Abstract
Mice homozygous for the Tyr208Asn amino acid substitution in the carboxy terminus of SHP-1 (referred to as Ptpn6spin mice) spontaneously develop a severe inflammatory disease resembling neutrophilic dermatosis in humans. Disease in Ptpn6spin mice is characterized by persistent footpad swelling and suppurative inflammation. Recently, in addition to IL-1α and IL-1R signaling, we demonstrated pivotal role for RIPK1, TAK1 and ASK1 in promoting inflammatory disease in Ptpn6spin mice. In the current study we have identified previously unknown role for CARD9 signaling as a critical regulator for Ptpn6spin-mediated footpad inflammation. Genetic deletion of CARD9 significantly rescued the Ptpn6spin-mediated footpad inflammation. Mechanistically, enhanced IL-1α mediated signaling in Ptpn6spin mice neutrophils was dampened in Ptpn6spinCard9−/− mice. Collectively, this study identifies SHP-1 and CARD9 crosstalk as a novel regulator of IL-1α driven inflammation and opens future avenues for finding novel drug targets to treat neutrophilic dermatosis in humans.
Keywords: IL-1α, SHP-1, CARD9, autoinflammation, macrophage, neutrophils, neutrophilic dermatosis
Introduction
The neutrophilic dermatoses encompass disorders, including Sweet’s Syndrome and Pyoderma Gangrenosum and subcorneal pustular dermatosis, that are characterized by neutrophilic infiltration in the tissues not associated with infection (1). Mice with a Tyr208Asn missense mutation in the Src homology region 2 (SH2) domain-containing phosphatase-1 (SHP-1) protein (encoded by Ptpn6) exhibit spontaneous skin lesions that are infiltrated with neutrophils and closely resemble neutrophilic dermatosis in humans (2–5). Ptpn6 is known for its role as a negative regulator of signal transduction in a variety of immune cell types (6). Furthermore, PTPN6 gene analyses from human patients with neutrophilic dermatosis such as Sweet’s syndrome and pyoderma gangrenosum have revealed the presence of PTPN6 splice variants or heterozygous mutations in affected individuals (3). Using Ptpn6spin mice as a model of inflammatory disease, we previously showed that IL-1α but not IL-1β, is a central cytokine that promotes neutrophilic footpad inflammation (4). Mechanistically, receptor interacting protein kinase (RIPK) 1 has been shown to regulate IL-1α expression independently of RIPK3, suggesting a role for RIPK1 and IL-1α signaling axis in driving footpad inflammation (4). Several studies including ours have demonstrated that hematopoietic or neutrophil specific blockade of Ptpn6 is sufficient to promote neutrophilic footpad inflammation (4, 7).
We have used a systematic genetic approach to delineate the molecular mechanisms and signaling pathways that are regulated by SHP-1 to modulate inflammation. Our recent studies demonstrated a central role for IL-1α, IL-1R, MyD88, tumor growth factor-β activated kinase 1 (TAK1), spleen tyrosine kinase (SYK), RIPK1 and Apoptosis signal-regulating kinase 1 (ASK1) in promoting inflammatory disease in Ptpn6spin mice, independent of Interferon-α/β receptor (IFNAR), Stimulator of interferon genes (STING), Integrin beta-3 (ITGB3) and NOD2-RIPK2 signaling (4, 8–10). The excessive inflammatory responses and persistent tissue damage in Ptpn6spin mice are driven by SYK and TAK1 mediated mitogen-activated protein kinases (MAPKs) and NF-κB signaling in hematopoietic cells (4, 8). SYK is upstream of Caspase-associated recruitment domain 9 (CARD9) and involved in signal transduction of a number of tyrosine kinase-coupled receptors including β2-integrins and various Fc-receptors (11, 12) thereby regulating NF-κB signaling (13, 14). Given that CARD9-mediated gene expression changes within neutrophils play important roles in non-infectious inflammation (15) and protects against fungal invasion of the central nervous system (16), we hypothesized that CARD9 plays a crucial role in provoking the inflammatory skin disease in Ptpn6spin mice.
