Abstract
In inflammatory diseases, the 5-lipoxygenase (5-LO) pathway contributes to epithelial damage and fibrosis by catalyzing the production of leukotrienes (LTs). Antagonists of the 5-LO pathway are currently approved for use in patients and are well tolerated. We found that expression of 5-LO is strongly induced in three models of chronic kidney disease: unilateral ureteral obstruction (UUO), folate nephropathy, and an orthologous mouse model of polycystic kidney disease. Immunohistochemistry showed that macrophages are the dominant source of 5-LO. Zileuton, a US Food and Drug Administration-approved antagonist of 5-LO, significantly reduced fibrosis at 7 and 14 days after UUO; these findings were confirmed using a genetically modified [5-LO-associated protein-knockout (Alox5ap−/−)] mouse strain. Inhibition of 5-LO did not appear to change infiltration of leukocytes after UUO as measured by flow cytometry. However, fluorescence-lifetime imaging microscopy showed that 5-LO inhibitors reversed the glycolytic switch in renal tubular epithelial cells after UUO. Two downstream enzymes of 5-LO, LTA4 hydrolase (LTA4H) and LTC4 synthase (LTC4S), are responsible for the synthesis of LTB4 and cysteinyl LTs, respectively. Fibrosis was reduced after UUO in Ltc4s−/−, but not Lta4h−/−, mice. In contrast, using the folate nephropathy model, we found reduced fibrosis and improved renal function in both Ltc4s−/− and Lta4h−/− mice. In summary, our studies suggest that manipulation of the 5-LO pathway may represent a novel treatment approach for chronic kidney disease.
Keywords: fibrosis, fluorescence-lifetime imaging microscopy, kidney, leukotrienes, 5-lipoxygenase
INTRODUCTION
Chronic kidney disease (CKD) is a highly prevalent disorder that is associated with significant morbidity and mortality. Despite optimal treatment, CKD often progresses to end-stage renal disease, highlighting the need for new therapies. One potential treatment target in CKD is renal fibrosis (8). Renal fibrosis is a universal finding in CKD and predicts CKD progression. The development of renal fibrosis is tightly regulated and involves cell-cell interactions between renal tubular epithelial cells, macrophages, and matrix-producing cells.
Macrophages can mediate epithelial damage through production of 5-lipoxygenase (5-LO) (27). 5-LO is the rate-limiting enzyme that catalyzes conversion of arachidonic acid to leukotriene (LT) A4 (LTA4). LTA4 is a short-lived eicosanoid that is converted to LTB4 by LTA4 hydrolase (LTA4H) or to cysteinyl LTs (LTC4, LTD4, and LTE4) by LTC4 synthase (LTC4S) (10, 32). LTB4 and cysteinyl LTs bind to distinct cell surface receptors and mediate diverse biological responses. LTB4 increases neutrophil and T cell influx and promotes the antimicrobial actions of neutrophils and macrophages. However, persistent LTB4 production also leads to sterile inflammation in chronic inflammatory disease (31). The cysteinyl LTs are elevated in type 2 inflammation and in patients with asthma (7). Antagonists of the 5-LO pathway (zileuton) and cysteinyl LT receptor type 1 (montelukast and pranlukast) are approved for the treatment of asthma and are well tolerated.
Although LTs have potent effects on leukocytes, they also contribute to disease progression through other cell types. Cell surface receptors for LTs are found on many cell types, including leukocytes, epithelial cells, fibroblasts, and endothelial cells. In epithelial cells, LTs can directly modify proliferation, survival, and apoptosis through activation of MAP kinases and the phosphoinositide 3-kinase/Akt pathway (13, 20, 34). LTs also activate fibroblast proliferation and migration, myofibroblast transformation, and synthesis of extracellular matrix (15, 26, 33).
In addition to allergic diseases such as asthma, LTs play key roles in other inflammatory diseases. In bleomycin-induced lung fibrosis, cysteinyl LTs play a significant role (1, 2). LTB4 inhibition may also reduce pulmonary fibrosis in a neutrophil-independent fashion (19). In ischemia-reperfusion injury of the kidneys, 5-LO plays a key role in neutrophil recruitment and epithelial damage (22, 25). LTs also contribute to cisplatin-mediated renal injury and tubular damage in the setting of albumin overload (4, 17). However, while these studies show that 5-LO can mediate acute injury, whether 5-LO promotes interstitial fibrosis and CKD progression is unknown. We hypothesized that 5-LO inhibition would reduce renal fibrosis by modifying inflammation or through direct effects on resident renal cells.
MATERIALS AND METHODS
Animal experiments.
All animal experiments were reviewed and approved by the University of Colorado Institutional Animal Care and Use Committee. All mice undergoing unilateral ureteral obstruction (UUO) or receiving folate were 10–15 wk of age. In experiments using zileuton, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were randomly assigned to zileuton or vehicle. Zileuton (Cayman, Ann Arbor, MI) was dissolved in a solution consisting of 25% DMSO (Sigma, St. Louis, MO) and 75% saline and administered once daily (30 mg/kg) by intraperitoneal injection starting 1 day before UUO. Control mice received vehicle (25% DMSO and 75% saline) once daily by intraperitoneal injection. Alox5ap−/− [5-LO-associated protein (FLAP)-knockout], Ltc4s−/− (LTC4 synthase-knockout), and Lta4h−/− (LTA4 hydrolase-knockout) mice were gifts from the laboratory of Robert Murphy (University of Colorado). All strains were fully backcrossed into the C57BL/6J strain. UUO was performed as previously described (21). Isoflurane anesthesia and sterile technique were used to expose and ligate the right ureter with a 4-0 silk suture. The surgical area was irrigated with 1 ml of sterile saline, and the wound was closed in two layers. For induction of folate nephropathy, mice were injected intraperitoneally with folic acid (Sigma). Mice were given a single dose of folic acid (60 mg/kg, dissolved in 0.3 M sodium bicarbonate) or sodium bicarbonate alone as a control. Blood was sampled using a right ventricular puncture, the right atrium was clipped, and mice were perfused with 10 ml of phosphate-buffered saline with heparin (Sigma).
