Abstract
Parkinson’s disease (PD) is characterized by a profound loss of dopaminergic neurons in the substantia nigra, accompanied by chronic neuroinflammation, mitochondrial dysfunction, and widespread accumulation of α-synuclein-rich protein aggregates in the form of Lewy bodies. However, the mechanisms linking α-synuclein pathology and dopaminergic neuronal death to chronic microglial neuroinflammation have not been completely elucidated. We show that activation of the microglial NLR family pyrin domain containing 3 (NLRP3) inflammasome is a common pathway triggered by both fibrillar α-synuclein and dopaminergic degeneration in the absence of α-synuclein aggregates. Cleaved caspase-1 and the inflammasome adaptor protein apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) were elevated in the substantia nigra of the brains of patients with PD and in multiple preclinical PD models. NLRP3 activation by fibrillar α-synuclein in mouse microglia resulted in a delayed but robust activation of the NLRP3 inflammasome leading to extracellular interleukin-1β and ASC release in the absence of pyroptosis. Nanomolar doses of a small-molecule NLRP3 inhibitor, MCC950, abolished fibrillar α-synuclein-mediated inflammasome activation in mouse microglial cells and extracellular ASC release. Furthermore, oral administration of MCC950 in multiple rodent PD models inhibited inflammasome activation and effectively mitigated motor deficits, nigrostriatal dopaminergic degeneration, and accumulation of α-synuclein aggregates. These findings suggest that microglial NLRP3 may be a sustained source of neuroinflammation that could drive progressive dopaminergic neuropathology and highlight NLRP3 as a potential target for disease-modifying treatments for PD.
One Sentence Summary
Oral treatment with a brain-penetrant NLRP3 inhibitor has protective effects in preclinical models of Parkinson’s disease
INTRODUCTION
Parkinson’s disease (PD) is the most prevalent synucleinopathy and the second most common neurodegenerative disorder worldwide, affecting around 2% of the population over the age of 60 (1). Although symptomatic treatments exist, no current therapies can effectively slow or halt disease progression (1). The pathological hallmark of PD is a profound loss of nigrostriatal dopaminergic neurons that is preceded by the accumulation and spread of characteristic Lewy body and neurite inclusions, consisting primarily of misfolded fibrillar α-synuclein (1). Accumulating evidence suggests that pathological fibrils are the major neurotoxic form of α-synuclein, which can self-propagate through interconnected brain regions and drive progressive dopaminergic degeneration (2–7). Chronic microglial neuroinflammation is evident early in the nigrostriatal system in living PD patients and remains a prominent feature in postmortem PD brains (8–10). Compelling evidence from clinical, pre-clinical and epidemiological studies supports a pathogenic role for chronic neuroinflammation during dopaminergic degeneration (10–15). However, a clear mechanism linking fibrillar α-synuclein pathology, progressive dopaminergic degeneration, and the underlying chronic neuroinflammation evident in clinical and experimental PD has not been completely defined.
Inflammasomes are multi-protein complexes that function as intracellular sensors of environmental and cellular stress (16–18). The NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome is composed of the NLRP3 sensor, the signaling adapter apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and the caspase-1 protease. Assembly of the NLRP3 complex in immune cells upon cellular stress triggers caspase-1 activation and caspase-1-mediated release of interleukin-1β (IL-1β) and interleukin-18 (IL-18) thereby initiating inflammatory responses (18). In neurodegenerative conditions such as Alzheimer’s disease (AD), persistent accumulation of misfolded protein aggregates can trigger and sustain inflammasome activation and thereby drive central nervous system (CNS) inflammation and neuropathology (19). Recent findings in AD models have demonstrated that microglia-derived inflammasome components such as ASC specks can cross-seed pathogenic amyloid fibrils (20). In PD patient brains, the inflammasome pathway can potentially be activated by oxidative stress and insoluble α-synuclein aggregates (18, 21, 22). However, the interplay between chronic NLRP3 activation, ASC release and progressive α-synuclein pathology in PD is still unclear.
We provide herein direct evidence of NLRP3 inflammasome activation in postmortem PD brains and in multiple rodent models involving fibrillar α-synuclein pathology as well as mitochondrial dysfunction and oxidative stress. We also confirm that reactive microglia are the source of NLRP3 inflammasomes in human PD brains and provide new insight into the mechanisms of α-synuclein-mediated NLRP3 activation and ASC release in microglia that have implications for PD pathophysiology. The potent small molecule NLRP3 inhibitor MCC950 is active in the CNS following oral delivery and can functionally inhibit NLRP3 activation in multiple mouse models of PD. Further, daily oral dosing with MCC950 is neuroprotective and reduces the loss of striatal dopamine and nigrostriatal dopaminergic degeneration in PD mice. We show that chronic NLRP3 inhibition with MCC950 protects against motor deficits, nigrostriatal dopaminergic degeneration, and accumulation of hyper-phosphorylated α-synuclein aggregates in multiple brain regions in the PD mice. Our results suggest that chronic NLRP3 activation might be a key pathological mechanism that drives PD pathology, and that NLRP3 inhibition with orally-active and CNS-penetrant drugs, such as MCC950, may represent a promising therapeutic strategy to mitigate progressive dopaminergic degeneration in PD.
RESULTS
Extensive inflammasome activation and ASC upregulation in PD patients and multiple PD models
To determine whether inflammasome activation occurs in clinical PD, we first assessed key inflammasome components in the substantia nigra of postmortem PD patient brains. We found substantially increased cleaved caspase-1 (p20) and adapter protein ASC in PD patient brains compared to age-matched controls (Fig. 1, A and B). Immunohistochemistry studies confirmed NLRP3 and ASC upregulation in PD substantia nigra sections, which was almost exclusively localized to Iba1-positive microglia (Fig. 1, C and D, fig. S1, A and B), consistent with previous reports linking chronic inflammasome activation to persistent reactive microgliosis (18, 19). A proportion of ASC was also identified outside of microglial cells (fig. S1B, blue arrows), supporting similar findings recently documented in AD patients (20). These results clearly show that increased inflammasome activation is evident at the sites of dopaminergic cell loss in end-stage PD patients. Downstream inflammasome activation markers including IL-1β have been shown to be elevated systemically in PD patient blood and in cerebrospinal fluid (9, 23). Similarly, we also found significantly elevated circulating caspase-1 in a cohort of 21 PD patients (p = 0.023; fig. S1C) suggesting that systemic inflammasome activation might also be involved in PD pathophysiology (18, 20, 22).
Fig. 1. Extensive inflammasome activation and microglial NLRP3 expression is observed in PD patient brains and animal models.
(A) Western blot and (B) densitometric analysis for caspase-1 and ASC from substantia nigra tissue lysates obtained from PD patients and control subjects (n=5–6/group). (C, D) Immunohistochemistry of key inflammasome components NLRP3 (green in C) and ASC (red in D), and Iba-1-positive microglia in substantia nigra tissue sections of postmortem PD patients and age-matched controls. Magnification x40, scale bar 20μm. (E, F) Immunohistochemistry within the striatum of mice 3 days after 6-OHDA or PBS injection, showing NLRP3 (green in E) and ASC (red in F) localized to hypertrophic activated Iba-1-positive microglia in 6-OHDA mice. Magnification x40, scale bar 20μm. (G) Western blot and (H) densitometric analysis for cleaved caspase-1 and ASC in ipsilateral striatal tissue of 6-OHDA and PBS-injected mice at 3 days after injection (n=4 mice/group). (I) Western blot and (J) densitometric analysis for cleaved caspase-1 and ASC in substantia nigra tissue from MitoPark mice (MP) and littermate controls (Ctrl) at 12 and 24 weeks of age (n=2–4 mice/group). (K) Western blot and (L) densitometric analysis for cleaved caspase-1 and ASC in ipsilateral striatal tissue from PBS- and α-synuclein PFF-injected mice at 30 days after injection (n=9 mice/group). Data shown as means ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 by Student’s (panels H and J) or Mann-Whitney (panels B and L) t-tests.
