Abstract
The mechanism underlying premature ovarian insufficiency remains incompletely understood. Here we report that mice with Per1m/m; Per2m/m double mutations display a decrease in female fertility starting approximately at 20 weeks old, with significantly less pups born from 32 weeks old onwards. Histological analysis revealed that a significant reduction of ovarian follicles was observed in the Per1/Per2 mutants compared with the littermate controls examined at 26 and 52 weeks old, while the difference was not statistically significant between the two groups at 3 and 8 weeks old. We further showed that vascular development including the ovarian follicle associated vascular growth appeared normal in the Per1/Per2 mutant mice, although clock genes were reported to regulate angiogenesis in zebrafish. The findings imply that loss-of-function mutations with Per1/Per2 result in a premature depletion of ovarian follicle reserve leading to the decline of reproductive capacity.
Keywords: PER1, PER2, ovarian follicle, ovarian insufficiency, knockout mouse
Disruption of circadian rhythm or its underlying regulatory network contributes to the premature depletion of ovarian follicle reserve.
Introduction
Premature ovarian insufficiency (POI) is characterized by a loss of ovarian function in women at reproductive ages, and is a heterogeneous condition with genetic, environmental, and other causes such as iatrogenic factors [1, 2]. The cyclic process of ovarian follicular development includes the follicle growth and maturation, ovulation, as well as corpus luteum formation. The ovarian events are tightly controlled by the circadian system, as shown by the rhythmic hormone release in the hypothalamus–pituitary–ovary axis [3].
Circadian rhythms exist in diverse life forms and modulate the transcriptional activity of numerous genes in both suprachiasmatic nuclei (SCN) and peripheral tissues including the reproductive system [4–6]. The molecular machinery of the circadian clock is a feedback loop of core regulators including the transcriptional activators BMAL1 and CLOCK, as well as the repressors PER1–3 and CRY1–2 in mammals [4–6]. PER1 and PER2 are critical in the regulation of circadian rhythmicity, and mice deficient in Per1 or Per2 displayed a short circadian period [7, 8]. Double mutations of Per1/Per2 genes disrupted circadian rhythms in locomotor activity and the expression of key clock genes as well as clock-regulated genes [7–9]. Both Per1 and Per2 mRNA were found to have a rhythmic oscillation in rat ovaries [10, 11], and were expressed in pituitary and hypothalamus that may participate in the coordination of GnRH (gonadotropin-releasing hormone) and LH (luteinizing hormone) surge [3, 7, 12–14]. Consistently, loss-of-function mutations with mouse Per1 or Per2 were reported to have a lower reproductive success in female mutants after 9 months old [15]. Irregular estrous cycles and lack of a coordinated LH surge were also observed in Clock mutants [16]. Knockout of Bmal1 led to impaired fertility due to the disruption of steroidgenesis and implantation [17–19], and Bmal1 deficiency in ovarian thecal cells was found to disrupt ovulation due to the alteration of the phasic sensitivity to LH [20].
In the circulatory system, PER2 mutation was shown to impair vascular endothelial function [21], and PER2 and BMAL1 were reported to have a role in the regulation of developmental angiogenesis in zebrafish [22]. The ovarian follicular development is accompanied by active angiogenesis [23]. To investigate the mechanism underlying the reproductive defects of Per1 and Per2 mutants, we analyzed the vascular growth in ovaries and other tissues of Per1/Per2 doubly mutant mice in this study. We found that loss-of-function mutations with Per1/Per2 genes led to the reduced female fertility resulting from the premature decrease of ovarian follicles, but did not produce any obvious effect on vascular growth during the follicular development.
Materials and methods
Animal models
Mice with mutations targeting Per1 and Per2 genes were generated, as previously described [7, 8]. We obtained the breeding pairs of Per1 and Per2 single mutants, and the doubly mutant mice (Per1m/m; Per2m/m) were generated by two rounds of mating. The genetic background of Per1/Per2 doubly mutant and control mice are C57/BL6. All animal experiments were performed in accordance with the institutional guidelines of the Soochow University Animal Center. In all the phenotype analysis, littermates were used as control.