Hydroxyproline assay.
Inferior pole segments of kidney (20–30 mg of tissue) were homogenized in water using a bead homogenizer (TissueLyser LT, Qiagen, Germantown, MD). An aliquot was removed for direct protein measurement using a Bradford assay (Bio-Rad, Hercules, CA). The remaining solution was mixed with 12 N HCl (Fisher Scientific, Hampton, NH) and hydrolyzed at 120°C for 24 h. An equal amount of protein per sample was loaded into 2.0-ml tubes (Eppendorf, Hamburg, Germany), and the solvent was evaporated in a fume hood. Hydroxyproline standards (Sigma) and samples were analyzed together. An acetate-citrate buffer (pH 6.0) containing 1.4% chloramine T (Sigma) was first added to the samples. Ehrlich’s reagent, containing 60% perchloric acid and para-dimethylbenzaldehyde (Sigma), was added, and the samples were heated to 60°C for 25 min. The hydroxyproline content was measured by reading the absorbance at 560 nm.
Second-harmonic generation and two-photon excitation fluorescence microscopy.
Kidney tissues were fixed in 10% phosphate-buffered formalin (Fisher Scientific) and embedded in paraffin. Sections (5 μm thick) were scanned in 15 random regions of interest. Images were acquired at ×20 magnification using a laser-scanning confocal microscope (Zeiss 780, Carl Zeiss, Jena, Germany) equipped with a titanium-sapphire laser (Chameleon Ultra II, Coherent, Santa Clara, CA). The average laser power of 7% at 800 nm [tuned for second-harmonic generation (SHG)] with 140-fs pulse duration and 80-MHz repetition rate was used. After passage through the microscope optics, the pulse duration was ~300 fs. SHG signal was detected on a nondescanned detector following transmission through a filter cube containing a narrow-band 390- to 410-nm emission filter (product no. hq400/20m-2p, Chroma Technology, Bellows Falls, VT). Collagen was quantified using ImageJ (National Institutes of Health). The green (autofluorescence) and red (fibrillar collagens) channels were separated, and a threshold was set for the collagen. Percent area was quantified using the threshold value.
Flow cytometry.
Flow cytometry of kidney suspensions was done as previously described (21). Fresh kidneys were cut into ~1-mm pieces. Liberase TL (Roche, Basel, Switzerland) and DNase (Sigma) were added to the kidney pieces, and the samples were placed in a shaking water bath for 30 min at 37°C. The reaction was terminated with addition of ice-cold FA3 buffer (phosphate-buffered saline, 10 mM HEPES, 2 mM EDTA, and 1% fetal calf serum). Cells were sequentially passed through a 70-μm and a 40-μm cell strainer and treated with a red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA). The following antibodies were used for flow cytometry staining: CD45-phycoerythrin (1:100 dilution; clone 30F11, BD PharMingen, San Jose, CA), Cd11b-FITC (1:100 dilution; clone M1/70, BD PharMingen), and Ly6C-peridinin-chlorophyll-protein-Cy5.5 (1:100 dilution; clone HK1.4, BioLegend, San Diego, CA). Flow cytometry was performed on a Gallios flow cytometer (Beckman Coulter, Brea, CA). Compensation beads and single-antibody-stained cells were used for compensation. All analysis was performed using Kaluza software (Beckman Coulter).
Immunohistochemistry and histology.
Formalin-fixed, paraffin-embedded tissues were cut into 4-μm-thick sections. Masson’s trichrome staining was performed as previously described (28). For 5-LO immunohistochemistry (IHC), slides were hydrated and subjected to antigen retrieval using citric acid-based antigen-unmasking solution (Vector Laboratories, Burlingame, CA). For F4/80 IHC, slides were hydrated and subjected to antigen retrieval with trypsin digestion (Sigma). After they were blocked with hydrogen peroxide and a blocking buffer (Vector Laboratories), slides were incubated at 4°C overnight in 5-LO antibody (Cell Signaling Technology, Danvers, MA) or F4/80 antibody (Bio-Rad). The Vectastain kit (Vector Laboratories) was used to incubate the slides in secondary antibody and ABC reagent according to the manufacturer’s recommendations. Slides were developed for 5-LO using a horseradish peroxidase (HRP) reagent (ImmPACT NovaRed, Vector Laboratories) and mounted in aqueous mounting medium (Vector Laboratories). Slides were developed for F4/80 using 3,3′-diaminobenzidine reagent (Vector Laboratories), dehydrated, and mounted with Permount (Fisher Scientific).
Immunofluorescence.
Kidney sections (5 μm thick) were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA). Slides were fixed in chilled acetone for 5 min and blocked with an avidin-biotin blocking system (Vector Laboratories) and 5% goat serum. Slides were incubated in phosphohistone H3 antibody (Cell Signaling Technology) overnight at 4°C and then in biotinylated anti-rabbit antibody (Vector Laboratories) and avidin-rhodamine (Vector Laboratories). Epithelial cells were marked by incubation of the slides in FITC-conjugated E-cadherin antibody (Cell Signaling Technology). Slides were mounted in VectaMount with DAPI (Vector Laboratories). Images were acquired on a laser-scanning confocal microscope (Zeiss 780).
Western blotting.
Kidneys were homogenized in ice-cold mammalian protein extraction reagent (M-PER, Thermo Fisher Scientific). Solubilized proteins were centrifuged at 14,000 g (4°C) for 10 min. Protein concentration of supernatants was determined using a Bradford assay. Protein was separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon P membranes (Millipore, Billerica, MA). Membranes were blocked for 1 h at room temperature in Tris-buffered saline [10 mM Tris·HCl (pH 7.4) and 140 mM NaCl containing 0.1% Tween 20 (TTBS) and 5% milk] and then incubated with 5% milk in TTBS solution containing primary antibodies for 16 h at 4°C. Membranes were washed in TTBS, and HRP-coupled secondary antibodies were used according to the manufacturer’s directions (Cell Signaling Technology). The following antibodies were used: 5-LO (1:500 dilution; Cell Signaling Technology), GAPDH (1:10,000 dilution; Santa Cruz Biotechnology), and anti-rabbit HRP (1:6,000 dilution; Cell Signaling Technology).
