Abstract
Chemoresistance in cancer is linked to a subset of cancer cells termed “cancer stem cells” (CSCs), and in particular, those expressing the CD44 variant appear to represent a more aggressive disease phenotype. Herein, we demonstrate that CD44v6 represents a CSC population with increased resistance to chemotherapeutic agents, and its high expression is frequently associated with poor overall survival (OS) and disease-free survival (DFS) in patients with colorectal cancer (CRC). CD44v6+ cells showed elevated resistance to chemotherapeutic drugs and significantly high tumor initiation capacity. Inhibition of CD44v6 resulted in the attenuation of self-renewal capacity and resensitization to chemotherapeutic agents. Of note, miRNA profiling of CD44v6+ spheroid-derived CSCs identified a unique panel of miRNAs indicative of high self-renewal capacity. In particular, miR-1246 was overexpressed in CD44v6+ cells, and associated with poor OS and DFS in CRC patients. We demonstrate that CD44v6+ CSCs induced chemoresistance and enhance tumorigenicity in CRC cells, and this was in part orchestrated by a distinct panel of miRNAs with dysregulated profiles. These findings suggest that specific miRNAs could serve as therapeutic targets as well as promising prognostic biomarkers in patients with colorectal neoplasia.
Keywords: Gastroenterology, Oncology
Keywords: Cancer
MicroRNA profiling of sphere-derived colorectal cancer stem cells identified a unique panel of miRNAs indicative of high self-renewal capacity.
Introduction
Colorectal cancer (CRC) is the third most common cancer and ranks as the second leading cause of cancer-related deaths in the United States (1, 2). One of the major underlying causes of increased mortality in CRC patients is the intrinsic resistance to chemotherapeutic drugs. Accordingly, the therapeutic response rates from current treatment regimens in patients with advanced CRC are quite poor (3, 4), and patients who are initially responsive will eventually acquire secondary resistance to these anticancer drugs. Emerging evidence indicates that a subset of cancer cells termed “cancer stem cells” (CSCs) are one of the primary mediators that contribute to such chemoresistance (5, 6). Historically, CSCs were considered to be a small population of cells with high self-renewal capacity, resulting in the initiation of cancer. However, the discovery of interconversion between CSCs and non-stem cancer cells has broadened the definition of CSCs, and these are now being recognized as a “dynamic” cell population that is modulated by a combination of genetic, epigenetic, and microenvironmental factors (7). Not surprisingly, there is a lack of consensus on universally acceptable molecular markers that can definitively identify CSC populations in human cancers. While several putative molecular CSC markers are widely used in CRC, these typically identify nonspecific CSC populations (8). Interestingly, CSC markers such as CD44+ and CD133+ appear to identify a subset of CSCs with higher metastatic potential (9, 10), suggesting the existence of a unique subpopulation of cancer cells within the CSC pool.
CD44 is a cell-surface glycoprotein involved in cell-cell interaction, adhesion, and migration (11). The human CD44 gene comprises 19 exons, among which 10 can be alternatively spliced to generate multiple variant isoforms (12, 13). Strikingly, these CD44 variants are frequently overexpressed in human tumors (14–18), including CRC (18). Furthermore, the overexpression of CD44v4–10, but not of parent CD44, in ApcMIN mice has been shown to promote adenoma initiation, highlighting that its alternately spliced variant forms have important oncogenic functions (19). Interestingly, while different splice variants are overexpressed in each cancer type, variant 6 (CD44v6) has long been identified as the primary variant in CRC, appears to be involved in metastatic processes (20, 21), and was recently recognized as a CRC-specific CSC marker (22).
Specific targeting of CSCs is a well-established modern therapeutic concept (23), and it has been postulated that reversible epigenetic regulators such as miRNAs are potential therapeutic candidates for the targeting of CSCs (24, 25). miRNAs are 18 to 25 nucleotide-long noncoding RNAs that play a major role in the regulation of self-renewal and cellular differentiation (26). For instance, transcription factors required for the reprogramming of pluripotent cells (Yamanaka factors) can be fully substituted with a group of miRNAs (27); and at the same time, key tumor-suppressor miRNAs such as miR-34a and miR-145, can cause the differentiation of embryonic stem cells by suppressing Yamanaka factors and hence backsliding of pluripotency (28). Despite the widespread acceptance for the biological roles of miRNAs in CSC self-renewal, it remains unclear whether they participate in clinically important processes such as drug resistance (29, 30). Herein, we interrogate this hypothesis and report that CD44v6 is a key CD44 variant that is frequently overexpressed in spheroid-derived CSCs (SDCSCs), and in patients high expression of CD44v6 significantly associates with poor survival outcomes. Furthermore, CD44v6 represents a unique subset of the CSC population and confers higher stemness and increased resistance to chemotherapeutic drugs in this malignancy. In an effort to understand the role of miRNAs, if any, in this process, small RNA expression profiling of CD44v6+ CSCs identified a unique miRNA expression pattern indicative of enhanced stemness-like features. Finally, we discovered that upregulated miR-1246 expression in CRC patients serves as a potentially important prognostic biomarker in this disease.
Results
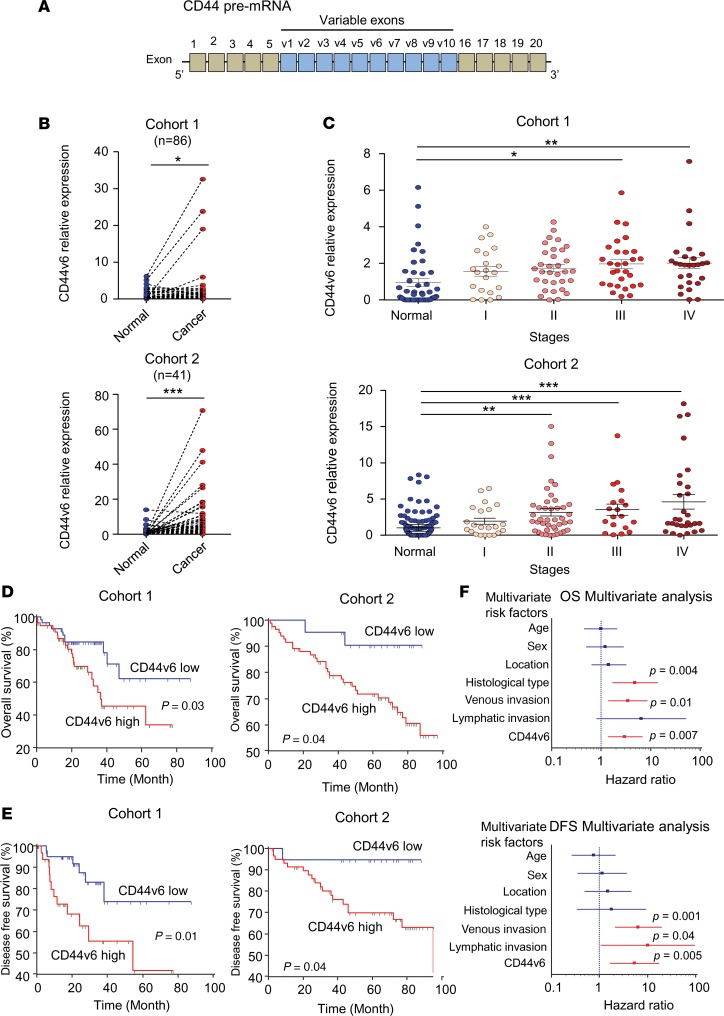
CD44v6 is frequently overexpressed and associates with poor prognosis in CRC patients.
