Abstract
Background:
Advanced platelet-rich fibrin (A-PRF) is an autogenous blood product with applications in dento-alveolar surgery. However, there is minimal information regarding its optimal clinical application or efficacy. The aim of this multi-arm parallel randomized controlled clinical trial was to evaluate the efficacy of A-PRF alone or with freeze-dried bone allograft (FDBA) in improving vital bone formation and alveolar dimensional stability during ridge preservation.
Methods:
Forty patients requiring extraction of non-molar teeth and replacement with dental implants were randomized into one of four ridge preservation approaches: A-PRF, A-PRF+FDBA, FDBA, or blood clot. A-PRF was prepared at 1,300 rpm for 8 minutes. Non-traumatic extractions and ridge preservation was performed. After an average of 15 weeks healing, bone core samples were harvested at the time of implant placement for micro-CT and histomorphometric analysis. Ridge dimensions were measured immediately after extraction and before implant placement.
Results:
Significantly greater loss of ridge height was noted in the blood clot group (3.8 ± 2.0 mm) compared to A-PRF (1.8 ± 2.1 mm) and A-PRF+FDBA (1.0 ± 2.3 mm) groups (P < 0.05). No significant differences in ridge width reduction were noted between groups. Significantly more vital bone was present in the A-PRF group (46% ± 18%) compared to the FDBA group (29% ± 14%) (P < 0.05). Bone mineral density was significantly greater in the FDBA group (551 ± 58 mg/cm3) compared to blood clot (487 ± 64 mg/cm3) (P < 0.05).
Conclusions:
This study demonstrates A-PRF alone or augmented with FDBA is a suitable biomaterial for ridge preservation. This study represents the first randomized controlled clinical trial comparing A-PRF with and without FDBA to FDBA alone for ridge preservation.
Keywords: biomaterials, bone regeneration, clinical trial, ridge preservation
1 |. INTRODUCTION
The use of dental implants has become a common treatment modality for the rehabilitation of the edentulous segments of the dentition.1 Since its first introduction, dental implantology has continued to evolve to meet the functional and esthetic demands of patients.1,2 One clinical challenge is in areas with inadequate alveolar bone volume to support implant placement after dental extraction. To overcome this challenge many surgical techniques and materials have been developed to preserve or regenerate tissues in order to provide an optimal site to place an implant of adequate size in the ideal position.3,4
The goal of alveolar ridge preservation is to minimize the reduction of the dimensions of the alveolus following tooth extraction by immediate placement of a biomaterial within the extraction socket5. Materials available for this purpose generally consist of matrix scaffolding materials and/or biologic agents. Matrix scaffolding materials are typically osteoconductive and are able to provide cell scaffolding and dimensional stability of the wound through space maintenance.6 These materials can be derived from allogeneic, xenogeneic, synthetic, or autogenic sources.4 Biologic agents are molecular mediators with typical osteoinductive properties to promote de novo bone formation.7 Matrix scaffolding materials and biologic agents can be used separately or together to achieve the desired surgical outcome.7,8 Of the available biomaterials, platelet-rich fibrin (PRF) has become increasingly popular since its first introduction in 2001.9 PRF is a platelet concentrate made of an autologous bioscaffold of a dense fibrin matrix with naturally integrated growth factors which are released from the scaffold over a sustained period to promote healing of hard and soft tissues.10–12 PRF has been shown to be a source of transforming growth factor β−1 (TGFβ−1), vascular endothelial growth factor (VEGF), and platelet derived growth factor (PDGF).11–13 These growth factors are bound within the fibrin matrix, resulting in a slow, sustained release through the natural maturation and reorganization of the clot.11,12,14 More recent modifications of the PRF preparation procedure have led to the development of advanced platelet rich fibrin (A-PRF) which uses lower G-forces to obtain higher growth factor release compared to PRF.15,16 There are several potential clinical applications of PRF, but to date the clinical outcomes of these applications have been evaluated with heterogeneous clinical approaches and inconsistent results.17
Therefore, the objective of this study was to conduct the first randomized controlled clinical trial evaluating four different ridge preservation approaches including A-PRF alone, or in combination with freeze-dried bone allograft (FDBA), FDBA alone, or no graft (blood clot). Evaluation of the ridge preservation approaches consisted of clinical measurement of the changes in alveolar dimensions (primary outcome) and histomorphometric and micro-CT analysis of bone core biopsies (secondary and tertiary outcomes, respectively) taken approximately four months after extraction at the time of implant placement.