In addition to the central role for α-synuclein in PD progression, mitochondrial dysfunction is considered a major underlying pathological mechanism in PD (24). To further investigate the contribution of NLRP3 during dopaminergic degeneration, we examined the activation of the NLRP3 inflammasome in distinct animal models of PD involving dopaminergic degeneration that is driven by mitochondrial dysfunction, oxidative stress or α-synuclein pathology. Consistent with our finding in human PD cases, immunohistochemistry studies revealed an almost exclusive localization of NLRP3 in hypertrophic reactive microglia (Fig. 1E), along with ASC expression (Fig. 1F) in the striatum of mice 3 days after 6-OHDA administration. Striatal NLRP3 expression after 6-OHDA treatment was also confirmed using NLRP3-GFP gene knock-in mice (fig. S2, A and B). Cleaved caspase-1 (p20) and ASC protein detected by western-blotting were also increased prior to motor dysfunction in 6-OHDA mice at day 3 (Fig. 1, G and H), as was Nlrp3, ASC, and Caspase-1 gene expression (fig. S2, C to E), suggesting that the upregulation and subsequent activation of inflammasome components occur at the early stages of dopaminergic degeneration in this model, correlating with the onset of reactive microgliosis. We also confirmed the lack of cleaved caspase-1 generation in 6-OHDA administered NLRP3 knockout mice (fig. S2F). Next, we investigated MitoPark mice, a levodopa-responsive genetic model of PD, where targeted inactivation of the mitochondrial transcription factor A (Tfam) gene in dopaminergic neurons leads to progressive degeneration over 32 weeks, accompanied by Parkinsonian motor deficits (25). Similar to 6-OHDA mice, we observed elevated Nlrp3 gene expression, and NLRP3 and ASC immunostaining (fig. S3, A and B), and increased generation of cleaved caspase-1 in the substantia nigra of 12- and 20-week old MitoPark mice (Fig. 1, I and J), before the onset of overt motor deficits. Together, these results suggest that robust microglial NLRP3 inflammasome activation occurs early in the disease process in mitochondrial dysfunction models of PD, and can occur in the absence of α-synuclein aggregates. Given the central role of neurotoxic α-synuclein fibrils in PD, we used the α-synuclein pre-formed fibril (PFF) model of PD (3) to determine whether inflammasome activation occurs with α-synuclein pathology in vivo. At 30 days after fibrillar α-synuclein infusion, we detected microglial NLRP3 immunostaining (fig. S4), and increases in ASC adaptor protein, and caspase-1 cleavage to the active p20 fragment (Fig. 1, K and L) in the striatum of PFF mice compared to phosphate-buffered saline (PBS)-injected controls, demonstrating that neurotoxic α-synuclein fibrils can indeed drive early inflammasome activation in vivo. Collectively, these results demonstrate that robust inflammasome activation occurs in PD patient brains and multiple disease models driven by mitochondrial dysfunction and α-synuclein pathology.
Fibrillar α-synuclein drives delayed microglial NLRP3 activation and extracellular ASC release in the absence of detectible pyroptosis
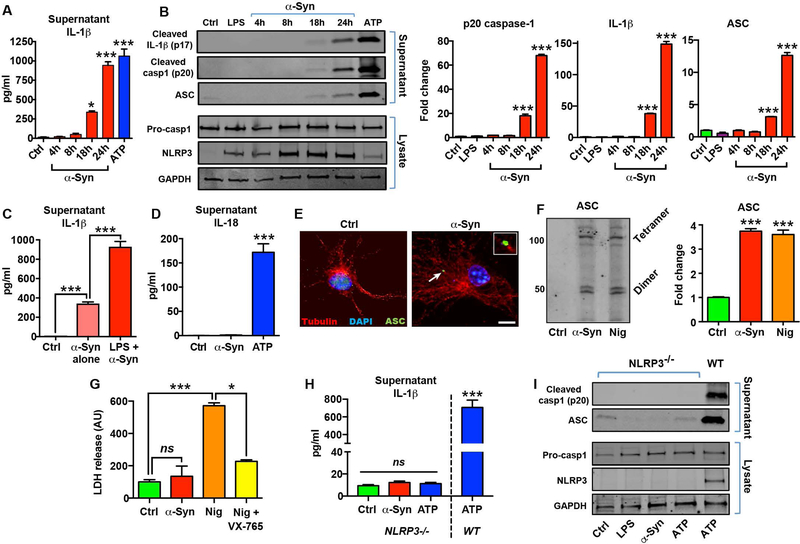
The pathological hallmark of PD is the presence of high molecular weight, misfolded α-synuclein-rich Lewy body inclusions (2, 5). Although persistent insoluble protein aggregates are considered the major triggers for chronic sterile inflammasome activation in the CNS (18, 19), the contribution of microglial neuroinflammation and the NLRP3 inflammasome pathway to α-synuclein pathology and its cell-to-cell transmission is still unclear. Furthermore, an emerging analogous paradigm in inflammasome biology is that prion-like activities of extracellular polymeric ASC can propagate chronic inflammation by spreading inflammasome signaling from cell-to-cell (20, 26). Previous studies in monocytic and immortalized microglial cell lines have shown that α-synuclein can trigger inflammasome activation similar to β-amyloid (21, 22), nonetheless the specific contribution of NLRP3 and ASC to inflammasome activation by α-synuclein is still not well defined. Hence, we evaluated the microglial response to fibrillar α-synuclein in primary microglia isolated from wild-type and NLRP3 knockout mice using highly characterized synthetic α-synuclein fibrils prepared by in vitro aggregation and sonication (fig. S5, A to C), which have been shown to drive α-synuclein pathology in vivo (3). In microglia primed with ultrapure lipopolysaccharide (LPS) and activated with fibrillar α-synuclein, we observed a time-dependent secretion of IL-1β that was detectable at 18 h (Fig. 2A). The amount of IL-1β secreted 24 h after α-synuclein treatment was equivalent to that obtained at 1 h with activation using the powerful NLRP3 activator ATP, suggesting that fibrillar α-synuclein mediates a delayed, but robust activation of the inflammasome pathway in microglia. We also confirmed the presence of caspase-1 p20 and cleaved IL-1β p17 in supernatants starting at 18 h, demonstrating delayed inflammasome activation (Fig. 2B). We found substantially increased extracellular ASC protein in the supernatants of α-synuclein-treated microglia (Fig. 2B), supporting our immunohistochemistry findings in PD patients, and suggesting that extracellular ASC might have a role in the propagation of inflammasome-driven pathology triggered by α-synuclein fibrils (16, 20, 26).
Fig. 2. Fibrillar α-synuclein drives delayed NLRP3 inflammasome activation and extracellular ASC release.