Here, we show that CARD9 plays a critical role in instigating the Ptpn6spin mediated inflammation by regulating the hallmark inflammatory cytokines. Genetic deletion of CARD9 in Ptpn6spin mice significantly rescues the cutaneous inflammatory disease. Furthermore, CARD9 in neutrophils regulates Ptpn6spin-mediated disease through the control of NF-κB, ERK and p-38 downstream of IL-1R pathway, thereby controlling the production of pro-inflammatory cytokines. Taken together, these data show that in addition to its well-known role in triggering various antimicrobial functions, CARD9 plays a major role in regulating Ptpn6spin-mediated inflammation and disease.
Methods
Mice
Card9−/− (17) and Ptpn6spin (18) mice have been described previously. 6–10 weeks old male and female mice (littermates) were used in this study unless otherwise mentioned. All mice were kept in specific pathogen-free conditions within the Animal Resource Center at St. Jude Children’s Research Hospital. All the animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of St. Jude Children’s Research Hospital, Memphis, TN.
Neutrophil isolation and in vitro stimulation
Bone marrow cells were isolated from the femurs of mice and neutrophils (CD11b+ Gr-1+) were purified by fluorescence activated cell sorting (FACS) as described elsewhere (4, 10). Neutrophils (1 × 106 cells/ml) were stimulated with 10 ng/ml recombinant murine IL-1α (Gold Biotechnology) for indicated time periods. Supernatant and cell lysates were collected, and ELISA and immunoblotting were performed.
Histopathology
Formalin-preserved feet were processed and embedded in paraffin according to standard procedures. Sections (5 μm) were stained with hematoxylin and eosin (H&E). For immunohistochemistry, formalin-fixed paraffin-embedded tissues were cut into 4 μm sections and slides were stained with anti-Ly–6G to stain neutrophils in the footpads, and the images were acquired using light microscopy.
Immunoblot analysis
Footpad protein lysates were collected in RIPA lysis buffer supplemented with complete protease inhibitor cocktail (Roche) and PhosSTOP (Roche) using a tissue homogenizer. Samples were quantified using Pierce™ BCA Protein Assay Kit as per manufacturer’s instructions and 40 μg of protein was resolved by SDS–PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 5% non-fat milk and incubated overnight at 4 °C with primary antibodies. The membranes were then probed with horseradish peroxidase (HRP)-tagged secondary antibodies at room temperature for 1 h. Immunoreactive proteins were visualized using the Luminata™ Western HRP chemiluminescence substrate. Antibodies to the following proteins were used from Cell signaling Technology (CST): phospho-ERK1/2 (CST 9101), ERK1 (CST #9102), phospho-p38 (CST 9211), p38 (CST #9212), phospho-IκBα (CST #2859), IκBα (CST #9242) and actin was from Proteintech (#66009–1-IG).
In vivo cytokine levels
Footpad protein lysates were processed as described above and cytokines were measured by ELISA.
ELISA
Cytokine ELISAs were performed according to the manufacturer’s instructions (Milliplex).
Flow Cytometry
CD11b (M1/70; Invitrogen), Gr-1 (RB6–8C5), CD3ε (145–2C11), CD19 (1D3), CD4 (RM4–5/GK1.5), CD8 (53–6.7), Ly-6G (1A8) were purchased from BD Biosciences or Biolegend. Flow cytometry data were acquired on LSR Fortessa (BD Biosciences) and analyzed using Flowjo software (Tree Star).
