Abstract
The cyclin kinase inhibitor p21 is acutely upregulated during acute kidney injury (AKI) and exerts cytoprotective effects. A proposed mechanism is oxidant stress-induced activation of p53, the dominant p21 transcription factor. Glycerol-induced rhabdomyolysis induces profound renal oxidant stress. Hence, we studied this AKI model to determine whether p53 activation corresponds with p21 gene induction and/or whether alternative mechanism(s) might be involved. CD-1 mice were subjected to glycerol-induced AKI. After 4 or 18 h, plasma, urinary, and renal cortical p21 protein and mRNA levels were assessed. Renal p53 activation was gauged by measurement of both total and activated (Ser15-phosphorylated) p53 and p53 mRNA levels. Glycerol evoked acute, progressive increases in renal cortical p21 mRNA and protein levels. Corresponding plasma (~25-fold) and urinary (~75-fold) p21 elevations were also observed. Renal cortical ratio of total to phosphorylated (Ser15) p53 rose three- to fourfold. However, the p53 inhibitor pifithrin-α failed to block glycerol-induced p21 gene induction, suggesting that an alternative p21 activator might also be at play. To this end, it was established that glycerol-induced AKI 1) dramatically increased plasma (~5-fold) and urinary (~75-fold) cortisol levels, 2) the glucocorticoid receptor antagonist mifepristone blocked glycerol-induced p21 mRNA and protein accumulation, and 3) dexamethasone or cortisol injections markedly increased p21 protein and mRNA in both normal and glycerol-treated mice, although no discernible p53 protein or mRNA increases were observed. We conclude that AKI-induced “systemic stress” markedly increases plasma and urinary cortisol, which can then activate renal p21 gene expression, at least in part, via a glucocorticoid receptor-dependent signaling pathway. Discernible renal cortical p53 increases are not required for this dexamethasone-mediated p21 response.
Keywords: cortisol, dexamethasone, mifepristone, pifithrin-α, p21, p53, rhabdomyolysis
INTRODUCTION
p21 is a potent cyclin-dependent kinase inhibitor and, as such, can induce cell cycle arrest (4, 9). It is predominantly regulated by activation of the p53 tumor suppressor gene (9). Cellular stimuli, most notably oxidant stress, increase p53 translation and, to a lesser extent, p53 transcription (9, 14). With p53 phosphorylation, nuclear translocation to p21 promoter regions occurs (26). This results in increased p21 transcription, with “downstream” p21 protein accumulation, in injured cells (9, 14).
Pioneering work by Price and colleagues (10, 15) and Safirstein and colleagues (24) demonstrated that renal tubular p21 levels are markedly, and acutely, upregulated by ischemia- and cisplatin-induced acute kidney injury (AKI). Given that oxidant stress occurs in virtually all forms of AKI, it seems logical that increased free radical formation drives renal p53 accumulation and phosphorylation, culminating in renal p21 gene activation and p21 accumulation in injured cells. However, this sequence of events remains speculative. For example, whereas p53 protein accumulation has been noted in the renal medulla following ischemia-induced AKI, no such change was observed in damaged renal cortical proximal tubule cells (7).
AKI-induced renal p21 elevations have been reported to exert diverse renal cytoprotective effects (5, 12). This has been most convincingly demonstrated in studies showing increased sensitivity to both toxic and ischemic renal damage in p21-deficient mice (10, 15, 24). These findings raise the possibility that, were it possible to pharmacologically upregulate renal tubular p21, new therapeutic opportunities might emerge.
As a prelude to achieving this goal, a better understanding of the factors that drive renal p21 expression during AKI is required. The present study using the well-characterized glycerol-induced rhabdomyolysis model of oxidant stress-induced AKI was undertaken to help achieve this goal (13, 25). The information gleaned from the following experiments offer five new insights into AKI-mediated p21 gene induction: 1) rhabdomyolysis-induced AKI increases both total and phosphorylated p53 content in the renal cortex, presumably stimulating p21 gene transcription; 2) an AKI-induced systemic “stress response” markedly increases glucocorticoid production; 3) the resultant hypercortisolism activates the renal p21 gene in a glucocorticoid receptor (GCR)-dependent manner; 4) marked renal p21 accumulation and increased plasma and urinary p21 result; and 5) AKI-induced systemic stress/hypercortisolism can activate the p21 gene in both normal and injured nephrons. Thus, renal tissue injury, per se, is not a prerequisite for stress-induced renal p21 production.
METHODS
All experiments were performed using male CD-1 mice (35–40 g body wt; Charles River Laboratories, Wilmington, DE) maintained under routine vivarium conditions with free access to food and water. The AKI models were approved by the Institutional Animal Care and Use Committee of Fred Hutchinson Cancer Research Center. All surgical procedures were conducted in animals under deep pentobarbital sodium anesthesia (40–50 mg/kg ip).
Glycerol-Induced AKI Model
Eighteen hours.
