Abstract
Heart failure with reduced ejection fraction (HFREF) increases neutral sphingomyelinase (NSMase) activity and mitochondrial reactive oxygen species (ROS) emission and causes diaphragm weakness. We tested whether a systemic pharmacological NSMase inhibitor or short-hairpin RNA (shRNA) targeting NSMase isoform 3 (NSMase3) would prevent diaphragm abnormalities induced by HFREF caused by myocardial infarction. In the pharmacological intervention, we used intraperitoneal injection of GW4869 or vehicle. In the genetic intervention, we injected adeno-associated virus serotype 9 (AAV9) containing shRNA targeting NSMase3 or a scrambled sequence directly into the diaphragm. We also studied acid sphingomyelinase-knockout mice. GW4869 prevented the increase in diaphragm ceramide content, weakness, and tachypnea caused by HFREF. For example, maximal specific forces (in N/cm2) were vehicle [sham 31 ± 2 and HFREF 26 ± 2 (P < 0.05)] and GW4869 (sham 31 ± 2 and HFREF 31 ± 1). Respiratory rates were (in breaths/min) vehicle [sham 61 ± 3 and HFREF 84 ± 11 (P < 0.05)] and GW4869 (sham 66 ± 2 and HFREF 72 ± 2). AAV9-NSMase3 shRNA prevented heightening of diaphragm mitochondrial ROS and weakness [in N/cm2, AAV9-scrambled shRNA: sham 31 ± 2 and HFREF 27 ± 2 (P < 0.05); AAV9-NSMase3 shRNA: sham 30 ± 1 and HFREF 30 ± 1] but displayed tachypnea. Both wild-type and ASMase-knockout mice with HFREF displayed diaphragm weakness. Our study suggests that activation of NSMase3 causes diaphragm weakness in HFREF, presumably through accumulation of ceramide and elevation in mitochondrial ROS. Our data also reveal a novel inhibitory effect of GW4869 on tachypnea in HFREF likely mediated by changes in neural control of breathing.
Keywords: ceramide, mitochondria, myocardial infarction, oxidants, skeletal muscle, tachypnea
INTRODUCTION
Heart failure with reduced ejection fraction (HFREF) causes diaphragm weakness that contributes to an abnormal breathing pattern, exercise intolerance, cardiovascular pathophysiology, and morbidity of disease (28, 32). However, there are no specific therapies for diaphragm weakness in HFREF. The only strategies currently available to circumvent the loss of diaphragm force in HFREF are inspiratory muscle and endurance training (14, 42). These approaches elicit beneficial effects (11), but are limited by patient compliance and supervision by qualified personnel and may not treat the specific diaphragm abnormality that results in weakness. This lack of specific therapies targeting the diaphragm is in part a consequence of limited understanding of the mechanisms leading to loss of force in HFREF.
HFREF increases the activity of the enzyme neutral sphingomyelinase (NSMase) in the diaphragm (19). Sphingomyelinases are a class of enzymes that catalyze the conversion of sphingomyelin into phosphocholine and ceramide (46, 49). Accordingly, HFREF promotes accumulation of ceramides in the diaphragm (19). Exogenous sphingomyelinase and ceramide cause diaphragm weakness (9, 23). However, the role of neutral sphingomyelinase on ceramide accumulation and diaphragm weakness in HFREF is unknown.
A common aspect of diaphragm weakness induced by HFREF and sphingomyelinase is the role of reactive oxygen species (ROS). Acute exposure to antioxidant compounds in vitro or knockout of ROS-producing enzymes prevent diaphragm weakness caused by recombinant sphingomyelinase (9, 23, 24, 38). Diaphragm from HFREF rats show that heightened mitochondrial ROS emission (35, 57) and chronic antioxidant treatment, either nonspecific (57) or targeting mitochondria (35), prevent diaphragm weakness in HFREF. In this context, actin oxidation has been proposed as putative mediator of diaphragm weakness due to excess ROS in HFREF (10).
In this study, we completed experiments to test the hypothesis that systemic administration of a pharmacological NSMase inhibitor prevents diaphragm weakness in HFREF. Our studies supported our hypothesis, which led us to conduct experiments with a genetic intervention to test the specific role of diaphragm NSMase on excess mitochondrial reactive oxygen species emission and loss of force in HFREF. There are three isoforms of NSMase expressed in the diaphragm, NSMase1, NSMase2, and NSMase3 (48). NSMase3 is the most abundant isoform in diaphragm from rodents and humans and mediates the increase in myocyte reactive oxygen species elicited by cytokines (48). Therefore, we targeted NSMase3 in our genetic intervention. NSMase3-knockout animals are not available. Hence, we used intra-diaphragm injection of recombinant adeno-associated virus (rAAV) to achieve selective transduction in the diaphragm tissue (54). An alternative mechanism of muscle weakness in HFREF is an elevated activity of secretory (circulating) sphingomyelinase (17), which is a product of the acid sphingomyelinase gene. To gain further insights into specific sphingomyelinases involved in the diaphragm dysfunction of HFREF, we conducted studies in acid sphingomyelinase knockout (ASMase−/−) mice.
METHODS
Animals, Surgical Procedures, and Interventions
All procedures performed in animals followed guidelines established by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Florida. Animals were housed at our institution’s facilities under a 12-h:12-h light-dark cycle with access to standard chow and water ad libitum. Our studies involved 100 male Wistar rats and 29 male mice, as detailed below. We used our previous data (3, 19) to determine the number of animals per group required to detect a 10–15% difference in maximal diaphragm force. Our analyses yielded sample size estimates of n = 3–7 animals/group.
Experiments focused on pharmacological targeting of NSMase included 36 male Wistar rats (12 wk old at time of surgery). Rats underwent either a myocardial infarction (MI; n = 25) or sham (n = 11) surgery. Eight weeks postsurgery, animals underwent echocardiography and were allocated into treatment groups. Treatment consisted of daily intraperitoneal injection of the neutral sphingomyelinase inhibitor GW4869 (1.5 mg·kg body wt−1·day−1, 5% DMSO in sterile saline; Cayman Chemicals, Ann Arbor MI) or vehicle (5% DMSO in sterile saline), and the intervention lasted 8–9 wk. The protocol for GW4869 preparation and in vivo injection was similar to that described previously (58).
In the genetic intervention, we used 64 male Wistar rats (13 sham, 51 MI) aged 16 wk at the time of surgery. Immediately before sham or MI surgeries, animals underwent a laparotomy for intradiaphragm injections of adeno-associated virus serotype 9 [adeno-associated virus (AVV9), 1 × 1011 vg/animal in 400–500 µl of sterile saline] that contained a U6 promoter, an eGFP segment, and either small-hairpin RNA (shRNA) targeting NSMase3 (SMPD4) mRNA or a scrambled sequence. The AAV9 was obtained from Vector Biolabs (SMPD4 shRNA sequence: VBLKO124164). The NSMase3 shRNA sequence was based on mouse NSMase3 mRNA (GenBank RefSeq NM_029945) and anticipated to also target rat NSMase3 mRNA based on sequence homology between the species. In pilot studies, we determined that intradiaphragm viral injection transduced 75–95% of the fibers, depending on the region (see example in results). These findings are consistent with previous data (54). In both pharmacological and genetic interventions, the inclusion criteria for HFREF rats in our studies was fractional shortening ≤ 40%, which corresponded to the mean − 2 × SD of the sham groups, as in our previous study (35).
To perform laparotomy and thoracotomy, rats were initially anesthetized with a 5% isoflurane-oxygen mixture. Immediately following induction of anesthesia and preparation of the skin, animals were intubated by mouth, placed on mechanical ventilation connected to a rodent respirator (Model 686, Harvard Apparatus), and maintained on a 1.5–3% isoflurane-oxygen mixture. We verified that animals were in the surgical plane of anesthesia immediately before and during the procedures. Using aseptic surgical procedures, we made incisions through the skin and abdominal wall. The abdominal wall was retracted laterally, and abdominal organs were carefully separated from the diaphragm. While the xyphoid process was lifted, the costal diaphragm was directly injected using a 27-gauge sterile tuberculin syringe (BD Cat. No. 305620, total volume of 500 µl) with an ∼90° angle (custom-made using sterile hemostast). We injected eight sites in the costal diaphragm with ∼50–60 µl/site. Following intradiaphragm injections, the abdominal muscles (3-0 PGA suture, Demesorb; Demetec, Miami, FL) and the skin (3-0 Nylon suture, Demelon; Demetec) were sutured and closed. While keeping the animal in the surgical plane of anesthesia and using aseptic procedures, we continued with myocardial infarction or sham surgery, as detailed below.