Quantification of litter and pup number
To assess reproductive performance, the doubly mutant (Per1m/m; Per2m/m, 8 weeks old) and littermate control female mice (Per1+/m; Per2+/m) were mated with age-matched male mice (B6 background). The total number of litters and pups for each female mouse were recorded up to 52 weeks old.
Histological analysis of ovarian follicles
Ovaries of Per1m/m; Per2m/m mutant and littermate control female mice (3, 8, 26, or 52 weeks old) were fixed in 4% PFA (paraformaldehyde) and embedded in paraffin. Serial sections (8 μm) were collected and stained with hematoxylin-eosin. The number of follicles with oocyte nuclei in every fifth section was counted and used for calculating the total number of follicles per ovary. The criteria for classifying developmental stages of ovarian follicles were as follows: primordial follicle, oocyte surrounded by a single layer of flattened follicular epithelial cells; primary follicle, oocyte surrounded by a single layer of cuboidal follicular cells; secondary follicle, oocyte surrounded by multiple layers of follicular cells; antral follicle: oocyte surrounded by granulosa cells containing also a fluid filled cavity.
Immunostaining
For whole-mount immunostaining with skin and retina, tissues were harvested and processed as previously described [24]. The antibodies used were rat anti-mouse PECAM-1 (platelet endothelial cell adhesion molecule 1; BD Pharmigen, 553370), rabbit anti-mouse LYVE-1 (lymphatic vessel endothelial hyaluronan receptor; Abcam, ab14917), and rabbit-anti-mouse cleaved Caspase-3 (Cell Signaling Technology, 9661S). Alexa488- and Alexa594 (Invitrogen)-conjugated secondary antibodies were used for staining. Slides were mounted with Vectashield (VectorLabs) and analyzed with the Olympus FluoView 1000 confocal microscope. For staining of frozen sections, ovary and uterus tissues were collected and processed as previously described [24]. Consecutive sections (10 μm in thickness) were incubated with antibodies against PECAM-1 and LYVE-1, followed by staining with the appropriate fluorochrome-conjugated secondary antibodies and mounted as described above. For the quantification of blood vessel parameters in the retina, fluorescent images were taken from similar regions in all samples and analyzed by using Image Pro Plus (Media Cybernetics).
Statistical analysis
Statistical analysis was performed with the unpaired t test. All statistical tests were two-sided. Data are presented as mean ± SD.
Results
Early onset of fertility decline with Per1/Per2 doubly mutant female mice
Per1/Per2 doubly mutant mice were shown to be arrhythmic [7, 25]. To investigate the effect and underlying mechanism of Per1/Per2 mutations on female reproduction, we set up mating using Per1m/m; Per2m/m doubly mutant female mice starting from 8 weeks old, and the heterozygous Per1+/m; Per2+/m littermate mice were used as control. Quantification of accumulated litter and pup number from the mutant and control mice is shown in Figure 1A and B, and Table 1. A trend of decrease of litter and pup number was already obvious starting from 20 weeks old, and a statistically significant difference in female fertility was observed from 32 weeks old onwards between the mutant and control mice. However, there was no significant difference in litter size (pups per litter recorded from 8 to 52 weeks old, Per1m/m; Per2m/m: 6.56 ± 0.99, n = 12; Per1+/m; Per2+/m: 6.85 ± 1.41, n = 11; P = 0.5714). It is also worth noting that few pups were born from Per1m/m; Per2m/m mutants after 44 weeks old (Table 1).
Figure 1.