2 |. MATERIALS AND METHODS
2.1 |. Patient population and enrollment
The Institutional Review Board at the University of California San Francisco approved the study design for this randomized controlled clinical trial, and the study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The study is registered with Clinicaltrials.gov (NCT03043885). Participants were recruited from the Dental Center at the University of California San Francisco between December 2014 and May 2016. All patients were screened for inclusion and exclusion criteria, and eligible patients were enrolled into the study after written consent was obtained. Patient inclusion criteria was based on having a single-rooted tooth requiring extraction and replacement with a dental implant supported restoration. In addition, all extracted single-rooted teeth were required to have a root position that was consistent with planned implant placement, and adequate space for a satisfactory implant restoration. Teeth were excluded if they demonstrated a buccal dehiscence of more than 25% of the length of the tooth or presence of acute infection of endodontic origin. Further exclusion criteria for enrollment included: patients that exhibited poor oral hygiene, pregnant woman or patients who intend to become pregnant, those who used tobacco, a medical condition that would be a contraindication to dental surgery or could alter healing such as an autoimmune disorder, immunosuppression, or uncontrolled diabetes.
Enrolled participants were randomized via a random sequence generator into one of four ridge preservation treatment protocols in a multi-arm parallel study design. The randomized sequence was concealed from the study clinicians enrolling and treating the patients, and assignments were revealed to the clinician on the day of treatment. The four treatment groups included A-PRF alone (A-PRF), A-PRF+FDBA, FDBA alone (FDBA), and blood clot alone (blood clot).
2.2 |. Ridge measurements
A measurement stent was created for each patient out of light cured resin* from an alginate impression. The stent was stably seated on the occlusal surfaces of multiple adjacent teeth to the extraction site. This measurement stent allowed for reproducible measurements of the alveolar ridge to be taken at two time points, immediately following extraction and before implant placement. Alveolar crest height was measured with a periodontal probe† from the stent to the mid-buccal wall crest. An indentation was made on the stent to guide the same position and angulation of the probe to be used at the second measurement time point. Alveolar width was measured with calipers‡ at the apical, middle, and coronal thirds of the ridge at the extraction site. To allow for reproducible width measurements at the second time point, demarcations were made on flanges that extended from the stent towards the apex of the tooth at the buccal and lingual aspects to indicate where the width measurements were initially made. All measurements using the periodontal probe and calipers were sounded to bone to avoid the confounding variables of tissue thickness. Ridge measurements were recorded by a single blinded examiner at two time points; immediately after extraction and immediately before implant placement.
2.3 |. Surgical protocol
Extractions and ridge preservation procedures were completed by three different residents at the UCSF Postgraduate Periodontology clinic. All patients were administered 600 mg ibuprofen and rinsed with chlorhexidine gluconate 0.12% mouth rinse* at the beginning of the surgical appointment. Local anesthetic was administered at the site. Non-traumatic tooth extraction was completed without the elevation of a mucoperiosteal flap. The socket was thoroughly curetted, irrigated with sterile saline, and inspected for the presence of perforations, fenestration, or dehiscence.
Patients were randomized into the A-PRF, A-PRF+FDBA, FDBA, or blood clot ridge preservation groups. For patients enrolled in the A-PRF or A-PRF+FDBA group, A-PRF was prepared according to the following protocol.16 Venous blood was collected via venipuncture of the forearm with a butterfly needle into a 10 mL sterile glass vacuum tube.† The blood sample was immediately centrifuged at 1,300 rpm (200 x g) for 8 minutes. The A-PRF clot was separated from the three distinct layers that had formed within the tube.