Statistical Analysis
All results are presented as Mean ± SEM. Disease curves were analyzed by performing Log-rank (Mantel-Cox) testing, and significant differences between two groups were determined by performing Mann-Whitney test. Statistical analysis between multiple samples were performed using the two-way ANOVA or Student’s t-test. All the analysis was done using Graph pad prism software (version 7.0). Differences were considered statistically significant when p < 0.05. ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Deletion of CARD9 ameliorates cutaneous inflammatory disease in Ptpn6spin mice
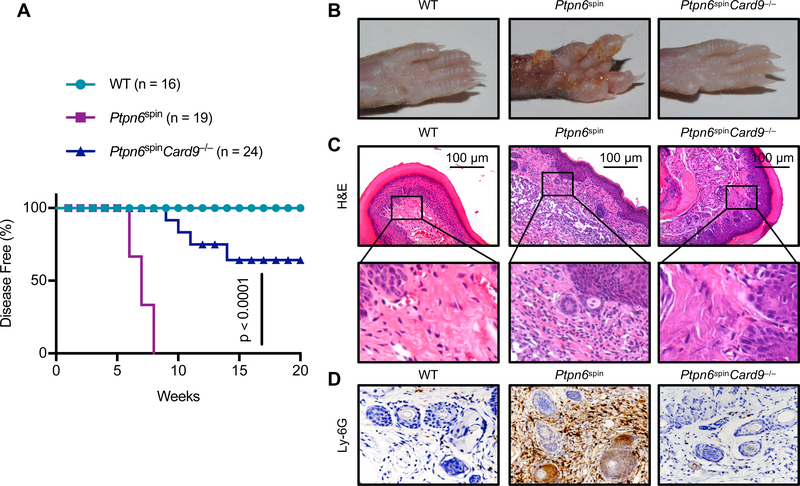
The role of CARD9 downstream of SYK in innate immune cells in response to fungal and bacterial infection has been well studied (16, 19, 20) but its role in causing inflammatory pathology in Ptpn6spin mediated neutrophil dermatosis is not known. Since Ptpn6fl/flSykfl/fl; LysM-Cre+ mice have showed protection from footpad inflammation as compared to Ptpn6fl/fl; LysM-Cre+ mice (8), we speculated that CARD9 might play a crucial role in exacerbating inflammatory surge in Ptpn6spin mice. To investigate the role of CARD9 in Ptpn6spin mediated pathology, we generated Ptpn6spinCard9−/− mice by crossing Card9−/− mice with Ptpn6spin mice. Consistent with our previous findings (10, 18, 21), Ptpn6spin mice spontaneously developed footpad inflammation between 6–10 weeks. Interestingly, Ptpn6spinCard9−/− mice demonstrated significant protection from the disease progression as compared to Ptpn6spin mice and approximately 60% of the Ptpn6spinCard9−/− mice remained disease free at the experimental end point of 20 weeks with no evident signs of footpad swelling (Fig. 1A, 1B). These data suggest that deletion of CARD9 is sufficient to provide significant protection in Ptpn6spin mice. Histological analysis confirmed that the extent of inflammation and lesions were attenuated in Ptpn6spinCard9−/− mice when compared to Ptpn6spin mice (Fig. 1C). Since this inflammation is characterized by heavy infiltration of neutrophils, we further analyzed the footpad sections by neutrophil specific immunostaining. Specific staining for neutrophils showed severe neutrophil infiltration in Ptpn6spin mice which was completely rescued in the Ptpn6spinCard9−/− mice (Fig. 1D). These findings establish CARD9 as a critical regulator of neutrophilic inflammation in the Ptpn6spin mice.
Fig. 1. Deletion of CARD9 ameliorates cutaneous inflammatory disease in Ptpn6spin mice.
(A) WT (n = 16), Ptpn6spin (n = 19) and Ptpn6spinCard9−/− (n = 24) crosses were monitored for disease progression. (B) Footpad images (C) H&E staining and (D) immunohistochemistry staining of neutrophils (Ly-6G) of WT, Ptpn6spin, and Ptpn6spinCard9−/− mice. Disease curves in (A) were analyzed by Log-rank (Mantel-Cox) test.
CARD9 deletion attenuates massive infiltration of myeloid cells observed in mouse models of neutrophilic dermatosis
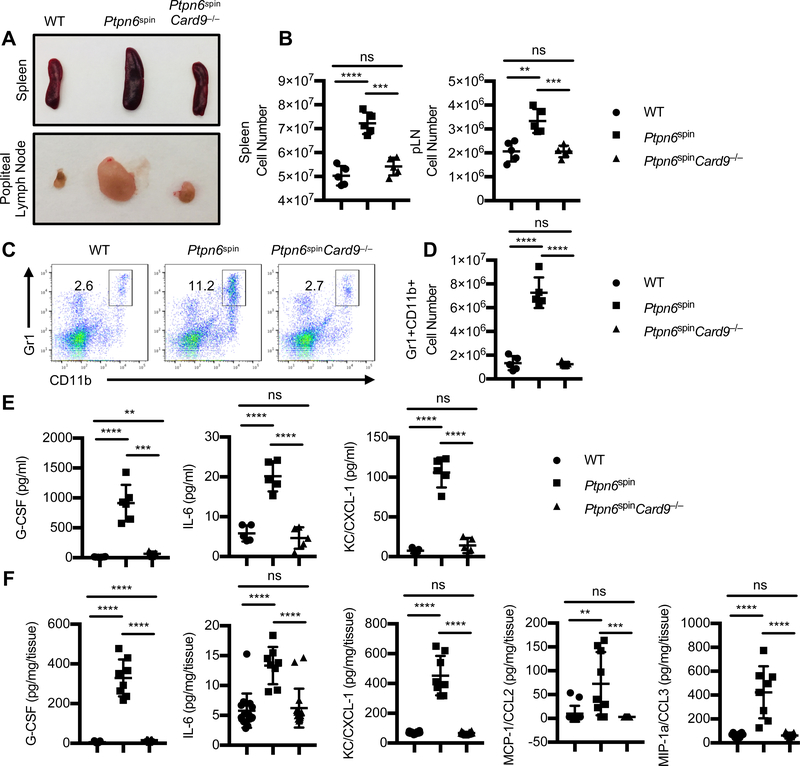