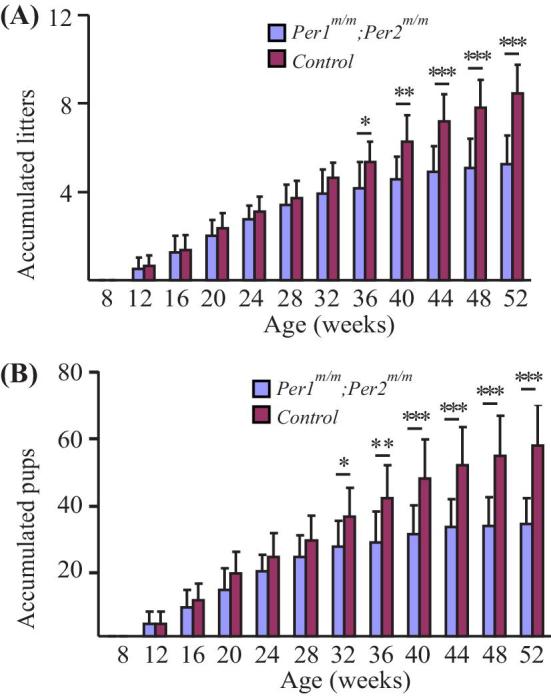
Quantification of accumulated litter and pup number of Per1m/m; Per2m/m doubly mutant and control mice. (A) Quantification of litter number of Per1m/m; Per2 m/m mutant and control mice. (B) Pup number of Per1m/m; Per2m/m mutant and control mice.
Table 1.
Litter size and pup number of Per1m/m; Per2m/m doubly mutant and control mice.
| Age (weeks) | Number of accumulated litters | n (Per1m/m;Per2m/m) | n (Control) | P value | |
|---|---|---|---|---|---|
| Per1m/m;Per2m/m | Control | ||||
| Number of accumulated litters | |||||
| 8 | 0 | 0 | 12 | 11 | |
| 12 | 0.50 ± 0.52 | 0.64 ± 0.50 | 12 | 11 | 0.53182939 |
| 16 | 1.25 ± 0.75 | 1.36 ± 0.67 | 12 | 11 | 0.70798791 |
| 20 | 2.00 ± 0.74 | 2.36 ± 0.67 | 12 | 11 | 0.23254607 |
| 24 | 2.75 ± 0.62 | 3.09 ± 0.70 | 12 | 11 | 0.22987128 |
| 28 | 3.42 ± 0.90 | 3.73 ± 0.79 | 12 | 11 | 0.39011313 |
| 32 | 3.92 ± 1.08 | 4.64 ± 0.67 | 12 | 11 | 0.07253613 |
| 36 | 4.17 ± 1.19 | 5.36 ± 0.92 | 12 | 11 | 0.01431332 |
| 40 | 4.58 ± 1.00 | 6.27 ± 1.19 | 12 | 11 | 0.00132172 |
| 44 | 4.92 ± 1.16 | 7.18 ± 1.25 | 12 | 11 | 0.00019715 |
| 48 | 5.08 ± 1.31 | 7.82 ± 1.25 | 12 | 11 | 4.6502E-05 |
| 52 | 5.25 ± 1.29 | 8.45 ± 1.29 | 12 | 11 | 6.6428E-06 |
| Number of accumulated pups | |||||
| 8 | 0 | 0 | 12 | 11 | |
| 12 | 3.58 ± 3.78 | 3.82 ± 3.79 | 12 | 11 | 0.88319599 |
| 16 | 8.58 ± 5.55 | 10.82 ± 5.13 | 12 | 11 | 0.32893069 |
| 20 | 14.08 ± 6.42 | 19.00 ± 6.45 | 12 | 11 | 0.0813109 |
| 24 | 19.75 ± 4.99 | 24.09 ± 7.12 | 12 | 11 | 0.10293094 |
| 28 | 24.08 ± 6.56 | 28.91 ± 7.42 | 12 | 11 | 0.11264827 |
| 32 | 27.17 ± 7.71 | 36.18 ± 8.75 | 12 | 11 | 0.01575296 |
| 36 | 28.42 ± 9.22 | 41.73 ± 10.04 | 12 | 11 | 0.00329245 |
| 40 | 30.83 ± 8.62 | 47.55 ± 11.80 | 12 | 11 | 0.00082026 |
| 44 | 33.00 ± 8.44 | 51.64 ± 11.58 | 12 | 11 | 0.00022759 |
| 48 | 33.42 ± 8.50 | 54.45 ± 12.13 | 12 | 11 | 8.5033E-05 |
| 52 | 33.92 ± 7.86 | 57.55 ± 12.23 | 12 | 11 | 1.604E-05 |