2.4 |. A-PRF treatment group
The entire A-PRF clot was removed from the tube and placed into the socket. Gauze were used to compress the clot lightly within the socket to be even with the level of the bony crest. If the A-PRF required modification to fit within the socket, the end of the A-PRF clot closest to the top of the tube, away from the red blood cell layer, was trimmed off. A resorbable collagen dressing‡ was placed over the socket, filling the space from the bony crest to the gingival margin. Vicryl sutures§ (4/0) were placed across the socket and cyanoacrylate¶ was applied to seal the margins.
2.5 |. A-PRF+FDBA treatment group
The A-PRF clot was cut into small pieces and freeze-dried bone allograft# was added to achieve a final volume with at 1:1 ratio of graft particulate to A-PRF. This was approximately 0.5 cc FDBA to a full A-PRF clot obtained from a single tube. The mixture was added to the socket up to the bony crest with light compression. The collagen dressing was applied in the same manner as described above.
2.6 |. FDBA treatment group
The same FDBA as used above was hydrated with sterile saline and added to the socket with light compression up to the bony crest. The collagen dressing was applied in the same manner as described above.
2.7 |. Blood clot treatment group
Further curettage of the socket walls was performed to allow the socket to fill with blood up to the bony crest. The collagen dressing was applied in the same manner as described above.
All patients for all groups received the same post-operative instructions. Chlorhexidine mouth rinse (0.12%) was prescribed to each patient for use twice daily for 2 weeks. For pain control, use of over-the-counter NSAIDs were recommended and prescription narcotics were prescribed as needed. Patients returned after 2 weeks for suture removal and again after one month for further evaluation of healing.
2.8 |. Bone core harvest
Patients were allowed to heal for an average of 15 weeks before returning to the clinic for placement of the implant. Necessary radiographs were taken to plan appropriately for implant placement. Local anesthetic was delivered and measurements of alveolar ridge height and width were taken with the measurement stent by a masked examiner as described above. An incision was made over the edentulous crest and a minimal mucoperiosteal flap was elevated for access. A trephine‖ was used first with a 2 mm internal diameter to obtain a core sample of the bone to the measured depth of the original socket using a surgical guide fabricated from the original cast to direct the trephine into the position of the socket. Harvested bone cores were immediately placed in 10% neutral buffered formalin. The osteotomy was then widened for implant placement according to standard protocol. One- or two-stage implant protocols were used based on the clinician’s preference, and patients were followed according to standard clinical protocols to completion of the implant restoration.
2.9 |. Bone core analysis
Bone core samples obtained from the patients of all groups were fixed in 10% neutral buffered formaldehyde for a minimum of 5 days before analysis. X-ray microtomography (micro-CT) scans for all bone cores were acquired by a high-resolution micro-CT system.* Scans were performed at 4x magnification (0.5 µm/pixel), 40 kV of voltage, and 8W of power using a LE#2 filter with a resolution of 5 µm pixels, and 1,200 slices were obtained per each volumetric reconstruction. The digitally reconstructed micro-CT volumetric data were further analyzed using visualization software.† Intensity segmentation was used to digitally differentiate mineralized tissues (new bone and graft particulate) from non-mineralized tissues (connective tissue, vascular tissue) based on the differential x-ray absorbance coefficient of the two tissue types. Analysis was performed to define mineral densities of segmented regions within and across groups.
After micro-CT scans, the cores were processed for histological analysis. Cores were dehydrated and decalcified in hydrochloric acid (HCl 10%). The cores were embedded in paraffin and sectioned at 4 µm along the apical-coronal axis. Sections were stained with hematoxylin and eosin. A single representative section from the center of the core was selected for analysis. The entirety of a section from the coronal to apical extent was viewed at 40x under a light microscope and converted to a digital format at 40x, 100x and 200x for quantitative analysis. Sections were analyzed for percentage of vital bone, residual graft material, and connective tissue (CT)/other tissue using an imaging processing program.‡ Vital bone and residual graft particulate was differentiated based upon the presence of osteocytes in the lacuna. Identification of CT/other consisted of all non-mineralized tissue in the section. Percentages of specified tissues were based on the calculated total area of the tissue section.
2.10 |. Statistical analysis
A power analysis was conducted to determine the sufficient number of patients in each treatment group to obtain a power of 80% to detect a 1.0 mm change in horizontal ridge dimensions using a type 1 (alpha) error rate of 0.05. The power analysis determined that a minimum of 10 patients would be required for each group, and 40 patients in total. An alpha of 0.05 was assumed after a Bonferroni correction for five relevant 2-way comparisons between the treatment groups.
