Abstract
Myocardial ischemia-reperfusion (I/R) results in the generation of free radicals, accumulation of lipid peroxidation-derived unsaturated aldehydes, variable angina (pain), and infarction. The transient receptor potential ankyrin 1 (TRPA1) mediates pain signaling and is activated by unsaturated aldehydes, including acrolein and 4-hydroxynonenal. The contribution of TRPA1 (a Ca2+-permeable channel) to I/R-induced myocardial injury is unknown. We tested the hypothesis that cardiac TRPA1 confers myocyte sensitivity to aldehyde accumulation and promotes I/R injury. Although basal cardiovascular function in TRPA1-null mice was similar to that in wild-type (WT) mice, infarct size was significantly smaller in TRPA1-null mice than in WT mice (34.1 ± 9.3 vs. 14.3 ± 9.9% of the risk region, n = 8 and 7, respectively, P < 0.05), despite a similar I/R-induced area at risk (40.3 ±8.4% and 42.2 ± 11.3% for WT and TRPA1-null mice, respectively) after myocardial I/R (30 min of ischemia followed by 24 h of reperfusion) in situ. Positive TRPA1 immunofluorescence was present in murine and human hearts and was colocalized with connexin43 at intercalated disks in isolated murine cardiomyocytes. Cardiomyocyte TRPA1 was confirmed by quantitative RT-PCR, DNA sequencing, Western blot analysis, and electrophysiology. A role of TRPA1 in cardiomyocyte toxicity was demonstrated in isolated cardiomyocytes exposed to acrolein, an I/R-associated toxin that induces Ca2+ accumulation and hypercontraction, effects significantly blunted by HC-030031, a TRPA1 antagonist. Protection induced by HC-030031 was quantitatively equivalent to that induced by SN-6, a Na+/Ca2+ exchange inhibitor, further supporting a role of Ca2+ overload in acrolein-induced cardiomyocyte toxicity. These data indicate that cardiac TRPA1 activation likely contributes to I/R injury and, thus, that TRPA1 may be a novel therapeutic target for decreasing myocardial I/R injury.
NEW & NOTEWORTHY Transient receptor potential ankyrin 1 (TRPA1) activation mediates increased blood flow, edema, and pain reception, yet its role in myocardial ischemia-reperfusion (I/R) injury is unknown. Genetic ablation of TRPA1 significantly decreased myocardial infarction after I/R in mice. Functional TRPA1 in cardiomyocytes was enriched in intercalated disks and contributed to acrolein-induced Ca2+ overload and hypercontraction. These data indicate that I/R activation of TRPA1 worsens myocardial infarction; TRPA1 may be a potential target to mitigate I/R injury.
Keywords: acrolein, cardiomyocytes, lipid peroxidation, myocardial infarction, transient receptor potential ankyrin 1 channel, unsaturated aldehydes
INTRODUCTION
Myocardial ischemia-reperfusion (I/R) induces angina and infarction. Although angina is a function of pain receptor activation (nociception) in the heart, relative lack of afferent innervation likely contributes to discordance between angina and infarct severity and referred pain (e.g., pain in the left arm and back of the neck during I/R). Recognition of the role of chest pain as a symptom of acute myocardial infarction has been an important health initiative, yet little is known about how pain receptors contribute to severity of myocardial injury. The transient receptor potential ankyrin 1 (TRPA1) is a pain receptor stimulated by unsaturated aldehydes that are products of lipid peroxidation in I/R (6). Moreover, remote pain stimuli appear to protect the cardiovascular system, suggesting that pain afferents can affect the I/R-stressed myocardium in significant ways (23). Nonetheless, the specific role of TRPA1 in myocardial I/R injury has not been assessed.
TRPA1 is widely distributed throughout the body in neural (sensory nerve fibers) (6) and non-neural tissues, including those in the cardiovascular system. Several studies have shown functional TRPA1 in the vasculature (16, 35) and in cardiomyocytes (2, 3). TRPA1 has been implicated in cardiovascular responses to a diversity of exogenous stimuli, including exposure to cold temperatures (4) and air pollution (12, 15, 26) and propofol (18, 34, 35), although the specific contribution of neuronal and non-neuronal TRPA1 in each setting is unclear. Activation of sensory nerve TRPA1 leads to pain signaling and the release of the vasoactive peptides substance P and calcitonin gene-related peptide (38), which affect local “neurogenic inflammation” (17, 39), yet these components may not be involved in all cardiovascular effects after TRPA1 activation (31, 32). Thus, neuronal and non-neuronal TRPA1 activation may contribute to coordinated and complex injury responses, such as infarction after I/R.
Because free radicals generated during I/R are short lived and limited by their high reactivity, tissue injury of I/R, including apoptosis, Ca2+ overload, and reentrant arrhythmias (24), is more likely the consequence of secondary, more stable species generated from lipid peroxidation (22). Recent work suggests that acrolein, a well-known toxic unsaturated aldehyde, is generated in the ischemic heart and contributes to myocardial injury (13, 37, 40). Acrolein is a known agonist of TRPA1 (6), and acrolein-induced activation of TRPA1 is linked to peripheral pain, increased blood flow, and edema as well as altered tracheal reactivity, airway resistance, and inflammation (1, 7, 15, 33). These studies have implicated TRPA1 as both a target of acrolein and a contributor of tissue inflammation and injury (12).
The present study was designed to test the hypothesis that myocardial I/R injury is mediated, in part, by TRPA1 channels in cardiomyocytes activated by unsaturated aldehydes, such as acrolein, that are formed during I/R. Consistent with our hypothesis, genetic deficiency of TRPA1 significantly diminishes I/R injury in situ, and a selective TRPA1 antagonist protects isolated cardiomyocytes from acrolein-induced Ca2+ entry and hypercontraction. These findings reveal a novel mechanism of cardiac injury mediated by TRPA1 and, moreover, support the broader notion that unsaturated aldehydes generated during I/R are a significant cause of cardiac injury.
MATERIALS AND METHODS
Mice
TRPA1-null mice (obtained from Dr. S. E. Jordt, Duke University), in which the TRPA1 gene in SV129 embryos was “knocked out” using a single construct, were bred back for 10 generations with C57BL/6 mice (6). TRPA1-null mice develop and breed normally. Breeding pairs of TRPA1 heterozygotes were used to generate homozygous littermate wild-type [WT (TRPA1+/+)] and null (TRPA1−/−) mice for this study; these mice reproduced in a Mendelian fashion. WT and TRPA1-null mice were genotyped using PCR products and primers, as previously described elsewhere (6). All four primers were mixed with tail DNA and amplified using Taq polymerase (Promega, Madison, WI), and products were run on 2% agarose gel with the WT band at 296 bp and the null band at 213 bp (25). Additional male C57BL/6 (WT) mice were either purchased from Jackson Laboratory (Bar Harbor, ME) or raised in house in a barrier facility with the TRPA1 mouse colony. Placental alkaline phosphatase (PLAP) transgenic/TRPA1-null mice (also known as Trpa1-null with IRES-PLAP reporter gene, B6;129P-Trpa1tm1Kykw/J) were purchased from Jackson Laboratory. This mouse model expresses the human PLAP gene (hPLAP) in place of the murine Trpa1 (mTrpa1) gene under control of the endogenous TRPA1 promoter (27). Mice were treated according to the American Physiological Society “Guiding Principles in the Care and Use of Animals,” and protocols were approved by the University of Louisville Institutional Animal Care and Use Committee.