The human CD44 gene comprises 19 exons, among which up to 10 are commonly alternatively spliced, resulting in the generation of multiple variant isoforms (12, 13) (Figure 1A). In particular, CD44v6 was recently recognized as one of the key isoforms that function as a CSC marker in CRC (22). Therefore, in order to understand its clinical significance, if any, we first examined whether the expression of CD44v6 is associated with clinical prognostic factors, as suggested previously (31). Using 2 independent cohorts of matched cancer and adjacent normal mucosa tissues, we found that CD44v6 is frequently overexpressed in tumor versus normal tissues (cohort 1, P < 0.05; cohort 2, P < 0.001; Figure 1B). We next evaluated the expression of CD44v6 between early- (stages I and II) and late-stage (stages III and IV) cancers using Fisher’s exact test, and observed that late-stage cancers had a significantly higher proportion with high expression of CD44v6, in both patient cohorts (cohort 1: CD44v6 low [17/53] vs. CD44v6 high [34/58] P < 0.01 and cohort 2: CD44v6 low [25/61] vs. CD44v6 high [28/45] P < 0.05, respectively; Figure 1C). These results are in line with a previous study that identified that CD44v6 was upregulated in advanced CRCs (18). Furthermore, in both cohorts, patients with tumors with high CD44v6 expression exhibited worse overall survival (OS) (both cohorts P < 0.05) and disease-free survival (DFS) (P = 0.01, P < 0.05, respectively; Figure 1, D and E). To evaluate the prognostic biomarker potential of CD44v6, we performed multivariate Cox regression analysis for survival outcomes in cohort 1 to determine whether CD44v6 is an independent prognostic factor for OS and DFS. Interestingly, we observed that CD44v6 emerged as an independent prognostic factor for both OS (HR: 3.04; CI: 1.35–6.85; P = 0.007) and DFS (HR: 5.30, CI: 1.64–17.12, P = 0.005), along with poor differentiation (HR: 4.82, CI: 1.66–14.05, P = 0.004) and presence of venous invasion (HR: 3.47, CI: 1.39–8.66, P = 0.01; Figure 1F and Table 1). Collectively, these data highlight that overexpression of CD44v6 is frequent in advanced CRCs, as well as its importance as a potential prognostic biomarker in this disease.
Figure 1. CD44v6 is a prognostic biomarker in CRC.
(A) Schematic representation of CD44 exons. (B) Gene expression of CD44v6 in matched cancer and adjacent normal mucosa tissues in 2 independent cohorts (Mann-Whitney U test). (C) CD44v6 gene expression categorized by tumor stages (cohort 1: n = 184, cohort 2: n = 150, 1-way ANOVA). (D) Kaplan-Meier analysis for overall survival (OS) in 2 independent cohorts (log-rank test). (E) Kaplan-Meier analysis of disease-free survival (DFS) in 2 independent cohorts (log-rank test). (F) Forest plots illustrate the Cox hazard proportional analysis based on overall survival and DFS stratified by CD44v6 expression and clinical factors. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1. Multivariate analysis for predictors of overall survival and disease-free survival in cohort 1.
CD44v6 expression is upregulated in colorectal CSCs, and mediates increased chemoresistance.
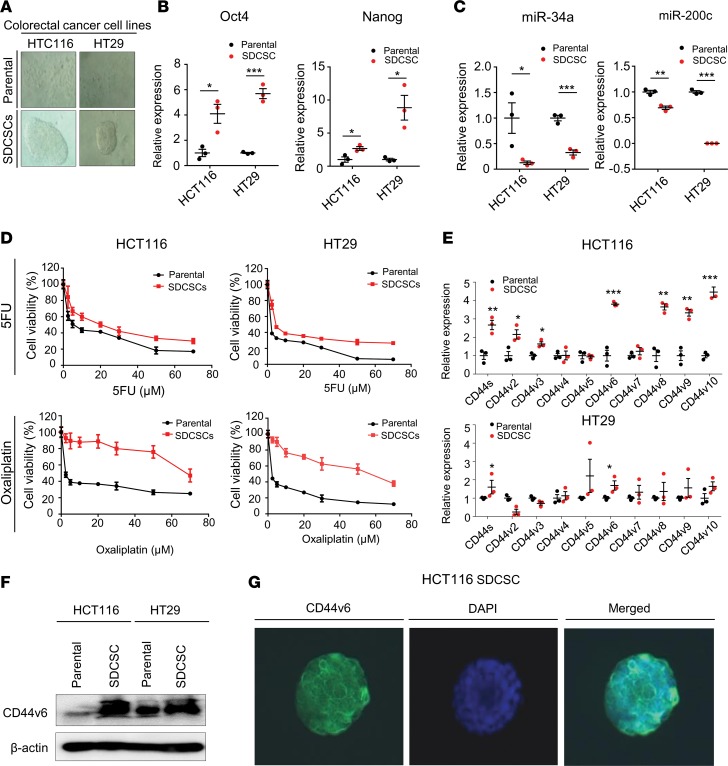
One of the primary reasons for the poor survival in patients with CRC is the primary or secondary resistance to chemotherapeutic drugs; a process that is intimately linked to the existence of CSCs (5, 6). Because CD44v6 was recently proposed to be CRC stem cell marker (32), we examined whether there were differences in chemotherapeutic resistance in parental CRC cell lines with regard to enriched cells with a higher stem cell fraction. Accordingly, we cultured CRC cell lines as spheroids — a well-established approach for the enrichment of CSC-like cells (33, 34). To ensure representation from both microsatellite-stable (MSS) and microsatellite-unstable (MSI) phenotypes, we generated SDCSCs from HCT116 (MSI) and HT29 (MSS) cell lines (Figure 2A). The expression of the stem cell markers OCT4 and Nanog was significantly increased in SDCSCs versus respective parental cell lines (all P < 0.05, Figure 2B). Furthermore, the expression of 2 major stemness-suppressive miRNAs, miR-34a and miR-200c (35, 36), was significantly downregulated in SDCSCs (all P < 0.05), demonstrating that these cells have altered epigenetic profiles, which encompasses the inhibition of stemness-suppressive miRNAs (Figure 2C). Collectively, based on the expression of these molecular markers, compared with parental cells, SDCSCs appear to have an increased stem cell population.
Figure 2. SDCSCs have high CD44v6 expression and increased tolerance to chemotherapeutic drugs.
