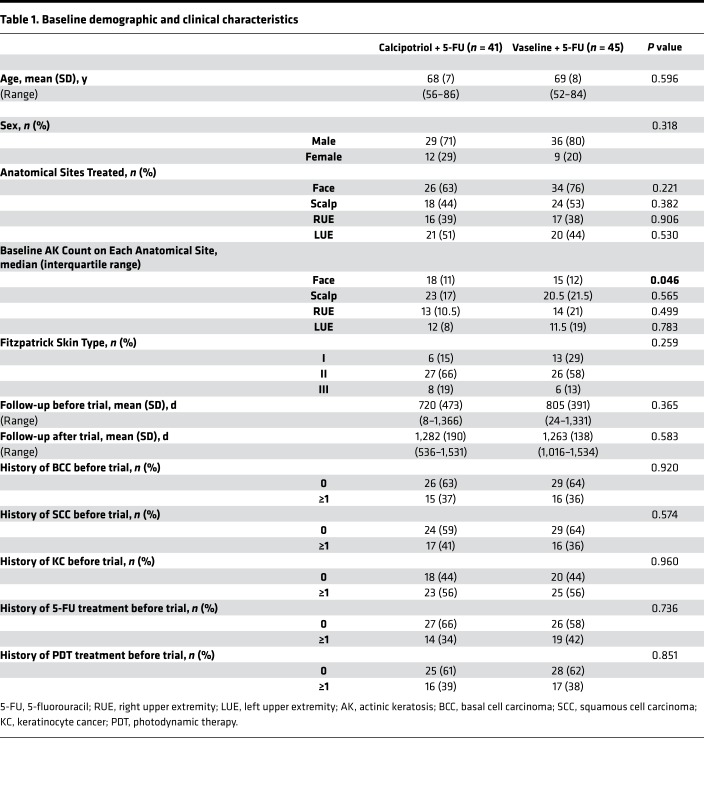
Demographic and clinical characteristics of test and control study participants (n = 86) were similar (Table 1). Participants in each treatment arm were similar in terms of age, skin type, and days of follow-up before and after the clinical trial. In both treatment arms, 56% of participants had a KC diagnosis within the period of interest before the trial. More specifically, 17 patients (41%) in the test arm and 16 patients (36%) in the control arm had a history of SCC during the period of interest. Nearly half of the participants also had a history of AK field treatment with 5-FU and/or PDT within the period of interest before the trial (Table 1). The treatment groups had similar AK counts on the scalp, right upper extremity (RUE), and left upper extremity (LUE) prior to initiating the trial (Table 1). The only statistically significant difference between groups was a higher baseline number of AKs on the face in the test group (Table 1). Demographic and clinical characteristics of the study participants who received treatment on the face and/or scalp (n = 72) were also similar between the 2 groups except for higher baseline AK counts on the face of the test group (Supplemental Table 1). There was no significant difference in the AK treatments that participants received after the trial (Supplemental Table 2).
Table 1. Baseline demographic and clinical characteristics.
Study outcomes.
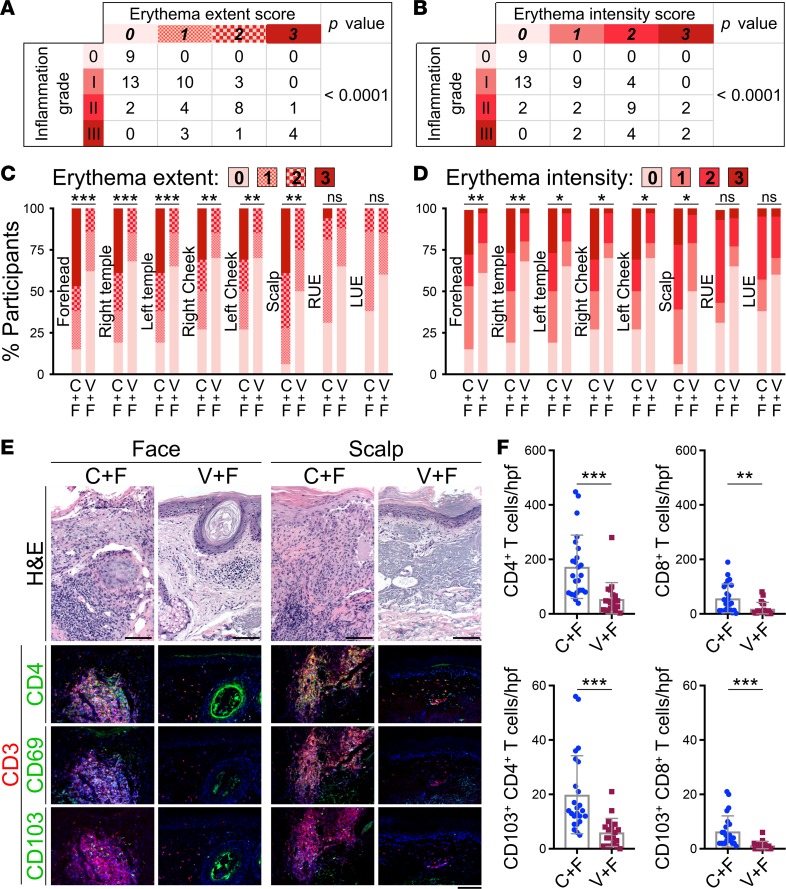
To investigate whether optimal immune activation upon calcipotriol plus 5-FU treatment was associated with long-term protection from skin cancer, we first examined whether the treatment-induced erythema extent and intensity on the treated anatomical sites could be used as a determinant of the immune activation against AKs (19). Among the prospective cohort study participants, the inflammation grades in the AK biopsies obtained 1 day after the treatment (Supplemental Figure 1) were tightly correlated with the erythema extent and intensity scores of the biopsied anatomical sites across the test and control groups (P < 0.0001; Figure 2, A and B). Next, we found that erythema extent and intensity were significantly higher on the treated face and scalp of the test compared with the control group (Figure 2, C and D). In contrast, no significant difference in erythema extent or intensity was observed between treatment groups