Abstract
In the last decade, it has become clear that epigenetic changes act together with genetic mutations to promote virtually every stage of tumorigenesis and cancer progression. This knowledge has triggered searches for “epigenetic drugs” that can be developed into new cancer therapies. Here we report that triptolide reduced lung cancer incidence from 70% to 10% in a Fen1 E160D transgenic mouse model and effectively inhibited cancer growth and metastasis in A549 and H460 mouse xenografts. We found that triptolide induced lung cancer cell apoptosis that was associated with global epigenetic changes to histone 3 (H3). These global epigenetic changes in H3 are correlated with an increase in protein expression of five Wnt inhibitory factors that include WIF1, FRZB, SFRP1, ENY2, and DKK1. Triptolide had no effect on DNA methylation status at any of the CpG islands located in the promoter regions of all five Wnt inhibitory factors. Wnt expression is implicated in promoting the development and progression of many lung cancers. Because of this, the potential to target Wnt signaling with drugs that induce epigenetic modifications provides a new avenue for developing novel therapies for patients with these tumor types.
Keywords: Triptolide, Lung Cancer, Demethylation, Wnt signaling, Histone 3
Introduction:
Lung cancer is the leading cause of cancer-related deaths world-wide in both men and women 1. Carcinogens and toxins in cigarette smoke can create inflammation and accumulation of DNA mutations and cellular damage which lead to lung cancer development 2. The most common form of lung cancer is non-small cell lung carcinoma (NSCLC), which accounts for 85% of all lung cancers. The 5 year overall survival of NSCLC is only about 15%, because most patients present with metastatic disease that is not curable 3. Unfortunately, only about 10% of patients with NSCLC harbor driver mutations that have approved targeted agents. Therefore, new therapies that have novel targets and mechanisms of action are needed 4.
Triptolide, an extract of Tripterygium wilfordii, has been used as an anti-inflammatory drug in traditional Chinese medicine, and has anti-cancer activity in a number of cancers, including lung cancer 5. Triptolide disrupts multiple signaling pathways including the HSP70, NFkB, and p53 pathways. Treatment with triptolide leads to decreased cancer cell proliferation, migration, invasion, and metastasis 6–10.
Surveys of the literature reveal the canonical Wnt signaling pathway promotes cancer initiation, growth, metastasis, and stem cell maintenance and is also elevated in NSCLC and many other adult cancers11. Inhibiting Wnt signaling in both normal and malignant cells can induce cellular senescence and apoptosis. In tumor cells, elevated Wnt expression is associated with the reduced expression of Wnt inhibitory factors12, this suggests the increased Wnt signaling is due to epigenetic changes. We sought to evaluate if triptolide is inducing cellular arrest and eventual apoptosis through activation of these Wnt inhibitory factors. Our research previously showed the triptolide derivative MRx102 induces its effects in lung cancer cells in part by increasing expression of Wnt inhibitory factor 1 (WIF1) 13. Other studies have demonstrated triptolide modulates the methylation status of H3 at several key lysine residues in multiple myeloma 7,14,15. The alteration of H3 methylation by triptolide has not yet been reported in solid tumors, including lung cancer.
We set out to determine the effect of triptolide on lung cancer formation in a transgenic mouse model of lung cancer, and the effects of triptolide on lung cancer proliferation and apoptosis in vitro and in vivo. Additionally, we sought to investigate the effects of triptolide on H3 methylation and Wnt pathway signaling and inhibition. Finally, we studied whether there was a correlation between response to triptolide in various lung cancer cell lines and specific Wnt pathway markers.
Methods:
Cell culture.
Human small airway epithelial cells (SAEC), A549, H460, H358, and H1299 cell lines were obtained from ATCC. SAEC cells were maintained in airway epithelial cell basal medium supplemented with ingredients from the Small Airway Epithelial Cell Growth Kit. Other cells were maintained in RPMI 1640 containing 10% fetal-bovine serum and 1% penicillin/streptomycin.
Antibodies and reagents.
Antibodies to β-catenin, WIF1, Histone 3, β-actin, Wnt3a, H3K4me2&3, H3K9me2&3, H3K27me1, 2&3, H3K36me2&3, H3K79 2&3, SFRP1, and DKK1 were purchased from Cell Signaling. Antibodies to FRZB and ENY2 were purchased from Abcam. Rabbit IgG, Mouse IgG were purchased from Abcam. Triptolide was purchased from Sigma.
Colony formation assay.
Cells were seeded at a density of 500 cells/well in a 6-well plate format, and allowed to adhere for 24 hr. The cells were then treated with the indicated concentrations of triptolide of 1, 3, and 10 nM or control DMSO. After 24 hrs, the medium was replaced with fresh medium and replaced again every 3 days thereafter. The cells were grown for 10 days, then fixed using methanol and stained with 1% crystal violet. Wells containing more than 50 cells were counted. Treatments with each dose were performed in triplicate and each experiment was performed at least three times. The relative numbers of colonies compared with untreated controls were plotted as cell viability.
DNA preparation.
DNA was isolated using an Allprep DNA/RNA mini kit (Qiagen) according to manufacturer’s instructions. For bisulfite DNA conversion we used the EZ DNA Methylation-Gold Kit (Zymo Research) according to the manufacturer’s instructions. For DNA methylation analysis, bisulfite-modified DNA was amplified with ZymoTaq™ DNA polymerase (Zymo Research) and gene-specific primers.
