Abstract
Fatty liver is the earliest response of the liver to excessive ethanol consumption. Central in the development of alcoholic steatosis is increased mobilization of nonesterified free fatty acids (NEFAs) to the liver from the adipose tissue. In this study, we hypothesized that ethanol-induced increase in ghrelin by impairing insulin secretion, could be responsible for the altered lipid metabolism observed in adipose and liver tissue. Male Wistar rats were fed for 5–8 wk with control or ethanol Lieber-DeCarli diet, followed by biochemical analyses in serum and liver tissues. In addition, in vitro studies were conducted on pancreatic islets isolated from experimental rats. We found that ethanol increased serum ghrelin and decreased serum insulin levels in both fed and fasting conditions. These results were corroborated by our observations of a significant accumulation of insulin in pancreatic islets of ethanol-fed rats, indicating that its secretion was impaired. Furthermore, ethanol-induced reduction in circulating insulin was associated with lower adipose weight and increased NEFA levels observed in these rats. Additionally, we found that increased concentration of serum ghrelin was due to increased synthesis and maturation in the stomach of the ethanol-fed rats. We also report that in addition to its effect on the pancreas, ghrelin can also directly act on hepatocytes via the ghrelin receptors and promote fat accumulation. In conclusion, alcohol-induced elevation of circulating ghrelin levels impairs insulin secretion. Consequently, reduced circulating insulin levels likely contribute to increased free fatty acid mobilization from adipose tissue to liver, thereby contributing to hepatic steatosis.
NEW & NOTEWORTHY Our studies are the first to report that ethanol-induced increases in ghrelin contribute to impaired insulin secretion, which results in the altered lipid metabolism observed in adipose and liver tissue in the setting of alcoholic fatty liver disease.
Keywords: adipose tissue, alcoholic fatty liver, ghrelin, insulin
INTRODUCTION
Alcohol-induced liver disease is a major health problem both in the United States and worldwide. In the United States alone, alcohol abuse is the leading cause for deaths from cirrhosis (39, 40). Alcoholic fatty liver disease, characterized by accumulation of lipids [primarily triglycerides (TGs)] in the hepatocytes is one of the earliest pathological changes in the progression of alcohol-induced liver disease (22). Ethanol abuse increases hepatocyte TG accumulation, partly from increased de novo fatty acid synthesis and increased flow of fatty acids to the liver from adipose tissue (20, 23, 42). In addition, alcohol-impaired fat transport out of the liver via reduced very-low-density lipoprotein secretion and decreased fatty acid oxidation contributes to the generation of fatty liver (15, 18, 24). The accumulation of fat in hepatocytes makes the liver susceptible to inflammatory mediators or toxic agents, leading to further progression to hepatitis and eventually fibrosis.
Adipose tissue is an important organ for energy homeostasis in the body. Adipose tissue serves as a storage site for the excess energy derived from food consumption. Studies have shown that chronic alcohol exposure reduces adipose tissue mass and adipocyte size in mice and rats (23, 42) by enhancing lipolysis of the adipose tissue. The free fatty acids (FFAs), thus released in to the circulation, are taken up by the liver and esterified to form TGs, leading to the development of fatty liver. Clinical studies have also demonstrated a negative correlation between liver fat and body fat mass, showing that alcoholics who have fatty liver have significantly lower body weight and lower fat mass than controls (1, 2, 30, 32). Thus, there is clearly a link between adipose tissue lipolysis and hepatic fat accumulation after alcohol exposure.
The adipose-liver axis is modulated by the hormone insulin, which influences lipid metabolism by promoting the export of lipoproteins from the liver and inhibiting lipolysis in adipocytes to facilitate fat storage in adipose tissue. Chronic ethanol exposure in rats has been shown to promote lipolysis in adipocytes by disrupting insulin-dependent signal transduction. In addition to increased insulin resistance, chronic ethanol administration also decreases serum insulin levels (23, 25, 26).
Insulin secretion from pancreatic β cells is tightly regulated by the nutrient status of the body. Although glucose, FFAs, and amino acids serve as stimuli for insulin release, several hormonal factors also regulate insulin secretion. Ghrelin, a hormone mainly secreted from the stomach (4), is reported to inhibit insulin secretion from pancreatic β cells in both humans and experimental animals (10, 11, 13, 36, 41). Interestingly, elevated ghrelin levels are reported in alcoholic subjects (19, 29). Chronic alcohol feeding significantly decreases serum insulin levels, which promotes adipocyte lipolysis and contributes to fat accumulation in the liver (23, 42). Furthermore, chronic infusion of ghrelin has been reported to increase hepatic lipid storage (5). Since it is known that ghrelin negatively regulates insulin secretion and ghrelin levels are increasing in alcoholics, we hypothesized that the alcohol-induced increase in circulating ghrelin contributes to decreased serum insulin and impaired lipid metabolism in adipose tissue and liver.
METHODS
Antibodies and reagents.
Antibodies (Abs) and reagents were purchased from the following companies. Ethanol was purchased from Pharmaco-AAPER (Brookfield, CT). IRDye infrared secondary Abs and blocking buffer were from Li-COR Biosciences (Lincoln, NE). Ghrelin rat/mouse synthetic peptide (cat. no. 494127) was purchased from Millipore Sigma (St. Louis, MO). Abs to ghrelin (cat. no. H-031-31) and ghrelin O-acyltransferase (GOAT; cat. no. H-032-12) were obtained from Phoenix Pharmaceuticals (Burlingame, CA). Ghrelin Ab (cat. no. ab85104) was from Abcam (Cambridge, MA). Collagenase P (cat. no. 112149002001) and histopaque (cat. nos. 1119 and 1077) were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were obtained from Sigma Chemical (St. Louis, MO) unless stated otherwise.
Animal maintenance and tissue collection.