Cardiovascular Phenotyping
Echocardiography.
A high-resolution micro-ultrasound imaging system (Vevo 770, VisualSonics) was used to perform two-dimensional M-mode echocardiography in anesthetized (2% isoflurane) male WT and TRPA1-null mice (12–16 wk old). Echocardiograms were analyzed using the VisualSonics Cardiac Measurement Package (version 17) (13).
Blood pressure.
Hemodynamic parameters [i.e., systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate (HR)] were measured in conscious male WT and TRPA1-null mice (12–16 wk old) by noninvasive pressure-volume tail-cuff methodology (CODA6, Kent Scientific, Torrington, CT). Blood pressure was measured via tail cuff in mice acclimated for ≥5 days to gentle heating with brief (<30-min) confinement in hooded Plexiglas tubes (14).
I/R Injury
Langendorff-perfused isolated heart.
Hearts were isolated from anesthetized mice (male littermates) via thoracotomy and maintained in Langendorff retrograde perfusion, as previously described in detail elsewhere (13). A custom left ventricular (LV) balloon was implanted in the heart for pressure recording during 30 min of perfusion before 30 min of ischemia and 45 min of reperfusion. Coronary flow (CF) was monitored by an in-line flow probe (Transonic), and baseline and reperfusion perfusates were measured for creatine kinase and lactate dehydrogenase activity (13).
In situ I/R.
Mice (male littermates) were subjected to 30 min of ischemia and 24 h of reperfusion (I/R) by left coronary artery ligation in situ. This model has been previously described in detail elsewhere (5, 43).
Molecular and Biochemical Detection of TRPA1
Histology and immunofluorescence.
Sections (4 µm) of formalin-fixed, paraffin-embedded heart and dorsal root ganglia (DRG) of WT and TRPA1-null and hPLAP mice and of the uninfarcted LV of a male human failing heart were stained with hematoxylin and eosin as well as TRPA1 antibody or hPLAP antibody as appropriate. Sections of the male human failing heart were also stained with isolectin B4 antibody to label capillaries. Immunofluorescence staining was performed with rabbit polyclonal antibody against human TRPA1 (1:200 dilution, catalog no. ACC-037, Alomone Labs, Jerusalem, Israel) without or with blocking peptide [1:1 dilution, peptide sequence: (C)NSTGIINETSDHSE, corresponding to amino acid residues 747–760 of the first extracellular loop of human TRPA1, accession no. O75762, Alomone Labs] or rabbit monoclonal antibody against hPLAP (1:100 dilution, catalog no. ab133602, Abcam) and Alexa Fluor 647-conjugated goat anti-rabbit secondary antibody (1:400 dilution, catalog no. 21244, Invitrogen). Briefly, slides were deparaffinized, and heat-mediated antigen retrieval was performed in 10 mM citrate buffer (pH 6.0) for 20 min at 95°C. Slides were cooled to room temperature and then incubated with blocking buffer (10% normal goat serum, 1% BSA, and 0.025% Triton X-100 in 1× Tris-buffered saline) for 2 h at room temperature. Slides were then incubated with either TRPA1 antibody in blocking buffer with and without blocking peptide against TRPA1 (preincubated for 1 h at room temperature before addition to tissue sections) or PLAP antibody in blocking buffer at 4°C overnight. Slides were incubated with Alexa Fluor 647-conjugated goat anti-rabbit secondary antibody for 1 h in darkness at room temperature. Slides were washed three times for 5 min with 1× Tris-buffered saline + Tween 20 after every step. Tissue sections incubated with secondary antibody only served as the negative control. Slides were covered with DAPI containing Slow Fade Gold antifade reagent (catalog no. S36938, Invitrogen), and fluorescence was visualized using NIS-Elements software (Nikon, Tokyo, Japan) on a Nikon eclipse Ti fluorescence microscope at ×20 with a DAPI filter for nuclear staining and a Cy5 filter for TRPA1 or PLAP staining. Thick sections of the human small intestine stained with the hPLAP monoclonal antibody were used as a positive control [see Supplemental Figure S2 (doi:10.6084/m9.figshare.7597466)]. Light-level microscopy was performed using a Spot digital camera on an Olympus microscope.
To assess TRPA1 cellular distribution, freshly isolated cardiomyocytes from WT mice were fixed [3% paraformaldehyde-PBS (pH 7.4) for 10 min at room temperature], spotted onto glass slides, permeabilized (0.1% Triton X-100-PBS for 3 min at room temperature), and incubated for 30 min in 5% milk or 1% BSA-PBS as appropriate. Cells were washed three times for 5 min in PBS + Tween 20 and then incubated with primary antibody against the extracellular loop of TRPA1 (1:75 dilution in 5% milk, Alomone Labs) with or without blocking peptide or primary antibody against the intracellular amino terminus of TRPA1 (1:200 dilution in 1% BSA, rabbit TRPA1 antibody, catalog no. NB 110-40763, Novus Biologicals) overnight at 4°C. Connexin43 antibody (1:200 dilution in 5% milk, mouse monoclonal antibody, catalog no. ab11369, Abcam) was used to label intercalated disks. Cells were then incubated with Alexa Fluor 647-conjugated goat anti-rabbit secondary antibody for TRPA1 or Alexa Fluor 594-conjugated goat anti-mouse secondary antibody for connexin 43 (1:400 dilution, Invitrogen) for 1 h at room temperature in darkness. Slides were covered with DAPI containing Slow Fade Gold antifade reagent (catalog no. S36938, Invitrogen), and fluorescence was visualized on a Nikon A1 confocal microscope with a ×60 oil-immersion objective lens. TRPA1-specific staining in cardiomyocytes stained with or without peptide block was discerned under identical acquisition parameter settings (i.e., laser power, photomultiplier tube gain, and pinhole size) in NIS-Elements software.
TRPA1 quantitative RT-PCR.