Analysis of cell death, cell cycle and senescence.
To detect apoptosis, we used the Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit from ThermoFisher Scientific according to the manufacturer’s instructions. Counterstaining was performed with a PI/RNase solution. Apoptosis and the cell cycle were analyzed on a FACScan using CellQuest software (Becton-Dickinson).
Cell Harvesting and Protein Analysis, Immunoblotting blotting.
Cell lysates were prepared in Laemmli sample buffer and analyzed by immunoblotting as previously described 13.
Transfections and luciferase assays.
A549 cells were transfected with ShWnt3a or ShWIF-1 plasmids (Origene), using Lipofectamine 3000 reagent (Life technologies), according to the manufacturer’s instructions. The luciferase reporter activity for Wnt signaling was determined using the Luciferase Assay System (Promega), according to the manufacturer’s instructions. A549and H460 cells were co-transfected using TOPflash or FOPflash (Addgene), and the pGL4.50[luc2/CMV/Hygro] Vector (Renilla luciferase, Promega) was included as the internal control. Cells were treated with triptolide for 48 hrs after transfection. Luciferase activities were assayed using a dual luciferase reporter assay system (Promega), following the manufacturer’s instructions, and measured using a GloMax luminometer (Promega). Firefly luciferase expression was normalized against Renilla luciferase expression to determine the relative luciferase activities. Duplicate wells were assayed for each transfection, and three independent transfection assays were performed.
Animal studies.
E160D mouse model: The FEN1 E160D mice background has been previously described 16. Thirteen-month-old E160D mice received subcutaneous saline (mock) or Triptolide (1mg/kg), four times over a two-week period which we deemed as a “cycle.” This same protocol was repeated for eight cycles, as outlined in Supplementary Figure S2d (upper panel). After this period the mice were sacked and analysis was performed. Twenty mice were used for each group. NOD SCID Gamma (NSG) mouse tumorigenesis model: A549 (1×106) and H460 (0.5×106) cells were injected subcutaneously into eight-week-old NSG mice. After one week (H460) or three weeks (A549), the mice received subcutaneous saline (mock) or Triptolide (1.5mg/kg), three times per week for five weeks (H460) or six weeks (A549), as outlined in Supplementary Figure S2d (middle panel). Six mice were used per treatment group. Tumors were measured weekly and the volume was calculated as LxWxW/2 (mm3). NSG mouse tumor metastasis model: A549 (1X105) and H460 (0.5X105) cells were injected into tail vein of eight-week-old NSG mice. After four weeks, treatment injection received subcutaneous saline (mock) or triptolide (1.5mg/kg), three times per week for eight weeks, as outlined in Supplementary Figure S2d (lower panel). NSG mice were purchased from the Jackson Laboratory. Protocols for all animal experiments conducted at City of Hope Medical Center were approved by the City of Hope Medical Center Animal Care and Use Committee, guidelines were strictly enforced.
Immunohistochemistry and Immunofluorescence staining.
Tissues from the indicated animal experiments were fixed in 10% formalin, routinely processed and then paraffin embedded. We stained 5-µm sections with hematoxylin and eosin and then analyzed them for regular morphology. The tissue slides were deparaffinized, incubated overnight at 37°C in 0.01 M sodium citrate buffer and pretreated for 10 min. in a microwave. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide, followed by incubating in PBS containing 10% normal goat serum. Slides were incubated with Wnt3a, β-catenin, H3K79me2 and H3K79me3 antibodies overnight and washed with 3X PBS. Slides were incubated with Alexa Flour 555 goat anti-rabbit IgG or Oregon Green 488 goat anti-mouse IgG (Life Technologies) in 1% BSA/PBS for one hour at RT to stain the primary antibodies. The slides were washed three times with PBS, and mounted with Prolong Gold antifade mounting medium containing DAPI (Life Technologies). Slides were imaged using an Immunofluorescence microscope and histological analysis was performed in a double-blind fashion. We used the Image-Pro premier (Media Cybernetics) to fill colors in immunofluorescence images and prepare merged views of the images.
Analysis of publicly available datasets.
Alterations to Wnt signaling in NSCLCs were obtained from TCGA, and analyzed using the indicated tools on the TCGA home page (tcga-data.nci.nih.gov/ and Kaplan-Meier Plotter).
Statistical analysis.
The statistical significance of differences in proliferation was determined by using an ANOVA. Differences of P<0.05 were considered statistically significant. The data represent the mean values and standard deviations from three replicates.