on the treated upper extremities in our prospective cohort study participants (Figure 2, C and D). The high erythema scores on the face and scalp correlated with an intense CD4+ T cell response, which was much more robust than the CD8+ T cell response, and the induction of CD69+ and CD103+ Trm cells against the AKs on the test group’s face and scalp immediately after calcipotriol plus 5-FU treatment (Figure 2, E and F, and Supplemental Figure 2). Interestingly, CD4+ T cells colocalized with the premalignant keratinocytes in AKs, while CD8+ T cells were mostly found deep in the dermis, suggesting a bystander role for CD8+ T cells in the immunity induced by calcipotriol plus 5-FU against AKs (Supplemental Figure 3 and ref. 19).
Figure 2. Immune activation, erythema, and Trm induction by calcipotriol plus 5-FU treatment.
(A and B) The inflammation grades of AK biopsies are compared with the (A) erythema extent and (B) erythema intensity on the biopsied anatomical sites 1 day after completion of a 4-day treatment course. Note that the data include all participants in the prospective cohort study who contributed an AK biopsy during the clinical trial. P values were calculated by Fisher’s exact test. (C) Stacked bar chart compares the distribution of participants’ erythema extent in each treatment group for each treated anatomical site. **P < 0.001, ***P < 0.0001 by Fisher’s exact test. ns, not significant. (D) Stacked bar chart compares the distribution of participants’ erythema intensity in each treatment group for each treated anatomical site. *P < 0.01, **P < 0.001, ***P < 0.0001 by Fisher’s exact test. ns, not significant. (E) Representative histologic images show AKs that were biopsied on the face and scalp 1 day after treatment with calcipotriol plus 5-FU (C+F) or Vaseline plus 5-FU (V+F). Tissue samples were stained with H&E and with antibodies against CD3 plus CD4, CD69, or CD103 to detect induction of CD4+ T cell immunity and CD69+CD103+ Trm cells in the lesional skin. Erythema intensity associated with the site of the biopsies: face C+F, 3; face V+F, 0; scalp C+F, 2; scalp V+F, 1. Scale bars: 100 μm. (F) CD4+ T, CD8+ T, CD103+CD4+ Trm, and CD103+CD8+ Trm cells are quantified across 24 high-power field (hpf) images of 3 test (C+F) AKs and 20 hpf images of 3 control (V+F) AKs biopsied 1 day after treatment completion. **P < 0.001, ***P < 0.0001 by Wilcoxon’s rank-sum test.