The neutrophilic infiltration and footpad swelling is often characterized by enlargement of spleen and popliteal lymph nodes that drain the inflamed feet (4, 8, 10). The enlarged spleen and popliteal lymph nodes observed in Ptpn6spin mice were completely reversed in the Ptpn6spinCard9−/− mutant mice (Fig. 2A). In concurrence with the rescue of spleen and popliteal lymph node sizes, the cell numbers in the spleen and popliteal lymph nodes of Ptpn6spinCard9−/− mutant mice were significantly reduced than in Ptpn6spin mice (Fig. 2B). Flow cytometric analysis of spleen (Supplemental Fig. 1A–1F) and popliteal lymph nodes (Supplemental Fig. 1G–1J) did not show any significant difference in total B and T cell numbers between WT and Ptpn6spin mice. Interestingly, splenic B cell numbers were marginally increased in Ptpn6spinCard9−/− mice as compared to Ptpn6spin mice. Furthermore, percentages of Gr1+CD11b+ neutrophils in the spleen of Ptpn6spinCard9−/− mice tend to be comparable to that in WT mice and were significantly lower than in Ptpn6spin mice (Fig. 2C, 2D), indicating that CARD9-dependent increase in the numbers of infiltrating and circulating myeloid cells is responsible for causing this suppurative inflammation in the footpads of Ptpn6spin mice.
Fig. 2. Deletion of CARD9 limits hyperactivation of SYK-mediated signaling in Ptpn6spin mice.
(A and B) Representative images and cell numbers of spleen and popliteal lymph node tissues, in WT (n = 7), Ptpn6spin (n = 7) and Ptpn6spinCard9−/− (n = 7) mice. (C) Flow cytometry analysis of Gr-1+CD11b+ neutrophil population in spleen of WT, Ptpn6spin and Ptpn6spinCard9−/− mice. (D) Total cell number of Gr-1+CD11b+ neutrophils in spleen of WT, Ptpn6spin and Ptpn6spinCard9−/− mice. (E) Serum concentration of cytokines (G-CSF, IL-6) and chemokine (KC/CXCL-1) were measured by ELISA from WT (n = 6), Ptpn6spin (n = 6) and Ptpn6spinCard9−/− (n = 6) mice. (F) Footpads from WT (n = 11), Ptpn6spin (n = 10) and Ptpn6spinCard9−/− (n = 11) mice were homogenized and concentration of cytokines (G-CSF, IL-6) and chemokine (KC/CXCL-1, CCL2 and CCL3) were measured by ELISA in the quantified lysates. Each point represents an individual mouse, and the line represents Mean ± SEM. Two-way ANOVA was used to determine the significance between the two groups analyzed. ns not significant, **p < 0.01, ***p < 0.001, ****p < 0.0001.
We next investigated the cytokine and chemokine concentrations in the serum and footpads of these mice. The levels of G-CSF, IL-6 and CXCL1/KC were significantly reduced in the serum and footpads from Ptpn6spinCard9−/− mice when compared to Ptpn6spin mice (Fig. 2E, 2F). In addition to chemoattractant CXCL-1, the levels of CCL2 (MCP-1) and CCL3 (MIP-1α) were significantly reduced in the footpads from Ptpn6spinCard9−/− mice when compared to Ptpn6spin mice (Fig. 2F). These results indicate that CARD9 plays a crucial role in Ptpn6spin mediated inflammation by regulating aberrant production of pro-inflammatory cytokines and chemokines.