(A) Images of sphere-derived cancer stem cells (SDCSCs) and their respective parental cells. (B) Gene expression of pluripotency markers Oct4 and Nanog in SDCSCs and their parental cell lines (n = 3, Mann-Whitney’s U test). (C) Expression of the stemness-suppressive miRNAs miR-34a and miR-200c in SDCSCs and their parental cell lines (n = 3, Mann-Whitney U test). (D) Cytotoxicity from various doses of 5FU (top 2 panels) and oxaliplatin (bottom 2 panels) was assessed for SDCSCs and their respective parental cells lines, HCT116 and HT29. (E) Relative gene expression of CD44 variants in SDCSCs and their parental cell lines (n = 3, Mann-Whitney U test). (F) CD44v6 protein expression in SDCSCs and their parental cell lines, HCT116 and HT29. (G) Immunofluorescence assay of CD44v6 in SDCSCs from HCT116 cells with FITC staining (left) and DAPI nuclear staining (middle); a merged image is shown on the right. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we interrogated whether SDCSCs exhibit enhanced resistance to commonly used chemotherapeutic agents (5-fluorouracil [5FU] and oxaliplatin). The cytotoxicity of both chemotherapeutic agents was significantly attenuated in SDCSCs compared with that in the parental cells (all P < 0.05, Figure 2D), validating a higher tolerance of CSCs to cytotoxic chemotherapy. To examine the CD44 variants that are overexpressed in CSCs, we compared the expression of CD44 variants in SDCSCs concurrently with their respective parental cells. We observed that CD44v6 transcripts were consistently overexpressed in both cell types (both P < 0.05, Figure 2E), which was subsequently confirmed by corresponding changes in protein expression in Western immunoblotting and immunofluorescence experiments (Figure 2, F and G). Another key feature of CSCs is their high capacity to initiate tumor formation; hence, we used a xenotransplantation assay to evaluate their tumor-initiating capacity. While at higher concentrations of cells, differences between SDCSCs and parental cells were negligible, upon implantation of 1 × 103 cells, SDCSCs generated a markedly greater number of xenograft tumors than the parental cells, confirming their higher tumor-initiating capacity (Supplemental Table 2; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.125294DS1). Taken together, these findings highlight that CSCs express high levels of CD44v6, possess enhanced resistance to chemotherapeutic agents, and have an increased ability to form tumors.
While spheroids provide a useful model for studying CSCs, their inability to grow efficiently as a monolayer limits their use for functional evaluations. Therefore, to investigate the link between CSCs and chemoresistance, we generated 2 5FU-resistant (5FUR) cell lines from HCT116 and SW480 by continuously culturing these cells with increasing concentrations of 5FU over 9 months (37) (Supplemental Figure 1A). We found that 5FUR cells morphologically resembled cells with a mesenchymal origin (Supplemental Figure 1B) and had a higher tolerance to 5FU (Supplemental Figure 1C). Considering that previous studies have shown that 5FUR CRC cells have a higher expression of CSC markers such as CD44 and CD133 (38, 39), we examined whether CD44 variants were also overexpressed in our chemoresistant cell lines. While mRNA of most variants was overexpressed in the chemoresistant cells, we observed significantly higher overexpression of CD44 variants 4 and 6 in the 5FUR cells (both P < 0.05, Supplemental Figure 1D). When we examined expression of CD44 variants in both 5FUR cells and SDCSCs, we found that CD44v6 was the most consistently overexpressed variant in both cell types (Supplemental Figure 1E). To confirm that CD44v6 protein was overexpressed in the 5FUR cell lines as well, Western blotting and immunofluorescence analyses were undertaken, which substantiated our observation of increased expression of this variant in 5FUR cells (Supplemental Figure 1, F and G).
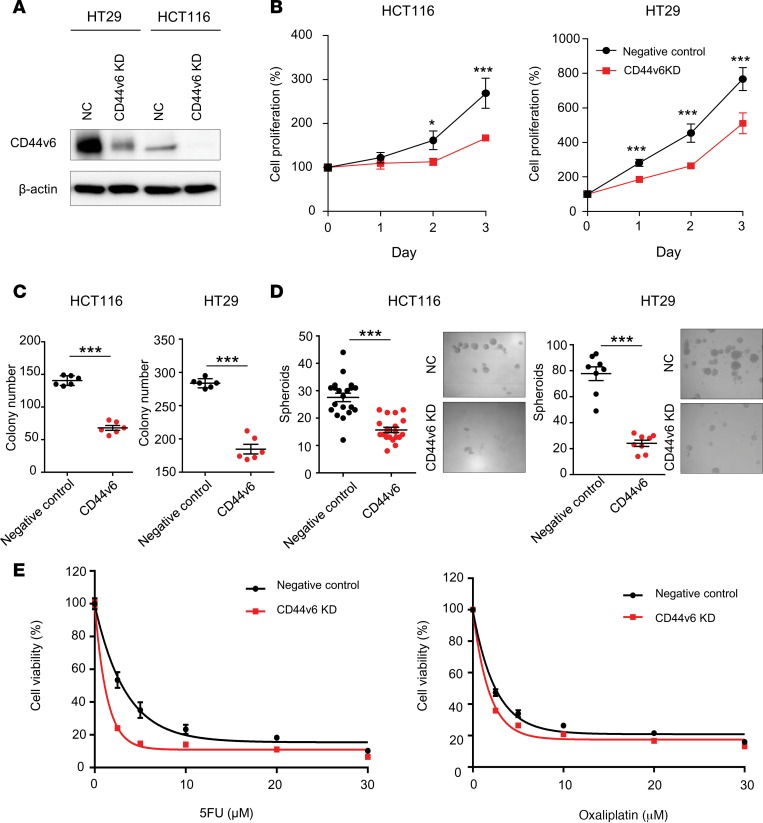
Downregulation of CD44v6 results in decreased stemness and enhanced sensitivity to chemotherapeutic drugs.
Considering that CD44v6 appears to be involved in the development of drug resistance in CSCs, we examined its functional role in CRC cell lines by inhibiting its expression (Figure 3A) using a previously validated siRNA (40). We wanted to ascertain whether suppression of CD44v6 alters key oncogenic traits in CSCs, such as cellular proliferation or colony-forming capacity. The inhibition of CD44v6 resulted in significantly reduced cellular proliferation and colony-forming capacity in both HCT116 and HT29 cell lines (all P < 0.05, Figure 3, B and C). To determine whether CD44v6 modulates the formation and proliferation of CSCs, we examined the spheroid-forming capacity of CD44v6-knockdown cells versus the parental cells. We found that the inhibition of CD44v6 suppresses spheroid-forming capacity, supporting the role of CD44v6 in tumor initiation and CSC expansion (both P < 0.001, Figure 3D). Next, we examined whether the inhibition of CD44v6 alters sensitivity to the chemotherapeutic drugs, and observed that the suppression of CD44v6 resulted in increased sensitivity to 5FU and oxaliplatin in both HCT116 and HT29 cell lines (P < 0.05, parental versus CD44v6-knockdown for doses from 2.5 to 10 μM; Figure 3E). Collectively, these findings indicate that CD44v6 regulates response to chemotherapeutic agents, in part through the regulation of cancer stemness.
Figure 3. Suppression of CD44v6 results in reduced oncogenicity and resistance to chemotherapeutic agents.