Mice were briefly anesthetized with isoflurane, and glycerol (50%) was injected intramuscularly in equally divided doses into each hindlimb. The glycerol dose was varied (6, 6.5, 7, 7.5, 8, 8.5, or 9 ml/kg, n = 2–3 mice per dose) to produce variable degrees of renal injury. At 4 h after glycerol injection, a tail vein blood sample (~100 µl) was obtained, and at 18 h, the mice were deeply anesthetized with pentobarbital sodium. The abdominal cavity was opened through a midline abdominal incision, a terminal blood sample was obtained from the vena cava, the kidneys were removed and iced, and renal cortical samples were cut and extracted for protein and total RNA assay (RNeasy Mini+, Qiagen, Germantown, MD). A terminal urine sample was collected from the urinary bladder. Plasma samples were assayed for blood urea nitrogen (BUN) and creatinine (6). Renal cortical, plasma, and urinary p21 concentrations were determined with a “sandwich” ELISA, which employs two distinct monoclonal antibodies (capture and detection) directed against two different p21 epitopes, thereby conferring p21 specificity (catalog no. 212072, Abcam, Cambridge, MA) (6). Renal cortical p21 and p53 mRNA levels were determined by RT-PCR, factored by GAPDH (6). Control plasma, urine, and tissue samples from 10 normal mice were used for comparisons. Total and activated (Ser15-phosphorylated) p53 levels (18) were determined by ELISA (catalog no. ab205713, Abcam).
Four hours.
To assess renal cortical changes at an earlier time point than 18 h after glycerol injection, an additional five mice were injected with glycerol (8.5 ml/kg), and renal cortical protein and RNA samples were obtained 4 h later for use in the assays described above (see Eighteen hours). The results were contrasted to those observed in five normal mice.
Pifithrin-α p53 Inhibition
Pifithrin-α is a widely used p53 inhibitor (3, 8) and, as such, has been widely used to differentiate p53-dependent versus p53-independent events (3, 8). To further explore the potential role of p53 in glycerol-mediated renal p21 gene activation, three mice were injected with pifithrin-α (10 mg/kg ip, 10% DMSO in saline; catalog no. 506154, EMD Millipore, Burlington, MA) (3). Three additional mice were injected with vehicle. Immediately thereafter, the mice were injected with glycerol (8.5 mg/kg). At 4 h after glycerol injection, renal cortical tissues were analyzed for p21 mRNA to assess whether pifithrin-α would lower p21 mRNA levels.
Impact of Dexamethasone on Glycerol-Induced p21 Elevations
Inflammation can induce p21 gene activation via p53-independent mechanisms (1, 2). Given that AKI evokes intrarenal and systemic inflammatory responses (6), the potential for the potent anti-inflammatory corticosteroid dexamethasone (DXM) to alter glycerol-induced p21 gene activation was assessed. Ten mice were injected with glycerol (8.5 ml/kg); half of these mice had been injected with DXM (250 µg ip in saline; catalog no. D1159, Sigma, St. Louis, MO) 30 min before the glycerol injection. At 4 h after the glycerol injection, renal cortical tissues were obtained and assayed for p21 mRNA and protein levels.
To determine the impact of DXM on renal p21 expression in the absence of renal injury, five normal mice were injected with DXM as described above, and after 4 h, p21 protein and mRNA levels were measured and compared with normal values. Activation of p53 was assessed by p53 [total and phosphorylated (Ser15) p53] protein assay and p53 mRNA levels, as described above.
Cortisol Treatment
To ascertain whether a second glucocorticoid could recapitulate DXM’s p21 effects on the glycerol-induced AKI model, five mice were injected with a biologically equivalent dose of cortisol (1 mg ip) and 30 min later with glycerol (8.5 ml/kg). After 4 h, p21 protein and mRNA levels were assessed and compared with both normal values and values in four kidneys obtained 4 h after glycerol injection.
Endogenous Urinary and Plasma Cortisol Levels
To determine whether the AKI-induced systemic stress response (6) is associated with hyperadrenalism, urinary free cortisol levels were measured by ELISA (catalog no. AD1-900-071, Enzo, Farmingdale, NY) in samples from normal mice and from mice 4 or 18 h after glycerol injection (n = 5 mice per group). Total plasma cortisol levels (normally ~95% protein-bound and ~5% free cortisol) were also measured 4 h after glycerol injection and compared with normal plasma values (n = 5 mice in each group; Clinical Chemistry Laboratories, University of Washington).
Impact of the GCR Antagonist Mifepristone on Glycerol-Induced Renal p21 and p21 mRNA Levels
Given the unexpected findings that corticosteroid injection increased, rather than suppressed, p21 expression and that endogenous cortisol levels were markedly elevated after glycerol injection (see results), we questioned whether GCR blockade would decrease renal p21 expression. Ten mice were injected with glycerol (8.5 mg/kg), half with and half without the GCR antagonist mifepristone (MFP, 30 mg/kg, in 85% propylene glycol; catalog no. AC459982500, Fisher Scientific) (11, 23). After 4 h, the kidneys were removed, and renal cortices were analyzed for p21 mRNA and protein levels. Eight mice, treated with and without MFP (n = 4 each) in the absence of glycerol injection, were used to evaluate MFP effects in the absence of glycerol-induced renal injury.