All animals received humane care in accordance with the guidelines established by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee at the Nebraska-Western Iowa Health Care System Veterans Affairs Research Service. Male Wistar rats weighing 175–200 g purchased from Charles River (Portage, MI) were weight-matched and pair-fed for 5–8 wk with control and ethanol-containing Lieber-DeCarli diets (33) as described previously (9). The ethanol diet contained 18% of total calories from protein, 35% from fat, 11% from carbohydrate, and 36% from ethanol. In the control diet, ethanol was replaced isocalorically with maltodextrin. Two hours before the euthanization, all rats were given their respective fresh diet to ensure that all rats were in the “fed” state. Rats were euthanized under isoflurane anesthesia. Blood was collected from the vena cava. Liver, epididymal adipose tissue, pancreas, and stomach tissues were excised and either processed for histopathological studies or immediately stored at −80°C until processed for subsequent biochemical analyses.
Liver TGs and serum nonesterified FFAs.
Liver TGs were extracted according to the Folch procedure (16) and saponified to quantify the TGs using the diagnostic kit no. TR22421 from Thermo Fisher Scientific (Middletown, VA). Serum nonesterified FFAs (NEFAs) were quantified using the NEFA-HR (2) diagnostic kit from Wako Life Sciences (Mountain View, CA).
INS-1E cell culture and insulin secretion assay.
Insulinoma-derived INS-1E β cells were provided by Dr. Pierre Maechler (University Medical Center, Geneva, Switzerland). As described previously (35), cells were cultured in RPMI-1640 media with 10% FCS. Upon reaching confluence, the cells were treated with 50 mM ethanol with or without 10 nM ghrelin in FCS-containing medium for 24 h. The ghrelin concentration used in this experiment was based on earlier published dose-dependent in vitro and in vivo studies (14, 38). After 24 h, the medium was replaced with Krebs-Ringer bicarbonate buffer, and the glucose-stimulated insulin secretion was measured following 30 min of exposure under the basal (2.5 mM glucose) or stimulated (15 mM glucose) condition in the presence or absence of ghrelin, as described previously (17, 35). The concentrations of glucose used for this insulin secretion assay were based on earlier studies that showed a dose-dependent increase in ATP generation, intracellular Ca2+ levels, and ultimately insulin secretion from β cells; the optimal concentration of 15 mM was regarded as the “stimulatory” dose of glucose that induced insulin secretion from INS-1E cells (17, 35, 37).
Pancreatic islet isolation.
Pancreatic islets were isolated and cultured as described previously (7, 27). Briefly, after collecting the pancreas from euthanized rats, the pancreas was washed with PBS solution three times and then cut into 1–2-mm pieces to maximize surface area for enzymatic digestion. The pieces were subjected to collagenase digestion (collagenase P at 5.5 mg/ml RIPA buffer). After 15 min, the digestion was terminated by the addition of cold RIPA buffer containing 1% BSA, and the islets were separated by gradient centrifugation using histopaque. After collecting the islets (from histopaque/media interface), they were suspended into RPMI media with 1% FBS in a petri dish and then individually picked under an inverted microscope using a plastic transfer pipette. After overnight culture in RPMI media, insulin secretion assay was performed on the isolated islets as described above.
Insulin and ghrelin levels.
Serum insulin was measured using the rat insulin ELISA kit (Mercodia AB, Sweden; cat. no. 10-1250-01). Acyl ghrelin was measured by the rat/mouse ghrelin (active) ELISA kit (EMD Millipore, Billerica, MA; cat. no. EZRGRA-90K). Serum levels of glucagon and glucose-dependent insulinotropic peptide (GIP) were measured using the Multiplex MAP Magnetic Bead-based immunoassay kits (Millipore). The assay was conducted according to the manufacturer’s instructions using a handheld magnetic separator block for 96-well flat bottom plates (Millipore) and analyzed using the Luminex 200 system (Luminex, Austin, TX).
Immunohistochemistry.
Paraffin-embedded tissue sections (5 µm thick) were deparaffinized in xylene and rehydrated in ethanol. Slides were then subjected to antigen retrieval by microwaving the sections in 10 mM sodium citrate buffer (pH 6) for 20 min. After cooling to room temperature, the sections were rinsed once in PBS (pH 7.4), permeabilized with 2% Triton X-100/PBS, and blocked for 1 h in 1% BSA/PBS. The sections were incubated overnight with Abs specific for insulin, ghrelin, and ghrelin receptor (1:200 dilution), followed by staining with appropriate Alexa Fluor-conjugated secondary Abs. Images were acquired using an LSM 710 Zeiss Confocal Microscope. Staining intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Gene expression analysis.
RNA was isolated from tissue/cell pellet samples using the PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA) and was reverse transcribed from 1 μg of total RNA using TaqMan Reverse Transcription Reagents (Applied Biosystems). Quantitative PCR was performed using a rat-specific TaqMan Gene Expression assay for ghrelin (cat. no. rn00572319, Applied Biosystems) and TaqMan Fast Universal PCR Master Mix (Applied Biosystems). SYBR Green quantitative PCR was performed using GOAT-specific primers. (Sense 5′-CGA GGC AGT GGA ACC GAA G-3′; Antisense 5′-GGC AAA AGT GTG GAT CAG ATA GTC-3′, from Integrated DNA Technologies) with iTaq Universal SYBR Green Supermix (Bio-Rad). The ∆∆Ct method was used to determine the fold change using actin for normalization.
Hepatocyte culture and treatments.
Primary hepatocytes from chow-fed rats were isolated by collagenase (Type 1V, Sigma, cat. no. C5138) perfusion method and cultured in Williams’ media with 5% FBS as described previously (8). Briefly, hepatocytes were seeded on sterile collagen-coated dishes. After 2 h, cells were washed with PBS, followed by incubation with serum-free Williams’ media containing oleic acid-BSA conjugate in the presence or absence of ghrelin and/or ethanol. After overnight incubation, the cellular TG levels were determined.
Western blot analysis.
Tissue samples were homogenized in ice-cold lysis buffer, consisting of 50 mM Tris·HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 1% NP-40 (pH 7.4) containing protease inhibitor cocktail (Sigma, cat. no. P2714-1BTL). Samples were separated by 12% SDS-PAGE and blotted on nitrocellulose, and proteins were detected with primary Abs and their appropriate secondary Abs. Protein bands were quantified using the Odyssey Infrared Imager and associated software.
Statistical analysis.