(A) CD44v6 protein expression following transient knockdown (KD) in HT29 and HCT116 cell lines. NC, negative control. (B) Cell proliferation of CD44v6 KD cells and parental cell lines (n = 8 per time point, Mann-Whitney U test). (C) Clonogenicity of HCT116 and HT29 cells with and without KD of CD44v6 (n = 6, Mann-Whitney U test). (D) Spheroid formation capacity of HCT116 and HT29 cells with and without KD of CD44v6 (HCT116: n = 20, HT29: n = 8, Mann-Whitney U test). (E) Cytotoxicity of various doses of 5FU (left) and oxaliplatin (right) was assessed for HCT116 cells with and without KD of CD44v6. *P < 0.05, ***P < 0.001.
CD44v6-positive SDCSCs demonstrate higher tumorigenicity and increased resistance to chemotherapeutic drugs.
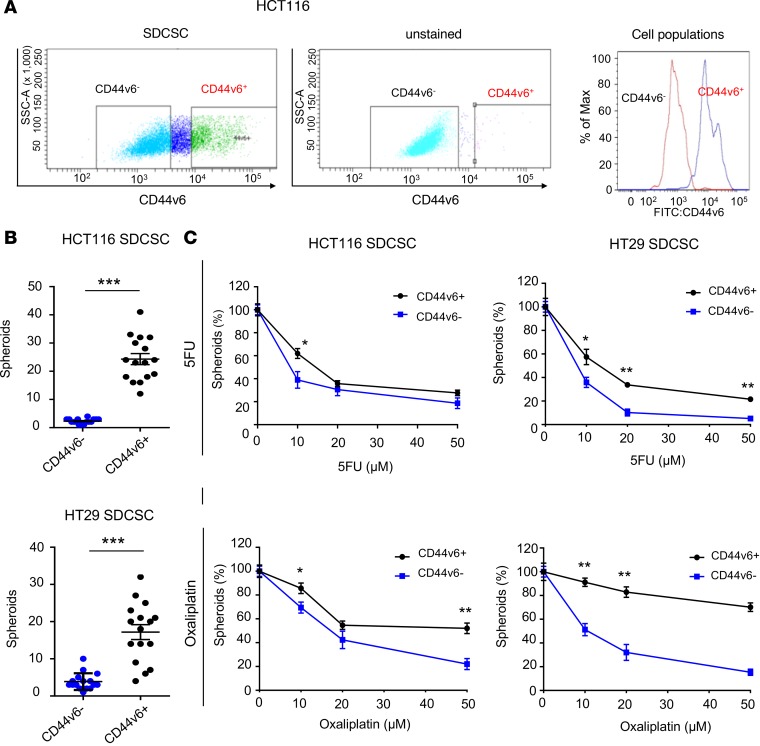
Next, we investigated whether increased resistance to chemotherapeutic agents by CSCs is due to CD44v6 expression or is simply a result of their stem cell–like characteristics. Using a fluorescence-conjugated CD44v6 antibody, we sorted SDCSCs into CD44v6+ and those lacking the protein (CD44v6−, Figure 4A) and examined whether these 2 subpopulations of SDCSCs have distinct functional attributes. Interestingly, CD44v6+ SDCSCs showed markedly higher spheroid-forming capacity than CD44v6− cells (both cell lines P < 0.001), indicating that SDCSCs with high expression of CD44v6 have more pronounced stem cell–like characteristics (Figure 4B). We thereafter assessed how the presence of 5FU or oxaliplatin might impact the spheroid-forming capacity of the 2 subpopulations of SDCSCs. We found that CD44v6+ SDCSCs derived from both cell lines formed markedly increased numbers of spheroids as well in the presence of 5FU or oxaliplatin than CD44v6− SDCSCs (both cell lines, both drugs, P <0.05, Figure 4C). In addition, xenotransplantation assays showed that CD44v6+ SDCSCs had a markedly higher ability to generate tumors compared with CD44v6− cells, indicating that CD44v6+ cells are CSCs with higher tumor initiation capacity (Supplemental Table 3). In summary, we demonstrate that CSCs that express CD44v6 are substantially less sensitive to chemotherapeutic drugs and show greater tumorigenicity than those that do not express CD44v6.
Figure 4. CD44v6+ compared with CD44v6– sphere-derived cancer stem cells have higher stemness.
(A) FACS analysis of CD44v6-positive and -negative sphere-derived cancer stem cells (SDCSCs) derived from a HCT116 cell line. (B) Spheroid-forming capacity of HCT116- and HT29-derived CD44v6+ and CD44v6– SDCSCs (n = 16, Mann-Whitney U test). (C) Spheroid-forming ability of HCT116 (left) and HT29 (right) CD44v6+ and CD44v6– SDCSCs in the presence of 5FU (top) and oxaliplatin (bottom; n = 6 per time point, Mann-Whitney U test). *P < 0.05, **P < 0.01, ***P < 0.001.
CD44v6 SDCSCs possess a distinct miRNA expression profile.
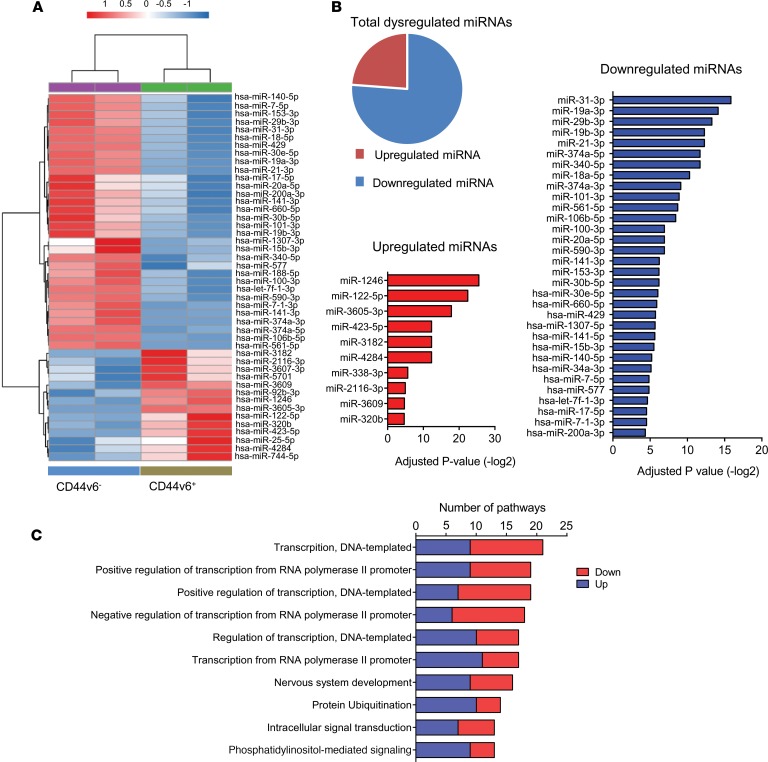
Since CD44v6 expression is associated with a higher resistance to chemotherapeutic agents, we performed small RNA sequencing for CD44v6+ and CD44v6− cells to examine whether any distinct molecular signatures can help explain these differences in chemotherapeutic tolerance (Figure 5A). In total, 42 differentially expressed miRNAs were identified, of which 10 were upregulated and 32 were downregulated (Figure 5B and Supplemental Table 4). Interestingly, several putative tumor-suppressor miRNAs that inhibit stemness and developmental pathways were identified in the list of downregulated miRNAs (28, 41, 42) (Figure 5B). These miRNAs included miR-101-3p, miR-34a-3p, let-7f-1-3p, and several miR-200 family members (miR-141-3p, miR-141-5p, miR-200a-3p, and miR429). Therefore, while expression levels of these stemness-suppressive miRNAs were generally downregulated in the total SDCSC population, their expression was significantly lower in CD44v6+ SDCSCs (all P < 0.05), indicating that these CSCs have an epigenetic profile indicative of their greater stemness. To establish their functional roles, we predicted putative target genes for the differentially expressed miRNAs using TargetScan and subsequently assessed the associated pathways through KEGG analysis (Figure 5C and Supplemental Figure 2). In addition, we analyzed associated pathways for both upregulated and downregulated miRNAs in the CD44v6+ population independently (Supplemental Figure 3, A and B). Interestingly, several transcription-associated pathways were found to be affected by miRNAs dysregulated in the CD44v6+ CSC population. Considering that miRNAs themselves are posttranscriptional regulators of gene expression, the identification of multiple transcription-associated pathways affected by this collection of miRNAs highlights the importance of these miRNAs in gene regulation within the cancer stem cells.