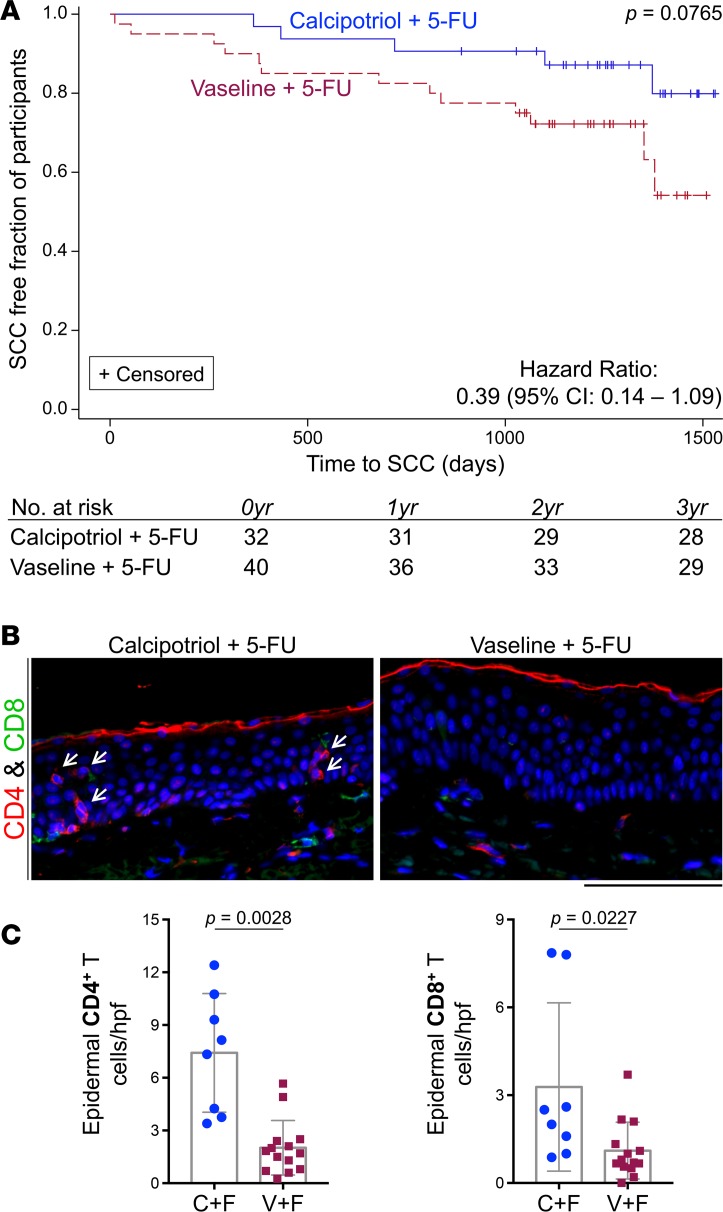
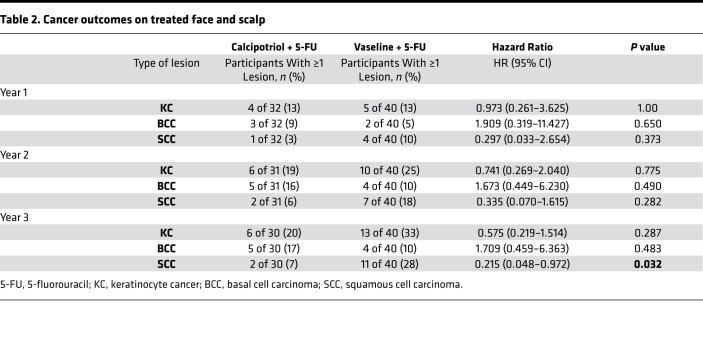
Consistent with this robust immune activation, Kaplan-Meier analysis revealed that a higher proportion of participants who received calcipotriol plus 5-FU treatment on their face and scalp remained SCC-free on these anatomical sites throughout the entire longitudinal study period, which reached greater than 1,500 days after the clinical trial initiation date. However, this trend did not reach statistical significance (P = 0.0765; Figure 3A). Importantly, only 2 out of 30 participants in the calcipotriol plus 5-FU group developed SCC on their treated face and scalp within 3 years following the clinical trial compared with 11 out of 40 in the control group (P = 0.032), corresponding to a hazard ratio (HR) of 0.215 (95% CI: 0.048–0.972; Table 2). There was no statistical difference in the percentage of patients in each group who developed SCC within 1 or 2 years after the trial, likely due to the paucity of events (Table 2).
Figure 3. Proportion of SCC-free participants on the treated face and scalp over time and the accompanying Trm cell persistence in the skin.