The results were presented as means ± SE. Data were analyzed by one-way ANOVA, followed by Student’s Newman-Keuls post hoc test. Comparison between two groups was analyzed using the Student’s t-test. P values of <0.05 were considered significant.
RESULTS
General parameters at euthanization after 6–8 wk of alcohol administration.
As shown in Table 1, we observed similar body weights in the ethanol-fed rats compared with their pair-fed controls. However, ethanol-fed rats exhibited a significant increase in liver weight and a significant decrease in adipose weight, resulting in an increased liver/body weight ratio and a decreased adipose/body weight ratio (P < 0.05). Additionally, ethanol-fed rats showed increased hepatic TGs and serum NEFA levels, indicating that there was a negative relationship between adipose tissue weight and serum NEFA (r = −0.817; n = 8). Chronic ethanol administration decreased circulating insulin levels and concurrently increased serum levels of acyl ghrelin (hereafter referred as ghrelin) levels significantly. The alcohol-induced decrease in serum insulin levels has also been reported by others (23, 25, 26). The decrease in serum insulin level was observed in as early as 2 wk of ethanol feeding, which persisted at 4 wk and thereafter. Note that these hormonal changes occurred despite similar glucose levels observed in both groups of rats. Furthermore, serum glucagon and glucose-dependent insulinotropic polypeptide (GIP) incretins that regulate serum insulin levels were not significantly different in both groups of rats.
Table 1.
Values of selected parameters at euthanization after 6–8 wk of alcohol feeding to the rats
| Control | Ethanol | |
|---|---|---|
| Body wt, g | 407.33 ± 5.48 | 397.23 ± 11.26 |
| Liver wt, g | 12.64 ± 0.22 | 15.87 ± 0.60* |
| Relative liver wt, g/100 g body wt | 3.11 ± 0.08 | 3.99 ± 0.09* |
| Relative adipose wt, g/100 g body wt | 2.77 ± 0.27 | 2.08± 0.21* |
| Serum triglycerides, mg/dl | 97.44 ± 10.04 | 157.60 ± 14.18* |
| Serum nonesterified free fatty acids, mmol/l | 0.20 ± 0.02 | 0.34 ± 0.05* |
| Liver triglycerides, mg/g tissue | 15.46 ± 1.45 | 47.24 ± 8.57* |
| Serum glucose, mg/dl | 211.66 ± 12.02 | 230.16 ± 17.00 |
| Serum insulin, ng/ml | 2.8 ± 0.35 | 1.8 ± 0.28* |
| Serum ghrelin, pg/ml | 6.56 ± 1.11 | 11.24 ± 2.56* |
| Glucagon, pg/ml | 12.04 ± 3.73 | 10.23 ± 1.80 |
| Glucose-dependent insulinotropic polypeptide, pg/ml | 124.91 ± 33.61 | 137.38 ± 27.96 |
Values are means ± SE; n = 10–14 rats.
P < 0.05.
Glucose tolerance test and insulin and ghrelin during fasting.
Since circulating insulin and ghrelin levels were determined at euthanization/under the fed conditions, we measured fasting serum insulin and ghrelin levels to verify whether the ethanol-induced hormonal imbalance persists even in the fasting conditions. As shown in Fig. 1, the ethanol-induced decrease in serum insulin and increase in ghrelin levels after 6 h of fasting was comparable to that observed under the fed-state, as presented in Table 1.
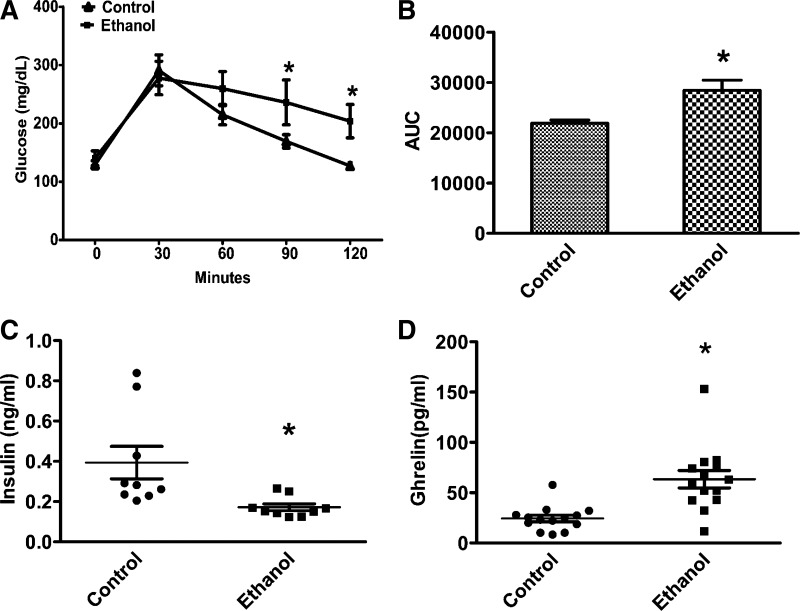
Fig. 1.
Chronic ethanol feeding is associated with impaired glucose tolerance and altered serum insulin and ghrelin levels after fasting. A: glucose tolerance test (GTT) was performed as described under experimental procedures. B: area under curve (AUC) from GTT. C: serum insulin level after 6 h of fasting. D: serum ghrelin level after 6 h of fasting. Values are expressed as means ± SE (n = 12–14). *P < 0.05.
The analysis of a glucose tolerance test (GTT) revealed an increased area under the curve for glucose in the ethanol-fed rats (Fig. 1B). Increased area under the curve during GTT and low levels of insulin clearly indicates that impaired glucose clearance is due to decreased insulin levels.