Results:
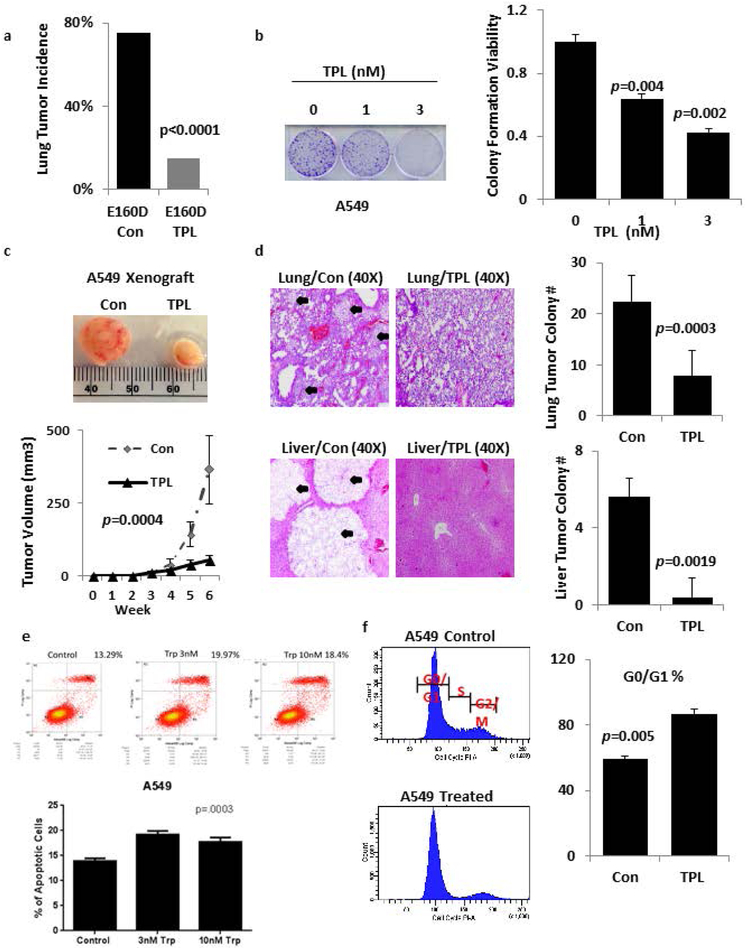
We tested the effects of triptolide on a previously established E160D FEN1 transgenic mouse model, which develops spontaneous lung tumors at high frequency (Figure 1a, P<0.001, Supplementary Figure S1a)16. Treating 13-month-old, E160D mice for three months with triptolide decreased lung tumor incidence from 70% to 10%. Furthermore, triptolide treatment significantly decreased the ability of A549 and H460 tumor colonies to form in vitro, and suppressed the growth of subcutaneous A549 and H460 xenografts (Figure 1b-c, Supplementary Figure S1b-c). Triptolide also significantly decreased the metastasis of A549 and H460 cells to the lung and liver in mouse xenografts after tail vein injection (Figure 1d, Supplementary Figure S1d).
Figure 1: Triptolide inhibits tumorigenicity and metastasis.
a) Spontaneous tumor incidence in E160D FEN1 mutant mice with or without subcutaneous triptolide treatment, n=20 for each group. The tumor incidence in each group was determined by anatomical analysis and histopathology. P-values were calculated using the Fisher Exact test. b) Colony formation assays of A549 lung cancer cells cultured in the indicated concentrations of triptolide. c) Representative tumor formation in NSG Xenograft mice untreated or treated subcutaneously with triptolide, n = 6 for each group. d) Haematoxylin and Eosin (H&E) staining of tumor or lung tissue after 8 weeks of subcutaneous triptolide treatment, n=6 for each group. Black arrows indicate metastatic nodules. e) A549 cells untreated or treated with 10 nM triptolide for 48h, then analyzed over FACS using a fluorescent annexin assay. f) A549 cells were treated with the indicated concentrations of triptolide for 48 h, stained with PI and analyzed by FACS. The proportion of DNA in the S-phase was calculated using ModfitLT Version 3.0 Software. Data are shown as the mean ± SD (error bars) from three independent experiments.
To identify the cellular events that inhibit A549 and H460 cell proliferation and invasion, we used a fluorescent annexin based assay with fluorescence activated cell sorting (FACS) to analyze population levels of apoptosis (Figure 1e, Supplementary Figure S1e). For the H460 cells, triptolide had a dose dependent effect on the apoptotic index and while this was not true of the A549 cell line, the increase in the apoptotic index was statistically significant for both cell lines after triptolide treatment. We next used propidium iodide staining to analyze cell cycle of both cell lines (P=0.005 Figure 1f, P<0.005 Supplementary Figure S1f), and determined triptolide treatment caused the G0/G1 fraction to increase from 59.3% (±1.5) to 86.7% (±3.2) in A549 cells, and to increase from 53.2% (±2.6) to 86.7% (±3.5) in H460 cells.
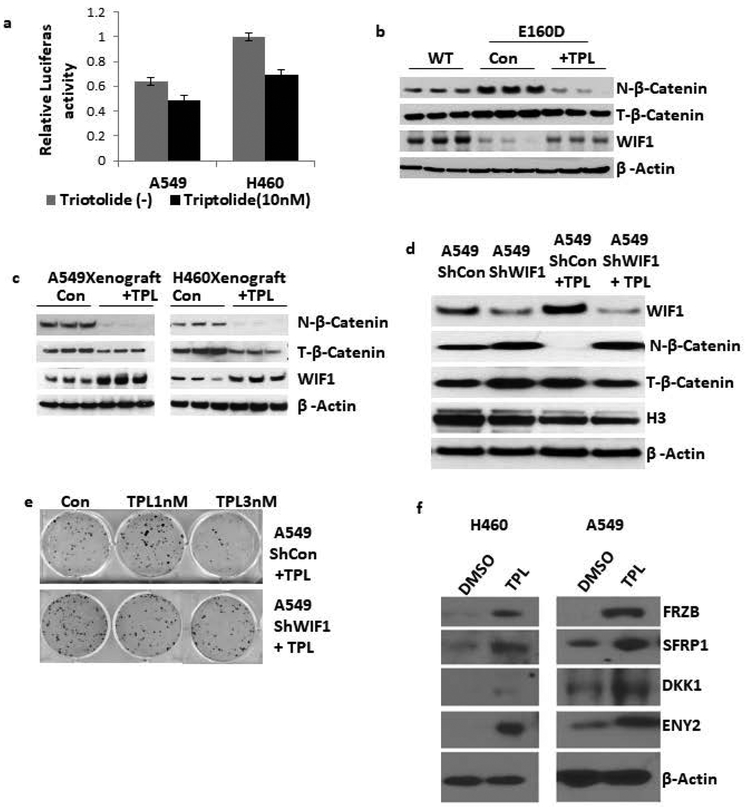
Restoring WIF1 expression in lung cancer cell lines inhibits their growth both in vitro and in vivo17. WIF1 has been identified as an important suppressor of cancer stemness and an inducer of cellular senescence 18. Moreover, expressing WIF1 and other Wnt inhibitory proteins, such as SFRP1, FRZB, DKK1, and ENY2, can suppress the proliferation and induce cellular senescence and apoptosis of various human cancers. Therefore, we first determined if our lung cancer cell lines had elevated levels of canonical Wnt signaling, and if the pathway could be inhibited by triptolide treatment using a T-cell factor/lymphoid enhancer factor (TCF/LEF)-responsive luciferase vector. TCF/LEF transcription factors are the major end-point mediators of Wnt signaling and are suitable for monitoring endogenous Wnt signaling in cells (Figure 2a). Upon triptolide treatment both the A549 and H460 cell lines had significant decreases in Wnt signaling.