To perform myocardial infarction, we performed a left thoracotomy to expose the heart, removed the pericardium, and ligated the left anterior descending (LAD) coronary artery near the left atrium with a 6-0 monofilament absorbable PGA suture (Demesorb; Demetec). Electrocardiogram and body temperature were monitored with Vmed Technology PC-Display software and a PC-VetChek wireless patient monitor (Vmed Technology, Mill Creek, WA). Once animals reached a steady condition postligation, the lungs were hyperinflated and the thoracic (3-0 PGA suture, Demesorb; Demetec) and skin (3-0 Nylon, Demelon; Demetec) incisions closed separately. We carried out sham procedure in the same manner as myocardial infarction but omitted the coronary artery ligation. Animals received bupivacaine and buprenorphine directly after surgery, and buprenorphine administration continued every 8–12 h for 72 h.
We also conducted studies in acid sphingomyelinase-knockout mice (ASMase−/−). We used frozen embryos from Jackson Laboratories with permission from Dr. Mariana Nikolova-Karakashian (15) to establish a colony of ASMase−/− mice originally described by Horinuchi et al. (30). Homozygous ASMase−/− and B6;129 mice (13 ± 1.5 wk old) underwent either MI (n = 17 ASMase−/−, n = 8 WT) or Sham surgery (n = 4 B6;129 mice) followed by terminal experiments 16–20 wk post-surgery. The surgical procedures in mice were similar to those described above for rats, except that the ribs were approximated using 6-0 PGA suture to close the thoracic incision (3). We completed a larger number of MI surgeries in ASMase−/− mice because the strain presents neurodegeneration and motor impairments (30, 41). All ASMase−/− mice in our colony showed signs of ataxia by 12 wk of age, but we selected for surgeries those with normal body weight and body condition score determined by visual inspection. Despite our efforts, we encountered difficulties with long-term survival in ASMase−/− mice (see results). We studied a smaller subset of wild type animals because diaphragm weakness post-MI has already been shown in mice (3, 42), and our experiments were aimed just at confirming weakness in the B6;129 strain.
Echocardiography.
Rats in the pharmacological studies underwent echocardiography 8 wk postsurgery and at the end of the intervention (within 1 wk of terminal experiments). Rats in the genetic intervention underwent echocardiography right before terminal experiments (16 wk postsurgery). Animals were maintained under 2% isoflurane anesthesia while two-dimensional M-mode ultrasound images were obtained at 7.5 MHz (Aplio; Toshiba America Medical Systems, Tusin, CA) in the parasternal short axis view. Measurements were performed using the leading edge-to-leading edge method. Fractional shortening (FS) was calculated using the following equation: %FS = (LVIDd – LVIDs) × 100/(LVIDd), where LVIDd is LV internal diameter end-diastole and LVIDs is LV internal diameter end-systole.
Breathing Measurements
We used whole body plethysmography to assess respiratory frequency, as described previously (29). Briefly, animals were placed in a sealed whole body plethysmograph (chamber volume 4 liters; Buxco Electronic, Wilmington, NC) that was ventilated with room air (21% O2) at a rate of 2 l/min. The plethysmograph was fitted with a pressure transducer (TRD5700; Buxco Electronic) for noninvasive monitoring of respiratory frequency (RF). Each rat was acclimated to the plethysmograph for 1 h, followed by respiratory activity and animal behavior (simultaneous video) recording for 1 h (Spike2 version 7; Cambridge Electronics Design). RF was determined from the average interval between peak inspiratory pressure drops for 15 successive breaths while the animal was resting quietly. Our system was not calibrated for measurement of volumes; therefore, tidal volume and minute ventilation are not reported herein.
Terminal experiments.
All animals were anesthetized with isoflurane (5% induction, 2–3% maintenance), and we performed a laparotomy to excise the diaphragm and heart. The right costal hemidiaphragm was cleaned of excess adipose tissue, snap-frozen in liquid nitrogen, and stored at −80°C for further analysis. The left costal hemi-diaphragm was further dissected for assessment of contractile function. In the genetic intervention, a bundle of the left diaphragm was separated for measurement of mitochondrial H2O2 emission in vitro, as described below. The heart was kept in ice-cold buffer until further dissection of right and left ventricles. We obtained an endocardial image of the left ventricle and determined infarct size by planimetry, as previously described (3, 22). The left and right ventricles were blotted dry briefly to obtain wet weight.
Sphingolipid content.
We homogenized the diaphragm in a commercially available cell lysis buffer (no. 9803; Cell Signaling Technology) with a protease and phosphatase inhibitor cocktail (no. 5872; Cell Signaling Technology). We sonicated and centrifuged the homogenate (15,000 g, 10 min, 4°C) and saved the supernatant for analysis of protein and ceramide content. We shipped the supernatant to the Lipidomics Core at the Medical University of South Carolina for analysis of ceramide content, which was performed via tandem mass spectrometry using a TSQ 7000 triple quadrupole mass spectrometer (7).
Mitochondrial hydrogen peroxide emission.
We cut small portions of the costal diaphragm (10–20 mg) and placed the tissue in a petri dish containing ice-cold buffer X with the following concentrations (in mM): 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 14.3 PCr, 6.56 MgCl2-6H2O, 50 K-MES, 0.5 glutamate, and 0.2 malate (pH = 7.1). The bundle was quickly teased apart along the longitudinal axis, with connective and adipose tissue removed in the process. The bundles were promptly placed in buffer X containing saponin (30 μg/ml) and incubated on a rotator (Argos Technologies, Elgin, IL) for 30 min at 4°C. Once incubation in saponin was complete, the bundles were washed in cold wash buffer that was composed of (in mM) 105 K-MES, 30 KCl, 10 K2HPO4, 5 MgCl2-6H2O, 0.5 mg/ml BSA, 0.1 EGTA, 0.5 glutamate, and 0.2 malate (pH = 7.1).
We measured rates of hydrogen peroxide emission from permeabilized fibers in a fluorometer (Fluorolog-3; Horiba, Edison, NJ) using a solution containing the following: 10 μM Amplex Ultra Red (Life Technologies), 25 μM blebbistatin, 105 mM K-MES, 30 mM KCl, 10 mM K2HPO4, 5 mM MgCl2-6H2O, 0.5 mg/ml BSA, 1 mM EGTA, and 1 U/ml horseradish peroxidase. We heated the solution to 37°C in a water bath and continuously stirred the solution using a magnetic stir bar. After measuring baseline fluorescence, we added 10 mM succinate to assess the rate of mitochondrial H2O2 emission (JH2O2). Amplex Ultra Red fluorescence was converted to H2O2 concentration using a standard curve. We then collected the diaphragm fiber bundle and washed in distilled water. Bundles were dried overnight in an incubator set to 37°C, and dry weight was measured to calculate H2O2 emission in pmol·min−1·mg bundle dry weight−1, which was normalized to citrate synthase activity (35).
Citrate synthase activity.
We measured citrate synthase (CS) activity using a commercially available colorimetric assay (MitoCheck; Cayman Chemical, Ann Arbor, MI), following the manufacturer’s recommendations and using the Synergy 2 Biotek microplate reader (Biotek Instruments, Winooski, VT). A separate bundle from the muscle region used for assessment of mitochondrial JH2O2 was homogenized using a tissue grinder and centrifuged at 5,000 g for 2 min. The supernatant was used for measurement of protein content, and equal amounts of protein were loaded in each well for assessment of enzymatic activity based on changes in absorbance at 412 nm at 25°C. Citrate synthase is a mitochondrial matrix enzyme whose activity is not different in subsarcolemmal and intermyofibrillar mitochondrial fractions (13) and is linearly related to mitochondria content measured by transmission electron microscopy, which is considered the gold standard method (e.g see Ref. 36).