Histologic, micro-CT, and alveolar ridge measurement data were analyzed first via an ANOVA F-test. A student’s t-test was used for comparisons of between group differences. Significance was determined at P < 0.05. All statistical analysis was performed using statistical software.§
3 |. RESULTS
Forty-five patients met all inclusion criteria and were enrolled into the study. Throughout the course of the study, two patients developed medical conditions that were a contraindication to implant placement. Three other patients were dismissed from the study due to non-compliance with study protocol. Forty patients successfully completed the study, with 10 patients in each treatment group included for all analyses (Figure 1). The mean age was 58 years old, and 18 men and 22 women completed the study with no significant demographic differences between groups.
FIGURE 1.

Study flow diagram
Healing time between extraction and implant placement across all groups averaged 107 days (± 19 days). The average healing times for each treatment group, A-PRF (106 ± 16 days), A-PRF+FDBA (107 ± 25 days), FDBA (110 ± 22 days), and blood clot (105 ±14 days) were not significantly different. Adequate healing to support implant placement was observed in all four treatment groups with no adverse events reported.
3.1 |. Primary outcome: Ridge preservation
Alveolar ridge dimensions were measured immediately after extraction and immediately before implant placement. No statistical differences between A-PRF and FDBA were noted (Table 1). The treatment groups using A-PRF and A-PRF+FDBA demonstrated significantly less ridge height reduction compared to treatment with blood clot alone (P < 0.05) (Table 1). Alveolar crest width reduction was greatest at the coronal third in all groups but with no significant differences noted between groups (Table 1).
TABLE 1.
Alveolar ridge dimensional change (mm) of four ridge preservation approaches
| Variable | Blood clot | A-PRF | A-PRF+FDBA | FDBA |
|---|---|---|---|---|
| Loss of ridge height | 3.8 ± 2.0 | 1.8 ± 2.1* | 1.0 ± 2.3† | 2.2 ± 1.8 |
| Loss of ridge width (coronal) | 2.9 ± 1.7 | 2.8 ± 1.2 | 1.9 ± 1.1 | 2.5 ± 1.1 |
| Loss of ridge width (middle) | 1.8 ± 1.3 | 1.8 ± 1.8 | 1.7 ± 1.2 | 1.5 ± 1.2 |
| Loss of ridge width (apical) | 1.5 ± 1.6 | 1.8 ± 1.5 | 1.6 ± 1.5 | 1.2 ± 1.3 |
Data are expressed as the mean ± SD
P < 0.05, statistically significant difference compared to blood clot (ANOVA and student’s t-test)
P < 0.01, statistically significant difference compared to blood clot (ANOVA and student’s t-test
3.2 |. Secondary outcome: Histologic analysis
Representative histological sections from each of the four treatment groups are presented in Figure 2. Generally, treatment with A-PRF or A-PRF+FDBA resulted in the formation of denser bone trabecular structure. An absence of mature trabecular structure was noted in the FDBA group. Residual graft particulate was identified in nine out of 10 samples from the FDBA treated group and five out of 10 samples in the A-PRF+FDBA group. In the FDBA group, three of the 10 samples demonstrated more residual graft particulate than vital bone. FDBA encapsulated by CT was observed more often in the FDBA group compared to the A-PRF+FDBA group.
FIGURE 2.