Fluorescence-lifetime imaging microscopy.
Changes in metabolism characterized by levels of free vs. bound NADH in kidney cortex were detected by fluorescence-lifetime imaging microscopy (FLIM) using a Zeiss 780 laser-scanning confocal/multiphoton-excitation fluorescence microscope with a 34-channel GaAsP QUASAR detection unit and nondescanned detectors for two-photon fluorescence (Zeiss, Thornwood, NY) equipped with a FastFLIM box (model A320, ISS, Champaign, IL) and a titanium-sapphire laser (Chameleon Ultra II). The phasor transformation and data analysis were carried out using Global SimFCS software (Laboratory for Fluorescence Dynamics, University of California, Irvine, CA), as described previously (5). Briefly, the fluorescence decay in each pixel of the FLIM line scan was transformed to sine and cosine components, which were then represented in a two-dimensional polar (phasor) plot. Each pixel of the FLIM line scan gave rise to a single point (phasor) in the phasor plot and, when used in reciprocal mode, enabled each point of the phasor plot to be mapped to each pixel of the FLIM line scan. Because phasors follow simple vector algebra, it is possible to determine the fractional contribution of two or more independent molecular species coexisting in the same pixel. Fractional analysis of free vs. bound NADH in each pixel was determined based on fluorescence lifetime corresponding to 0.4 ns (free) and 3.4 ns (bound) for NADH. Data for each condition were plotted using Prism 6.0 software (GraphPad, San Diego, CA).
Statistics.
Values are means ± SE. One-way ANOVA with Dunnett’s multiple comparisons was used to compare three or more groups. Statistical analysis was performed using GraphPad Prism 7.03.
RESULTS
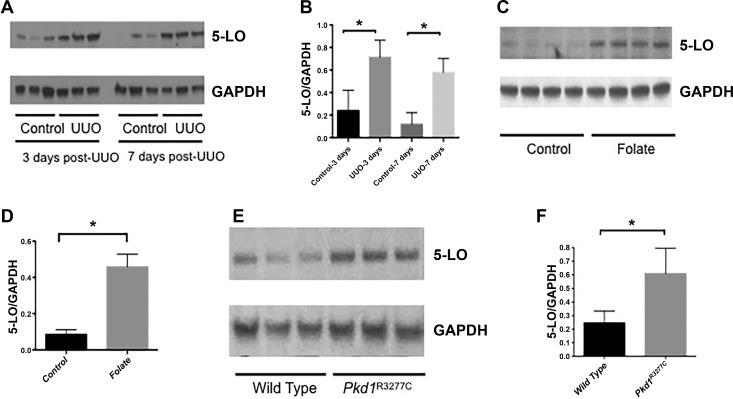
We first tested whether 5-LO was increased in models of renal fibrosis and CKD. We found that expression of 5-LO was significantly increased after UUO at 3 and 7 days (Fig. 1, A and B). To confirm these findings in another model of renal fibrosis, we measured 5-LO in the folic acid nephropathy model. We found a significant increase in 5-LO 35 days after administration of folic acid (Fig. 1, C and D). In the UUO and folic acid models, tubular damage and inflammation occur rapidly (9). To test whether 5-LO is activated in a slower model of CKD, we used an orthologous model of autosomal-dominant (AD) polycystic kidney disease (PKD): the Pkd1R3277C/R3277C mouse. The p.R3277C (RC) point mutation in the Pkd1 gene is also seen in some patients with ADPKD; mice in the C57BL/6 background develop progressive renal cysts and fibrosis but remain alive at 9 mo (12). In 6-mo-old mice, we found a significant increase in 5-LO in Pkd1RC/RC mice (Fig. 1, E and F).
Fig. 1.
Expression of 5-lipoxygenase (LO) in models of chronic kidney disease. A and B: representative Western blots and corresponding densitometry reveal increased 5-LO expression 3 and 7 days after unilateral ureteral obstruction (UUO, n = 3). C and D: representative Western blots and densitometry showing increased 5-LO expression 35 days after administration of folic acid (n = 4 mice). E and F: representative Western blots and densitometry showing increased 5-LO expression in an orthologous mouse model of autosomal-dominant polycystic kidney disease (PKD). For Western blots, GAPDH was used as a loading control. Values are means ± SE. *P < 0.05.
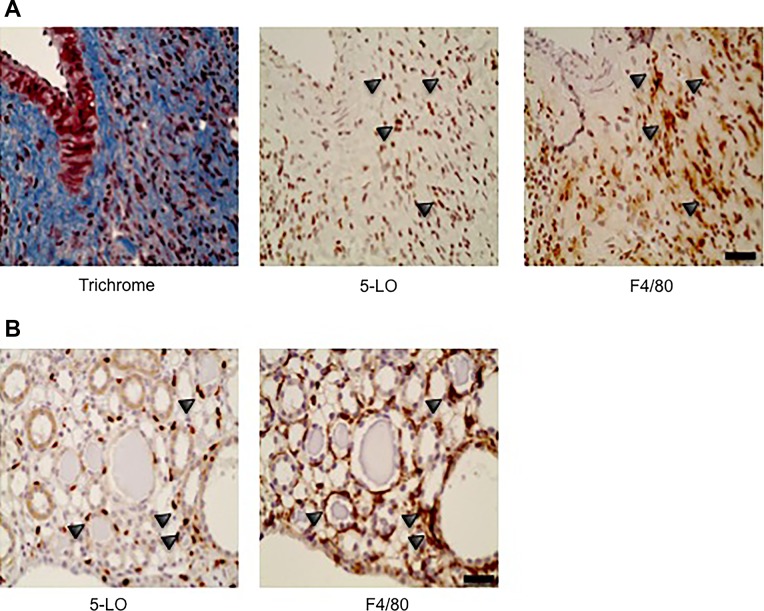
Although 5-LO has been detected in many cell types, it is mainly expressed in macrophages and neutrophils (27). We previously found that macrophages significantly outnumber neutrophils in the UUO model (21). Macrophages also play a key role in the development of renal fibrosis and PKD (14, 18). We therefore hypothesized that the macrophages are the predominant cell type expressing 5-LO in the UUO and ADPKD models. To determine if 5-LO is expressed on macrophages, we performed IHC on serial sections of post-UUO kidneys. In Fig. 2A, serial sections of a post-UUO kidney were stained using Masson’s trichrome, 5-LO IHC, and F4/80 IHC to identify macrophages. We found numerous clusters of 5-LO-expressing cells in fibrotic regions. The majority of these cells also expressed F4/80, supporting the notion that macrophages are a major source of 5-LO after UUO. Similarly, we found a large number of 5-LO-positive cells in the peritubular regions in Pkd1RC/RC mice; a large number of these cells were also positive for F4/80 (Fig. 2B).