Unilateral Renal Ischemia Model
Eight mice were anesthetized with pentobarbital sodium and subjected to a midline abdominal incision, exposing the renal pedicles. Four mice were subjected to left renal pedicle occlusion at 37°C for 22 min by application of an atraumatic microvascular clamp. The remaining four mice served as surgical controls. After completion of unilateral ischemia, vascular clamps were removed, the abdominal cavity was closed, and mice were allowed to recover from anesthesia. After 4 h, the mice were anesthetized again, and both kidneys were resected. Control tissue samples were obtained from four normal mice. p21 protein and mRNA levels were compared between 1) postischemic left kidneys, 2) contralateral, uninjured, right kidneys, 3) kidneys from sham-operated mice, and 4) kidneys from normal (nonsurgical) mice. Three plasma samples from each group were also assayed for total cortisol concentrations (see Endogenous Urinary and Plasma Cortisol Levels).
Statistics
Values are means ± SE. Statistical comparisons were made by unpaired Student’s t-test, with Bonferroni’s correction applied for multiple comparisons. P < 0.05 was considered statistically significant.
RESULTS
Glycerol-Induced Changes in p21 Expression
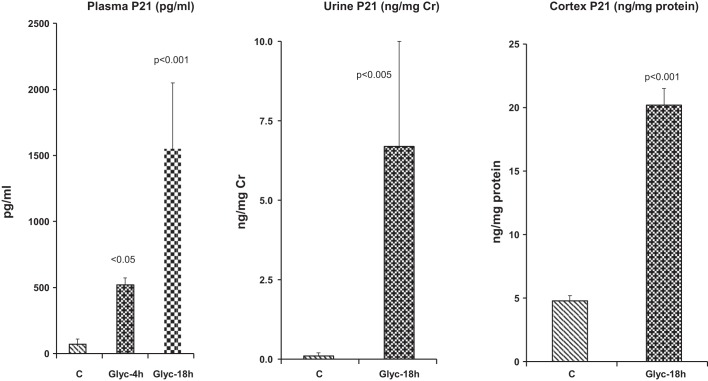
Within 4 h of glycerol injection, plasma p21 levels increased ∼10-fold (Fig. 1, left). This change was progressive in nature, with plasma p21 levels reaching values (by 18 h) that were ~25-fold greater than those seen in controls. The plasma p21 increases were matched by ~75-fold increases in urinary p21 levels, e.g., as factored by urinary creatinine (Fig. 1, middle).
Fig. 1.
Expression of p21 is upregulated by glycerol-induced acute kidney injury. Left: plasma p21 concentrations rose markedly by 4 and 18 h after glycerol (Glyc) injection. C, controls. Middle: increases in plasma concentrations were reflected by dramatic elevations in urinary p21-to-creatinine (Cr) ratios, as assessed 18 h after glycerol injection (P < 0.005 after log base 10 conversion). Right: renal cortical p21 protein levels also rose by 18 h after glycerol injection, but increases were relatively modest (~4-fold) compared with plasma and urinary elevations. Values are means ± SE; n = 15, glycerol doses ranging from 6 to 9 ml/kg, 2–3 at each dose; n = 10 control values.
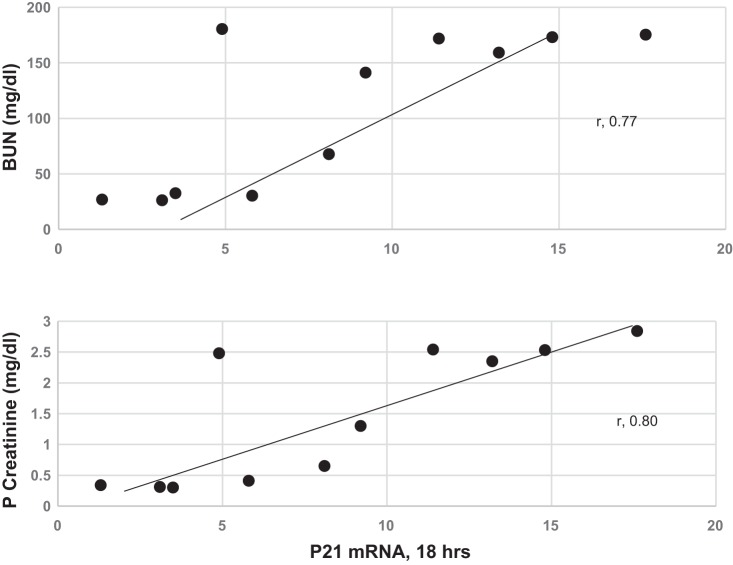
Renal cortical p21 protein elevations were also observed 18 h after glycerol injection (Fig. 1, right) and were modestly correlated with plasma p21 concentrations (r = 0.62, P < 0.02; data not shown). As shown in Fig. 2, renal cortical p21 mRNA values correlated with the severity of glycerol-induced AKI, as gauged by 18-h elevations in BUN and creatinine (r = 0.80 for p21 mRNA vs. 18-h creatinine and r = 0.77 for p21 mRNA vs. 18-h BUN).