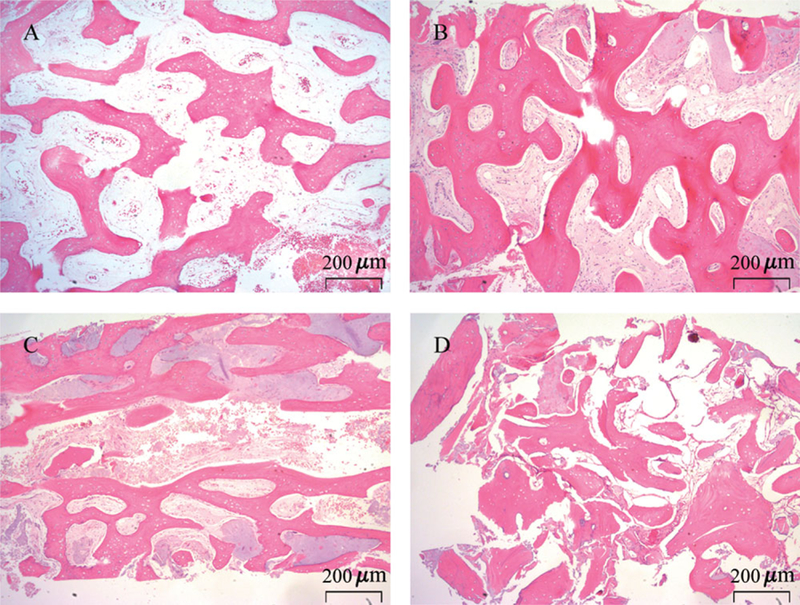
Representative hematoxylin and eosin histological sections from bone cores taken from healed extraction sites after ridge preservation using blood clot (A), A-PRF (B), A-PRF+FDBA (C), FDBA (D). Original magnification is 40x
Histomorphometric analysis was performed on one representative section from bone cores obtained from all patients (n = 10/treatment group). Percentage of vital bone, residual graft particulate, and CT/other tissue was compared across treatment groups (Figure 3). The treatment group using A-PRF demonstrated the highest percentage of vital bone (46% ± 18%) of all groups and was significantly greater than the treatment group using FDBA (P < 0.05), which demonstrated the least amount of vital bone (29% ± 14%). No other significant differences between groups were noted for percentage of vital bone or CT/other. The percentage of residual graft particulate was 11% ± 9% in the FDBA group and 3% ± 3% in the A-PRF+FDBA group.
FIGURE 3.

Histomorphometric analysis of bone cores taken from healed extraction sites after ridge preservation using blood clot, A-PRF, A-PRF+FDBA, or FDBA. Percentage of vital bone, connective tissue (CT)/other tissue, and residual graft particulate over total area was analyzed and plotted as mean ± SD. One representative histological section stained with hematoxylin and eosin from each core was analyzed at 40x. Ten cores were analyzed for each ridge preservation group. *significantly greater vital bone compared to FDBA (P < 0.05)
3.3 |. Tertiary outcome: Bone mineral density
Micro-CT analysis of bone cores from healed sockets (n = 10/treatment group) demonstrated no significant difference in bone mineral density for treatment groups using A-PRF (493 ± 70 mg/cm3) compared to A-PRF+FDBA (521 ± 58 mg/cm3) or FDBA (551 ± 58 mg/cm3). Significantly less bone mineral density was noted using blood clot alone (487 ± 64 mg/cm3) compared to FDBA alone (P < 0.05) (Figure 4).
FIGURE 4.

Bone mineral density of bone cores taken from healed extraction sites after ridge preservation using blood clot, A-PRF, A-PRF+FDBA, or FDBA. Ten cores were analyzed for each ridge preservation group and data are presented as mean± SD. *significantly greater bone mineral density compared to blood clot (P < 0.05)
4 |. DISCUSSION
This study tested the effectiveness of A-PRF as a biomaterial for ridge preservation by comparing A-PRF to FDBA, to a mixed preparation of A-PRF and FDBA, and to a natural blood clot in a randomized controlled clinical trial. Changes in ridge dimensions and quantity and quality of bone formation were evaluated across all groups, which together are able to provide a clinically relevant evaluation of the utility of the materials for ridge preservation. This study represents the first randomized controlled clinical trial to compare A-PRF to a common mineralized grafting material, FDBA, for applications in ridge preservation, and the first evaluation of a mixed application of A-PRF+FDBA for ridge preservation.
A-PRF demonstrated a desired characteristic of a biomaterial for ridge preservation by providing space maintenance. Ridge preservation using A-PRF+FDBA demonstrated the least amount of reduction of ridge height (1.0 ± 2.3 mm) and width (1.9 ± 1.1 mm) compared to all other groups. Sites treated with A-PRF alone demonstrated comparable amounts of ridge reduction to those treated with FDBA. All ridge preservation approaches outperformed the control blood clot group, but only A-PRF and A-PRF+FDBA demonstrated significantly less ridge height reduction compared to blood clot.