(A) Time course of IL-1β secretion in control (Ctrl), or LPS-primed primary microglia exposed to fibrillar α-synuclein (α-Syn; 10 μM). ATP (5 mM) treatment for 1 h was used as a positive control. (B) Western blot (left) and densitometric analysis (right) for cleaved caspase-1 (p20), cleaved IL-1β, and ASC in the supernatants of primed microglia treated with α-synuclein for 24 h. Expression of pro-caspase-1, NLRP3 and GAPDH were determined in cell lysates and 1 h ATP treatment (5 mM) was used as a positive control. (C) Comparison of α-synuclein-mediated IL-1β secretion in un-primed or LPS-primed primary microglia at 24 h. (D) IL-18 secretion in microglia treated with α-synuclein for 24 h compared with ATP (1 h). (E) Immunofluorescence staining for ASC (green) in control or α-synuclein-activated microglia showing the formation of a characteristic inflammasome ASC speck in green. Scale bar 10μm. Inset (top right) shows magnified view of ASC speck identified by arrow. (F) Western blot (left) and band quantification (right) for detection of oligomeric ASC following chemical crosslinking. Nigericin (Nig; 10 μM, 1 h) was used as a positive control. (G) LDH release assay for quantification of caspase-1 dependent pyroptosis (ns, not significant). Nigericin and VX-765 (20 μM) were used as a positive controls. (H) Supernatant IL-1β and (I) western blots for cleaved caspase-1 and ASC release from NLRP3−/− microglia. Wildtype (WT) microglia activated with ATP was used for comparison. Data represented as mean ± SEM from at least 3 independent experiments. *P <0.05, ***P < 0.001 by one-way ANOVA with Bonferroni’s post-hoc test (panels B, C, F, H), or Kruskal-Wallis test with Dunn’s post-hoc test (panels A, D, G).
Inflammasome ‘priming’ induces transcriptional upregulation of inflammasome components, which facilitates the assembly and activation of the inflammasome complex (18). To determine whether priming was a requirement for α-synuclein-mediated inflammasome activation, we repeated the experiments in the absence of the LPS-priming step. We found that α-synuclein fibrils alone were able to induce IL-1β release at 24 h, although only to ~35% of the level of primed cells (Fig. 2C). As IL-18 is also a key inflammatory mediator that is released during inflammasome activation (17, 27), we quantified IL-18 concentrations in α-synuclein-activated microglia. Under the same conditions, no IL-18 was detectible with α-synuclein (Fig. 2D), despite ATP stimulation releasing substantial amounts of IL-18 from microglia. We also confirmed the formation of characteristic ASC specks in α-synuclein treated microglia at 24 h (Fig. 2E), as well as the presence of oligomeric ASC in crosslinking experiments (Fig. 2F). Because α-synuclein fibrils caused increased extracellular ASC together with the release of inflammasome components, we evaluated the potential mechanisms of cellular ASC release. In macrophages and other immune cells stimulated with NLRP3 activators such as nigericin or monosodium urate crystals, the inflammasome complex is released during caspase-1-dependent cell lysis by a process termed pyroptosis (16). Extracellular release of ASC by pyroptosis has been shown to be the primary mechanism by which inflammasome activation propagates inflammation in systemic inflammatory models (20, 26). Therefore, we assayed whether inflammasome activation by α-synuclein fibrils triggered pyroptosis in microglia. Even at 24 h, when inflammasome activation was maximal, α-synuclein did not cause detectable pyroptosis, which was quantified using lactate dehydrogenase (LDH) release (Fig. 2G). In contrast, nigericin treatment readily triggered caspase-1 dependent pyroptosis in microglia within 1 h, with substantial LDH release that could be blocked with the caspase-1 inhibitor VX-765 (Fig. 2G).
Although prior reports have shown that α-synuclein can elicit inflammasome activation in monocyte and microglial cell lines, resulting in IL-1β and caspase-1 release (21), the role of NLRP3 in this process has not been clearly defined, and recent studies suggest that alternate inflammasomes may be involved in CNS pathologies (28, 29). To determine the specific contribution of the NLRP3 pathway during inflammasome activation by α-synuclein, we used microglia isolated from NLRP3 knockout mice. α-synuclein could not stimulate the release of IL-1β, caspase-1 or ASC from NLRP3 knockout microglia even at 24 h after treatment (Fig. 2, H and k), indicating that fibrillar α-synuclein specifically activates the NLRP3 pathway in microglia. This suggests that α-synuclein fibrils trigger robust microglial NLRP3 inflammasome activation without pyroptosis, resulting in a delayed, but substantial release of ASC, caspase-1 p20 and cleaved IL-1β, but not IL-18. Collectively, these findings provide mechanistic insight into inflammasome activation by pathological α-synuclein fibrils.
Orally administered NLRP3 inhibitor MCC950 is active in the CNS and blocks inflammasome activation in multiple preclinical PD models
Having confirmed that the NLRP3 inflammasome is activated in both clinical and experimental PD, we evaluated the therapeutic potential of blocking NLRP3 activation using the potent small molecule NLRP3 inhibitor MCC950 (30). We confirmed that MCC950 blocked ATP- and nigericin-dependent NLRP3 activation in primary microglia, with an IC50 of 7.7 nM (Fig. 3A and fig. S6, A and B), similar to the potency reported for macrophages (30). Further, pre-treatment with 100 nM MCC950 completely blocked the release of active IL-1β p17, caspase-1 p20 and ASC by ATP and nigericin treatment in microglia (fig. S6 C to F). Nanomolar doses of MCC950 abrogated α-synuclein-mediated IL-1β release into the supernatant (Fig. 3B) as well as the release of cleaved caspase-1, cleaved IL-1β and ASC in western blots of supernatants (Fig. 3, C and D). MCC950 also effectively blocked ASC oligomerization (Fig. 3, E and F) induced by α-synuclein fibrils in microglia. Together, these results show that nanomolar concentrations of the small molecule NLRP3 inhibitor MCC950 are sufficient to block several pathological mediators of microglial inflammasome activation by α-synuclein aggregates. We next determined whether MCC950 could be effective in vivo to block CNS NLRP3 inflammasome activation in PD models. First, CNS pharmacokinetic studies were performed, which confirmed that oral dosing with 20 mg/kg was well tolerated and resulted in brain concentrations substantially above the IC50 for NLRP3 activation over a 24-hour period (Fig. 3G). Daily oral administration of MCC950 at 20 mg/kg blocked the generation of cleaved caspase-1 in the striatum of α-synuclein PFF-injected mice at 30 days (Fig. 3H). Oral dosing with MCC950 also blocked cleaved caspase-1 generation in MitoPark mice (Fig. 3I) and in the 6-OHDA model of PD (Fig. 3J). These results demonstrate that MCC950 is orally-active in the CNS and can effectively block nigrostriatal inflammasome activation in distinct PD models driven by both mitochondrial dysfunction and α-synuclein pathology.
Fig. 3. Oral dosing of the potent NLRP3 inhibitor MCC950 is active in the CNS and blocks inflammasome activation in multiple pre-clinical PD models.