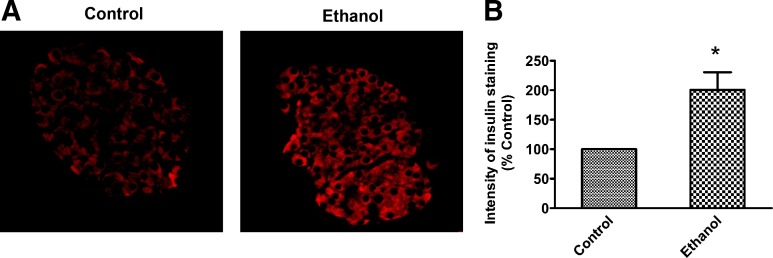
Ghrelin hormone is known to inhibit insulin secretion from pancreatic β cells in both in vitro and in vivo conditions (10, 36, 41). This information, combined with our observation that serum insulin is dramatically reduced in ethanol-fed rats, led us to investigate its level in the pancreas. We conducted quantitative analysis for insulin by immunostaining the pancreata from rats that were in the fed condition. We observed a significant accumulation of intracellular insulin in pancreatic islets of ethanol-fed rats (Fig. 2, A and B), indicating that insulin secretion is impaired in the ethanol-fed rats. Histogram analysis revealed that there is ~twice more insulin retained inside the islets of ethanol-fed rats compared with control. These data combined with data on serum insulin levels shown in Table 1 suggest a negative correlation between the insulin content in the islet versus the circulating serum levels in the ethanol-fed rats (r = −0.866; n = 3). Collectively, these data suggested that increased serum ghrelin levels may be a key factor in alcohol-associated impaired plasma insulin levels in rats.
Fig. 2.
Chronic ethanol treatment is associated with accumulation of insulin in the pancreatic islets. A: immunohistochemistry of insulin in pancreatic islets of control and ethanol-fed rats, demonstrating insulin accumulation in islets of ethanol-fed rats. B: graphical representation of the intensity of immunohistochemical staining as a percent of control. Values are means ± SE of five individual islets from each section of three sets of control and ethanol-fed animals. *P < 0.05.
Ghrelin inhibits insulin secretion.
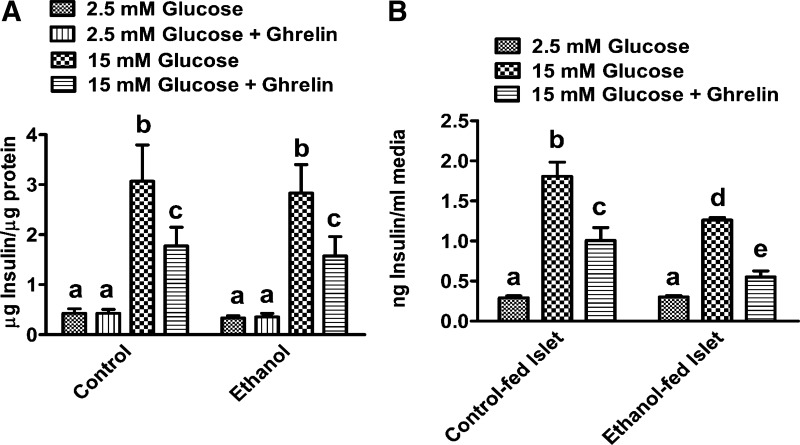
To confirm the effects of ghrelin on insulin secretion, we first conducted studies on INS-1E β cells. These cells depict many important characteristics of the pancreatic β cells and secrete physiologic levels of insulin in response to glucose by utilizing similar trafficking pathways as observed in vivo (38). Also, important to these studies, these cells express ghrelin receptor, namely growth hormone secretagogue receptor type 1a (GHS-R1a) (43). As explained in methods, we first incubated the cells with 2.5 mM glucose (45 mg/dl) and then stimulated with 15 mM glucose in the presence or absence of ghrelin. Consistent with previous reports (38), ghrelin had no effect on insulin release from INS-1E β cells under basal (2.5 mM) glucose level but significantly decreased glucose-stimulated insulin release (Fig. 3A). These data indicate that ghrelin requires a stimulatory level of glucose for inhibiting insulin secretion. These results corroborate previous studies, showing that ghrelin impairs membrane potential and suppresses only 15 mM glucose-stimulated Ca2+ influx and insulin secretion (10, 11). Although not statistically significant, ethanol treatment caused a 15–20% decrease in insulin secretion compared with ghrelin alone. We also examined the effects of ghrelin on insulin secretion in an ex vivo model of isolated pancreatic islets from experimental rats. As explained in methods, islets were isolated from control and ethanol-fed rats and cultured for 24 h before performing the insulin secretion assay. As expected, ghrelin treatment significantly decreased the stimulated insulin secretion from islets isolated from both control and ethanol-fed rats (Fig. 3B). However, an additive effect on impairment in insulin secretion was observed when we treated the islets of ethanol-fed rats with ghrelin (Fig. 3B).
Fig. 3.
Ghrelin significantly inhibits glucose-stimulated insulin secretion from β cells. A: INS-1E cells were cultured with or without 50 mM ethanol or 10 nM ghrelin for 24 h. After 24 h of treatment, for the insulin secretion assay, cells were exposed to 15 mM glucose with or without 10 nM ghrelin for 30 min. Insulin secretion was determined after 30 min by measuring insulin in the media. B: islets were isolated from control and ethanol-fed rats and cultured in RPMI media for 24 h, and then insulin secretion was determined after 30 min of exposure to 15 mM glucose with and without 10 nM ghrelin. Values are means ± SE (n = 4–6). Values not sharing a common letter (i.e., a, b, c, d, or e) are statistically different. P < 0.05.
Increased serum ghrelin after alcohol administration is due to increased synthesis and maturation.