CARD9 in neutrophils regulate IL-1α-mediated inflammatory signaling in Ptpn6spin mutant mice
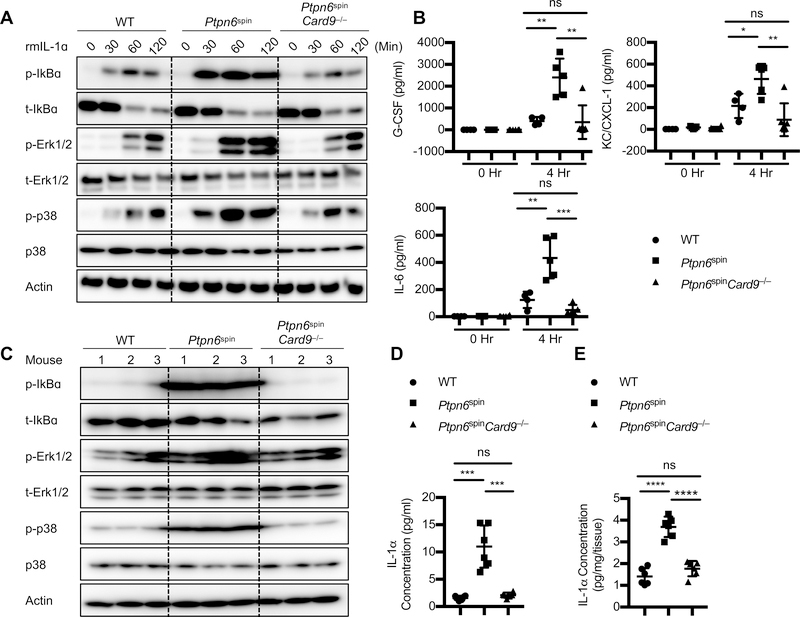
We had previously generated Ptpn6spinIl1α−/− DKO mice and shown that IL-1α regulates inflammatory disease and wound healing responses in Ptpn6spin mice (4, 8). Since Ptpn6spinCard9−/− mice showed significant protection from the Ptpn6spin mediated inflammatory disease, we speculated the involvement of CARD9 in IL-1α mediated signaling. In order to delineate the underlying mechanism, we stimulated neutrophils from WT, Ptpn6spin and Ptpn6spinCard9−/− mice with recombinant murine IL-1α. Stimulation of neutrophils with IL-1α led to increased MAPK and NF-κB signaling in Ptpn6spin mice that was significantly dampened in the Ptpn6spinCard9−/− mice (Fig. 3A). Consistent with increased MAPK and NF-κB signaling, cytokine and chemokine levels were also increased in Ptpn6spin neutrophils stimulated with IL-1α (Fig. 3B). These levels were significantly reduced in the Ptpn6spinCard9−/− neutrophils (Fig. 3B). These results suggest that IL-1α can signal through the SYK-CARD9 axis to instigate inflammatory cascade in the Ptpn6spin mice.
Fig. 3. CARD9 in neutrophils regulates IL-1α-mediated inflammatory signaling in Ptpn6spin mutant mice.
(A) Neutrophils from bone marrow of respective mice strains were stimulated with recombinant mouse IL-1α (10 ng/ml) for the indicated time period. Whole cell lysates were prepared and protein expression of p-IκBα, IκBα, p-ERK1/2, ERK1/2, p-p38, and p38 were determined by western blotting. β-actin was used as an internal control. (B) Cytokines (G-CSF, IL-6) and chemokine (KC/CXCL-1) concentrations were measured by ELISA in the supernatants of neutrophils stimulated with IL-1α for indicated time period. (C) Footpads were harvested from respective mice strains. Lysates were prepared and protein expression of p-IκBα, IκBα, p-ERK1/2, ERK1/2, pp38, and p38 were determined by western blotting. β-actin was used as an internal control. (D and E) IL-1α concentration was measured in (D) serum and (E) footpads of WT (n = 5), Ptpn6spin (n = 7) and Ptpn6spinCard9−/− (n = 5) mice by ELISA. Each point represents an individual mouse, and the line represents Mean ± SEM. Two-way ANOVA was used to determine the significance between the two groups analyzed. ns not significant, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Since CARD9 has been shown to be involved in regulating activation of MAPK and NF-κB signaling downstream of TLRs and CLRs (11, 14, 19), we hypothesized that the targeted MAP kinase and NF-κB signaling drives Ptpn6spin associated inflammation. In agreement, we found that in vivo CARD9 deletion markedly dampened local activation of MAPK and NF-κB signaling in the footpads from Ptpn6spinCard9−/− mutant mice (Fig. 3C). In order to confirm whether IL-1α is being produced at the site of inflammation, we measured the levels of IL-1α in the serum and footpads of these mice. Consistent with the above findings, IL-1α levels were increased in the Ptpn6spin mice that was significantly dampened in the Ptpn6spinCard9−/− mice (Fig. 3D, 3E). Taken together, these results are consistent with the hypothesis that CARD9 plays a critical role in promoting inflammation and disease in a mouse model of neutrophilic dermatoses.