Fig. 2.
Elevations in p21 mRNA in the renal cortex correlated with severity of renal injury, as assessed by degrees of blood urea nitrogen (BUN) and plasma (P) creatinine elevations. Glycerol dose of 6–9 ml/kg induced a wide spectrum of BUN and creatinine elevations. Renal cortical p21 mRNA correlated with these elevations in BUN and creatinine. Normal BUN and creatinine concentrations were 18 ± 1 and 0.35 ± 0.05 mg/dl, respectively (not shown).
Glycerol-Induced Changes in p21 mRNA, p53 mRNA, and p53 Protein Levels
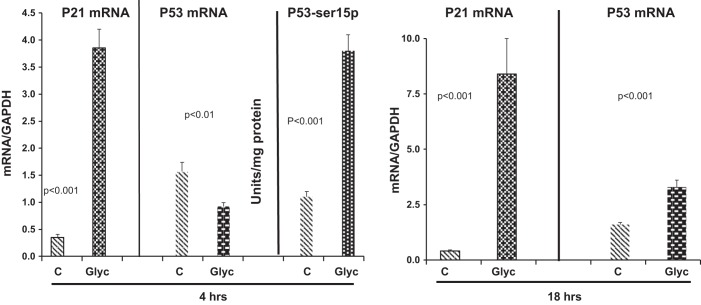
As shown in Fig. 3, marked and progressive increases in p21 mRNA were observed 4 and 18 h after glycerol injection. This finding supports the concept that increased p21 gene transcription was, at least in part, responsible for the time-matched increases in p21 protein levels, as shown in Fig. 1. Conversely, no increase in p53 gene transcription was apparent at 4 h, given that a modest decrease, rather than an increase, in p53 mRNA was observed 4 h after glycerol injection (Fig. 3, left). In contrast, by 4 h after glycerol injection, a threefold increase in total renal cortical p53 protein had occurred: 0.37 ± 0.007 versus 0.12 ± 0.025 (control) units/mg tissue protein (P = 0.001). Furthermore, a doubling of the percent phosphorylation (Ser15) of p53 was observed: 98 ± 5% versus 48 ± 1% (control) (P < 0.001). Absolute increases in phosphorylation (Ser15) of p53 are depicted in Fig. 3. The increases in p53 protein in the absence of an increase in p53 mRNA imply increased p53 translation and stabilization, which are the known prime determinants of tissue p53 levels (9, 14, 26).
Fig. 3.
Levels of p21 and p53 mRNA 4 and 18 h after glycerol (Glyc) injection and phosphorylated (Ser15) p53 (p53-ser15p) levels 4 h after glycerol injection. Marked elevations in p21 mRNA were observed 4 h after glycerol injection, despite a significant decrease in p53 mRNA levels at 4 h. C, controls. By 18 h after glycerol injection, p21 mRNA increased further (note change in y-axis for 4 h vs. 18 h). Despite a small increase in p53 mRNA at 18 h, there was no correlation between p21 mRNA values and the corresponding p53 mRNA values (r = −0.02). Phosphorylated (Ser15) p53 protein increased 3- to 4-fold 4 h after glycerol injection. Values are means ± SE; n = 5–10 mice per group.
At 18 h after glycerol injection, slight elevations in p53 mRNA were observed (Fig. 3, right), suggesting increased p53 transcription. However, no correlations were observed between p21 mRNA and these p53 mRNA increases (r = −0.02).
Pifithrin-α
The p53 inhibitor pifithrin-α failed to suppress the glycerol-induced p21 mRNA increases: 0.34 ± 0.1 (control), 1.43 ± 0.11 (4 h after glycerol), and 1.71 ± 0.08 (4 h after glycerol + pifithrin). However, given the fact that the glycerol model caused a marked increase in both total and activated [phosphorylated (Ser15)] p53, it appears that 1) pifithrin failed to suppress these p53 increases, e.g., due to its alternative biological effects (22), or 2) alternative molecules were able to induce p21, even in a state of pifithrin-induced p53 inhibition.
Dexamethasone Treatment
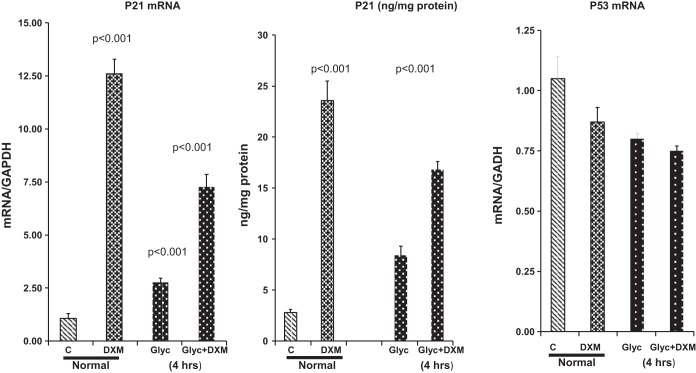
Within 4 h of DXM injection into normal mice, dramatic increases in p21 protein (10-fold) and mRNA (15-fold) levels were observed in the renal cortex. DXM also evoked nearly two- to threefold increases in p21 mRNA and protein in kidneys 4 h after glycerol injection (Fig. 4). In neither the normal mice nor these glycerol-injected mice could these DXM-induced increases in p21 mRNA and protein be ascribed to p53 gene transcription, given that p53 mRNA levels tended to fall, rather than rise, with DXM treatment (Fig. 4, right). Furthermore, p53 total protein levels and the extent of p53 phosphorylation were not impacted by DXM treatment: 0.12 ± 0.02 units/mg protein and 48% phosphorylated in controls and 0.15 ± 0.04 units/mg protein and 50% phosphorylated 4 h after DXM injection (not significant for both comparisons).