(A) Dose-response curve for inhibition of ATP-induced NLRP3 inflammasome activation by MCC950 in LPS-primed microglia (n=3–4). (B) Fibrillar α-synuclein (α-Syn)-mediated microglial IL-1β secretion in the presence or absence of MCC950 (100 nM) (n=8–11). (C) Western blots and (D) densitometric analysis for cleaved caspase-1 (p20), cleaved IL-1β (p17) and ASC in the supernatants of α-synuclein-activated microglia co-treated with MCC950 (n=3). (E) Western blot and (F) densitometric quantification of oligomeric ASC (both dimers (D) and tetramers (T)) in microglia treated as indicated (n=3). (G) Pharmacokinetics of MCC950 over 24 h in plasma (ng/ml) or perfused mouse brain (ng/g) following oral gavage (20 mg/kg) (n=3–4 mice/time point). The equivalent microglial IC50 of MCC950 (~3 ng/ml) is indicated by the dashed line. (H-J) Western blot and densitometric quantification of cleaved caspase-1 in (H) ipsilateral striatal tissue lysates of α-synuclein PFF-injected mice at 30 days (n=6 mice/group); (I) substantia nigra tissue lysates of 12-week old MitoPark (MP) mice (n=5–7 mice/group); and (J) ipsilateral striatal tissue lysates of 6-OHDA (6-OH) lesioned mice at 7 days (n=9 mice/group). Data shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Bonferroni’s post-hoc test (panels F, H-J), or Kruskal-Wallis test with Dunn’s post-hoc test (panels B, D).
Daily oral dosing with MCC950 protects against nigrostriatal dopaminergic degeneration in experimental PD
Since MCC950 was orally bioavailable and crossed the blood brain barrier at therapeutically relevant concentrations, we next examined whether daily MCC950 dosing protects against neuropathology and dopaminergic degeneration. The unilateral 6-OHDA model induces a partial striatal lesion with progressive retrograde nigrostriatal pathology, and allows assessment based on behavioral and neurochemical parameters relevant to PD (31). At 21 days after 6-OHDA lesioning, MCC950-treated mice displayed a marked reduction in amphetamine-induced ipsilateral rotations (Fig. 4A, and movie S1). Mice that received MCC950 also displayed improved balance-beam performance compared to untreated 6-OHDA mice (Fig. 4B). Quantification of striatal dopamine and metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) confirmed that MCC950-treated mice were protected against the loss of dopamine that results from nigrostriatal degeneration and dopaminergic neuron loss in this model (Fig. 4, C to E). We also found higher striatal tyrosine hydroxylase (TH) expression in MCC950-treated mice (Fig. 4, F and G). The neuroprotective effect of oral MCC950 treatment was further confirmed by unbiased stereological counts of TH-positive dopaminergic neurons in the substantia nigra (Fig. 4, H and I), which demonstrated partial preservation of the nigrostriatal system in drug-treated mice. We also verified these findings using NLRP3 knockout mice, which showed substantially improved amphetamine-induced rotational behavior and prevented loss of dopamine and its metabolites DOPAC and HVA after 6-OHDA administration (Fig. 4, J to M). These results demonstrate that pharmacological inhibition of NLRP3 with an orally-active, CNS-permeable inhibitor can protect against dopaminergic degeneration in vivo in rodent models of PD, confirming a pathologic role for NLRP3 inflammasome activation in driving disease progression in mice.
Fig. 4. NLRP3 inhibition with oral MCC950 treatment protects against nigrostriatal dopaminergic degeneration and behavioral deficits in the 6-OHDA model of PD.
(A) Amphetamine-induced ipsilateral rotations quantified at 21 days in PBS-injected, 6-OHDA (6-OH)-injected, or 6-OHDA-injected mice treated with MCC950 (20mg/kg, daily oral gavage) (n=8–16 mice/group). (B) Balance beam footslips quantified 14 days after PBS or 6-OHDA injection (n=8–12 mice/group). (C-E) Striatal dopamine and its metabolites DOPAC and HVA 28 days after PBS or 6-OHDA injection (n=7–8 mice/group). (F) Striatal denervation in 6-OHDA mice shown with DAB immunohistochemistry for tyrosine hydroxylase (TH). Representative images are shown. (G) Western blot and quantitation for TH in the ipsilateral striatum of PBS or 6-OHDA injected mice at 28 days (n=3 mice/group). (H) Representative images and (I) stereological estimates for TH-positive substantia nigra dopaminergic neurons in MCC950-treated and untreated 6-OHDA mice (n=4 mice/group). (J) Amphetamine-induced ipsilateral rotations at 21-days after PBS or 6-OHDA injection in age-matched wildtype (WT) and NLRP3−/− mice (n=4/mice group). (K-M) Dopamine, DOPAC, and HVA in the ipsilateral striatum of wildtype and NLRP3−/− mice (n=6/group) 28-days after PBS or 6-OHDA injection. Data shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s (panel J, L) or Mann-Whitney (panel K, M) t-tests, or by one-way ANOVA with Bonferroni’s post-hoc test (panel A, B, D, G) or Kruskal-Wallis test with Dunn’s post-hoc test (panels C, E), or by two-way ANOVA with Bonferroni’s post-hoc test (panel I). Ns, not significant.
Chronic dosing with MCC950 protects against dopaminergic degeneration induced by pathological α-synuclein aggregates
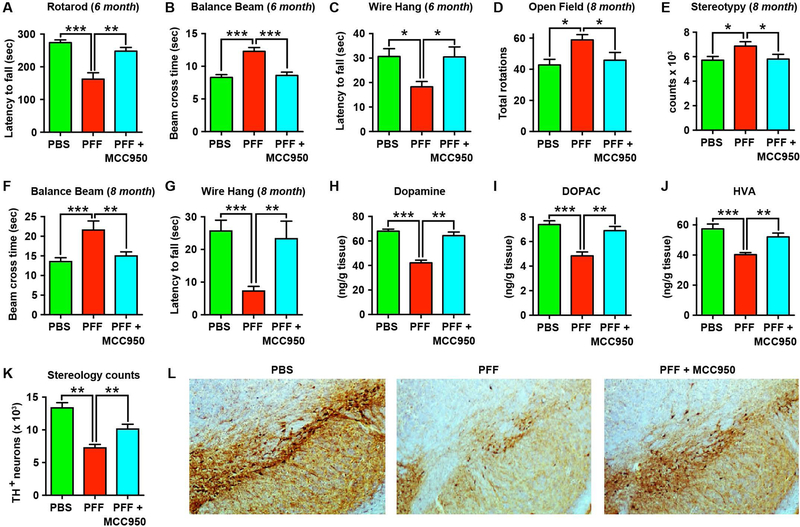
Having confirmed the neuroprotective efficacy of MCC950 in an acute neurotoxicant model in which pathology is driven by oxidative stress and mitochondrial dysfunction, we next evaluated the efficacy of MCC950 in a chronic progressive model of α-synuclein pathology. For these studies, we utilized the PFF model in which a single striatal injection of synthetic α-synuclein fibrils drives transmission of pathological misfolded α-synuclein resulting in Lewy body-like pathology in the interconnected nigrostriatal system (3). PFF mice develop progressive motor deficits, loss of striatal dopamine and degeneration of TH-positive dopaminergic neurons in the substantia nigra. For our studies, we used synthetic human α-synuclein fibrils prepared by in vitro aggregation and sonication as previously described (3). We first confirmed that drinking water administration of MCC950 could enter the brain, by treating mice (0.3 mg/ml) for 5 days and confirming presence of drug in perfused brain tissue (Table S1). Separate mice were then inoculated with 8 μg of pre-formed α-synuclein fibrils in the right dorsal striatum and were administered MCC950 in their drinking water. Control mice received an equivalent intra-striatal injection of PBS, the vehicle used for preparing α-synuclein fibrils. At six months, we performed a battery of standard motor function tests to evaluate Parkinsonian motor deficits in PFF mice. Mice undergoing chronic MCC950 treatment showed improved performance on the Rotarod (Fig. 5A). MCC950-treated PFF mice also displayed improved performance on the balance beam test, requiring less time to cross the beam compared to untreated PFF mice (Fig. 5B). Similar results were obtained with the wire-hang test (Fig. 5C) in which MCC950-treated PFF mice had latency times comparable to those of PBS-injected controls. We also used an automated open-field activity monitor to measure motor behavior parameters and subsequently quantified striatal dopamine and its metabolites at 8 months. In open-field assessments, we found increased rotational behavior in PFF mice compared to PBS-injected controls, which was reduced in MCC950-treated PFF mice (Fig. 5D). Similarly, stereotypic behavior was increased in PFF mice compared to PBS controls, which was reduced in the MCC950-treated PFF group (Fig. 5E). Other open-field parameters such as average velocity, total distance travelled, or time spent in the center, were not significantly different between any of the groups tested (P > 0.05; fig. S7, A to C), which is consistent with previous reports using this model (3). A separate cohort of PFF mice were also treated with MCC950 and motor deficits were examined at 8-months before neurostereology. Similar to the first cohort of mice, balance beam and wire-hang performances were found to be improved in MCC950-treated PFF mice at this 8-month termination point (Fig. 5, F and G, and movies S2 and S3).