Other studies have also compared PRF in various ridge preservation approaches.17–19 Improved alveolar width preservation using PRF compared to a blood clot alone has been previously demonstrated18. Also comparable to the current study, Thakkar et al. reported ridge preservation with PRF mixed with demineralized freeze-dried bone allograft (DFDBA) resulted in significantly less ridge dimensional changes compared to treatment with DFDBA alone.19 Further, ridge dimensional changes using A-PRF or A-PRF+FDBA in the current study compare favorably with a systematic review of reports for ridge preservation using various mineralized bone substitutes4. That review of eight included studies reported ridge height reductions ranging from 0.5 ± 1.1 mm to 1.6 ± 0.9 mm and ridge width reductions ranging from 1.1 ± 1.1 mm to 3.48 ± 2.68 mm using various ridge preservation protocols.4 The findings in the current study on ridge dimensional changes compare favorably with the reported literature and provide further support of A-PRF or A-PRF+FDBA as a ridge preservation material.
This study additionally validated A-PRF as a desired ridge preservation biomaterial by demonstrating promotion of vital bone formation at the treated sockets. Histomorphometric analysis demonstrated that ridge preservation using A-PRF resulted in the most vital bone formation (46% ± 18%) across all four groups, and this bone formation was significantly greater than observed in the FDBA treatment group which demonstrated the least amount of vital bone formation (29% ± 14%) (P < 0.05). The addition of FDBA to A-PRF did not significantly decrease vital bone compared to A-PRF alone. The amount of vital bone found across all treatment groups was similar to findings in other studies that demonstrated vital bone formation to range from 27%−68% using mineralized allografts for ridge preservation.5,20–22 To date no previous study has evaluated vital bone formation using A-PRF mixed with mineralized graft particulate for ridge preservation in a randomized controlled clinical trial. However, in a study using an external sinus lift procedures Tatullo et al. reported an average of 1.4 times more vital bone using xenograft mixed with PRF compared to xenograft alone.23
A-PRF performed similarly to FDBA in providing space maintenance but significantly outperformed FDBA in the formation of vital bone. Without a mineralized graft material, the space maintenance characteristics provided by A-PRF are likely due to the dense fibrin matrix that forms during the processing protocol.10 A denser fibrin matrix may resorb more slowly and thereby provide the scaffolding required to maintain dimensions of the ridge as new tissues form and mature. The presence of mineralized graft material may have contributed to the decreased vital bone formation at sites treated with FDBA. Residual graft particulate was found at 9 of the 10 sites treated with FDBA. The presence of residual graft particulate in healed ridge preserved sites has previously been reported at the time of implant placement.2,4,24,25 A slowly resorbing graft material can be beneficial by providing good space maintenance throughout the entire time course of healing. However, proper healing requires neovascularization and ingrowth of new tissues into the wound space that the graft material occupies. It is possible that the addition of graft material such as FDBA may impede osteogenesis by delaying healing or by requiring additional time for resorption. Additionally, increased vital bone formation may also be due to the intrinsic and concentrated growth factors present within A-PRF.11,15,16 The growth factors are bound within the fibrin matrix, and during reorganization of the wound growth factors are released from the fibrin.14 This ensures a concentrated and sustained release of growth factors at the appropriate time during healing to promote bone formation.