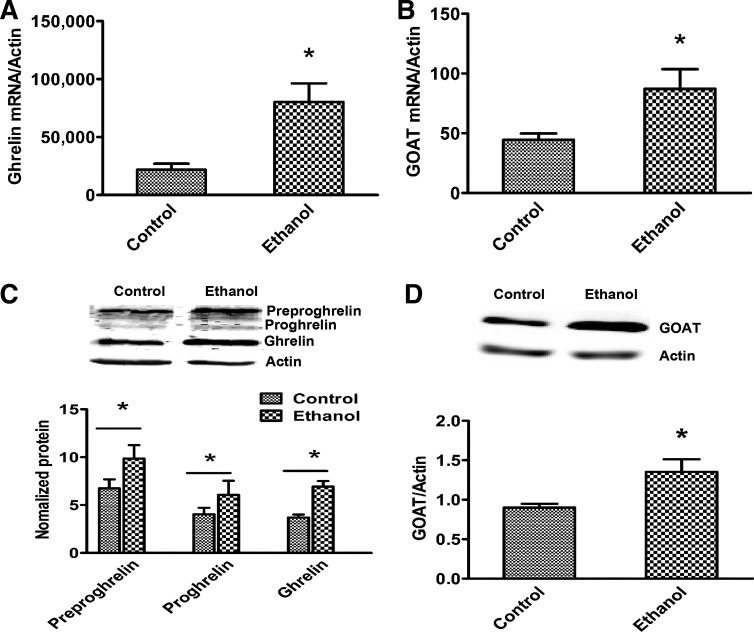
The stomach is the main site for ghrelin production. However, lower amounts have also been detected in the intestine, pancreas, kidney, and hypothalamus (4). Since the stomach is the predominate site for ghrelin synthesis, we excised different parts of the stomach and measured ghrelin gene expression in the fundus, corpus, and pylorus regions in control and ethanol-fed rats. Gene expression and Western blot data indicated that the corpus part of the stomach is the main site, whereas pylorus is the minor site of ghrelin production. As we expected, ghrelin mRNA expression was significantly increased in the corpus portion of stomach from ethanol-fed rats compared with control rats (Fig. 4A). Even though the pylorus region expresses less ghrelin levels than corpus, a similar trend in increased expression of ghrelin was also observed in the pylorus region of the ethanol-fed rats (data not shown). We did not observe ghrelin protein expression in the fundus portion of the stomach in rats of either experimental group. This gene expression data corroborated our Western blot results (Fig. 4C). Similar to ghrelin gene expression and protein content results, we also observed a significant increase in the ethanol-fed rats in the gene and protein levels of GOAT, an enzyme responsible for maturation of ghrelin, (Fig. 4, B and D).
Fig. 4.
Increased serum ghrelin levels are due to increased synthesis and maturation in ethanol-fed rats. Control and ethanol-fed rat stomach (corpus region) ghrelin (A) and ghrelin O-acyltransferase (GOAT; B) gene expression. Immunoblot analysis of stomach ghrelin (C) and GOAT (D) protein levels. Values are means ± SE (n = 6–8). *P < 0.05.
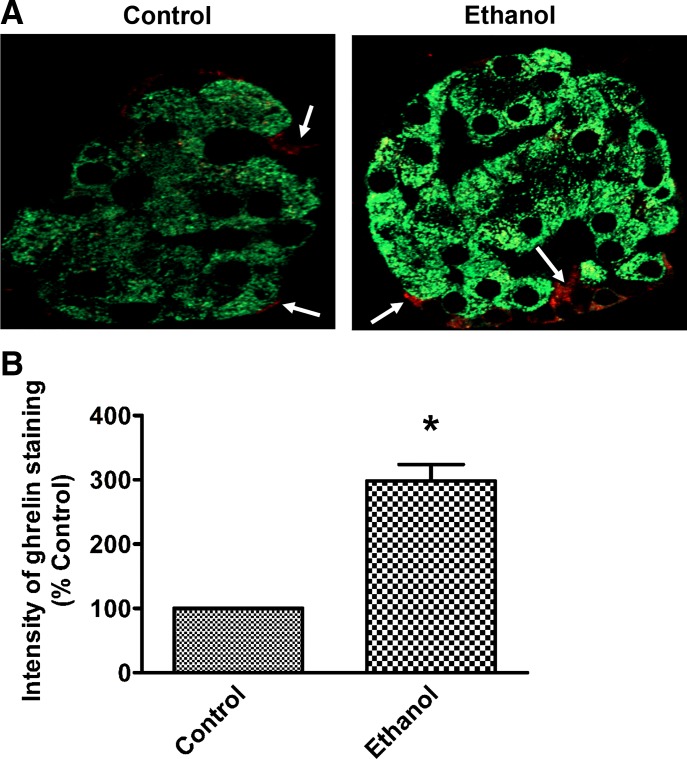
It is known that the 1% of pancreatic islets cell population called epsilon cells expresses ghrelin (3). This information, combined with our observations that alcohol administration dramatically increases ghrelin stomach content and circulating levels, directed us to compare ghrelin levels in pancreatic islets. As shown in Fig. 5A, immunohistochemical staining demonstrated that ghrelin is indeed expressed in a small fraction of islet cells, mainly localized in the periphery of islets (as indicted with arrow marks in Fig. 5A), and this expression is significantly increased in islets of ethanol-fed rats (Fig. 5B).
Fig. 5.
Chronic ethanol treatment increased pancreatic ghrelin content. A: representative images of control and ethanol-fed rat pancreas immunostained for insulin (green) and ghrelin (red), depicting increased ghrelin synthesis in epsilon cells of islets of chronic ethanol-fed rats. B: graphical representation of the intensity of immunohistochemical staining expressed as a percent of control. Values are means ± SE of four individual islets from each section of three sets of control and ethanol-fed animals. *P < 0.05.
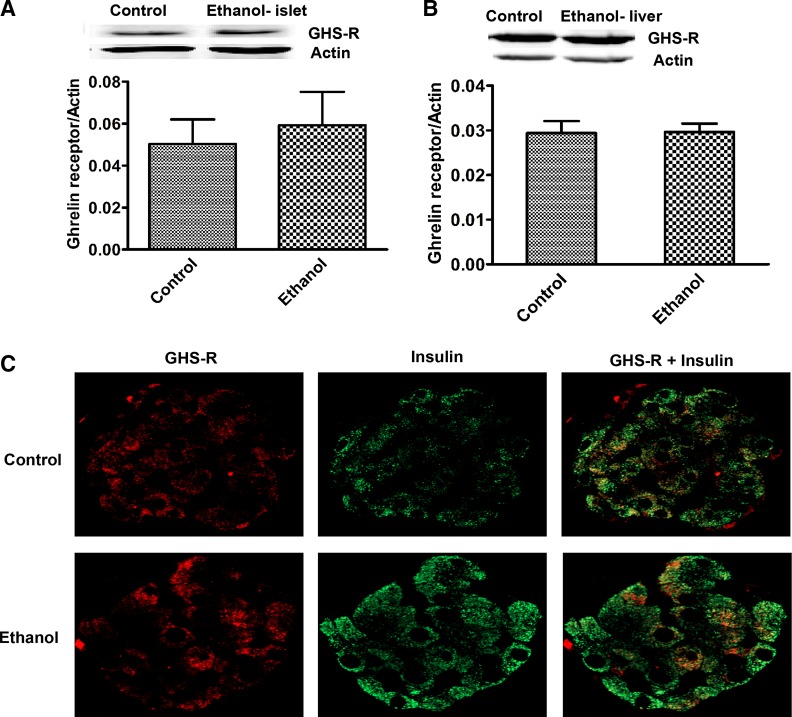
Ghrelin receptor, known as growth hormone secretagogue receptor (GHS-R), is distributed in many tissues, including the pancreatic islets and liver. In this study, we examined whether the increased circulating ghrelin levels affect ghrelin receptor content in the pancreas and liver. Western blot analysis of total liver homogenates and isolated pancreatic islets from control and ethanol-fed rats revealed that GHS-R protein level is not changed in these tissues after ethanol treatment (Fig. 6, A and B). To confirm that GHS-R is present on β cells, we conducted immunohistochemical staining of the islets with insulin and GHS-R. We found that GHS-R colocalized with insulin, indicating that GHS-R is present on β cells (Fig. 6C).