Fig. 2.
Localization of cells expressing 5-lipoxygenase (5-LO). A: immunohistochemistry of serial sections of formalin-fixed paraffin-embedded tissues stained for fibrosis using Masson’s trichrome, 5-LO, and F4/80. Arrowheads point to corresponding cells in the serial sections and show a high level of coexpression of 5-LO and F4/80. B: serial sections of autosomal-dominant polycystic kidney disease tissues show coexpression of 5-LO and F4/80. Scale bars = 20 μm.
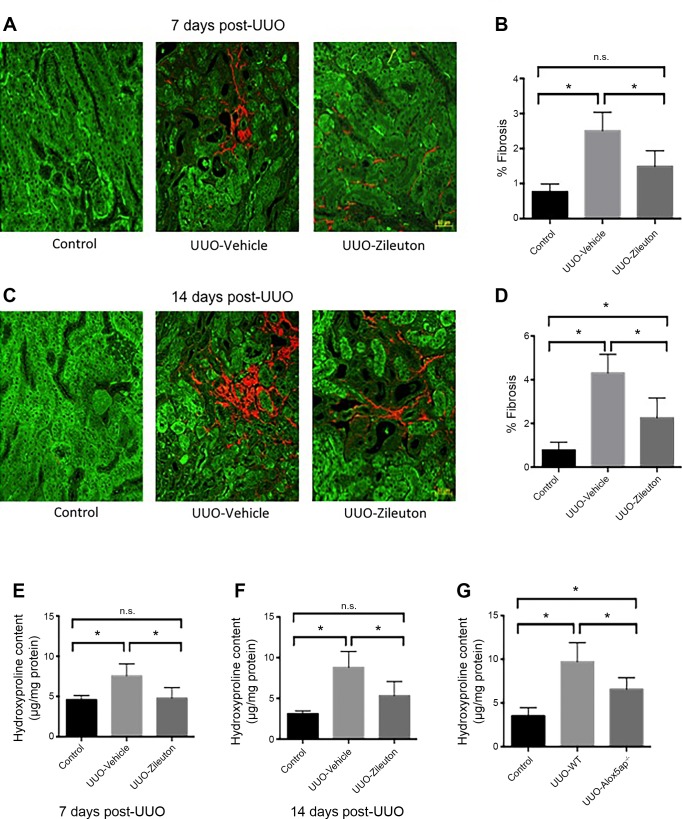
To determine if 5-LO promoted renal fibrosis after injury, we used a US Food and Drug Administration-approved pharmacological antagonist of 5-LO: zileuton. Zileuton injections (30 mg·kg−1·day−1 ip) were started 1 day before initiation of UUO, and mice were collected at 7 and 14 days after UUO. To quantify collagen deposition, we used SHG microscopy. Fibrillar collagen heterotrimers are noncentrosymmetric structures that can be accurately imaged using SHG microscopy. We previously showed that SHG is a very sensitive and specific measure of collagen deposition after UUO (28). Compared with vehicle control, zileuton decreased collagen deposition by ~50% at 7 and 14 days after UUO (Fig. 3, A–D). We also used hydroxyproline measurements to corroborate these data. Similar to SHG imaging, hydroxyproline deposition was decreased in zileuton-treated mice (Fig. 3, E and F). To confirm these findings, we used a genetically modified mouse. FLAP presents substrate to 5-LO, a necessary step in LT synthesis; mice deficient in FLAP exhibit complete abrogation of LT production (11). UUO was performed in FLAP-knockout (Alox5ap−/−) mice, and kidneys were collected at 14 days; hydroxyproline measurements showed that FLAP-knockout mice were also protected from fibrosis after UUO (Fig. 3G).
Fig. 3.
Inhibition of 5-lipoxygenase (5-LO) reduces renal fibrosis after unilateral ureteral obstruction (UUO). A: microscopy for second-harmonic generation (SHG, red) and autofluorescence (green) in control kidneys and kidneys 7 days after UUO. B: quantification of SHG signal at 7 days (%fibrosis area) reveals a decrease in collagen in zileuton-treated mice (n = 5 mice/group). C and D: SHG/autofluorescence images from 14 days after UUO (n = 3 mice/group). E and F: hydroxyproline assays from homogenized kidneys at 7 and 14 days after UUO in zileuton-treated mice (n = 7 mice/group). G: hydroxyproline assay 14 days after UUO in 5-LO-associated protein knockout (Alox5ap−/−) and wild-type (WT) mice (n = 7 mice/group). Values are means ± SE. *P < 0.05; ns, not significant. Scale bars = 50 μm.