Bone mineral density was measured in this study by micro-CT. Bone mineral density in this context provides a measure of the extent of mineralization of the bone. As new bone becomes more mineralized as it matures, a higher bone mineral density represents more mature bone. In this study bone mineral density was similar at sites treated with A-PRF compared to sites treated with FDBA alone. It should be noted that FDBA has a high mineral density by itself, thus residual FDBA particulate at the healing site would elevate the bone mineral density measure. Therefore, a comparable bone mineral density measure between A-PRF and FDBA reflects the advanced healing and maturity of vital bone from A-PRF treated sites. A study of alveolar bone in cadavers demonstrated mature non-grafted bone had bone mineral densities above 800 mg/cm3, while in this study the healed grafted bone was below 600 mg/cm3 across all treatment groups.26
Other studies have utilized micro-CT to evaluate bone formation at grafted sites.27–29 It would have been possible to use micro-CT to evaluate percentage of vital bone throughout the entire volume of the bone core in this current study. However, at the digital resolution that the samples were scanned, vital bone and residual graft particulate could not be accurately differentiated digitally. The histological analysis employed in this study could more accurately differentiate vital bone from residual graft particulate. Therefore, the histological method was chosen over micro-CT to report percentage of vital bone, as distinguishing between vital and residual graft particulate is an important criterion for evaluating the efficacy of a biomaterial in grafting approaches.
Differences in the surgical protocol of this study may have resulted in findings that differed from other ridge preservation and PRF studies. It was surprising to observe no significant differences in change in ridge width at the coronal third across all treatment groups. This may have been a result of using a resorbable collagen dressing without the same occlusive characteristics of other membranes popularly used for ridge preservation. The resorbable collagen dressing and cyanoacrylate were used in this study to provide initial stability for the wound and have been reported to be used in other ridge preservation procedures.5,28 By using the resorbable collagen dressing, any benefit in new bone formation could be attributed to the graft material itself. This was important as one of the potential benefits of A-PRF is the dense fibrin structure which may have some cell occlusive properties itself. However, the lack of a membrane may have resulted in increased dimensional changes, especially at the coronal third. Secondarily, preparation of PRF used in this study followed the A-PRF protocol. The A-PRF protocol uses lower G-forces which has been shown to allow for greater release of growth factors and leukocytes from the clots in vitro.15,16 Multiple PRF preparation protocols have been reported throughout the literature.10,15,16,19 However, it is unknown how differences in reported in vitro characteristics of PRF correlate to meaningful clinical findings.
5 |. CONCLUSIONS
Findings from this randomized controlled clinical trial have demonstrated A-PRF to be a suitable biomaterial for ridge preservation. The use of A-PRF produced significantly more vital bone compared to FDBA, while also preserving ridge dimensions similarly to FDBA and better than blood clot alone. Modest improvements in ridge dimensional changes were demonstrated when using A-PRF+FDBA without a significant decrease in vital bone formation. These findings demonstrate the regenerative potential of A-PRF in a healing extraction site and suggest wider applications of A-PRF outside of ridge preservation. Future studies should extrapolate the osteogenic potential of A-PRF in more extensive ridge augmentation procedures and investigate further regenerative capacities in periodontal regeneration.
ACKNOWLEDGMENTS
This study was supported in part by a grant from Dentis, USA (La Palma, CA). Drs. Clark, Rajendran, Paydar, and Dollard have received travel reimbursement from Dentis, USA.
Footnotes
Triad, Dentsply, York, PA.
UNC-15, G. Hartzell and Son, Concord, CA.
Ridge Mapping Calipers, Salvin Dental Specialties, Charlotte, NC.
Peridex, 3M, Minneapolis, MN.
Plain Vacuum Tube, Process for PRF, Nice, France.
Collaplug, Zimmer Dental, Warsaw, IN.
Ace Surgical Supply, Brockton, MA.
PeriAcryl90, GluStitch, Delta, Canada
AlloOss, Ace Surgical Supply, Brockton, MA.
Ace Surgical Supply, Brockton, MA
MicroXCT-200, Carl Zeiss, Oberkochen, Germany.
Avizo 3D, LEI, Hillsboro, OR
Image J, National Institutes of Health, Bethesda, MD.
JMP, SAS, Cary, NC.
All other authors report no conflicts of interest.