Figure 5. CD44v6+ sphere-derived cancer stem cells have a distinct miRNA profile.
(A) Heatmap of differentially expressed miRNAs between CD44v6+ and CD44v6– sphere-derived cancer stem cells (SDCSCs). (B) List of upregulated and downregulated miRNAs ranked by P values. (C) List of pathways identified by KEGG analysis based on predicted downstream target genes for differentially expressed miRNAs between CD44v6+ and CD44v6– SDCSCs. Down, downregulated; Up, upregulated.
CD44v6-associated miR-1246 as a potential prognostic biomarker in CRC patients.
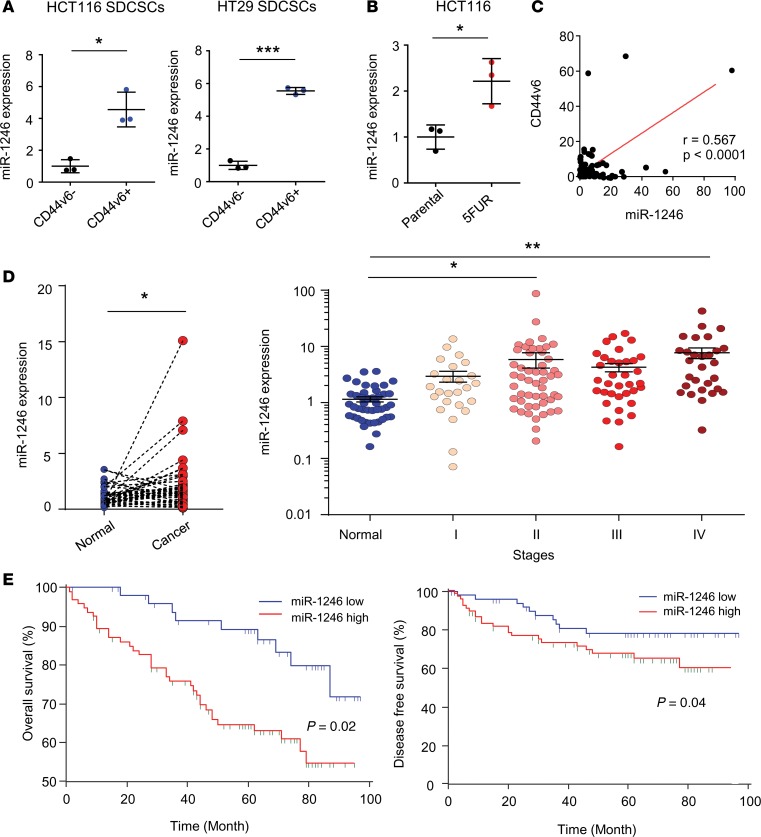
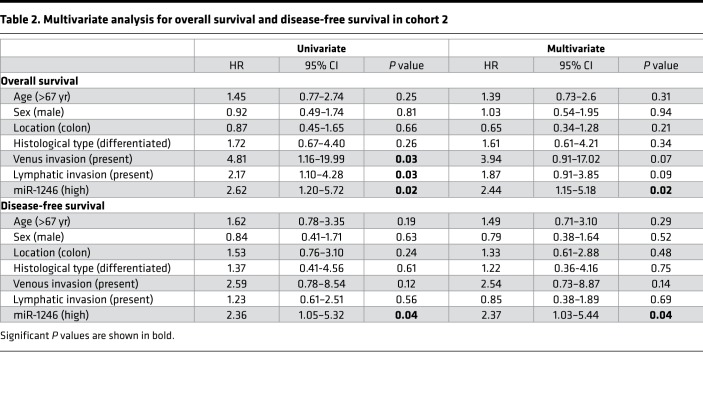
When we examined the list of miRNAs differentially expressed between CD44v6+ and CD44v6− SDCSCs, we identified miR-1246 as the most significantly overexpressed miRNA in CD44v6+ SDCSCs (Figure 5, A and B). Interestingly, miR-1246 has been classified as a putative oncogenic miRNA that promotes stemness and enhances drug resistance in other cancer types (43, 44). To evaluate its role in CRC, we first validated its overexpression by quantitative PCR (qPCR) in CD44v6+ SDCSCs (Figure 6A), as well as in the 5FUR cell lines (Figure 6B). Next, we investigated the clinical significance of miR-1246 in CRC patients by examining whether its expression correlated with CD44v6 status in CRC specimens. Intriguingly, Pearson’s correlation analysis in CRC specimens demonstrated that miR-1246 and CD44v6 were positively correlated with one another (Figure 6C; r = 0.567, P < 0.0001). To further evaluate the clinical significance of this CSC-related miRNA, we compared its expression levels in CRC and normal tissues, and found that this putative oncogene was significantly overexpressed in CRC tissues (P < 0.05, Figure 6D). Furthermore, when comparing the expression of miR-1246 in different stages of CRC in cohort 2, we noted that it appeared to increase with increasing cancer stage, with the highest overexpression in stage IV CRCs (P < 0.01, Figure 6D). To examine whether expression of miR-1246 also had any prognostic potential in patients with CRC (as was the case with CD44v6 overexpression), Kaplan-Meier survival analysis revealed that patients with high miR-1246 expression exhibited worse OS and DFS (both P < 0.05, Figure 6E). Furthermore, we used Cox regression multivariate analysis to examine whether miR-1246 was an independent prognostic factor for OS and DFS. Intriguingly, the analysis revealed that miR-1246 was an independent prognostic factor for both OS (HR: 2.44, CI: 1.15–5.18, P = 0.02) and DFS (HR: 2.37, CI: 1.03–5.44, P = 0.04; Table 2). Collectively, these data highlight the clinical importance of this miRNA, which was systematically identified from molecular profiling of CSCs, and its potential as a prognostic biomarker in CRC patients.