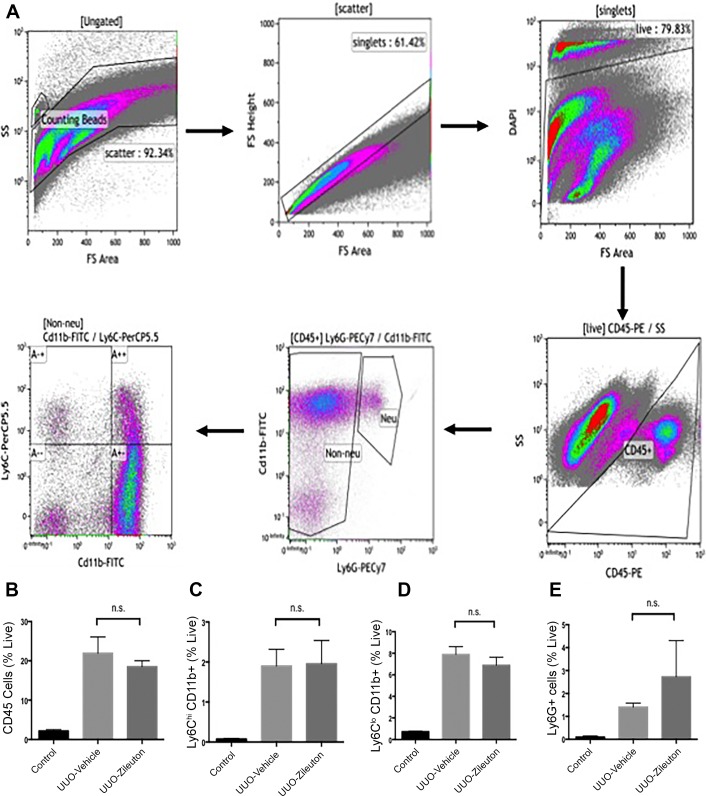
These results suggest that 5-LO metabolites play a crucial role in development of tubulointerstitial fibrosis. In some disease states, LTB4 and cysteinyl LTs promote leukocyte infiltration into tissues. Given the importance of macrophages in renal fibrosis, we tested whether zileuton modified macrophage recruitment after UUO. Using flow cytometry, we assessed macrophage recruitment 3 days after UUO. The gating strategy for single-cell kidney suspensions is shown in Fig. 4A. Flow cytometry showed no difference in recruited CD45+ cells (Fig. 4B). Previous studies showed that distinct macrophage populations (Ly6ChiCD11b+ and Ly6CloCD11b+) have different roles in renal repair and fibrosis. We found that zileuton did not affect levels of either population (Fig. 4, C and D). LTB4 has been shown to recruit neutrophils after renal ischemia. However, we found that zileuton did not change neutrophil number after UUO (Fig. 4E).
Fig. 4.
Inhibition of 5-lipoxygenase (5-LO) does not change myeloid infiltration after unilateral ureteral obstruction (UUO). A: gating strategy for flow cytometry. Singlets were separated into live and dead cells using DAPI. Ly6G−CD45+ cells (nonneutrophils) were analyzed for CD11b and Ly6C expression. B: analysis of single-cell kidney suspensions by flow cytometry shows no significant change in CD45+ cell recruitment with zileuton treatment. C and D: flow cytometry for Ly6ChiCD11b+ and Ly6CloCD11b+ cells reveals no significant change with zileuton. E: flow cytometry for neutrophils reveals no change in Ly6G+ cells. Values are means ± SE; n = 3 mice/group. ns, Not significant.
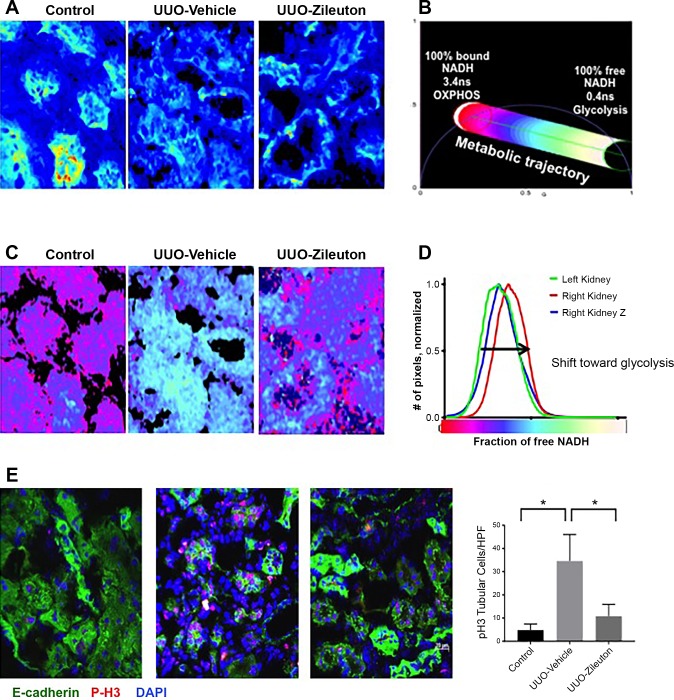
LTs can also exert direct effects on epithelial cells, independent of inflammation. Through specific cell surface receptors, LTs potently regulate epithelial proliferation and apoptosis (24). Furthermore, LTs can activate metabolic signaling that is crucial to a glycolytic switch (23). Since a persistent glycolytic switch has been linked to renal fibrosis (16), we next tested whether 5-LO inhibition in mice undergoing UUO affected epithelial metabolism. To test whether 5-LO altered renal tubular epithelial cell metabolism after UUO, we used FLIM. Certain endogenous autofluorescent molecules, such as NADH, have characteristic decay times. Free NADH and protein-bound NADH have different decay times, and this difference can be quantified by FLIM (5). Previous studies showed that FLIM can accurately detect increases in glycolysis by measuring a shift toward free NADH (29, 30). We compared changes in renal tubular NADH levels in mice 3 days after UUO. Figure 5A shows the imaged portion of the renal cortex and demonstrates the fluorescence intensity and cellular localization of NADH. We focused the FLIM quantification to renal tubules, as seen in the images in Fig. 5A. Using the phasor approach, we then used FLIM to measure a range of free NADH and bound NADH and color-transformed the images according to the metabolic trajectory in Fig. 5B. We found that UUO induced a change in NADH consistent with a glycolytic shift (Fig. 5C). This was largely abrogated in the zileuton-treated mice (Fig. 5C). These data suggest that 5-LO metabolites may play a direct role in modifying renal tubular epithelial metabolism. To determine if this glycolytic switch affected epithelial phenotype, we performed immunofluorescence for phosphohistone H3, a marker of mitosis (Fig. 5E). At 14 days after UUO, we found clusters of mitotic tubules in control mice but significantly fewer phosphohistone H3-positive cells in zileuton-treated mice. This suggests that 5-LO promotes glycolysis and epithelial proliferation but is ineffective in epithelial repair.