(A) Kaplan-Meier analysis shows that at any given time over more than 1,500 days of follow-up, a higher proportion of participants in the calcipotriol plus 5-FU treatment arm remained free of squamous cell carcinoma (SCC) on the treated face and scalp compared with controls. This trend approaches statistical significance. (B) Representative immunostaining images depict epidermal CD4+ and CD8+ Trm cells in the perilesional skin regions of AK-SCC spectrum biopsies obtained from the face and scalp of the participants after trial (arrows point to epidermal CD4+ Trm cells). Scale bar: 100 μm. (C) Bar graphs show the number of epidermal CD4+ and CD8+ Trm cells in the perilesional normal skin from 22 participants, counted blindly and averaged across 10 random hpf per sample. Note that every AK-SCC spectrum biopsy that was obtained from the face and scalp of the participants during the >3-year period after trial completion was included in this analysis. P values were calculated by Wilcoxon’s rank-sum test.
Table 2. Cancer outcomes on treated face and scalp.
In contrast to SCC risk reduction on the face and scalp, there was no significant difference in the percentage of participants who developed SCC or BCC across all the treated anatomical sites at 1, 2, or 3 years after the trial (Supplemental Figure 4 and Supplemental Table 3). In addition, there was no significant difference in the percentage of participants who developed BCC on their treated face and/or scalp within 1, 2 or 3 years after the trial (Table 2). Likewise, a similar proportion of participants in both groups remained without a BCC diagnosis on their treated face and/or scalp over the >1,500-day follow-up period after the trial (P = 0.1936; Supplemental Figure 5).
To explain the lack of treatment efficacy against BCC, we examined the immune microenvironment of BCCs that had been biopsied during routine clinical follow-up after the trial. In contrast to the substantial T cell infiltration detected in SCC, BCC from the same participant that developed on the same anatomical site (face) and was biopsied at the same time had minimal T cell infiltrate, which predominantly consisted of Foxp3+ regulatory T cells (Supplemental Figure 6). A T cell–poor tumor microenvironment was present in BCCs of the face and scalp in both the test and control participants compared with their AK-SCC spectrum counterparts after the trial (Supplemental Figure 7).
Trm cell persistence in the calcipotriol plus 5-FU–treated skin.
We performed immunostaining on the skin biopsies obtained for clinical diagnostic purposes after the trial to determine the contribution of the Trm cells to the chemopreventive effect of calcipotriol plus 5-FU treatment on the face and scalp. We obtained tissue sections from 22 participants who had been treated on the face or scalp during the trial and had AK-SCC spectrum biopsies from the treated sites obtained during routine clinic visits after the trial. Blinded analysis revealed markedly increased epidermal CD4+ Trm (7.4 versus 2 cells per high-power field [hpf] on average, P = 0.0028) and CD8+ Trm cells (3.3 versus 1.1 cells per hpf on average, P = 0.0227) in the perilesional normal skin of the test group compared with control group (Figure 3, B and C). As expected, the majority of epidermal T cells were CD69+ and/or CD103+, confirming their Trm status (Supplemental Figure 8 and ref. 23).
Discussion
Our prospective cohort study has revealed that a 4-day course of calcipotriol plus 5-FU immunotherapy, which effectively eliminated AKs in a double-blind randomized controlled trial (19), is associated with a reduced long-term risk of SCC. Prior to our findings, it had been reported that a 4-week course of topical 5-FU monotherapy for AK field treatment reduces SCC risk on the face and ears at 1 year following treatment; this effect, however, completely dissipates by 2 years (18). In contrast, our results suggest that calcipotriol plus 5-FU treatment is effective in preventing SCC development on the face and scalp within 3 years after the treatment. This chemopreventive effect is associated with the induction of a long-lasting T cell immunity in the skin. Therefore, our findings have broad implications by establishing a previously unrecognized concept that an immunotherapeutic agent effective in eliminating precancerous lesions can potentially yield long-term cancer prevention in humans.