Fig. 5. Chronic NLRP3 inhibition with oral MCC950 protects against motor deficits and dopaminergic degeneration in the α-synuclein PFF model of PD.
(A) Rotarod test in α-synuclein PFF-injected mice at 6 months following treatment with or without MCC950 in the drinking water (0.3 mg/ml) (n=15–17 mice/group). PBS-injected mice were used as controls. (B) Balance beam performance measured as time taken to cross the beam in PFF mice at 6 months (n=15–17 mice/group). (C) Wire-hang test in PFF mice (n=15–17 mice/group) at 6 months. (D, E) Open-Field activity at 8 months post PBS or PFF inoculation measuring rotational counts (D) and stereotypic behaviour (E) (n=10 mice/group). (F, G) Balance beam, and wire-hang tests on a separate cohort of PBS-injected control mice, or MCC950-treated and untreated PFF mice at 8 months (n=6–8 mice/group). (H-J) Dopamine, DOPAC, and HVA in the ipsilateral striatum of PFF mice at 8 months (n=8 mice/group). (K) Stereological estimates and (L) representative images for tyrosine hydroxylase (TH)-positive dopaminergic neurons in the substantia nigra of 8-month old PFF mice (n=5–6 mice/group). Data shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Bonferroni’s post-hoc test (panels B-E, G, K), or Kruskal-Wallis test with Dunn’s post-hoc test (panels A, F, H-J).
Previous studies using the PFF model have shown that there is a progressive loss of dopaminergic neurons in the substantia nigra, accompanied by a reduction in striatal dopamine on the injected side (3). Consistent with these reports, in untreated PFF mice we found a substantial reduction in striatal dopamine and dopamine metabolites at 8 months (Fig. 5H). In contrast, MCC950-treated PFF mice had significantly higher striatal dopamine concentrations (P < 0.01; Fig. 5H), indicating that they were protected against dopaminergic degeneration induced by α-synuclein pathology. MCC950-treated mice also had higher dopamine metabolites DOPAC (Fig 5I) and HVA (Fig. 5J) compared to the untreated PFF mice. Next, unbiased stereological counts of TH-positive dopaminergic neurons in the substantia nigra were performed. About 45% loss in TH-positive neurons was observed in PFF mice compared to PBS-injected control mice (Fig. 5, K and L), which is comparable to previous reports in this model (3). MCC950-treated PFF mice had significantly more surviving TH+ neurons than untreated PFF mice (P < 0.01; Fig. 5, K and L). Together, these results indicate that chronic MCC950 administration can protect mice against progressive motor deficits and dopaminergic degeneration induced by pathological fibrillar α-synuclein aggregates in vivo.
Pharmacological inhibition with MCC950 prevents α-synuclein pathology in the CNS
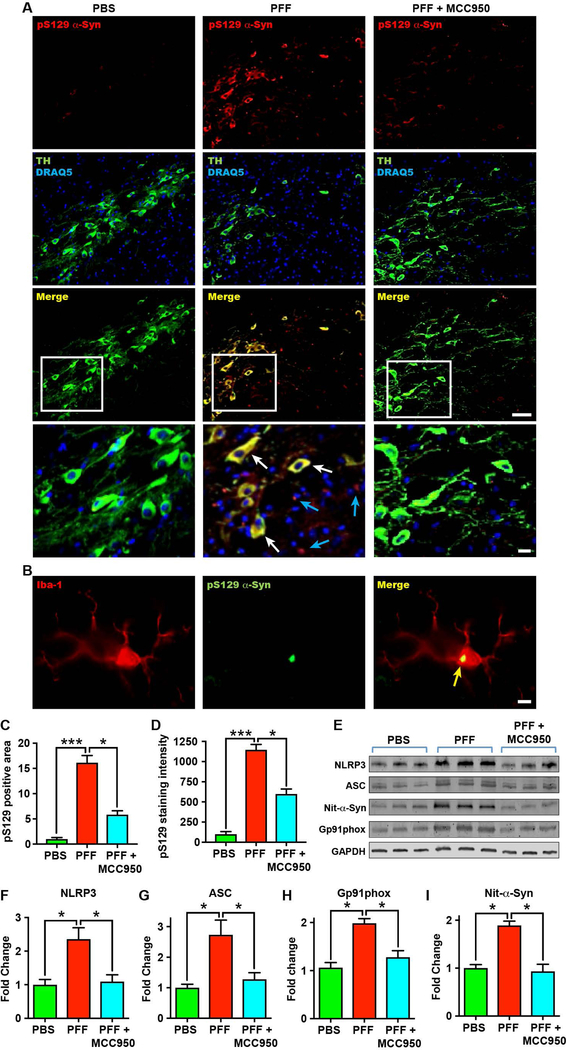
The propagation of α-synuclein pathology in the CNS is considered to be crucial to the disease process in clinical PD (2–5, 32). The transmission of Lewy body pathology seen in PD is recapitulated in the PFF model after the inoculation of synthetic α-synuclein fibrils in wildtype mice, and can be followed by quantifying the deposition of hyper-phosphorylated serine 129 (pS129) α-synuclein (3, 5, 32). We examined the transmission of pathological α-synuclein to regions outside of the striatum in our PFF mouse model, by evaluating the presence of hyper-phosphorylated α-synuclein in the substantia nigra and cortex at 8 months after PFF-injection. We detected pS129 α-synuclein both in the nigral tract and in the extra-nigral regions of PFF mice (fig. S8, A and B, and fig. S9, A and B). Most pS129-positive α-synuclein inclusions in the substantia nigra not only were localized to TH-positive dopaminergic neurons (Fig. 6A, and fig. S10, A to F), but were also found localized to Iba-1 stained microglia (Fig. 6B). Furthermore, pS129 α-synuclein co-localized with ubiquitin-positive aggregates (fig. S10, G to I), in line with previous findings in this model and in human PD (3). To evaluate whether inhibition of NLRP3 inflammasome activation could mitigate pathological hyper-phosphorylated α-synuclein accumulation, we examined the brains of MCC950-treated PFF mice. PFF mice that were chronically dosed with MCC950 had markedly fewer pS129-positive α-synuclein inclusions both within the nigrostriatal system (Fig. 6, A, C, and D, and fig. S8), abd in cortical brain regions (fig. S9) compared to untreated PFF mice. Total α-synuclein expression was not altered by MCC950-treatment (fig. S11). These findings therefore indicate that chronic NLRP3 activation contributes to the propagation of pS129 α-synuclein aggregates in the PFF mouse model in vivo, and that pharmacological NLRP3 inhibition with MCC950 can effectively mitigate this central pathological process.