Protein Carbonylation
We determined protein carbonyls using the Oxi-Select protein carbonyl immunoblot kit (no. STA-308), following the manufacturer’s instructions as described by our group (35) and others (31). The experiment was similar to the immunoblot protocol described below, with the only difference being a DNPH derivatization step performed on the membrane after transfer. We blocked the membranes with Odyssey blocking buffer and incubated the membrane with an anti-DNPH antibody (1:1,000) overnight at 4°C. Membranes were washed with Tris-buffered saline with Tween (TBS-T) four times (5 min each) and incubated with secondary antibody (IR Dye, 1:20,000; LI-COR, Lincoln, NE,) for 1 h at RT. Membranes were washed with TBS-T (4 × 5 min), once with TBS (5 min), and were promptly scanned on an Odyssey CLx Imaging system (LI-COR).
Immunoprecipitation
We homogenized ∼15 mg of diaphragm in RIPA buffer containing the following: 20 mM Tris·HCl (pH = 8), 137 mM NaCl, 2 mM EDTA, and 1% Triton X-100. We measured protein content with a DC assay and diluted the samples to ∼2.5 µg/μl and used the Catch and Release version 2.0 immunoprecipitation kit (no. 17-500; Millipore), following the manufacturer’s recommendations with slight modifications. Briefly, we centrifuged the spin columns 30 s at 2,000 g to remove resin and washed the columns twice with 1× wash buffer (400 μl). For immunoprecipitation, we added wash buffer, tissue homogenate (∼250 μg), anti-actin monoclonal antibody (∼3 μg; DSHB, no. JLA-20), and antibody capture affinity ligand. We rotated the spin column end over end for 20 h at 4°C. We then centrifuged the spin columns for 30 s at 2,000 g to collect the flow-through, washed the column three times with 1× wash buffer (400 μl), and added 70 μl of 1× denaturing elution buffer with 5% vol/vol β-mercaptoethanol. We incubated the beads with the denaturing buffer for 15 min on a vortex shaker and spun the columns for 1 min at 5,000 g to collect the eluent. The eluent was stored at −80°C until use for immunoblots.
SDS-PAGE and Immunoblotting
We homogenized diaphragm samples in a buffer consisting of 20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and protease/phosphatase inhibitor cocktail (no. 5872; Cell Signaling Technology). We used a Bullet Blender Storm (Next Advance, Averill Park, NY) to homogenize the samples in tubes filled with 25-μl SSB05 beads, ∼25 μl pf SSB14B beads (Next Advance), and 10× volume of cell lysis buffer by diaphragm weight. We homogenized samples at a speed of 12 for 1 min. The samples were then centrifuged at 5,000 g for 2 min in an Eppendorf 5424 centrifuge (Eppendorf, Hauppauge, NY), and the supernatant (S1) was rotated end over end in an Argos RotoFlex Plus (Argos Technologies, Elgin, IL) for 1 h at 4°C. We then sonicated S1 three times for 3 s at 20% amplitude using a Branson Ultrasonics SLPe Digital sonifier and centrifuged the samples (15,000 g, 30 min, 4°C; Eppendorf) to obtain supernatant S2, which was saved to determine total protein content (DC protein assay, Bio-Rad) and perform gel electrophoresis and immunoblotting.
We mixed the supernatant S2 with 2× Laemmli buffer (Bio-Rad) at a 1:1 ratio, loaded similar amounts of protein (20–30 μg) onto a 4–20% Criterion TGX stain-free gel (Bio-Rad), and performed electrophoresis at 200 V for 50 min on ice. We activated and scanned the gel in a Gel Doc EZ Imager (Bio-Rad), incubated the gel in transfer buffer for 15 min, and then transferred proteins onto a nitrocellulose membrane at 200 mA for 2 h at room temperature. After transfer, we imaged the membrane using Gel Doc EZ Imager to determine protein content in each lane. The membrane was incubated in LI-COR Odyssey blocking buffer for 1 h at room temperature, washed with TBS for 5 min, and probed with primary antibodies overnight at 4°C. The primary antibodies we used were from Santa Cruz Biotechnology (actin: SC-7210; GFP: SC-8334). After primary antibody incubation, membranes were washed in TBS-T (4 × 5 min) and incubated in secondary antibodies (LI-COR) for 1 h at room temperature. Subsequently, membranes were washed with TBS-T (4 × 5 min) and rinsed in TBS (5 min) before scanning on an Odyssey CLx Imaging system (LI-COR). We normalized the signals to total protein content in the sample’s respective lane (2). To determine carbonylated actin, we probed the same membrane for carbonyls and actin using two fluorescence channels (λ = 700 and 800 nm) for secondary antibodies. This approach revealed several carbonyl bands in the actin immunoprecipitate, but only one band corresponded to the actin signal (see results). Because there were two carbonylated bands near the actin signal, yet only one correspondent to actin, we used Gel-band fitter software (47) to define the carbonylated actin signal. Carbonylated actin data are expressed as carbonyl signal (λ = 800 channel) corresponding to actin band normalized to actin signal (λ = 700 channel). We probed diaphragm homogenates for protein expression of NSMase3 as described in myotubes (48, 56) but could not detect a reliable signal consistent with NSMase3 (not shown).
Diaphragm Contractile Function
A diaphragm muscle strip was dissected along with a rib and the central tendon. The rib was tied to a glass rod, and the central tendon was attached to a Dual-Mode Muscle Lever System (300C-LR; Aurora Scientific, Aurora, ON, Canada) using a 4-0 silk suture. The bundle was placed in a water-jacketed organ bath containing Krebs buffer and d-turbocurarine (25 μM) and continuously gassed with 95 O2-5% CO2. The fiber bundle was placed at optimal length (Lo) for twitch force production at room temperature and then equilibrated at 37°C for measurements of isometric force. We stimulated the muscle with frequencies ranging from 1 to 200 Hz (current: 600 mA; pulse duration: 0.25 ms; train duration: 0.5 s), using a biphasic high-power stimulator (701C; Aurora Scientific) controlled by automated software (DMC; Aurora Scientific). Diaphragm strips from animals in the genetic intervention were stimulated only at 200 Hz for assessment of maximal isometric force. Peak isometric force was measured using the “high-throughput” mode of DMA software (Aurora Scientific) and normalized to bundle cross-sectional area (CSA), which was computed using muscle weight and Lo. We used a four-parameter Hill equation to analyze the force-frequency relationships from each animal.
Statistics
All data passed normality and homogeneity of variance tests (SigmaPlot version 13; Systat Software). We then compared treatment and groups using a two-way ANOVA (factor 1, treatment: vehicle vs. GW4869 or scrambled- vs. NSMase3-shRNA; factor 2, condition: sham vs. HFREF). We used a one-way ANOVA to compare data from mice. Bonferroni’s test was adopted for post hoc comparisons when appropriate. In the case of a two-way ANOVA, the post hoc test was focused on comparison of groups within each treatment. We also used unpaired Student’s t-test as needed. Statistical tests used for each variable are indicated in tables and figure legends. In all cases, we used two-tailed tests and declared significance when P < 0.05. We used Prism version 6.0 (GraphPad) to perform group comparisons.
RESULTS
Pharmacological Intervention
Fifteen animals (60%) that underwent MI surgery survived until the start of pharmacological interventions. Four animals that underwent MI surgery and survived for ≥8 wk did not meet the inclusion criteria based on echocardiography. Overall, the number of animals and treatment days in the pharmacological intervention were as follows: sham vehicle (n = 5; 62 ± 1 days), HFREF vehicle (n = 6; 64 ± 2 days), sham GW4869 (n = 6; 65 ± 2 days), and HFREF GW4869 (n = 5; 59 ± 1 days). Echocardiography data and ventricle weights were consistent with the development of HFREF and showed similar characteristics for animals in the vehicle and GW4969 treatments, with no changes in cardiac function from pre- to posttreatment assessments (Table 1).
Table 1.