Figure 6. miR-1246 is overexpressed in CD44v6+ CSCs and is a prognostic biomarker for CRC.
(A) Expression of miR-1246 in CD44v6+ and CD44v6– sphere-derived cancer stem cells (SDCSCs) derived from HCT116 and HT29 cell lines (n = 3, Mann-Whitney U test). (B) Expression of miR-1246 in 5FUR and parental HCT116 cell line (n = 3, Mann-Whitney U test). (C) Correlation analysis between CD44v6 and miR-1246 expression in CRC tissue from clinical cohort 1. (D) miR-1246 expression in cancer tissues versus adjacent normal mucosa (left), and in normal tissue and CRC at different stages (right; 1-way ANOVA). (E) Kaplan-Meier analysis of overall survival and disease-free survival in cohort 2 (log-rank test). *P < 0.05, **P < 0.01, ***P < 0.001.
Table 2. Multivariate analysis for overall survival and disease-free survival in cohort 2.
Discussion
CD44v6 is a well-recognized CRC-associated oncogene that was recently identified to be a marker of CSCs, and has been postulated to be involved in metastatic processes (22). Herein, we for the first time to our knowledge systematically evaluated its biological and clinical significance, and confirmed that CD44v6 is frequently overexpressed in advanced CRCs and that patients with higher expression of CD44v6 demonstrate significantly worse prognosis. We thereafter used a series of experiments to demonstrate that CD44v6 confers chemotherapeutic resistance in CRC, and this effect was in part attributable to CSC-like characteristics. Subsequently, we reveal that a subset of CSCs with high levels of CD44v6 display marked resistance to chemotherapeutic agents and that these cells exhibit a unique miRNA profile, which includes downregulation of several putative stemness-suppressive miRNAs. We finally identified miR-1246 as one of the most differentially overexpressed miRNAs in CD44v6+ CSCs, and that patients with increased expression of miR-1246 demonstrated poor OS and DFS — highlighting that CSC-associated miRNA could serve as a promising prognostic biomarker in CRC patients.
Accumulating evidence indicates that CSCs play a major role in multiple processes of cancer progression, including metastasis and drug resistance (5, 6). A recent study demonstrated the prominence of stem cell features in metastatic tumors and that stemness was associated with oncogenic dedifferentiation (45). Accordingly, we have focused specifically on variants of CD44, which have long been recognized as one of several bona fide stem cell markers (46). Several recent studies have also suggested that variants of CD44 appear to play critical roles in cancers in general (19, 21). In particular, CD44v6 is postulated to be a key regulator of tumor progression and metastasis in CRC and was recently identified as a CRC CSC marker (22). While there is an unclear understanding on the clinical significance of CD44v6, a recent meta-analysis showed that overexpression of CD44v6 correlates with worse prognosis (31). In line with these findings, using 2 independent clinical patient cohorts, we demonstrated that CD44v6 is frequently overexpressed in late-stage CRCs and high expression of this variant is associated with worse OS and DFS. From a mechanistic standpoint, we demonstrated that downregulation of CD44v6 enhances sensitivity to chemotherapeutic drugs. Consistent with our results, overexpression of CD44v6 has been shown to attenuate apoptotic responses to both 5FU and oxaliplatin in a colon cancer cell line (47). Our present study for the first time to our knowledge demonstrates that a subpopulation of CSCs marked by the overexpression of CD44v6 displays higher sphere-forming capacity, as well as increased resistance to chemotherapeutic drugs. Based on the miRNA and gene expression profiles derived from differentially expressed miRNAs using a predictive algorithm, CD44v6+ cells appear to have distinct functional properties. Collectively, these findings indicate that not all CSCs contribute equally to drug resistance or metastatic processes, and targeting of a specific CSC subpopulation may constitute a more effective means for a cancer therapeutic strategy. In fact, an inhibitor of CD44v6 has already been shown to be effective in a preclinical model of pancreatic cancer (48), which could be used to target a subset of aggressive CSCs.
One of the key findings of the present study is that the miRNA expression profiling of CD44v6+ CSCs showed a distinct epigenetic profile with downregulation of several well-characterized cancer stemness-suppressive miRNAs, including miR-101-3p, miR-34a-3p, let-7f-1-3p, and several miR-200 family members (miR-141-3p, miR-141-5p, miR-200a-3p, and miR-429) (28, 41, 42). The inhibition of these stemness-suppressive miRNAs in CD44v6+ CSCs is consistent with our in vitro data showing that the CD44v6+ subpopulation exhibits significantly higher sphere-forming capability, as well as enhanced resistance to chemotherapeutic agents. With a growing interest in miRNA-based cancer therapies, replenishing these downregulated miRNAs might be a potential therapeutic strategy in eradicating the highly oncogenic CSC populations (24, 49). In addition, we identified several unique miRNAs that were overexpressed in CD44v6+ CSCs. One such miRNA, miR-1246, which was previously reported to be oncogenic and promoted stemness and enhanced drug resistance (43, 44), was significantly overexpressed in CD44v6+ CSCs. It has also been reported that miR-1246 is overexpressed in cancer exosomes and promotes cellular motility and invasiveness, highlighting its functional importance (50). We demonstrated that the expression of miR-1246 correlates with CD44v6 in clinical specimens, and Cox regression analysis showed that miR-1246 was an independent prognostic factor for both OS and DFS and appeared to be involved in self-renewal processes. Although further validation is required using a larger clinical cohort, our data suggest that stemness-associated miRNAs could be used as potential targets for CRC prognosis. In addition, we have identified several potential pathways, including pathways associated with gene transcription, regulated by miRNAs in the CD44v6+ population. Considering that miRNAs are involved in the transcriptional regulation of gene expression, identification of these pathways highlights the importance of these miRNAs in gene regulation within cancer stem cells. Furthermore, we demonstrated that miR-1246, one of most highly differentially expressed miRNAs in the CD44v6+ population, is involved in self-renewal processes and can be used as a promising prognostic biomarker in CRC patients.
In conclusion, we demonstrate that the CD44v6 variant is an important regulator of cancer stemness and resistance to chemotherapeutic agents in CRC. In particular, CD44v6+ CSCs exhibit higher resistance to chemotherapeutic agents and increased self-renewal capability, which correlates with a unique miRNA expression profile. Taken together, these findings imply that miRNAs dysregulated in CRC could be targeted as important prognostic biomarkers, as well as potential therapeutic targets in this malignancy.
Methods
Cell lines and materials.
The CRC cell lines HCT116, HT29, and SW480 were purchased from ATCC, and 5FUR cell lines were established by a method described previously (51). The cells were grown in DMEM (Gibco) with 10% FBS and 1% penicillin-streptomycin and maintained at 37°C in a humidified incubator at 5% CO2. CRC SDCSCs were generated from HCT116 and HT29 cell lines in serum-free medium (DMEM/F12) supplemented with B27 and N2 supplements (Gibco), 10 ng/ml human recombinant basic FGF (Gibco), and 20 ng/ml EGF (Sigma-Aldrich) cultured in a Costar Ultra-Low attachment flask (Corning). 5FU and oxaliplatin (Sigma-Aldrich) were dissolved in DMSO and diluted to appropriate experimental concentrations with the culture medium.