Fig. 6. NLRP3 therapeutic inhibition with MCC950 ameliorates pathological α-synuclein accumulation in PFF mice.
(A) Representative immunohistochemistry images for phosphorylated serine 129 α-synuclein (pS129 α-Syn) (red), dopaminergic neurons labelled with tyrosine hydroxylase (TH, green), and nuclei stained with DRAQ5 (blue), in the substantia nigra. Images show PBS-injected mice, α-synuclein PFF-injected mice, and α-synuclein PFF-injected mice treated with MCC950 (drinking water, 0.3 mg/ml) for 8 months. Scale bar 30μm. Bottom panels indicate magnified section outlined by white box demonstrating pS129 α-Syn inclusions within dopaminergic neurons (white arrows – yellow merge), as well as not associated with dopaminergic neurons (blue arrows) in untreated PFF mice (scale bar 10μm). (B) Representative immunohistochemistry image for Iba-1 (microglia, red) and pS129 α-Syn (green) in PFF mice showing co-localization with microglia (yellow arrow) (scale bars 5μm). (C, D) Quantification of pS129 α-Syn-positive staining area (C) and staining intensity (D) in the substantia nigra region of stained sections of PFF mice after 8 months (n=7–8 mice/group). (E) Western blot and (F-I) quantitation for NLRP3, ASC, gp91phox and nitrosylated α-synuclein (Nit-α-Syn) in the substantia nigra of PFF mice after 8 months (n=4 mice/group). Data shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Kruskal-Wallis test with Dunn’s post-hoc test.
Because we previously found NLRP3 and ASC upregulation at the site of α-synuclein PFF infusion at early stages of this model, we also examined the substantia nigra from 8 month PFF mice and found increased NLRP3 and ASC expression (Fig. 6, E to G), suggesting that there is a concomitant inflammasome pathology in line with the propagation of hyperphosphorylated α-synuclein. In addition, mice treated with MCC950 had reduced NLRP3 and ASC expression (Fig. 6, E to G). We also examined other key markers associated with neuroinflammation and α-synuclein pathology in PD and found reduced amounts of substantia nigra gp91phox (NOX2) and nitrated α-synuclein in MCC950-treated mice (Fig. 6, H and I). These results indicate that chronic NLRP3 inflammasome activation can contribute to downstream mechanisms of α-synuclein pathology.
DISCUSSION
The pathological hallmarks of progressive neurodegenerative diseases are the persistent accumulation and spread of disease-specific misfolded protein aggregates such as α-synuclein-rich Lewy bodies in PD. Although pathological involvement of misfolded protein aggregates in driving neurodegeneration and the accompanying motor and/or cognitive deficits are now well recognized, the associated mechanisms remain unclear, making it difficult to design therapeutic approaches that are effective in vivo. Chronic activation of the NLRP3 inflammasome, which occurs in response to persistent misfolded proteins in the CNS, has emerged as a central neuroinflammatory mechanism that can drive neurodegeneration, making it an important therapeutic target (18–20). In PD, α-synuclein aggregates have been implicated as potential activators of the NLRP3 inflammasome (21), although direct in vivo evidence has been lacking. The clinical and experimental results outlined here suggest that the NLRP3 inflammasome might play a role in PD pathophysiology and progression. They also provide insight into the mechanisms of α-synuclein-mediated NLRP3 activation in mouse microglia that might be relevant to PD pathophysiology. In addition, our results demonstrate that in multiple rodent models of PD, pharmacological inhibition of the NLRP3 inflammasome using an orally active CNS-permeable drug, can protect against fibrillar α-synuclein pathology, and prevent nigrostriatal dopaminergic degeneration driven by Lewy-body like pathology, as well as independently by mitochondrial dysfunction and oxidative stress.
Our results provide compelling clinical evidence for inflammasome activation in postmortem PD brains, confirming that key inflammasome markers are elevated in the substantia nigra of PD patients. We have also confirmed that both NLRP3 and ASC localize to hypertrophic reactive microglia in PD patients. Our mechanistic studies with α-synuclein fibrils demonstrate that the kinetics and NLRP3 activation profile induced by α-synuclein fibrils in mouse microglia are markedly different from conventional activators such as ATP and nigericin, indicating that α-synuclein fibrils trigger a strong, but delayed, activation of the NLRP3 inflammasome. The delayed activation observed with α-synuclein is likely due to the requirement for phagocytosis of α-synuclein to trigger the intracellular NLRP3 sensor (19, 33). Furthermore, α-synuclein-mediated NLRP3 activation failed to elicit IL-18 production from primary mouse microglia even at 24 h, whereas NLRP3 activation with ATP resulted in IL-18 secretion within 1 h. Conventional NLRP3 activation typically results in cell lysis via caspase-1-dependent pyroptosis causing the release of stable polymeric ASC specks into the extracellular space. This, in turn, can drive further inflammasome activation and thereby propagate the inflammatory response (16, 26). We observed substantial extracellular ASC release following NLRP3 activation with α-synuclein fibrils. However, α-synuclein treatment did not induce detectible microglial pyroptosis even at 24 h, when the highest amounts of cleaved caspase-1, IL-1β and ASC are detectible in the supernatant. In contrast, activation with nigericin readily induced caspase-1 dependent pyroptosis in microglia within 1 h, similar to the macrophage response (16, 30). This suggests that the release of active IL-1β p17, caspase-1 p20 and ASC in the absence of pyroptosis is unique to NLRP3 activation with α-synuclein fibrils, and that microglia are not intrinsically resistant to pyroptosis. Collectively, these findings provide insight into the neuroinflammatory pathology of PD, with potential therapeutic relevance given the emerging paradigm whereby extracellular ASC propagates inflammatory responses in vivo and can increase the formation of amyloid-beta aggregates by acting as a cross-seed for misfolded proteins in the CNS (16, 20, 26). Our in vitro results using the NLPR3 inhibitor MCC950 demonstrate that nanomolar doses of drug can prevent ATP and nigericin-mediated NLRP3 activation in microglia. Pre-treatment with 100 nM of MCC950 almost completely blocked fibrillar α-synuclein-mediated NLRP3 activation and release of active IL-1β and caspase-1 p20. In addition, MCC950 also blocked ASC release from microglia following NLRP3 activation by α-synuclein fibrils. Given that extracellular ASC released by microglia has been shown to cross-seed amyloid protein aggregates and propagate neuropathology (20), our mechanistic findings suggest that inhibition with MCC950 might be effective in blocking this key downstream pathological process.