Characteristics and echocardiography variables of animals receiving vehicle or GW4869
| Vehicle Sham |
Vehicle HFREF |
GW4869 Sham |
GW4869 HFREF |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Infarct area, % | 37.5 ± 20 | 36 ± 25 | ||||||
| Body wt, g | 391 ± 36 | 572 ± 49 | 304 ± 59 | 515 ± 71 | 366 ± 44 | 503 ± 49 | 320 ± 40 | 520 ± 40 |
| LVEDD, mm | 7.5 ± 0.4 | 7.9 ± 0.4 | 8.6 ± 1 | 9 ± 2 | 7.4 ± 1 | 7.3 ± 1 | 9.2 ± 1.3* | 9.1 ± 0.7* |
| LVESD, mm | 3.5 ± 0.3 | 4.0 ± 0.2 | 6.2 ± 2* | 6.5 ± 3* | 3.6 ± 1 | 3.7 ± 0.5 | 6.3 ± 1.3* | 6.3 ± 0.7* |
| FS, % | 54 ± 4 | 48 ± 4 | 29 ± 12* | 29 ± 12* | 53 ± 10 | 49 ± 5 | 32 ± 4* | 31 ± 4* |
| LV wt, mg | 960 ± 89 | 1,160 ± 108† | 850 ± 103 | 1,190 ± 358* | ||||
| RV wt, mg | 140 ± 25 | 200 ± 56* | 140 ± 27 | 180 ± 29† | ||||
| TL, mm | 46 ± 1 | 45 ± 1 | 45 ± 1 | 44 ± 1 | ||||
| LV wt/TL, mg/mm | 20 ± 2 | 26 ± 2† | 19 ± 2 | 27 ± 9* | ||||
| RV wt/TL, mg/mm | 3.0 ± 0.7 | 4.5 ± 1* | 3.1 ± 0.5 | 4.1 ± 1† | ||||
Values are means ± SD; n = 5–6 animals/group. FS, fractional shortening; HFREF, heart failure with reduced ejection fraction; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; RV, right ventricle; TL, tibial length.
Significantly different from sham within treatment group (Bonferroni’s post hoc test).
Significantly different from sham within treatment group (t-test).
Diaphragm ceramide data are shown in Fig. 1. The predominant ceramide species in the diaphragm was C18, in agreement with previous reports (19, 40). Two-way ANOVA showed no significant interaction effect for any ceramide subspecies or total ceramide. There was a condition effect for C14, C16, C18, C18:1, C20:1, C22:1, and total ceramide. There was also a treatment effect on C14, C16, C22, C22:1, C24:1, C26, and total ceramide. Post hoc tests revealed that ceramide contents were 22–53% higher in HFREF within vehicle treatment and not significantly different between sham and HFREF within GW4869 treatment.
Fig. 1.
Diaphragm ceramide subspecies in sham (open bars) and heart failure with reduced ejection fraction (HFREF; gray bars). Total ceramide content calculated as sum of individual ceramide subspecies. Each graph shows 2-way ANOVA P values for each factor and interaction. *P < 0.05, significant differences between conditions (sham vs. HFREF) were tested separately for each treatment (Bonferroni post hoc test). Data are means ± SD from n = 4–6 rats/group.
Total protein carbonyl was not affected by either HFREF or GW4869 (Fig. 2, A and B). We further examined actin carbonylation as putative mechanism of diaphragm weakness caused by myocardial infarction (10). There was no effect of HFREF or GW4869 on actin carbonyls assessed using immunoprecipitation and immunoblotting (Fig. 2, C and D).
Fig. 2.
Diaphragm total protein and actin carbonyls are unchanged with heart failure with reduced ejection fraction (HFREF) or GW4869. A: immunoblots of total protein carbonyls (top) and representative region of total protein in membrane (bottom). B: optical density data from protein carbonyl from each lane normalized to total protein signal in membrane. Data are means ± SE from vehicle sham (n = 5), vehicle HFREF (n = 6), GW4869 sham (n = 6), and GW4869 HFREF (n = 5) analyzed by 2-way ANOVA. C: sample immunoblot images from actin immunoprecipitation. IB, immunoblot; IP, immunoprecipitation. D: optical density of carbonyl band corresponding to actin (lower band on carbonyl blot) normalized to actin signal in Western blot images. Individual data (scatterplots) and group mean (bars) from sham (n = 4) and vehicle HFREF (n = 4), GW4869 sham (n = 6), and GW4869 HFREF (n = 5). Data analyzed by 2-way ANOVA. ● and ■, Data from individual animals within each group.
At the functional level, we found that HFREF decreased diaphragm maximal specific force ∼15% in vehicle-treated animals, whereas GW4869 prevented diaphragm weakness induced by HFREF (Fig. 3A). However, peak twitch force was not different among groups. Nonlinear regression using the four-parameter Hill equation showed that neither HFREF nor GW4869 altered the slope of the force-frequency relationship (vehicle: sham 3.8 ± 0.4, HFREF 3.6 ± 0.3; GW4869: sham 3.6 ± 0.1, HFREF 3.5 ± 0.3) or frequency (F50 in Hz) that elicits 50% maximal force (vehicle: sham 40 ± 2, HFREF 39 ± 0.3; GW4869: sham 40 ± 1, HFREF 39 ± 2). For slope and F50, two-way ANOVA statistics showed no significant effect for interaction, treatment, or condition terms (P > 0.05; details not shown). The loss of diaphragm maximal force was accompanied by increased respiratory rate in HFREF animals within vehicle treatment, which was prevented by GW4869 (Fig. 3B).
Fig. 3.
GW4869 prevents diaphragm weakness and tachypnea in animals with heart failure with reduced ejection fraction (HFREF). A: isometric force normalized to bundle cross-sectional area. Data analyzed using nonlinear regression. Two-way ANOVA results shown in graph are for maximal force. Regarding peak twitch force, 2-way ANOVA showed no significant effect for interaction (P = 0.646), treatment (P = 0.137), or condition (P = 0.108). Data are shown as means ± SE in each frequency for clarity of visualization. B: respiratory rate in breaths/min. Results from 2-way ANOVA are shown above bar graph. *P < 0.05, significant differences between conditions (sham vs. HFREF) were tested separately for each treatment (Bonferroni post hoc test). Individual data (scatterplots) and group mean (bars) from sham (n = 5) and vehicle HFREF (n = 6), GW4869 sham (n = 6), and GW4869 HFREF (n = 5). ● and □, Data from individual animals within each group.
Genetic Intervention
Nine rats injected with NSMase3-shRNA (43%) and 10 rats injected with scrambled shRNA (33%) survived until terminal experiments (16–18 wk postsurgery). Two animals in the NSMase3-shRNA and three animals in the scrambled-shRNA group did not meet the echocardiography inclusion criteria for HFREF. Overall, the number of animals and days from surgery to terminal experiment were as follows: sham SCRM-shRNA (n = 6; 119 ± 3 days), HFREF SCRM-shRNA (n = 6; 113 ± 1 days), sham NSMase3-shRNA (n = 7; 113 ± 1 days), and HFREF NSMase3-shRNA (n = 7; 113 ± 1 days).
Echocardiography data and ventricle weights were consistent with the presence of HFREF in animals injected with scrambled- and NSMase3-shRNA (Table 2). There was a trend (P = 0.10) toward greater infarct area in HFREF animals injected with NSMase3-shRNA. These animals also displayed an ∼80% higher right ventricle-to-tibial length ratio compared with sham (P < 0.05), which was not present in animals that received scrambled shRNA. All other variables from echocardiography and ventricle weights were similar when groups between treatments were compared.
Table 2.
Characteristics and echocardiography variables of animals receiving intradiaphragm injection of adeno-associated virus containing scrambled shRNA or NSMase3-shRNA
| Scrambled shRNA |
NSMase3 shRNA |
|||
|---|---|---|---|---|
| Sham | HFREF | Sham | HFREF | |
| Infarct area, % | 30 ± 5 | 37 ± 8 | ||
| Body wt, g | 599 ± 27 | 617 ± 56 | 625 ± 74 | 632 ± 56 |
| LVEDD, mm | 7.6 ± 1 | 9.4 ± 0.5* | 7.1 ± 0.8 | 8.9 ± 1.3 |
| LVESD, mm | 4.4 ± 1 | 7.3 ± 0.7* | 3.4 ± 0.5 | 6.7 ± 1.3* |
| FS, % | 42 ± 5 | 23 ± 5* | 51 ± 5 | 25 ± 5* |
| LV wt, mg | 961 ± 61 | 1,182 ± 98* | 1,033 ± 127 | 1,232 ± 53* |
| RV wt, mg | 225 ± 27 | 308 ± 147 | 235 ± 29 | 417 ± 185* |
| TL, mm | 44 ± 1 | 45 ± 1 | 44 ± 1 | 44 ± 1 |
| LV wt/TL, mg/mm | 22 ± 1 | 27 ± 3.2* | 23 ± 3 | 28 ± 1* |
| RV wt/TL, mg/mm | 5 ± 0.5 | 7.0 ± 3 | 5 ± 0.5 | 9 ± 4* |
Values are means ± SD; n = 6–7 rats/group. FS, fractional shortening; HFREF, heart failure with reduced ejection fraction; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; NSMase3, neutral sphingomyelinase 3; RV, right ventricle; shRNA, small-hairpin RNA; TL, tibial length.