Figure 2: Triptolide restores multiple Wnt inhibitory factors expression to inhibit Wnt signaling.
a) Triptolide inhibits β-catenin/TCF-dependent transcription. TOPflash and FOPflash together with the pLG4.5 vector were co-transfected into A549 and H460 cells, and then treated with or without triptolide for 48 hours. The luciferase activity was normalized against Renilla activity and the activity of control cells transfected with TOPflash and FOPflash is designated as 100%. The experiments were performed in triplicate and representative data are shown. Results are expressed as mean±s.d. b) Inactivation of Wnt signaling in E160D mice. Immunoblot of total β-catenin, nuclear β-catenin and WIF1 protein in whole cell lysates, plus nuclear extracts from E160D mouse lung tissue. We compared seventeen-month-old wild type mice, E160D control mice, and E160D mice treated with triptolide. β-actin and H3 were used as the loading controls. c) Inactivation of Wnt signaling in A549 and H460 Xenograft mice. Immunoblot of total β-catenin, nuclear β-catenin, WIF1 protein in whole cell lysates, plus nuclear extracts of tumor tissue from mice treated with or without triptolide. β-actin and H3 were used as the loading controls. The actin loading control is the same as that in Figure 4b. d) A549 cells were stably transfected with shWIF1 or a scrambled shRNA control plasmid and then treated with 10 nM triptolide for 48 hours. Cell extracts were analyzed by immunoblot to determine the expression levels of WIF1, nuclear β-catenin, total β-catenin and nuprotein respectively. e) Colony formation assay of A549 lung cancer cells transfected with the shWIF1 or control plasmid cultured in the indicated concentrations of triptolide. f) A549 and H460 cells were treated with 2nM triptolide for 6 days and blotted against the indicated Wnt inhibitory factors using β-Actin as a loading control.
To investigate the potential role of the Wnt signaling pathway in triptolide-mediated apoptosis, we analyzed WIF1 protein in tumors from the E160D transgenic and NSG xenograft mice, as well as in A549, H460, H1299, and H358 lung cancer cell lines (Figure 2b-c, Supplementary Figure S2a). We found triptolide treatment induced expression of WIF1 in tumors from the E160D transgenic, NSG xenograft mice, and in the A549, H460, H1299, and H358 lung cancer cell lines. Furthermore, nuclear levels of β-catenin in the E160D transgenic mice, NSG xenograft mice, and the A549 and H460 lines were reduced by triptolide treatment (Supplementary Figure S2b). This shows the Wnt signaling pathway was being perturbed. To determine if WIF1 expression was essential for the anti-cancer activity of triptolide WIF1 was knocked down by shRNA in A549 cells (Figure 2d). Triptolide treatment of the A549 WIF1 knockdown cells could not restore WIF1 expression nor decrease the levels of nuclear β-catenin. Additionally, the A549 WIF1 knockdown cells were significantly less sensitive to the induction of apoptosis by triptolide as revealed by a colony formation assay (Figure 2e).
We suspected triptolide was causing Wnt pathway inhibition in cells on a global scale and through multiple factors. Protein expression levels of four more known Wnt inhibitory factors were analyzed including FRZB, SFRP1, DKK1, and ENY2 (Figure 2f). Consistent with our WIF1 findings, all four factors were significantly upregulated in both A549 and H460 cells after triptolide treatment. In summary, our results show triptolide inhibits Wnt signaling through induction and upregulation of multiple Wnt inhibitory factors.