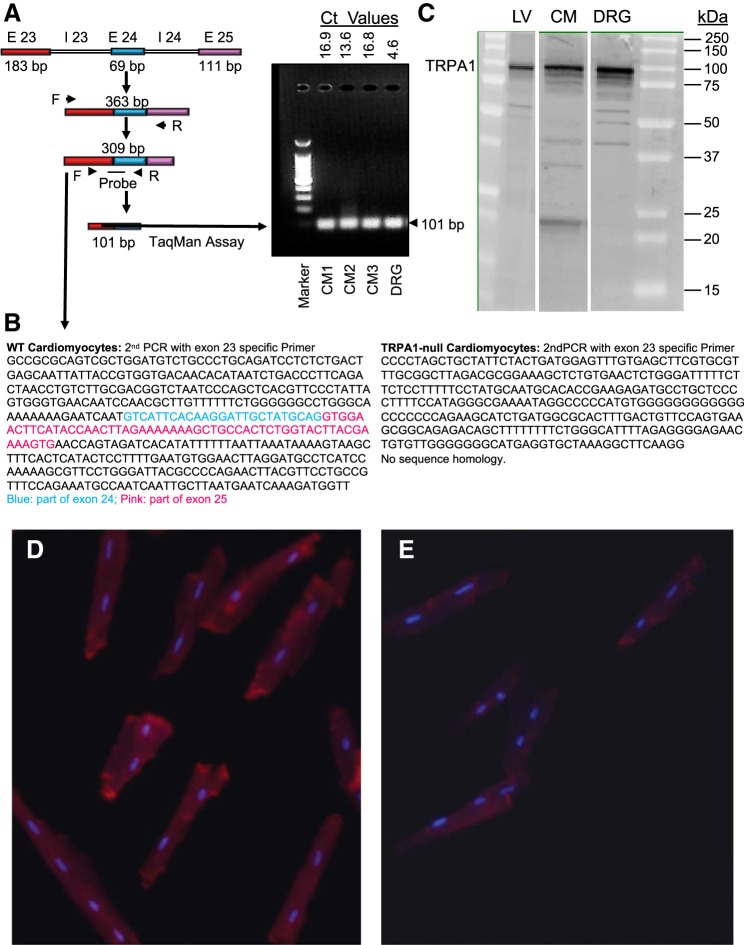
Isolation of total RNA from ∼500,000 cardiomyocytes from WT and TRPA1-null hearts and DRG (positive control) from WT mice was accomplished using TRIzol (Invitrogen) and an RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. RNase-free DNase (catalog no. 79254, Qiagen) was used to remove any contaminating DNA. RNA was eluted from RNeasy minicolumns into RNase-free water (30 μl), and the concentration was measured using a spectrophotometer (NanoDrop 2000C, Thermo Scientific). Total cDNA was synthesized using an iScript cDNA synthesis kit (catalog no. 170-8891, Bio-Rad) according to the manufacturer’s instructions. Briefly, denatured RNA (1 µg, 15 μl), 5× iScript reaction mix (4 μl), and 5× iScript reverse transcriptase (1 μl) were mixed (20 μl), and cDNA synthesis was carried out in a thermocycler (iCycler, Bio-Rad) under the following conditions: 42°C for 60 min and 94°C for 5 min. The resultant cDNA was target amplified using the following TRPA1-specific primers: 5′-GATGCCTTCAGCACCCCATTG-3′ (forward) and 5′-CACTTTGCGTAAGTACCAGAGTGG-3′ (reverse). Real-time quantitative PCR was performed using murine-specific TaqMan gene expression assays. Briefly, 2× TaqMan Fast Universal PCR Master Mix (5 μl, catalog no. 4440040), undiluted cDNA (2 μl) from target-specific PCR, and murine primers (3 μl) for mTrpa1 (Mm01227447_m1, catalog no. 4351372, Applied Biosystems) or β-actin (Mm00607939_m1, catalog no. 4331182, Applied Biosystems) were added sequentially to each well of a 384-well plate for TaqMan assays (10-μl final volume), and samples were subjected to quantitative RT-PCR using standard protocols on a real-time PCR system (model 7900 HT, Applied Biosystems). For each RNA sample, cDNAs were run in duplicate for each assay set in the same plate along with β-actin as the loading control. The relative expression level of mTrpa1 in both cardiomyocytes and DRG is shown as a cycle threshold (Ct) value (see Fig. 4).
Fig. 4.
Molecular evidence of transient receptor potential ankyrin 1 (TRPA1) in cardiomyocytes. A: mRNA of wild-type (WT) cardiomyocytes was probed for the specific TRPA1 sequence bridging exons (E) 23 and 24 (101-bp product), run on gel electrophoresis, and quantified by RT-PCR. CM, cardiomyocytes; Ct, cycle threshold; I, intron; F, forward; R, reverse. B: sequencing of cDNA from WT and TRPA1-null cardiomyocytes. The WT group matched the mTRPA1 sequence, whereas the TRPA1-null group (as expected) did not. C: Western blot for TRPA1 protein (relative molecular mass ≈ 100,000) in lysates of the left ventricle (LV), isolated cardiomyocyte (CM), and dorsal root ganglion (DRG; positive control) of WT mice. D and E: representative epifluorescence micrographs of isolated WT (D) and TRPA1-null (E) cardiomyocytes stained for TRPA1.
Agarose gel electrophoresis and DNA sequencing.
PCR products from the mTrpa1 TaqMan assay were run on 1.5% agarose gel and stained with ethidium bromide to visualize the expected 101-bp band in WT cardiomyocytes and DRG. Also, PCR products of target-specific mTrpa1 amplification (309 bp) in WT and TRPA1-null cardiomyocytes and WT DRG were sequenced on Illumina NextSeq 500 by the Center for Genetic and Molecular Medicine DNA Sequencing Core at the University of Louisville.
Western blot analysis.
LV, cardiomyocyte membrane, and DRG preparations of WT mice were homogenized in RIPA buffer (150 mM sodium chloride, 1.0% Nonidet P-40 or Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) and separated by SDS-PAGE. Immunoblots were analyzed using the polyclonal TRPA1 antibody (1:1,000 dilution, Alomone Labs). Blots were developed using ECL Plus reagent and detected with a Typhoon 9400 variable-mode imager.
Cardiomyocyte Isolation, Electrophysiology, Ca2+ Imaging, and Hypercontraction Assay
Isolation.
Ca2+-tolerant cardiomyocytes were isolated from the LV by collagenase digestion, as previously described (11). Briefly, WT hearts were perfused for 10 min and then digested with Liberase Blendzyme I (Roche) to release myocytes. Isolated myocytes were introduced into Tyrode bicarbonate buffer with increasing Ca2+ concentration. Viable cells were layered onto laminin-coated plates overnight.
Electrophysiology.