Premature ovarian follicle insufficiency in Per1/Per2 mutants
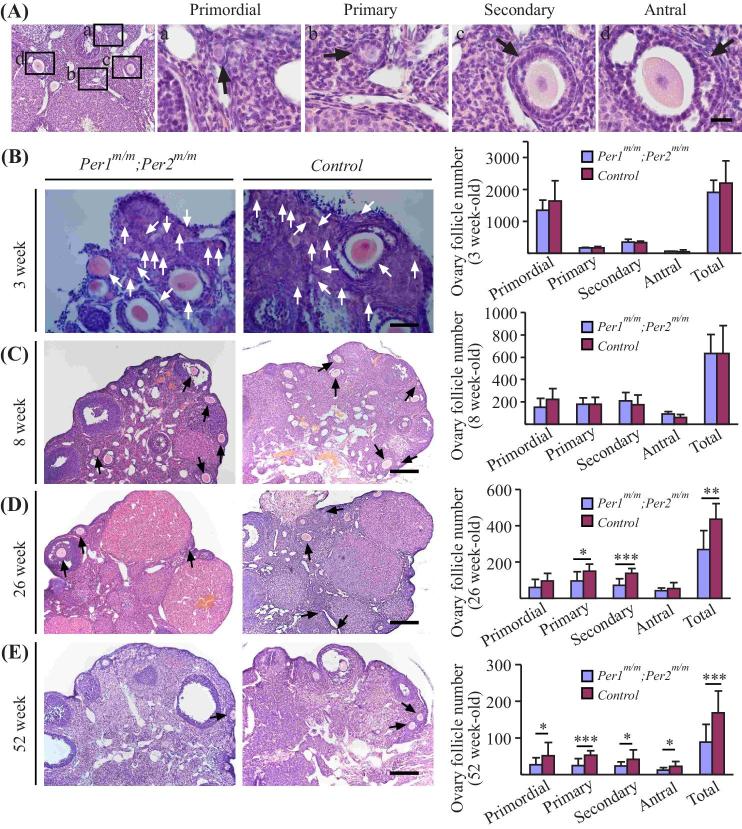
Although a number of genes and nongenetic factors have been identified contributing to the occurrence of POI, the involvement of circadian clock and its regulators in this pathogenesis is unclear. To analyze the effect of double Per1/Per2 mutations on ovarian follicle development, ovaries from different ages of mice (3, 8, 26, and 52 weeks old) were collected for histological analysis of follicle number at primordial, primary, secondary, and antral stages (Figure 2A). Quantification of follicle number revealed that Per1m/m; Per2m/m doubly mutant mice had similar number of primordial, primary, secondary, or antral ovarian follicles at the stages of 3 weeks old (Figure 2B) in comparison with those of control littermates. There was also no significant difference in the total number of ovairan follicles observed in the Per1/Per2 mutant ovaries of 8-week-old mice (Figure 2C). However, a significant decrease in the number of ovarian follicles was detected at ages of 26 weeks old (Figure 2D) and 52 weeks old (Figure 2E) between the two groups (Table 2). Analysis by immunostaining with cleaved caspase-3 showed that there was no statistically significant difference in apoptotic cell number between the Per1m/m; Per2m/m doubly mutant and control mice (Supplemental Figure S1; Per1m/m; Per2m/m: 53.35 ± 2.03, n = 3; Control: 51.69 ± 2.68, n = 3; P = 0.4406).
Figure 2.
Analysis of ovarian follicle development in Per1m/m; Per2m/m mutant and control mice. (A) Ovarian follicular development in wild-type mice at different stages including primordial, primary, secondary, and antral stages. (B–E) Histological analysis and quantification of ovarian follicle number at different ages, including 3, 8, 26, and 52 weeks old. Arrows indicate the follicles with oocyte nuclei for quantification. Scale bar: 20 μm in A, 50 μm in B, 200 μm in C–E.
Table 2.