Cell sorting.
SDCSCs derived from HCT116 and HT26 cell lines were stained with CD44v6-FITC monoclonal antibody (catalog MA5-16966; Invitrogen), and propidium iodide was used to eliminate dead cells. Cell sorting was performed using a BD FACSAria II (BD Biosciences) with a 100-μm nodule. The number of sorted cells was monitored with an antibody specific for CD44v6-FITC, and stained cells were deemed to be positive for CD44v6.
Cell viability, colony formation, and spheroid formation assays.
Cells were incubated with various concentrations of 5FU or oxaliplatin for 72 hours in 96-well plates, and cell proliferation was measured using an MTT assay, as described previously (52). For the comparison between parental cells and SDCSCs, we cultured the same number of parental cells and SDCSCs in stem cell medium in 96-well plates. For the spheroid formation assay, HCT116 and HT29 cells were dissociated into single cells using TrypLE (Gibco), filtered using a 40-μm cell strainer (Corning), and seeded in Ultra-Low attachment 96-well plates (Sigma-Aldrich) in serum-free stem cell medium. Spheroids were treated with 5FU or oxaliplatin 24 hours after seeding. Spheres were counted using a light microscope (Olympus) following 4-day incubation.
Gene expression, miRNA expression, and transfection analyses.
Total RNA was extracted using miRNeasy Mini Kits (QIAGEN) following the manufacturer’s instructions. For the analysis of mRNA expression, 1 μg of total RNA was reverse transcribed to complementary DNA using an Advantage RT-for-PCR kit (Clontech Laboratories Inc.). Power SYBR Green (Applied Biosystems) real-time PCR was performed using a StepOnePlus system (Applied Biosystems). The primers used were as follows: OCT4 (forward: 5′-ACATCAAAGCTCTGCAGAAAGAACT, reverse: 5′-CTGAATACCTTCCCAAATAGAACCC); Nanog (forward: 5′-CCGAAGAATAGCAATGGTGTGACG, reverse: 5′-AGGAGAATTTGGCTGGAACTGC); and GAPDH (forward: 5′-ACCCAGACTGTGGTATGG, reverse: 5′-CAGTGAGCTTCCCGTTCAG). qPCR for CD44 variants was performed in accordance with a previously described method (53). All qPCR gene expression was normalized to the expression of GAPDH and analyzed using the ΔΔCt method. For miRNA expression analysis, we used a TaqMan Real-Time PCR Assay Kit (Applied Biosystems) and TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems), as described previously (54). The expression of all miRNAs was analyzed using the ΔΔCt method and normalized using RNU6B for cell lines and miR-16 for clinical samples. All TaqMan primers were purchased from Ambion. To inhibit the expression of CD44v6, siRNA was used in HCT116 and HT29 cell lines using siRNA sequences described previously (40) with siPort NeoFX (Gibco) transfection reagent, in accordance with the manufacturer’s instructions.
Western immunoblotting.
Western immunoblotting experiments were performed as described previously (55). Cells were treated with various concentrations of 5FU or oxaliplatin for 24 hours and lysed using 100 μl of 1× SDS sample buffer containing β-mercaptoethanol. The primary antibodies used were a monoclonal mouse anti–human CD44v6 antibody (ab78960; Abcam) and a monoclonal rabbit anti–human BMI1 antibody (6964P; Cell Signaling Technology). Anti-mouse IgG or anti-rabbit IgG secondary antibodies were purchased from Santa Cruz Biotechnology Inc. A monoclonal mouse β-actin antibody (Sigma-Aldrich) was used as the loading control.
Xenotransplantation assay.
Five-week-old male athymic nude mice (Harlan Laboratories) were housed under 12-hour controlled conditions of light and fed ad libitum. The SDCSCs and CRC cell lines, at various concentrations (102 to 105 cells), were injected subcutaneously into the abdominal flanks of the mice, and tumor development was assessed weekly, for up to 2 months.
Clinical specimens.
A cohort of 344 fresh frozen tissue specimens comprising 127 normal mucosa and 217 CRC tissues were included in this study. These tissues were collected from patients enrolled at Mie University Hospital, Tsu, Japan, and the Tokyo Medical Dental University, Tokyo, Japan. Detailed information on patient demographics and clinicopathological characteristics is provided in Supplemental Table 1.
Small RNA sequencing and miRNA analysis.
Next-generation sequencing library construction for miRNAs from tissue was performed using a TruSeq Small RNA Kit (Illumina) with up to 1 μg total RNA input, in accordance with the manufacturer’s protocol. The quality of individual libraries was assessed using a High Sensitivity DNA Kit (Agilent). Libraries were pooled together prior to size selection (~148 nt) by gel electrophoresis using a Pippin HT instrument (Sage Science Inc.). The efficiency of size selection was assessed using the High Sensitivity DNA Kit, and the pooled libraries were thereafter quantified by qPCR using a KAPA Library Quantification Kit, Universal (KAPA Biosystems), prior to sequencing on an Illumina HighSeq 2500 (Illumina) instrument, with single-end 35-base read lengths. Small RNA-Seq data were processed as described previously (56). In brief, the raw sequence image files from the Illumina HiSeq 2500 in the bcl format were converted to the fastq format using CASAVA v1.8.2, and the adapters from the 3′ end were clipped using cutadapt v.1.10 (http://cutadapt.readthedocs.io/en/stable/guide.html). Additionally, all of the reads shorter than 15 nt and 3′ bases below a quality score of 30 were discarded. sRNABench (http://bioinfo5.ugr.es/sRNAbench) was used to map the reads to RNA libraries, and alignments were performed with Bowtie v0.12.9. The algorithm used in sRNABench was based on mirAnalyzer, where the reads with the same sequence were collapsed and mapped to the human genome and the miRBase v21 miRNA database (http://www.mirbase.org/). Differentially expressed miRNAs were identified using DESeq2 (57) and selected based on adjusted P values (P < 0.05), absolute value of log2 fold change >1, and a base mean value >20. Gene ontology (GO) biological process pathways (http://geneontology.org) for the network figure (Supplemental Figure 2) were selected in the following way: for each dysregulated miRNA, the list of target transcripts annotated by TargetScan version 7.1 (http://www.targetscan.org/vert_71/) was uploaded into the Database for Annotation, Visualization and Integrated Discovery (https://david.ncifcrf.gov) for functional annotation. A GO pathway was selected if it was significant (P ≤ 0.05) across all up- or downregulated miRNAs. These criteria resulted in the final selection of 15 GO pathways. The miRNA expression sequencing data from our study have been deposited in the GEO public domain with the accession number GSE125904.
Statistical analysis.