By inducing the expression of TSLP in the skin, calcipotriol synergizes with the cytotoxic effects of 5-FU and leads to a robust CD4+ T cell immunity against skin carcinogenesis (19, 21, 22, 24). Accordingly, AKs biopsied after this combination treatment exhibit increased TSLP expression and massive CD4+ T cell infiltrates compared with AKs biopsied before treatment or after control treatment (19). Further, we have demonstrated that CD4+ T cell immunity induced by calcipotriol plus 5-FU treatment generates Trm cells associated with durable protection against SCC development. Following their induction by calcipotriol plus 5-FU treatment in the skin, CD4+ and CD8+ Trm cells persist in the epidermal niche. We propose that the effectiveness of this immunological memory is evident by the reduced 3-year risk of SCC on the face and scalp of our participants treated with calcipotriol plus 5-FU compared with Vaseline plus 5-FU.
The lack of protection against BCC development on the face and scalp after treatment with calcipotriol plus 5-FU can be attributed to the immune-based mechanism of this combination therapy. First, the 4-day course of calcipotriol plus 5-FU immunotherapy is directed against AKs, which carry a mutational burden and an antigenic composition similar to SCC (25). This is distinct from the mutations found in BCC, particularly the driver mutations in the sonic hedgehog signaling pathway (25). As such, the lack of protection against a genetically distinct cancer such as BCC could be anticipated given that the Trm cell response induced by calcipotriol plus 5-FU combination is likely antigen-specific. Second, BCCs that developed in our participants were immunologically cold and contained minimal effector T cell infiltrates compared with SCCs. This lack of immunogenicity plus the recognized immunosuppressive microenvironment including regulatory T cells surrounding BCCs (26) may contribute to the lack of efficacy observed for BCC immunoprevention.
Our results are consistent with previous murine studies in which treatment with calcipotriol was shown to reduce the number of new epithelial neoplasms and delay the onset of malignant tumor development in a TSLP-dependent manner (19, 24). Notably, the 4-day calcipotriol plus 5-FU treatment was most associated with a reduction in SCC development on the face and scalp, where it potently activated the adaptive immune response. In contrast, calcipotriol plus 5-FU treatment caused a modest increase in erythema extent and intensity on the upper extremities of the participants in our clinical trial (19). Erythema induced by calcipotriol plus 5-FU treatment is a manifestation of immune activation against premalignant keratinocytes (19). The more robust immune induction in the face and scalp upon calcipotriol plus 5-FU treatment is potentially due to a severely sun-damaged state of these anatomical sites resulting in a higher mutational burden that could be more readily targeted by T cells (25). Another possible explanation for this observed difference may relate to the higher penetration of topical medications into the skin of the face and scalp compared with that of the upper extremities (27). Therefore, a longer calcipotriol plus 5-FU treatment course may provide durable protection against SCC development on the upper extremities.
In conclusion, we report that a 4-day course of calcipotriol plus 5-FU immunotherapy directed against AKs on the face and scalp reduces the risk of SCC 3 years following treatment. The higher number of AKs on the face of the participants in the test group prior to the clinical trial suggests that participants in the test group may have had more sun damage and been at higher risk of developing SCC on the face at baseline, lending further support to the validity of our conclusions. A higher proportion of patients in the calcipotriol plus 5-FU treatment group remained SCC-free on their face and scalp at any given time point during the follow-up period compared with the control group. However, this trend did not reach statistical significance, likely due to our limited sample size and follow-up duration. Further studies are warranted to determine the optimal calcipotriol plus 5-FU treatment duration required to induce a chemopreventive effect at all anatomical sites at risk for SCC development. In addition, longer follow-up is required to determine whether the chemopreventive effect of calcipotriol plus 5-FU treatment extends beyond 3 years. Finally, it will be critical to examine the efficacy of calcipotriol plus 5-FU immunotherapy for skin cancer prevention in high-risk populations including organ transplant recipients. Despite these limitations, our study establishes calcipotriol plus 5-FU combination as a short, well-tolerated topical treatment with the potential to reduce the incidence of SCC, therefore addressing a major public health problem. These remarkable findings substantiate the use of immunotherapeutic agents with minimal side effects and high efficacy against precancerous lesions in order to reduce the risk of cancer development and recurrence, which may be broadly applicable to skin and internal malignancies.
Methods
Clinical study design
Clinical trial.
The current study includes a post hoc analysis of a double-blind, randomized controlled trial, which was conducted at Washington University Medical Center between October 2013 and March 2015 (ClinicalTrials.gov number NCT02019355; ref. 19). Study participants included 130 immunocompetent adults with 4 to 15 actinic keratoses within a 25-cm2 area on the face, scalp, RUE, and/or LUE. Blinded participants were randomized to self-administer a 4-day course of twice-daily 5% 5-FU cream mixed in equal parts with either Vaseline or 0.005% calcipotriol ointment applied to the entire surface area of their qualified anatomical sites. Clinical assessment for AK clearance, erythema, and adverse effects was performed on the day immediately after treatment (day 5) and at weeks 2, 4, and 8 (19). AK biopsies were performed prior to treatment and 1 day following treatment completion.