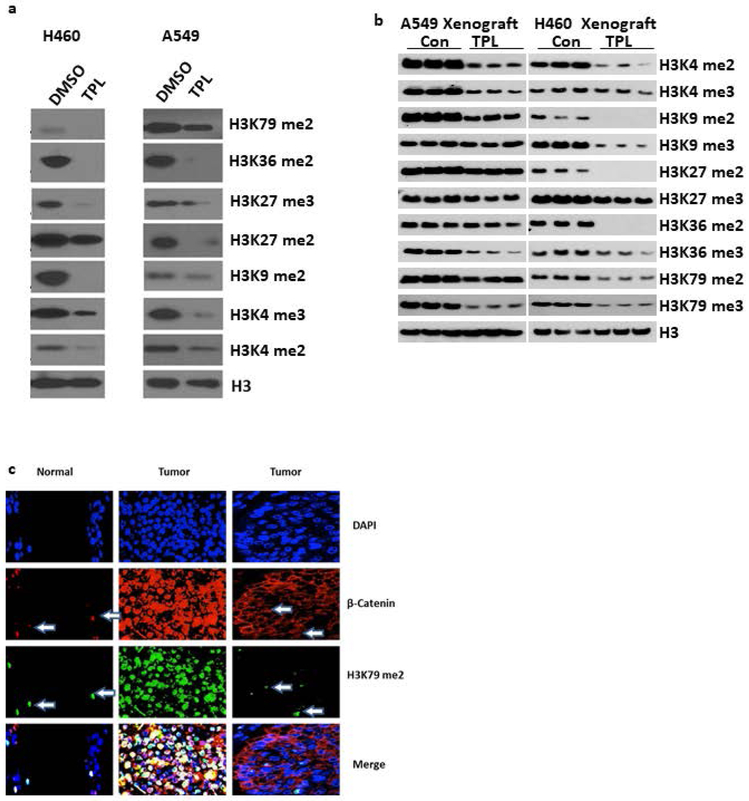
Several studies have revealed that triptolide alters the methylation status of H3 lysine residues in multiple myeloma and male germ cells. In male germ cells triptolide reduces the methylation of H3K919. In multiple myeloma, triptolide reduces the methylation of H3K4, H3K9, H3K27 and H3K36 through the down regulation of the SMYD3, SUV39H1, EZH2 and H3 methyltransferases14,15. Therefore, we examined the effect of triptolide on the methylation status of specific H3 lysine residues in lung cancer cell lines and in our xenograft model (Figure 3a-b). Triptolide treatment decreased the di-methylation and tri-methylation of H3K4, H3K9, H3K27, H3K36 and H3K79 in both A549 and H460 cell lines along with the xenograft model. There were no significant DNA methylation status changes in the CpG islands at any of the 5’ promoter elements of the Wnt inhibitory factors including WIF1, FRZB, SFRP1, DKK1, and ENY2 in both A549 and H460 cell lines after triptolide treatment (data not shown).
Figure 3: Triptolide induces epigenetic changes in H3 methylation and B-catenin nuclear translocation.
a) A549 and H460 cells were treated with 2nM triptolide for 6 days and blotted against the indicated H3 epigenetic marks using total H3 as a loading control. b) Blotting against H3 epigenetic marks in A549 and H460 xenograft mice after triptolide treatment. H3 was used as the loading control. c) Representative images of immunostained normal and cancer tissue showing elevated levels of H3K79me2 are positively correlated with increased nuclear β-catenin translocation. DAPI is used to outline cell nuclei. Arrows point to cells with high H3K79me2 expression and the subsequent β-catenin nuclear localization.
Our data in lung cancer cell lines is consistent with studies showing that elevated levels of methylation of H3K79 by the Dotcom methylation complex has been linked to Wnt signaling in mammalian cells. Consistent with our findings, when we analyzed the association between H3K79 methylation and nuclear β-catenin levels in human lung cancer tissues (Figure 3c), we found increased levels of H3K79me2 correlated with higher levels of nuclear β-catenin, which leads to increased Wnt signaling.
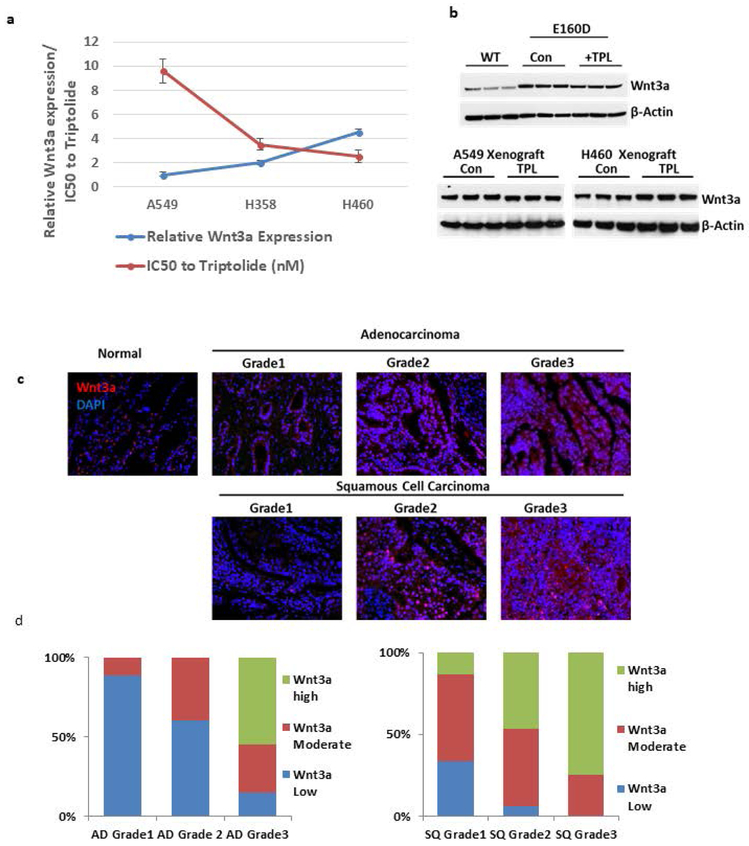
Finally, we examined the expression of specific Wnt ligands to determine if they could predict the response to Wnt-targeted therapy. Analysis of 1088 different NSCLC samples in the TCGA database revealed that Wnt2, Wnt3a, Wnt5b, Wnt7a, and Wnt9a were the most overexpressed Wnt ligands (Supplementary Figure S2c). Quantitative PCR (qPCR) was used to evaluate the relationship between Wnt3a expression and the efficacy of triptolide treatment on the growth of the A549, H358, and H460 lung cancer cell lines (Figure 4a, Supplementary Figure S2e-f). This showed increased Wnt3a mRNA expression correlated with a lower triptolide IC50. In addition, we observed Wnt3a mRNA expression did not change with response to triptolide treatment in cell lines, E160D mice, or the xenograft models (Figure 4b). This suggests Wnt3a mRNA expression levels may be useful to predict response to triptolide, but that Wnt3a is not part of the pathway for triptolide function. To further understand the prevalence of Wnt3a overexpression in NSCLC immunofluorescent staining was performed on the Wnt3a protein to determine its expression levels in NSCLC tissues (Figure 4c, Supplementary Figure 2g). Wnt3a protein expression was low in normal and grade 1 lung tissues, but the levels were significantly increased in higher grade samples. Wnt3a was overexpressed (2+ to 3+) in 51% of adenocarcinomas, and 43% of squamous cell carcinomas (Figure 4d).