Significantly different from sham within treatment group (Bonferroni’s post hoc test).
Our measurements of green fluorescent protein (GFP) expression confirmed predominant AAV transduction in the diaphragm compared with the heart (Fig. 4, A and B), with 75–95% of diaphragm fibers being transduced (e.g., see Fig. 4C and data from pilot studies). We also determined that GFP was expressed in the diaphragms of all animals included in the study (data not shown), which confirms transduction of the virus.
Fig. 4.
Green fluorescent protein (GFP) abundance in diaphragm and heart of animals injected with adeno-associated virus (AAV). A: representative Western blot image showing diaphragm-specific expression of GFP with intramuscular injection of AAV containing GFP and small-hairpin (sh)RNA plasmids. B: quantification of GFP optical density from image in A. Data show that intradiaphragm injection of AAV predominantly targeted the diaphragm, with negligible effects on the heart. *P < 0.05 using Student’s t-test. C: sample cross-sectional image from control (noninjected) and diaphragm injected with AAV9-U6-eGFP. Images were acquired, as part of pilot studies of AAV9, injection with the same exposure time and processed equally for removal of background fluorescence (ZenPro Software; Carl Zeiss Microscopy). Red shows wheat-germ agglutinin staining of glycoproteins to define space surrounding the sarcolemmal envelope. ● and ■, Data from individual animals within each group.
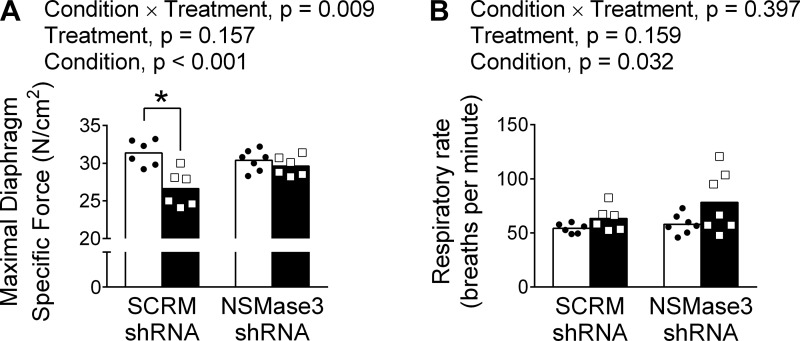
Diaphragm mitochondria H2O2 emission in HFREF increased nearly threefold compared with sham in animals treated with scrambled shRNA (Fig. 5). NSMase3-shRNA prevented the increase in mitochondrial H2O2 emission induced by HFREF. Measurements of isometric contractile function confirmed that maximal diaphragm force was diminished in HFREF animals that received scrambled shRNA (Fig. 6A). Importantly, maximal diaphragm force was preserved in animals with HFREF injected with NSMase3 shRNA. We also observed increased respiratory rate in animals with HFREF receiving either scrambled or NSMase3 shRNA (Fig. 6B).
Fig. 5.
Diaphragm-specific transduction of adeno-associated virus (AAV) with neutral sphingomyelinase 3 (NSMase3) small-hairpin (sh)RNA prevented excess mitochondrial hydrogen peroxide emission (JH2O2) emission in rats with heart failure with reduced ejection fraction (HFREF). JH2O2 measured in permeabilized diaphragm fiber bundles exposed to succinate. Data are normalized to citrate synthase (CS) activity, a marker of mitochondrial content. CS activity [arbitrary units (AU)·min−1·mg protein−1] was not different among groups (data not shown). *P < 0.05, significant differences between conditions (sham vs. HFREF) were tested separately within each treatment (Bonferroni post hoc test). Individual data (scatterplots) and group mean (bars) from n = 6 rats/group within scrambled (SCRM) shRNA and n = 6–7 rats/group within NSMase3-shRNA. ● and □, Data from individual animals within each group.
Fig. 6.
Intradiaphragm injection of adeno-associated virus (AAV) with neutral sphingomyelinase 3 (NSMase3) small-hairpin (sh)RNA prevents diaphragm weakness but not tachypnea in rats with heart failure with reduced ejection fraction (HFREF). Open and black bars represent sham and HFREF groups, respectively. A: maximal diaphragm-specific force measured in intact fiber bundles. B: respiratory rate by whole body plethysmography. *P < 0.05 by Bonferroni post hoc test within each treatment. Unpaired t-tests vs. respective sham showed P = 0.09 within each treatment. Individual data (scatterplots) and group mean (bars) from n = 6 rats/group within scrambled (SCRM) shRNA and n = 7 rats/group within NSMase3-shRNA. ● and □, Data from individual animals within each group.
Acid Sphingomyelinase Knockout
Four ASMase−/− mice (25%) and four WT mice (50%) that underwent MI surgery survived until terminal experiments. One WT mouse had a small infarct area (7%) and was not included in the study. Infarct area was greater in ASMase−/− than in WT mice (Table 3). Wild-type and ASMase−/− mice showed signs of LV and RV hypertrophy (Table 3), which are consistent with HFREF. Because of technical problems, we were unable to measure diaphragm function in one ASMase−/− mouse. Overall, our data showed decreased diaphragm maximal force in both WT and ASMase−/− mice (Fig. 7).
Table 3.
Animal characteristics and heart morphology
| HFREF |
|||
|---|---|---|---|
| Sham (WT) | WT | ASMase−/− | |
| Infarct area, % | 29 ± 9 | 41 ± 10† | |
| Body weight, g | 35 ± 2 | 36 ± 5 | 37 ± 9 |
| LV weight, mg | 117 ± 12 | 184 ± 43* | 235 ± 35* |
| RV weight, mg | 24 ± 4 | 37 ± 10† | 47 ± 9* |
Values are means ± SD; WT sham: n = 4 mice; HFREF animals: n = 3 mice/group. ASMase−/−, acid sphingomyelinase knockout; HFREF, heart failure with reduced ejection fraction; LV, left ventricle; RV, right ventricle; WT, wild type.
Significantly different (P < 0.05) from WT sham;
P = 0.07 vs. WT sham.
Fig. 7.
Heart failure with reduced ejection fraction (HFREF) decreases maximal diaphragm-specific force in wild-type (WT) and acid sphingomyelinase-knockout (ASMase−/−) mice. Individual data (scatterplots) and group mean (bars) (WT: sham, n = 4 mice; HFREF: WT and ASMase−/−, n = 3 mice/group). One-way ANOVA result was F(2,7) = 14.47, P = 0.003. *Significant (P < 0.05, Bonferroni post hoc) difference compared with WT Sham. In separate experiments, we established that maximal diaphragm force was not different in adult ASMase−/− mice (28 ± 0.3 N/cm2, n = 3) or WT animals. ●, □, and △, Data from individual animals within each group.
DISCUSSION
There are several novel and important findings in our study. Systemic administration of the NSMase inhibitor GW4869 mitigated diaphragm abnormalities caused by HFREF induced by myocardial infarction. This was evident by lowered levels of ceramide and protection against loss of maximal specific force. Concurrent with effects on the diaphragm, GW4869 prevented tachypnea induced by HFREF. Intradiaphragm injection of AAV-NSMase3-shRNA, designed to circumvent systemic effects, prevented the increase in diaphragm mitochondrial ROS and loss of maximal force but had no effect on the tachypnea caused by HFREF. Importantly, myocardial infarction induced diaphragm weakness in ASMase-knockout mice.