Prospective cohort study patient selection.
Postclinical trial study patient selection was performed between August 2017 and December 2017. The duration of follow-up after the trial was calculated for each patient as the number of days from the patient’s trial treatment start date to the most recent skin exam documented in the patient’s electronic medical record. For patients who indicated via phone interview that they had been diagnosed with skin cancer by a provider outside of the Washington University system, diagnoses were confirmed by clinical records and pathology reports. Patients who had less than 365 days of follow-up or who were immunocompromised due to a new medical condition or institution of an immunosuppressive drug at any point after the trial were excluded from analysis.
Study assessment
Clinical data analysis.
Data collection and analysis were performed at Washington University between September 2017 and September 2018. Demographic and clinical characteristics of the study population (age, sex, Fitzpatrick skin type, baseline AK counts per anatomical site, AK treatment history, SCC and BCC history) were extracted from medical records and trial data. A detailed skin cancer and AK treatment history was collected for the available follow-up period after clinical trial initiation. In order to compare baseline skin cancer risk and AK treatment history between the 2 treatment groups prior to the trial, the same information was collected for a similar number of days before trial initiation (rounded to the nearest clinic visit). Primary study outcomes included the presence or absence of a histopathologic diagnosis of primary SCC (including invasive and in situ lesions), BCC, and any KC (SCC or BCC), located in any of the anatomical sites (face, scalp, RUE, and LUE) that were treated during the clinical trial. Cancer outcomes were assessed in a blinded manner at 1, 2, and 3 years following the clinical trial initiation date and were characterized as binary variables (diagnosis of 0 versus ≥1 skin cancer in that category since the trial). The date of the first SCC diagnosis following the clinical trial was recorded for time-to-event analysis.
Tissue analysis.
Tissue analysis was performed at Massachusetts General Hospital between July 2018 and December 2018. To determine the inflammation grades and the induction of Trm cell formation following calcipotriol plus 5-FU versus Vaseline plus 5-FU treatment, we performed H&E and immunostaining of formalin-fixed paraffin-embedded 5-μm sections of the AK biopsy tissue obtained during the trial. To determine whether Trm cells persisted in the skin, we evaluated lesional and perilesional tissue from all trial participants who had been treated on the face and/or scalp during the trial and had an AK-SCC spectrum biopsy performed at the treated site during routine clinical follow-up at any time during the >3-year follow-up after the trial. For immunofluorescent staining, tissue sections were incubated with primary antibodies against CD3 (clone CD3-12, Abcam), CD4 (clone SP35, Spring Bioscience), CD8 (clone C8/144B, Cell Signaling Technology), CD69 (clone FN50, Biolegend), and CD103 [clone EPR4166(2), Abcam] followed by secondary antibodies. All slides were counterstained with DAPI nuclear stain (Thermo Fisher Scientific). CD4 and CD8 automated immunohistochemical staining was performed using a Ventana Ultra automated immunostainer (Ventana Medical Systems). Cell counts were performed blindly on randomly selected histological images at ×200 magnification (hpf).
Clinical study oversight
The clinical trial and prospective cohort study protocols were approved by the academic IRBs of Washington University in St. Louis, Missouri and Massachusetts General Hospital in Boston, Massachusetts. All participants provided written informed consent to participate in the clinical trial and were contacted via phone to obtain verbal informed consent for access to their protected health information (PHI) and tissue samples for this prospective cohort study. The study was conceived, designed, initiated, and performed by the academic investigators. The authors confirm the accuracy and completeness of the data and analysis and the fidelity of the study to the protocol. All authors agreed to submit the manuscript for publication.
Statistics
Baseline demographic and clinical characteristics were characterized by mean and standard deviation for continuous variables and by frequency distribution and percentage of total for categorical variables. SAS 9.4 (SAS Institute) was used to perform 2-sample Student’s t tests for continuous variables, Pearson’s χ2 tests and Fisher’s exact test for categorical variables, and time-to-event analysis for Kaplan-Meier curves. Wilcoxon’s rank-sum test was used as the test of significance for T cell counts. A P value less than 0.05 was considered significant.