Fig. 4.
Glucocorticoid effects on p21 mRNA and protein expression. Administration of dexamethasone (DXM) to control (C) (normal) mice increased p21 mRNA and protein levels >10-fold within 4 h. DXM also evoked ~2- to 3-fold increases in p21 mRNA and protein levels 4 h after glycerol injection. These changes were observed despite slight decreases, rather than increases, in p53 mRNA levels over this 4-h time period. Values are means ± SE; n = 5–8 mice per group.
Cortisol injection fully recapitulated DXM’s effect on the glycerol model, raising p21 mRNA and protein levels 4 h after glycerol injection to the same extent as did DXM injection, nearly doubling baseline levels (data not shown).
Cortisol Measurements After Glycerol Injection
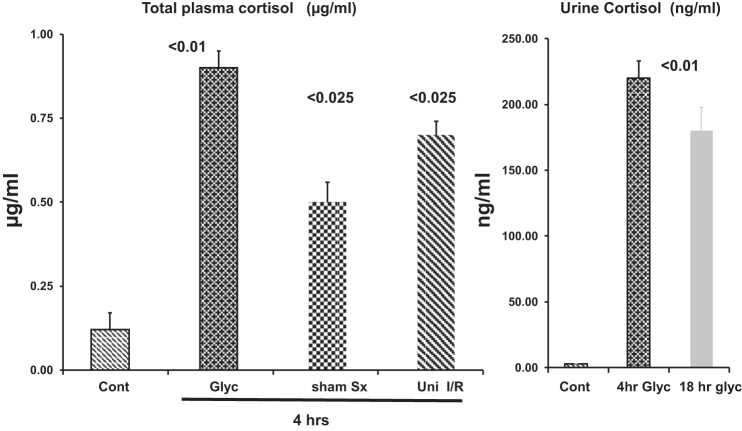
Within 4 h of glycerol injection, plasma total cortisol levels rose nearly fivefold over control values (P < 0.0001; Fig. 5). These changes were accompanied by massive (~75-fold) increases in urinary free cortisol concentrations 4 and 18 h after glycerol injection (P < 0.01 versus normal values for both time points).
Fig. 5.
Plasma and urinary cortisol levels in response to renal injury. Left: glycerol (Glyc) injection raised plasma cortisol levels ~6-fold within 4 h. Marked plasma cortisol increases were also observed 4 h after unilateral ischemia-reperfusion (Uni I/R) or sham surgery (Sx). Right: urinary free cortisol concentrations increased ~75-fold 4 or 18 h after glycerol injection. Cont, control. Values are means ± SE; n = 5 mice per group.
Effects of the Glucocorticoid Receptor Antagonist Mifepristone
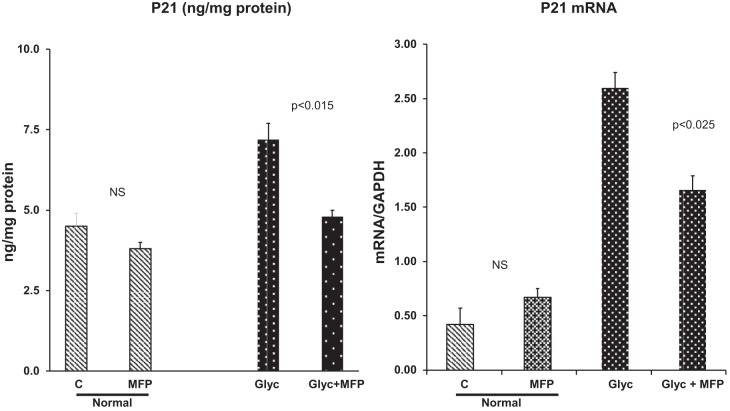
By 4 h after normal mice were treated with MFP, no significant changes in p21 protein or mRNA values were observed (Fig. 6). In contrast, in glycerol-treated mice, MFP normalized (i.e., returned to control) p21 protein levels (Fig. 6, left). In glycerol-treated mice, MFP reduced p21 mRNA levels, indicating that, blocking the GCR caused a decrease in p21 gene transcription.
Fig. 6.