Isolated cardiomyocytes were plated in a recording chamber on an inverted microscope and allowed to adhere to a glass coverslip for recordings in the cell-attached configuration of the patch-clamp technique. Borosilicate glass micropipettes pulled to a resistance of 5–8 MΩ were used to form gigaohm seals at the intercalated disk region of individual myocytes. Bath and pipette solutions consisted of (in mM) 10 Na-pyrophosphate, 110 Na-methanesulfonate, 2 MgSO4, 2 EGTA, 2 Cs-methansulfonate, and 10 HEPES, pH 7.4. Data were acquired at a sampling rate of 100 µs and low-pass filtered at 2 kHz using an Axopatch 200B amplifier and a Digidata 1440 digitizer (Molecular Devices). Recordings were performed at room temperature under continuous perfusion of bath solution containing DMSO (vehicle), cinnamaldehyde (50 µM), or cinnamaldehyde + HC-030031 (10 µM). Data were analyzed using Clampfit 10 software.
Ca2+ imaging.
Isolated cardiomyocytes were plated onto laminin-coated glass-bottom culture dishes at a density of 75,000 cells/well and incubated with 2 ml culture medium (37°C, overnight). Cells were loaded with 5 µM fluo 8-AM (catalog no. ab142773, Abcam) in HBSS for 1 h at room temperature. Some dishes were incubated in HBSS without fluo 8-AM as a negative control. After fluo 8 loading, a period of 30 min was allowed for deesterification of fluo 8-AM. In a set of dishes, cardiomyocytes were preincubated with 10 µM HC-030031 (a TRPA1 channel inhibitor) for 30 min at room temperature. Cardiomyocytes were visualized with a scanning confocal microscope using an oil-immersion ×60 objective lens. Experiments were performed at 37°C. After basal Ca2+ was monitored for 3 min, freshly prepared HBSS containing acrolein (25 µM) or acrolein + HC-030031 (10 µM) was added to the dish, and fluorescence at 488-nm excitation/510-nm emission was monitored continuously for 1 h or until cells were hypercontracted. Images were analyzed in Fiji software (National Institutes of Health), and intracellular Ca2+ concentration was expressed as change in fractional fluorescence (F488) relative to baseline.
Hypercontraction assay.
After isolation, myocytes (≈75,000) were incubated in serum-free MEM overnight in laminin-coated plates. To measure acrolein sensitivity, myocytes were superfused in an oxygenated HBSS buffer without or with acrolein for 60 min. To test for TRPA1 dependence, cells were preincubated with the TRPA1 inhibitor HC-030031 (10 μM) for 30 min without or with the Na+/Ca2+ exchanger 1 (NCX1) inhibitor SN-6 (10 μM) for 30 min (30) before superfusion with acrolein (10 or 25 μM) for 60 min in HBSS. Digital images of myocytes (60–100 myocytes/field) were used for quantification of the total number of rod-shaped cells, as previously described (9, 11). Control treatments were included each time cardiomyocyte hypercontraction was assayed. Effects of HBSS, HC-030031, and SN-6 alone on cardiomyocyte viability over 1 h were minimal and indistinguishable.
Mass spectrometry.
To assess whether the inhibitors HC-030031 and SN-6 directly reacted with acrolein, we made separate reaction mixtures of 100 µM inhibitor and 100 µM acrolein in 50 mM ammonium acetate (pH 7.4) and incubated the mixtures at 37°C. Aliquots of the reactions at 0, 5, 30, 45, and 60 min were analyzed with a mass spectrometer (Quattro LC, Waters, Milford, MA) in positive and negative ion modes.
Statistical Analysis
Data are presented as means ± SE. For different treatment groups, data were compared using paired or unpaired t-tests or one-way ANOVA with Student-Newman-Keuls or Bonferroni’s post test where appropriate (SigmaStat, SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
RESULTS
Cardiovascular Phenotyping
Overall, cardiac dimensions, cardiac function, and blood pressure were similar in TRPA1-null and aged-matched WT male mice (Table 1). The finding of no significant differences in LV-to-body weight ratios or HR between TRPA1-null and aged-matched WT male mice indicates that genetic deficiency of TRPA1 does not markedly alter baseline cardiovascular function in young adult male mice.
Table 1.
Cardiovascular parameters of WT and TRPA1-null mice
| Parameter | WT Mice | TRPA1-Null Mice |
|---|---|---|
| Body weight, g | 29 ± 1 | 30 ± 1 |
| LV/body weight, mg/g (corrected) | 4.3 ± 0.3 | 4.0 ± 0.2 |
| Heart rate, beats/min | 470 ± 19 | 454 ± 17 |
| Stroke volume, µl | 37 ± 1 | 38 ± 2 |
| Cardiac output, ml/min | 17 ± 1 | 17 ± 1 |
| LV end-diastolic volume, µl | 55 ± 4 | 60 ± 4 |
| LV end-systolic volume, µl | 18 ± 4 | 22 ± 3 |
| Ejection fraction, % | 68 ± 4 | 64 ± 3 |
| Blood pressure,* mmHg | ||
| Systolic | 106 ± 4 | 105 ± 3 |
| Mean | 88 ± 3 | 88 ± 4 |
| Diastolic | 78 ± 3 | 80 ± 4 |
Values are means ± SE; n = 7 wild-type (WT) mice and 9 transient receptor potential ankyrin 1 (TRPA1)-null mice except as noted. LV, left ventricle.
Determined by a noninvasive tail-cuff system (n = 4 WT mice and 6 TRPA1-null mice).
TRPA1 and I/R Injury
Ex vivo Langendorff perfusion.
To explore how TRPA1 contributed to ischemic injury, hearts isolated from WT and TRPA1-null mice (littermates) were isolated for Langendorff perfusion. No differences were observed in baseline parameters (HR, LV pressure, LV developed pressure, rate of rise of LV pressure, and CF; Fig. 1). However, after 30 min, HR recovered significantly more in TRPA1-null hearts than in WT hearts (Fig. 1A), yet contractile recovery (e.g., LV developed pressure) was minimal and indistinguishable between TRPA1-null and WT hearts (Fig. 1, B and C). Slightly greater hypercontracture in WT than TRPA1-null hearts was detected as elevated peak and sustained LV pressure during reperfusion, although it was not statistically different (Fig. 1D). HR recovery was paralleled, in general, by CF in TRPA1-null and WT hearts (Fig. 1E), although total release of creatine kinase and lactate dehydrogenase (markers of myocyte injury) was not significantly different between TRPA1-null and WT hearts (Table 2).
Fig. 1.