HFREF increases diaphragm NSMase activity and ceramide content (19), a finding that is supported by our present study (Fig. 1). Ceramide accumulation is mainly a result of SMase activation or de novo synthesis (49). GW4869 prevented the increase in diaphragm ceramide content induced by HFREF (Fig. 1), which suggests heightened NSMase activity as the cause of elevated ceramide. GW4869 inhibits NSMases and not ASMase in cultured skeletal muscle and nonmuscle cells (39, 48, 58). We selected a dose of GW4869 that maintains normal levels of ceramide content in skeletal muscle of healthy animals (58). Accordingly, GW4869 had no effect on ceramide content in diaphragm of sham animals but restored ceramide content in the diaphragms of HFREF animals to levels seen in the sham group. This suggests that intraperitoneal injection of GW4869 at a dose of 1.5 mg·kg−1·day−1 lowers muscle ceramide content only in conditions associated with elevated NSMase activity.
Sphingomyelinase activation and ceramide signaling increase diaphragm oxidants (23, 38). We measured protein carbonyls to test the effect of NSMase inhibition on oxidants in the diaphragm. Neither HFREF nor GW4869 affected total protein carbonyls (Fig. 2). These findings are consistent with previous studies (3) but disagree with data from mice in early stage (57) or patients in very advanced stages of HFREF (4). The reason for the discrepancy between our current study and previous data in humans may be related to patients being in end-stage disease, which is very difficult to reproduce in animal models. Moreover, assessment of total protein carbonyls can mask changes in carbonylation of individual proteins. For instance, carbonylation of diaphragm actin is elevated within 72 h postmyocardial infarction (10). Thus, we measured diaphragm actin carbonyls and found it to be unaffected by HFREF and GW4869 (Fig. 2) despite persistent increases in diaphragm ROS emission, as seen previously (57), and in our genetic intervention herein (Fig. 5). However, we cannot discard the possibility that a larger number of animals would reveal differences in total or actin carbonyls. In aggregate, our observations suggest that our measurements of protein carbonyls were insensitive to changes in oxidant signaling caused by HFREF and GW4869.
The specific link between heart failure and diaphragm neutral sphingomyelinase activation is unknown. Inflammatory cytokines that are elevated in HFREF also increase NSMase activity in muscle cells (48, 56). Three NSMase isoforms have been reported in the diaphragm (NSMase1, NSMase2, and NSMase3) (48). NSMase3 is the predominant isoform in diaphragm from rodents and humans (48). NSMase3 is a C-tailed anchored protein that localizes to the ER, Golgi, and mitochondria membranes (34, 48). NSMase3 gene expression increases in skeletal muscle of patients with statin-induced myopathy (18), and enzymatic activity increases in C2C12 myotubes exposed to TNFα (48). In cultured skeletal muscle cells, both NSMase2 and NSMase3 contribute to cytokine-mediated increase in oxidants (48, 56). To resolve the tissue-specific effects and NSMase isoform involved in diaphragm abnormalities in HFREF, we used intradiaphragm injection of AAV with shRNA targeting NSMase3. In agreement with others (54), intradiaphragm AAV injection elicited transduction predominantly in the diaphragm tissue, with negligible effects on the heart (Fig. 4).
Excess mitochondrial ROS emission is causally linked to diaphragm weakness in sepsis (37), hyperglycemia (12), mechanical ventilation (50, 55), and exposure to recombinant SMase in vitro (24). Downstream of NSMase, ceramide is the presumed second messenger leading to excess mitochondrial ROS. Ceramide in the cell membrane can form platforms that invaginate and fuse with mitochondria (5) or bind to ceramide transfer proteins that lead to mitochondrial incorporation of ceramide (8). At the mitochondria, ceramide promotes ROS formation through increase in fission and inhibition of electron transport chain complexes (25, 27, 53). Mitochondria-targeted antioxidants prevent diaphragm weakness induced by HFREF (35) suggesting that mitochondrial ROS mediate the diaphragm weakness. Our novel finding was that NSMase3 shRNA prevented excess diaphragm mitochondrial H2O2 emission and weakness in HFREF (Figs. 5 and 6). The increase in diaphragm mitochondrial H2O2 emission in HFREF animals that received scrambled shRNA is in agreement with previous findings in permeabilized fibers (35) and isolated mitochondria (57). In general, our findings support the notion that NSMase3 activation mediates the increase in diaphragm mitochondrial ROS that causes weakness in HFREF.
The secretory isoform of SMase, a product of the ASMase gene, is increased in serum of patients with HFREF and correlates with limb muscle weakness (17). In a previous study, we observed that diaphragm ASMase activity was similar in HFREF and sham rats 16 wk postsurgery (19). However, that observation does not exclude an involvement of ASMase at an earlier stage of the disease development. To test the contribution of ASMase to diaphragm abnormalities in HFREF, we completed studies in germ line ASMase-knockout mice. Despite difficulties with survival postsurgery that led to a small sample size, presumably because of neurodegenerative disorder (30, 41), our experiments revealed that ASMase knockout did not prevent diaphragm weakness postmyocardial infarction (Fig. 7). Importantly, the extent of diaphragm weakness in B6;129 mice was similar to that reported in the C57BL6 strain (3, 42). Overall, our findings suggest that neither circulating (secretory) nor diaphragm ASMase mediate the loss of diaphragm force caused by HFREF secondary to myocardial infarction.
Diaphragm weakness induced by NSMase activation may have relevance to aging and diseases involving systemic or muscle inflammation. Aging causes diaphragm weakness (26, 33) and increases NSMase abundance in limb muscles (51). If NSMase activity is elevated in aged diaphragm, NSMase inhibition might be a suitable approach to prevent weakness therein. Sepsis is an inflammatory condition that heightens NSMase activity and causes diaphragm weakness (56). In agreement with our study, the NSMase inhibitor GW4869 prevented excess activation of diaphragm NSMase and weakness in sepsis (56). Therefore, NSMase inhibition might be a suitable treatment against diaphragm weakness in multiple conditions.
Loss of diaphragm force impacts ventilatory and nonventilatory behaviors (43, 44). Based on the model proposed by Mantilla and Sieck (44), ventilation at rest and during exercise will most likely involve the recruitment of slow and fast fatigue-resistant motor units, with a possible contribution of fast-intermediate motor units during very high-intensity and/or prolonged exercise. Experiments in single fibers show that HFREF causes loss of force in type I, IIA, and IIX/B fibers (60). Therefore, a potential consequence of diaphragm weakness is a change in breathing pattern to maintain minute ventilation with a lower tidal volume and higher respiratory rate (32, 61). In pompe disease, intrapleural AAV9 treatment that corrects diaphragm neuromuscular deficits improve ventilatory function (21, 59). HFREF causes tachypnea as shown by others (16, 52) and our current data. A recent study in patients with HFREF suggests that respiratory muscle weakness contributes to elevated dead space ventilation, particularly during exercise (28). Elevated dead space ventilation due to tachypneic breathing pattern is a key determinant of impaired gas exchange during exercise in HFREF (62). We found that protection of diaphragm function with GW4869 administration in HFREF occurred alongside prevention of tachypnea (Fig. 4). In contrast, AAV9-NSMase3 shRNA prevented diaphragm weakness but not tachypnea in animals with HFREF. These findings suggest that diaphragm weakness in HFREF had no effect on resting respiratory rate. Importantly, we cannot rule out an impact of diaphragm weakness in HFREF on the tachypneic breathing pattern and elevated dead space ventilation during exercise reported by other groups (28, 62).
Overall, our data suggest that the impact of GW4869 on tachypnea of HFREF reflects a drug effect on the neural control of breathing in HFREF. An important and perhaps the main determinant of tachypnea in HFREF at rest is elevated peripheral chemoreceptor sensitivity (16, 52). Enhanced angiotensin II signaling in carotid body chemoreceptors contributes to hyperpnea in HFREF (52). GW4869 prevents vasoconstriction induced by angiotensin II in rat kidneys (6). Thus, GW4869 may have inhibited angiotensin II signaling in carotid body chemoreceptors. It also appears that GW4869 crosses the blood-brain barrier (58) and could thereby have affected the respiratory control center. Overall, the effect of GW4869 on breathing patterns are novel and suggest that NSMase and ceramide signaling are involved in the enhanced peripheral chemoreflex sensitivity that causes hyperpnea in HFREF.