Mifepristone (MPF) effects on glycerol-induced p21 gene induction (p21 mRNA) and renal p21 accumulation. MFP markedly diminished post-glycerol p21 protein and mRNA increases 4 h after glycerol + MFP injection. Conversely, MFP did not significantly impact p21 expression in non-glycerol-injected (normal) mice. Values are means ± SE; n = 4 mice group. NS, not significant.
Expression of p21 in Sham-Operated Mice and Mice Subjected to Unilateral Renal Ischemia
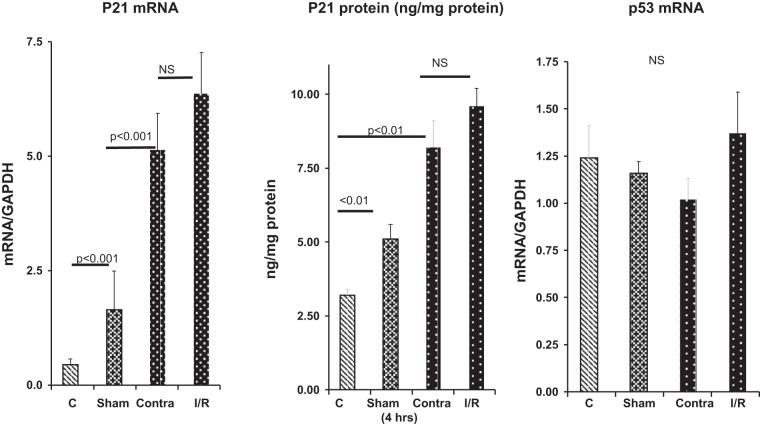
As shown in Fig. 7, left, within 4 h of sham surgery, renal cortical p21 mRNA increased fourfold. By 4 h after unilateral ischemic renal injury, p21 mRNA levels were markedly elevated in the postischemic left kidneys and contralateral right kidneys (10- and 15-fold, respectively). These p21 mRNA increases corresponded with marked increases in p21 protein levels (Fig. 7, middle). Of note was the lack of statistically significant differences in the degree of p21 protein or mRNA increases in the right contralateral kidneys compared with their left postischemic kidney counterparts. As with the glycerol-induced AKI model, both the unilateral ischemic injury model and the sham surgery model caused five- to sevenfold increases in plasma cortisol concentrations (Fig. 5).
Fig. 7.
Effects of surgical stress and renal ischemia-reperfusion (I/R) on p21 gene expression. Sham surgery caused significant elevations in renal cortical p21 mRNA and protein levels compared with control (C) values. Renal ischemia followed by 4 h of reperfusion also markedly increased p21 mRNA and protein levels. However, the contralateral uninjured kidneys (Contra) also showed marked p21 mRNA and protein increases, which did not significantly differ from values in their postischemic counterparts (n = 5 mice per group). p53 mRNA values did not significantly change as a result of these interventions. As shown in Fig. 5, both sham surgery and unilateral ischemia-reperfusion caused acute ~6- to 7-fold increases in plasma cortisol levels compared with control. Values are means ± SE; n = 4 mice per group. NS, not significant.
DISCUSSION
Heme iron-driven oxidative stress is the dominant mechanism underlying the glycerol model of rhabdomyolysis-induced acute renal failure (13, 25). The present study indicates, for the first time, that a concomitant of this oxidant injury is a dramatic increase in p21 protein levels, whether assessed in plasma, urine, or renal cortex. It is noteworthy that these changes were associated with nearly fourfold increases in renal cortical p21 mRNA, a change that mirrored AKI severity (assessed by BUN and creatinine levels, r ~ 0.8). Thus, increased renal cortical p21 gene transcription and translation were almost certainly at play. However, we previously observed that the systemic effects of AKI can increase p21 gene activity in extrarenal organs (e.g., heart and brain) (6), resulting in systemic p21 release. Given that circulating p21 undergoes glomerular filtration, both renal as well as extrarenal p21 production likely contributed to the increases in renal cortical and urinary p21 concentrations (6).
p53 is the dominant transcription factor that drives p21 gene expression (9, 14, 18, 26). In response to stressful stimuli (e.g., oxidant stress), tissue p53 levels rise, predominantly due to posttranscriptional events (e.g., increased mRNA translation and mRNA and protein stabilization). The results of these studies indicate that, in response to glycerol injection, a doubling of total p53 with near-complete (98%) Ser15 phosphorylation/activation occurs. Thus it is reasonable to assume that these elevated p53 levels played a role in p21 gene activation. Given these p53 results, it was surprising that the widely used p53 inhibitor pifithrin-α (22) failed to mitigate the glycerol-mediated p21 increases. There are two possible explanations for this paradox: 1) pifithrin may have failed to suppress p53, either due to postinjection compound instability or countervailing nonspecific biological effects (22); or 2) alternative molecules were able to drive p21 gene transcription, despite pifithrin injection. This latter consideration led us to search for such potential molecules.