Fig. 6.
Increased ghrelin levels do not promote ghrelin receptor [growth hormone secretagogue receptor (GHS-R)] increase. Immunoblot analysis of protein levels of GHS-R in isolated islets (A) and liver homogenates (B). Values are means ± SE (n = 4–6). Control and ethanol-fed rat pancreas (C) immunostained for insulin (green) and GHS-R (red).
Ghrelin treatment induces TG accumulation in primary hepatocytes.
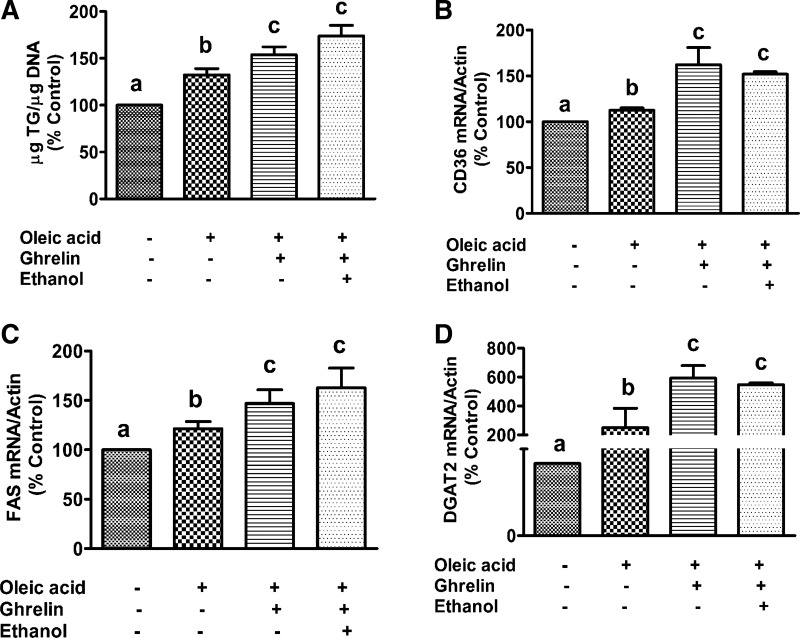
To determine whether ghrelin has any direct effect on hepatic fat accumulation, we treated primary cultures of rat hepatocytes overnight with 250 µM oleic acid in the presence or absence of ghrelin and ethanol. Treatment of hepatocytes with 10 nM ghrelin in the presence of oleic acid significantly increased TG content (Fig. 7A). These results indicate that in addition to inhibiting insulin secretion from the pancreas, ghrelin directly promotes fat accumulation in hepatocytes. We also observed an increased expression of fatty acid synthase (FAS), diacylglycerol acyltransferase (DGAT2), and fatty acid transporter CD36 in primary hepatocytes after treatment with ghrelin and oleic acid (Fig. 7, B–D). Collectively, these results indicate that the direct effect of ghrelin on hepatic TG increase is by upregulating increased FFA uptake as well as de novo lipogenesis. Although not statistically different, ethanol plus ghrelin treatment modestly increased the oleic acid-induced TG accumulation by 10–15% compared with ghrelin alone. Similar to TG content, FAS gene expression in the ethanol and ghrelin combined treated group was also modestly increased compared with ghrelin alone treatment. However, we did not observe any additional effect of ethanol on CD36 and DGAT2 gene expression. Note that the increased fat accumulation with ghrelin or ghrelin plus ethanol treatment occurred despite no change in hepatocyte GHS-R content.
Fig. 7.
Ghrelin itself induces triglyceride accumulation in hepatocytes. Primary hepatocytes from chow-fed rats were treated overnight with 10 nM ghrelin in the presence or absence of 250 µM oleic acid and ethanol (50 mM) and analyzed for triglyceride (TG) levels (A) and fatty acid transporter CD36 (B), fatty acid synthase (FAS; C), and diacylglyceride acyltransferase (DGAT2; D) gene expression. Values are means ± SE (n = 4–6). Values not sharing a common letter (i.e., a, b, or c) are statistically different. P < 0.05.
DISCUSSION
Fatty liver is the earliest and most common response of the liver to excessive ethanol consumption. As shown before, chronic ethanol administration results in increased liver TG levels, liver-to-body weight ratios, and produced a significant decrease in adipose-to-body weight ratio (Table. 1). This decreased adipose tissue weight was likely due to induction of adipose tissue lipolysis and increased FFA release (1, 2) for delivery to the liver (42, 45). It is also generally accepted that this is due to impaired insulin signaling that fails to inhibit adipose lipolysis (23). However, in this study, we observed that chronic ethanol administration to rats significantly decreased circulating insulin levels, while at the same time it significantly increased serum ghrelin levels. Interestingly, these hormonal changes occurred despite similar serum glucose, glucagon, and GIP incretin levels in both groups of rats, indicating that the alcohol-induced decreases in insulin levels are not modulated by the incretins measured. Rather, it is the alcohol-induced increase in ghrelin levels that regulates circulating insulin levels. Furthermore, immunostaining also revealed an increased accumulation of insulin in islets, which likely resulted from impaired secretion, ultimately causing decreased circulating levels of insulin. We saw an ~50% decrease in serum insulin level as early as 2 wk of ethanol feeding, which persisted at 4 wk and thereafter. The alcohol-induced decrease in serum insulin levels has also been reported by others in both human and animal models (23, 25, 26). Several clinical studies have reported an increased serum ghrelin in humans who chronically abuse alcohol (19, 29). These studies collectively portend a negative correlation between insulin and ghrelin levels in alcoholics.