Limitations and Methodological Considerations
Important methodological aspects to consider in our study are twofold: 1) systemic nature of GW4869 treatment and potential GW4869 effects in the failing heart and 2) mortality in animals receiving intradiaphragm injections of AAV. Cardiac NSMase activity is increased 2–3 mo postmyocardial infarction in rats (1). We started GW4869 treatment 2 mo postmyocardial infarction. Thus, we cannot discard the possibility that the effects of GW4869 on diaphragm function and breathing were secondary to the beneficial effects on cardiac remodeling. However, data from our genetic intervention suggest that the impact of GW4869 on diaphragm function was independent of cardiac effects. Our GFP data (Fig. 4) and another study (54) suggest that the intradiaphragm muscle injection method promoted viral transduction in the diaphragm but not the heart. Therefore, protective effects of intradiaphragm NSMase3 shRNA suggest independent effects of GW4869 on the heart, if any, and the diaphragms of animals with HFREF. Importantly, our approach for injection of AAV9 with shRNA expression under the U6 promoter targeted the diaphragm tissue, not muscle cells. Cell type-specific promoters are dependent on polymerase II, which are not ideal for expression of shRNA. Therefore, we were limited to using the RNA polymerase III promoter and opted for localized injections to obtain tissue- but not cell-specific effects.
Animals receiving intra-diaphragm injection of AAV NSMase3 shRNA had a higher survival postmyocardial infarction than those in the scrambled shRNA group. We consider that this finding reflects the experimental design of our study. We chose to perform intradiaphragm injections, which elicit an inflammatory response, soon before ligation of the coronary artery to minimize animal pain and distress associated with multiple survival surgeries. Intradiaphragm injections lowered survival postinfarct (33% survival) compared with noninjected animals in the pharmacological intervention (60% survival). NSMase signaling mediates the detrimental effects of inflammation on muscle cells (48, 49, 56). Thus, injection of NSMase3 shRNA may have attenuated the inflammatory responses to intradiaphragm injections, preserved diaphragm contractile function, and promoted higher survival. Therefore, our data cannot resolve the direct implications of diaphragm weakness and NSMase signaling for the systemic pathophysiology and mortality in the acute phase postmyocardial infarction. A potential alternative approach to transduce the diaphragm would be intrapleural injection of AAV (45). However, with shRNA expression under control of a nonspecific promoter, intrapleural injection results in cardiac transduction (20), which we wanted to avoid in our study.
Conclusions
Our study shows that shRNA and pharmacological targeting of neutral sphingomyelinase prevent diaphragm weakness in HFREF. The data suggest that NSMase3 is the isoform responsible for the diaphragm weakness in HFREF, whereas the cascade of events downstream of diaphragm NSMase3 activation that appear to mediate weakness consists of accumulation of ceramide and heightened mitochondrial ROS emission. Tachypnea is an important component of the integrative pathophysiology of HFREF. Our data suggest that NSMase signaling in areas involved in neural control of breathing contributes to tachypnea in HFREF. However, our findings suggest that diaphragm weakness does not contribute to tachypnea at rest in HFREF.
GRANTS
Experiments were funded by American Heart Association Grant 13GRNT17160000 and National Heart, Lung, and Blood Institute Grant R00-HL-098453. L. F. Ferreira is currently supported by National Heart, Lung, and Blood Institute Grant R01-HL-130318.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.F.F. conceived and designed research; P.D.C., B.A., L.F.H., J.-K.Y., D.D.C., and L.F.F. performed experiments; P.D.C., B.A., L.F.H., J.-K.Y., D.D.C., and L.F.F. analyzed data; P.D.C., B.A., L.F.H., J.-K.Y., D.D.C., and L.F.F. interpreted results of experiments; P.D.C., B.A., and L.F.F. prepared figures; P.D.C., B.A., and L.F.F. drafted manuscript; P.D.C., B.A., L.F.H., J.-K.Y., D.D.C., and L.F.F. edited and revised manuscript; P.D.C., B.A., L.F.H., J.-K.Y., D.D.C., and L.F.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Gregory Frye and Nikhil Patel for assistance with animal injections and care.
REFERENCES
- 1.Adamy C, Mulder P, Khouzami L, Andrieu-abadie N, Defer N, Candiani G, Pavoine C, Caramelle P, Souktani R, Le Corvoisier P, Perier M, Kirsch M, Damy T, Berdeaux A, Levade T, Thuillez C, Hittinger L, Pecker F. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J Mol Cell Cardiol 43: 344–353, 2007. doi: 10.1016/j.yjmcc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Ahn B, Beaver T, Martin T, Hess P, Brumback BA, Ahmed S, Smith BK, Leeuwenburgh C, Martin AD, Ferreira LF. Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery. Am J Respir Crit Care Med 190: 837–839, 2014. doi: 10.1164/rccm.201405-0993LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn B, Beharry AW, Frye GS, Judge AR, Ferreira LF. NAD(P)H oxidase subunit p47phox is elevated, and p47phox knockout prevents diaphragm contractile dysfunction in heart failure. Am J Physiol Lung Cell Mol Physiol 309: L497–L505, 2015. doi: 10.1152/ajplung.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn B, Coblentz PD, Beharry AW, Patel N, Judge AR, Moylan JS, Hoopes CW, Bonnell MR, Ferreira LF. Diaphragm abnormalities in patients with end-stage heart failure: nadph oxidase upregulation and protein oxidation. Front Physiol 7: 686, 2017. doi: 10.3389/fphys.2016.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babiychuk EB, Atanassoff AP, Monastyrskaya K, Brandenberger C, Studer D, Allemann C, Draeger A. The targeting of plasmalemmal ceramide to mitochondria during apoptosis. PLoS One 6: e23706, 2011. doi: 10.1371/journal.pone.0023706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista-Pérez R, del Valle-Mondragón L, Cano-Martínez A, Pérez-Méndez O, Escalante B, Franco M. Involvement of neutral sphingomyelinase in the angiotensin II signaling pathway. Am J Physiol Renal Physiol 308: F1178–F1187, 2015. doi: 10.1152/ajprenal.00079.2014. [DOI] [PubMed] [Google Scholar]
- 7.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv Exp Med Biol 688: 46–59, 2010. doi: 10.1007/978-1-4419-6741-1_3. [DOI] [PubMed] [Google Scholar]
- 8.Bockelmann S, Mina JGM, Korneev S, Hassan DG, Müller D, Hilderink A, Vlieg HC, Raijmakers R, Heck AJR, Haberkant P, Holthuis JC. A search for ceramide binding proteins using bifunctional lipid analogs yields CERT-related protein StarD7. J Lipid Res 59: 515–530, 2018. doi: 10.1194/jlr.M082354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bost ER, Frye GS, Ahn B, Ferreira LF. Diaphragm dysfunction caused by sphingomyelinase requires the p47(phox) subunit of NADPH oxidase. Respir Physiol Neurobiol 205: 47–52, 2015. doi: 10.1016/j.resp.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen TS, Mangner N, Werner S, Glaser S, Kullnick Y, Schrepper A, Doenst T, Oberbach A, Linke A, Steil L, Schuler G, Adams V. Diaphragm muscle weakness in mice is early-onset post-myocardial infarction and associated with elevated protein oxidation. J Appl Physiol (1985) 118: 11–19, 2015. doi: 10.1152/japplphysiol.00756.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalin LP, Arena R, Guazzi M, Myers J, Cipriano G, Chiappa G, Lavie CJ, Forman DE. Inspiratory muscle training in heart disease and heart failure: a review of the literature with a focus on method of training and outcomes. Expert Rev Cardiovasc Ther 11: 161–177, 2013. doi: 10.1586/erc.12.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan LA, Supinski GS. Hyperglycemia-induced diaphragm weakness is mediated by oxidative stress. Crit Care 18: R88, 2014. doi: 10.1186/cc13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol 264: C383–C389, 1993. doi: 10.1152/ajpcell.1993.264.2.C383. [DOI] [PubMed] [Google Scholar]