Discussion
Polymorphisms in the human PTPN6 gene are associated with a wide spectrum of autoinflammatory diseases (5, 22, 23). Therefore, understanding the basic biology and molecular mechanisms of these inflammatory disorders is crucial for developing improved treatment options. Using Ptpn6spin as a well-established mouse model for neutrophilic dermatosis, we have identified several novel checkpoint regulators that drive this inflammatory disease.
We have used a genetic approach to dissect the molecular mechanisms and signaling pathways that are regulated by SHP-1 to modulate inflammation. We previously demonstrated critical roles for IL-1α, IL-1R, MyD88, SYK, RIPK1, TAK1 and ASK1 in promoting inflammatory disease in Ptpn6spin mice (mice with reduced SHP-1 phosphatase activity) (8, 10). Importantly, in addition to demonstrating a central role for these molecules in the IL-1R signaling pathway, we also excluded the role of several other signaling pathways. Our studies showed that the disease in Ptpn6spin mice is independent of IFNAR, STING (involved in DNA sensing pathway), ITGB3 and NOD2-RIPK2 signaling axis (8). These studies, although negative, are critical in reinforcing the specific role of IL-1α signaling axis in provoking inflammatory disease in Ptpn6spin mice.
Furthermore, SYK represents a common point in the signaling pathways of Dectin-1, Dectin-2 and TLRs (24, 25). Downstream of SYK activation, an adapter protein known as CARD9 forms a trimolecular complex with BCL10 and MALT1, which is required for signaling from these receptors (26). The steps linking SYK and CARD9 downstream of IL-1α are currently undefined. This study opens avenues to further explore these pathways to study inflammation and auto-immune disorders independent of infection.
In conclusion, we have shown that IL-1α is an apical cytokine that regulates production of G-CSF, IL-6 and CXCL-1/KC to drive inflammatory disease in Ptpn6spin mice. Our results highlight a critical role for CARD9-mediated signaling in neutrophils in driving an inflammatory circuit that triggers excessive inflammatory response and persistent tissue damage in a mouse model of neutrophilic dermatosis. We have demonstrated that CARD9 signaling plays an important role in instigating the inflammatory disease in Ptpn6spin mice, as its absence resulted in the significant resolution of inflammation. The lack of CARD9 in Ptpn6spin neutrophils, led to a marked downregulation of MAPK and NF-κB signaling cascades resulting in a dramatic decrease in the inflammatory cytokine production. In summary, our study defines a previously undescribed role for CARD9 in promoting inflammation and disease in a mouse model of neutrophilic dermatoses. Consequently, inhibition of CARD9 by blocking its activity may provide a novel approach to design effective therapeutic strategies to treat inflammatory skin disorders.
Supplementary Material
Acknowledgments
We would like to thank Dr. Xin Lin for Card9−/− mice and Dr. Bruce Beutler for Ptpn6 mutant mice. We also thank members of Kanneganti lab for their comments and suggestions.
Grant: This work was supported by the National Institutes of Health Grants CA163507, AR056296, AI124346, and AI101935 and the American Lebanese Syrian Associated Charities (ALSAC) to T.-D.K.