Effects of ischemia and reperfusion on Langendorff-perfused hearts from male wild-type (WT) and transient receptor potential ankyrin 1 (TRPA1)-null mice. Isolated hearts were perfused for 30 min and then subjected to 30 min of ischemia followed by 45 min of reperfusion. Cardiac parameters, including heart rate (HR, beats/min; A), left ventricular (LV) developed pressure (LVDP; B), maximum and minimum rate of rise and fall of LV developed pressure (dP/dt; C), LV pressure minimum (LVPmin; D), and coronary flow (E), were monitored in real time by a LV implanted balloon and an in-line flow probe, respectively. Values are means ± SE. *P < 0.05, TRPA1-null vs. WT mice.
Table 2.
Effects of ischemia-reperfusion on injury markers and total flow in Langendorff-perfused hearts from male WT and TRPA1-null mice
| Injury Markers |
|||
|---|---|---|---|
| Creatine Kinase, IU | Lactate Dehydrogenase, IU | Total Flow, ml | |
| WT mice | 37.4 ± 5.5 | 7.3 ± 1.0 | 55.4 ± 8.1 |
| TRPA1-null mice | 36.0 ± 3.3 | 7.2 ± 0.7 | 66.0 ± 7.4 |
Values are means ± SE; n = 5 wild-type (WT) mice and 5 transient receptor potential ankyrin 1 (TRPA1)-null mice.
In vivo.
Because we observed modest TRPA1 dependence of I/R injury in the brief reperfusion (45 min) of the Langendorff model, we proceeded to test whether TRPA1 contributed to the more prolonged myocardial I/R injury in vivo. Male WT and TRPA1-null mice (littermates) were subjected to 30 min of left coronary artery occlusion followed by 24 h of reperfusion. In WT mice, this protocol resulted in large LV infarcts that were, on average, 34.1 ± 9.3% of the area at risk (Fig. 2). TRPA1-null mice were less sensitive to ischemia, with infarct size of only 14.3 ± 9.9% of the area at risk (Fig. 2). These observations suggest that genetic deficiency of TRPA1 results in less myocardial injury induced by coronary ligation. Because of these findings, we next examined the distribution of cardiac TRPA1.
Fig. 2.
Transient receptor potential ankyrin 1 (TRPA1) contributes to ischemia-reperfusion injury in mouse heart. A: representative images of triphenyltetrazolium chloride (TTC)- and phthalocyanine blue-stained midventricular cross sections of hearts from male wild-type (WT; i) and TRPA1-null (ii) mice 24 h after myocardial ischemia (30 min). The unstained (white) area in the risk region (TTC, red) represents the infarcted myocardium. B: summary data of the risk region [% of the left ventricle (LV); left y-axis) and infarct size (% of the risk region; right y-axis). Values are means ± SE. *P < 0.05, TRPA1-null vs. WT mice.
Cardiac distribution of TRPA1 protein.
TRPA1 expression is abundant in afferent sensory nerve endings associated peripherally with pain reception and pulmonary irritant responses (unmyelinated C-fibers). Although the heart has a relatively low level of nociceptive innervation, we immunostained for TRPA1 in the LV of mouse (Fig. 3, A–C) and human (heart failure; Fig. 3, D–F) hearts. Positive TRPA1 immunofluorescence was associated with cardiomyocytes and coronary blood vessels (Fig. 3, B and E). Positive TRPA1 staining was absent in the presence of TRPA1-specific blocking peptide (Fig. 3, C and F) or when primary antibody was absent (data not shown). To confirm expression of TRPA1 in mouse hearts and, specifically, in cardiomyocytes, we performed quantitative RT-PCR, DNA sequencing, Western blot analysis, and fluorescence microscopy (Fig. 4). Our targeted PCR amplified a mRNA segment across exons 23 and 24 (i.e., targeted deletion in TRPA1-null mice) (6), and this PCR product was visualized by gel electrophoresis (Fig. 4A). TRPA1 transcript levels were substantially lower in cardiomyocytes than in isolated DRG, as reflected by Ct values (Fig. 4A). Sequencing of this PCR product confirmed TRPA1 identity in isolated cardiomyocytes of WT but not TRPA1-null mice (Fig. 4B). Western blot analysis provided evidence that TRPA1 protein (relative molecular mass ≈100,000) was present in both cardiomyocytes and DRG (Fig. 4C). Antibodies generated against TRPA1 peptide fluorescently labeled isolated cardiomyocytes of WT but not TRPA1-null mice (Fig. 4, D and E). Together, quantitative RT-PCR, DNA sequencing, Western blot analysis, and immunostaining indicated that TRPA1 is present in isolated cardiomyocytes from WT but not TRPA1-null mice.
Fig. 3.
Transient receptor potential ankyrin 1 (TRPA1) in the healthy mouse heart and failing human heart. A–C: mid left ventricular cross sections of male wild-type mouse stained with hematoxylin and eosin (A), fluorescently labeled TRPA1 antibody (red) and DAPI [blue (nuclear stain)] (B), and fluorescently labeled TRPA1 antibody, specific TRPA1 blocking peptide, and DAPI (C). D–F: ventricular sections of the uninfarcted failing human myocardium stained with hematoxylin and eosin (D), fluorescently labeled TRPA1 antibody (green) and DAPI (E), and fluorescently labeled TRPA1 antibody, specific TRPA1 blocking peptide, isolectin B4 [red (stains capillaries)], and DAPI (F). Cardiomyocytes and the coronary vasculature stained positive with TRPA1 antibody.
To better define the location and function of TRPA1 in cardiomyocytes, we performed confocal microscopy and electrophysiology. Immunostaining of isolated cardiomyocytes revealed TRPA1-associated fluorescence that largely overlapped that of connexin 43, a gap junction protein enriched in intercalated disks (Fig. 5, A–D). Similarly, two different TRPA1 antibodies (i.e., one antibody targeting the extracellular loop and one antibody targeting the intracellular terminus) and epifluorescence were used to localize positive TRPA1 staining in intercalated disks [see Supplemental Figure S5 (doi:10.6084/m9.figshare.7597466) and Supplemental Movie S1 (doi:10.6084/m9.figshare.7597466)]. TRPA1 staining was prevented by coincubation of TRPA1 antibody with a blocking peptide (shared sequence with the targeted extracellular loop of TRPA1), which decreased TRPA1 staining [Fig. 5E; see Supplemental Figure S3C (doi:10.6084/m9.figshare.7597466)] but not connexin43 staining (Fig. 5C). The DRG served as positive control for TRPA1 staining [see Supplemental Figure S1 (doi:10.6084/m9.figshare.7597466)]. Because these data show that TRPA1 is localized in the membrane, we assessed membrane functionality of TRPA1 channels in isolated adult cardiomyocytes. Using the cell-attached patch configuration of patch-clamp technique, we performed single channel recordings. When targeting the intercalated disk region of isolated cardiomyocytes, we obtained recordings of single channel opening events that had amplitudes similar to those previously reported for heterologous expression of TRPA1 channels (29, 44). We observed single channel activity in sarcolemmal patches that was significantly potentiated upon application of cinnamaldehyde, a known TRPA1 agonist (Fig. 5F, iv–vi) (28). Consistent with the notion that functional TRPA1 channels reside in the sarcolemma of adult mouse cardiomyocytes, channel activity that was enhanced by application of cinnamaldehyde also was inhibited by application of the selective TRPA1 antagonist HC-030031 (Fig. 5F, vii–ix).