In general, the β cells sense the changes in nutritional status and correspondingly release insulin. β cells respond to many nutrients in the blood circulation, but glucose is the primary stimuli for the insulin release from the pancreas. Furthermore, fasting or food intake, respectively, increase or decrease the secretion of ghrelin from the stomach. Since both ghrelin and insulin hormone secretions are primarily controlled by the feeding status and metabolite levels, we further measured fasting serum insulin and ghrelin levels and conducted a GTT in experimental rats after 6 h of fasting. Consistent with initial results at euthanization, ethanol-fed rats similarly exhibited higher levels of ghrelin and lower levels of insulin in serum compared with their pair-fed control rats. In addition, ethanol-fed rats also showed impaired glucose clearance during a GTT. This decrease in glucose clearance could be due to decreased insulin levels, as shown in this and other studies.
Ghrelin is synthesized as preproghrelin, which is first cleaved to proghrelin and then cleaved again to form ghrelin. Ghrelin only becomes active when octanoic acid is linked to serine at the three-position by the enzyme GOAT (21). Acylated ghrelin binds to GHS-R, which is distributed in a variety of tissues (28). As shown in Fig. 6, GHS-R colocalizes with insulin on β cells, but the receptor level is not changed with alcohol administration. Once ghrelin binds to its receptor on β cells, it activates voltage-dependent K+ channels that suppress Ca2+ influx necessary for glucose-stimulated insulin secretion to consequently impair insulin release (13). As shown in Fig. 3A, acute treatment with ghrelin significantly inhibits insulin release from β cells. However, acute ethanol treatment did not significantly inhibit the insulin release from the pancreatic β cells. Our studies are the first to report that it is the increased circulating level of ghrelin in ethanol-fed rats that contributes to impairing insulin secretion from the pancreas causing increased islet content and decreased circulating insulin levels (Fig. 2).
In this study, besides measuring serum ghrelin levels in both fed and fasting conditions, we also demonstrated that increased levels of acyl ghrelin are because of an increased ghrelin synthesis and maturation by increasing GOAT enzyme in stomachs of alcohol-fed rats. A very small amount of ghrelin can also be produced in pancreatic islets, but this ghrelin might not contribute to the circulating ghrelin levels, instead it could serve as a local regulator of insulin release (12). In this study, we were not able to detect ghrelin secretion from pancreatic islet in ex vivo experimental condition. It is possible that the ghrelin level may be below the detection limit of the commercial ELISA used. However, we did observe increased content of ghrelin in pancreatic islets of ethanol-fed rats by immunohistochemical staining of the pancreas (Fig. 5). Note, those pancreatic tissues were from rats those were in fed condition and showed an increased content of both insulin and ghrelin in islets of ethanol-fed rats. This finding suggests that impaired glucose-induced insulin secretion from islets of chronic ethanol-fed rats in ex vivo conditions (Fig. 3B) likely results from an increased content of islet ghrelin that can act as a local inhibitor for glucose-stimulated insulin release.
Because the liver expresses abundant ghrelin receptors, we hypothesized that ghrelin may directly affect energy metabolism in hepatocytes. Thus, we treated primary cultures of rat hepatocytes with 250 µM oleic acid in the presence or absence of acyl ghrelin. Overnight treatment of hepatocytes with ghrelin in the presence of oleic acid significantly increased TG content and fatty acid transporter (CD36) and DGAT2 levels. Consistent with our observations, Li et al. (31) also demonstrated that genetic disruption of either ghrelin or ghrelin receptor genes reduces the incidence of obesity and hepatic steatosis in mice. Furthermore, they showed that the ghrelin directly increases hepatic lipogenesis by activating the mTOR-PPARγ signaling pathway. Barazzoni et al. (5) also reported that chronic infusion of ghrelin increases hepatic lipid accumulation. Our results indicate that in addition to inhibiting insulin secretion and consequently increasing adipose tissue lipolysis and circulating NEFA level, ghrelin promotes the liver uptake of circulating NEFAs by upregulating CD36 expression as well as by promoting de novo fatty acid synthesis (FAS mRNA increase) and esterification (DGAT2 mRNA increase) to ultimately increase fat accumulation. Although, it is known that chronic ethanol treatment increases hepatic fat accumulation by increasing FAS and other lipid-synthesizing enzyme expression (6, 34, 44), the combination of ethanol and ghrelin treatment did not show any significant increase in oleic acid-induced TG accumulation and expression of lipid-synthesizing enzymes compared with ghrelin alone. These results suggest that both ghrelin and ethanol are likely using similar mechanisms for regulating lipid metabolism in hepatocytes.
To summarize, we have presented compelling evidence that the alcohol-induced elevation of circulating ghrelin levels impairs insulin secretion. Consequently, reduced circulating insulin levels likely contribute to increased fatty acid mobilization from adipose tissue to liver, thereby contributing to hepatic steatosis. We further show that an increase in ghrelin can directly modulate hepatic lipid metabolism to favor fat accumulation. Thus, modulating ghrelin and/or its receptor could be a favorable therapeutic option for treating alcoholic fatty liver disease.
GRANTS
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant K01-AA-024254-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.R. conceived and designed research; K.R., P.G.T., J.L.K., E.N.H., and C.A.C. performed experiments; K.R. analyzed data; K.R. and K.K.K. interpreted results of experiments; K.R. prepared figures; K.R., K.K.K., and C.A.C. drafted manuscript; K.R., K.K.K., and C.A.C. edited and revised manuscript; K.R., K.K.K., and C.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Pierre Maechler (University Medical Center, Geneva, Switzerland) for providing INS-1E pancreatic β cells.