Fig. 5.
5-Lipoxygenase (5-LO) contributes to glycolytic shift after unilateral ureteral obstruction (UUO). A: fluorescence-lifetime imaging microscopy (FLIM) after UUO in vehicle- and zileuton-treated mice reveals fluorescence intensity. B: color-coded metabolic trajectory demonstrates lifetimes for both bound NADH and free NADH. C: representative FLIM analysis with FLIM map to represent metabolic status. D: fractional analysis of NADH signal from control and UUO kidneys from vehicle- and zileuton-treated (Z) mice. E: immunofluorescence for phosphohistone H3 (P-H3) and E-cadherin shows a large number of proliferating epithelial cells 14 days after UUO in vehicle-treated mice. HPF, high-power field. Values are means ± SE. *P < 0.05.
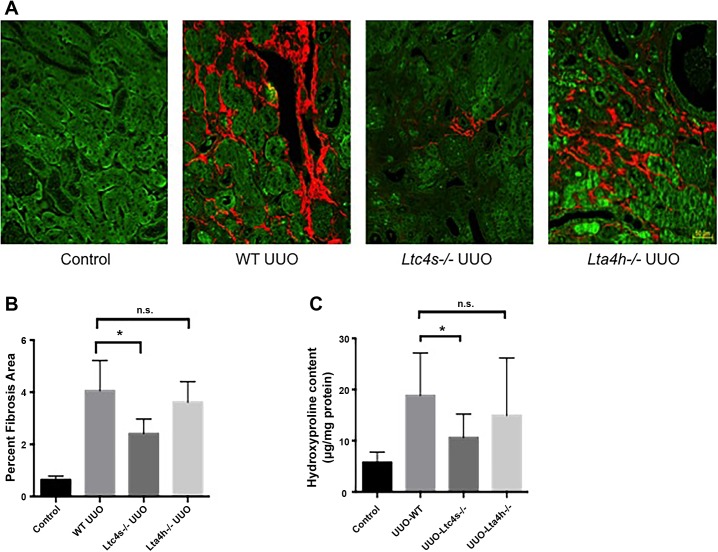
5-LO catalyzes the first step in LT synthesis. The immediate product, LTA4, is metabolized by LTA4 hydrolase to generate LTB4 or by LTC4 synthase to generate cysteinyl LTs. To determine which LTs contributed to renal fibrosis during UUO, we compared wild-type mice with both Lta4h−/− and Ltc4s−/− mice. UUO was performed in the three groups, and kidneys were collected at 14 days. SHG images showed that Ltc4s−/−, but not Lta4h−/−, mice had significantly less collagen deposition after UUO (Fig. 6, A and B). Hydroxyproline measurements confirmed that Ltc4s−/− mice were protected from renal fibrosis (Fig. 6C).
Fig. 6.
Leukotriene (LT) C4 synthase contributes to fibrosis after unilateral ureteral obstruction (UUO). A: second-harmonic generation (SHG)/autofluorescence microscopy was performed 14 days after UUO in wild-type (WT), LTC4 synthase-knockout (Ltc4s−/−), and LTA4 hydrolase-knockout (Lta4h−/−) mice. B: quantification of SHG signal reveals a decrease in collagen in Ltc4s−/− mice only (n = 4 mice/group). C: hydroxyproline assays performed 14 days after UUO also show a reduction in collagen deposition in Ltc4s−/− mice (n = 8 mice/group). Values are means ± SE. *P < 0.05; ns, not significant. Scale bar = 50 μm.
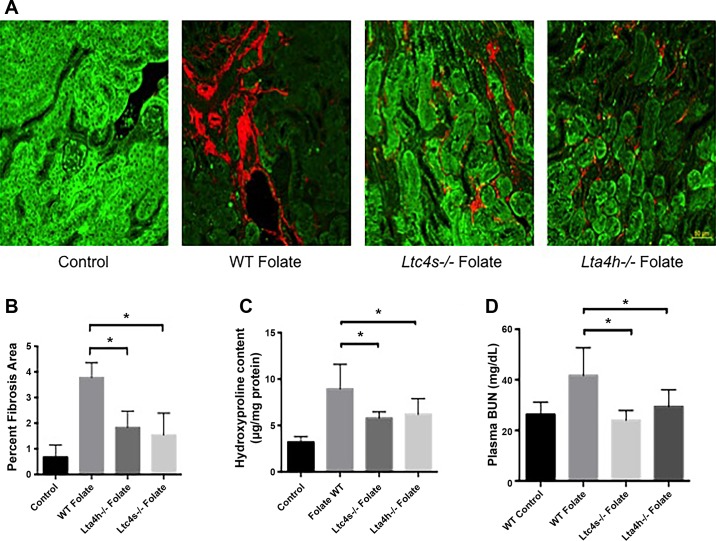
We next determined whether LTs played an important role in a different model of renal fibrosis, the folic acid nephropathy model. This model also allows for the assessment of renal function. After a single dose of intraperitoneal folic acid or vehicle, kidneys were collected at 84 days. Collagen deposition, as measured by SHG, was less in folate-treated Lta4h−/− and Ltc4s−/− mice than wild-type controls exposed to folate (Fig. 7, A and B). Hydroxyproline measurements confirmed less collagen deposition in both groups of mice (Fig. 7C). To determine if the mice had improved renal function, plasma blood urea nitrogen (BUN) was measured at 84 days. Plasma BUN was significantly increased in wild-type mice exposed to folate; however, neither Lta4h−/− nor Ltc4s−/− mice exposed to folate exhibited an elevated BUN (Fig. 7D).
Fig. 7.
Leukotrienes (LTs) contribute to fibrosis and renal dysfunction in folic acid nephropathy model. A: second-harmonic generation (SHG)/autofluorescence microscopy was performed 84 days after folic acid administration in wild-type (WT), LTC4 synthase-knockout (Ltc4s−/−), and LTA4 hydrolase-knockout (Lta4h−/−) mice. B: quantification of SHG signal shows a reduction of collagen in Ltc4s−/− and Lta4h−/− mice (n = 4 mice/group). C: hydroxyproline assay at 84 days shows a reduction of collagen deposition in Ltc4s−/− and Lta4h−/− mice (n = 6 mice/group). D: plasma blood urea nitrogen (BUN) 84 days after folic acid administration shows significant reduction of BUN in Ltc4s−/− and Lta4h−/− mice (n = 8 mice/group). Values are means ± SE. *P < 0.05. Scale bar = 50 μm.