Fig. 5.
Transient receptor potential ankyrin 1 (TRPA1) in cardiomyocyte membranes colocalized to the intercalated disk. A–E: isolated male wild-type (WT) mouse cardiomyocytes stained with DAPI [blue (nuclear stain); A], fluorescently labeled TRPA1 antibody (green; B), connexin 43 antibody (red; C), and combined antibodies without (D; white arrows indicate colocalization) or with (E) TRPA1-blocking peptide. F: representative cell-attached patch recordings (holding potential: −40 mV) of single channel events at the intercalated disk region of an isolated adult cardiomyocyte in the absence [control (i, ii, and iii)] and presence of cinnamaldehyde (50 µM; iv, v, and vi) and HC-030031 (10 µM; vii, viii, and ix). Open probabilities (nPo) are shown for each condition. Recordings are representative of two independent experiments.
TRPA1-dependent cardiomyocyte injury.
Because TRPA1 forms a tetrameric cation channel protein, we considered channel-dependent Ca2+ entry to be a likely mechanism by which activating TRPA1 could induce injury (hypercontraction) in cardiomyocytes. Hence, we tested whether acrolein induced Ca2+ entry in a TRPA1-dependent manner. Consistent with our hypothesis, we found that treatment of isolated fluo 8-loaded cardiomyocytes elevated intracellular Ca2+ (Fig. 6A). Acrolein-induced elevation of intracellular Ca2+, although not abolished, was delayed significantly when cells were preincubated with the TRPA1 antagonist HC-030031 for 30 min and also present during acrolein treatment, suggesting that TRPA1 channels may contribute to changes in Ca2+ entry into cardiomyocytes upon exposure to cytotoxic aldehydes. To determine whether elevation of intracellular Ca2+ was related to cell death, myocytes were either untreated or preincubated with the TRPA1 antagonist HC-030031, the NCX1 inhibitor SN-6 (30), or HC-030031 + SN-6 and then superfused with acrolein. Superfusion with acrolein (10 or 25 μM) led to increased hypercontracture (shortening and rounding) of myocytes (>85% contracted, 15% viable) within 60 min (Fig. 6B). However, in the presence of HC-030031 or SN-6, the percentage of viable acrolein-treated myocytes was significantly increased to 55% and 65%, respectively (Fig. 6C), consistent with the notion that acrolein-induced hypercontracture was mediated by Ca2+ overload. When HC-030031 and SN-6 antagonists were combined, no greater protection was observed, indicating likely convergence of protective mechanisms against Ca2+ overload (Fig. 6C). Neither HC-030031 nor SN-6 formed adducts with acrolein when incubated for ≤60 min at 37°C, as measured by mass spectrometry, indicating that the inhibitors were acting specifically (data not shown).
Fig. 6.

Acrolein-induced transient receptor potential ankyrin 1 (TRPA1)-dependent Ca2+ entry and cardiomyocyte hypercontracture. Cardiomyocytes isolated from hearts of male wild-type (WT) mice were plated overnight and superfused with acrolein (10 or 25 µM), and hypercontraction was monitored over 60 min. A: time course of acrolein-induced changes in intracellular Ca2+ [fluorescence at 488 nm (F488)] in isolated fluo 8-loaded cardiomyocytes. Acrolein (25 µM) was added at time indicated by arrow in the absence or presence of HC-030031. B: representative images of cardiomyocytes at time 0 or 60 min after acrolein exposure (rounded myocyte in the bottom left corner). C: isolated cardiomyocyte survival after superfusion with acrolein alone or in the presence of TRPA1 antagonist (HC-030031; 10 µM), SN-6 (10 µM), or HC-030031 + SN-6 over 60 min. Values are means ± SE; n = 2–4 separate experiments. *P < 0.05 vs. acrolein alone.
DISCUSSION
This study reveals a novel role of TRPA1 in cardiac I/R injury; thus, we propose that TRPA1 may be a therapeutic target for intervention in I/R (and, potentially, heart failure), which so far has been elusive. TRPA1 is expressed in cardiomyocytes and other cells of the healthy murine and diseased human heart and not just in pain sensory (nociceptive) fibers (afferents). Although TRPA1 activation in other cell types besides cardiomyocytes may contribute to cardiac I/R injury in vivo, our results show that TRPA1 in cardiomyocytes, especially localized to the sarcolemma and intercalated disks, is a likely target of lipid peroxidation-derived aldehydes, such as acrolein generated during I/R (12). Moreover, TRPA1 activation by acrolein contributes directly to cardiomyocyte cell death via Ca2+ overload. A corollary inference is that because TRPA1 is a receptor of unsaturated aldehydes, our findings further support the idea that cardiac I/R injury may be attributed to secondary oxidants such as aldehydes (22).
As previously reported (3), our current data provide complementary evidence that TRPA1 is present and functional in murine cardiomyocytes and is present in the failing human heart. Our findings substantiate evidence from a recent study using immunofluorescence to localize TRPA1 in cardiomyocyte sarcomeres of WT mice (3). Similarly, in both studies, TRPA1 was observed selectively in intercalated disks, and our demonstration of increased channel open probability in single channel patch preparations with cinnamaldehyde, a known TRPA1 agonist, and subsequent inhibition of channel activity by a TRPA1 antagonist supports the idea of a functional TRPA1 channel (Fig. 5E). Moreover, using a humanized transgenic mouse expressing the hPLAP gene under control of the mouse TRPA1 promoter (i.e., hPLAP mice) (27), we found that TRPA1 is expressed in the adult mouse DRG, heart, and cardiomyocytes [see Supplemental Figures S2 and S3 (doi:0.6084/m9.figshare.7597466)]. This evidence removes uncertainty regarding antibody specificity, because it uses a completely different antibody (i.e., anti-human PLAP), yet we still observe similar organ distribution of TRPA1. Notably, immunofluorescence in organs of humanized (hPLAP) mice is diffuse, because PLAP is a cytosolic enzyme, rather than a transmembrane protein.