Figure 4: Wnt3a expression is predictive of triptolide’s effectiveness.
a) Triptolide IC50 values and the relative expression of Wnt3a mRNA in multiple cell lines. Wnt3a expression was normalized to mRNA levels in the A549 cell line. b) Immunoblot of Wnt3a expression in total protein samples extracted from the lung tissue of WT, E160D, and A549/H460 xenograft mice treated with/without triptolide. β-actin was used as the loading control. The actin loading control is the same as that in Figure 2c. c) Immunofluorescence staining of adeno- and squamous cell carcinomas grades 1-3. Cell nuclei are stained with DAPI in blue and Wnt3a is stained in red. Normal tissue sample staining is on the left. d) Fluorescent quantitation of Wnt3a over the total cell population of adeno- and squamous cell carcinoma differentiated by grade.
Discussion:
In summary, our findings suggest triptolide mediates its anti-cancer activities in lung cancer through epigenetic changes that arrest cells in G1-phase and could potentially lead to apoptosis. These results are unique from previous descriptions of the effects of triptolide on various cancers in that we show triptolide prevents lung cancer formation in a transgenic mouse model; that triptolide inhibits lung cancer proliferation, apoptosis, and metastasis; and that triptolide leads to decreased Wnt pathway signaling through overexpression of several Wnt inhibitory proteins. This is particularly important in lung cancer, where the Wnt pathway plays a significant role in cancer development and proliferation. The Wnt pathway has been difficult to target therapeutically, and triptolide seems to effectively inhibit Wnt signaling in a variety of lung cancer models that we tested through multiple Wnt inhibitory factors. Ectopic overexpression of WIF1 in salivary gland tumors has been shown to inhibit cancer stemness and induce cellular senescence18, and because of this it is significantly downregulated in many cancer types20,21. These previous findings and our data suggest triptolide is mediating its anti-cancer activity through upregulation of multiple Wnt inhibitory factors which can lead to inhibition of cell growth, cellular senescence, and apoptosis. It is known that WIF1 binds directly to extracellular Wnt ligands and prevents their interaction with Wnt receptors leading to degradation of β-catenin22. This is consistent with our data showing nuclear β-catenin levels in the E160D transgenic mice, NSG xenograft mice, and the A549 and H460 lines were reduced by triptolide treatment (Supplementary Figure S2b). We hypothesize that the significant upregulation of Wint inhibitory factors massively destabilize β-catenin signaling which results in cell cycle arrest and apoptosis. Further study is needed to elucidate the exact mechanism for how this occurring and what apoptotic machinery is being activated upon triptolide treatment.
Based on our results, we propose that, in lung cancer cells, triptolide induces global reduction in methylation at key H3 residues that include H3K4, H3K9, H3K27, H3K36, and H3K79, which is consistent with reports in multiple myeloma and breast cancer (Figure 3a) 7,14,15,23,24. The mechanism of action behind these epigenetic changes on H3 has been suggested to be mediated through altering expression of the histone demethylases LSD1 and JMJD2B 7. Conversely, triptolide reduces the expression of the methyltransferase EZH2 in prostate cancer cell lines 9. The exact mechanism of how triptolide is modulating demethylase/methyltransferase expression has not been fully elucidated. One hypothesis is that triptolide is exerting its effects through epigenetic changes of histone methylation at the gene promoter regions of LSD1, JMJD2B, and EZH2. As of now studies have only shown triptolide is globally affecting histone methylation status7,9. Additional research is needed to understand how triptolide is exerting its effects on histone methylation on a gene by gene basis.
Canonical Wnt pathway signaling is crucial for embryonic development, controlling cell migration, differentiation, and proliferation 21. Wnt signaling also facilitates and maintains adult stem cells 25. De-regulation and hyper-activation of the Wnt pathway is thought to contribute to carcinogenesis in many cancers including NSCLC 26. Along with H3 de-methylation, we observed significant increase in expression of multiple Wnt inhibitory factors including WIF1, FRZB, SFRP1, ENY2, and DKK1 after triptolide treatment. Our previous work showed the triptolide derivative MRx102 upregulated WIF1 in lung cancer cell lines through promoter hypomethylation 13. However, we did not observe this same phenomenon at any of the promoter regions of the five Wnt inhibitory factors studied even at low doses of triptolide (data not shown).