DISCUSSION
In this study we have found that inhibition of the 5-LO pathway strongly reduces renal fibrosis in two separate animal models, UUO and folic acid nephropathy. Our experiments also demonstrate that cysteinyl LTs are important mediators of damage in both models, while LTB4 is important in the folic acid nephropathy model. Previous studies showed that LTs are produced in models of renal tubular injury. Our study expands on this work by showing a potent antifibrotic effect in two models as well as defining groups of LTs that mediate pathogenic effects. We also tested whether a novel microscopy technique, FLIM, could assess metabolic changes in injured kidneys. We found that FLIM could detect a glycolytic shift after UUO, a shift that was reversed with 5-LO inhibition.
Macrophages play a major role in the development of renal fibrosis (8). Since LTs are potent regulators of leukocyte recruitment, we predicted that monocyte recruitment would be decreased with a 5-LO antagonist. However, flow cytometry revealed no difference in recruitment of monocyte-derived cells, suggesting that the protective effects are not mediated by a reduction of macrophage number. There are many possible explanations for these findings. First, 5-LO metabolites may act directly on nonleukocyte cell types, such as renal tubular epithelial cells or matrix-producing cells. This would be consistent with previous studies showing that LTs can modify epithelial and fibroblast phenotype. Our FLIM experiments also suggest a direct effect on renal tubular epithelial metabolism. It is also possible that LTs do not change the number of macrophages but do change the phenotype. Previous studies showed that two major myeloid populations emerge after UUO or ischemia: Ly6ChiCD11b+ and Ly6CloCD11b+ cells (3, 18). Neither large population changes with zileuton; however, it is possible that small subgroups do change.
LTB4 and cysteinyl LTs have distinct roles in many inflammatory diseases, such as asthma and pulmonary fibrosis. Our experiments show that LTC4 synthase mediates fibrosis in both UUO and folic acid nephropathy but that LTA4 hydrolase appears to be involved only in folic acid nephropathy. This may be related to the different pathogenic events in the two different models. The folic acid model consists of one discrete renal tubular insult followed by either renal repair or development of CKD. Promotion of renal repair is likely related to renal tubular regeneration as well as effects on matrix-producing cells and endothelial cells. With the UUO model, there is persistent renal obstruction and ischemia. Since there is no opportunity for renal epithelial repair, the extent of renal fibrosis may be more dependent on myofibroblast activation and matrix synthesis.
Alterations in epithelial metabolism, especially glucose metabolism, have been linked to renal fibrosis (6, 16). Healthy proximal tubular cells do not use glycolysis for energy production; renal tubular injury often leads to a glycolytic shift in renal tubular epithelial cells. We have applied a novel microscopy technique that accurately and reproducibly measures metabolic changes seen with a glycolytic shift. This technique is performed with unstained tissue in a semiautomated fashion that significantly reduces observer bias. FLIM can also be applied to the medullary region and the interstitium and may represent an effective approach to monitor changes in metabolism with different renal injury models.
The 5-LO pathway has many attractive therapeutic targets. Since pharmacological therapies for CKD will need to be administered for years, the therapies should be very well tolerated. Antagonists of 5-LO and the cysteinyl LT type 1 receptor are approved for another chronic disease, asthma, and have shown an excellent safety profile in all patients, including children. Clinical trials are ongoing for 5-LO inhibitors as well LTB4 receptor antagonists. Future studies in patients with CKD may be able to determine if 5-LO is activated in human CKD and whether metabolite levels (urinary LTE4) may be used to guide therapy in patients with CKD.
GRANTS
This project was supported by a grant from the Consortium for Fibrosis Research and Translation at the University of Colorado (to S. B. Furgeson). Imaging experiments were performed in the University of Colorado-Anschutz Medical Campus Advanced Light Microscopy Core, supported in part by National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Institute Grant UL1-TR-001082. Flow cytometry experiments were performed using the University of Colorado Cancer Center flow cytometry core facility, supported by National Cancer Institute Support Grant P30-CA-046934. Kidney tissue processing and histology were performed using the University of Colorado Denver Research Histology Shared Resource (National Cancer Institute Cancer Center Support Grant P30-CA-046934). J. R. Montford is supported by VA Career Development Award BX003839.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.H., M.L., M.C.W.-E., R.A.N., and S.B.F. conceived and designed research; J.R.M., C.B., E.D., K.H., and S.B.F. performed experiments; C.B., E.D., R.A.N., and S.B.F. analyzed data; E.D., M.C.W.-E., R.A.N., and S.B.F. interpreted results of experiments; S.B.F. prepared figures; S.B.F. drafted manuscript; J.R.M., C.B., E.D., K.H., M.L., M.C.W.-E., R.A.N., and S.B.F. edited and revised manuscript; S.B.F. approved final version of manuscript.