Quantification of ovarian follicle number at different development stages of Per1m/m; Per2m/m doubly mutant and control mice (3, 8, 26, 52 weeks old).
| Ovarian follicle stages | Ovarian follicle number (3 weeks old) | n (Per1m/m;Per2m/m) | n (Control) | P value | |
|---|---|---|---|---|---|
| Per1m/m;Per2m/m | Control | ||||
| Ovarian follicle number (3 weeks old) | |||||
| Primordial | 1356.00 ± 307.05 | 1642.00 ± 634.34 | 5 | 5 | 0.39068631 |
| Primary | 162.00 ± 23.61 | 165.00 ± 53.85 | 5 | 5 | 0.911982313 |
| Secondary | 343.00 ± 102.02 | 337.00 ± 45.36 | 5 | 5 | 0.90731292 |
| Antral | 55.00 ± 12.75 | 53.00 ± 54.15 | 5 | 5 | 0.937904244 |
| Total | 1916.00 ± 370.18 | 2197.00 ± 692.08 | 5 | 5 | 0.446499944 |
| Ovarian follicle number (8 weeks old) | |||||
| Ovarian follicle stages | Ovarian follicle number (8 weeks old) | n (Per1m/m;Per2m/m) | n (Control) | P value | |
| Per1m/m;Per2m/m | Control | ||||
| Primordial | 151.67 ± 80.04 | 222.00 ± 95.43 | 9 | 5 | 0.185634764 |
| Primary | 177.78 ± 57.29 | 177.00 ± 61.85 | 9 | 5 | 0.98228293 |
| Secondary | 211.11 ± 70.70 | 174.00 ± 86.16 | 9 | 5 | 0.422737236 |
| Antral | 91.11 ± 24.21 | 61.00 ± 25.38 | 9 | 5 | 0.057257555 |
| Total | 631.67 ± 172.92 | 634.00 ± 248.26 | 9 | 5 | 0.984694705 |
| Ovarian follicle number (26 weeks old) | |||||
| Ovarian follicle stages | Ovarian follicle number (26 weeks old) | n (Per1m/m;Per2m/m) | n (Control) | P value | |
| Per1m/m;Per2m/m | Control | ||||
| Primordial | 60.00 ± 43.09 | 95.91 ± 41.76 | 11 | 8 | 0.085424818 |
| Primary | 94.38 ± 51.79 | 148.18 ± 40.64 | 11 | 8 | 0.021065468 |
| Secondary | 72.50 ± 35.36 | 138.18 ± 24.73 | 11 | 8 | 0.000173887 |
| Antral | 41.88 ± 13.61 | 53.64 ± 32.41 | 11 | 8 | 0.350178113 |
| Total | 268.75 ± 103.71 | 435.91 ± 85.81 | 11 | 8 | 0.001301834 |
| Ovarian follicle number (52-week-old) | |||||
| Ovarian follicle stages | Ovarian follicle number (52 weeks old) | n (Per1m/m;Per2m/m) | n (Control) | P value | |
| Per1m/m;Per2m/m | Control | ||||
| Primordial | 27.14 ± 19.19 | 51.25 ± 35.87 | 14 | 12 | 0.039192918 |
| Primary | 25.00 ± 19.12 | 52.50 ± 12.88 | 14 | 12 | 0.000299226 |
| Secondary | 23.93 ± 11.12 | 41.67 ± 25.35 | 14 | 12 | 0.026081444 |
| Antral | 12.14 ± 7.26 | 22.50 ± 13.23 | 14 | 12 | 0.018619029 |
| Total | 88.21 ± 48.70 | 167.92 ± 59.29 | 14 | 12 | 0.00095254 |
Angiogenesis in ovaries of Per1/Per2 doubly mutant mice
It has been shown that morpholino-mediated knockdown of per2 or bmal1 altered the developmental angiogenesis in zebrafish [22]. To find out whether Per1/Per2 mutations would affect vascular development including the cyclic angiogenesis during ovarian follicular development, we performed the fluorescence immunostaining with ovaries and several other tissues of Per1m/m; Per2m/m mutant and control mice at different development stages. Quantification of retina vascularization area (P5, postnatal day 5) showed that there was no significant difference between Per1m/m; Per2m/m mutant and wild-type (P = 0.3644) or the heterozygous control mice (P = 0.5886; WT: 46.25 ± 2.93%, n = 6; Per1+/m; Per2+/m: 46.97 ± 5.16%, n = 6; Per1m/m; Per2m/m: 48.52 ± 5.39, n = 9; Supplemental Figure S2A). Constistently, there was no difference detected in blood vascular and lymphatic vessels of skin (P5) between Per1/Per2 mutant and controls (Supplemental Figure S2B). At the adult stage, blood vascular and lymphatic vessels also appeared normal in the ear skin, trachea, and uterus of Per1m/m; Per2m/m mutant mice compared with those of the control (Supplemental Figure S3A–C). Consistently, there was no obvious difference in both blood vascular and lymphatic vessel growth associated with the ovarian follicles between Per1/Per2 mutants and control mice (Figure 3).