- 14.Dall’Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 47: 757–763, 2006. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 15.Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB, Wei J, Daugherty A, Smart EJ, Reid MB, Merrill AH Jr, Nikolova-Karakashian M. Acid Sphingomyelinase Deficiency Prevents Diet-induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J Biol Chem 284: 8359–8368, 2009. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, Tschope C, Ponikowski P, Claus RA, Anker SD. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J 28: 821–828, 2007. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 18.Elam MB, Majumdar G, Mozhui K, Gerling IC, Vera SR, Fish-Trotter H, Williams RW, Childress RD, Raghow R. Patients experiencing statin-induced myalgia exhibit a unique program of skeletal muscle gene expression following statin re-challenge. PLoS One 12: e0181308, 2017. doi: 10.1371/journal.pone.0181308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Empinado HM, Deevska GM, Nikolova-Karakashian M, Yoo JK, Christou DD, Ferreira LF. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur J Heart Fail 16: 519–525, 2014. doi: 10.1002/ejhf.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk DJ, Mah CS, Soustek MS, Lee KZ, Elmallah MK, Cloutier DA, Fuller DD, Byrne BJ. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther 21: 1661–1667, 2013. doi: 10.1038/mt.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk DJ, Soustek MS, Todd AG, Mah CS, Cloutier DA, Kelley JS, Clement N, Fuller DD, Byrne BJ. Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice. Mol Ther Methods Clin Dev 2: 15007, 2015. doi: 10.1038/mtm.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006. doi: 10.1111/j.1748-1716.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol 299: C552–C560, 2010. doi: 10.1152/ajpcell.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira LF, Moylan JS, Stasko S, Smith JD, Campbell KS, Reid MB. Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J Appl Physiol (1985) 112: 1538–1545, 2012. doi: 10.1152/japplphysiol.01269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272: 11369–11377, 1997. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 26.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem 272: 24154–24158, 1997. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 28.Hamazaki N, Masuda T, Kamiya K, Matsuzawa R, Nozaki K, Maekawa E, Noda C, Yamaoka-Tojo M, Ako J. Respiratory muscle weakness increases dead-space ventilation ratio aggravating ventilation-perfusion mismatch during exercise in patients with chronic heart failure. Respirology 24: 154–161, 2019. doi: 10.1111/resp.13432. [DOI] [PubMed] [Google Scholar]
- 29.Hayward LF, Castellanos M, Noah C. Cardiorespiratory variability following repeat acute hypoxia in the conscious SHR versus two normotensive rat strains. Auton Neurosci 171: 58–65, 2012. doi: 10.1016/j.autneu.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet 10: 288–293, 1995. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 31.Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell 13: 92–101, 2014. doi: 10.1111/acel.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev 22: 191–207, 2017. doi: 10.1007/s10741-016-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley RC, McDonagh B, Ferreira LF. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Exp Gerontol 103: 69–79, 2018. doi: 10.1016/j.exger.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Krönke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem 281: 13784–13793, 2006. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- 35.Laitano O, Ahn B, Patel N, Coblentz PD, Smuder AJ, Yoo JK, Christou DD, Adhihetty PJ, Ferreira LF. Pharmacological targeting of mitochondrial reactive oxygen species counteracts diaphragm weakness in chronic heart failure. J Appl Physiol (1985) 120: 733–742, 2016. doi: 10.1152/japplphysiol.00822.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao WC, Chen YH, Li HY, Wang TT, Lan P, Pan KH, Ge HQ, Xie QM, Zhou JC. Diaphragmatic dysfunction in sepsis due to severe acute pancreatitis complicated by intra-abdominal hypertension. J Int Med Res 46: 1349–1357, 2018. doi: 10.1177/0300060517747163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loehr JA, Abo-Zahrah R, Pal R, Rodney GG. Sphingomyelinase promotes oxidant production and skeletal muscle contractile dysfunction through activation of NADPH oxidase. Front Physiol 5: 530, 2015. doi: 10.3389/fphys.2014.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, Hazen-Martin DJ, Obeid LM, Hannun YA, Smith GK. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem 277: 41128–41139, 2002. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 40.Lukaszuk B, Miklosz A, Zendzian-Piotrowska M, Wojcik B, Gorski J, Chabowski A. Changes in the diaphragm lipid content after administration of streptozotocin and high-fat diet regime. J Diabetes Res 2017: 1–12, 2017. doi: 10.1155/2017/3437169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macauley SL, Sidman RL, Schuchman EH, Taksir T, Stewart GR. Neuropathology of the acid sphingomyelinase knockout mouse model of Niemann-Pick A disease including structure-function studies associated with cerebellar Purkinje cell degeneration. Exp Neurol 214: 181–192, 2008. doi: 10.1016/j.expneurol.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Mangner N, Bowen TS, Werner S, Fischer T, Kullnick Y, Oberbach A, Linke A, Steil L, Schuler G, Adams V. Exercise Training Prevents Diaphragm Contractile Dysfunction in Heart Failure. Med Sci Sports Exerc 48: 2118–2124, 2016. doi: 10.1249/MSS.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 43.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantilla CB, Sieck GC. Impact of diaphragm muscle fiber atrophy on neuromotor control. Respir Physiol Neurobiol 189: 411–418, 2013. doi: 10.1016/j.resp.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 82: 27–44, 2004. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 47.Mitov MI, Greaser ML, Campbell KS. GelBandFitter–a computer program for analysis of closely spaced electrophoretic and immunoblotted bands. Electrophoresis 30: 848–851, 2009. doi: 10.1002/elps.200800583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moylan JS, Smith JD, Wolf Horrell EM, McLean JB, Deevska GM, Bonnell MR, Nikolova-Karakashian MN, Reid MB. Neutral sphingomyelinase-3 mediates TNF-stimulated oxidant activity in skeletal muscle. Redox Biol 2: 910–920, 2014. doi: 10.1016/j.redox.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal 15: 2501–2517, 2011. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39: 1749–1759, 2011. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russ DW, Wills AM, Boyd IM, Krause J. Weakness, SR function and stress in gastrocnemius muscles of aged male rats. Exp Gerontol 50: 40–44, 2014. doi: 10.1016/j.exger.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Schultz HD, Marcus NJ, Del Rio R. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol 100: 124–129, 2015. doi: 10.1113/expphysiol.2014.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith ME, Tippetts TS, Brassfield ES, Tucker BJ, Ockey A, Swensen AC, Anthonymuthu TS, Washburn TD, Kane DA, Prince JT, Bikman BT. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem J 456: 427–439, 2013. doi: 10.1042/BJ20130807. [DOI] [PubMed] [Google Scholar]
- 54.Smuder AJ, Falk DJ, Sollanek KJ, Nelson WB, Powers SK. Delivery of recombinant adeno-associated virus vectors to rat diaphragm muscle via direct intramuscular injection. Hum Gene Ther Methods 24: 364–371, 2013. doi: 10.1089/hgtb.2013.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smuder AJ, Sollanek KJ, Nelson WB, Min K, Talbert EE, Kavazis AN, Hudson MB, Sandri M, Szeto HH, Powers SK. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med 115: 179–190, 2018. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Supinski GS, Alimov AP, Wang L, Song XH, Callahan LA. Neutral sphingomyelinase 2 is required for cytokine-induced skeletal muscle calpain activation. Am J Physiol Lung Cell Mol Physiol 309: L614–L624, 2015. doi: 10.1152/ajplung.00141.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Supinski GS, Callahan LA. Diaphragmatic free radical generation increases in an animal model of heart failure. J Appl Physiol (1985) 99: 1078–1084, 2005. doi: 10.1152/japplphysiol.01145.2004. [DOI] [PubMed] [Google Scholar]
- 58.Tabatadze N, Savonenko A, Song H, Bandaru VV, Chu M, Haughey NJ. Inhibition of neutral sphingomyelinase-2 perturbs brain sphingolipid balance and spatial memory in mice. J Neurosci Res 88: 2940–2951, 2010. doi: 10.1002/jnr.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todd AG, McElroy JA, Grange RW, Fuller DD, Walter GA, Byrne BJ, Falk DJ. Correcting neuromuscular deficits with gene therapy in pompe disease. Ann Neurol 78: 222–234, 2015. doi: 10.1002/ana.24433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007. doi: 10.1152/ajpheart.00085.2007. [DOI] [PubMed] [Google Scholar]
- 61.Wilcox PG, Pardy RL. Diaphragmatic weakness and paralysis. Hai 167: 323–341, 1989. doi: 10.1007/BF02714961. [DOI] [PubMed] [Google Scholar]
- 62.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail 16: 835–842, 2010. doi: 10.1016/j.cardfail.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]