Although we did not observe any significant toxicities (the most common being slight weight loss, decreased appetite, and increased stool frequency and diarrhea) in the mouse models we tested, triptolide is toxic at higher doses and has relatively poor pharmacokinetics. At least two triptolide derivatives which have different pharmacodynamics are currently under investigation for other cancers including pancreatic, liver, and acute myeloid leukemia 27,28.
In summary, we found triptolide treatment decreases lung cancer cell proliferation, tumor formation, and metastasis which is associated with decreased Wnt signaling. Additional research into the mechanisms of triptolide’s effects of histone demethylation is needed. Triptolide and triptolide-like drugs are promising lung cancer therapies.
Supplementary Material
Supplementary Figure 1: Triptolide inhibits tumorigenicity and metastasis. a) Representative images of lung tissue from E160D mice with or without subcutaneous triptolide treatment (1 mg/kg for three months. b) Colony forming assays of H460 lung cancer cells cultured in the indicated concentrations of triptolide. c) Representative tumors formed from H460 cells xenografts in NSG mice with or without subcutaneous triptolide treatment (n = 6 for each group). d) Haematoxylin and Eosin (H&E) staining of tumor or lung tissue after eight weeks of subcutaneous triptolide treatment (n=6 for each group.) Black arrows indicate metastatic nodules. e) H460 cells untreated or treated with 10 nM triptolide for 48h, then analyzed by FACS using a fluorescent annexin assay. f) H460 cells were treated with the indicated concentrations of triptolide for 48 h, stained with PI and analyzed by FACS. The proportion of DNA in the S-phase was calculated using ModfitLT Version 3.0 Software. Data are shown as the mean ± SD (error bars) from three independent experiments.
Supplementary Figure 2: Triptolide induces upregulation of WIF1 and its effectiveness in cancer cells is predictive on Wnt3a mRNA expression. a) Immunoblot of WIF1 protein from whole cell lysates of lung cancer cell lines (A549, H460, H1299, and H358) treated with or without triptolide. β-actin was used as the loading control. b) Immunoblotting was used to determine changes in the cellular expression and location of β-catenin caused by treatment with triptolide. The immunoblot shows total β-catenin, nuclear β-catenin and WIF1 protein in whole cell lysates from the lung cancer cells treated with/without triptolide. The nuclear extract of β-actin and H3 were used as loading controls. c) The UCSC Cancer Genomics Browser was used to compare the expression of Wnt ligands in 1088 NSCLC samples. d) Injection, harvesting, and analysis plan for mouse xenografts and for the E160D mouse model. e) A549, H358, and H460 cell lines were treated or untreated with 10 nM triptolide for 48 hours. At that time the plates were confluent and were stained with 0.5% typan blue. Representative stained plates are shown from the experiment, which was performed in triplicate. f) Triptolide IC50 values and the relative expression of Wnt3a mRNA in multiple cell lines. Wnt3a expression was normalized to mRNA levels in the A549 cell line. g) Wnt3a expression levels for differing grades of adeno- and squamous cell carcinoma tumor tissues.
Novelty and Impact: This research provides critical insight into triptolide’s effects in lung cancer cells by inhibition of the Wnt pathway by upregulation of key Wnt inhibitory factors. Furthermore, we show relative Wnt3a expression is a viable predictor of triptolide effectiveness in treating lung cancer. This additional information will allow doctors to make an informed decision on whether triptolide is an effective drug on a patient-by-patient basis.
Acknowledgements
We thank the Integrative Functional Genomics, Light Microscopy, Flow Cytometry and Pathology Core Facilities at City of Hope for their assistance on the DNA sequencing, histopathology, and immunofluorescence analyses. We thank Margaret Morgan for expert editing of this manuscript.
Research reported in this publication is supported by the National Cancer Institute of the National Institutes of Health under award numbers NIH 5K12CA001727–20 (Raz) and then through the use of the City of Hope Survey Research Core. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the generous support of the Baum Family Foundation in support of this research. We would also like to thank Youqiang Li for his valuable contributions to the manuscript. Lastly, we would like to thank Keqiang Zhang at City of Hope for his invaluable research and intellectual contributions.
Footnotes
Supplementary Information is available in the online version of the paper.
Competing Financial Interests Statement
D.R.: Cireca, Consultant; Merck, grant funding.
Disclosure of Potential of Conflicts of Interest:
DR: Cireca, consultant; Merck, grant funding.