In addition to p53, it is notable that multiple inflammation-induced transcription factors (e.g., NF-κB) and cytokines (e.g., IL-6) can also activate p21 independent of p53 (1, 2). Given that AKI induces both renal and extrarenal inflammatory responses (21), we next questioned whether the potent anti-inflammatory agent dexamethasone would suppress glycerol-mediated renal p21 gene induction. Surprisingly, the exact opposite was the case: dexamethasone dramatically increased, rather than decreased, renal cortical p21 mRNA and protein levels in normal and glycerol-injured kidneys. Cortisol administration reproduced this dexamethasone response in glycerol-induced AKI mice, indicating that a glucocorticoid class effect, rather than a dexamethasone-specific action, was at play. To the best of our knowledge, the present results are the first to demonstrate that glucocorticoids can activate the renal p21 gene. It is notable that an extensive literature indicates that glucocorticoids dramatically suppress, rather than increase, p53-driven gene activity (16, 17, 20). Furthermore, dexamethasone did not increase the levels of total p53, p53 serine phosphorylation, or p53 mRNA. Thus it seems clear that dexamethasone-mediated p21 upregulation does not require an increase in p53 levels. However, it remains possible that even normal p53 levels might play a role in glucocorticoid-driven p21 increases due to a variety of factors, e.g., MDM2 and MDMX, and not just absolute p53 levels, ultimately determine p53 activity (19).
To assess whether endogenous glucocorticoid increases might be at least partly responsible for the glycerol-induced increases in renal p21, it was critical to determine whether the glycerol-induced AKI model does, in fact, increase glucocorticoid production. To make this determination, both plasma total cortisol and urinary “free” cortisol levels were assessed. Within 4 h of glycerol injection, total plasma cortisol increased five- to sevenfold (Fig. 5). Direct renal cortisol access was confirmed by a ~75-fold increase in urinary free cortisol levels both 4 and 18 h after glycerol injection. To test the physiological relevance of these AKI-induced cortisol increases to the augmented p21 expression, the impact of the glucocorticoid receptor (GCR) antagonist mifepristone was assessed. As shown in Fig. 6, MFP administration almost completely blocked glycerol-induced renal p21 protein increases and significantly decreased p21 transcription, as assessed by reductions in p21 mRNA. Thus these data strongly support the concept that endogenously generated cortisol stimulates p21 gene expression, and via the GCR pathway. In sum, our results suggest a novel mechanism for renal p21 accumulation via glycerol-induced AKI: 1) AKI induces a systemic stress response; 2) glucocorticoid synthesis increases; 3) circulating cortisol gains ready renal access (i.e., increased urinary free cortisol levels); and 4) via interaction with the GCR pathway, renal p21 gene activation increases, and p21 protein is produced. Finally, it is interesting to speculate that, in addition to activating p21, an increase in plasma cortisol during AKI could mitigate evolving AKI by exerting its anti-inflammatory effect. In this regard, it is well accepted that intrarenal inflammation represents a postinjury amplification loop that could be attenuated by increased cortisol production (21).
Finally, we assessed whether an AKI-induced stress response can activate the renal p21 gene, even in the absence of direct tissue injury. Indeed, this appears to be the case based on observations that mice subjected to unilateral ischemic injury manifested comparable p21 protein and mRNA increases in postischemic and contralateral (uninjured) kidneys. Furthermore, surgical stress, as induced by sham renal ischemia, also raised, albeit to lesser degrees, renal cortical p21 mRNA and protein levels. Concomitant with these findings, both the unilateral ischemia protocol and the sham surgery protocols caused five- to sevenfold increases in plasma cortisol levels. Thus these findings indicate that systemic stress, independent of direct renal injury, is sufficient to stimulate the renal corticosteroid-p21 pathway. The relative contribution(s) of corticosteroid- versus potential non-corticosteroid-mediated “stress reactants” (e.g., catechols) to the renal cortical p21 gene response remains to be defined.
In conclusion, dramatic and sustained p21 gene activation occurs with the glycerol-induced AKI model, a process that is reflected by concomitant dramatic increases in plasma and urinary p21 concentrations. During this process, p53 accumulation/activation occur (a well-known stimulus for increased p21 gene transcription). However, alternative (non-p53) activators, most notably AKI-induced increases in glucocorticoid production/GCR binding, also appear to play a crucial role. To our knowledge, this is the first report of glucocorticoid-driven renal p21 gene upregulation with resultant striking renal p21 accumulation. Given that glucocorticoids remain a mainstay in the treatment of multiple forms of acute and chronic renal injuries, the present observations suggest a novel pathway by which these agents may impact the kidney. Hence, further study of this glucocorticoid-p21 pathway seems warranted from a basic science, as well as a clinical/therapeutic, perspective.
GRANTS
This work was supported by a sponsored research agreement with Renibus Therapeutics (Southlake, TX) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-1 DK-38432.
DISCLOSURES
R. A. Zager and A. C. M. Johnson have taken out a provisional patent concerning the potential therapeutic utility of targeted renal glucocorticoid-induced p21 upregulation.