Although α-synuclein pathology is now considered central to the spread of disease pathophysiology in clinical PD, there is also strong clinical and experimental evidence for mitochondrial dysfunction, oxidative stress, and proteasomal impairment as key mechanisms that can initiate and drive dopaminergic neuron loss (2, 4, 34). For example, large-scale genome-wide association studies have shown that peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-responsive genes, which regulate mitochondrial biogenesis and cellular bioenergetics, are specifically down-regulated in PD patients (24). Similarly, the loss of functional mitochondrial TFAM signaling results in progressive dopaminergic degeneration (25). Our findings in the 6-OHDA and MitoPark models confirm that NLRP3 inflammasome activation in the nigrostriatal system can also occur in the absence of α-synuclein pathology. Oral MCC950 treatment effectively blocked NLRP3 activation in all PD models that we tested, irrespective of the distinct upstream triggers in these models. Furthermore, functional inhibition of NLRP3 with MCC950 resulted in substantial neuroprotection in the 6-OHDA model, demonstrating that NLRP3 is also a key driver of neuropathology triggered by the mitochondrial dysfunction and reactive oxygen species.
Although neurotoxicant models are useful for pre-clinical validation of new therapeutic agents, they do not recapitulate the relentless spread of α-synuclein-mediated Lewy body pathology that occurs in human PD and that is considered central to disease progression (35). Most disease-modifying PD therapeutics that have been developed and validated using neurotoxicant models have failed to show efficacy in the clinic, underscoring the importance of targeting α-synuclein-driven pathology in PD (35). Until recently, the lack of robust pre-clinical models that reproduced the clinical features of α-synuclein pathology and progressive dopaminergic degeneration has limited the development of effective therapeutic strategies (35). The PFF model recapitulates many of the cardinal features of Lewy body pathology in PD and has been used to model the mechanisms of α-synuclein pathology that drive dopaminergic degeneration (3, 32). Our results demonstrate that NLRP3 inflammasome activation occurs in the PFF model at early stages of neuropathology and support a crucial role for chronic microglial NLRP3-driven mechanisms in the propagation of fibrillar α-synuclein pathology (8). These findings are consistent with previous studies in transgenic AD mice lacking NLRP3 and ASC, which reported reduced amyloid-β deposition and enhanced microglial clearance of amyloid-β plaques (19, 20). Chronic NLRP3 inhibition with orally active MCC950 protected against motor deficits and nigrostriatal dopaminergic degeneration, and markedly reduced the amount of hyperphosphorylated α-synuclein aggregates both within, and outside of, the nigrostriatal system in PFF mice, further underscoring the importance of chronic NLRP3 activation in propagating α-synuclein pathology in this pre-clinical model.
The NLRP3 inhibitor used in our studies, MCC950 (originally referred to as CP-465773), comes from a class of sulfonylurea-containing compounds initially reported as inhibitors of IL-1β post-translational processing (36). Compared to other chemical classes reported to inhibit NLRP3 activation with micromolar potency, MCC950 and its analogs (37, 38) have nanomolar potency, with very high target selectivity. Data obtained from a U.S. Environmental Protection Agency ToxCast/Tox21 screen (39) indicate that MCC950/CP-465773 and a close chemical analog have extremely limited and very weak (> 10 μM) off-target activity (Table S2). Our data clearly demonstrate that the compound can effectively cross the blood-brain barrier, rendering it a good lead for medicinal chemistry optimization toward an optimized lead candidate suitable for CNS diseases. Although MCC950 itself cannot be commercialized because of previous lapsed patents, we have developed numerous analogs (37, 38) in novel chemical space with improved chemical properties (Table S3). These could lay the groundwork for future clinical translation of a therapy to treat neurodegenerative diseases.
There are important limitations and caveats with our study and findings. For example, our results do not establish the precise mechanisms by which sustained NLRP3 activation can drive α-synuclein pathology and dopaminergic degeneration. Recent reports have shown that activated inflammasome components such as prionoid ASC can directly interact with and seed misfolded proteins (20). Similarly, studies in NLRP3 knockout mice indicate that sustained inflammasome activation can impair the clearance of pathological protein aggregates, causing their accumulation (19). It is also plausible that secreted inflammasome mediators could be directly neurotoxic to dopaminergic neurons. Additional studies would be required to clarify these mechanisms. Another important consideration with our study is that MCC950 was administered before the induction of neuropathology in rodents, therefore treatment studies initiated after disease onset would be required to conclusively establish whether MCC950 can rescue or halt ongoing neuropathology, which would be more clinically relevant in late stage PD.
Current therapies for PD, including levodopa treatment and deep brain stimulation, can manage symptoms, but have little to no impact on the underlying disease pathology. Therefore, there is an urgent need to develop therapeutic strategies that halt or impede disease progression (1). Our results demonstrate that the NLRP3 inflammasome plays a key role in PD-like pathophysiology in rodents and might represent a feasible therapeutic target to mitigate neurotoxic α-synuclein pathology, and the resulting nigrostriatal dopaminergic neuron loss in PD. The potency and specificity of MCC950 for NLRP3 inhibition in the CNS, combined with its neuroprotective efficacy and safety profile after long-term oral dosing, make it an excellent lead for optimization of drug candidates and potential clinical translation in PD.
MATERIALS AND METHODS
Study design
Studies were primarily designed (i) to determine whether increased NLRP3 inflammasome activation occurs in PD patients and in distinct experimental PD models; and (ii) to test the efficacy and CNS penetration of the small molecule NLRP3 inhibitor, MCC950, in experimental models of α-synuclein pathology and dopaminergic degeneration relevant to PD. For in vivo studies, age-matched littermates were randomly assigned to drug or vehicle treatment groups. Animal numbers for each study type were determined by the investigators on the basis of previous experience with the standard disease models that were used, or from pilot studies. For in vitro studies with isolated primary microglia, experiments were performed with at least three replicates per study in addition to pilot optimization studies for dose and time-point determination. Mice were humanely euthanized at defined study endpoints and all experimental procedures were carried out in accordance with local institutional animal ethics approvals.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad). Normally distributed data were analyzed using a two-tailed Student’s t test when comparing two groups, or a one-way or two-way ANOVA with Bonferroni’s multiple comparison post-test when comparing more than two groups. For non-normally distributed data (Shapiro-Wilk test, P < 0.05), a nonparametric two-tailed Mann-Whitney U test was used when comparing two groups, or a Kruskal-Wallis test with Dunn’s multiple comparison post-test when assessing more than two groups. Differences with P < 0.05 were considered statistically significant. All data are presented as the mean ± Standard Error of the Mean (SEM) if not mentioned otherwise.
Supplementary Material
Movie S2. Representative balance-beam videos of PFF mice with, and without MCC950 treatment.
Movie. S1. Representative amphetamine-induced rotation videos of 6-OHDA mice with, and without MCC950 treatment.
Movie S3. Representative wire-hang videos of PFF mice with, and without MCC950 treatment.
Fig. S1. Inflammasome components are upregulated in PD patients.
Fig. S2. Inflammasome components are upregulated, and activation occurs via NLRP3 in the 6-OHDA mouse model of Parkinson’s disease.
Fig. S3. NLRP3 and ASC are upregulated in the MitoPark mouse model of PD.
Fig. S4. NLRP3 is expressed by microglia in the α-synuclein pre-formed fibril (PFF) mouse model.
Fig. S5. Validation of α-synuclein pre-formed fibrils (PFF).
Fig. S6. Validation of MCC950 as a potent inhibitor of NLRP3 inflammasome activation in microglia.
Fig. S7. Additional open-field activity measurements in PFF mice.
Fig. S8. Representative images of hyperphosphorylated α-synuclein staining in the substantia nigra of PFF mice.