Cardiac injury as a result of LV pacing, I/R, and heart failure generates lipid peroxidation products, including unsaturated aldehydes, such as acrolein and 4-hydroxynonenal, which are TRPA1 agonists (36, 41). Recently, using an ultraperformance LC-MS and the multiple-reaction-monitoring method, we measured free acrolein in perfused hearts subjected to global ischemia by 15 min of exposure to N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium iodide, which binds acrolein, and showed that myocardial ischemia results in acrolein formation (13). Acrolein reacts readily with cellular nucleophiles, including reduced glutathione, histidine, lysine, and cysteine, leading to the formation of covalent adducts. TRPA1 protein contains multiple free intracellular cysteine residues, which are thought to be covalently modified by strong electrophiles, such as acrolein, as part of the mechanism to activate TRPA1 channels (28). Accumulation of acrolein-modified proteins in the ischemic heart is consistent with a role of protein modification in injury (13). A significant role of acrolein in I/R injury is supported by results showing that the sensitivity of cardiomyocytes to acrolein-induced cell death is increased in myocytes deficient in acrolein metabolism (13). Our observation of decreased acrolein-induced hypercontraction in the presence of a TRPA1 antagonist, as well as by SN-6, an inhibitor of NCX (Fig. 4), further supports this pathway.
Our previous investigation into the mechanism of acrolein-induced cell death revealed that acrolein treatment caused changes in Na+ current similar to those reported for oxidants such as tert-butyl hydroperoxide (11) and lipid peroxidation products such as HNE (9) and isoketal (19). In cells exposed to 4-hydroxynonenal (9) and peroxide (8), the increase in the window current was accompanied by prolongation of the action potential, increased arrhythmogenesis, and elevation of intracellular Na+. This increase in intracellular Na+ has been suggested to increase intracellular Ca2+ concentration, presumably via NCX, and could lead to Ca2+-induced rigor and hypercontracture (11). Our current results support convergence on this common pathway, because a TRPA1 antagonist, HC-030031, delayed both acrolein-induced Ca2+ accumulation and myocyte hypercontraction, implicating Ca2+ overload as a major trigger of cell death, as previously suggested (42). Both dysregulation of Na+ current and Ca2+ overload are important features of myocardial ischemic injury that leads to contractile filament hypercontracture, myocyte necrosis (21), and arrhythmogenesis. Thus, together, our observations imply that, by increasing Ca2+ overload, I/R-induced lipid peroxidation products such as acrolein could contribute, at least in part, to myocardial necrosis and sudden cardiac death. TRPA1 activation by aldehydes such as acrolein may contribute to I/R injury directly by enhancing Ca2+ entry and overload in cardiomyocytes, as shown in heterologous systems (1). Thus, perhaps a TRPA1 inhibitor may be provided post-I/R to reduce infarction and/or angina mediated by lipid-derived aldehydes that directly activate cardiomyocyte TRPA1. Additionally, a similar anti-TRPA1 approach may be beneficial in human heart failure where lipid peroxidation products (unsaturated aldehydes) and inflammation are implicated in worsening outcomes (36), and we show that TRPA1 is widely expressed in human failing heart (Fig. 3, D–F).
The present study has important limitations. Because our in vivo model of I/R uses a whole body deficient (TRPA1-null) mouse, we cannot conclude that the beneficial cardioprotection against I/R in vivo is solely a function of the absence of TRPA1 in cardiomyocytes, e.g., compensatory changes (other TRP proteins and TRPV1) (3) and loss of neutrally mediated effects (catecholamines). Nonetheless, we infer from our overall data that cardiomyocyte-specific TRPA1 is a critical contributor to I/R injury for the following reasons. First, TRPA1-null mice are not protected against other sources of aldehyde stress. For example, we show that TRPA1-null mice are more sensitive than WT mice to inhaled toxic levels of acrolein (15). Second, I/R by LV coronary artery ligation is specific to the heart and, as such, is a well-controlled form of cardiomyocyte stress and injury. Third, hearts of TRPA1-deficient mice are similar in structure and function to hearts of WT mice. Fourth, activation of vascular TRPA1 is known to induce dilation and increase blood flow (16), a response that likely promotes reperfusion and preservation of the myocardium after ischemia but should not induce greater injury. We found no evidence of diminished CF after I/R in Langendorff-perfused TRPA1-null hearts compared with WT hearts (Fig. 6E), suggesting little to no contribution (albeit perhaps surprisingly) of differences in vascular function to the differences in the extent of I/R-induced injury between groups. Of course, reperfusion enhances oxidative stress and aldehyde formation (10), so the deleterious contribution of neuronal and nonneuronal TRPA1 (e.g., vascular) in I/R injury remains unclear. Finally, we acknowledge the possibility that the effects of I/R in TRPA1-null mice in vivo are complicated because of different physiological functions of TRPA1 in different cell types; thus, this uncertainty provides fertile ground for future studies to elucidate more precisely the contribution of TRPA1 to I/R using models with cell-specific deletion (or targeted inhibition) of TRPA1.
GRANTS
This work was supported by American Heart Association Grant 16SDG27260070, National Institutes of Health Grants GM-103492, HL-122676, HL-142710, and ES023716; and a grant from the University of Louisville School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
D. J. Conklin is the guarantor of this work. As such, he had full access to all data and takes responsibility for the integrity and accuracy of the data.
AUTHOR CONTRIBUTIONS
D.J.C. and A.B. conceived and designed research; D.J.C., Y.G., G.J., M.A.N., P.J.K., L.G., and J.D.H. performed experiments; D.J.C., Y.G., G.J., M.A.N., D.O., P.J.K., and J.D.H. analyzed data; D.J.C., Y.G., M.A.N., J.D.H., R.B., and A.B. interpreted results of experiments; D.J.C., Y.G., G.J., M.A.N., and L.G. prepared figures; D.J.C. drafted manuscript; D.J.C., Y.G., G.J., M.A.N., D.O., R.B., and A.B. edited and revised manuscript; D.J.C., Y.G., G.J., M.A.N., D.O., P.J.K., L.G., R.B., and A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. S.-E. Jordt (Duke University) for the donation of breeding pairs of TRPA1-null mice, F. Li, D. Mosley, G. Shirk, and the Diabetes and Obesity Center’s Imaging and Physiology Core at the University of Louisville for technical assistance, and Dr. P. Boor (University of Texas Medical Branch at Galveston) and B. Bishop (University of Louisville) for the donation of fixed specimens of the human heart and human small intestine, respectively.