Figure 3.
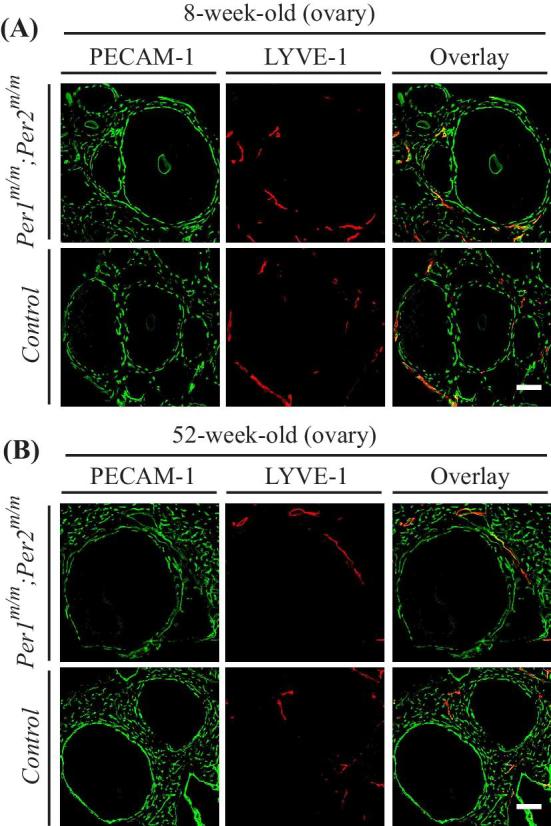
Analysis of vascular formation in ovaries of Per1m/m; Per2m/m doubly mutant mice. (A, B) Immunostaining analysis of blood vascular and lymphatic vessels in ovaries of 8 weeks old (A) and 52 weeks old (B) Per1m/m; Per2m/m mutant and control mice (PECAM-1, green; LYVE-1, red). Scale bar: 100 μm.
Discussion
We show in this study that mice with Per1/Per2 double mutations displayed an early onset of decrease in the female fertility. However, there was no obvious change with vascular growth associated with the ovarian follicles and in other tissues examined in the mutant mice. Interestingly, we found that there was a significant reduction of the ovarian follicle number in the Per1/Per2 mutants from the middle-aged stage onwards compared with the littermate controls. The process of ovarian follicle development is tightly controlled by many factors. It is possible that Per1/Per2 loss-of-function mutations may alter gene expression involved in the follicular development, leading to the depletion of the ovarian follicle reserve and the decline of reproductive capacity.
It was previously demonstrated that deletion of Per1 or Per2 affected female fertility at the age of 9–12 months old displaying abnormal oestrous cycle and reduced implantation success, but there was no obvious difference observed with young adult mutants (2–6 months old) [15]. In this study, we found that the doubly Per1/Per2 mutations caused an earlier onset of decrease in the female fertility than that of single mutation, suggesting a synergistic effect of PER1 and PER2 in reproduction. The phenotype was observed starting from 20 weeks old with significantly less pups delivered at the stage of 32 weeks old. Consistently, there was a significant decrease of ovarian follicles in the Per1/Per2 double mutants compared with the control mice examined at 26 and 52 weeks old, while the total number of ovarian follicles was similar between the two groups at young ages (3 and 8 weeks old). This suggests that the abnormal decline of ovarian follicle reserve observed in the adult Per1/Per2 mutants contributes to their reduced fertility, in addition to other causes as previously reported [15].