REFERENCES
- 1.Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res 21: 962–967, 1997. doi: 10.1097/00000374-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Addolorato G, Capristo E, Greco AV, Stefanini GF, Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med 244: 387–395, 1998. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 3.Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 52: 486–493, 2009. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- 4.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 5.Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288: E228–E235, 2005. [Erratum in Am J Physiol Endocrinol Metab 291: E428, 2006.] doi: 10.1152/ajpendo.00115.2004. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco MP, Marco C, Segovia JL. Chronic ingestion of ethanol stimulates lipogenic response in rat hepatocytes. Life Sci 68: 1295–1304, 2001. doi: 10.1016/S0024-3205(00)01035-3. [DOI] [PubMed] [Google Scholar]
- 7.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11: 3–31, 2009. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey CA, Kragskow SL, Sorrell MF, Tuma DJ. Chronic ethanol administration impairs the binding and endocytosis of asialo-orosomucoid in isolated hepatocytes. J Biol Chem 262: 2704–2710, 1987. [PubMed] [Google Scholar]
- 9.Casey CA, McVicker BL, Donohue TM Jr, McFarland MA, Wiegert RL, Nanji AA. Liver asialoglycoprotein receptor levels correlate with severity of alcoholic liver damage in rats. J Appl Physiol (1985) 96: 76–80, 2004. doi: 10.1152/japplphysiol.00375.2003. [DOI] [PubMed] [Google Scholar]
- 10.Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 53: 3142–3151, 2004. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 11.Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 56: 2319–2327, 2007. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 12.Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 55: 3486–3493, 2006. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 13.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 118: 239–249, 2008. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146: 241–244, 2002. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 17.Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ. Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol 251: 103–111, 2006. doi: 10.1016/j.mce.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Gearing KL, Göttlicher M, Widmark E, Banner CD, Tollet P, Strömstedt M, Rafter JJ, Berge RK, Gustafsson JA. Fatty acid activation of the peroxisome proliferator activated receptor, a member of the nuclear receptor gene superfamily. J Nutr 124 Suppl 8: 1284S–1288S, 1994. doi: 10.1093/jn/124.suppl_8.1284S. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear SJ, Mottershead M, Sung EZ, Wong LS, McTernan PG, Kumar S, Nwokolo CU. Dysregulation of plasma ghrelin in alcoholic cirrhosis. Clin Endocrinol (Oxf) 73: 323–329, 2010. doi: 10.1111/j.1365-2265.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- 20.Grunnet N, Kondrup J. The effect of ethanol on the beta-oxidation of fatty acids. Alcohol Clin Exp Res 10, Suppl: 64S–68S, 1986. doi: 10.1111/j.1530-0277.1986.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105: 6320–6325, 2008. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic and clinical aspects. Alcohol Clin Exp Res 15: 45–66, 1991. doi: 10.1111/j.1530-0277.1991.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 23.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 282: 28465–28473, 2007. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- 24.Kharbanda KK, Todero SL, Ward BW, Cannella JJ 3rd, Tuma DJ. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem 327: 75–78, 2009. doi: 10.1007/s11010-009-0044-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Hwang JY, Lee DY, Song EH, Park KJ, Kim GH, Jeong EA, Lee YJ, Go MJ, Kim DJ, Lee SS, Kim BJ, Song J, Roh GS, Gao B, Kim WH. Chronic ethanol consumption inhibits glucokinase transcriptional activity by Atf3 and triggers metabolic syndrome in vivo. J Biol Chem 289: 27065–27079, 2014. doi: 10.1074/jbc.M114.585653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Song EH, Lee HJ, Oh YK, Park YS, Park JW, Kim BJ, Kim DJ, Lee I, Song J, Kim WH. Chronic ethanol consumption-induced pancreatic beta-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem 285: 37251–37262, 2010. doi: 10.1074/jbc.M110.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinasiewicz A, Juszczak M, Pachecka J, Fiedor P. Pancreatic islets isolation using different protocols with in situ flushing and intraductal collagenase injection. Physiol Res 53: 327–333, 2004. [PubMed] [Google Scholar]
- 28.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 29.Kraus T, Schanze A, Gröschl M, Bayerlein K, Hillemacher T, Reulbach U, Kornhuber J, Bleich S. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res 29: 2154–2157, 2005. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- 30.Levine JA, Harris MM, Morgan MY. Energy expenditure in chronic alcohol abuse. Eur J Clin Invest 30: 779–786, 2000. doi: 10.1046/j.1365-2362.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, Xiang X, Li Y, Zhao J, Mulholland M, Zhang W. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proc Natl Acad Sci USA 111: 13163–13168, 2014. doi: 10.1073/pnas.1411571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liangpunsakul S, Crabb DW, Qi R. Relationship among alcohol intake, body fat, and physical activity: a population-based study. Ann Epidemiol 20: 670–675, 2010. doi: 10.1016/j.annepidem.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989. [PubMed] [Google Scholar]
- 34.McVicker BL, Rasineni K, Tuma DJ, McNiven MA, Casey CA. Lipid droplet accumulation and impaired fat efflux in polarized hepatic cells: consequences of ethanol metabolism. Int J Hepatol 2012: 978136, 2012. doi: 10.1155/2012/978136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145: 667–678, 2004. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- 36.Meyer C. Final answer: ghrelin can suppress insulin secretion in humans, but is it clinically relevant? Diabetes 59: 2726–2728, 2010. doi: 10.2337/db10-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murdock DJ, Clarke J, Flatt PR, Barnett YA, Barnett CR. Role of CYP2E1 in ketone-stimulated insulin release in pancreatic B-cells. Biochem Pharmacol 67: 875–884, 2004. doi: 10.1016/j.bcp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144: 916–921, 2003. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 39.Singal AK, Anand BS. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis (Hoboken) 2: 53–56, 2013. doi: 10.1002/cld.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 362: k2817, 2018. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59: 2145–2151, 2010. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, Zhou Z, Zhang X. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS One 8: e55382, 2013. doi: 10.1371/journal.pone.0055382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52: 301–310, 2004. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 44.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277: 29342–29347, 2002. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 45.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 180: 998–1007, 2012. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]