Differences between groups were evaluated by the Mann-Whitney U test (2-tailed), Fisher’s exact test, and 1-way ANOVA, as appropriate. For time-to-event analyses, survival estimates were calculated using Kaplan-Meier analysis, and groups were compared with the log-rank test. The thresholds between low and high expression groups for CD44v6 and miR-1246 were defined by Youden’s index. OS was based on the time assessed from the date the patient underwent surgery until the date of death resulting from any cause, or last known follow-up for patients who were still alive. DFS was based on the time measured from the date the patient underwent curative surgery to the date of disease recurrence, death from any cause, or last contact with the patient. In univariate and multivariate analyses, a Cox proportional hazards model was used to estimate HRs and 95% CI for death or recurrence. Assumption of proportionality were confirmed for the Cox proportional hazards analyses by generating Kaplan-Meier survival curves (e.g., high- vs. low-expression groups) and by ensuring that the 2 curves did not intersect each other. All P values were 2-sided, and those less than 0.05 were considered statistically significant. All error bars in the figures represented mean ± SEM.
Study approval.
Written informed consent was obtained from all patients, and the Institutional Review Boards of Mie University Hospital and the Tokyo Medical Dental University approved the study. The animal protocol was approved by the Institutional Animal Care and Use Committee of the Baylor Scott & White Research Institute.
Author contributions
ST and AG conceived and designed the study. ST and SK acquired data.ST, SK, EH, JC, JG, KVKJ, YT, and AG analyzed and interpreted data (e.g., statistical analysis, biostatistics, computational analysis). ST, SK, and AG wrote, reviewed, and/or revised the manuscript. AG, YT, and HU provided administrative, technical, or material support. AG supervised the study.
Supplementary Material
Acknowledgments
We thank Margaret M. Hinshelwood, Baylor Scott & White Research Institute, Charles A. Sammons Cancer Center at Dallas, for critical suggestions and editing, further improving the quality of this article. We also thank Tadanobu Shimura, Baylor Scott & White Research Institute, Charles A. Sammons Cancer Center at Dallas, for technical assistance. This work was supported by grants CA72851, CA184792, CA202797 and CA187956 from the National Cancer Institute, NIH; RP140784 from the Cancer Prevention Research Institute of Texas; and grants from the Baylor Foundation and Baylor Scott & White Research Institute.
Version 1. 03/21/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(6):e125294. https://doi.org/10.1172/jci.insight.125294.
Contributor Information
Shusuke Toden, Email: Shusuke.toden@gmail.com.
Jacob Cardenas, Email: jacob.cardenas@bswhealth.org.
Jinghua Gu, Email: jinghua.gu@bswhealth.org.
Kendall Van Keuren-Jensen, Email: kjensen@tgen.org.
Hiroyuki Uetake, Email: h-uetake.srg2@tmd.ac.jp.
Yuji Toiyama, Email: ytoi0725@clin.medic.mie-u.ac.jp.
Ajay Goel, Email: Ajay.Goel@baylorhealth.edu.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Eng J Med. 2005;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 3.Giacchetti S, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18(1):136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 5.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120(1):41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 7.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Chu P, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int J Cancer. 2009;124(6):1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 11.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39(6):527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 12.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 13.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279(26):26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 14.Koopman G, et al. Activated human lymphocytes and aggressive non-Hodgkin’s lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med. 1993;177(4):897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heider KH, et al. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol. 1993;120(1):227–233. doi: 10.1083/jcb.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura Y, Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-Z. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe KK, Ellis LM, Saya H. Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet. 1993;341(8847):725–726. doi: 10.1016/0140-6736(93)90490-8. [DOI] [PubMed] [Google Scholar]
- 18.Wielenga VJ, et al. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53(20):4754–4756. [PubMed] [Google Scholar]
- 19.Zeilstra J, et al. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene. 2014;33(5):665–670. doi: 10.1038/onc.2012.611. [DOI] [PubMed] [Google Scholar]
- 20.Gotley DC, Fawcett J, Walsh MD, Reeder JA, Simmons DL, Antalis TM. Alternatively spliced variants of the cell adhesion molecule CD44 and tumour progression in colorectal cancer. Br J Cancer. 1996;74(3):342–351. doi: 10.1038/bjc.1996.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder JW, et al. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344(8935):1470–1472. doi: 10.1016/S0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 22.Todaro M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14(3):342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007;21(14):3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, et al. MicroRNAs and cancer stem cells: the sword and the shield. Oncogene. 2014;33(42):4967–4977. doi: 10.1038/onc.2013.492. [DOI] [PubMed] [Google Scholar]
- 25.Widschwendter M, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39(2):157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 26.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi N, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Jain AK, et al. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012;10(2):e1001268. doi: 10.1371/journal.pbio.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar FH, Li Y, Wang Z, Kong D, Ali S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat. 2010;13(3):57–66. doi: 10.1016/j.drup.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep. 2017;7(1):814. doi: 10.1038/s41598-017-00812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8(5):486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bu P, et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell. 2013;12(5):602–615. doi: 10.1016/j.stem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toden S, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36(3):355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallas NA, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69(5):1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paschall AV, et al. CD133+CD24lo defines a 5-Fluorouracil-resistant colon cancer stem cell-like phenotype. Oncotarget. 2016;7(48):78698–78712. doi: 10.18632/oncotarget.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20(13):1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konno Y, et al. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5(15):6049–6062. doi: 10.18632/oncotarget.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 43.Zhang WC, et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun. 2016;7:11702. doi: 10.1038/ncomms11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa S, et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br J Cancer. 2014;111(8):1572–1580. doi: 10.1038/bjc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malta TM, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173(2):338–354.e15. doi: 10.1016/j.cell.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, et al. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. 2014;146(4):1108–1118. doi: 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lv L, et al. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumour Biol. 2016;37(7):8811–8824. doi: 10.1007/s13277-015-4755-6. [DOI] [PubMed] [Google Scholar]
- 48.Matzke-Ogi A, et al. Inhibition of tumor growth and metastasis in pancreatic cancer models by interference with CD44v6 signaling. Gastroenterology. 2016;150(2):513–25.e10. doi: 10.1053/j.gastro.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi RU, Miyazaki H, Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2016;6:38750. doi: 10.1038/srep38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl Oncol. 2009;2(4):321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi M, et al. The clinical significance of MiR-148a as a predictive biomarker in patients with advanced colorectal cancer. PLoS ONE. 2012;7(10):e46684. doi: 10.1371/journal.pone.0046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Chen K, Jiang P, Zhang X, Li X, Li Z. CD44v/CD44s expression patterns are associated with the survival of pancreatic carcinoma patients. Diagn Pathol. 2014;9:79. doi: 10.1186/1746-1596-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hur K, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62(9):1315–1326. doi: 10.1136/gutjnl-2011-301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jascur T, Fotedar R, Greene S, Hotchkiss E, Boland CR. N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) triggers MSH2 and Cdt2 protein-dependent degradation of the cell cycle and mismatch repair (MMR) inhibitor protein p21Waf1/Cip1. J Biol Chem. 2011;286(34):29531–29539. doi: 10.1074/jbc.M111.221341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeri A, et al. Total Extracellular small RNA profiles from plasma, saliva, and urine of healthy subjects. Sci Rep. 2017;7:44061. doi: 10.1038/srep44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.