AUTHOR CONTRIBUTIONS
R.A.Z. conceived and designed research; A.C.M.J. performed experiments; R.A.Z. and A.C.M.J. analyzed data; R.A.Z. and A.C.M.J. interpreted results of experiments; R.A.Z. prepared figures; R.A.Z. drafted manuscript; R.A.Z. edited and revised manuscript; R.A.Z. and A.C.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of A. C. M. Johnson: Fred Hutchinson Cancer Research Center, Seattle, WA 98109 (e-mail: acwillia@fredhutch.org).
REFERENCES
- 1.Basile JR, Eichten A, Zacny V, Münger K. NF-κB-mediated induction of p21Cip1/Waf1 by tumor necrosis factor-α induces growth arrest and cytoprotection in normal human keratinocytes. Mol Cancer Res 1: 262–270, 2003. [PubMed] [Google Scholar]
- 2.Bellido T, O’Brien CA, Roberson PK, Manolagas SC. Transcriptional activation of the p21WAF1,CIP1,SDI1 gene by interleukin-6 type cytokines. A prerequisite for their pro-differentiating and anti-apoptotic effects on human osteoblastic cells. J Biol Chem 273: 21137–21144, 1998. doi: 10.1074/jbc.273.33.21137. [DOI] [PubMed] [Google Scholar]
- 3.Dagher PC, Mai EM, Hato T, Lee SY, Anderson MD, Karozos SC, Mang HE, Knipe NL, Plotkin Z, Sutton TA. The p53 inhibitor pifithrin-α can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F284–F291, 2012. doi: 10.1152/ajprenal.00317.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E. Biology of the cell cycle inhibitor p21CDKN1A: molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89: 155–178, 2015. doi: 10.1007/s00204-014-1430-4. [DOI] [PubMed] [Google Scholar]
- 5.Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, Alam J, Nath KA. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int 60: 2181–2191, 2001. doi: 10.1046/j.1523-1755.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AC, Zager RA. Plasma and urinary p21: potential biomarkers of AKI and renal aging. Am J Physiol Renal Physiol 315: F1329–F1335, 2018. doi: 10.1152/ajprenal.00328.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. p53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol 14: 128–138, 2003. doi: 10.1097/01.ASN.0000040596.23073.01. [DOI] [PubMed] [Google Scholar]
- 8.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285: 1733–1737, 1999. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Xu Y. p53, oxidative stress, and aging. Antioxid Redox Signal 15: 1669–1678, 2011. doi: 10.1089/ars.2010.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megyesi J, Andrade L, Vieira JM Jr, Safirstein RL, Price PM. Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Renal Physiol 283: F810–F816, 2002. doi: 10.1152/ajprenal.00078.2002. [DOI] [PubMed] [Google Scholar]
- 11.Meijer OC, Koorneef LL, Kroon J. Glucocorticoid receptor modulators. Ann Endocrinol (Paris) 79: 107–111, 2018. doi: 10.1016/j.ando.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Nath KA. Provenance of the protective property of p21. Am J Physiol Renal Physiol 289: F512–F513, 2005. doi: 10.1152/ajprenal.00224.2005. [DOI] [PubMed] [Google Scholar]
- 13.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brate A, Giannakakou P. The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist Updat 6: 313–322, 2003. doi: 10.1016/j.drup.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sengupta S, Vonesch J-L, Waltzinger C, Zheng H, Wasylyk B. Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells. EMBO J 19: 6051–6064, 2000. doi: 10.1093/emboj/19.22.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta S, Wasylyk B. Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes Dev 15: 2367–2380, 2001. doi: 10.1101/gad.202201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev 11: 3471–3481, 1997. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: alone and together in regulation of p53. Transl Cancer Res 1: 88–89, 2012. [PMC free article] [PubMed] [Google Scholar]
- 20.Sjögren S, Jaako P, Karlsson S, Flygare J. Glucocorticoids reduce expression of p53 target genes and reverse anemia in a mouse model of Diamond-Blackfan anemia. Blood 122: 3703–3709, 2013. doi: 10.1111/bjh.13632 [DOI] [Google Scholar]
- 21.Tonnus W, Gembardt F, Latk M, Parmentier S, Hugo C, Bornstein SR, Linkermann A. The clinical relevance of necroinflammation—highlighting the importance of acute kidney injury and the adrenal glands. Cell Death Differ 26: 68–82, 2019. doi: 10.1038/s41418-018-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walton MI, Wilson SC, Hardcastle IR, Mirza AR, Workman P. An evaluation of the ability of pifithrin-α and -β to inhibit p53 function in two wild-type p53 human tumor cell lines. Mol Cancer Ther 4: 1369–1377, 2005. doi: 10.1158/1535-7163.MCT-04-0341. [DOI] [PubMed] [Google Scholar]
- 23.Wannachalee T, Turcu AF, Auchus RJ. Mifepristone in the treatment of the ectopic adrenocorticotropic hormone syndrome. Clin Endocrinol (Oxf) 89: 570–576, 2018. doi: 10.1111/cen.13818. [DOI] [PubMed] [Google Scholar]
- 24.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21WAF1/CIP1 that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005. doi: 10.1152/ajprenal.00101.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 49: 314–326, 1996. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, St Clair DK. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res 65: 3745–3750, 2005. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]