Author contributions
LAC and SD conceived and designed the clinical study. ARR, MT, and KHN performed the clinical study and related data collection. ARR, LAC, and SD interpreted the data and wrote the manuscript. ISR contributed to clinical data. MW performed the statistical analysis.
Supplementary Material
Acknowledgments
We thank Barbara Gilchrest, David Fisher, Keith Flaherty, and Ethan Lerner for critically reading the manuscript. Shadmehr Demehri holds a Career Award for Medical Scientists award from the Burroughs Wellcome Fund. KHN and SD were supported by grants from the Burroughs Wellcome Fund, Sidney Kimmel Foundation, Cancer Research Institute and NIH (K08AR068619 and DP5OD021353).
Version 1. 03/21/2019
Electronic publication
Funding Statement
SD is supported by this grant.
SD is supported by this grant.
SD is supported by this grant.
SD is supported by this grant.
SD is supported by this grant.
Footnotes
Conflict of interest: LAC and SD are co-inventors on a filed patent for the use of calcipotriol plus 5-fluorouracil for the treatment of precancerous skin lesions (PCT/US2015/049434).
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(6):e125476. https://doi.org/10.1172/jci.insight.125476.
Contributor Information
Abby R. Rosenberg, Email: rosenberg.a@wustl.edu.
Mary Tabacchi, Email: mtabacchi@wustl.edu.
Michael Wallendorf, Email: mwallendorf@wustl.edu.
Ilana S. Rosman, Email: irosman@wustl.edu.
Lynn A. Cornelius, Email: lcorneli@dom.wustl.edu.
References
- 1.Cakir BÖ, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20(4):419–422. doi: 10.1016/j.fsc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Eigentler TK, Leiter U, Häfner HM, Garbe C, Röcken M, Breuninger H. Survival of patients with cutaneous squamous cell carcinoma: results of a prospective cohort study. J Invest Dermatol. 2017;137(11):2309–2315. doi: 10.1016/j.jid.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Clayman GL, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23(4):759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 5.Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363–374. doi: 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- 6.Migden MR, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 7.Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17(5):491–508. doi: 10.1007/s40257-016-0207-3. [DOI] [PubMed] [Google Scholar]
- 8.Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi: 10.1136/bmj.f6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48(2):183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012;122(2):464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JL. Actinic keratosis treatment as a key component of preventive strategies for nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2010;3(6):39–44. [PMC free article] [PubMed] [Google Scholar]
- 12.Criscione VD, et al. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 13.Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systematic review. Br J Dermatol. 2013;169(3):502–518. doi: 10.1111/bjd.12420. [DOI] [PubMed] [Google Scholar]
- 14.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795–797. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415. doi: 10.1002/14651858.CD004415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 Suppl 1):S28–S38. doi: 10.1016/j.jaad.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Sotiriou E, Apalla Z, Vrani F, Lallas A, Chovarda E, Ioannides D. Photodynamic therapy vs. imiquimod 5% cream as skin cancer preventive strategies in patients with field changes: a randomized intraindividual comparison study. J Eur Acad Dermatol Venereol. 2015;29(2):325–329. doi: 10.1111/jdv.12538. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock MA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154(2):167–174. doi: 10.1001/jamadermatol.2017.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham TJ, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127(1):106–116. doi: 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce DJ, Camacho F, Balkrishnan R, Fleischer AB, Feldman SR. Trends in on and off-label calcipotriene use. J Dermatolog Treat. 2006;17(5):308–313. doi: 10.1080/09546630600813659. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103(31):11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato-Deguchi E, Imafuku S, Chou B, Ishii K, Hiromatsu K, Nakayama J. Topical vitamin D3 analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167(1):77–84. doi: 10.1111/j.1365-2133.2012.10917.x. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe R, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7(279):279ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22(4):494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martincorena I, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omland SH, Nielsen PS, Gjerdrum LM, Gniadecki R. Immunosuppressive environment in basal cell carcinoma: the role of regulatory T cells. Acta Derm Venereol. 2016;96(7):917–921. doi: 10.2340/00015555-2440. [DOI] [PubMed] [Google Scholar]
- 27.Rougier A, Dupuis D, Lotte C, Roguet R, Wester RC, Maibach HI. Regional variation in percutaneous absorption in man: measurement by the stripping method. Arch Dermatol Res. 1986;278(6):465–469. doi: 10.1007/BF00455165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.