Fig. S9. Representative images and quantitation of hyperphosphorylated α-synuclein staining in the cortex of PFF mice.
Fig. S10. Hyperphosphorylated α-synuclein is present within dopaminergic neurons, and is associated with ubiquitin in the PFF mouse model.
Fig. S11. Total α-synuclein is not altered in PFF mice treated with MCC950.
Table S1. Brain and plasma concentrations of MCC950 following 5-day drinking water administration (0.3 mg/mL) to mice.
Table S2. Off-target activity of MCC950 and a structural analogue from ToxCast/Tox21 datasets.
Table S3. Comparison of chemical and pharmacological properties of novel MCC950 analogues.
Table S4. Primary data (Excel file).
Acknowledgements
We thank Ruby Pelingon for experimental assistance, and the Centre for Microscopy & Microanalysis core facility at The University of Queensland.
Funding: Supported by the National Health and Medical Research Council of Australia (NHMRC, Project grant 1086786 to T.M.W., M.A.C., L.A.O., K.S., and A.A.B.R.), The Michael J. Fox Foundation for Parkinson’s Research and The Shake It Up Australia Foundation (grants 9916 and 12626 to T.M.W., M.A.C., K.S., R.G. and A.A.B.R.), and the National Institutes of Health (R01 grants NINDS NS100090, NIEHS ES026892, NIEHS NS088206 to A.G.K). T.M.W. is supported by a NHMRC Career Development Fellowship (1105420), R.G. is supported by an Advance Queensland Mid-Career Fellowship, and K.S. is supported by the Australian Research Council and the National Health and Medical Research Council of Australia (Fellowships FT130100361 and 1141131).
Footnotes
Competing interests: A.A.B.R., L.A.O., K.S., and M.A.C., are a co-inventors on patent applications for NLRP3 inhibitors which have been licensed to Inflazome Ltd, a biotechnology company that is developing drugs to address clinical unmet needs in inflammatory disease by targeting inflammasomes (WO/2016/131098 and WO/2017/140778, Sulfonylureas and related compounds and use of same). M.A.C. and L.A.O. are co-founders and shareholders of Inflazome, and are acting as CEO and CSO respectively. M.A.C also holds a fractional Professorial Research Fellow appointment at The University of Queensland. K.S. was a member of the Scientific Advisory Board for Inflazome Ltd from 2016 to 2017. The remaining authors declare no competing interests.
Data and materials availability: All the data are included in the main text or in the supplementary material.
REFERENCES AND NOTES
- 1.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE, Parkinson disease. Nat Rev Dis Primers 3, 17013 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ, Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16, 109–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM, Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G, Missing pieces in the Parkinson’s disease puzzle. Nat Med 16, 653–661 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V, alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM, Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong YC, Krainc D, alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ, In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21, 404–412 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P, Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202, 17–20 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M, Neuroimmunological processes in Parkinson’s disease and their relation to alpha-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro 5, 113–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soria FN, Engeln M, Martinez-Vicente M, Glangetas C, Lopez-Gonzalez MJ, Dovero S, Dehay B, Normand E, Vila M, Favereaux A, Georges F, Lo Bianco C, Bezard E, Fernagut PO, Glucocerebrosidase deficiency in dopaminergic neurons induces microglial activation without neurodegeneration. Hum Mol Genet 26, 2603–2615 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R, Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Koprich JB, Reske-Nielsen C, Mithal P, Isacson O, Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation 5, 8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM, Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28, 7687–7698 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vroon A, Drukarch B, Bol JG, Cras P, Breve JJ, Allan SM, Relton JK, Hoogland PV, Van Dam AM, Neuroinflammation in Parkinson’s patients and MPTP-treated mice is not restricted to the nigrostriatal system: microgliosis and differential expression of interleukin-1 receptors in the olfactory bulb. Exp Gerontol 42, 762–771 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P, The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15, 738–748 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Zhou R, Tschopp J, The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Walsh JG, Muruve DA, Power C, Inflammasomes in the CNS. Nat Rev Neurosci 15, 84–97 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT, NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT, Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552, 355–361 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M, Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One 8, e55375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G, MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener 11, 28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T, Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180, 147–150 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR, Global PDGEC, PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med 2, 52ra73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG, Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104, 1325–1330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmuller W, Latz E, The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 15, 727–737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tschopp J, Schroder K, NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10, 210–215 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, Ghetti B, Koller BH, LeBlanc AC, Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 22, 1676–1686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E, Rainone V, Nemni R, Mancuso R, Clerici M, The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener 11, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA, A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21, 248–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heuer A, Smith GA, Lelos MJ, Lane EL, Dunnett SB, Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: motor impairments identify extent of dopamine depletion at three different lesion sites. Behav Brain Res 228, 30–43 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM, Brundin P, Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213, 1759–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT, The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9, 857–865 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM, Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Beal MF, Parkinson’s disease: a model dilemma. Nature 466, S8–10 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Perregaux DG, McNiff P, Laliberte R, Hawryluk N, Peurano H, Stam E, Eggler J, Griffiths R, Dombroski MA, Gabel CA, Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J Pharmacol Exp Ther 299, 187–197 (2001). [PubMed] [Google Scholar]
- 37.O’Neill L, Coll R, Cooper M, Robertson A, Schroder K. (2016), vol. WO/2016/131098, chap. PCT/AU2016/050103.
- 38.O’Neill L, Coll R, Cooper M, Robertson A, Schroder K, Macleod AM, Miller DJ. (2017), vol. WO/2017/140778, chap. PCT/EP2017/053498.
- 39.Sipes NS, Martin MT, Kothiya P, Reif DM, Judson RS, Richard AM, Houck KA, Dix DJ, Kavlock RJ, Knudsen TB, Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem Res Toxicol 26, 878–895 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S2. Representative balance-beam videos of PFF mice with, and without MCC950 treatment.
Movie. S1. Representative amphetamine-induced rotation videos of 6-OHDA mice with, and without MCC950 treatment.
Movie S3. Representative wire-hang videos of PFF mice with, and without MCC950 treatment.
Fig. S1. Inflammasome components are upregulated in PD patients.
Fig. S2. Inflammasome components are upregulated, and activation occurs via NLRP3 in the 6-OHDA mouse model of Parkinson’s disease.
Fig. S3. NLRP3 and ASC are upregulated in the MitoPark mouse model of PD.
Fig. S4. NLRP3 is expressed by microglia in the α-synuclein pre-formed fibril (PFF) mouse model.
Fig. S5. Validation of α-synuclein pre-formed fibrils (PFF).
Fig. S6. Validation of MCC950 as a potent inhibitor of NLRP3 inflammasome activation in microglia.
Fig. S7. Additional open-field activity measurements in PFF mice.
Fig. S8. Representative images of hyperphosphorylated α-synuclein staining in the substantia nigra of PFF mice.
Fig. S9. Representative images and quantitation of hyperphosphorylated α-synuclein staining in the cortex of PFF mice.
Fig. S10. Hyperphosphorylated α-synuclein is present within dopaminergic neurons, and is associated with ubiquitin in the PFF mouse model.
Fig. S11. Total α-synuclein is not altered in PFF mice treated with MCC950.
Table S1. Brain and plasma concentrations of MCC950 following 5-day drinking water administration (0.3 mg/mL) to mice.
Table S2. Off-target activity of MCC950 and a structural analogue from ToxCast/Tox21 datasets.
Table S3. Comparison of chemical and pharmacological properties of novel MCC950 analogues.
Table S4. Primary data (Excel file).