Pathogenesis underlying POI is still incompletely understood and may involve a wide spectrum of causes. Of the causative factors of genetic origin, more than 50 genes have been identified as POI candidates, which are involved in various processes such as oogenesis and folliculogenesis [1, 2]. Among the causative candidates, abnormal hormonal signaling including the follicle-stimulating hormone (FSH) receptor-mediated pathway may be one of the major contributors of POI [1]. It is known that the primary circadian pacemaker in the SCN provides a central control for the expression of core clock genes as well as clock-controlled genes, including the release of FSH and LH with a diurnal rhythm [14]. Furthermore, rhythmic expression of Per1 and Per2 was detected in the steroidogenic cells of preantral, antral, and preovulatory follicles as well as corpora lutea [10]. Accumulating evidences indicate that the circadian clock plays an important role in the amplitude and timing of ovarian steroid hormone synthesis, oocyte maturation, and ovulation [14]. Alteration of estrogen-mediated signaling could affect the follicular pool [26, 27]. Consistently, it has been found that disruption of circadian rhythm produces a negative effect on fertility in rodent models and women exposed to shiftwork schedules [14]. It is therefore likely that disruption of circadian rhythm or clock-regulated gene network by the Per1/Per2 mutation may contribute to the occurrence of POI by affecting the reproductive endocrine system at possibly each level of the hypothalamic–pituitary–ovarian axis.
While clock genes were reported to regulate angiogenesis in zebrafish [22], we did not observe any obvious difference in vascular development in several tissues examined including retina, skin, trachea, and uterus from the Per1/Per2 mutant and control mice. Consistently, the reduced female fertility caused by Per1/Per2 double mutations is not due to the alteration of vascular components associated with ovarian follicle development. It is possible that the distinct vascular phenotype may result from the species difference between zebrafish and mouse in the regulation of vascular formation by circadian clock genes. However, due to the potential for off-target effects by the use of antisense morpholinos [28], it is necessary to validate the vascular phenotypes using knockout models targeting zebrafish per2 or bmal1.
In summary, we found in this study that Per1/Per2 double mutations caused a significant decrease of ovarian follicles in mutant mice from the middle-aged stage onwards. Although the detailed mechanism remains a topic for further investigation, findings of this study showed that the alteration of circadian rhythm and its underlying regulatory network could be an important factor contributing to the pathogenesis of POI. Future studies in this direction may yield more mechanistic insights into POI and provide potential therapeutic targets for the disease.
Supplemental Data
Supplemental Figure S1. Analysis of apoptosis in ovaries of Per1m/m; Per2m/m doubly mutant and control mice. (A) Immunostaining analysis of apoptotic cells in ovaries (3 weeks old) of Per1m/m; Per2m/m mutant and control mice (cleaved caspase 3, red; PECAM-1, green; LYVE-1, red; DAPI, blue). (B) Quntification of apoptotic cells (per grid, ×40 magnification). Scale bar: 50 μm in A.
Supplemental Figure S2. Analysis of vascular growth in retina and skin of Per1m/m; Per2m/m doubly mutant and control neonatal mice (P5). (A, B) Immunostaining analysis of blood vascular and lymphatic vessels in retina (A) and skin (B) of Per1m/m; Per2m/m mutant and control mice (P5, postnatal day 5; PECAM-1, green; LYVE-1, red). Quantification of retina vascularization area showed that there was no significant difference detected among Per1/Per2 mutant and control mice (A; WT: 46.25 ± 2.93%, n = 6; Per1+/m; Per2+/m: 46.97 ± 5.16%, n = 6; Per1m/m; Per2m/m: 48.52 ± 5.39, n = 9). Scale bar: 100 μm in A and B.