REFERENCES
- 1.Esposito M, Maghaireh H, Grusovin MG, Ziounas I, Worthington HV. Interventions for replacing missing teeth: management of soft tissues for dental implants. Cochrane Database Syst Rev 2012;15:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arunyanak SP, Pollini A, Ntounis A, Morton D. Clinician assessments and patient perspectives of single-tooth implant restorations in the esthetic zone of the maxilla: a systematic review. J Prosthet Dent 2017;118:10–17. [DOI] [PubMed] [Google Scholar]
- 3.Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol 2003;74:990–999. [DOI] [PubMed] [Google Scholar]
- 4.Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res 2014;93:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck TM, Mealey BL. Histologic analysis of healing after tooth extraction with ridge preservation using mineralized human bone allograft. J Periodontol 2010;81:1765–1772. [DOI] [PubMed] [Google Scholar]
- 6.Susin C, Wikesjö UM. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol 2000 2013;62:232–242. [DOI] [PubMed] [Google Scholar]
- 7.Suárez-López Del Amo F, Monje A, Padial-Molina M, Tang Z, Wang HL. Biologic Agents for Periodontal Regeneration and Implant Site Development. Biomed Res Int 2015;2015: 957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padial-Molina M, Rios HF. Stem cells, scaffolds and gene therapy for periodontal engineering. Curr Oral Health Rep 2014;1:16–25. [Google Scholar]
- 9.Choukroun J, Adda F, Schoeffler C, Vervelle A. An opportunity in perio-implantology: the PRF. Implantodontie 2001;42:55–62. (French). [Google Scholar]
- 10.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:37–44. [DOI] [PubMed] [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:45–50. [DOI] [PubMed] [Google Scholar]
- 12.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:51–55. [DOI] [PubMed] [Google Scholar]
- 13.Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009;27:63–69. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001;936:355–367. [DOI] [PubMed] [Google Scholar]
- 15.Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 2014;40:679–689. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: growth Factor Release, Biocompatibility, and Cellular Response. J Periodontol 2017;88:112–121. [DOI] [PubMed] [Google Scholar]
- 17.Castro AB, Meschi N, Temmerman A, et al. Regenerative potential of leucocyte and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J Clin Periodontol 2017;44:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, Ammann P. Clinical and histological evaluation of postextraction plateletrich fibrin socket filling: a prospective randomized controlled study. Implant Dent 2013;22:295–303. [DOI] [PubMed] [Google Scholar]
- 19.Thakkar DJ, Deshpande NC, Dave DH, Narayankar SD. A comparative evaluation of extraction socket preservation with demineralized freeze-dried bone allograft alone and along with platelet-rich fibrin: a clinical and radiographic study. Contemp Clin Dent 2016;7:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotek PD, Neiva RF, Wang HL. Comparison of dermal matrix and polytetrafluoroethylene membrane for socket bone augmentation: a clinical and histologic study. J Periodontol 2009;80:776–785. [DOI] [PubMed] [Google Scholar]
- 21.Wang HL, Tsao YP. Histologic evaluation of socket augmentation with mineralized human allograft. Int J Periodontics Restorative Dent 2008;28:231–237. [PubMed] [Google Scholar]
- 22.Trombelli L, Farina R, Marzola A, Bozzi L, Liljenberg B, Lindhe J. Modeling and remodeling of human extraction sockets. J Clin Periodontol 2008;35:630–639. [DOI] [PubMed] [Google Scholar]
- 23.Tatullo M, Marrelli M, Cassetta M, et al. Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 2012;9:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol 1998;69:414–421. [DOI] [PubMed] [Google Scholar]
- 25.Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-OssA. Clin Oral Impl Res 2003;14:137–143. [DOI] [PubMed] [Google Scholar]
- 26.Blok Y, Gravesteijn FA, van Ruijven LJ, Koolstra JH. Microarchitecture and mineralization of the human alveolar bone obtained with microCT. Arch Oral Bio 2013;58:621–627. [DOI] [PubMed] [Google Scholar]
- 27.Temmerman A, Vandessel J, Castro A, et al. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol 2016;43:990–999. [DOI] [PubMed] [Google Scholar]
- 28.Brownfield LA, Weltman RL. Ridge preservation with or without an osteoinductive allograft: a clinical, radiographic, microcomputed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J Periodontol 2012;83:581–589. [DOI] [PubMed] [Google Scholar]
- 29.Goh BT, Teh LY, Tan DBP, Zhang Z, Teoh SH. Novel 3D polycaprolactone scaffold for ridge preservation – a pilot randomised controlled clinical trial. Clin Oral Impl Res 2015;26:271–277. [DOI] [PubMed] [Google Scholar]