Abbreviations
- SHP-1
Src homology region 2 (SH2) domain-containing phosphatase-1
- CARD9
Caspase-associated recruitment domain 9
- SYK
Spleen tyrosine kinase
- MAPK
mitogen-activated protein kinase
- RIPK1
Receptor interacting protein kinase 1
- TAK1
Tumor growth factor-β activated kinase 1
References
- 1.Lear JT, Atherton MT, and Byrne JP. 1997. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med J 73: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nesterovitch AB, Szanto S, Gonda A, Bardos T, Kis-Toth K, Adarichev VA, Olasz K, Ghassemi-Najad S, Hoffman MD, Tharp MD, Mikecz K, and Glant TT. 2011. Spontaneous insertion of a b2 element in the ptpn6 gene drives a systemic autoinflammatory disease in mice resembling neutrophilic dermatosis in humans. Am J Pathol 178: 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesterovitch AB, Gyorfy Z, Hoffman MD, Moore EC, Elbuluk N, Tryniszewska B, Rauch TA, Simon M, Kang S, Fisher GJ, Mikecz K, Tharp MD, and Glant TT. 2011. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am J Pathol 178: 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, and Kanneganti TD. 2013. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature 498: 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao H, and Hegele RA. 2002. Identification of polymorphisms in the human SHP1 gene. J Hum Genet 47: 445–447. [DOI] [PubMed] [Google Scholar]
- 6.Pao LI, Badour K, Siminovitch KA, and Neel BG. 2007. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol 25: 473–523. [DOI] [PubMed] [Google Scholar]
- 7.Abram CL, Roberge GL, Pao LI, Neel BG, and Lowell CA. 2013. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 38: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurung P, Fan G, Lukens JR, Vogel P, Tonks NK, and Kanneganti TD. 2017. Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1alpha-Mediated Inflammatory Disease. Immunity 46: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurung P, and Kanneganti TD. 2016. Autoinflammatory Skin Disorders: The Inflammasomme in Focus. Trends Mol Med 22: 545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tartey S, Gurung P, Dasari TK, Burton A, and Kanneganti TD. 2018. ASK1/2 signaling promotes inflammation in a mouse model of neutrophilic dermatosis. J Clin Invest 128: 2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocsai A, Zhou M, Meng F, Tybulewicz VL, and Lowell CA. 2002. Syk is required for integrin signaling in neutrophils. Immunity 16: 547–558. [DOI] [PubMed] [Google Scholar]
- 12.Mocsai A, Zhang H, Jakus Z, Kitaura J, Kawakami T, and Lowell CA. 2003. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood 101: 4155–4163. [DOI] [PubMed] [Google Scholar]
- 13.Zhong X, Chen B, Yang L, and Yang Z. 2018. Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth S, and Ruland J. 2013. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol 34: 243–250. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth T, Futosi K, Sitaru C, Ruland J, and Mocsai A. 2016. Neutrophil-specific deletion of the CARD9 gene expression regulator suppresses autoantibody-induced inflammation in vivo. Nat Commun 7: 11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EM, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, and Lionakis MS. 2015. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog 11: e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, and Lin X. 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol 8: 198–205. [DOI] [PubMed] [Google Scholar]
- 18.Croker BA, Lawson BR, Rutschmann S, Berger M, Eidenschenk C, Blasius AL, Moresco EM, Sovath S, Cengia L, Shultz LD, Theofilopoulos AN, Pettersson S, and Beutler BA. 2008. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc Natl Acad Sci U S A 105: 15028–15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond RA, Saijo S, Iwakura Y, and Brown GD. 2011. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol 41: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhang R, Wu W, Song Y, Wan Z, Han W, and Li R. 2018. Impaired Specific Antifungal Immunity in CARD9-Deficient Patients with Phaeohyphomycosis. J Invest Dermatol 138: 607–617. [DOI] [PubMed] [Google Scholar]
- 21.Lukens JR, and Kanneganti TD. 2014. SHP-1 and IL-1alpha conspire to provoke neutrophilic dermatoses. Rare Dis 2: e27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christophi GP, Hudson CA, Gruber R, Christophi CP, and Massa PT. 2008. Promoter-specific induction of the phosphatase SHP-1 by viral infection and cytokines in CNS glia. J Neurochem 105: 2511–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christophi GP, Hudson CA, Gruber RC, Christophi CP, Mihai C, Mejico LJ, Jubelt B, and Massa PT. 2008. SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients. Lab Invest 88: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, and Sousa C. Reis e. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206: 2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, and Geijtenbeek TB. 2009. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol 10: 203–213. [DOI] [PubMed] [Google Scholar]
- 26.Bertin J, Guo Y, Wang L, Srinivasula SM, Jacobson MD, Poyet JL, Merriam S, Du MQ, Dyer MJ, Robison KE, DiStefano PS, and Alnemri ES. 2000. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem 275: 41082–41086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.