Supplemental Figure S3. Analysis of vascular growth in adult tissues of Per1m/m; Per2m/m doubly mutant and control mice. (A–C) Immunostaining analysis of blood vascular and lymphatic vessels in ear skin (A, 7 weeks old), trachea (B, 7 weeks old), and uterus (C, 8 and 52 weeks old) of Per1m/m; Per2m/m mutant and control mice (PECAM-1, green; LYVE-1, red). Scale bar: 100 μm in A, B, and C.
Supplementary Material
Acknowledgment
We thank the staff in Animal facility of Soochow University for technical assistance.
Notes
YZ, CL and YL contributed equally to this work.
Footnotes
Grant Support: This work was supported by grants from the Ministry of Science and Technology of China (2012CB947600), the National Natural Science Foundation of China (91539101, 81770489, 91739304), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Reference
- 1. Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev 2016; 37:609–635. [DOI] [PubMed] [Google Scholar]
- 2. Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab 2018; 29:795–807. [DOI] [PubMed] [Google Scholar]
- 3. de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 2006; 147:1148–1153. [DOI] [PubMed] [Google Scholar]
- 4. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012; 35:445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 2012; 74:246–260. [DOI] [PubMed] [Google Scholar]
- 6. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003; 4:649–661. [DOI] [PubMed] [Google Scholar]
- 7. Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001; 105:683–694. [DOI] [PubMed] [Google Scholar]
- 8. Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999; 400:169–173. [DOI] [PubMed] [Google Scholar]
- 9. Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptacek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 2007; 128:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 2006; 147:3769–3776. [DOI] [PubMed] [Google Scholar]
- 11. Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod 2006; 75:624–632. [DOI] [PubMed] [Google Scholar]
- 12. Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol 2005; 17:119–130. [DOI] [PubMed] [Google Scholar]
- 13. Sitzmann BD, Lemos DR, Ottinger MA, Urbanski HF. Effects of age on clock gene expression in the rhesus macaque pituitary gland. Neurobiol Aging 2010; 31:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sellix MT. Circadian clock function in the mammalian ovary. J Biol Rhythms 2015; 30:7–19. [DOI] [PubMed] [Google Scholar]
- 15. Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction 2008; 135:559–568. [DOI] [PubMed] [Google Scholar]
- 16. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 2004; 14:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1–/– mice. Endocrinology 2009; 150:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuji T, Kiyosu C, Akiyama K, Kunieda T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol Reprod Dev 2012; 79:795–802. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Johnson BP, Shen AL, Wallisser JA, Krentz KJ, Moran SM, Sullivan R, Glover E, Parlow AF, Drinkwater NR, Schuler LA, Bradfield CA. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci USA 2014; 111:14295–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, Sellix MT. Conditional deletion of Bmal1 in ovarian theca cells disrupts ovulation in female mice. Endocrinology 2016; 157:913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007; 115:2188–2195. [DOI] [PubMed] [Google Scholar]
- 22. Jensen LD, Cao Z, Nakamura M, Yang Y, Brautigam L, Andersson P, Zhang Y, Wahlberg E, Lanne T, Hosaka K, Cao Y. Opposing effects of circadian clock genes Bmal1 and Period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep 2012; 2:231–241. [DOI] [PubMed] [Google Scholar]
- 23. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25:581–611. [DOI] [PubMed] [Google Scholar]
- 24. Chu M, Li T, Shen B, Cao X, Zhong H, Zhang L, Zhou F, Ma W, Jiang H, Xie P, Liu Z, Dong N et al.. Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. eLife 2016; 5:e21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001; 30:525–536. [DOI] [PubMed] [Google Scholar]
- 26. Zangen D, Kaufman Y, Zeligson S, Perlberg S, Fridman H, Kanaan M, Abdulhadi-Atwan M, Abu Libdeh A, Gussow A, Kisslov I, Carmel L, Renbaum P et al.. XX ovarian dysgenesis is caused by a PSMC3IP/HOP2 mutation that abolishes coactivation of estrogen-driven transcription. Am J Hum Genet 2011; 89:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol 2009; 202:407–417. [DOI] [PubMed] [Google Scholar]
- 28. Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S et al.. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 2015; 32:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.