REFERENCES
- 1.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei SR, Ghosh M, Sinharoy P, Dey S, Bratz IN, Damron DS. TRPA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway. Channels (Austin) 11: 587–603, 2017. doi: 10.1080/19336950.2017.1365206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrei SR, Sinharoy P, Bratz IN, Damron DS. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: co-localization at z-discs, costameres and intercalated discs. Channels (Austin) 10: 395–409, 2016. doi: 10.1080/19336950.2016.1185579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES, Bevan S, Brain SD. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun 5: 5732, 2014. doi: 10.1038/ncomms6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cε interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res 92: 873–880, 2003. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Benjebria A, Crozet Y, Eskew ML, Rudeen BL, Ultman JS. Acrolein-induced smooth muscle hyperresponsiveness and eicosanoid release in excised ferret tracheae. Toxicol Appl Pharmacol 135: 35–44, 1995. doi: 10.1006/taap.1995.1206. [DOI] [PubMed] [Google Scholar]
- 8.Beresewicz A, Horackova M. Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J Mol Cell Cardiol 23: 899–918, 1991. doi: 10.1016/0022-2828(91)90133-7. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar A. Electrophysiological effects of 4-hydroxynonenal, an aldehydic product of lipid peroxidation, on isolated rat ventricular myocytes. Circ Res 76: 293–304, 1995. doi: 10.1161/01.RES.76.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 11.Castro GJ, Bhatnagar A. Effect of extracellular ions and modulators of calcium transport on survival of tert-butyl hydroperoxide exposed cardiac myocytes. Cardiovasc Res 27: 1873–1881, 1993. doi: 10.1093/cvr/27.10.1873. [DOI] [PubMed] [Google Scholar]
- 12.Conklin DJ. Acute cardiopulmonary toxicity of inhaled aldehydes: role of TRPA1. Ann NY Acad Sci 1374: 59–67, 2016. doi: 10.1111/nyas.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conklin DJ, Guo Y, Jagatheesan G, Kilfoil PJ, Haberzettl P, Hill BG, Baba SP, Guo L, Wetzelberger K, Obal D, Rokosh DG, Prough RA, Prabhu SD, Velayutham M, Zweier JL, Hoetker JD, Riggs DW, Srivastava S, Bolli R, Bhatnagar A. Genetic deficiency of glutathione S-transferase P increases myocardial sensitivity to ischemia-reperfusion injury. Circ Res 117: 437–449, 2015. doi: 10.1161/CIRCRESAHA.114.305518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conklin DJ, Haberzettl P, Jagatheesan G, Baba S, Merchant ML, Prough RA, Williams JD, Prabhu SD, Bhatnagar A. Glutathione S-transferase P protects against cyclophosphamide-induced cardiotoxicity in mice. Toxicol Appl Pharmacol 285: 136–148, 2015. doi: 10.1016/j.taap.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin DJ, Haberzettl P, Jagatheesan G, Kong M, Hoyle GW. Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol Appl Pharmacol 324: 61–72, 2017. doi: 10.1016/j.taap.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 104: 987–994, 2009. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipović MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM, Khalil M, Siklosi N, Nau C, Ivanović-Burmazović I, Neuhuber WL, Becker C, Neurath MF, Reeh PW. TRPA1 and substance P mediate colitis in mice. Gastroenterology 141: 1346–1358, 2011. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Fischer MJ, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem 285: 34781–34792, 2010. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ II, Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res 97: 1262–1269, 2005. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 21.Hale SL, Leeka JA, Kloner RA. Improved left ventricular function and reduced necrosis after myocardial ischemia/reperfusion in rabbits treated with ranolazine, an inhibitor of the late sodium channel. J Pharmacol Exp Ther 318: 418–423, 2006. doi: 10.1124/jpet.106.103242. [DOI] [PubMed] [Google Scholar]
- 22.Hill BG, Bhatnagar A. Beyond reactive oxygen species: aldehydes as arbitrators of alarm and adaptation. Circ Res 105: 1044–1046, 2009. doi: 10.1161/CIRCRESAHA.109.209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 120, Suppl: S1–S9, 2009. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation 80: 1115–1127, 1989. doi: 10.1161/01.CIR.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 25.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340–350, 2010. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, Hazari M. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol 324: 51–60, 2017. doi: 10.1016/j.taap.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545, 2007. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 29.Niforatos W, Zhang XF, Lake MR, Walter KA, Neelands T, Holzman TF, Scott VE, Faltynek CR, Moreland RB, Chen J. Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597). Mol Pharmacol 71: 1209–1216, 2007. doi: 10.1124/mol.106.033621. [DOI] [PubMed] [Google Scholar]
- 30.Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol 573: 161–169, 2007. doi: 10.1016/j.ejphar.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 31.Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res 87: 760–768, 2010. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- 32.Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemény Á, Materazzi S, Nassini R, Helyes Z, Szolcsányi J, Pintér E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol 689: 56–64, 2012. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 33.Simon SA, Liedtke W. How irritating: the role of TRPA1 in sensing cigarette smoke and aerogenic oxidants in the airways. J Clin Invest 118: 2383–2386, 2008. doi: 10.1172/JCI36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha S, Sinharoy P, Bratz IN, Damron DS. Propofol causes vasodilation in vivo via TRPA1 ion channels: role of nitric oxide and BKCa channels. PLoS One 10: e0122189, 2015. doi: 10.1371/journal.pone.0122189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinharoy P, Bratz IN, Sinha S, Showalter LE, Andrei SR, Damron DS. TRPA1 and TRPV1 contribute to propofol-mediated antagonism of U46619-induced constriction in murine coronary arteries. PLoS One 12: e0180106, 2017. doi: 10.1371/journal.pone.0180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: role of aldose reductase. Am J Physiol Heart Circ Physiol 283: H2612–H2619, 2002. doi: 10.1152/ajpheart.00592.2002. [DOI] [PubMed] [Google Scholar]
- 37.Takabe W, Niki E, Uchida K, Yamada S, Satoh K, Noguchi N. Oxidative stress promotes the development of transformation: involvement of a potent mutagenic lipid peroxidation product, acrolein. Carcinogenesis 22: 935–941, 2001. doi: 10.1093/carcin/22.6.935. [DOI] [PubMed] [Google Scholar]
- 38.Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E, Marone IM, Ferreira J, Geppetti P, Nassini R. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139: 1361–1377, 2016. doi: 10.1093/brain/aww038. [DOI] [PubMed] [Google Scholar]
- 39.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104: 13519–13524, 2007. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med 9: 109–113, 1999. doi: 10.1016/S1050-1738(99)00016-X. [DOI] [PubMed] [Google Scholar]
- 41.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 95: 4882–4887, 1998. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Sun Y, Asahi M, Otsu K. Acrolein, an environmental toxin, induces cardiomyocyte apoptosis via elevated intracellular calcium and free radicals. Cell Biochem Biophys 61: 131–136, 2011. doi: 10.1007/s12013-011-9169-5. [DOI] [PubMed] [Google Scholar]
- 43.West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 118: 1970–1978, 2008. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci 27: 605–611, 2008. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]