REFERENCES
- 1.Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA 101: 3047–3052, 2004. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem 279: 46129–46134, 2004. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- 3.Clements M, Gershenovich M, Chaber C, Campos-Rivera J, Du P, Zhang M, Ledbetter S, Zuk A. Differential Ly6C expression after renal ischemia-reperfusion identifies unique macrophage populations. J Am Soc Nephrol 27: 159–170, 2016. doi: 10.1681/ASN.2014111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng B, Lin Y, Ma S, Zheng Y, Yang X, Li B, Yu W, Xu Q, Liu T, Hao C, He R, Ding F. The leukotriene B4-leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney Int 92: 89–100, 2017. doi: 10.1016/j.kint.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Digman MA, Caiolfa VR, Zamai M, Gratton E. The phasor approach to fluorescence lifetime imaging analysis. Biophys J 94: L14–L16, 2008. doi: 10.1529/biophysj.107.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, Luo J, Zen K, Yang J. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am J Physiol Renal Physiol 313: F561–F575, 2017. doi: 10.1152/ajprenal.00036.2017. [DOI] [PubMed] [Google Scholar]
- 7.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 340: 197–206, 1999. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 8.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddy AA, López-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol 27: 1233–1247, 2012. doi: 10.1007/s00467-011-1938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh A, Chen F, Thakur A, Hong H. Cysteinyl leukotrienes and their receptors: emerging therapeutic targets in central nervous system disorders. CNS Neurosci Ther 22: 943–951, 2016. doi: 10.1111/cns.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths RJ, Smith MA, Roach ML, Stock JL, Stam EJ, Milici AJ, Scampoli DN, Eskra JD, Byrum RS, Koller BH, McNeish JD. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J Exp Med 185: 1123–1129, 1997. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci 103: 24–32, 2007. doi: 10.1254/jphs.FP0060651. [DOI] [PubMed] [Google Scholar]
- 14.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly MM, Chakir J, Vethanayagam D, Boulet LP, Laviolette M, Gauldie J, O’Byrne PM. Montelukast treatment attenuates the increase in myofibroblasts following low-dose allergen challenge. Chest 130: 741–753, 2006. doi: 10.1378/chest.130.3.741. [DOI] [PubMed] [Google Scholar]
- 16.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27: 3356–3367, 2016. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landgraf SS, Silva LS, Peruchetti DB, Sirtoli GM, Moraes-Santos F, Portella VG, Silva-Filho JL, Pinheiro CS, Abreu TP, Takiya CM, Benjamin CF, Pinheiro AA, Canetti C, Caruso-Neves C. 5-Lypoxygenase products are involved in renal tubulointerstitial injury induced by albumin overload in proximal tubules in mice. PLoS One 9: e107549, 2014. doi: 10.1371/journal.pone.0107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 19.Lv J, Xiong Y, Li W, Yang W, Zhao L, He R. BLT1 mediates bleomycin-induced lung fibrosis independently of neutrophils and CD4+ T cells. J Immunol 198: 1673–1684, 2017. doi: 10.4049/jimmunol.1600465. [DOI] [PubMed] [Google Scholar]
- 20.Mezhybovska M, Wikström K, Ohd JF, Sjölander A. The inflammatory mediator leukotriene D4 induces β-catenin signaling and its association with antiapoptotic Bcl-2 in intestinal epithelial cells. J Biol Chem 281: 6776–6784, 2006. doi: 10.1074/jbc.M509999200. [DOI] [PubMed] [Google Scholar]
- 21.Montford JR, Lehman AMB, Bauer CD, Klawitter J, Klawitter J, Poczobutt JM, Scobey M, Weiser-Evans M, Nemenoff RA, Furgeson SB. Bone marrow-derived cPLA2α contributes to renal fibrosis progression. J Lipid Res 59: 380–390, 2018. doi: 10.1194/jlr.M082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noiri E, Yokomizo T, Nakao A, Izumi T, Fujita T, Kimura S, Shimizu T. An in vivo approach showing the chemotactic activity of leukotriene B4 in acute renal ischemic-reperfusion injury. Proc Natl Acad Sci USA 97: 823–828, 2000. doi: 10.1073/pnas.97.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohd JF, Wikström K, Sjölander A. Leukotrienes induce cell-survival signaling in intestinal epithelial cells. Gastroenterology 119: 1007–1018, 2000. doi: 10.1053/gast.2000.18141. [DOI] [PubMed] [Google Scholar]
- 24.Paruchuri S, Sjölander A. Leukotriene D4 mediates survival and proliferation via separate but parallel pathways in the human intestinal epithelial cell line Int 407. J Biol Chem 278: 45577–45585, 2003. doi: 10.1074/jbc.M302881200. [DOI] [PubMed] [Google Scholar]
- 25.Patel NS, Cuzzocrea S, Chatterjee PK, Di Paola R, Sautebin L, Britti D, Thiemermann C. Reduction of renal ischemia-reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol Pharmacol 66: 220–227, 2004. doi: 10.1124/mol.66.2.220. [DOI] [PubMed] [Google Scholar]
- 26.Peng J, Zhou H, Kuang G, Xie L, Tian T, Liu R. The selective cysteinyl leukotriene receptor 1 (CysLT1R) antagonist montelukast regulates extracellular matrix remodeling. Biochem Biophys Res Commun 484: 474–479, 2017. doi: 10.1016/j.bbrc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med 357: 1841–1854, 2007. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 28.Ranjit S, Dobrinskikh E, Montford J, Dvornikov A, Lehman A, Orlicky DJ, Nemenoff R, Gratton E, Levi M, Furgeson S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int 90: 1123–1128, 2016. doi: 10.1016/j.kint.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stringari C, Cinquin A, Cinquin O, Digman MA, Donovan PJ, Gratton E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci USA 108: 13582–13587, 2011. doi: 10.1073/pnas.1108161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stringari C, Edwards RA, Pate KT, Waterman ML, Donovan PJ, Gratton E. Metabolic trajectory of cellular differentiation in small intestine by phasor fluorescence lifetime microscopy of NADH. Sci Rep 2: 568, 2012. doi: 10.1038/srep00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian BC, Majumdar R, Parent CA. The role of the LTB4-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin Immunol 33: 16–29, 2017. doi: 10.1016/j.smim.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan M, Tang X, Stsiapanava A, Haeggström JZ. Biosynthesis of leukotriene B4. Semin Immunol 33: 3–15, 2017. doi: 10.1016/j.smim.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Woo CH, You HJ, Cho SH, Eom YW, Chun JS, Yoo YJ, Kim JH. Leukotriene B4 stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J Biol Chem 277: 8572–8578, 2002. doi: 10.1074/jbc.M104766200. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Dou Y, Zheng X, Tan Y, Cheng J, Li L, Du Y, Zhu D, Lou Y. Cysteinyl leukotrienes synthesis is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Toxicology 287: 38–45, 2011. doi: 10.1016/j.tox.2011.05.014. [DOI] [PubMed] [Google Scholar]