References:
- 1.Siegel R, Ma J, Zou Z & Jemal A Cancer statistics, 2014. CA. Cancer J. Clin 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS Tobacco smoke carcinogen and lung cancer. J. Natl. Cancer Inst 91, 1194–1210 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Crinò L, Weder W, van Meerbeeck J & Felip E Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol 21, (2010). [DOI] [PubMed] [Google Scholar]
- 4.Cetin K, Ettinger DS, Hei Y-J & O ‘malley CD survival by histologic subtype in stage iV nonsmall cell lung cancer based on data from the surveillance, Epidemiology and End results Program. Clin. Epidemiol 3, 139–148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng C, Xhu H, Song H et al. Targets and molecular mechanisms of triptolide in cancer therapy. Chinese J. cancer Res 26, 622–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang XH, Wong BC, Lin MC et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene 20, 8009–18 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Wen L, Chen Y, Zeng L-I et al. Triptolide induces cell-cycle arrest and apoptosis of human multiple myeloma cells in vitro via altering expression of histone demethylase LSD1 and JMJD2B. Acta Pharmacol. Sin 33, 109–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerheide SD, Kawahara TLA, Orton K & Morimoto RI Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J. Biol. Chem 281, 9616–9622 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Tamgue O, Chai CS, Hao L et al. Triptolide inhibits histone methyltransferase EZH2 and modulates the expression of its target genes in prostate cancer cells. Asian Pacific J. Cancer Prev 14, 5663–5669 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Reno TA, Kim JY & Raz DJ Triptolide inhibits lung cancer cell migration, invasion, and metastasis. Ann. Thorac. Surg 100, 1817–1825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polakis P Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol 4, 9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malinauskas T & Jones EY Extracellular modulators of Wnt signalling. Curr. Opin. Struct. Biol 29, 77–84 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Reno TA, Tong SW, Wu J et al. The triptolide derivative MRx102 inhibits Wnt pathway activation and has potent anti-tumor effects in lung cancer. BMC Cancer 16, 439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao F, Chen Y, Zeng L et al. Role of triptolide in cell proliferation, cell cycle arrest, apoptosis and histone methylation in multiple myeloma U266 cells. Eur. J. Pharmacol 646, 1–11 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Zhao F, Chen Y, Li R et al. Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology 267, 70–79 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, Dai H, Zhou M et al. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med 13, 812–819 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Kim J, You L, Xu Z et al. Wnt inhibitory factor inhibits lung cancer cell growth. J. Thorac. Cardiovasc. Surg 133, 733–737 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran I, Ganapathy V, Gillies E et al. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. 5, e1246–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong J, Wang H, Guangming G et al. Male Germ Cell Apoptosis and Epigenetic Histone Modification Induced by Tripterygium wilfordii Hook F. PLoS One 6, e20751 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran I, Thavathiru E, Ramalingam S et al. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 31, 2725–2737 (2012). [DOI] [PubMed] [Google Scholar]
- 21.MacDonald BT, Tamai K & He X Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh JC Kodjbachian L, Rebbert ML et al. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nature 398, 431 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Mohan M, Herz HM, Takahashi YH et al. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom). Genes Dev. 24, 574–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Deng L, Chen F et al. Inhibition of histone H3K79 methylation selectively inhibits proliferation, self-renewal and metastatic potential of breast cancer. Oncotarget 5, 10665–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nusse R Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol 73, 59–66 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Stewart DJ Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst 106, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Fidler JM, An J, Cater BZ & Andreeff M Preclinical antileukemic activity, toxicology, toxicokinetics and formulation development of triptolide derivative MRx102. Cancer Chemother. Pharmacol 73, 961–974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee S & Saluja A Minnelide, a novel drug for pancreatic and liver cancer. Pancreatology 15, S39–S43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Triptolide inhibits tumorigenicity and metastasis. a) Representative images of lung tissue from E160D mice with or without subcutaneous triptolide treatment (1 mg/kg for three months. b) Colony forming assays of H460 lung cancer cells cultured in the indicated concentrations of triptolide. c) Representative tumors formed from H460 cells xenografts in NSG mice with or without subcutaneous triptolide treatment (n = 6 for each group). d) Haematoxylin and Eosin (H&E) staining of tumor or lung tissue after eight weeks of subcutaneous triptolide treatment (n=6 for each group.) Black arrows indicate metastatic nodules. e) H460 cells untreated or treated with 10 nM triptolide for 48h, then analyzed by FACS using a fluorescent annexin assay. f) H460 cells were treated with the indicated concentrations of triptolide for 48 h, stained with PI and analyzed by FACS. The proportion of DNA in the S-phase was calculated using ModfitLT Version 3.0 Software. Data are shown as the mean ± SD (error bars) from three independent experiments.
Supplementary Figure 2: Triptolide induces upregulation of WIF1 and its effectiveness in cancer cells is predictive on Wnt3a mRNA expression. a) Immunoblot of WIF1 protein from whole cell lysates of lung cancer cell lines (A549, H460, H1299, and H358) treated with or without triptolide. β-actin was used as the loading control. b) Immunoblotting was used to determine changes in the cellular expression and location of β-catenin caused by treatment with triptolide. The immunoblot shows total β-catenin, nuclear β-catenin and WIF1 protein in whole cell lysates from the lung cancer cells treated with/without triptolide. The nuclear extract of β-actin and H3 were used as loading controls. c) The UCSC Cancer Genomics Browser was used to compare the expression of Wnt ligands in 1088 NSCLC samples. d) Injection, harvesting, and analysis plan for mouse xenografts and for the E160D mouse model. e) A549, H358, and H460 cell lines were treated or untreated with 10 nM triptolide for 48 hours. At that time the plates were confluent and were stained with 0.5% typan blue. Representative stained plates are shown from the experiment, which was performed in triplicate. f) Triptolide IC50 values and the relative expression of Wnt3a mRNA in multiple cell lines. Wnt3a expression was normalized to mRNA levels in the A549 cell line. g) Wnt3a expression levels for differing grades of adeno- and squamous cell carcinoma tumor tissues.